Abstract
Candida albicans is a commensal colonizer of the gastrointestinal tract of humans, where it coexists with highly diverse bacterial communities. It is not clear whether this interaction limits or promotes the potential of C. albicans to become an opportunistic pathogen. Here we investigate the interaction between C. albicans and three species of streptococci from the viridans group, which are ubiquitous and abundant oral commensal bacteria. The ability of C. albicans to form biofilms with Streptococcus oralis, Streptococcus sanguinis, or Streptococcus gordonii was investigated using flow cell devices that allow abiotic biofilm formation under salivary flow. In addition, we designed a novel flow cell system that allows mucosal biofilm formation under conditions that mimic the environment in the oral and esophageal mucosae. It was observed that C. albicans and streptococci formed a synergistic partnership where C. albicans promoted the ability of streptococci to form biofilms on abiotic surfaces or on the surface of an oral mucosa analogue. The increased ability of streptococci to form biofilms in the presence of C. albicans could not be explained by a growth-stimulatory effect since the streptococci were unaffected in their growth in planktonic coculture with C. albicans. Conversely, the presence of streptococci increased the ability of C. albicans to invade organotypic models of the oral and esophageal mucosae under conditions of salivary flow. Moreover, characterization of mucosal invasion by the biofilm microorganisms suggested that the esophageal mucosa is more permissive to invasion than the oral mucosa. In summary, C. albicans and commensal oral streptococci display a synergistic interaction with implications for the pathogenic potential of C. albicans in the upper gastrointestinal tract.
INTRODUCTION
Candida albicans is a pleomorphic fungus that colonizes the gastrointestinal (GI) and genitourinary mucosal surfaces of humans, persisting in these niches as a commensal in up to 60% of healthy individuals (5, 50). Alterations in host immunity, bacterial flora or local environmental factors, such as oral salivary flow, are believed to determine C. albicans transition from a commensal to an opportunistic pathogen, capable of causing a wide range of superficial mucosal or life-threatening systemic infections (10, 36, 44). At mucosal environments, C. albicans coexists with a highly diverse bacterial flora. For instance, in the human oral cavity, more than 700 different bacterial species have been described to exist, with a single individual harboring several hundreds of bacterial phylotypes (15). Resident oral microorganisms colonize hard surfaces such as teeth and prosthetic devices forming complex polymicrobial biofilm structures (42, 65). In health, mucosal biofilm growth in the oral cavity is limited by a rapid epithelial turnover and host innate immune defenses at the mucosal interface (18). However, immunosuppression allows the formation of mucosal biofilms, leading to the clinical appearance of thrush (14). Our group has shown that these biofilms are not only comprised by a dense network of Candida cells but also by commensal oral bacteria in close association with Candida (20). Thus, the interaction of C. albicans with commensal resident bacteria is bound to be an important determinant of C. albicans colonization and persistence at mucosal niches and is likely to modulate its virulence at these sites.
Oral streptococci from the viridans group are the most ubiquitous and abundant primary colonizers of oral surfaces (1, 16, 38). These streptococci are considered commensal organisms of limited virulence and are generally associated with oral health. However, viridans streptococci can cause life-threatening systemic infections if the oral mucosa is disrupted and the host defense mechanisms are compromised (28, 34). C. albicans has the ability to coaggregate with a variety of oral bacteria, including most species from the viridans group of streptococci (26, 30, 33). Physically associated cells of C. albicans and streptococci have been demonstrated in vivo, in tooth-associated biofilms, via fluorescence in situ hybridization (FISH), with streptococcal cells forming “corn-cob-like” structures around C. albicans hyphae (65). The mechanisms mediating coaggregation between C. albicans and oral streptococci, specifically Streptococcus gordonii, have been characterized as adhesin-receptor interactions. The adhesins on the streptococcal surface have been identified as the cell surface-associated, antigen I/II family adhesins, SspA and SspB (4, 31), while the hyphal wall protein Als3p has been shown to serve as receptor for the streptococcal adhesin SspB (52). Furthermore, C. albicans has been demonstrated to form mixed-species biofilms with S. gordonii on plastic wells under static conditions, and contact with S. gordonii has been shown to enhance C. albicans filamentation (4).
Based on the known in vitro and in vivo interactions between C. albicans and oral streptococci, it is likely that the two organisms form an interkingdom partnership that promotes mucosal colonization or infection. Since hyphal formation is promoted by streptococci, while it is also a prerequisite for tissue invasion (35, 51), it is possible that contact of C. albicans and streptococci may alter the invasive phenotype of the former. The goal of our work was to characterize the role of the interaction between C. albicans and streptococci in the pathogenesis of mucosal infection using biologically relevant in vitro model systems. The interaction of C. albicans and streptococci as abiotic surface biofilms was studied in standard flow cell devices under salivary flow. However, since no system existed that allowed the study of mucosal biofilms under flow conditions, we designed a novel flow cell device able to harbor an organotypic mucosal tissue analogue, where microorganisms can form biofilms using saliva-supplemented medium as nutrient source, simulating environmental conditions in the upper GI tract. Since the type of mucosal epithelium that lines the alimentary tract mucosa may serve as an ecological determinant of invasive infection, we tested Candida-streptococci interactions in two organotypic mucosal models, one of the oral, and one of the esophageal mucosa. We used these systems to test the hypothesis that C. albicans and streptococci form a synergistic partnership when forming mixed-species biofilms under conditions similar to their in vivo niche.
MATERIALS AND METHODS
Microorganisms used and microbiological media.
The microorganisms used in the present study were Candida albicans SC5314, Streptococcus oralis 34 (kindly provided by P. E. Kolenbrander), Streptococcus gordonii Challis CH1 (kindly provided by J. M. Tanzer), and Streptococcus sanguinis SK36 (ATCC BAA-1455). C. albicans was routinely maintained in yeast extract-peptone-dextrose (YPD) agar and grown in YPD medium, aerobically, at room temperature, in a rotor shaker. YPD medium consisted of 5 g of yeast extract (Fisher Scientific, Pittsburgh, PA) liter−1, 10 g of peptone (Fisher Scientific) liter−1, and 20 g dextrose (Fisher Scientific) liter−1. Streptococci were routinely grown in brain heart infusion (BHI) medium (Oxoid, Ltd., Cambridge, United Kingdom) under aerobic static conditions at 37°C. In some experiments, Fusobacterium nucleatum strain ATCC 10953 was used as a positive control for induction of apoptosis in the oral epithelial three-dimensional (3-D) system (17).
Saliva, used to supplement the biofilm growth medium, was collected from 10 systemically healthy volunteers according to a protocol approved by the Institutional Review Board of the University of Connecticut Health Center (IRB 10-216-2). Briefly, whole stimulated saliva was collected in polypropylene tubes on ice, pooled, and treated with 2.5 mM dithiothreitol (Sigma-Aldrich, St. Louis, MO) for 10 min to reduce salivary protein aggregation. The saliva was then centrifuged at 7,500 × g, at 4°C, for 20 min, and supernatants were diluted with Dulbecco phosphate-buffered saline (D-PBS; Mediatech, Inc., Manassas, VA) to obtain a 25% (vol/vol) saliva/D-PBS solution. Diluted saliva was then filtered through a 0.22-μm-pore-size polyethersulfone low-protein-binding filter (Nalgene; Thermo Fisher Scientific, Rochester, NY), divided into aliquots, and frozen at −80°C until further use.
Abiotic surface biofilms of C. albicans and streptococci.
Biofilms of streptococci and C. albicans, either as monospecies or as mixed species were allowed to develop on glass surfaces under flow using saliva-supplemented medium (22.5% sterile human saliva, 10% BHI, 67.5% [vol/vol] D-PBS) as nutritional source. Standard flow cell chambers for abiotic biofilms were constructed according to the method of Palmer (45) and fabricated by the machine shop at The School of Engineering, University of Connecticut. Each flow cell track (40 mm long, 3 mm wide, and 2 mm deep) was milled into a high-density polytetrafluoroethylene block (MSC Industrial Direct, Inc., Melville, NY). A 24-by-60-mm glass coverslip, secured to the top of the flow cell with a silicone adhesive, served as attachment site for the growing biofilm. Prior to each experiment, the flow cells were cleaned overnight with 0.1 M HCl and rinsed with sterile distilled water. Flow cells were sterilized by flowing 10% hypochlorite for 2 h using a peristaltic pump, followed by continuous rinsing with sterile distilled water for an additional 2 h. Flow cells were then placed at 37°C and treated with saliva-supplemented medium for 15 min to allow formation of a salivary pellicle on the glass surface. In order to prepare the inoculum, overnight stationary-phase cultures of each organism were used to inoculate new cultures that were allowed to grow until the late logarithmic phase. The cultures were normalized to an optical density at 600 nm of 1, and the microbial cells were washed in salivary growth medium. The inoculum for each flow cell consisted of 106 cells of C. albicans or 107 cells of either S. oralis, S. sanguinis, or S. gordonii or a combination of C. albicans and each type of streptococcus. After injecting the inoculum, the flow cells were inverted, and the microorganisms were allowed to attach for 30 min under static conditions. Flow cells were then placed upright, and the pump started with a flow rate for all experiments of 100 μl min−1 to approximate in vivo oral salivary flow (13). Biofilms were allowed to develop for 0, 4, or 16 h. For some experiments, PBS was flowed through the flow cells instead of salivary medium to evaluate the retention of streptococci in biofilms in the absence of nutrients.
The effect of C. albicans on streptococcal accretion was tested, in a closed system, in the absence of nutrients. For these experiments, 107 cells of S. oralis were suspended in PBS and added alone or in combination with 106 cells of C. albicans on saliva-conditioned glass coverslips placed on multiwell plates. Plates were incubated with 125 rpm agitation at 37°C for 0.5, 1.5, and 3 h, after which the cells were washed, stained, and quantified using microscopy. In some experiments C. albicans, suspended in salivary medium, was allowed to attach to saliva-conditioned coverslips for 3 h, followed by a PBS rinse and the addition of S. oralis.
Fabrication and assembly of tissue-harboring flow cells.
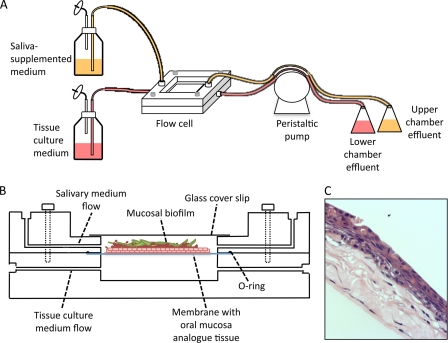
In order to grow mucosal biofilms under an environment that resembles the upper GI tract, we designed a flow cell system able to harbor a mucosal tissue analogue, pregrown in a porous membrane, with saliva flowing over the tissue and bathing its apical surface and mammalian cell culture medium flowing underneath and feeding the tissue basally (Fig. 1). The flow cell apparatus consists of two discrete pieces that can be assembled to form two flow chambers separated by a membrane. For convenient visualization, a window was built into the upper flow cell chamber. This window is sealed by attaching a 22-by-22-mm glass coverslip to the outside flow cell surface. The flow cell is assembled with an O-ring placed between the membrane and the upper component in order to seal the system. The upper and lower components are held in place by screws. Flow cells with these characteristics were fabricated according to our specifications by Sirois Tool Company, Inc. (Berlin, CT). The body of the upper and lower flow cell components was milled from a block of polytetrafluoroethylene (MSC Industrial Direct, Inc.). The connectors used between the components and the tubing were 0.0625-in. hose barb connectors with a #10-32 threaded port made of Kynar polyvinylidene fluoride (Small Parts, Inc.). The flow cell apparatus was assembled as illustrated in Fig. 1 by connecting two medium reservoirs to the upper and lower components of the flow cell via silicone manifold tubing (Watson-Marlow, Inc., Wilmington, MA). One medium reservoir was filled with saliva-supplemented medium, which flowed through the upper chamber. The second medium reservoir consisted of a 3:1 (vol/vol) mixture of Dulbecco's modified Eagle's medium containing 4.5 g of glucose liter−1, 0.58 g of l-glutamine liter−1, and 0.11 g of sodium pyruvate liter−1 and Ham's F-12 medium containing 0.14 g of l-glutamine liter−1 (Mediatech, Inc.). HEPES buffer to a final concentration of 15 mM was also added to this medium, which flowed through the lower chamber. A peristaltic pump model 205S/CA (Watson-Marlow Inc., Wilmington, MA) was connected downstream of the flow cells to establish a flow rate of 100 μl min−1.
Fig 1.
Schematic representation of flow cell device that supports mucosal biofilm formation in the presence of salivary flow. (A) Components of the assembled flow cell system. (B) Cross-section view of the flow cell device, which consists of two pieces of polytetrafluoroethylene juxtaposed to each other and held together by screws. A membrane containing a pregrown oral mucosa analogue tissue is placed in the middle, supported by an o-ring, forming two separate chambers for independent saliva-supplemented medium and tissue culture medium flow. (C) Example of an H&E-stained section of an oral mucosa analogue tissue over which microorganisms are inoculated to form mucosal biofilms in the device.
Oral and esophageal mucosa analogue tissues.
A detailed protocol that describes the procedures used to culture the oral mucosa analogue has been previously published (19). Briefly, the system consists of an immortalized human oral keratinocyte cell line (OKF6/TERT-2) seeded on collagen type I-embedded fibroblasts (3T3 fibroblasts). The tissues, grown in transwell inserts, are then airlifted to ensure epithelial differentiation and stratification. The procedure takes approximately 2 to 3 weeks to complete. The human cell line EPC2 (kindly provided by A. K. Rustgi, University of Pennsylvania), established from a biopsy specimen from a healthy esophageal mucosa and immortalized similarly to the OKF6 cells, was used to construct an esophageal mucosa analogue, according to previously published protocols (27). The 3-D system derived from this cell line has striking similarities with the esophageal mucosal tissue morphology in vivo (3, 27). Both mucosal 3-D systems represent healthy, nonkeratinizing stratified squamous epithelia (19, 27). After tissue maturation, membranes containing 3-D mucosa analogues were cut from transwell rings and used for flow cell biofilm experiments.
Mucosal biofilm formation by C. albicans and S. oralis in tissue-harboring flow cells.
Prior to each experiment, the flow cells were sterilized as described above for standard flow cells. The flow cells were connected to the media reservoirs and salivary and mammalian cell culture medium were pumped through the chambers. A piece of polyethylene terephthalate (PET) membrane was placed between the two chambers for the initial flow of media. A membrane containing a pregrown tissue analogue was then placed between the two chambers to replace the PET membrane. The flow was allowed to proceed for 15 min to condition the mucosal surface for the attachment of microorganisms. Tissue-harboring flow cell inocula were prepared as in standard flow cells and consisted of a similar inoculum size, i.e., 106 cells of C. albicans or 107 cells of S. oralis or a combination of both organisms in 500 μl of salivary medium. Microorganisms were inoculated into the upper flow cell chamber and left to attach to the mucosal surface for 30 min under static conditions. Flow was then reestablished at 100 μl min−1. Biofilms were allowed to form for 4, 16, or 24 h at 37°C, after which flow cells were disassembled and the tissue was removed and used for further analysis. A noninfected tissue, placed in the running flow cell under the same flow conditions, was used as a control to evaluate tissue viability under salivary flow.
Staining, imaging, and quantification of abiotic and mucosal biofilms via confocal microscopy.
Abiotic surface and mucosal surface biofilms were fixed in 4% paraformaldehyde for 1 h. C. albicans was visualized after staining for 1.5 h, at room temperature, with a fluorescein isothiocyanate (FITC)-labeled anti-Candida polyclonal antibody (Meridian Life Science, Saco, ME). For biofilms containing streptococci, this was followed by FISH with the streptococcus-specific oligonucleotide probe STR405 (54), labeled with Alexa 546, as previously described (20). Biofilms were visualized with a Zeiss LSM 510 confocal scanning laser microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY) equipped with an argon (488- and 543-nm) laser, using a water immersion C-Apochromat ×40 objective (NA1.2). Stacks of z-plane images from at least eight different fields of view per sample were acquired and later reconstructed into 3-D images using IMARIS software (Bitplane, Inc., Saint Paul, MN). Surface reconstructions using the surpass mode were used to calculate the biovolume (in μm3) of each microorganism.
Quantification of tissue invasion by biofilm microorganisms.
Tissue invasion was evaluated in 5-μm-thick paraformaldehyde-fixed, paraffin-embedded tissue sections. Prior to staining, the tissues were deparaffinized and rehydrated in a series of ethanol washes. Immunofluorescence for C. albicans and FISH for S. oralis were then performed as described above for intact biofilms. Sections were counterstained with the nucleic acid stain Hoechst 33258 (Invitrogen, Carlsbad, CA). Fluorescence images were acquired using a Zeiss Axio Imager M1 microscope and an EC Plan-Neofluar ×20 NA 0.5 air objective. Phase-contrast images were also acquired in order to facilitate visualization of the epithelial compartment. Images were quantified using ImageJ (49). Invasion was defined as presence of microorganisms below the epithelial apical margin. The percentage of invasion was determined by measuring the area (in μm2) in which a specific microorganism appeared below the epithelial surface layer and dividing by the total area (above and below the epithelial surface) occupied by the microorganism in the image. At least eight different fields of view were quantified per sample.
Evaluation of mucosal tissue apoptosis under flow.
Mucosal cell apoptosis was evaluated at 4, 16, and 24 h by immunofluorescence staining for active caspase-3 (Abcam, Cambridge, MA), an early apoptosis marker. Briefly, deparaffinized and rehydrated sections were subjected to antigen retrieval by immersion in a solution of 10 mM citric acid and 0.05% Tween 20 (pH 6.0) at 95°C for 20 min, followed by blocking for 30 min with 4% normal donkey serum (Sigma-Aldrich). Sections were then incubated for 1.5 h at room temperature with a rabbit polyclonal antibody to active caspase-3 and for 1 h with a goat anti-rabbit secondary antibody labeled with Alexa 546 (Abcam). Sections were then counterstained with Hoechst 33258 (Invitrogen). Fluorescence images were acquired using a Zeiss Axio Imager M1 microscope and an EC Plan-Neofluar ×20 NA 0.5 air objective. The images were manually quantified. As a positive control for the induction of apoptosis, we inoculated the oral mucosal model with F. nucleatum ATCC 10953, followed by incubation under static conditions in an anaerobic chamber for 16 h, since these conditions are known to trigger high levels of apoptotic cell death in 3-D tissue constructs (2, 17). The results were expressed as the percentage of caspase-3-positive epithelial cells from the total number of epithelial nuclei quantified in the same image.
Quantification of C. albicans and S. oralis planktonic growth as monocultures and in coculture.
Saliva-supplemented medium (22.5% saliva, 30% BHI in D-PBS) or 30% BHI (in D-PBS) was inoculated with 105 cells of C. albicans or 106 cells of each streptococcus or a combination of C. albicans and streptococci and then incubated at 37°C under static conditions. Growth was evaluated by sampling liquid broths, starting 30 min after inoculation and from then on every ∼1.5 h, followed by a 10-s sonication at 15% amplitude (in a Branson sonicator model 4C15), demonstrated to disrupt aggregates without affecting viability. Microbial cultures were then serially diluted and plated in YPD agar incubated at room temperature (for C. albicans quantification) or in BHI agar supplemented with 1 mg of amphotericin B liter−1 and incubated at 37°C (for streptococcal quantification).
Quantification of C. albicans biomass via real-time RT-PCR.
Quantification of C. albicans cells in mucosal biofilms was performed via a protocol developed by our group based on the absolute quantification of the translation elongation factor EF-1β (EFB1) (63). RNA was isolated from mucosal biofilms by a modification of the protocol described by Park et al. (47) to enrich for C. albicans RNA. Briefly, biofilm-containing tissues were scraped off the membrane and homogenized in lysis buffer (4 M guanidine thiocyanate, 25 mM sodium citrate, 0.5% Sarkosyl N-lauroyl-sarcosine, 0.1 M β-mercaptoethanol) by repeated passage through a 20.5-gauge needle. These conditions effectively lysed mammalian cells but not C. albicans. RNA was then isolated from pelleted microorganisms using the RiboPure yeast kit (Applied Biosystems/Ambion, Austin, TX), followed by real-time reverse transcription-PCR (RT-PCR) starting with equal volumes (10 μl) of RNA from each sample. Standard curves constructed with a linearized plasmid containing the target sequence (pEFB) were used to determine EFB1 transcript number. Detailed methods and assay validation have been previously published (63).
RESULTS
C. albicans enhances the ability of oral streptococci to form abiotic surface biofilms but does not affect planktonic growth.
We first tested the ability of C. albicans to form biofilms on abiotic surfaces with three species of Streptococcus commonly found in the oral cavity. We utilized a saliva-supplemented flow cell system, which mimics oral salivary flow conditions and allows in situ visualization of biofilms grown on a glass surface (Fig. 2). We observed that C. albicans and the three species of streptococci tested were able to form mixed-species biofilms, with streptococci benefiting from this interaction by displaying increased biofilm mass in the presence of C. albicans.
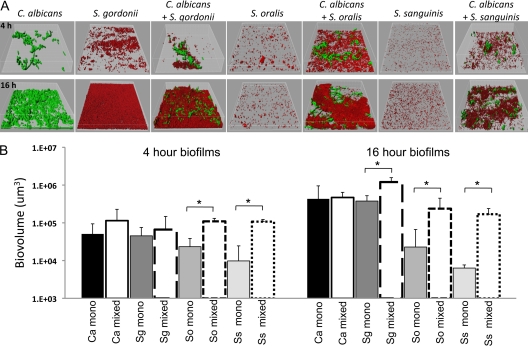
Fig 2.
Four- and sixteen-hour abiotic surface biofilms of C. albicans and commensal oral streptococci grown as monospecies or in C. albicans-streptococci mixed-species biofilms. Biofilms were grown in standard flow cells using saliva-supplemented medium as nutritional source. (A) 3-D reconstructions of representative confocal laser scanning microscopy (CLSM) images of 4-h (top) and 16-h (bottom) biofilms. C. albicans (green) was visualized after staining with a FITC-conjugated anti-Candida antibody. S. gordonii, S. oralis, and S. sanguinis (red) were visualized after fluorescence in situ hybridization (FISH) with a Streptococcus-specific probe conjugated to Alexa 546. (B) Average biovolumes (in μm3) for each species as measured in eight different CLSM image stacks from two independent experiments. C. albicans mixed-species biovolumes represent the average biovolumes of C. albicans when grown with each Streptococcus sp. since biovolumes of C. albicans did not differ among biofilms with the three streptococci tested. Columns: Ca, C. albicans; Sg, S. gordonii; So, S. oralis; Ss, S. sanguinis. *, P < 0.05 when monospecies biovolumes were compared to mixed-species biovolumes using the t test.
The monospecies biofilm forming ability and biofilm growth capacity of the three species of streptococci tested varied and was consistent with existing reports in the literature (46). For example, S. gordonii was able to grow as a monospecies biofilm from 4 h to 16 h (P < 0.001 for 4-h versus 16-h biovolumes). S. gordonii was also able to grow from 4 h to 16 h in the presence of C. albicans (P < 0.001 for 4-h versus 16-h biovolumes), reaching greater biovolumes at 16 h, in mixed-species biofilms than as a monospecies. This result suggested that C. albicans increased the ability of S. gordonii to form biofilms by increasing growth and/or accretion. In contrast, S. oralis and S. sanguinis did not show statistically significant biofilm growth from 4 to 16 h, in both monospecies and dual-species biofilms (Fig. 2). However, compared to monospecies, biofilms of C. albicans and S. oralis or of C. albicans and S. sanguinis showed a dramatic increase in the streptococcal biofilm biomass, an effect evident at both time points evaluated.
To explore the mechanism of Candida-triggered streptococcal biomass increase, we first compared the interactions of S. oralis with C. albicans in saliva-supplemented growth medium and nutrient-free medium (PBS) under conditions of flow. As demonstrated in Fig. 3A and B, in the presence of nutrients, C. albicans increased the initial attachment of S. oralis (0 h). This increase appeared to be a direct consequence of coaggregation, as demonstrated by the close physical association between the two organisms. Furthermore, under these conditions, S. oralis was able to grow as a biofilm from 0 to 4 h, in the presence or absence of C. albicans (P < 0.001), while no further growth occurred from 4 to 16 h regardless of C. albicans presence. These results suggested that C. albicans allows streptococci to accumulate in biofilms mainly by retaining streptococcal cells in the biofilm via direct physical interaction. When all nutrients were withdrawn from the influent medium (Fig. 3A and B, PBS), no growth of either S. oralis or C. albicans/S. oralis biofilms was observed; however, C. albicans was able to maintain its initial biomass, even at 16 h, and its presence allowed S. oralis to be retained in biofilms, as opposed to monospecies S. oralis biofilms which detached by 16 h. This confirmed that physical interaction with C. albicans is important for S. oralis biofilm retention under conditions of flow.
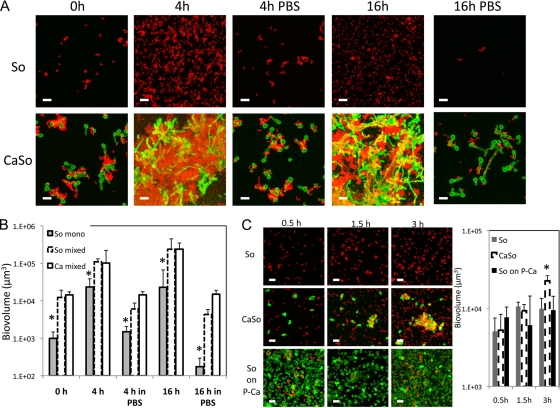
Fig 3.
Time course of abiotic surface biofilm formation by S. oralis (red) as monospecies or in the presence of C. albicans (green) and in the presence or absence of nutrients. In panels A and B, microorganisms were allowed to attach to flow cell surfaces in the presence of saliva for 30 min, after which (0 h) saliva supplemented-medium or PBS were flown through the flow cells for either 4 or 16 h. (A) Representative 3-D image projections from biofilms grown with salivary medium (4 h and 16 h) or with PBS flown through the flow cells (4 h in PBS, 16 h in PBS). (B) Biovolume measurements (in μm3) from all conditions shown in panel A. (C) Attachment of S. oralis to saliva-coated glass surfaces in a closed system, under agitation and in the absence of nutrients, in the presence or absence of Candida. “So” represents a condition in which S. oralis as a monospecies was added. “CaSo” represents a condition in which C. albicans and S. oralis were added. “So on P-Ca” represents a condition in which S. oralis was added to preattached Candida. Bars, 10 μm. *, P < 0.05 when monospecies biovolumes of S. oralis were compared to mixed-species biovolumes at the same time point using the t test.
Next, accretion of streptococci on a saliva-conditioned glass substratum in the presence or absence of C. albicans was evaluated, in PBS, in a closed system under constant agitation. As shown in Fig. 3C, when C. albicans was mixed with S. oralis in the planktonic state, it did not increase the ability of S. oralis to attach to glass surfaces after 30 min. This is in contrast to the data shown in Fig. 3A and B (at time point 0 h), where saliva was present in the system. However, after 3 h, C. albicans increased the attachment of S. oralis, forming communities where a close physical interaction was evident. Interestingly, preattached C. albicans did not affect the attachment of planktonic S. oralis at any of the time points evaluated, possibly because the metabolic state of the preadhered organisms did not promote adhesin-ligand interactions that might lead to “tightly knit” communities. These results strengthen the notion that physical interaction between C. albicans and streptococci in the planktonic state is important for subsequent increased streptococcal biofilm formation. These findings also demonstrate that although saliva facilitates the initial interaction, it is not absolutely necessary for biofilm formation. These experiments do not rule out, however, that in the presence of nutrients, C. albicans stimulates streptococcal growth or that another type of interaction, metabolic in nature, may be taking place in the microenvironment of the two closely associated organisms.
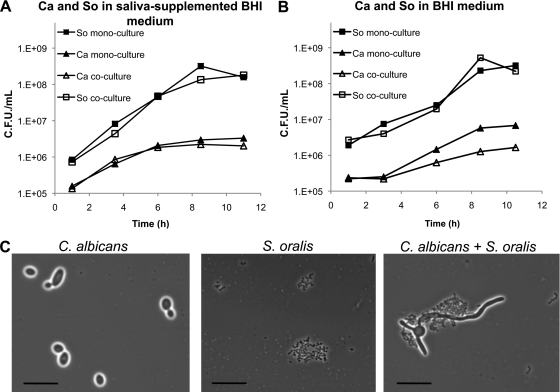
To further explore the effect of C. albicans on streptococcal growth, we quantified microbial growth in single- and mixed-species planktonic cultures inoculated at a similar ratio as in flow cell experiments (C. albicans/S. oralis 1:10). As seen in Fig. 4, the presence of C. albicans did not affect the planktonic growth of S. oralis either in the presence or absence of saliva, despite microscopic observations of planktonic coaggregation between the two organisms (Fig. 4C, similar results observed with S. gordonii and S. sanguinis [data not shown]). These results further suggested that the beneficial influence of C. albicans on S. oralis in biofilms is not a consequence of growth stimulation. These planktonic experiments also showed that the yield of C. albicans was lower in the presence of S. oralis, in the absence of saliva (Fig. 4B). Such inhibition of late planktonic growth was presumably due to nutrient exhaustion since further experiments showed that spent media from streptococcal cultures did not inhibit C. albicans planktonic growth (data not shown). Finally, some differences were noted in the growth curves of monocultures of C. albicans, which did not exhibit a lag phase in the presence of saliva (Fig. 4A and B).
Fig 4.
(A and B) Planktonic growth of C. albicans and S. oralis as monocultures or in coculture in saliva-supplemented medium (22.5% saliva, 30% BHI in PBS) as depicted in panel A or in 30% BHI (in PBS) medium as depicted in panel B. Ca, C. albicans; So, S. oralis. S. oralis and C. albicans were inoculated simultaneously at a ratio of 10:1. Graphs show data from one representative experiment out of three independent replicates. (C) Representative phase-contrast micrographs of monospecies and mixed-species cultures after 7 h of growth in saliva-supplemented medium. Bar, 20 μm.
Mucosal biofilm formation by C. albicans under salivary flow.
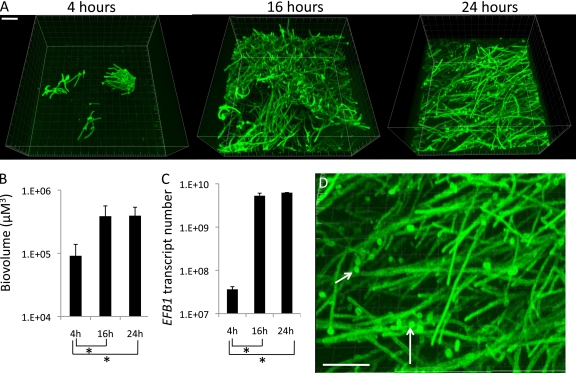
Although C. albicans can colonize hard oral surfaces such as teeth and dental prostheses, the major niche of this organism and systemic portal of entry are mucosal surfaces. Thus, our next goal was to test the ability of C. albicans and commensal oral bacteria to form mucosal biofilms and evaluate the potential of mixed biofilms to trigger tissue damage and invasion. Therefore, we developed a novel in vitro flow cell system that allows biofilm formation on a mucosal surface under salivary flow to simulate microbial interactions on the oral mucosa in vivo (Fig. 1). We first tested the ability of C. albicans to form mucosal biofilms in this device and characterized the kinetics of mucosal biofilm formation. The newly designed tissue harboring flow cell device (Fig. 1) was able to support mucosal biofilm growth by C. albicans. Figure 5 shows representative 3-D micrographs of 4-, 16-, and 24-h monospecies biofilms of C. albicans formed on an oral mucosa tissue analogue under salivary flow. As the biovolume and real-time RT-PCR quantification of C. albicans biomass indicates, most of the biofilm growth occurred from the early to the 16-h time point, whereas no further growth was seen from 16 to 24 h. The finding that EFB1 transcription levels were stable between 16 and 24 h indicates that the viability of C. albicans biofilms was maintained over time. We also observed that 4- and 16-h biofilms were mainly comprised of hyphae, while at 24 h yeast cells appeared in the external layer of the biofilm mass, a finding consistent with biofilm maturation (Fig. 5D, arrows) (23). These results indicate that C. albicans follows a similar pattern of biofilm formation on the oral mucosa as that described for abiotic surfaces with an initial attachment stage, followed by a growth phase in which hyphae are the main component of the biofilm, followed by a mature stage characterized by the appearance of yeast cells with dispersal capacity (57).
Fig 5.
Kinetics of C. albicans mucosal biofilm formation under salivary flow. (A) Representative 3-D reconstructions of C. albicans biofilms at different time points imaged after staining with a FITC-labeled anti-Candida antibody (green). (B) Biovolume quantifications of biofilms at each time point as measured in eight different CLSM image stacks. (C) Quantification of C. albicans biomass via real-time RT-PCR for EFB1. (D) High-magnification image of a 24-h biofilm showing yeast cells among the biofilm hyphae, consistent with the biofilm entering a dispersal stage. Bar, 40 μm. *, P < 0.05 as determined by t test.
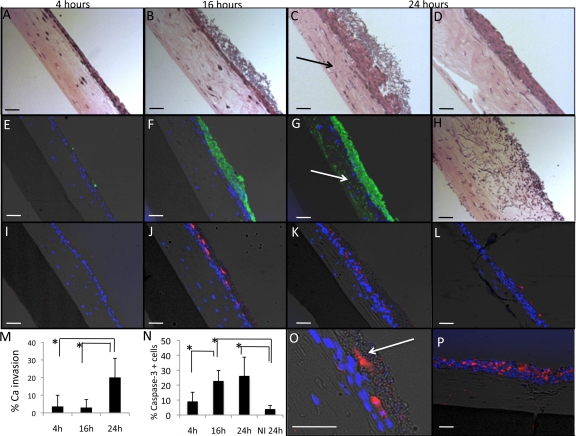
We also evaluated the ability of C. albicans biofilms to invade the oral mucosa under salivary flow. Figure 6A to C shows sections of oral mucosa harboring C. albicans biofilms, stained with hematoxylin and eosin (H&E), that were grown for different periods of time in the flow cell system. Figure 6E to G show tissue sections in which C. albicans was stained via immunofluorescence. As can be appreciated from these representative micrographs and from quantification of C. albicans invasion (Fig. 6M), at 16 h, C. albicans had formed a superficial adherent biofilm mass with minimal invasion of the adjacent mucosa. Tissue invasion was not highly evident until the biofilm entered a mature phase (at 24 h).
Fig 6.
Tissue sections showing kinetics of C. albicans mucosal biofilm formation, C. albicans invasion capacity, and C. albicans-induced apoptosis of the oral mucosa, under salivary flow. (A to C) H&E-stained sections of oral mucosa analogue tissue after 4 h (A), 16 h (B), and 24 h (C) of C. albicans biofilm formation. (D) Noninfected oral mucosa analogue tissue after 24 h of salivary flow. (E to G) Overlay images of tissue sections containing C. albicans biofilms stained with an FITC-labeled anti-Candida antibody (green) counterstained with the nucleic acid stain Hoechst 33258 (blue) and also visualized via phase-contrast light microscopy. Notice that at 16 h there is minimal invasion of the oral mucosa by C. albicans (B and F), while at 24 h C. albicans has invaded the epithelium and is now visible in the collagen compartment (C and G, arrows). (H) C. albicans was inoculated on a collagen/fibroblast layer with no epithelium present and was allowed to form a biofilm for 16 h under salivary flow. Despite lack of mucosal invasion of C. albicans at 16 h (B), C. albicans readily invades a collagen layer at this same time point (H). (I to L) Overlay images of tissue sections containing 4 h (I), 16 h (J), and 24 h (K) C. albicans mucosal biofilms and a noninfected control tissue after 24 h of salivary flow (L) stained for the early apoptosis marker caspase-3 (red) and the nucleic acid stain Hoechst 33258 (blue) and also visualized via phase-contrast light microscopy. (M) Quantification of C. albicans invasion in immunofluorescence micrographs at different time points. (N) Quantification of tissue viability at different time points of C. albicans biofilm formation and in a noninfected control after 24 h of flow (NI 24 h). (O) Caspase-3-positive cells (red) integrated into the biofilm biomass. (P) A positive control for the caspase-3 assay consisting of a tissue infected with the apoptosis-inducing microorganism Fusobacterium nucleatum, under anaerobic conditions, for 16 h. Bars in all images, 50 μm. *, P < 0.05 as determined by t test.
We then questioned whether lack of tissue invasion at 16 h occurred because C. albicans acquires “a noninvasive phenotype” under the environmental conditions imposed in the flow cell system or because the epithelial layer is still impenetrable at this time. For these experiments, we inoculated C. albicans on a fibroblast-collagen layer (without epithelium) allowing biofilm growth for 16 h. As Fig. 6H shows, C. albicans readily penetrated the collagen layer at this time point, demonstrating that salivary flow allows an invasive phenotype to develop if the appropriate substratum is provided. These results also highlight the protective capacity of the oral epithelium against C. albicans invasion into the submucosa.
We then evaluated the effect of C. albicans biofilms on mucosal cell apoptosis, since farnesol, a quorum-sensing alcohol produced in abundance by Candida biofilms, is known to trigger mammalian cell apoptosis (62). Tissue sections were used to evaluate apoptosis via immunostaining for the early apoptosis marker caspase-3 (Fig. 6I to L and N to P). Figure 6L demonstrates that only a minimal proportion of epithelial cells appeared apoptotic in uninfected tissues after 24 h of salivary flow. Interestingly, these cells were localized to the most superficial epithelial layer, a pattern compatible with physiologic epithelial desquamation. An early 4-h C. albicans biofilm induced minimal apoptosis, while mucosa harboring a biofilm for 16 h demonstrated a significant increase in the proportion of caspase-3-positive cells compared to a 4-h-infected mucosae or an noninfected control. At 24 h, there was a trend for a further increase in apoptosis; however, the results were not statistically significant compared to 16-h samples. Overall, at the end of the incubation period the mean percentage of apoptotic cells did not exceed 30% of the total, which represents a mild apoptotic effect compared to F. nucleatum, which under anaerobic conditions triggered apoptosis in up to 70% of mucosal cells (positive control, Fig. 6P). We also observed that apoptotic cells were incorporated into the biofilm mass (Fig. 6O), a finding consistent with our previous observations in an in vivo oral mucosal biofilm model (20).
C. albicans promotes mucosal biofilm formation by S. oralis, while S. oralis enhances the ability of C. albicans to invade the oral mucosa.
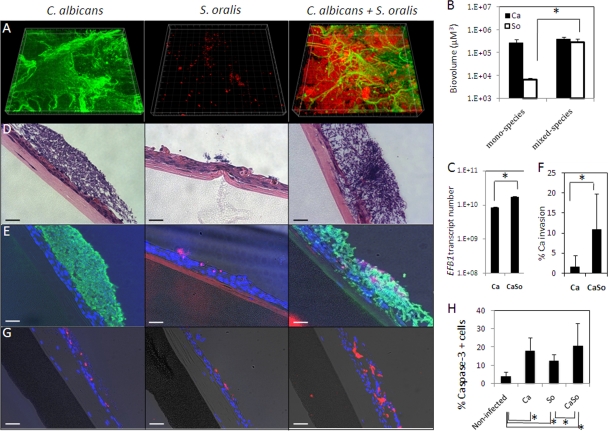
Next, we used tissue-harboring flow cell devices to test the ability of C. albicans and streptococci to form mucosal biofilms under salivary flow. Among oral viridans streptococci, S. oralis and its close phylogenetic relative Streptococcus mitis are commonly found in the oral mucosa and are frequently reported as a cause of septicemia in immunosuppressed patients, a population also afflicted by systemic Candida infections (6, 34). Thus, we chose S. oralis for further experiments in order to test the interaction of this organism with the oral mucosa in the presence or absence of C. albicans. We first evaluated the ability of S. oralis to form mucosal biofilms as monospecies. As shown in Fig. 7A, S. oralis was not capable of forming a single species mucosal biofilm. However, in the presence of C. albicans there was an ∼45-fold increase in the biomass of S. oralis. Biovolume measurements showed that the biofilm biomass of C. albicans was not significantly affected by the presence of S. oralis (Fig. 7B), while quantification of C. albicans via real-time RT-PCR showed a slight increase in C. albicans in mixed-species biofilms (Fig. 7C). Tissue sections were then used to evaluate invasion of the microorganisms into the oral mucosa after 16 h of flow. Figure 7D shows H&E-stained sections of oral mucosa harboring C. albicans and S. oralis monospecies and mixed-species biofilms, while Fig. 7E depicts immunofluorescence staining for C. albicans and FISH for S. oralis. As shown by these representative images and the corresponding invasion quantification data (Fig. 7F), C. albicans monospecies biofilms displayed minimal ability to invade the oral mucosa, while in the presence of S. oralis there is an ∼7-fold increase in the relative invasion of C. albicans. No evidence of S. oralis invasion of the oral mucosa was observed either in the presence or absence of C. albicans, although in tissues monoinfected with S. oralis, endocytosis of the microorganism by superficial epithelial cells was occasionally seen.
Fig 7.
Synergistic interactions between C. albicans and S. oralis in biofilms formed over an oral mucosa analogue under salivary flow. (A) Representative 3-D reconstructions of monospecies and mixed-species mucosal biofilms of C. albicans and S. oralis after 16 h of biofilm formation. C. albicans was stained with an FITC-labeled anti-Candida antibody (green), and S. oralis was visualized after FISH with an Alexa 546-labeled Streptococcus-specific probe (red). (B) Biovolume quantifications from CLSM image stacks of C. albicans and S. oralis as monospecies or in mixed-species mucosal biofilms. (C) Quantification of C. albicans biomass in monospecies and mixed-species biofilms via real-time RT-PCR for EFB1. (D) H&E-stained sections of oral mucosa analogue tissue after 16 h of monospecies (two left images) or mixed-species (right image) biofilm growth. Panel E shows overlay images of tissue sections containing monospecies or mixed-species C. albicans and S. oralis biofilms stained with a FITC-labeled anti-Candida antibody (green), followed by FISH with an Alexa 546-labeled Streptococcus-specific probe (red), counterstained with the nucleic acid stain Hoechst 33258 (blue), and also visualized via phase-contrast light microscopy. (F) Quantification of C. albicans invasion as a monospecies or in mixed-species biofilms. The percentage of invasion (% invasion) was determined by measuring the fraction of the area (in μm2) in which C. albicans appears below the epithelial apical margin in comparison to the total area occupied by C. albicans in each condition. (G) Overlay images of tissue sections containing monospecies and mixed-species mucosal biofilms stained for the early apoptosis marker caspase-3 (red) and the nucleic acid stain Hoechst 33258 (blue) and also visualized via phase-contrast light microscopy. (H) Quantification of cells positive for caspase-3 in noninfected oral mucosa after 16 h of salivary flow and in tissues harboring monospecies and mixed-species biofilms of C. albicans and S. oralis. Bars in all images, 50 um. *, P < 0.05 as determined by t test. Ca, C. albicans; So, S. oralis; CaSo, mixed species.
Evaluation of cellular apoptosis via caspase-3 revealed that the presence of both C. albicans and S. oralis triggered apoptosis of the oral mucosa. Interestingly, despite increased tissue invasion by C. albicans in mixed-species biofilms, there was no statistically significant difference in the number of apoptotic epithelial cells between tissues harboring C. albicans and tissues harboring C. albicans-S. oralis biofilms. Since epithelial cell apoptosis triggered by C. albicans has been associated with endocytosis (60), this finding suggests that the increased invasion of C. albicans fundamentally takes place by degradation of the intercellular epithelial junction proteins as we previously reported (59) and not by intracellular invasion.
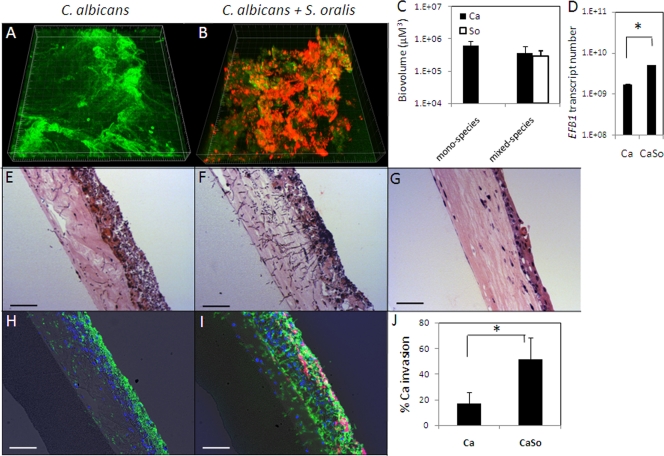
We then questioned whether the type of alimentary tract epithelium is an ecological determinant of growth and tissue invasion of single species C. albicans biofilms or C. albicans-streptococcal biofilms. To explore this further, we inoculated 3-D esophageal mucosal tissues with C. albicans or a combination of C. albicans and S. oralis, and subjected them to salivary flow for 16 h. Figure 8 demonstrates that C. albicans can form monospecies and mixed-species biofilms with S. oralis on an esophageal mucosa analogue. Importantly, as previously seen on an oral mucosa analogue, we also observed increased tissue invasion by C. albicans in the presence of S. oralis. Interestingly, invasion by C. albicans into the submucosal compartment of the esophageal mucosa was significantly higher than that in the oral mucosa in both single-species and dual-species biofilms (P < 0.05), despite the fact that C. albicans biomass was higher in the oral mucosa (P < 0.05).
Fig 8.
Interaction of C. albicans and S. oralis in biofilms formed over an esophageal mucosa analogue. (A and B) Representative 3-D reconstructions of monospecies and mixed-species mucosal biofilms of C. albicans and S. oralis at 16 h. C. albicans was stained with an FITC-labeled anti-Candida antibody (green), and S. oralis was visualized after FISH with an Alexa 546-labeled Streptococcus-specific probe (red). (C) Biovolume quantifications of C. albicans and S. oralis in monospecies or mixed-species mucosal biofilms. (D) Quantification of C. albicans biomass in monospecies and mixed-species biofilms via real-time RT-PCR for EFB1. (E and F) H&E-stained sections of esophageal mucosa after 16 h of C. albicans monospecies or mixed-species biofilm growth. (G) An uninfected esophageal mucosa analogue. (H and I) Overlay images of tissue sections containing monospecies or mixed-species C. albicans and S. oralis biofilms stained with an FITC-labeled anti-Candida antibody (green), followed by FISH with an Alexa 546-labeled Streptococcus-specific probe, counterstained with the nucleic acid stain Hoechst 33258 (blue) and also visualized via phase-contrast light microscopy. (J) Quantification of C. albicans invasion as a monospecies or in mixed-species biofilms. The percentage of invasion (% invasion) was determined by measuring the fraction of the area (in μm2) in which C. albicans appears below the epithelial apical margin in comparison to the total area occupied by C. albicans in each condition. Bars in all images, 50 μm. *, P < 0.05 as determined by t test. Ca, C. albicans; So, S. oralis; CaSo, mixed species.
DISCUSSION
Our understanding of mixed species biofilms and their impact in human health is still in its infancy, although it is clear that microorganisms exist as polymicrobial communities in nature. The interkingdom interactions of C. albicans and bacteria in the context of human disease have received increased attention in recent years (8, 44, 48, 61). Interactions between C. albicans and bacteria are likely to play a role not only during C. albicans colonization of mucosal surfaces but also during the course of mucosa-associated or invasive infections (8, 58). However, it is not clear to what extent C. albicans-bacterial interactions promote or prevent disease. While the interaction of C. albicans with bacterial species such as Pseudomonas aeruginosa has been demonstrated to be antagonistic in nature, C. albicans seems able to coexist with Staphylococcus sp. and with oral streptococci (4, 9, 29, 48). Few studies, however, have tried to characterize such multispecies interactions in biologically relevant models. The emergence of the human immunodeficiency virus, the development of newer intensive chemotherapy regimens for malignancy, and the rising number of organ transplant recipients over the past decades has resulted in an increase in the number of patients at risk for invasive fungal infections (32, 43, 55). Possible synergistic or competitive interactions between fungi and bacteria and their implications for therapy are thus of great importance in order to understand infections afflicting these patient populations.
We present here evidence that supports the hypothesis that the interaction between C. albicans and commensal oral streptococci is mutualistic in nature. Although we observed slight suppression of C. albicans growth by high streptococcal cell numbers while in a closed planktonic coculture system, this negative effect was not evident in an open flow cell system, with a constant nutrient supply. Moreover, we demonstrate that C. albicans facilitates streptococcal biofilm formation, whereas certain streptococcal species, such as S. oralis, display a poor capacity to form single-species biofilms regardless of the experimental system used (abiotic or mucosal). On the other hand, the presence of streptococci enhances the ability of C. albicans to invade oral and esophageal mucosa analogues. These results contradict the long held belief that the commensal bacterial flora protects us against oral candidiasis (39). Such protection may still be in effect in the lower GI tract and vaginal mucosa, where colonization by Candida is reported to be enhanced by antibiotic-mediated suppression of the resident bacterial flora (41). On the contrary, the oropharynx has been demonstrated to be more resistant to C. albicans colonization than the lower GI tract and vagina following antibiotic depletion of resident bacteria (40). Moreover, reports indicating an emergence of oral Candida infections after antibiotic treatment are rare and commonly associated with pharmacologically induced immunosuppression (25). The main determinant of oropharyngeal candidiasis seems to be the lack of a protective immune response, as evidenced by increased oral candidiasis in asthmatic patients using locally immunosuppressive corticosteroid inhalers, or during the early stages of AIDS, situations in which oral bacterial load is thought to remain for the most part unaltered (22, 24). We thus propose that colonization of C. albicans in the upper GI tract is facilitated by interactions with oral commensal bacteria in which the resident bacterial flora continuously “prime” Candida to a more invasive phenotype, while the immune system is constantly limiting C. albicans invasion. This hypothesis will need further testing in a system where the immunological component is present.
Our work indicates that coaggregation-mediated attachment, rather than growth stimulation, plays a crucial role facilitating streptococcal biofilm formation in the presence of C. albicans. Coaggregation seems to facilitate initial adhesion and retention, but it could also allow a metabolic interaction between the two organisms that promotes streptococcal biofilm formation. This is supported by the observation that although not all streptococcal cells in mixed biofilms under salivary flow were directly attached to C. albicans, at 4 and 16 h of biofilm formation, luxuriant streptococcal accretion was mostly observed in areas of physical proximity between the two organisms, a finding that could suggest the presence of a local microenvironment with conditions different from those in the surrounding fluid phase. Increased local concentrations of diffusible signals produced by C. albicans may alter the streptococcal phenotype allowing its increased biofilm formation. It is possible that C. albicans quorum-sensing molecules, such as farnesol or tyrosol, may have a role in these events. However, unpublished data from our laboratory suggest that (E,E)-farnesol inhibits streptococcal growth and cannot account for the phenotype observed in the present studies. Thus, functional studies investigating the physical interaction, signaling events or other mechanisms mediating streptococcal biofilm formation in the presence of Candida remain a focus of future investigations. Moreover, our data suggest that the increased streptococcal biomass in mixed-species biofilms was not a result of growth stimulation as planktonic growth curves of streptococci in the presence or absence of C. albicans remained unchanged. It is noteworthy that microscopic examination of planktonic cocultures of C. albicans and streptococci showed the formation of coaggregates, especially in the presence of saliva, whereby streptococcal cells associated with hyphae (Fig. 4). Thus, the absence of a growth stimulatory effect of C. albicans on streptococcal planktonic growth is not likely attributable to lack of physical proximity in suspension cultures. There is still a possibility that a growth enhancing effect is only present in the biofilm growth state, although time course biovolume data with S. oralis and S. sanguinis dispute this.
Candida biofilms triggered a low level of apoptosis in the oral mucosa model which was not significantly increased by the addition of oral streptococci. The low levels of Candida-triggered oral epithelial cell apoptosis are consistent with previous reports in nonbiofilm systems (60). Although farnesol has been reported to trigger mammalian cell apoptosis (62), it is possible that it did not reach the concentration needed to enhance apoptotic cell death in our open flow system. In addition to apoptosis, other forms of cell death, independent of caspase-3 activation, could take place in our system that were not tested in this work. For example, cell necrosis mediated by Candida phospholipases could be triggering significant levels of cell death, provided that physiologically relevant levels accumulate (37).
In the present study, we also show that C. albicans displays increased invasion capacity in the presence of streptococci. Recent findings suggest that contact of C. albicans and bacteria, particularly streptococci, induces changes in C. albicans phenotype that could explain our results. For example, coincubation of S. gordonii with C. albicans has been shown to induce activation of the mitogen-activated protein kinase, Cek1p, which is involved in starvation-specific hyphal development but is also important during systemic candidiasis (4, 11). Moreover, it has been demonstrated that bacterial peptidoglycans, specifically muramyl dipeptides, promote hyphae induction in C. albicans via activation of cyclic AMP intracellular signaling (64). The result of these and other signaling events will be dissected in future experiments that will characterize the global changes in transcriptional profiles of C. albicans cells growing in mixed-species biofilms in comparison to monospecies biofilms.
The experiments described here were facilitated by the development of a novel flow cell device capable of harboring upper GI mucosal tissue analogues. Thus, this model serves as an in vitro analogue of the upper GI tract, allowing the investigation of interactions among microorganisms within a biologically relevant environment. Importantly, the impact of the presented model extends beyond the study of mucosal infection by Candida biofilms since the system can be adapted to harbor different tissue types, while the microbial component can be modified to represent a variety of resident communities. Using this model we investigated whether the type of mucosal epithelium that lines the alimentary tract mucosa is an ecological determinant of invasive infection. When comparing invasion of C. albicans either as a monospecies or in mixed species, we found increased invasion in esophageal mucosa than in oral mucosa (Fig. 7 and 8). This finding is in agreement with work by our group and others demonstrating that the oral mucosa of mice is less susceptible to C. albicans invasion than the esophageal mucosa (21). The ability of C. albicans to invade the esophageal but not the oral submucosa has also been documented in immunocompromised human hosts (53). It is unclear why the esophageal mucosa is more permissive to Candida invasion than the oral mucosa. Although the two epithelia share many morphological features (both are stratified squamous epithelia), they may have differences in secretory or cell surface-expressed products with innate immune recognition, or effector function, that could differentially control the assembly and survival of microorganisms on their surface. Compared to esophageal cells, oral epithelial cells express higher levels of several Toll-like and NOD receptors, functionally linked to higher ability of these cells to synthesize antimicrobial peptides (56). It is possible that higher production of such peptides (e.g., β-defensins) in the oral mucosa limit the ability of the organism to trigger localized invasion compared to esophageal tissue (7, 12). Alternatively, different cell type substrates and different host GI tract niches may modulate the transcriptional response of the organism, such that Candida acquires an invasive phenotype in one mucosal tissue but not the other (47). Future work will focus on the role of different epithelia on polymicrobial biofilm formation and susceptibility to infection.
In conclusion, we have developed a novel model system of the upper alimentary tract mucosa which allows the examination of microbial interactions in a biologically relevant environment. Using this system, we present evidence of synergy between C. albicans and oral streptococci with direct implications in the pathogenesis of C. albicans-associated infections. We also show experimentally that the esophageal mucosa is more permissive to invasive infection, which may explain the more frequent systemic dissemination of C. albicans through the esophageal mucosa in animals and humans. Further molecular characterization of these interactions is under way, which may lead to novel strategies in preventing invasive infections in immunocompromised hosts.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institute of Dental and Craniofacial Research, National Institutes of Health (R21DE020166 to P.I.D. and RO1DE013986 to A.D.-B.).
Footnotes
Published ahead of print 21 November 2011
REFERENCES
- 1. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. 2005. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 43:5721–5732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. An SS, et al. 2011. Hypoxia-induced expression of VEGF in the organotypic spinal cord slice culture. Neuroreport 22:55–60 [DOI] [PubMed] [Google Scholar]
- 3. Andl CD, et al. 2003. Epidermal growth factor receptor mediates increased cell proliferation, migration, and aggregation in esophageal keratinocytes in vitro and in vivo. J. Biol. Chem. 278:1824–1830 [DOI] [PubMed] [Google Scholar]
- 4. Bamford CV, et al. 2009. Streptococcus gordonii modulates Candida albicans biofilm formation through intergeneric communication. Infect. Immun. 77:3696–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnett JA. 2008. A history of research on yeasts 12: medical yeasts part 1, Candida albicans. Yeast 25:385–417 [DOI] [PubMed] [Google Scholar]
- 6. Barrett AP. 1989. Recognition and management of invasive pharyngeal candidiasis in acute leukemia. Oral Surg. Oral Med. Oral Pathol. 67:275–278 [DOI] [PubMed] [Google Scholar]
- 7. Bowdish DM, Davidson DJ, Hancock RE. 2006. Immunomodulatory properties of defensins and cathelicidins. Curr. Top. Microbiol. Immunol. 306:27–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carlson E. 1983. Enhancement by Candida albicans of Staphylococcus aureus, Serratia marcescens, and Streptococcus faecalis in the establishment of infection in mice. Infect. Immun. 39:193–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carlson E. 1982. Synergistic effect of Candida albicans and Staphylococcus aureus on mouse mortality. Infect. Immun. 38:921–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coogan MM, Fidel PL, Jr, Komesu MC, Maeda N, Samaranayake LP. 2006. (B1) Candida and mycotic infections. Adv. Dent. Res. 19:130–138 [DOI] [PubMed] [Google Scholar]
- 11. Csank C, et al. 1998. Roles of the Candida albicans mitogen-activated protein kinase homolog, Cek1p, in hyphal development and systemic candidiasis. Infect. Immun. 66:2713–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dale BA, Fredericks LP. 2005. Antimicrobial peptides in the oral environment: expression and function in health and disease. Curr. Issues Mol. Biol. 7:119–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dawes C, Watanabe S, Biglow-Lecomte P, Dibdin GH. 1989. Estimation of the velocity of the salivary film at some different locations in the mouth. J. Dent. Res. 68:1479–1482 [DOI] [PubMed] [Google Scholar]
- 14. de Repentigny L, Lewandowski D, Jolicoeur P. 2004. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin. Microbiol. Rev. 17:729–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dewhirst FE, et al. 2010. The human oral microbiome. J. Bacteriol. 192:5002–5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diaz PI, et al. 2006. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 72:2837–2848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dickinson BC, et al. 2011. Interaction of oral bacteria with gingival epithelial cell multilayers. Mol. Oral Microbiol. 26:210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dongari-Bagtzoglou A, Fidel PL., Jr 2005. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J. Dent. Res. 84:966–977 [DOI] [PubMed] [Google Scholar]
- 19. Dongari-Bagtzoglou A, Kashleva H. 2006. Development of a highly reproducible three-dimensional organotypic model of the oral mucosa. Nat. Protoc. 1:2012–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dongari-Bagtzoglou A, Kashleva H, Dwivedi P, Diaz P, Vasilakos J. 2009. Characterization of mucosal Candida albicans biofilms. PLoS One 4:e7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dwivedi PP, Mallya S, Dongari-Bagtzoglou A. 2009. A novel immunocompetent murine model for Candida albicans-promoted oral epithelial dysplasia. Med. Mycol. 47:157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fidel PL., Jr 2011. Candida-host interactions in HIV disease: implications for oropharyngeal Candidiasis. Adv. Dent. Res. 23:45–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat. Rev. Microbiol. 9:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukushima C, et al. 2003. Oral candidiasis associated with inhaled corticosteroid use: comparison of fluticasone and beclomethasone. Ann. Allergy Asthma Immunol. 90:646–651 [DOI] [PubMed] [Google Scholar]
- 25. Gligorov J, et al. Prevalence and treatment management of oropharyngeal candidiasis in cancer patients: results of the French CANDIDOSCOPE study. Int. J. Radiat. Oncol. Biol. Phys. 80:532–539 [DOI] [PubMed] [Google Scholar]
- 26. Grimaudo NJ, Nesbitt WE, Clark WB. 1996. Coaggregation of Candida albicans with oral Actinomyces species. Oral Microbiol. Immunol. 11:59–61 [DOI] [PubMed] [Google Scholar]
- 27. Harada H, et al. 2003. Telomerase induces immortalization of human esophageal keratinocytes without p16INK4a inactivation. Mol. Cancer Res. 1:729–738 [PubMed] [Google Scholar]
- 28. Herzberg MC, Meyer MW, Kilic A, Tao L. 1997. Host-pathogen interactions in bacterial endocarditis: streptococcal virulence in the host. Adv. Dent. Res. 11:69–74 [DOI] [PubMed] [Google Scholar]
- 29. Hogan DA, Kolter R. 2002. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 296:2229–2232 [DOI] [PubMed] [Google Scholar]
- 30. Holmes AR, Gopal PK, Jenkinson HF. 1995. Adherence of Candida albicans to a cell surface polysaccharide receptor on Streptococcus gordonii. Infect. Immun. 63:1827–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holmes AR, McNab R, Jenkinson HF. 1996. Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect. Immun. 64:4680–4685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hsueh PR, Teng LJ, Yang PC, Ho SW, Luh KT. 2002. Emergence of nosocomial candidemia at a teaching hospital in Taiwan from 1981 to 2000: increased susceptibility of Candida species to fluconazole. Microb. Drug Resist. 8:311–319 [DOI] [PubMed] [Google Scholar]
- 33. Jenkinson HF, Lala HC, Shepherd MG. 1990. Coaggregation of Streptococcus sanguis and other streptococci with Candida albicans. Infect. Immun. 58:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khan SA, Wingard JR. 2001. Infection and mucosal injury in cancer treatment. J. Natl. Cancer Inst. Monogr. 29:31–36 [DOI] [PubMed] [Google Scholar]
- 35. Koh AY, Kohler JR, Coggshall KT, Van Rooijen N, Pier GB. 2008. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 4:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lai CC, Tan CK, Huang YT, Shao PL, Hsueh PR. 2008. Current challenges in the management of invasive fungal infections. J. Infect. Chemother. 14:77–85 [DOI] [PubMed] [Google Scholar]
- 37. Leidich SD, et al. 1998. Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J. Biol. Chem. 273:26078–26086 [DOI] [PubMed] [Google Scholar]
- 38. Liljemark WF, Gibbons RJ. 1972. Proportional distribution and relative adherence of Streptococcus miteor (mitis) on various surfaces in the human oral cavity. Infect. Immun. 6:852–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liljemark WF, Gibbons RJ. 1973. Suppression of Candida albicans by human oral streptococci in gnotobiotic mice. Infect. Immun. 8:846–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maraki S, et al. 2003. Effects of doxycycline, metronidazole and their combination on Candida species colonization of the human oropharynx, intestinal lumen and vagina. J. Chemother. 15:369–373 [DOI] [PubMed] [Google Scholar]
- 41. Maraki S, et al. 2001. Prospective evaluation of the impact of amoxicillin, clarithromycin and their combination on human gastrointestinal colonization by Candida species. Chemotherapy 47:215–218 [DOI] [PubMed] [Google Scholar]
- 42. Marsh PD, Moter A, Devine DA. 2011. Dental plaque biofilms: communities, conflict and control. Periodontol. 2000 55:16–35 [DOI] [PubMed] [Google Scholar]
- 43. Martin GS, Mannino DM, Eaton S, Moss M. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348:1546–1554 [DOI] [PubMed] [Google Scholar]
- 44. Morales DK, Hogan DA. 2010. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog. 6:e1000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Palmer RJ. 1999. Microscopy flowcells: perfusion chambers for real-time study of biofilms. Biofilms 310:160–166 [DOI] [PubMed] [Google Scholar]
- 46. Palmer RJ, Jr, Kazmerzak K, Hansen MC, Kolenbrander PE. 2001. Mutualism versus independence: strategies of mixed-species oral biofilms in vitro using saliva as the sole nutrient source. Infect. Immun. 69:5794–5804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park H, et al. 2009. Transcriptional responses of Candida albicans to epithelial and endothelial cells. Eukaryot. Cell 8:1498–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peters BM, et al. 2010. Microbial interactions and differential protein expression in Staphylococcus aureus-Candida albicans dual-species biofilms. FEMS Immunol. Med. Microbiol. 59:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rasband WS. 1997-2011, posting date ImageJ. National Institutes of Health, Bethesda, MD: http://rsbweb.nih.gov/ij/ [Google Scholar]
- 50. Sanchez-Vargas LO, et al. 2005. Oral Candida isolates colonizing or infecting human immunodeficiency virus-infected and healthy persons in Mexico. J. Clin. Microbiol. 43:4159–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2:1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Silverman RJ, Nobbs AH, Vickerman MM, Barbour ME, Jenkinson HF. 2010. Interaction of Candida albicans cell wall Als3 protein with Streptococcus gordonii SspB adhesin promotes development of mixed-species communities. Infect. Immun. 78:4644–4652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thom K, Forrest G. 2006. Gastrointestinal infections in immunocompromised hosts. Curr. Opin. Gastroenterol. 22:18–23 [DOI] [PubMed] [Google Scholar]
- 54. Thurnheer T, Gmur R, Giertsen E, Guggenheim B. 2001. Automated fluorescent in situ hybridization for the specific detection and quantification of oral streptococci in dental plaque. J. Microbiol. Methods 44:39–47 [DOI] [PubMed] [Google Scholar]
- 55. Tortorano AM, et al. 2006. Candidaemia in Europe: epidemiology and resistance. Int. J. Antimicrob. Agents 27:359–366 [DOI] [PubMed] [Google Scholar]
- 56. Uehara A, Fujimoto Y, Fukase K, Takada H. 2007. Various human epithelial cells express functional Toll-like receptors, NOD1 and NOD2 to produce anti-microbial peptides, but not proinflammatory cytokines. Mol. Immunol. 44:3100–3111 [DOI] [PubMed] [Google Scholar]
- 57. Uppuluri P, et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 6:e1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Venkatesh MP, Pham D, Fein M, Kong L, Weisman LE. 2007. Neonatal coinfection model of coagulase-negative Staphylococcus (Staphylococcus epidermidis) and Candida albicans: fluconazole prophylaxis enhances survival and growth. Antimicrob. Agents Chemother. 51:1240–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Villar CC, Kashleva H, Nobile CJ, Mitchell AP, Dongari-Bagtzoglou A. 2007. Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect. Immun. 75:2126–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Villar CC, Zhao XR. 2010. Candida albicans induces early apoptosis followed by secondary necrosis in oral epithelial cells. Mol. Oral Microbiol. 25:215–225 [DOI] [PubMed] [Google Scholar]
- 61. Wargo MJ, Hogan DA. 2006. Fungal-bacterial interactions: a mixed bag of mingling microbes. Curr. Opin. Microbiol. 9:359–364 [DOI] [PubMed] [Google Scholar]
- 62. Wiseman DA, Werner SR, Crowell PL. 2007. Cell cycle arrest by the isoprenoids perillyl alcohol, geraniol, and farnesol is mediated by p21Cip1 and p27Kip1 in human pancreatic adenocarcinoma cells. J. Pharmacol. Exp. Ther. 320:1163–1170 [DOI] [PubMed] [Google Scholar]
- 63. Xie Z, Thompson A, Kashleva H, Dongari-Bagtzoglou A. 2011. A quantitative real-time RT-PCR assay for mature C. albicans biofilms. BMC Microbiol. 11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Xu XL, et al. 2008. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe 4:28–39 [DOI] [PubMed] [Google Scholar]
- 65. Zijnge V, et al. 2010. Oral biofilm architecture on natural teeth. PLoS One 5:e9321. [DOI] [PMC free article] [PubMed] [Google Scholar]