Abstract
Neutrophils have recently been shown to release DNA-based extracellular traps that contribute to microbicidal killing and have also been implicated in autoimmunity. The role of neutrophil extracellular trap (NET) formation in the host response to nonbacterial pathogens has received much less attention. Here, we show that the protozoan pathogen Toxoplasma gondii elicits the production of NETs from human and mouse neutrophils. Tachyzoites of each of the three major parasite strain types were efficiently entrapped within NETs, resulting in decreased parasite viability. We also show that Toxoplasma activates a MEK-extracellular signal-regulated kinase (ERK) pathway in neutrophils and that the inhibition of this pathway leads to decreased NET formation. To determine if Toxoplasma induced NET formation in vivo, we employed a mouse intranasal infection model. We found that the administration of tachyzoites by this route induced a rapid tissue recruitment of neutrophils with evidence of extracellular DNA release. Taken together, these data indicate a role for NETs in the host innate response to protozoan infection. We propose that NET formation limits infection by direct microbicidal effects on Toxoplasma as well as by interfering with the ability of the parasite to invade target host cells.
INTRODUCTION
Neutrophils have long been regarded as one of the most important of the induced host innate defenders primarily because they are the earliest cells to arrive at sites of infection or inflammation in response to chemotactic signals. They can rapidly accumulate in large numbers and deploy a diverse arsenal of weapons aimed at eliminating invading pathogens. The resolution of inflammation and infection involves the apoptosis of neutrophils and phagocytic elimination by macrophages. As such, neutrophils are often regarded as potent but short-lived cells with a limited ability to affect adaptive immunity (34). Nonetheless, their biological significance may extend beyond this view insofar as neutrophils can also display immunoregulatory activity on other cells of the immune system (2, 6, 14, 34, 48, 54).
Neutrophils engulf pathogens through phagocytosis, and the resulting microbe-carrying phagosome fuses with lysosomes, where the microorganism can be degraded. Microbial killing relies on both oxidative and nonoxidative mechanisms. Oxidative mechanisms involve the production of reactive oxygen species through the activity of the NADPH oxidase enzyme complex, while nonoxidative mechanisms rely on the release of antimicrobial peptides and proteases (22, 23). Together, these mechanisms were until recently thought to encompass the whole antimicrobial activity of neutrophils.
A landmark study by Brinkmann et al. identified a previously unrecognized neutrophil antimicrobial mechanism involving the release of nuclear DNA that can entrap and kill extracellular pathogens (11). Originally discovered in neutrophils, the formation of extracellular traps has now been described for eosinophils and mast cells (50, 55). Neutrophil extracellular traps (NETs) have been shown to be composed of a DNA backbone studded with histones and laced with various antimicrobial peptides that together can kill microbial pathogens. Several bacterial and fungal pathogens have been shown to be susceptible to NET killing, including Staphylococcus aureus, Streptococcus pyogenes, Streptococcus pneumoniae, Escherichia coli, Mycobacterium tuberculosis, Listeria monocytogenes, and Candida albicans (5, 11, 13, 27, 38, 47). There is also evidence that some bacterial pathogens avoid killing by NETs by releasing nucleases (5, 13). At present, much less is known about the extent to which neutrophils form NETs in response to protozoan pathogens, although there is recent evidence for Leishmania-induced NET formation (25, 28).
The ability of another major protozoan parasite, Toxoplasma gondii, to elicit neutrophil recruitment during infection raises the question of whether or not NET formation is elicited by this important human opportunistic pathogen. Toxoplasma normally causes asymptomatic infection in immunocompetent individuals, but the parasite can cause serious clinical disease in immunocompromised hosts (21, 31). Immunity to T. gondii consists of a strong Th1 response that provides protection to the host. However, this inflammatory response can become pathological if not appropriately controlled by downmodulatory cytokines (26, 43). There is evidence that neutrophils play a role during Toxoplasma infection inasmuch as they are rapidly recruited to the site of infection, the lack of recruitment in the absence of CXCR2 correlates with increased susceptibility, and they are capable of producing several cytokines and chemokines in response to the parasite (8, 10, 17, 18). The ability of Toxoplasma to elicit the formation of NETs has not been addressed.
Here, we show for the first time that both murine and human neutrophils release NETs in response to all three major clonal lineages of Toxoplasma and that the parasites become physically entrapped by these macromolecular structures. The formation of NETs (also called NETosis) is invasion independent but partially depends upon the activation of the extracellular signal-regulated kinase 1/2 (ERK1/2) mitogen-activated protein kinase (MAPK). With a mouse intranasal infection model that stimulates rapid neutrophil accumulation in the lungs, we provide in vivo evidence for the release of NETs in response to the parasite. We hypothesize that the NETs released by neutrophils function as an innate mechanism of parasite killing and that by trapping parasites, NETs interfere with the ability to infect host cells and establish infection.
MATERIALS AND METHODS
Mice.
Female C57BL/6 and Swiss Webster mice were purchased from the Jackson Laboratory (Bar Harbor, ME) or Taconic Farms (Germantown, NY) and used at 6 to 8 weeks of age. C57BL/6 LYS-eGFP knock-in mice, expressing enhanced green fluorescent protein (eGFP) under the control of the lysozyme (LYS) promoter, were a generous gift from David Sacks (National Institutes of Health). All mice were maintained in the Transgenic Mouse Core Facility at the Cornell University College of Veterinary Medicine, a specific-pathogen-free (SPF) facility which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. All animal experiments were approved by the Cornell University Institutional Animal Care and Use Committee.
Mouse neutrophil isolation.
Mouse neutrophils were isolated as previously described (1). Briefly, mice were injected intraperitoneally (i.p.) with 0.5 ml of 10% thioglycolate (Becton Dickinson, Franklin Lakes, NJ), and 20 h later, peritoneal exudate cells (PEC) were collected by peritoneal cavity lavage with phosphate-buffered saline (PBS) (Cellgro, Manassas, VA). Neutrophils were subsequently purified by continuous Percoll gradient centrifugation as described elsewhere previously (44). Briefly, Percoll (GE Healthcare, Fairfield, CA), adjusted to pH 7.4, was mixed at a ratio of 1:9 with PEC resuspended in PBS. The mixture was then transferred into a 10-ml polycarbonate centrifuge tube, and ultracentrifugation was performed at 60,000 × g for 65 min at 4°C using a 50 Ti rotor (Beckman Centrifuges, Brea, CA). The layer enriched for neutrophils was washed twice with PBS and resuspended in complete Dulbecco's modified Eagle's medium (DMEM), consisting of 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, 30 mM HEPES (all purchased from Invitrogen Life Technologies, Carlsbad, CA), 2% bovine growth serum (HyClone, Logan, UT), and 0.05 mM β-mercaptoethanol in DMEM. Neutrophil preparations were routinely over 95% pure.
Human neutrophils.
Polymorphonuclear leukocytes (PMN) were purified as described previously (4). Briefly, human peripheral blood was collected from healthy adult donors following informed consent. Neutrophils were isolated by centrifugation at 500 × g for 50 min at 23°C in a Sorvall ST 16 centrifuge (Fisher Scientific, Pittsburgh, PA) using 1-Step Polymorphs (Accurate Chemical and Scientific Corporation, Westbury, NY). The neutrophil layer was collected and washed twice in Ca2+- and Mg2+-free Hanks balanced salt solution (HBSS), and any remaining red blood cells were subjected to hypotonic lysis by brief incubation in Dulbecco's PBS (DPBS)–endotoxin-free water (ratio of 1:6), followed by osmotic restabilization with 4× DPBS. The cells were resuspended in HBSS containing 0.5% human serum albumin (HSA), 2 mM Ca2+, and 10 mM HEPES, buffered to pH 7.4.
HL-60 cell differentiation.
The generation of differentiated HL-60 cells was performed as described previously (15). Briefly, HL-60 cells (4 × 105 cells/ml) were treated with 1.25% dimethyl sulfoxide (DMSO; Calbiochem) in RPMI 1640 supplemented with 5% heat-inactivated fetal bovine serum (FBS) for 4 days. Differentiated cells were harvested by centrifugation at 500 × g for 10 min at room temperature and washed once with the medium used for NET experiments.
Picogreen DNA measurement.
Extracellular DNA was quantitated by using a previously described method (49). Briefly, 2 × 105 neutrophils were seeded in triplicate into a 96-well cell culture plate (Costar, Corning, NY). Medium, phorbol myristate acetate (PMA), or parasites were added to the cells, and the plate was briefly centrifuged to synchronize the infection. At the indicated time points, 50 μl of 500 mU/ml micrococcal nuclease (Worthington Biochemical, Lakewood, NJ) was added, and the culture was incubated for 10 min at 37°C. Enzymatic digestion was terminated by using 5 mM EDTA, and cultures were centrifuged at 200 × g for 8 min. One hundred microliters of the cell-free supernatant was transferred into a flat-bottom 96-well plate for the quantification of double-stranded DNA using the Quant-iT Picogreen assay (Invitrogen, Carlsbad, CA). One hundred microliters of Picogreen reagent was added to the samples, which were then incubated at room temperature in the dark for 4 min. Extracellular DNA was measured with a spectrofluorometer at 480-nm excitation and 520-nm emission.
Sytox Green viability assay.
Mouse neutrophils were incubated with parasites tagged with Tomato Red protein (generated by B. Striepen, University of Georgia, and kindly provided by E. Robey, University of California—Berkeley) in the presence of 1 μM cytochalasin D (Calbiochem, Darmstadt, Germany) to inhibit parasite invasion. After 4 h at 37°C, the medium was aspirated, and coverslips were incubated with Sytox Green live-cell exclusion dye (Invitrogen, Carlsbad, CA) for 10 min at room temperature. Coverslips were then rinsed in PBS, mounted by using ProLong Antifade containing 4′,6-diamidino-2-phenylindole (DAPI) (Molecular Probes, Carlsbad, CA), and visualized by fluorescence microscopy.
ERK1/2 inhibition.
To inhibit ERK phosphorylation, cells were pretreated with 10 μM the MEK1/2 inhibitor U0126 [1,4-diamino-2,3-dicyano-1,4-bis(2-aminophenylthio)butadiene; Cell Signaling Technology, Danvers, MA] for 2 h at 37°C. Parasites (multiplicity of infection [MOI] of 5:1) or PMA (60 nM) was then added to the cells, and samples were subsequently collected for fluorescence microscopy or were lysed with SDS sample reducing buffer for Western blot analysis.
Western blotting.
Cell lysates were resolved by SDS-PAGE and immunoblotted by using anti-phospho-p44/42 MAPK (ERK1/2) and anti-phospho-P38 antibody (Ab) (Cell Signaling Technology, Danvers, MA). Blots were stripped and reprobed for total p44/42 ERK and p38 antibodies (Cell Signaling). Blots were visualized by using an ECL-based detection system (Thermo Scientific, Rockford, IL).
Immunofluorescence microscopy.
Neutrophils were incubated on poly-l-lysine-treated glass coverslips in a 24-well plate with medium, PMA, or Toxoplasma and centrifuged for 5 min at 200 × g to initiate infections, followed by incubation at 37°C for the indicated times. Samples were collected by gently removing coverslips, fixing them with 3% paraformaldehyde (20 min at room temperature), and were then blocked in PBS supplemented with normal mouse serum and fetal calf serum for 1 h at room temperature. Coverslips were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-Toxoplasma SAG-1 Ab (Argene, Massapequa, NY) and then washed and mounted with ProLong Antifade containing DAPI (Molecular Probes). Staining with anti-histone H3 Ab was accomplished according to the manufacturer's instructions (Cell Signaling Technology). Briefly, coverslips were fixed as described above and then permeabilized by using ice-cold 100% methanol for 10 min at −20°C, followed by washing in PBS. Coverslips were blocked for 1 h at room temperature and then incubated overnight with anti-histone H3 Ab. Coverslips were then washed three times with PBS and incubated with Alexa Fluor 647 goat anti-rabbit IgG(H+L) Ab (Invitrogen, Carlsbad, CA) for 2 h at room temperature. After rinsing in PBS, coverslips were mounted with ProLong Antifade containing DAPI. Images were collected with an Olympus BX51 fluorescence microscope equipped with a DP 70 camera using Olympus DP controller software and Olympus DP manager software or a Leica SP5 confocal microscope.
Mouse intranasal infection model.
A total of 5 × 107 RH strain parasites in PBS were administered to Swiss Webster mice intranasally in a total volume of 50 μl. Control mice were administered the same volume of sterile PBS intranasally. Mice were euthanized at 6 h postinfection, and lungs were perfused with 3% paraformaldehyde through tracheal injection. Lungs were then excised and further fixed by immersion in 3% paraformaldehyde overnight. Lungs were then paraffin embedded and sectioned for staining. Bronchoalveolar lavage fluid (BALF) was collected by washing the lungs with 200 μl of PBS twice. Samples were spun down at 1,000 rpm for 8 min, and supernatants were assessed for double-stranded DNA (dsDNA) content using the Picogreen assay described above.
Neutrophil depletion in vivo.
Mice (3 per group) were injected i.p. with 1 mg control rat IgG or monoclonal Ab (MAb) 1A8 (BioXCell, Lebanon, NH) on day −2 and day 0. On day 0, mice were administered 107 tachyzoites intranasally, and 6 h later, BALF (200 μl) was collected.
Statistical analyses.
Statistical analyses were performed by using Prism software. Two-way analysis of variance (ANOVA) unpaired tests with Turkey's posttest analysis and one-way ANOVAs with Bonferroni posttest analysis were used to determine statistical significance. P values of <0.05 were considered significant.
RESULTS
Mouse neutrophils undergo NETosis in response to PMA.
Most studies to date that examined NET formation have employed human neutrophils (11, 24, 25, 30). We wanted to determine whether mouse PMN could also release NETs after appropriate stimulation, particularly in response to T. gondii. We used thioglycolate-elicited, Percoll gradient-purified mouse neutrophils as a cell source (1). In addition, we employed cells isolated from LYS-eGFP knock-in mice, in which green fluorescent protein (GFP)-tagged lysozyme is constitutively expressed. The reasoning for this was that it was shown previously that lysozyme is retained inside cells undergoing NETosis (46). Since NETs are composed of a DNA backbone with histone molecules studded across the DNA fibers, we stained for both DNA and histones as a positive identifier of NET formation. As shown in Fig. 1A, neutrophils incubated in medium alone did not spontaneously extrude extracellular traps, as determined by the absence of staining for extracellular DNA and histone H3. However, we show in Fig. 1B that neutrophils incubated with PMA for 4 h formed extracellular traps, as determined by the release of DAPI-positive strand-like material (Fig. 1Ba) that also stained positive for histone H3 (Fig. 1Bb). Further confirmation for NETosis was that, as predicted, there was no concurrent release of intracellular lysozyme (Fig. 1Bc). To quantify NET formation over time, cells were stimulated with PMA, and they were then treated at specific time points with micrococcal nuclease to solubilize extracellular DNA. Cell-free supernatants were tested for nucleic acid content by using Picogreen, a fluorochrome that binds specifically to double-stranded DNA. As shown in Fig. 2A, PMA induced a robust release of DNA, which could be detected within 2 h of PMN stimulation.
Fig 1.

Thioglycolate-elicited mouse neutrophils produce NETs in response to PMA. Purified PMN from LYS-eGFP mice were incubated for 4 h in medium alone (A) or in medium with 600 μM PMA (B). NETs were visualized by staining with DAPI (a) and histone H3 (b). Lysozyme was retained within cells, confirming that NET formation was a regulated event rather than nonspecific cell lysis (c). Merged images are shown in panel d. The scale bar indicates 20 μm. These experiments were repeated at least 3 independent times, with similar results.
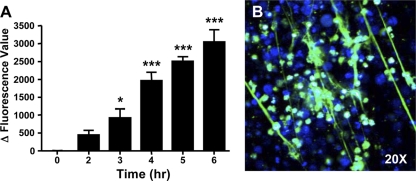
Fig 2.
Quantitative measurement of PMA and Toxoplasma-induced NET formation. Mouse neutrophils were either stimulated with 600 μM PMA (A) or infected with RH strain tachyzoites at an MOI of 5:1 (B), and NET formation was measured by solubilizing extracellular DNA and measuring Picogreen fluorescence in supernatants as an indicator of double-stranded DNA. Data for both experiments have been corrected to account for the contribution to the fluorescence signal by either cells incubated with medium alone or parasites incubated with medium alone. Experiments were repeated at least 3 independent times, with similar results. ∗, P < 0.05; ∗∗∗, P < 0.005 (relative to time zero).
Toxoplasma elicits NET formation by mouse neutrophils.
To determine whether Toxoplasma triggers the release of extracellular traps from mouse neutrophils, we incubated thioglycolate-elicited PMNs with tachyzoites and determined NET formation 4 h later. As shown in Fig. 3A to D, coincubation with the type I lineage RH strain stimulated the formation of NETs, as determined by DNA release (Fig. 3A) and staining for histone H3 (Fig. 3B). Furthermore, staining for Toxoplasma (Fig. 3C and D) showed evidence for parasite entrapment within the NETs. As with neutrophil stimulation with PMA (Fig. 2A), a rapid release of double-stranded DNA during coincubation with tachyzoites could also be quantitatively measured by Picogreen binding (Fig. 2B). There are 3 major clonal lineages of T. gondii that differ in the host responses elicited (32, 41), prompting us to ask whether they differ in their abilities to induce NETs. However, we found that, like the type I RH strain (Fig. 3A to D), both type II (Fig. 3E and F) and type III (Fig. 3G and H) parasite strains triggered the release of NETs in which parasites appeared to be entrapped.
Fig 3.
Release of NETs occurs in a parasite strain-independent manner. Purified mouse neutrophils were added to type I (RH) (A to D), type II (PTG) (E and F), and type III (CTG) (G and H) strains of Toxoplasma, and NET formation was assessed 4 h later by staining for DNA (A, F, and H) and histone H3 (B). Tachyzoites were visualized by staining with Ab to the parasite surface protein SAG-1 (C, E, and G). The insets in panels D, F, and H are merged and expanded images showing the entrapment of parasites within NETs. The scale bars indicate 20 μm. Experiments were repeated at least 3 independent times, with similar results.
Active invasion is not required for NET formation.
We envisioned that Toxoplasma could stimulate NET release during the invasion of host cells or that neutrophil DNA release could be triggered by soluble factors derived from extracellular parasites. To distinguish between these possibilities, we asked whether NET formation occurred in the presence of cytochalasin D, a drug blocking actin polymerization that is required for parasite entry into the host cell (20). We confirmed that this drug also blocked the invasion of neutrophils (data not shown). Figure 4A shows the formation of NETs when coincubation with parasites (shown in Fig. 4B) was carried out in the presence of cytochalasin D. The merged image shown in Fig. 4C also reveals clear evidence of parasite entrapment within NETs (Fig. 4C). We conclude that the invasion of host neutrophils is not required for NET induction by T. gondii.
Fig 4.
NET formation in murine neutrophils does not require invasion. Neutrophils were incubated with Toxoplasma (RH strain at an MOI of 5:1) for 4 h in the presence of cytochalasin D (1 μM). (A) DAPI staining of DNA revealing the presence of NETs. (B) The same image showing parasite SAG-1 staining. (C) Merged image. The merged image in panel C and the expanded area within the red box reveal SAG-1-positive material entrapped within a NET. The scale bar in panel C represents 10 μm. This experiment was performed on three independent occasions.
Killing of T. gondii in NETs.
We next assessed the viability of parasites entrapped within NETs, since these structures are known to contain antimicrobial components. To address this issue, we made use of Sytox Green, a live-cell exclusion dye which binds to dsDNA within dead cells or parasites and which would also be expected to bind NETs. These experiments also employed a transgenically modified RH parasite strain that constitutively expresses Red Tomato fluorescent dye. In the presence of cytochalasin D, neutrophils generated NETs (Fig. 5A) during coincubation with fluorescent tachyzoites (Fig. 5B). Staining with Sytox Green revealed that many tachyzoites were nonviable (Fig. 5C and D). In this experiment, fewer than 1% of tachyzoites were stained with this live-cell exclusion dye in the absence of neutrophils, but after coincubation, approximately 25% of parasites were Sytox Green positive (Fig. 5I). To determine whether parasite death was dependent upon NET formation as opposed to some other mechanism of killing, parallel experiments were performed in the presence of DNase. As expected, the inclusion of this nuclease prevented the formation of NETs (Fig. 5E and G). Strikingly, tachyzoites that were present in these cultures (Fig. 5F) failed to take up Sytox Green (Fig. 5G and H). In this experiment, fewer than 1% of parasites took up this dye when DNase was included to prevent NET formation (Fig. 5I). We conclude that NETs promote the extracellular killing of parasites.
Fig 5.
Parasites are killed in the presence of NETs. (A to D) Neutrophils were incubated with transgenic RH strain parasites expressing Tomato Red fluorescent protein in the presence of cytochalasin D, and 4 h later, cells were stained with the live-cell exclusion DNA dye Sytox Green. (A) DAPI stain; (B) red fluorescence associated with parasites; (C) Sytox Green staining; (D) Merged image. Panel D and the enlarged field show the merged image. Yellow arrows indicate parasites whose nuclei were stained with Sytox Green (nonviable), and red arrows indicate tachyzoites (TZ) that exclude the dye (viable). (E to H) In parallel, the experiment was carried out in the presence of DNase (1 μg/ml). Under this condition, NETs failed to form (E and G), and parasites (F) excluded Sytox Green (G and H). (I) Quantitation of Sytox Green-positive parasites identified by red fluorescence, incubated alone (TZ), in the presence of neutrophils (PMN/TZ), and in the presence of neutrophils with DNase (PMN/TZ/DNase). ∗, P < 0.05. This experiment was performed on three independent occasions.
We performed plaque assays to confirm the inactivation of tachyzoites by NETs. As shown schematically in Fig. 6A, tachyzoites were cultured with or without neutrophils in the presence of cytochalasin D to prevent invasion and phagocytosis. After 6 h, parasites were recovered and plated onto fibroblast monolayers, and plaques (indicative of zones of lysis) were then enumerated 5 to 7 days later. Figure 6B shows a plaque assay carried to completion, indicating that RH strain parasites recovered from the neutrophil coculture were impaired in their ability to cause fibroblast lysis compared to parasites incubated without neutrophils. In Fig. 6C, we counted plaques prior to complete lysis and found an approximately 50% decrease in numbers of plaque-forming tachyzoites of both the RH and type II PTG strains after coincubation with neutrophils.
Fig 6.
Decreased parasite viability following incubation with neutrophils. (A) Schematic showing the experimental setup. Parasites were incubated in the presence of cytochalasin D with or without neutrophils for 6 h. The contents of the wells were collected, washed, and added to fibroblast (Fϕ) monolayers to measure parasite infectivity. The formation of plaques, indicative of foci of infection, was visualized by the staining of monolayers with Diff-Quik. (B) Example of fibroblast monolayers in which lysis was allowed to proceed to completion in RH-infected cultures compared in parallel to the same number of tachyzoites coincubated with neutrophils. (C) Plaque formation after the addition of RH and PTG tachyzoites preincubated in the presence or absence of neutrophils. Plaques were enumerated on day 5 (RH) and day 7 (PTG) following inoculation onto fibroblast monolayers.
Human neutrophils release NETs in response to T. gondii.
Next, we sought to determine whether human neutrophils also responded to Toxoplasma with the formation of extracellular traps. To address this question, we first assessed the extracellular release of nuclear DNA by employing the human promyelocytic leukemia cell line HL-60, which can be induced to differentiate into mature, neutrophil-like myeloid cells when treated with dimethyl sulfoxide (15). We determined whether differentiated HL-60 cells responded to parasites by extruding DNA by using the Picogreen DNA assay. As shown in Fig. 7A, PTG parasites stimulated a robust release of extracellular DNA, with kinetics similar to those seen with mouse PMN (Fig. 2A). An examination of the cells revealed the presence of Sytox Green-positive DNA strands similar to that with stimulated mouse neutrophils (Fig. 7B). In our hands, parasites provided an even stronger stimulus for NET formation than lipopolysaccharide (LPS) or PMA (data not shown).
Fig 7.
Differentiated HL-60 cells form extracellular traps in response to Toxoplasma. HL-60 cells were differentiated into neutrophil-like cells with DMSO and then subsequently cultured with T. gondii. (A) Extracellular DNA release relative to medium controls was measured by a Picogreen binding assay. ∗, P < 0.05; ∗∗∗, P < 0.005. (B) Image collected 6 h after culture showing Picogreen-positive NET-like structures induced by Toxoplasma. The experiments were repeated at least 3 independent times, with similar results.
We then sought to determine whether or not freshly isolated human peripheral blood neutrophils also released NETs upon parasite stimulation. Human neutrophils activated with PMA underwent a robust, time-dependent release of extracellular DNA, as measured by Picogreen staining (Fig. 8A). Cells infected with Toxoplasma also underwent a similar release of extracellular DNA, and as predicted from the behavior of mouse neutrophils, the blocking of invasion with cytochalasin D had no effect on DNA release (Fig. 8A). NET release by human neutrophils was confirmed visually by an immunofluorescence assay (Fig. 8B to E). Cells were incubated with T. gondii in the presence of cytochalasin D for 4 h and then fixed and stained for parasites and DNA. Figure 8B and C dramatically shows an extensive network of extracellular traps formed in response to Toxoplasma. In the merged image (Fig. 8D), evidence for the entrapment of parasites is clearly visible.
Fig 8.
Human peripheral blood neutrophils produce NETs in response to Toxoplasma. (A) Purified neutrophils were incubated with 60 nM PMA or tachyzoites (MOI of 5:1) with or without cytochalasin D (CytD) (1 μM), and the release of DNA was measured over time by using a Picogreen fluorescence assay. Tg, Toxoplasma gondii. ∗, P < 0.05; ∗∗, P <0.01; ∗∗∗, P < 0.005. (B and C) Visual confirmation of NET formation in response to parasites at 4 h postincubation as determined by DNA release (B) in the proximity of parasites (C). (D and E) The merged image (D) and the expanded view (E) show extensive parasite entrapment within NETs. Results are representative of at least 3 independent experiments.
Inhibition of ERK1/2 MAPK activation blocks Toxoplasma-induced NET formation.
The intracellular control of NET formation is still not well understood, but there is evidence for the involvement of a Raf-MEK-ERK signaling pathway (30). Therefore, we sought to determine if Toxoplasma activates the ERK1/2 pathway and whether this signaling kinase is involved in parasite-induced NETosis. Accordingly, we infected human neutrophils with parasites and collected samples for Western blot analysis of ERK1/2 activation. Figure 9A demonstrates that Toxoplasma triggers ERK1/2 phosphorylation as early as 5 min postinfection and that phosphorylation was sustained for at least 1 h. The incubation of the cells with medium alone did not induce any noticeable phosphorylation of ERK. To determine whether ERK1/2 phosphorylation could be blocked, we used U0126, a well-known chemical inhibitor of MEK1/2 that serves as the upstream MAPK kinase phosphorylating ERK1/2. Cells were treated for 2 h with U0126 and then infected with RH strain parasites in the continued presence of the inhibitor. As expected, the inhibitor completely blocked ERK1/2 phosphorylation triggered by Toxoplasma infection (Fig. 9A). In the same cell lysates, we found high levels of p38 MAPK activation even in the absence of parasites, and the phosphorylation of this MAPK was not affected by the inhibitor.
Fig 9.
Activation of ERK1/2 in response to Toxoplasma controls NET extrusion. (A) Human peripheral blood neutrophils were incubated in medium alone, with Toxoplasma (Tg) alone, or with the MEK inhibitor U0126. Western blotting was performed to determine the phosphorylation of ERK and p38 MAPK, and blots were stripped and reprobed for total MAPK levels. (B) Images over time from DAPI-stained cells incubated with parasites (top) or with parasites and the ERK inhibitor (bottom). (C) NET formation over time by neutrophils either treated or not treated with the MEK1/2 inhibitor using the Picogreen DNA measuring kit. These experiments were repeated 3 times, with similar results. ∗, P < 0.05; ∗∗, P <0.01; ∗∗∗, P < 0.005; ns, not significant.
We next asked whether the inhibition of ERK1/2 activation affected NETosis triggered by Toxoplasma. Neutrophils in the presence of the MEK1/2 inhibitor or an equimolar concentration of DMSO were infected with Toxoplasma, and NET formation was monitored. As expected, NETs were formed in the presence of parasites in medium alone (Fig. 9B and C). NETosis was decreased when ERK1/2 activation was blocked. Nevertheless, despite the potency of U0126 in blocking ERK activation, NET formation was not completely inhibited, and there was no significant difference in the presence and absence of the inhibitor at later time points (Fig. 9C). Similar results were obtained with another MEK inhibitor (PD98059) (data not shown). We conclude that optimal NET formation requires ERK1/2 signaling but that this MAPK is not absolutely needed for the formation of extracellular traps.
We addressed the role of Toll-like receptor (TLR) signaling in mouse NET formation by comparing the responses of neutrophils from MyD88−/− mice to those of neutrophils from MyD88+/+ littermates. However, in multiple experiments (n = 4), we found that NETs were formed by MyD88-negative neutrophils at levels comparable to those of wild-type cells in response to Toxoplasma (data not shown). Therefore, it is most likely that TLR signaling in mouse neutrophils does not drive NET formation in response to the parasite.
Evidence that Toxoplasma induces NET formation in vivo.
Finally, we asked whether T. gondii could elicit NET formation by PMN recruited at a site of acute inflammation. To address this question, we employed a Toxoplasma mouse infection model in which parasites were administered intranasally, and lungs were assessed 6 h later for parasite colonization, neutrophil recruitment, and NET induction. We employed this model because we wanted to introduce a single dose of parasites in a tightly controlled situation. However, we also note that T. gondii does indeed disseminate to the lungs during human and animal infections (19, 37). Lungs from mice injected with PBS displayed normal pulmonary architecture with minimal cellular infiltration (Fig. 10A), no detectable myeloperoxidase (MPO)-positive cells (Fig. 10B), and no Toxoplasma Ab-reactive cells (Fig. 10C). However, lungs from infected mice lost the normal pulmonary architecture, and this was associated with massive cellular infiltration (Fig. 10D). The infiltrating cells were composed mostly of neutrophils, as determined by the expression of MPO in serial sections (Fig. 10E). Large numbers of parasites were also present in the lung tissue (Fig. 10F). Therefore, in this in vivo model of parasite-induced inflammation, neutrophils and Toxoplasma are brought into close proximity in infected tissue.
Fig 10.
(A to F) Neutrophils form NETs in vivo during Toxoplasma infection. Mice were injected intranasally with PBS as a control (A to C) or infected intranasally with PTG strain parasites (D to F). Six hours later, lungs were collected and sectioned, and serial sections were stained with hematoxylin and eosin (HE) (A and D), Ab to myeloperoxidase (MPO) (B and E), and an anti-T. gondii antiserum (TOXO) (C and F). (G) Mice were administered control IgG or neutrophil-depleting MAb 1A8 and then administered PBS or RH strain tachyzoites intranasally. After 6 h, BALF was collected, and dsDNA was measured in cell-free supernatants. (H) BALF cell pellets from the intranasal infection were plated onto fibroblast monolayers, and 5 days later, plaques were enumerated. The experiments were repeated three times, with similar results.
Next, bronchoalveolar lavage fluid (BALF) was collected from the lungs of mice administered PBS and Toxoplasma-infected mice that had also been administered control rat IgG. After a spin-down step to remove cells, supernatants were tested for dsDNA as a measure of NET formation. Samples from Toxoplasma-infected mice displayed greatly increased amounts of dsDNA compared to control mice injected with PBS, consistent with the release of NETs in response to infection (Fig. 10G). To determine whether this was due to the presence of neutrophils, mice were administered MAb 1A8 to deplete Ly6G-positive neutrophils. As shown in Fig. 10G, this treatment resulted in an inability to recover soluble DNA from the lungs of infected mice. Finally, we recovered BALF from infected control and neutrophil-depleted mice and assessed parasite infectivity by a plaque assay. As shown in Fig. 10H, there was a major increase in the recovery of infective parasites in neutrophil-depleted mice. Together, the data suggest that in this model, neutrophil recruitment to the site of infection results in NET release that negatively impacts the infectivity of parasites.
DISCUSSION
Neutrophil extracellular traps have recently emerged as powerful weapons used by PMN to combat microbial pathogens. The results of the present study demonstrate that both mouse and human PMN undergo NET production in response to the protozoan pathogen T. gondii. We report that neutrophils respond to Toxoplasma infection by releasing extracellular traps and that DNA release is not dependent on active invasion by the parasite or host cell phagocytic function. PMN formed extracellular traps in response to all 3 clonal lineages of T. gondii. We also show that parasite-induced NET release involves a MEK-ERK pathway. Finally, we found evidence for NET formation during in vivo infection with Toxoplasma.
Neutrophil extracellular traps are composed primarily of DNA, histones, and other antimicrobial components, forming an extracellular mesh that can trap and inactivate pathogens (52). In addition to histones, the antimicrobial components have been shown to include bacterial permeability-increasing protein (BPI), myeloperoxidase, as well as neutrophil elastase (11, 53), which are effective in killing Gram-positive and Gram-negative bacteria (11, 24). It has also been shown that NETs are an important protective mechanism during fungal infection and that they contain calprotectin, a molecule involved in defense against Candida albicans (46, 47). We also obtained evidence for tachyzoite killing that depended upon the formation of NETs. At present, we do not know what specific components of the NETs mediate parasite killing.
We hypothesize that another major functional consequence of NET formation in the context of T. gondii infection may be that NETs interfere with host cell invasion by ensnaring parasites. As an obligate intracellular protozoan, it is essential for Toxoplasma to establish itself within its intracellular niche so that it can scavenge the host cell for essential nutrients, enabling parasite survival and replication. Therefore, any parasite entrapped within a NET will ultimately die as a result of the failure to successfully invade a host cell.
The results reported here are among the first to show NET formation in response to protozoan parasites. Elsewhere, it was reported previously that Leishmania triggers NET formation. In the case of Leishmania amazonensis, NET entrapment resulted in parasite killing that was partly dependent upon the presence of histones (28). Other studies with Leishmania donovani promastigotes showed the formation of NETs, but in this case, parasites evaded killing in dependence upon the expression of lipophosphoglycan (25). There is also evidence for Plasmodium falciparum-associated NET formation, and the elicitation of antinuclear antibodies as a result of DNA release may play a role in pathology in infected children (3). Collectively, these studies contribute to an emerging view that the release of extracellular traps by neutrophils is a critical innate defense mechanism that is elicited by a broad range of microbial pathogens.
NETosis is a newly recognized pathway of programmed fatality that enables neutrophils to exert antimicrobial activity even after the cells have died. The process of NETosis appears to involve histone citrullination-dependent chromatin decondensation and the disintegration of the nuclear membrane, followed by the mixing of nuclear and cytoplasmic effector proteins before eventual release into the extracellular milieu (12, 24, 36, 51). The molecular mechanisms underlying this novel type of programmed cell death are an area of intense investigation (39). NETosis has been shown to depend on the generation of reactive oxygen species and a fully functional NADPH complex (40). Neutrophils isolated from patients with chronic granulomatous disease (CGD), who have defects in NADPH oxidase activity, fail to exhibit NET formation (7, 24). Also, myeloperoxidase and neutrophil elastase have been shown to regulate the formation of neutrophil extracellular traps (33, 35). Recently, Hakkim et al. identified a signaling pathway involved in extracellular trap formation that involves a Raf-MEK-ERK pathway (30). We previously reported that Toxoplasma triggers Jun N-terminal protein kinase (JNK) MAPK activation in neutrophils (44), prompting us to examine whether ERK activation could mediate parasite-induced NET extrusion. Indeed, we found evidence for parasite-triggered ERK phosphorylation, and the pharmacological inhibition of this cascade blocked, at least partially, the NET response to T. gondii infection.
We considered the possibility that NET formation was triggered from within infected cells. However, the blocking of invasion with cytochalasin D had no effect on the formation of NETs. In addition, a soluble sonicated parasite lysate could also trigger NET release although not as efficiently as live parasites (data not shown). Taken together, it seems most likely that the production of extracellular traps is mediated by factors released by extracellular tachyzoites. Nevertheless, while cytochalasin D blocks invasion, parasites are still able to attach to the host cell surface and discharge proteins into the host cell (29, 42). It is possible that this phenomenon is involved in stimulating NET extrusion.
The in vivo relevance of NET formation during infection with Toxoplasma and other microorganisms is not yet clear. However, we used a mouse intranasal infection model to obtain evidence for a role of NET formation in innate pulmonary defense against T. gondii. While this is not a natural route of infection, it is nevertheless well established that parasites disseminate to the lungs and cause pneumonia in humans and animals (19, 37). Furthermore, the MyD88-dependent recruitment of neutrophils to the lamina propria occurs after oral infection with the parasite, and there is evidence that neutrophils protect against infection (9, 16, 45). Whether protection is a result of interference with invasion by NET entrapment or other neutrophil antimicrobial functions is not known and may be difficult to determine until more is known about the control of NET extrusion. Regardless, our data and those of others demonstrate that NET release is an event that is triggered upon an encounter with both prokaryotic and eukaryotic microbial pathogens. How this functions in host defense against infection or possibly might contribute to the disease process will be important issues to address in the future.
ACKNOWLEDGMENTS
We thank M. Hossain for expert technical assistance.
This work was supported by Public Health Services grants AI47888 (E.Y.D.), AI085332 (G.E.D.), and HL018208 (M.R.K.).
Footnotes
Published ahead of print 21 November 2011
REFERENCES
- 1. Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. 2011. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int. Immunol. 23:317–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Appelberg R. 2007. Neutrophils and intracellular pathogens: beyond phagocytosis and killing. Trends Microbiol. 15:87–92 [DOI] [PubMed] [Google Scholar]
- 3. Baker VS, et al. 2008. Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age. Malar. J. 7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ball CJ, King MR. 2011. Role of c-Abl in L-selectin shedding from the neutrophil surface. Blood Cells Mol. Dis. 46:246–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beiter K, et al. 2006. An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr. Biol. 16:401–407 [DOI] [PubMed] [Google Scholar]
- 6. Bennouna S, Bliss SK, Curiel TJ, Denkers EY. 2003. Cross-talk in the innate immune system: neutrophils instruct early recruitment and activation of dendritic cells during microbial infection. J. Immunol. 171:6052–6058 [DOI] [PubMed] [Google Scholar]
- 7. Bianchi M, et al. 2009. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114:2619–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bliss SK, Butcher BA, Denkers EY. 2000. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J. Immunol. 165:4515–4521 [DOI] [PubMed] [Google Scholar]
- 9. Bliss SK, Gavrilescu LC, Alcaraz A, Denkers EY. 2001. Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology. Infect. Immun. 69:4898–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bliss SK, Marshall AJ, Zhang Y, Denkers EY. 1999. Human polymorphonuclear leukocytes produce IL-12, TNF-a, and the chemokines macrophage-inflammatory protein-1a and -1b in response to Toxoplasma gondii antigens. J. Immunol. 162:7369–7375 [PubMed] [Google Scholar]
- 11. Brinkmann V, et al. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532–1535 [DOI] [PubMed] [Google Scholar]
- 12. Brinkmann V, Zychlinsky A. 2007. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 5:577–582 [DOI] [PubMed] [Google Scholar]
- 13. Buchanan JT, et al. 2006. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 16:396–400 [DOI] [PubMed] [Google Scholar]
- 14. Charmoy M, et al. 2010. Neutrophil-derived CCL3 is essential for the rapid recruitment of dendritic cells to the site of Leishmania major inoculation in resistant mice. PLoS Pathog. 6:e1000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Collins SJ, Ruscetti FW, Gallagher RE, Gallo RC. 1979. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J. Exp. Med. 149:969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Del Rio L, Bennouna S, Salinas J, Denkers EY. 2001. CXCR2 deficiency confers impaired neutrophil recruitment and increased susceptibility during Toxoplasma gondii infection. J. Immunol. 167:6503–6509 [DOI] [PubMed] [Google Scholar]
- 17. Del Rio L, et al. 2004. Toxoplasma gondii triggers myeloid differentiation factor 88-dependent IL-12 and chemokine ligand 2 (monocyte chemoattractant protein 1) responses using distinct parasite molecules and host receptors. J. Immunol. 172:6954–6960 [DOI] [PubMed] [Google Scholar]
- 18. Denkers EY, Del Rio LD, Bennouna S. 2003. Neutrophil production of IL-12 and other cytokines during microbial infection. Chem. Immunol. Allergy 83:95–114 [DOI] [PubMed] [Google Scholar]
- 19. Derouin F, Garin YJ. 1991. Toxoplasma gondii: blood and tissue kinetics during acute and chronic infections in mice. Exp. Parasitol. 73:460–468 [DOI] [PubMed] [Google Scholar]
- 20. Dobrowolski JM, Sibley LD. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 84:933–939 [DOI] [PubMed] [Google Scholar]
- 21. Dubey JP. 1998. Advances in the life cycle of Toxoplasma gondii. Int. J. Parasitol. 28:1019–1024 [DOI] [PubMed] [Google Scholar]
- 22. Faurschou M, Borregaard N. 2003. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 5:1317–1327 [DOI] [PubMed] [Google Scholar]
- 23. Faurschou M, Sorensen OE, Johnsen AH, Askaa J, Borregaard N. 2002. Defensin-rich granules of human neutrophils: characterization of secretory properties. Biochim. Biophys. Acta 1591:29–35 [DOI] [PubMed] [Google Scholar]
- 24. Fuchs TA, et al. 2007. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176:231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gabriel C, McMaster WR, Girard D, Descoteaux A. 2010. Leishmania donovani promastigotes evade the antimicrobial activity of neutrophil extracellular traps. J. Immunol. 185:4319–4327 [DOI] [PubMed] [Google Scholar]
- 26. Gazzinelli RT, et al. 1996. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent upon CD4+ T cells and accompanied by overproduction of IL-12, IFN-g, and TNF-a. J. Immunol. 157:798–805 [PubMed] [Google Scholar]
- 27. Grinberg N, Elazar S, Rosenshine I, Shpigel NY. 2008. Beta-hydroxybutyrate abrogates formation of bovine neutrophil extracellular traps and bactericidal activity against mammary pathogenic Escherichia coli. Infect. Immun. 76:2802–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guimaraes-Costa AB, et al. 2009. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U. S. A. 106:6748–6753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hakansson S, Charron AJ, Sibley LD. 2001. Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J. 20:3132–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hakkim A, et al. 2011. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 7:75–77 [DOI] [PubMed] [Google Scholar]
- 31. Hill D, Dubey JP. 2002. Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infect. 8:634–640 [DOI] [PubMed] [Google Scholar]
- 32. Howe DK, Sibley LD. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human diseases. J. Infect. Dis. 172:1561–1566 [DOI] [PubMed] [Google Scholar]
- 33. Metzler KD, et al. 2011. Myeloperoxidase is required for neutrophil extracellular trap formation: implications for innate immunity. Blood 117:953–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nathan C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6:173–182 [DOI] [PubMed] [Google Scholar]
- 35. Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. 2010. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191:677–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Papayannopoulos V, Zychlinsky A. 2009. NETs: a new strategy for using old weapons. Trends Immunol. 30:513–521 [DOI] [PubMed] [Google Scholar]
- 37. Peterson E, Liesenfeld O. 2007. Clinical disease and diagnostics, p 81–100 In Weiss LM, Kim K. (ed), Toxoplasma gondii. The model apicomplexan: perspectives and methods. Academic Press, Amsterdam, Netherlands [Google Scholar]
- 38. Ramos-Kichik V, et al. 2009. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis (Edinb.) 89:29–37 [DOI] [PubMed] [Google Scholar]
- 39. Remijsen Q, et al. 2011. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 18:581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Remijsen Q, et al. 2011. Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 21:290–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saeij JP, Boyle JP, Boothroyd JC. 2005. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends Parasitol. 21:476–481 [DOI] [PubMed] [Google Scholar]
- 42. Saeij JP, et al. 2007. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 445:324–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sher A, et al. 1992. Role of T cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol. Rev. 127:183–204 [DOI] [PubMed] [Google Scholar]
- 44. Sukhumavasi W, Egan CE, Denkers EY. 2007. Mouse neutrophils require JNK2 MAPK for Toxoplasma gondii-induced IL-12p40 and CCL2/MCP-1 release. J. Immunol. 179:3570–3577 [DOI] [PubMed] [Google Scholar]
- 45. Sukhumavasi W, et al. 2008. TLR adaptor MyD88 is essential for pathogen control during oral Toxoplasma gondii infection but not adaptive immunity induced by a vaccine strain of the parasite. J. Immunol. 181:3464–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Urban CF, et al. 2009. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5:e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Urban CF, Reichard U, Brinkmann V, Zychlinsky A. 2006. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 8:668–676 [DOI] [PubMed] [Google Scholar]
- 48. van Gisbergen KP, Geijtenbeek TB, van Kooyk Y. 2005. Close encounters of neutrophils and DCs. Trends Immunol. 26:626–631 [DOI] [PubMed] [Google Scholar]
- 49. von Kockritz-Blickwede M, Chow O, Ghochani M, Nizet V. 2010. Visualization and functional evaluation of phagocyte extracellular traps. Methods Microbiol. 37:139–160 [Google Scholar]
- 50. von Kockritz-Blickwede M, et al. 2008. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 111:3070–3080 [DOI] [PubMed] [Google Scholar]
- 51. Wang Y, et al. 2009. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 184:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wartha F, Beiter K, Normark S, Henriques-Normark B. 2007. Neutrophil extracellular traps: casting the NET over pathogenesis. Curr. Opin. Microbiol. 10:52–56 [DOI] [PubMed] [Google Scholar]
- 53. Weinrauch Y, Drujan D, Shapiro SD, Weiss J, Zychlinsky A. 2002. Neutrophil elastase targets virulence factors of enterobacteria. Nature 417:91–94 [DOI] [PubMed] [Google Scholar]
- 54. Yang D, de la Rosa G, Tewary P, Oppenheim JJ. 2009. Alarmins link neutrophils and dendritic cells. Trends Immunol. 30:531–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yousefi S, et al. 2008. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 14:949–953 [DOI] [PubMed] [Google Scholar]