Abstract
Acute inflammatory activation of macrophages by Toll-like and related receptors is characterized by transient activation of MAPK-, NF-κB- and IRF-mediated signaling pathways and expression of pro-inflammatory genes. This activation state is inherently unstable and often transitions into a state of `tolerance' characterized by diminished signaling, repressive chromatin modifications, and an alternative gene expression program. This Viewpoint describes signaling and epigenetic mechanisms associated with transition to tolerant states, which are proposed to correspond to alternative activation states programmed by the original inflammatory stimuli.
Keywords: Alternative activation, Epige netics, Macrophages, Signal transduction, Tolerance
Acute inflammatory state
Rapid activation of an acute inflammatory response in macrophages is a key part of innate immunity and host defense. Acute macrophage activation is mediated by receptors that sense microbial products and activate potent inflammatory signaling pathways. Key activating receptors that sense microbial products include TLRs, NOD-like receptors (NLRs), RIG-I-like receptors and C-type lectin receptors [1]. These receptors, and ITAM-associated receptors and inflammasomes, also sense endogenous `danger' signals, such as those resulting from tissue damage/degradation and cell necrosis. In addition, acute macrophage activation is mediated by receptors for inflammatory cytokines such as TNF. Although proximal signaling by these receptors varies, and complex signals are generated to fine tune and focus functional responses, these receptors all activate a core inflammatory signaling program important for acute activation [1]. This core response consists of activation of MAPKs and of NF-κB and IRF transcription factors, leading to transient expression of key cytokines, chemokines and inflammatory mediators (Fig. 1A).
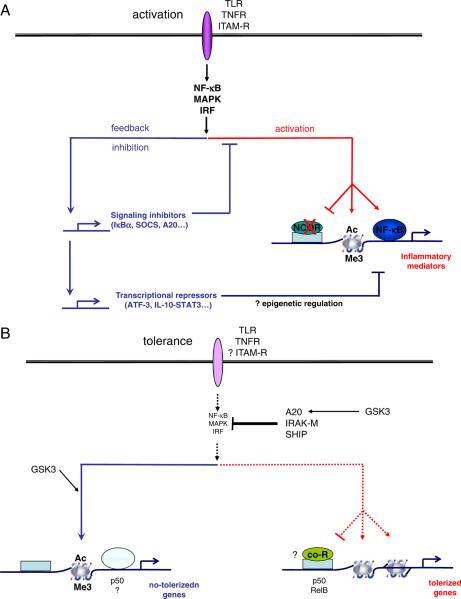
Figure 1.
(A) Acute macrophage activation. Core inflammatory signaling downstream of activating receptors that is mediated by MAPKs, NF-κB and IRFs activates inflammatory gene expression by inducing transcription factors to bind to inflammatory gene loci, chromatin modifications such as acetylation (Ac) or trimethylation (Me3) of histones, and dismissal of transcriptional repressors such as NCoR. At the same time, the core inflammatory signals induce feedback inhibition mechanisms that restrain the magnitude of activation and terminate the acute phase of signaling and gene expression. Feedback inhibition is mediated by signaling inhibitors including IκBa, SOCS1 and A20, and transcriptional repressors including STAT3 and ATF-3. (B) Transition to tolerance and alternative activation. Several hours after strong activation as depicted in (A), upon rechallenge with the same activating stimuli inflammatory signaling by MAPKs, NF-κB and IRFs is suppressed by signaling inhibitors that include A20, IRAK-M, and SHIP. In addition, chromatin at inflammatory gene loci assumes a repressive configuration, including diminished acetylation and methylation of select histone lysine residues. Expression of nontolerized genes is maintained and further induced by inflammatory stimulation. Mechanisms regulating expression of nontolerized genes are not well understood, but appear to involve low-level core inflammatory signals (MAPKs), alternative signaling pathways (GSK3, p50) and permissive chromatin remodeling.
More recently, the importance of transmitting signals to transcription factors and chromatin at inflammatory gene loci has been appreciated [2, 3] (Fig. 1). Post-translational modification of transcription factors and chromatin proteins (the latter termed `epigenetic regulation' in this context) plays a key role in regulating inflammatory gene expression. For example, one function of MAPK signaling in inflammatory gene induction is the modification of chromatin, such as phosphorylation of histone 3 on serine 10 (H3S10) by p38-MSK signaling that facilitates the removal of repressive chromatin marks. In addition, MAPKs and NF-κB play a role in the dismissal of prebound transcriptional repressors and corepressors, such as Bcl6, NCoR and SMRT, from inflammatory gene loci [4, 5]. This `dismissal' of prebound repressors removes a set of brakes that prevents baseline expression of inflammatory genes, and such removal facilitates responses to positive gene-activating signals. Inflammatory gene expression is associated with additional chromatin modifications such as histone acetylation and methylation and nucleosome remodeling that open gene loci and facilitate transcription and transcript elongation [2, 3, 6–8]. Thus, chromatin is a target and transducer of inflammatory signaling, and specific chromatin proteins can be therapeutically targeted to suppress inflammation [9]. An important question is how the epigenetic landscapes that are induced during development and prior environmental stimulation of macrophages influence cell responses to incoming signals to achieve gene-specific and finely tailored gene expression responses ([10] and discussed in this Viewpoint series [11, 12]).
Post-activation transition state: Feedback inhibition and termination of acute inflammatory signaling
Strong inflammatory signals such as those induced by TLRs concomitantly activate feedback inhibitory mechanisms that restrain the magnitude of inflammatory responses by attenuating inflammatory signaling and gene expression [13] (Fig. 1A). Thus, expression of inflammatory genes such as Tnf and Il6 is transient and returns to baseline several hours after TLR stimulation. Feedback inhibition of inflammatory receptor signaling has been studied extensively and conceptually falls into two categories: (i) termination of the input signal and (ii) active repression of inflammatory gene expression. The input signal is terminated by receptor desensitization and by induction of signaling inhibitors that can also suppress signaling by heterologous activating receptors. Prominent examples of induced feedback inhibitors of signaling are the SOCS and A20 proteins that inhibit proximal signaling by multiple receptors, and IκBα and MAPK phosphatases (termed MKPs or DUSPs) that inhibit more downstream signaling events. SOCS, A20, IκBα and MKP expression are induced by the same NF-κB and MAPK pathways that they feed back to inhibit (Fig. 1A).
In addition to termination of positive signaling, the acute inflammatory response induces active repressive mechanisms that downregulate inflammatory gene expression [13]. The most established of these mechanisms is induction of IL-10 that activates STAT3 to suppress inflammatory gene expression. The mechanism of STAT3 action has remained elusive, but STAT3 likely works indirectly by inducing transcriptional repressors that in turn directly suppress inflammatory genes [14]. The importance of the IL-10-STAT3 feedback mechanism is highlighted by the substantially increased cytokine production and inflammatory pathology that occur in its absence. Inflammatory signaling also directly induces transcriptional repressors of inflammatory genes, such as ATF-3 [15]; IκBα also terminates NF-κB target gene expression by promoting export of NF-κB components from the nucleus to the cytoplasm [16]. Finally, inflammatory gene expression is restrained by induction of micro RNAs that promote mRNA degradation and suppress protein translation ([17] and this Viewpoint series [18]). One interesting question that remains to be investigated is whether epigenetic changes in chromatin play an active or causal role in termination of inflammatory gene transcription.
Transition to tolerant and alternative activation states
The above described initial and feedback inhibition phases of `classical' macrophage activation have been well studied and are mediated by the same core signaling pathways. Although activating signals can oscillate, for the most part the initial phase of signaling terminates after two to four hours. Less is known about the subsequent phases of `classical' macrophage activation and the signaling events that mediate activation and differentiation. A common functional outcome after exposure of macrophages to strong inflammatory stimuli, such as TLR ligands, is the development of a tolerant state that begins approximately six hours after stimulation and lasts for several days [19]. This state, termed endotoxin tolerance, is characterized by refractoriness of inflammatory gene induction to activation by subsequent challenge by TLR ligands. Thus, many genes that were activated during the initial activation phase, especially those encoding inflammatory cytokines, are repressed and are no longer inducible (termed tolerizable genes). Tolerization is gene-specific, as other genes, for example genes encoding anti-microbial peptides, are expressed and further induced on LPS challenge of tolerized macrophages. A well-described mechanism of endotoxin tolerance is suppressed TLR signaling; this signaling block is mediated by desensitization of TLR4 and induction of molecules such as IRAK-M, SHIP, SOCS1 and A20 that inhibit proximal TLR signaling [19] (Fig. 1B). Recent reports have shown that regulation at the level of chromatin plays an important role in endotoxin tolerance [20–22]. Thus, in tolerized macrophages, induction of permissive chromatin marks and nucleo-some remodeling at inflammatory gene loci are suppressed, possibly by transcriptional repressors such as NF-κB p50 homodimers or chromatin-modifying enzymes such as G9a and HP-1 [20–23] (Fig. 1B). In contrast, nontolerized genes exhibit a permissive open chromatin environment that allows ongoing gene expression. Thus, epigenetic regulation allows fine tuning of incoming signals and gene-specific regulation.
An interesting question concerns the functional phenotype of tolerized macrophages. Gene expression studies demonstrating expression of nontolerizable genes encoding antimicrobial proteins have suggested that tolerized macrophages can participate in host defense while avoiding the excessive toxicity associated with high cytokine production. Tolerized macrophages, prior and subsequent to challenge with LPS, express a large number of genes involved in various macrophage functions at higher levels than naive macrophages [21, 23]. Thus, tolerized cells may also mediate additional macrophage functions related to metabolism, homeostasis, regulation of adaptive immunity and tissue remodeling. Indeed, LPS-tolerized macrophages express genes, such as Ccl2, Ccl17, Ccl22 and Arginase1, that are typically expressed by `M2' (alternatively activated macrophages) [23]. Combined initial stimulation of TLRs and ITAM-associated receptors leads to high IL-10 production and an M2-related phenotype with expression of a subset of genes characteristic of alternatively activated or regulatory macrophages ([24, 25] and discussed in this Viewpoint series [26]). Thus, TLR-tolerized macrophages express aspects of alternative activation and may correspond to a subtype on the spectrum of M2 macrophages [25–28]. In contrast to direct polarization towards alternative activation after exposure to IL-4/IL-13, IL-10 or glucocorticoids, macrophages stimulated by TLR ligands first pass through an inflammatory `M1-like' phase before transitioning to an alternative activation phenotype.
Addition of LPS-tolerized macrophages to the alternative activation continuum raises questions about the extent of overlap between `tolerant' and `alternatively activated' states, and whether stimuli other than TLR ligands can induce `tolerant' macrophages that are `alternatively activated'. We refer to these cells as tolerant/alternatively activated macrophages and define them as cells that do not produce inflammatory cytokines on inflammatory challenge but express various other genes and mediate alternative functions. Recent work has shown that immune complexes, which acutely activate a transient inflammatory `M1” macrophage phenotype by activation of ITAM-associated Fc receptors, generate alternatively activated macrophages that promote angiogenesis in the more chronic setting of anti-tumor immunity [29]. Tumor-associated macrophages produce low amounts of pro-inflammatory cytokines and thus express aspects of tolerance, which may contribute to the immunosuppressed tumor environment [27]. Ligation of ITAM-associated β2 integrins, which has acute inflammatory effects, also potently induces expression of IL-10 and signaling inhibitors that block TLR responses and may lead to a tolerant/alternatively activated phenotype [30]. Finally, the classical inflammatory `M1' cytokine TNF induces a tolerant state in macrophages, with a block in TLR4 signaling and suppressed chromatin remodeling at inflammatory gene loci [31]. Collectively, these studies support the idea that various microbial products and endogenous inflammatory ligands induce a transient inflammatory state that transitions to complex related, but partially distinct, phenotypes that combine elements of tolerance (to avoid inflammatory toxicity) and alternative activation (to perform necessary functions). Thus, rather than necessarily existing on the opposite end of a spectrum, alternative activation is intimately linked with, and can follow, inflammatory `M1 activation'.
An important question that is mostly unexplored is what are the signals that specify the transition from acute inflammation to a tolerant state? Some of the mechanisms discussed above that contribute to the initial downregulation of inflammatory gene expression may contribute to the development of tolerance, but can not explain: (i) the persistent silenced state of inflammatory gene loci nor (ii) the transcription of `nontolerizable' genes that is active in tolerized calls and is increased after LPS challenge. One explanation for the persistence of repressed gene expression is that epigenetic processes, namely induction of a stable nonpermissive chromatin state, play a role; however, the mechanisms that lead to a nonpermissive chromatin environment are not well understood. Given that induction of tolerance at the epigenetic level requires new gene expression, it will be important to identify the mediators of gene silencing that are induced as part of the initial inflammatory response.
Another clue to the persistence of tolerance is that inhibitors that suppress TLR signaling, such as SHIP, IRAK-M and A20, and repressors of inflammatory gene loci, such as p50 and RelB, appear themselves to be encoded by nontolerizable genes [19, 22, 23, 31]. Expression of these inhibitors increases steadily during the tolerization period and can be further superinduced by secondary LPS challenge. The super-induction of nontolerizable genes by LPS is dependent on core inflammatory MAPK signaling [21], but emerging evidence suggests that signals other than the core MAPK/canonical NF-κB/IRF signaling module contribute to the gradual and sustained increase in gene expression that occurs during tolerization. For example, noncanonical NF-κB signaling contributes to LPS-induced tolerance [22, 23], and recent work implicates the GSK3 kinase, which is typically regulated by PI3K-Akt signaling, in sustained A20 expression and TNF-induced tolerance [31] (Fig. 1B). In addition, TNF stimulation results in a second wave of signaling after 1–3 days, with induction of Jun, canonical and noncanonical NF-κB components, and calcineurin-NFAT signaling [32]. Collectively, this work suggests that, in parallel with attenuation of core inflammatory signaling, there is the emergence of a second wave of alternative signaling cascades that lead to tolerant/alternatively activated macrophages. Understanding the mechanisms that induce and regulate late-phase signaling and the functional correlates of alternative signals represent an important challenge in the field.
Regulation of macrophage transitions in vitro and in vivo
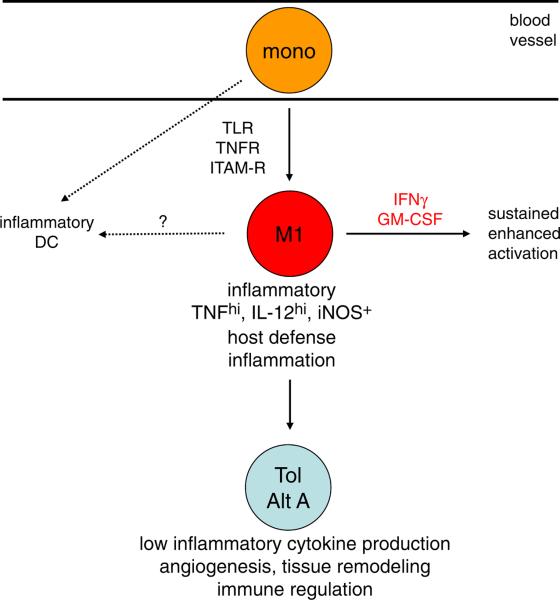
The transition from an acute inflammatory into a tolerant/alternatively activated state is beneficial for the resolution of acute inflammation. If tolerance is a consequence of strong inflammatory signaling, how is inflammation maintained in the setting of ongoing infection or in chronic inflammatory diseases? One answer is that weak activating stimuli may not effectively engage feedback inhibition, or that M1 cytokines, such as IFN-γ and GM-CSF, can suppress feedback inhibitory mechanisms [33], and also prevent differentiation into a tolerant/alternative state ([20] and the references therein). Diminished feedback inhibition and tolerance induction would prolong the duration of the macrophage inflammatory state. Thus, cytokines produced during innate and acquired immune responses and expressed in the inflammatory microenvironment will regulate the transition of acute inflammatory macrophages into tolerant/alternatively activated macrophages. Another answer is that, in vivo during ongoing inflammation, there is a steady migration of monocytes from the circulation into inflammatory sites. We propose that the newly arrived monocytes that become acutely and classically activated are important contributors to inflammation, whose actions can be augmented by M1 cytokines and by differentiation of monocytes into inflammatory Dcs [34] (Fig. 2); however, in the setting of resolving or sterile inflammation, as the activated monocytes/macrophages migrate and differentiate in inflammatory tissues they can transition into tolerant and alternatively activated states. Such transitions have been observed in vivo [35], and would ensure that the amount of inflammatory cytokines and toxicity are limited and that cells evolve to assume new functions, such as tissue repair. This model predicts that macrophages at inflammatory sites, particularly in chronic inflammation, will assume several phenotypes that may be associated with different anatomic subregions of inflamed tissues. Evidence supporting this idea includes the phenotypic heterogeneity associated with the anatomic location of myeloid cells in lupus nephritis and rheumatoid arthritis [36, 37]. In the latter case, recently emigrated perivascular and sublining macrophages may represent the classical inflammatory phenotype, whereas cells that have migrated into the lining layer or synovial fluid may assume aspects of a tolerant or alternatively activated phenotype.
Figure 2.
Differentiation of monocytes and macrophages after trafficking into inflammatory sites. Monocytes that enter inflammatory sites and are strongly activated by TLRs, TNFRs or ITAM-associated receptors will enter an acute inflammatory state (M1) and can also differentiate into inflammatory DCs. Cytokines such as IFN-γ and GM-CSF will maintain the inflammatory state. With time and migration through inflamed tissues, inflammatory monocytes/macrophages transition to tolerant alternatively activated (Tol/Alt A) states. At alternative inflammatory sites characterized by M2 cytokine expression monocytes can directly transition to alternatively activated states (not depicted).
Concluding remarks
The current paradigms of macrophage differentiation recognize the plasticity of macrophage phenotypes but posit that polarization into different phenotypes is regulated directly by varying environmental cues [25–28, 38]. In these models, alternative activation states characterized by low inflammatory cytokine production are induced directly by suppressive factors such as IL-4/13, IL-10 and glucocorticoids, and are readily reversible, including changes in chromatin marks [38–40]. Moreover, classical endotoxin tolerance is not typically included in macrophage differentiation schemes. We suggest that the acute inflammatory activation state of macrophages is transient and inherently unstable, and evolves into a tolerant state associated with features of alternative activation. This transition is at least in part `programmed' by the initial inflammatory stimulus and this programming is maintained by induced refractoriness to environmental cues and by epigenetic modifications that determine patterns of gene expression. We suggest extending the current models of macrophage polarization/differentiation to include tolerant phenotypes in the spectrum of classical to alternative activation. Future investigation of the signaling mechanisms and epigenetic modifications that control tolerant phenotypes will be necessary to yield important insights into regulation of macrophage function.
Acknowledgements
This work was supported by grants from the National Institutes of Health, U.S.A.
Footnotes
Conflict of interest: The author has no financial or commercial conflict of interest.
References
- 1.Takeuchi O, Akira S. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R, Horng T. Nat. Rev. Immunol. 2009;9:692–703. doi: 10.1038/nri2634. [DOI] [PubMed] [Google Scholar]
- 3.Smale ST. Cell. 2010;140:833–844. doi: 10.1016/j.cell.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barish GD, et al. Genes Dev. 2010;24:2760–2765. doi: 10.1101/gad.1998010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass CK, Saijo K. Nat. Rev. Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- 6.Hargreaves DC, et al. Cell. 2009;138:129–145. doi: 10.1016/j.cell.2009.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramirez-Carrozzi VR, et al. Cell. 2009;138:114–128. doi: 10.1016/j.cell.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Santa F, et al. Cell. 2007;130:1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Nicodeme E, et al. Nature. 2010;468:1119–1123. doi: 10.1038/nature09589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Natoli G, et al. Genes Dev. 2011;25:101–106. doi: 10.1101/gad.2018811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ostuni R, Natoil G. Eur. J. Immunol. 2011;41 doi: 10.1002/eji.201141706. in press. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi O, Akira S. Eur. J. Immunol. 2011;41 doi: 10.1002/eji.201141792. in press. [DOI] [PubMed] [Google Scholar]
- 13.Liew FY, et al. Nat. Rev. Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 14.Smith AM, et al. J. Biol. Chem. 2011;286:23582–23590. doi: 10.1074/jbc.M111.249235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilchrist M, et al. Nature. 2006;441:173–178. doi: 10.1038/nature04768. [DOI] [PubMed] [Google Scholar]
- 16.Hayden MS, Ghosh S. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Sheedy FJ, et al. Nat. Immunol. 2009;11:141–147. doi: 10.1038/ni.1828. [DOI] [PubMed] [Google Scholar]
- 18.Alam A, O'Neill LA. Eur. J. Immunol. 2011;41 doi: 10.1002/eji.201141740. in press. [DOI] [PubMed] [Google Scholar]
- 19.Biswas SK, Lopez-Collazo E. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Ivashkiv LB. Proc. Natl. Acad. Sci. USA. 2010;107:19438–19443. doi: 10.1073/pnas.1007816107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foster SL, et al. Nature. 2007;447:972–978. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 22.Liu TF, et al. J. Biol. Chem. 2011;286:9856–9864. doi: 10.1074/jbc.M110.196790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porta C, et al. Proc. Natl. Acad. Sci. USA. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerber JS, Mosser DM. J. Immunol. 2001;166:6861–6868. doi: 10.4049/jimmunol.166.11.6861. [DOI] [PubMed] [Google Scholar]
- 25.Mosser DM, Edwards JP. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fleming BD, Mosser DM. Eur. J. Immunol. 2011;41 doi: 10.1002/eji.201141717. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantovani A, Sica A. Curr. Opin. Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Martinez FO, et al. Annu. Rev. Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 29.Andreu P, et al. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, et al. Immunity. 2010;32:518–530. doi: 10.1016/j.immuni.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S-H, et al. Nat. Immunol. 2011;12:607–615. doi: 10.1038/ni.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarilina A, et al. Proc. Natl. Acad. Sci. USA. 2011;108:1573–1578. doi: 10.1073/pnas.1010030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X, Ivashkiv LB. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serbina NV, et al. Annu. Rev. Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnold L, et al. J. Exp. Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bethunaickan R, et al. J. Immunol. 2011;186:4994–5003. doi: 10.4049/jimmunol.1003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamilton JA, Tak PP. Arthritis Rheum. 2009;60:1210–1221. doi: 10.1002/art.24505. [DOI] [PubMed] [Google Scholar]
- 38.Murray PJ, Wynn TA. J. Leukoc. Biol. 2011;89:557–563. doi: 10.1189/jlb.0710409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishii M, et al. Blood. 2009;114:3244–3254. doi: 10.1182/blood-2009-04-217620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satoh T, et al. Nat. Immunol. 2010;11:936–944. doi: 10.1038/ni.1920. [DOI] [PubMed] [Google Scholar]