Abstract
The robust cell culture systems for hepatitis C virus (HCV) are limited to those using cell culture-adapted clones (HCV in cell culture [HCVcc]) and cells derived from the human hepatoma cell line Huh7. However, accumulating data suggest that host factors, including innate immunity and gene polymorphisms, contribute to the variation in host response to HCV infection. Therefore, the existing in vitro systems for HCV propagation are not sufficient to elucidate the life cycle of HCV. A liver-specific microRNA, miR122, has been shown to participate in the efficient replication of HCV. In this study, we examined the possibility of establishing a new permissive cell line for HCV propagation by the expression of miR122. A high level of miR122 was expressed by a lentiviral vector placed into human liver cell lines at a level comparable to the endogenous level in Huh7 cells. Among the cell lines that we examined, Hep3B cells stably expressing miR122 (Hep3B/miR122) exhibited a significant enhancement of HCVcc propagation. Surprisingly, the levels of production of infectious particles in Hep3B/miR122 cells upon infection with HCVcc were comparable to those in Huh7 cells. Furthermore, a line of “cured” cells, established by elimination of HCV RNA from the Hep3B/miR122 replicon cells, exhibited an enhanced expression of miR122 and a continuous increase of infectious titers of HCVcc in every passage. The establishment of the new permissive cell line for HCVcc will have significant implications not only for basic HCV research but also for the development of new therapeutics.
INTRODUCTION
Hepatitis C virus (HCV) infects over 170 million people worldwide and frequently leads to persistent infection, which in turn can lead to chronic hepatitis, cirrhosis, and hepatocellular carcinoma (34). HCV belongs to the Flaviviridae family and has a single-stranded positive RNA genome of approximately 9.6 kb. The genome of HCV is translated into a single polyprotein at the endoplasmic reticulum (ER) membrane and is then cleaved by host- and virus-encoded proteases, resulting in 10 structural and nonstructural proteins (41, 44). Due to the lack of a small-animal model and an efficient cell culture system, efforts to understand the HCV life cycle as well as development of anti-HCV drugs have been hampered (42). In a major breakthrough, HCV replicon cells, in which HCV RNA autonomously replicates, were established by Lohmann et al. (37). Afterwards, the infectious HCV in cell culture (HCVcc), based on the genotype 2a JFH1 strain in combination with the human hepatocellular carcinoma cell line Huh7, was developed (36, 64, 70). On the basis of the results obtained with these in vitro systems, the life cycle of HCV was clarified, and substantial progress has been made in screening host factors involved in HCV propagation as well as anti-HCV drug candidates (20, 51). Among them, a liver-specific microRNA (miRNA), miR122, has been shown to be one of the most important host factors for HCV replication.
miRNAs are small noncoding RNAs that consist of 20 to 25 nucleotides and modulate gene expression in plants and animals (3, 26). Most miRNAs negatively regulate translation through interaction with the 3′ untranslated region (UTR) of mRNA in a sequence-specific manner. Some of them have been shown to play important roles in the viral life cycle (56). Interestingly, miR122 has been shown to bind to HCV 5′ UTRs and to enhance translation and replication of HCV RNA (23, 28, 29, 38, 52). In addition, enhancement of HCVcc propagation through the direct interaction of miR122 with HCV 5′ UTR has been demonstrated (27). Recently, intravenous administration of the locked nucleic acid (LNA) complementary to miR122 was shown to suppress the propagation of HCV in chimpanzees chronically infected with HCV, suggesting that miR122 is a promising therapeutic target for chronic hepatitis C (31).
It has been shown that HCV exploits various host factors to form a replication complex for efficient replication (43). In vitro propagation of HCV is limited to Huh7 cells and their derivatives, and thus, it is important to confirm the data obtained in Huh7 cells by using other human liver cell lines, because the patterns of gene expression vary among cell lines. Although establishment of an HCV replicon system based on liver cell lines has been reported (11, 66), robust propagation of HCVcc in well-characterized human liver cell lines other than Huh7 cells has not succeeded yet. The gene expression profile of mice xenotransplanted with human hepatocytes from different donors inoculated with a single source of HCV revealed that host factors contributed to the variation in host response to HCV infection, including the activation of innate antiviral signaling pathways (65). Furthermore, gene polymorphism in interleukin 28B (IL-28B) was shown to be associated with natural clearance (62) and response to combination therapy with interferon (IFN) and ribavirin (19, 58, 59). Therefore, the solely available in vitro propagation system for HCVcc, employing Huh7-derived cells, is not sufficient. The establishment of alternative HCV strains and permissive cell lines is needed to elucidate molecular mechanisms of propagation and pathogenesis of HCV in more detail.
Although there have been several attempts to generate chimeric HCVs based on the JFH1 strain (21) and an infectious clone of genotype 1a, H77S, that produces fewer infectious particles than the genotype 2a JFH1 strain (68), propagation of HCV was still limited to Huh7 cells. Exogenous expression of miR122 has been shown to support HCV RNA replication in a human embryonic kidney epithelial cell line and mouse embryonic fibroblasts (7, 35), and we therefore thought that the possibility of complete propagation of HCVcc in various human liver cell lines by the expression of miR122 needed to be examined. Among the cell lines that we examined, Hep3B cells, which were established from human liver tumor biopsy samples in 1976 (1) and have been well characterized as model liver cells in various fields of research (47, 55, 63, 67), were shown to support the efficient propagation of HCVcc comparable to that in Huh7 cells by the expression of miR122. Establishment of novel cell culture systems through the exogenous expression of miR122 provides a clue to understanding the precise roles of miR122 in the life cycle of HCV.
MATERIALS AND METHODS
Plasmids.
The cDNA clones of wild-type miR122 (WT-miR122), single mutant miR122 (sMT-miR122), double mutant miR122 (dMT-miR122), Aequorea coerulescens green fluorescent protein (AcGFP), and claudin-1 (CLDN) were inserted between the XhoI and XbaI sites of a lentiviral vector, pCSII-EF-RfA, which was kindly provided by M. Hijikata, and the resulting plasmids were designated pCSII-EF-WT-miR122, pCSII-EF-sMT-miR122, pCSII-EF-dMT-miR122, pCSII-EF-AcGFP, and pCSII-EF-Claudin1, respectively. pHH-JFH1 was kindly provided by T. Wakita (39). pHH-JFH1-E2p7NS2mt contains three adaptive mutations in pHH-JFH1 (53). pFGR-JFH1 and pSGR-JFH1 encoded a full-length and a subgenomic cDNA of the JFH1 strain, respectively. The complementary sequence of miR122 was inserted into the PmeI site of the pmirGLO vector (Promega, Madison, WI), and the resulting plasmid was designated pmirGLO-miR122comp. pIFNβ-Luc and pISRE-Luc carrying a firefly luciferase gene under the control of the beta IFN (IFN-β) and interferon-sensitive response element (ISRE) promoters, respectively, were kindly provided by T. Kawai and S. Akira. The internal control plasmid encoding a Renilla luciferase (pRL-TK) was purchased from Promega. The plasmids used in this study were confirmed by sequencing with an ABI Prism 3130 genetic analyzer (Applied Biosystems, Tokyo, Japan).
Cells.
All cell lines were cultured at 37°C under the condition of a humidified atmosphere and 5% CO2. The human embryonic kidney 293T cell line and hepatocellular carcinoma cell lines Huh7, Huh6/CLDN, HepG2/CD81, Hep3B, and PKC/PRL/5 were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma, St. Louis, MO) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% fetal calf serum (FCS). HepG2/CD81 cells were generated as described previously (60). Huh6 cells were transduced with a lentiviral vector expressing claudin-1, and the resulting cells were designated Huh6/CLDN. The Huh7-derived cell line Huh7.5.1 was kindly provided by F. Chisari and was maintained in DMEM containing nonessential amino acids (NEAA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% FCS. Hep3B replicon cells harboring the subgenomic HCV RNA were maintained in DMEM containing 10% FCS, NEAA, and 400 μg/ml G418 (Nakalai Tesque, Kyoto, Japan).
Viruses.
pHH-JFH1-E2p7NS2mt was transfected into Huh7.5.1 cells, and the culture supernatants were collected after serial passages. The infectivity of HCVcc was determined by focus-forming assay and expressed in focus-forming units (FFU) (64). The lentiviral vectors and ViraPower lentiviral packaging mix (Invitrogen, San Diego, CA) were cotransfected into 293T cells, and the supernatants were recovered at 48 h posttransfection. The culture supernatants were centrifuged at 1,000 × g for 5 min and cleared through a 0.45-μm-pore-size filter. The lentivirus titer was determined by a Lenti-X quantitative reverse transcription (qRT)-PCR titration kit (Clontech, Mountain View, CA). The vesicular stomatitis virus (VSV) variant NCP12.1, derived from the Indiana strain, was kindly provided by M. Whitt. Pseudotype VSVs bearing the HCV E1 and E2 glycoproteins (HCVpv) and VSV G protein (VSVpv) were prepared as described previously (60). The infectivity of the pseudotype viruses was assessed by the expression of luciferase, determined by a Bright-Glo luciferase assay system (Promega) following a protocol provided by the manufacturer and expressed in relative light units (RLU).
Reagents and antibodies.
Cyclosporine (CsA) and human recombinant IFN-α2 were purchased from Sigma and R&D Systems (Minneapolis, MN), respectively. BODIPY 558/568 lipid probe was purchased from Invitrogen. Poly(I·C) was purchased from InvivoGen (San Diego, CA). LNAs complementary to miR122 (LNA-miR122; 5′-CcAttGTcaCaCtCC-3′) and its negative control (LNA-Cont; 5′-CcAttCTgaCcCtAC-3′) (LNA in capital letters, DNA in lowercase letters; sulfur atoms in oligonucleotide phosphorothioates are substituted for nonbridging oxygen atoms; capital C indicates LNA methylcytosine) (14) were purchased from Gene Design (Osaka, Japan). miScript miRNA mimics hsa-miR122 and its negative control were purchased from Qiagen (Valencia, CA). Mouse monoclonal antibodies to HCV NS5A and β-actin were purchased from Austral Biologicals (San Ramon, CA) and Sigma, respectively. Mouse anti-apolipoprotein E (anti-ApoE), rabbit anti-diacylglycerol acyltransferase 1 (DGAT1), rabbit anti-signal transducer and activators of transcription 2 (anti-STAT2), and rabbit anti-IFN regulatory factor 3 (anti-IRF3) antibodies were purchased from Santa Cruz (Santa Cruz, CA). Rabbit anti-HCV core protein was prepared as described previously (45). Phycoerythrin (PE)-conjugated anti-human CD81 (anti-hCD81) and anti-mouse IgG antibodies were purchased from BD Biosciences (Franklin Lakes, NJ). Mouse anti-double-stranded RNA (anti-dsRNA) IgG2a (J1 and K2) antibodies were from Biocenter Ltd. (Szirak, Hungary). Alexa Fluor 488 (AF488)-conjugated anti-mouse and -rabbit IgG and AF594-conjugated anti-rabbit IgG antibodies were from Invitrogen.
Quantitative RT-PCR.
For quantitation of HCV RNA, total RNA was prepared from cells by using an RNeasy minikit (Qiagen). The synthesis of a first-stranded cDNA and quantitative RT-PCR were performed using TaqMan EZ RT-PCR core reagents and an ABI Prism 7000 system (Applied Biosystems) according to the manufacturer's protocol. For quantitation of miRNA, total RNA was prepared from cells by using an miRNeasy minikit (Qiagen), and miR122 was estimated by using miR122-specific RT primers and amplified using specific primers provided in the TaqMan MicroRNA assays (Applied Biosystems) according to the manufacturer's protocol. U6 small nuclear RNA (snRNA) was used as an internal control. Fluorescent signals were analyzed by an ABI Prism 7000 system (Applied Biosystems).
Transfection and immunoblotting.
Cells were transfected with the plasmids by using Trans IT LT-1 (Mirus, Madison, WI) or Lipofectamine 2000 (Invitrogen) according to the manufacturers' protocols. Cells were lysed on ice in Triton lysis buffer (20 mM Tris-HCl [pH 7.4], 135 mM NaCl, 1% Triton X-100, 10% glycerol) supplemented with a protease inhibitor mix (Nacalai Tesque). The samples were boiled in loading buffer and subjected to 5 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA) and reacted with primary antibody and then secondary horseradish peroxidase-conjugated antibody. The immunocomplexes were visualized with Super Signal West Femto substrate (Pierce, Rockford, IL) and detected by using an LAS-3000 image analyzer (Fujifilm, Tokyo, Japan).
Indirect immunofluorescence assay.
Cells cultured on glass slides were fixed with 4% paraformaldehyde in phosphate-buffered saline (PBS) at room temperature for 30 min. After washing three times with PBS, the cells were permeabilized for 20 min at room temperature with PBS containing 0.25% saponin and blocked with phosphate buffer containing 2% bovine serum albumin (BSA) for 1 h at room temperature. The cells were incubated with blocking buffer containing mouse anti-dsRNA, rabbit anti-NS5A, rabbit anti-core, rabbit anti-IRF3, or rabbit anti-STAT2 at room temperature for 1 h, washed three times with PBS, and incubated with blocking buffer containing appropriate AF488-conjugated and AF594-conjugated secondary antibodies at room temperature for 1 h. Finally, the cells were washed three times with PBS and observed with a FluoView FV1000 laser scanning confocal microscope (Olympus, Tokyo, Japan).
Flow cytometry.
Cultured cells were detached with 0.25% trypsin-EDTA and incubated with PE-conjugated anti-hCD81 antibody or anti-mouse IgG antibody for 1 h at 4°C. After being washed twice with PBS containing 1% BSA, the cells were analyzed by a BD FACSCalibur flow cytometry system (BD Biosciences).
In vitro transcription, RNA transfection, and colony formation.
The plasmids pSGR-JFH1 and pFGR-JFH1 were linearized with XbaI and treated with mung bean exonuclease. The linearized DNA was transcribed in vitro by using a MEGAscript T7 kit (Applied Biosystems) according to the manufacturer's protocol. The in vitro-transcribed RNA (10 μg) was electroporated into Hep3B cells at 106 cells/0.4 ml under conditions of 270 V and 960 μF using a Gene Pulser apparatus (Bio-Rad, Hercules, CA) and plated on DMEM containing 10% FCS and NEAA. The medium was replaced with fresh DMEM containing 10% FCS, NEAA, and 400 μg/ml G418 at 24 h posttransfection. The remaining colonies were fixed with 4% paraformaldehyde and stained with crystal violet at 1 month postelectroporation.
Luciferase assay.
Cells were seeded onto 24-well plates at a concentration of 5 × 104 cells/well and transfected with 250 ng of each of the plasmids. At 24 h posttransfection, cells were stimulated with the appropriate ligands for 24 h and then lysed in 100 μl of passive lysis buffer (Promega). Luciferase activity was measured in 20-μl aliquots of the cell lysates using a dual-luciferase reporter assay system (Promega). Firefly luciferase activity was standardized with that of Renilla luciferase cotransfected with the internal control plasmid pRL-TK and was expressed as RLU.
RESULTS
Expression of miR122 facilitates replication of HCVcc in various liver cell lines.
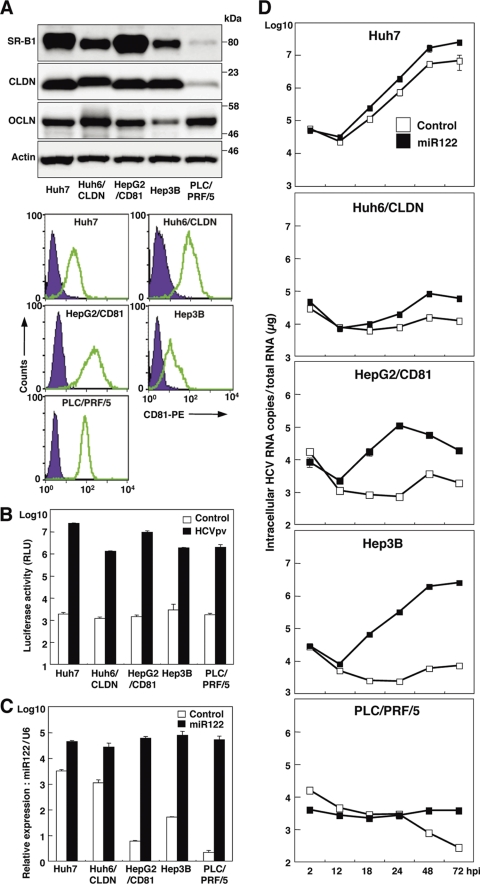
The robust in vitro cell culture systems for HCV use the HCV genotype 2a isolate JFH1 and Huh7-derived cell lines (64). To expand the host range of HCVcc to gain more insight into the host-virus interaction, we examined the effect of expression of miR122, a liver-specific microRNA that was shown to be crucial for the efficient replication of HCV (27–29, 38, 52), in several well-characterized liver cell lines: Huh6, HepG2, Hep3B, and PLC/PRF/5. Although hCD81, SR-B1, claudin-1 (CLDN), and occludin (OCLN) are known to be crucial for entry of HCVcc (15, 48, 49, 54), the Huh6 and HepG2 cell lines express little or no CLDN and hCD81 (10, 22), respectively. Therefore, CLDN and hCD81 were exogenously expressed in the cell lines, and the resulting lines were designated Huh6/CLDN and HepG2/CD81, respectively. Expression of the receptor molecules in the cell lines was confirmed by immunoblot and fluorescence-activated cell sorter (FACS) analyses (Fig. 1A). To further examine the susceptibility to HCV infection, pseudotyped VSV bearing the HCV envelope protein, HCVpv, was inoculated into these cell lines. Significant expression of luciferase was observed in these cell lines upon infection with HCVpv but not upon infection with the control virus (Fig. 1B), suggesting that the liver cell lines express functional receptors required for entry of HCV. To determine the effect of miR122 on the replication of HCVcc, we next assessed the level of miR122 in the liver cell lines by qRT-PCR. Although miR122 is highly expressed in the liver (13), the expression level of miR122 varied among the liver cell lines (Fig. 1C, white bars). To examine the effect of the exogenous expression of miR122 in the liver cell lines on the replication of HCVcc, miR122 was expressed in the cell lines by the lentiviral vector. The expression level of miR122 in the liver cell lines, including Huh7 cells, was shown to be upregulated to a significantly greater extent than that in Huh7 cells alone (Fig. 1C, black bars). To examine the effect of miR122 on the replication of HCV, HCVcc was inoculated into the cell lines (Fig. 1D). Although Huh7 cells exhibited an efficient HCV replication, a slight enhancement of the replication was observed by the expression of miR122. No HCV replication was observed in PLC/PRF/5 cells irrespective of miR122 expression. Hep3B and HepG2/CD81 cells exhibited a significant enhancement of HCV replication by the expression of miR122, in contrast to a slight increase in Huh6/CLDN cells. Notably, HCV RNA levels were drastically increased by more than 300-fold at 72 h postinfection in Hep3B cells by the expression of miR122, suggesting that Hep3B is the most suitable cell line for investigating the biological significance of miR122 on the propagation of HCV and for establishing a permissive cell line for HCVcc. Therefore, we used Hep3B cells overexpressing miR122 (Hep3B/miR122 cells) for further experiments.
Fig 1.
Expression of miR122 facilitates replication of HCVcc in various liver cell lines. (A) Human liver cell lines Huh7, Huh6/CLDN, HepG2/CD81, Hep3B, and PLC/PRF/5 were lysed and subjected to immunoblotting using appropriate antibodies. The expression levels of hCD81 in the liver cell lines were determined by flow cytometry. (B) The human liver cell lines were inoculated with HCVpv or control virus and washed three times after 2 h of incubation. Luciferase activities were determined at 24 h postinfection. (C) The cell lines were transduced with lentiviral vectors expressing miR122 or AcGFP as a control. After serial passages, total RNA was extracted from the cells and relative expression of miR122 was determined by qRT-PCR by using U6 snRNA as an internal control. (D) The cells expressing miR122 or control were infected with HCVcc at an MOI of 1. Total RNA was extracted from the cells at the indicated time and subjected to qRT-PCR analysis. The data are representative of three independent experiments. Error bars indicate the standard deviation of the mean.
Expression of biologically active miR122 facilitates replication of HCVcc in Hep3B cells.
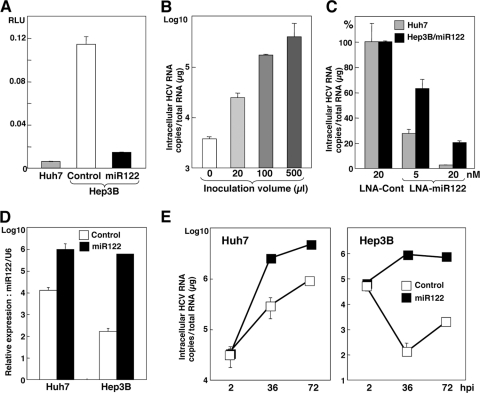
To confirm the activity of endogenously and exogenously expressed miR122 to suppress the translation in cells, a pmirGLO vector carrying the complementary sequence of miR122 under the luciferase gene was transfected into Huh7 cells, Hep3B cells expressing AcGFP (Hep3B/Cont), and Hep3B/miR122 cells. Suppression of luciferase expression was observed in Huh7 and Hep3B/miR122 cells but not in Hep3B/Cont cells (Fig. 2A), suggesting that miR122 exogenously expressed in Hep3B cells is as biologically active as that endogenously expressed in Huh7 cells. To determine the effect of miR122 on the propagation of HCVcc, Hep3B cells were infected with the lentiviral vector expressing miR122 and then inoculated with HCVcc. The levels of HCV RNA in Hep3B cells upon infection with HCVcc were increased in proportion to the amount of lentiviral vector (Fig. 2B). Recently, an inhibitor for miR122, SPC3649, which is an LNA in which 2′ oxygen and 4′ carbon are connected via methylene units, has been shown to possess potent anti-HCV activity in chimpanzees chronically infected with HCV (31). We next examined the effect of LNA on the replication of HCVcc in Huh7 and Hep3B/miR122 cells. HCV RNA replication in Huh7 and Hep3B/miR122 cells was significantly and dose-dependently decreased by treatment with LNA-miR122 but not treatment with LNA-Cont (Fig. 2C). We further investigated the effect of the mimic miR122, the synthetic double-stranded RNA oligonucleotides that mimic endogenous miRNA function, on the propagation of HCV. Huh7 and Hep3B cells transfected with mimic miR122 but not those transfected with the negative control exhibited a high level of expression of miR122 (Fig. 2D) and enhanced RNA replication upon infection with HCVcc (Fig. 2E). Collectively, these results clearly indicate that expression of biologically active miR122 plays a crucial role in the replication of HCV in Hep3B cells.
Fig 2.
Expression of biologically active miR122 facilitates replication of HCVcc in Hep3B cells. (A) Huh7, Hep3B/Cont, and Hep3B/miR122 cells were transfected with pmirGLO-miR122comp, and luciferase activity was determined at 24 h posttransfection. (B) Hep3B cells were transduced with the lentiviral vector expressing miR122 in a dose-dependent manner and then infected with HCVcc at an MOI of 1 at 48 h postransduction. Total RNA was extracted from the cells at 72 h postinfection and subjected to qRT-PCR. (C) LNA-Cont (20 nM) or LNA-miR122 (5 nM or 20 nM) was introduced into Hep3B/miR122 cells and infected with HCVcc at an MOI of 1 at 12 h posttransfection. Total RNA was extracted from the cells at 24 h postinfection and subjected to qRT-PCR. (D) Huh7 and Hep3B cells were transfected with mimic miR122 (20 nM) or a negative control (20 nM), and total miRNA was determined by qRT-PCR at 24 h posttransfection. (E) Huh7 and Hep3B cells were transfected with mimic miR122 (20 nM) or a negative control (20 nM) and infected with HCVcc at an MOI of 1 at 12 h posttransfection. Total RNA was extracted from the cells at the indicated time (hpi, hours postinfection) and subjected to qRT-PCR.
Establishment of a novel permissive cell line for robust propagation of HCVcc by expression of miR122 in Hep3B cells.
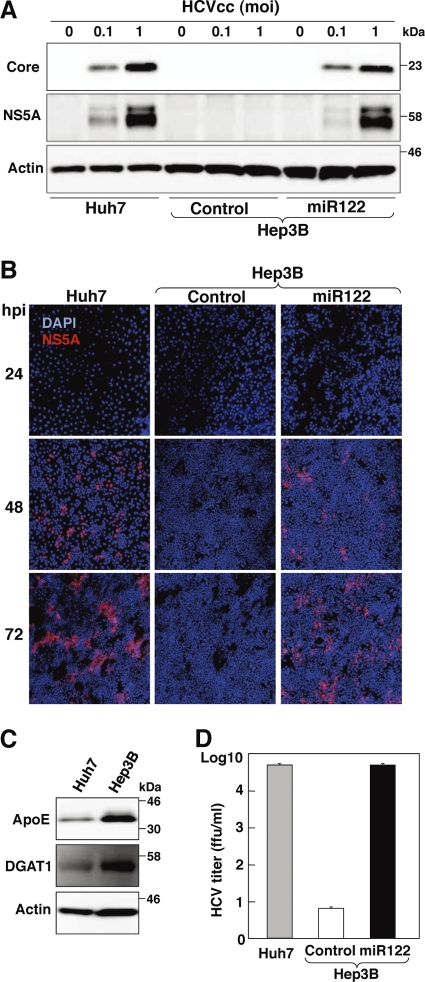
We next examined the possibility of establishing a permissive cell line for the robust propagation of HCVcc by the expression of miR122 in Hep3B cells. Huh7, Hep3B/miR122, and Hep3B/Cont cells were infected with HCVcc, and the levels of expression of HCV NS5A and core proteins were assessed by immunoblotting at 72 h postinfection. Expression of the viral proteins in Hep3B/miR122 cells was almost comparable to that in Huh7 cells, in contrast to no expression in Hep3B/Cont cells (Fig. 3A). Small foci stained by immunofluorescence assay appeared at 24 h postinfection in Hep3B/miR122 and Huh7 cells but not in Hep3B/Cont cells and grew into large foci at 72 h postinfection, indicating that infectious particles are generated in Hep3B/miR122 cells and the progeny particles expand infection to the neighboring cells (Fig. 3B). The morphology of Hep3B cells is completely different from that of Huh7 cells, and thus, these results are not due to contamination of Huh7 cells. DGAT1 and ApoE have been shown to play crucial roles in the recruitment of core protein to the lipid droplets and viral infectivity, respectively (9, 24). Higher levels of expression of ApoE and DGAT1 were detected in Hep3B cells than in Huh7 cells (Fig. 3C). Furthermore, the concentration of infectious particles recovered in the culture supernatant of Hep3B/miR122 cells infected with HCVcc at a multiplicity of infection (MOI) of 1 at 72 h postinfection was approximately 5 × 104 FFU/ml, which was comparable to that in Huh7 cells, and was in clear contrast to the significantly lower titer in Hep3B/Cont cells (less than 10 FFU/ml). These results clearly indicate that expression of miR122 in Hep3B cells enables the establishment of a novel permissive cell line for the robust propagation of HCVcc.
Fig 3.
Establishment of a novel permissive cell line for robust propagation of HCVcc by expression of miR122 in Hep3B cells. (A) Huh7, Hep3B/Cont, and Hep3B/miR122 cells were infected with HCVcc at an MOI of 0.1 or 1, and the levels of expression of viral proteins were determined by immunoblotting using appropriate antibodies at 72 h postinfection. (B) Huh7, Hep3B/Cont, and Hep3B/miR122 cells were infected with HCVcc at an MOI of 1 and incubated with 1% methylcellulose in DMEM containing 5% FCS for the indicated time. Cells were fixed with 4% paraformaldehyde and subjected to indirect immunofluorescence assay using anti-NS5A antibody, followed by AF594-conjugated anti-rabbit IgG (red). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). (C) Huh7 and Hep3B cells were lysed and subjected to immunoblotting using appropriate antibodies. (D) Huh7, Hep3B/Cont, and Hep3B/miR122 cells were infected with HCVcc at an MOI of 1, the culture supernatants were collected at 72 h postinfection, and the viral titers of the supernatants were determined by focus-forming assay using Huh7.5.1 cells.
Establishment of an HCV RNA replicon in Hep3B/miR122 cells.
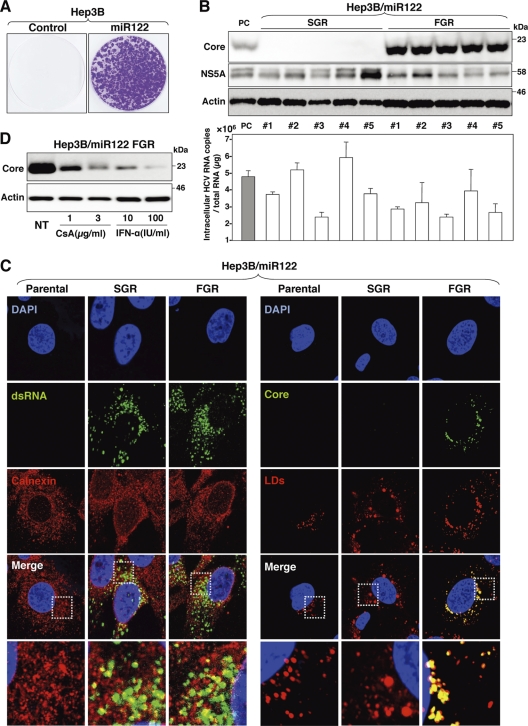
It has been shown that “cured” cells established through the elimination of the HCV genome from replicon cells by treatment with IFN-α exhibited more potent propagation of HCVcc than the original Huh7 cells (4). To establish a cured cell line derived from Hep3B/miR122 cells for further improvement of HCVcc propagation, we first established HCV replicon cells in Hep3B/miR122 cells. In vitro-transcribed sub- or full-genomic HCV RNA of the JFH1 strain was electroporated into Hep3B/miR122 and Hep3B/Cont cells, the cells were cultured with 400 μg/ml of G418 for 1 month, and subgenomic replicon (SGR) and full-genomic replicon (FGR) cells were established. Hep3B/miR122 cells electroporated with viral RNA generated a large number of colonies, in contrast to the complete absence of colony formation in Hep3B/Cont cells (Fig. 4A). High levels of HCV RNA comparable to those in the Huh7 cells harboring SGR of the JFH1 strain were detected in Hep3B/miR122 cells harboring either SGR or FGR of the JFH1 strain (Fig. 4B, lower). Expression of NS5A was detected in all of the clones of Hep3B/miR122 cells harboring either SGR or FGR, and that of the core protein was detected in all of the FGR clones (Fig. 4B, upper). HCV core protein and RNA were shown to localize mainly on the lipid droplets and on the cytoplasmic face of ER, respectively (40, 61). Immunofluorescence analyses revealed that dsRNA was colocalized with calnexin, an ER marker, in both SGR and FGR cells and HCV core protein was colocalized with lipid droplets in the FGR cells, as previously described (Fig. 4C). Treatment of Hep3B/miR122 cells harboring an FGR of the JFH1 strain with either CsA or IFN-α decreased the expression of core protein in a dose-dependent manner (Fig. 4D), suggesting that the Hep3B/miR122 replicon cells can be used for screening antiviral compounds for HCV.
Fig 4.
Establishment of an HCV RNA replicon in Hep3B/miR122 cells. (A) Full-genomic replicon RNA of HCV was electroporated into Hep3B/Cont and Hep3B/miR122 cells, and the medium was replaced with DMEM containing 10% FCS and 400 μg/ml G418 at 24 h posttransfection. Colony formation was determined as indicated in Materials and Methods. (B) (Upper) Sub- and full-genomic HCV replicons (SGR and FGR) in Hep3B/miR122 cells were subjected to immunoblotting using the appropriate antibodies. Huh7.5.1 cells infected with HCVcc were used as a positive control (PC). (Lower) Intracellular HCV copy number in replicon clones. SGR in Huh7 cells was used as a positive control. (C) SGR and FGR in Hep3B/miR122 cells were fixed with 4% paraformaldehyde and subjected to indirect immunofluorescence assay using the appropriate antibodies. Lipid droplets (LDs) were stained red with BODIPY. Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). The boxed regions in the merged images are magnified. (D) Hep3B/miR122 FGR cells were treated with DMEM containing 10% FCS and the indicated concentrations of CsA and IFN-α and then subjected to immunoblotting using appropriate antibodies at 48 h posttransfection. NT, no treatment.
Elimination of HCV RNA from HCV replicon RNA from Hep3B/miR122 cells enhances propagation of HCVcc.
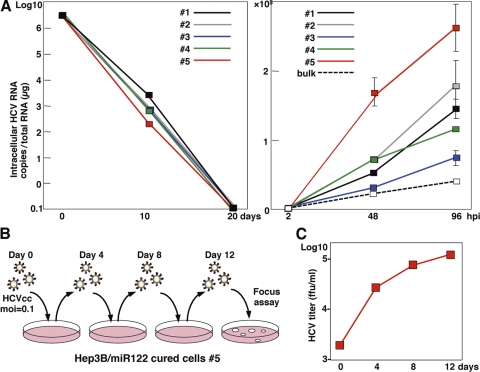
To establish cured Hep3B/miR122 cells, five clones of the Hep3B/miR122 replicon cells harboring FGR of the JFH1 strain were treated with 100 IU/ml of IFN-α to eliminate viral RNA, and viral RNA was gradually decreased and completely eliminated at 20 days posttreatment (Fig. 5A, left). We then examined the sensitivity of the cured cell clones for propagation of HCVcc. All of the cured cell clones exhibited enhancement of propagation of HCVcc, especially clone 5, which achieved a level of replication of HCVcc more than 6-fold higher than that in the parental Hep3B/miR122 cells (Fig. 5A, right). To examine the effect of serial passage of HCVcc in the cured Hep3B/miR122 cells, HCVcc was inoculated into the cured cells at an MOI of 0.1, and the culture supernatants harvested at 4 days postinfection were reinoculated into the naïve cured cells (Fig. 5B). Infectious titers in the culture supernatants were continuously increased in accord with the number of passages (Fig. 5C). These results indicate that a novel cell line capable of complete propagation of HCVcc was established by the introduction of miR122 and the curing process, as in the case of Huh7 cells by using Hep3B cells.
Fig 5.
Elimination of HCV RNA from HCV replicon RNA from Hep3B/miR122 cells enhances propagation of HCVcc. (A) (Left) Hep3B/miR122 FGR cell clones were treated with IFN-α (100 IU/ml), and HCV RNA was determined by qRT-PCR at 10 and 20 days posttreatment; (right) Hep3B/miR122 parental cells (bulk) and the cured cells were infected with HCVcc at an MOI of 0.1, and HCV RNA was determined by qRT-PCR at 48 and 96 h postinfection. (B) Schematic diagram of the experimental procedure for serial passage of HCVcc in Hep3B/miR122 cured cells. The cured cells were infected with HCVcc at an MOI of 0.1. (C) The infectious titers in the culture supernatants of the Hep3B/miR122 cured cells were determined at the indicated time points by focus-forming assay using Hep3B/miR122 cells.
Cured Hep3B/miR122 cells facilitate efficient propagation of HCVcc through enhanced expression of miR122.
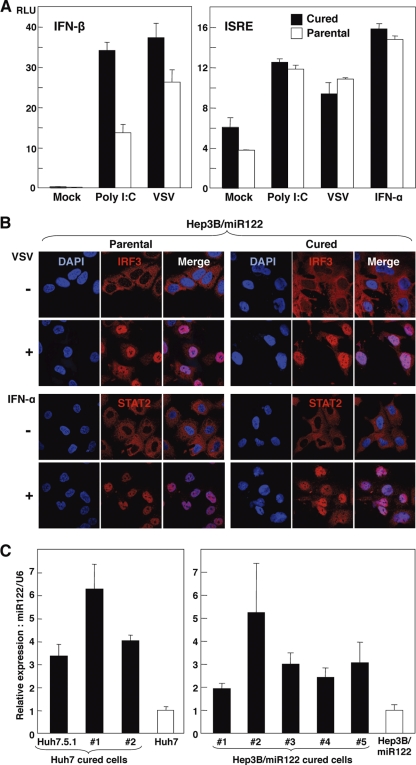
It has been reported that one of the reasons for the high susceptibility of the cured cell line Huh7.5 to the propagation of HCVcc is the disruption of the innate immune responses caused by mutation in RIG-I, a key sensor for viral RNA in the cytoplasm (57, 69). To examine the innate immune response in the cured Hep3B/miR122 cells, reporter plasmids encoding the luciferase gene under the control of either the IFN-β (Fig. 6A, left) or ISRE (Fig. 6A, right) promoter were transfected into the cured or parental Hep3B/miR122 cells and stimulated with poly(I·C), VSV, or IFN-α. Activation of these promoters in the cured Hep3B/miR122 cells was not impaired but rather was enhanced upon stimulation with poly(I·C) or VSV compared with that in the parental cells. To further assess the authenticity of viral RNA recognition and ISG induction pathways in the cured Hep3B/miR122 cells, nuclear localization of IRF3 and STAT2 upon stimulation was determined by immunofluorescence analysis. IRF3 and STAT2 in both cured and parental Hep3B/miR122 cells were translocated into the nucleus upon stimulation with VSV and IFN-α, respectively (Fig. 6B). These results suggest that the efficient propagation of HCVcc in the cured Hep3B/miR122 cells might be attributable to reasons other than impairment of the innate immune response. Therefore, we hypothesized that the Hep3B/miR122 cells harboring the HCV genome are capable of surviving in the presence of a high concentration of G418 by amplification of the viral genome through enhancement of miR122 expression and that once HCV RNA was eliminated, the cured cells would acquire the ability to propagate HCV due to the high expression of miR122. To test this hypothesis, the levels of miR122 in both Huh7- and Hep3B/miR122-derived cured cells were compared with those in the parental cells. Intriguingly, both cured cell lines exhibited a significant increase of miR122 expression (approximately 2- to 6-fold) in comparison with that in the parental cells (Fig. 6C). These results suggest that the efficient propagation of HCVcc in the cured Hep3B/miR122 cells was partially attributable to an enhanced expression of miR122, rather than an impairment of the signaling pathway of innate immunity.
Fig 6.
Cured Hep3B/miR122 cells facilitate efficient propagation of HCVcc through enhanced expression of miR122. (A) (Left) Hep3B/miR122 parental cells and cured cells of clone 5 were cotransfected with pIFNβ-Luc and pRL-TK and then infected with the VSV NCP mutant at an MOI of 0.01 or transfected with 1 μg of poly(I·C) at 24 h posttransfection, and luciferase activities were determined at 48 h posttreatment; (right) the cells were cotransfected with pISRE-Luc and pRL-TK and then infected with VSV at an MOI of 0.01 or treated with IFN-α (100 IU/ml) at 24 h posttransfection, and luciferase activities were determined at 48 h posttreatment. (B) (Upper) Hep3B/miR122 parental cells and the cured cells were infected with VSV at an MOI of 0.01, fixed with 4% phosphonoformic acid at 18 h postinfection, and subjected to indirect immunofluorescence assay using rabbit anti-IRF3 antibody, followed by AF488-conjugated anti-rabbit IgG (red); (lower) the cells were treated with IFN-α (100 IU/ml), fixed with 4% paraformaldehyde at 1 h postinfection, and subjected to indirect immunofluorescence assay using rabbit anti-STAT2 antibody, followed by AF488-conjugated anti-rabbit IgG (red). Cell nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). (C) Total RNA was extracted from parental Huh7 and Hep3B/miR122 cells and their cured cells, and the relative expression of miR122 was determined by qRT-PCR by using U6 snRNA as an internal control.
Specific interaction of miR122 with viral RNA is crucial for efficient propagation of HCVcc.
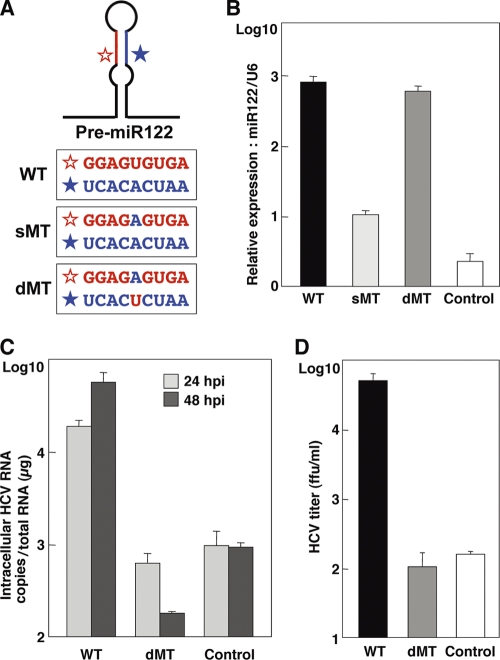
To evaluate the effect of a specific interaction of miR122 with the target sequence in the 5′ UTR of HCV RNA on the enhancement of viral propagation, we generated two mutant pre-miR122s: sMT-miR122 has a substitution of uridine to adenosine, and dMT-miR122 carries an additional complementary substitution of adenosine to uridine to stabilize the expression. These substitutions have been shown to abrogate interaction with the target sequence (27) (Fig. 7A). A high level of expression of dMT-miR122 comparable to that of WT-miR122 was detected in Hep3B cells, in contrast to the low level of expression of sMT-miR122 (Fig. 7B). As described above, the expression level of miR122 in Hep3B cells was significantly lower than that in Huh7 cells (Fig. 1B). Taking advantage of this low level of miR122 expression, WT-miR122 and dMT-miR122 were exogenously expressed in Hep3B cells by the lentiviral vector to assess the importance of the specific interaction of miR122 with viral RNA. Not only intracellular viral RNA levels but also infectious titers in the culture supernatants were enhanced by the expression of WT-miR122, but they were not enhanced by the expression of dMT-miR122 (Fig. 7C and D). These results suggest that specific interaction of miR122 with the 5′ UTR of HCV is crucial for the efficient replication and propagation of HCV.
Fig 7.
Specific interaction of miR122 with viral RNA is crucial for efficient propagation of HCVcc. (A) Diagram of pre-miR122 and partial nucleotide sequences of wild type (WT) miR122 and mutant miR122 carrying a single mutation (sMT) and double mutations (dMT). (B) Hep3B cells were transduced with lentiviral vectors expressing either WT-, sMT-, or dMT-miR122 or with a control, and the relative expression of miR122 was determined by qRT-PCR by using U6 snRNA as an internal control. (C) Hep3B cells expressing WT- or dMT-miR122 or the control cells were infected with HCVcc at an MOI of 1, and the level of HCV RNA was determined by qRT-PCR at 24 and 48 h postinfection. (D) The culture supernatants were collected at 72 h postinfection, and the viral titers of the supernatants were determined by focus-forming assay using Huh7.5.1 cells.
DISCUSSION
Most miRNAs utilize the normal RNA interfering pathway and repress translation of the target mRNAs (3, 26). For instance, miR122 targets the 3′ UTR of the cytoplasmic polyadenylation element binding protein (CPEB) (5), hemochromatosis (Hfe) and hemojuvelin (Hjv) (6), a disintegrin and metalloprotease family 10 (ADAM10) (2), and cationic amino transporter 1 (CAT-1) (8) and represses their translation. In contrast, HCV uniquely exploits the liver-specific miR122 to stimulate viral translation (23, 27–29, 38, 52). In this study, we assessed the possibility of establishment of human liver cell lines that are susceptible to HCVcc propagation through exogenous expression of miR122 by a lentiviral vector. Although Huh7 cells and their derived cell lines are highly susceptible to propagation of HCVcc, they intrinsically express an abundant amount of miR122. Among the cell lines that we investigated, Hep3B cells exhibit a high sensitivity to HCVcc propagation by expression of miR122 compared to that of Huh7 cells, whereas no sensitivity to HCVcc was observed in the parental Hep3B cells. Therefore, the Hep3B cell line was suggested to be an ideal tool to investigate miR122 function in the life cycle of HCV.
RNA viruses replicate in host cells with high error rates, generating a broad population diversity, which allows rapid adaptation to new environments (33). HCV propagates in the liver of patients with quasispecies heterogeneity and transmits to a new host through contaminated blood or blood products (16). It is known that the complexity of HCV clones significantly decreases during transmission through a genetic bottleneck, resulting in a more homogeneous population. This selection of certain clones is mainly caused by the host factors required for viral replication and immune pressure in a new host and is involved in the early phase of HCV infection in the new environment (18, 25, 32). A sole cell line, Huh7, has been employed in most of the experiments for in vitro studies of entry, RNA replication, and particle formation of HCV. Therefore, it has not been possible to assess propagation of HCVcc in human liver cell lines other than Huh7 cells and transmission of HCVcc to liver cell lines of different origins. The establishment of a novel human liver cell line, Hep3B/miR122, for propagation of HCVcc would help to generate new insights into the mutual interaction between HCV and human hepatocytes. Although we are not able to evaluate the effects of the acquired immunity on the induction of the adaptive mutations in cell culture systems, we can assess the host factors involved in the generation of the adaptive mutations by using two different human liver cell lines that support continuous propagation of HCVcc. Further studies are needed to determine the adaptive mutations in the HCV genome by passage in either Hep3B/miR122 or Huh7 cells and in one after the other.
At least seven major HCV genotypes and numerous subtypes have been identified (21), but laboratory strains capable of replicating in vitro are limited (36, 64, 68, 70). It is important to establish cell lines that permit the complete propagation of a wide range of HCV genotypes for further understanding of the life cycle of HCV. Although the partial replication of serum-derived HCV in primary hepatocytes in a specialized culture system has been reported (50), development of a simpler and more user-friendly system is required for promotion of research on HCV. It might be feasible to establish new cell culture systems for not only various genotypes of infectious HCV clones but also serum-derived HCV by the expression of miR122 in various human liver cell lines.
While preparing the manuscript, Narbus et al. reported that the expression of miR122 enhances HCV replication in HepG2/CD81 cells (46). Our data also demonstrated that the expression of miR122 increased HCV replication in HepG2/CD81 cells, as shown in Fig. 1D. However, the impact of miR122 expression on the production of infectious particles in HepG2/CD81 cells is significantly lower than that in Huh7 cells (46). Although LH86 (71) and Li23 (30) cell lines derived from human hepatocellular carcinoma have been shown to permit propagation of HCVcc, these cell lines are not well characterized. In contrast, the Hep3B cell line has been utilized in a wide range of research fields for a long time, resulting in the accumulation of many sources of data from genomic and proteomic analyses (1, 47, 55, 63, 67). Moreover, the Hep3B cell line is available from the major cell banks all over the world, which should readily allow reevaluation of the findings in this study. Comparison of the experimental data on HCVcc propagation between Huh7 and Hep3B/miR122 cells might provide a clue to understanding the host factors crucial for the efficient propagation of HCV in human liver cells.
The higher susceptibility to HCVcc propagation of the cured cells derived from Huh7 cells than the parental cells was suggested to be attributable to impairment of the innate immune response (57). However, this is not the only reason for efficient propagation of HCVcc in the Huh7-based cured cell lines (17). It has been shown that cured cell lines, such as Huh7.5.1 and Huh7-Lunet, express a higher level of miR122 than the parental Huh7 cells (13), suggesting that upregulation of miR122 in the cured cells participates in the efficient propagation of HCVcc. However, the level of miR122 expression in the cured Hep3B cells was not necessarily correlated with the replication efficiency of HCVcc in the present work (Fig. 6C). Most recently, Denard et al. reported that the expression of CREB3L1/OASIS, which specifically prevents division of virus-infected cells, in cured Huh7 cells was reduced compared to that in the parental cells (12), suggesting that CREB3L1/OASIS is also involved in the enhancement of HCVcc propagation in the cured cells.
In this study, we have shown that expression of miR122 confers susceptibility to human liver cell lines for the efficient propagation of HCVcc. Elimination of the HCV genome from the replicon cells of Hep3B/miR122 cells enhanced propagation of HCVcc in accord with the increment of miR122 expression, and propagation of HCVcc in the cured cells was continuously increased in every passage. Furthermore, the interaction between HCV RNA and miR122 was shown to be specific for production of infectious particles in Hep3B/miR122 cells. The establishment of a new permissive cell line for HCVcc allows us not only to investigate the biological function of miR122 on the life cycle of HCV but also to develop novel therapeutics for chronic hepatitis C.
ACKNOWLEDGMENTS
We thank M. Tomiyama for her secretarial work. We also thank M. Hijikata, T. Wakita, F. Chisari, T. Kawai, S. Akira, and M. Whitt for providing experimental materials.
This work was supported in part by grants-in-aid from the Ministry of Health, Labor, and Welfare (Research on Hepatitis); the Ministry of Education, Culture, Sports, Science, and Technology; and the Osaka University Global Center of Excellence Program.
Footnotes
Published ahead of print 23 November 2011
REFERENCES
- 1. Aden DP, Fogel A, Plotkin S, Damjanov I, Knowles BB. 1979. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature 282:615–616 [DOI] [PubMed] [Google Scholar]
- 2. Bai S, et al. 2009. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J. Biol. Chem. 284:32015–32027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bartel DP. 2009. MicroRNAs: target recognition and regulatory functions. Cell 136:215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blight KJ, McKeating JA, Rice CM. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 76:13001–13014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burns DM, D'Ambrogio A, Nottrott S, Richter JD. 2011. CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature 473:105–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castoldi M, et al. 2011. The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice. J. Clin. Invest. 121:1386–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang J, et al. 2008. Liver-specific microRNA miR-122 enhances the replication of hepatitis C virus in nonhepatic cells. J. Virol. 82:8215–8223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang J, et al. 2004. miR-122, a mammalian liver-specific microRNA, is processed from hcr mRNA and may downregulate the high affinity cationic amino acid transporter CAT-1. RNA Biol. 1:106–113 [DOI] [PubMed] [Google Scholar]
- 9. Chang KS, Jiang J, Cai Z, Luo G. 2007. Human apolipoprotein E is required for infectivity and production of hepatitis C virus in cell culture. J. Virol. 81:13783–13793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cormier EG, et al. 2004. CD81 is an entry coreceptor for hepatitis C virus. Proc. Natl. Acad. Sci. U. S. A. 101:7270–7274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Date T, et al. 2004. Genotype 2a hepatitis C virus subgenomic replicon can replicate in HepG2 and IMY-N9 cells. J. Biol. Chem. 279:22371–22376 [DOI] [PubMed] [Google Scholar]
- 12. Denard B, et al. 2011. The membrane-bound transcription factor CREB3L1 is activated in response to virus infection to inhibit proliferation of virus-infected cells. Cell Host Microbe 10:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ehrhardt M, et al. 18 April 2011. Profound differences of microRNA expression patterns in hepatocytes and hepatoma cell lines commonly used in hepatitis C virus studies. Hepatology. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 14. Elmen J, et al. 2008. LNA-mediated microRNA silencing in non-human primates. Nature 452:896–899 [DOI] [PubMed] [Google Scholar]
- 15. Evans MJ, et al. 2007. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446:801–805 [DOI] [PubMed] [Google Scholar]
- 16. Farci P, et al. 2000. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science 288:339–344 [DOI] [PubMed] [Google Scholar]
- 17. Feigelstock DA, Mihalik KB, Kaplan G, Feinstone SM. 2010. Increased susceptibility of Huh7 cells to HCV replication does not require mutations in RIG-I. Virol. J. 7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feliu A, Gay E, Garcia-Retortillo M, Saiz JC, Forns X. 2004. Evolution of hepatitis C virus quasispecies immediately following liver transplantation. Liver Transpl. 10:1131–1139 [DOI] [PubMed] [Google Scholar]
- 19. Ge D, et al. 2009. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461:399–401 [DOI] [PubMed] [Google Scholar]
- 20. Gentzsch J, et al. 2011. Hepatitis C virus complete life cycle screen for identification of small molecules with pro- or antiviral activity. Antiviral Res. 89:136–148 [DOI] [PubMed] [Google Scholar]
- 21. Gottwein JM, et al. 2009. Development and characterization of hepatitis C virus genotype 1-7 cell culture systems: role of CD81 and scavenger receptor class B type I and effect of antiviral drugs. Hepatology 49:364–377 [DOI] [PubMed] [Google Scholar]
- 22. Haid S, Windisch MP, Bartenschlager R, Pietschmann T. 2010. Mouse-specific residues of claudin-1 limit hepatitis C virus genotype 2a infection in a human hepatocyte cell line. J. Virol. 84:964–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henke JI, et al. 2008. microRNA-122 stimulates translation of hepatitis C virus RNA. EMBO J. 27:3300–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herker E, et al. 2010. Efficient hepatitis C virus particle formation requires diacylglycerol acyltransferase-1. Nat. Med. 16:1295–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hughes MG, Jr, et al. 2005. HCV infection of the transplanted liver: changing CD81 and HVR1 variants immediately after liver transplantation. Am. J. Transplant. 5:2504–2513 [DOI] [PubMed] [Google Scholar]
- 26. Huntzinger E, Izaurralde E. 2011. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat. Rev. Genet. 12:99–110 [DOI] [PubMed] [Google Scholar]
- 27. Jangra RK, Yi M, Lemon SM. 2010. Regulation of hepatitis C virus translation and infectious virus production by the microRNA miR-122. J. Virol. 84:6615–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jopling CL, Schutz S, Sarnow P. 2008. Position-dependent function for a tandem microRNA miR-122-binding site located in the hepatitis C virus RNA genome. Cell Host Microbe 4:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. 2005. Modulation of hepatitis C virus RNA abundance by a liver-specific microRNA. Science 309:1577–1581 [DOI] [PubMed] [Google Scholar]
- 30. Kato N, et al. 2009. Efficient replication systems for hepatitis C virus using a new human hepatoma cell line. Virus Res. 146:41–50 [DOI] [PubMed] [Google Scholar]
- 31. Lanford RE, et al. 2010. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327:198–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Laskus T, et al. 2004. Analysis of hepatitis C virus quasispecies transmission and evolution in patients infected through blood transfusion. Gastroenterology 127:764–776 [DOI] [PubMed] [Google Scholar]
- 33. Lauring AS, Andino R. 2010. Quasispecies theory and the behavior of RNA viruses. PLoS Pathog. 6:e1001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lavanchy D. 2009. The global burden of hepatitis C. Liver Int. 29(Suppl. 1):74–81 [DOI] [PubMed] [Google Scholar]
- 35. Lin LT, et al. 2010. Replication of subgenomic hepatitis C virus replicons in mouse fibroblasts is facilitated by deletion of interferon regulatory factor 3 and expression of liver-specific microRNA 122. J. Virol. 84:9170–9180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lindenbach BD, et al. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623–626 [DOI] [PubMed] [Google Scholar]
- 37. Lohmann V, et al. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110–113 [DOI] [PubMed] [Google Scholar]
- 38. Machlin ES, Sarnow P, Sagan SM. 2011. Masking the 5′ terminal nucleotides of the hepatitis C virus genome by an unconventional microRNA-target RNA complex. Proc. Natl. Acad. Sci. U. S. A. 108:3193–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Masaki T, et al. 2010. Production of infectious hepatitis C virus by using RNA polymerase I-mediated transcription. J. Virol. 84:5824–5835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miyanari Y, et al. 2007. The lipid droplet is an important organelle for hepatitis C virus production. Nat. Cell Biol. 9:1089–1097 [DOI] [PubMed] [Google Scholar]
- 41. Moradpour D, Penin F, Rice CM. 2007. Replication of hepatitis C virus. Nat. Rev. Microbiol. 5:453–463 [DOI] [PubMed] [Google Scholar]
- 42. Moriishi K, Matsuura Y. 2007. Evaluation systems for anti-HCV drugs. Adv. Drug Deliv. Rev. 59:1213–1221 [DOI] [PubMed] [Google Scholar]
- 43. Moriishi K, Matsuura Y. 2007. Host factors involved in the replication of hepatitis C virus. Rev. Med. Virol. 17:343–354 [DOI] [PubMed] [Google Scholar]
- 44. Moriishi K, Matsuura Y. 2003. Mechanisms of hepatitis C virus infection. Antivir. Chem. Chemother. 14:285–297 [DOI] [PubMed] [Google Scholar]
- 45. Moriishi K, et al. 2010. Involvement of PA28gamma in the propagation of hepatitis C virus. Hepatology 52:411–420 [DOI] [PubMed] [Google Scholar]
- 46. Narbus CM, et al. 2011. HepG2 cells expressing microRNA miR-122 support the entire hepatitis C virus life cycle. J. Virol. 85:12087–12092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park JG, et al. 1995. Characterization of cell lines established from human hepatocellular carcinoma. Int. J. Cancer 62:276–282 [DOI] [PubMed] [Google Scholar]
- 48. Pileri P, et al. 1998. Binding of hepatitis C virus to CD81. Science 282:938–941 [DOI] [PubMed] [Google Scholar]
- 49. Ploss A, et al. 2009. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457:882–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ploss A, et al. 2010. Persistent hepatitis C virus infection in microscale primary human hepatocyte cultures. Proc. Natl. Acad. Sci. U. S. A. 107:3141–3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Randall G, et al. 2007. Cellular cofactors affecting hepatitis C virus infection and replication. Proc. Natl. Acad. Sci. U. S. A. 104:12884–12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Roberts AP, Lewis AP, Jopling CL. 2011. miR-122 activates hepatitis C virus translation by a specialized mechanism requiring particular RNA components. Nucleic Acids Res. 39:7716–7729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Russell RS, et al. 2008. Advantages of a single-cycle production assay to study cell culture-adaptive mutations of hepatitis C virus. Proc. Natl. Acad. Sci. U. S. A. 105:4370–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Scarselli E, et al. 2002. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 21:5017–5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Seow TK, Liang RC, Leow CK, Chung MC. 2001. Hepatocellular carcinoma: from bedside to proteomics. Proteomics 1:1249–1263 [DOI] [PubMed] [Google Scholar]
- 56. Skalsky RL, Cullen BR. 2010. Viruses, microRNAs, and host interactions. Annu. Rev. Microbiol. 64:123–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sumpter R, Jr, et al. 2005. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 79:2689–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Suppiah V, et al. 2009. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat. Genet. 41:1100–1104 [DOI] [PubMed] [Google Scholar]
- 59. Tanaka Y, et al. 2009. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat. Genet. 41:1105–1109 [DOI] [PubMed] [Google Scholar]
- 60. Tani H, et al. 2007. Replication-competent recombinant vesicular stomatitis virus encoding hepatitis C virus envelope proteins. J. Virol. 81:8601–8612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Targett-Adams P, Boulant S, McLauchlan J. 2008. Visualization of double-stranded RNA in cells supporting hepatitis C virus RNA replication. J. Virol. 82:2182–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thomas DL, et al. 2009. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461:798–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vannucchi AM, et al. 1993. Effects of cyclosporin A on erythropoietin production by the human Hep3B hepatoma cell line. Blood 82:978–984 [PubMed] [Google Scholar]
- 64. Wakita T, et al. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Walters KA, et al. 2006. Host-specific response to HCV infection in the chimeric SCID-beige/Alb-uPA mouse model: role of the innate antiviral immune response. PLoS Pathog. 2:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Windisch MP, et al. 2005. Dissecting the interferon-induced inhibition of hepatitis C virus replication by using a novel host cell line. J. Virol. 79:13778–13793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wong N, et al. 2005. Transcriptional profiling identifies gene expression changes associated with IFN-alpha tolerance in hepatitis C-related hepatocellular carcinoma cells. Clin. Cancer Res. 11:1319–1326 [PubMed] [Google Scholar]
- 68. Yi M, Villanueva RA, Thomas DL, Wakita T, Lemon SM. 2006. Production of infectious genotype 1a hepatitis C virus (Hutchinson strain) in cultured human hepatoma cells. Proc. Natl. Acad. Sci. U. S. A. 103:2310–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yoneyama M, et al. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730–737 [DOI] [PubMed] [Google Scholar]
- 70. Zhong J, et al. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 102:9294–9299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhu H, et al. 2007. Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral defense system. Gastroenterology 133:1649–1659 [DOI] [PubMed] [Google Scholar]