Abstract
Respiratory syncytial virus (RSV) interaction with epithelial and dendritic cells (DCs) is known to require divalent cations, suggesting involvement of C-type lectins. RSV infection and maturation of primary human DCs are reduced in a dose-dependent manner by EDTA. Therefore, we asked whether RSV infection involves DC-SIGN (CD209) or its isoform L-SIGN (CD299) (DC-SIGN/R). Using surface plasmon resonance analysis, we demonstrated that the attachment G glycoprotein of RSV binds both DC- and L-SIGN. However, neutralization of DC- and L-SIGN on primary human DCs did not inhibit RSV infection, demonstrating that interactions between RSV G and DC- or L-SIGN are not required for productive infection. Thus, neither DC- nor L-SIGN represents a functional receptor for RSV. However, inhibition of these interactions increased DC activation, as evidenced by significantly higher levels of alpha interferon (IFN-α), MIP-1α, and MIP-1β in plasmacytoid DCs (pDCs) exposed to RSV after neutralization of DC-and L-SIGN. To understand the molecular interactions involved, intracellular signaling events triggered by purified RSV G glycoprotein were examined in DC- and L-SIGN-transfected 3T3 cells. RSV G interaction with DC- or L-SIGN was shown to stimulate ERK1 and ERK2 phosphorylation, with statistically significant increases relative to mock-infected cells. Neutralization of DC- and L-SIGN reduced ERK1/2 phosphorylation. With increased DC activation following DC- and L-SIGN neutralization and RSV exposure, these data demonstrate that the signaling events mediated by RSV G interactions with DC/L-SIGN are immunomodulatory and diminish DC activation, which may limit induction of RSV-specific immunity.
INTRODUCTION
Respiratory syncytial virus (RSV) infects most infants during the first year of life and is often the first infection experienced (9). However, RSV infection does not induce sustained, protective immunity, as reinfection occurs every 2 to 3 years throughout life. RSV has demonstrated the ability to interfere with the function of innate immunity and with both the cellular and humoral arms of adaptive immunity. Nonstructural proteins 1 and 2 (NS1/NS2) of RSV have multiple mechanisms for disrupting type 1 interferon (IFN) pathways (55). Antibody responses induced by natural infection in infants are of relatively low magnitude and short-lived (39), and RSV is known to suppress the proliferative capacity of lymphocytes (43). Additionally, inefficient function in memory T and B cell compartments has been described (4, 42, 51, 59). While primary tropism in the lung is for airway epithelial cells, RSV infection of both primary dendritic cells (DCs) (23, 27, 29) and monocyte-derived dendritic cells (moDCs) (8, 12, 23, 26, 30, 35) has been demonstrated in human, bovine, and ovine systems. Thus, indirect effects of RSV-infected epithelium on airway DCs or direct effects of RSV on DCs through infection or secreted RSV proteins may impact host immunity.
While RSV has been shown to bind to surfactants A (3, 20, 25) and D (33), Toll-like receptor 4 (TLR4) (31), CX3CR1 (57), and heparan sulfate/glycosaminoglycans (13, 14, 24), a specific protein receptor for RSV has yet to be reported. It has been demonstrated that calcium is required for infection of epithelial cells (47). We have recently demonstrated that infection of DCs is also blocked in the presence of EDTA (29), suggesting the involvement of calcium-dependent (C-type) lectins during RSV infection. C-type lectins commonly found on DCs include dendritic cell-specific ICAM-3 grabbing nonintegrin (DC-SIGN), mannose receptor (CD206), DEC 205 (CD205), dectin-1 and -2 (C-type lectin domain family member 7A [CLEC7A] and CLEC6A, respectively), CLEC12A, CLEC9A, DC immunoreceptor (DCIR) (CLEC4A), and Mincle (CLEC4E) (17).
DC-SIGN (CD209) and the closely related molecule L-SIGN (DC-SIGNR; CD299), collectively designated DC/L-SIGN, are tetrameric type II transmembrane proteins containing calcium-dependent carbohydrate recognition domains (CRDs) that recognize mannose- and fucose-containing oligosaccharides (54). Binding of endogenous ligands containing these oligosaccharides results in activation of host immunity at multiple levels, including DC maturation and migration, T cell priming, and immunomodulation (e.g., Th1/Th2 skewing) (16, 17, 54). However, DC/L-SIGN have been shown to function as pattern recognition receptors (PRRs) to detect and bind exogenous ligands on invading pathogens, including Mycobacterium tuberculosis (21), Helicobacter pylori (21), Porphyromonas gingivalis (60), Enterobacter sakazakii (38), HIV-1 (18, 21, 53), Ebola virus (2), hepatitis C virus (10), West Nile virus (11), Dengue virus (36, 45, 56), and severe acute respiratory syndrome coronavirus (28). In addition to immune activation, these interactions may also facilitate pathogen uptake and dissemination (11, 36, 56) and/or modulate host immunity (16, 17, 21, 38, 54, 60). While ligand binding induces cellular kinases and signaling cascades (7, 38, 48), recent studies demonstrate that signaling via DC/L-SIGN can also cooperate with signals transduced by other PRRs, such as TLRs, to facilitate infection or immunomodulation (16, 22, 34).
We have recently demonstrated that CD209 expression may be increased following RSV infection of primary human myeloid DCs (mDCs) and pDCs (29). Furthermore, we showed that, as with epithelial cells, RSV infection was inhibited in the presence of EDTA, suggesting a potential role for C-type lectins in RSV binding and/or infection of DCs. Modeling of the L-SIGN (DC-SIGNR) receptor tetramer predicted RSV G glycoprotein, the putative viral attachment protein, may bind L-SIGN (52). We therefore examined the ability of RSV G to bind and signal through DC/L-SIGN and the impact of DC/L-SIGN neutralization on RSV-mediated infection and activation of primary human DCs.
MATERIALS AND METHODS
Viruses and purified viral glycoproteins.
Recombinant RSV expressing green fluorescent protein (GFP) (rgRSV), a gift of Mark Peeples (Ohio State University) (24), was grown in HEp-2 cells with minimal essential medium (MEM) containing 10% fetal calf serum (FCS). Mock-infected HEp-2 cells were similarly processed to generate a mock control stock for infection. Supernatants from HEp-2 cells infected with vaccinia virus expressing secreted RSVG (vvGs, a gift of Gail Wertz, University of Virginia) (44) was used for purification of RSV G protein. RSV G glycoprotein was purified by fast protein liquid chromatography (FPLC) using lentil lectin (GE Healthcare) with minor modification of the manufacturer's protocols, eluting bound protein with 0.5 M NaCl–0.02 M Tris-HCl–0.5 M methyl-α-mannoside, pH 7.4. Supernatant from uninfected HEp-2 cells was similarly processed to generate a negative-control chromatography preparation. Purified RSV G was dialyzed against 1× Hanks' balanced salt solution (without calcium or magnesium) containing 10 mM HEPES and 0.005% Tween 20 overnight at 4°C. Immediately before binding studies were performed, CaCl2 was added to a final concentration of 1 mM.
Surface plasmon resonance.
DC-SIGN–Fc (CD209) or L-SIGN–Fc (CD299) was purchased from R&D Systems and covalently coupled to a CM5 chip at a density of approximately 6,000 response units. A blank surface with no antigen was created under identical coupling conditions for use as a reference. Purified RSV G was allowed to flow over the immobilized proteins and reference cell in 150 mM NaCl, 10 mM HEPES, pH 7.5, 0.005% Tween 20, and 10 mM CaCl2. The data were processed with SCRUBBER-2 and double referenced by subtraction of the blank surface and a blank injection (no analyte).
Isolation of primary DCs and generation of moDCs and macrophages.
Elutriated human monocytes were obtained from the NIH Department of Transfusion Medicine. The donors were healthy adults, and their serologic status for HIV-1 and cytomegalovirus (CMV) was determined. Additional donor information was limited to age, sex, and race. pDCs, and mDC were sequentially purified using BDCA-4 and then BDCA-1 magnetic isolation kits (Miltenyi Biotec). The isolated pDCs were incubated in 10% RPMI and interleukin 3 (IL-3) (1 ng/ml; BioWhittaker). The isolated mDC were cultured in 10% RPMI and granulocyte-macrophage colony-stimulating factor (GM-CSF) (2 ng/ml; PeproTech Inc.). The purity of the isolated DCs was examined by phenotypic analyses using a lineage cocktail (CD3, CD14, CD16, CD19, CD20, and CD56) and CD1c, CD11c, CD14, and CD123 (BD Biosciences). The cells were rested at 37°C overnight.
Infection and maturation of DCs with EDTA treatment or DC/L-SIGN neutralization.
Aliquots of isolated DCs were exposed to rgRSV or an equal volume of mock HEp-2 supernatant. The requirements for divalent cations in RSV infection were examined after treatment with dilutions of EDTA (50 mM to 0.005 mM) on ice for 30 min, and then the EDTA-treated rgRSV was added to mDCs or pDCs at a multiplicity of infection (MOI) of 3. DCs were similarly treated with EDTA (30 min at room temperature). rgRSV and DCs were then mixed, incubated, and analyzed 24 h postinfection (p.i.). Twenty-four hours p.i., the cells were pelleted, and the supernatants were removed and frozen. The cells were stained with “DC activation mix” (BD Biosciences) containing CD86-phycoerythrin (PE), CD80-allophycocyanin (APC), and CD209–peridinin chlorophyll protein (PerCP)-Cy5.5; fixed in 2% paraformaldehyde; and analyzed by flow cytometry. Monoclonal antibodies specific for DC-SIGN (clone 120507), L-SIGN (clone 120604), or both DC- and L-SIGN (clone 120526) were obtained from the NIH AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH). IgG2b isotype control antibody was purchased from R&D Systems. Isolated and rested mDCs and pDCs (2 × 105 to 4 × 105 cells) were incubated with DC- and L-SIGN antibodies (10 μg/ml each of clones 12057 and 120604 or 10 μg/ml of clone 15026) or isotype control antibody for 1 h at 37°C, and then rgRSV (MOI = 1) was added. The cells were incubated for 3 h or overnight, after which the cells were pelleted and the supernatants were removed. The cells were stained with a multiparameter flow cytometry panel developed to evaluate DC maturation in greater detail. RSV-exposed DCs were stained with ViViD viability dye (Molecular Probes) while being incubated with Fc block (BD Biosciences), followed by staining with a mixture of phenotypic and maturation cell surface markers, including CD11c-PE (BD Biosciences), CD14-Qdot605 (VRC Flow Cytometry Core), CD123–Cy5-PE (BD Biosciences), CD86-AX700 (VRC Flow Cytometry Core), CD40–Texas Red-PE (VRC Flow Cytometry Core), CD209–PerCP-Cy5.5 (BD Biosciences), and CD163-APC (R&D Systems). After staining, the cells were fixed and analyzed on the LSRII flow cytometer (BD Biosciences). Flow cytometry data were analyzed using FlowJo software (Tree Star, Inc.). Due to donor-to-donor variation in receptor expression levels, mean fluorescence intensities (MFI) for RSV-exposed samples were normalized to mock-infected samples for the same donor to determine the fold increase in receptor expression. The normalized data from pooled donors were then compared.
Cytokine and chemokine production.
Cytokine and chemokine concentrations in the reserved DC supernatants were measured by enzyme-linked immunosorbent assay (ELISA) according to the kit protocols (R&D Systems). Due to donor-to-donor variation, cytokine and chemokine concentrations (in pg/ml) for RSV-exposed samples were normalized to those of mock-infected samples for the same donor to determine the fold increase in protein production. When mock-treated samples were below the level of detection, the protein concentration designated as the limit of detection (as established by the manufacturer's kit protocols) was used for normalization. The normalized data from pooled donors were then compared.
RSV infection of DC-SIGN and L-SIGN stable transfectants.
Parental K562 and Raji cell lines and each cell line stably transfected with DC-SIGN or with L-SIGN were obtained from Theodore Pierson (NIAID NIH). Parental 3T3 cells and 3T3–DC-SIGN stable transfectants were obtained from Mary Marovich (Walter Reed Army Institute of Research), and 3T3–L-SIGN stable transfectants were obtained from the NIH AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH). The cells were infected with various MOIs of rgRSV and analyzed by flow cytometry 24 h p.i.
DC-SIGN/L-SIGN signaling.
Parental 3T3 cells, 3T3-DC-SIGN transfectants, and 3T3–L-SIGN transfectants were serum starved overnight, incubated with 10 μg/ml anti-DC/L-SIGN (clone 120526; NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) or IgG2a isotype control antibody (R&D Systems), and then exposed to 1 μg purified RSV G, purified HIV-1BaL gp120 (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) or HEp-2 negative-control chromatography preparation for 30 min. The cells were washed with cold phosphate-buffered saline (PBS) and lysed with RIPA lysis buffer (Thermo Scientific) supplemented with HALT phosphatase inhibitors and HALT protease inhibitors (Thermo Scientific). Protein concentrations were determined by microplate bicinchoninic acid (BCA) analysis (Pierce Chemical). Using 2 μg protein per well, the samples were electrophoresed and transferred to polyvinylidene difluoride (PVDF) membranes using the iBlot apparatus (Invitrogen). After blocking, the blots were incubated with phosphospecific ERK1/2 (Thr202/Tyr204; catalog number 9101) and Akt (Ser473; catalog number 9211) rabbit monoclonal antibodies (Cell Signaling Technologies) and then horseradish peroxidase (HRP)-labeled mouse anti-rabbit IgG (Jackson ImmunoResearch) and developed with ECL-Plus chemiluminescent reagent (GE Healthcare). The blot was stripped with Restore Western Blot Stripping Buffer (Thermo Scientific), probed with panspecific ERK1/2 (catalog number 9102), Akt (catalog number 9272), and α-tubulin rabbit monoclonal antibodies (catalog number 2125; Cell Signaling Technologies) and then with HRP-conjugated mouse anti-rabbit IgG, and developed with ECL-Plus substrate. The films were scanned, and densitometry was performed using ImageJ software. The density of each ERK1/2 and Akt band was normalized to the α-tubulin band for each sample to control for sample loading, and then the ratio between the anti-DC/L-SIGN-treated sample and the isotype control-treated sample for each treatment condition was calculated.
Statistical analyses.
Maturation and cytokine data are represented as the geometric mean ± the standard deviation for 6 donors. Statistical comparisons were evaluated by analysis of variance (ANOVA) on rank sums (Mann-Whitney or Wilcoxon rank sum tests) using SigmaStat software.
RESULTS
RSV requires divalent cations for infection of primary human DCs.
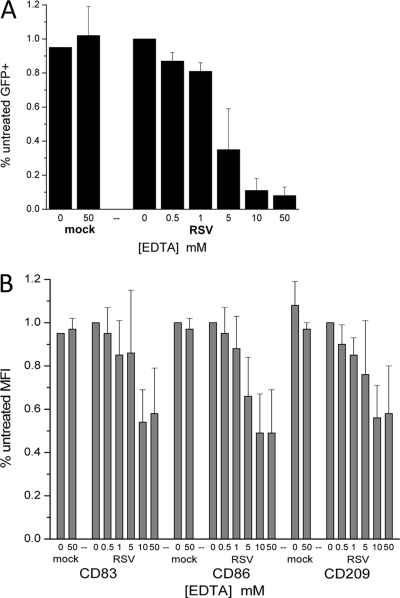
Requirements for divalent cations in RSV infection were examined after EDTA treatment of rgRSV or of primary mDCs or pDCs. In all donors, RSV infection of DCs was completely inhibited at ≥10 mM EDTA with partial inhibition at 5 mM EDTA (Fig. 1). At EDTA concentrations of ≤1 mM, the ability of rgRSV to infect either DC subset was unaffected. No difference in the ability of EDTA to inhibit RSV infection was observed whether the virus stock or the cells were treated. As with epithelial cells, presumably the loss of calcium was the critical factor in the inhibition of infection, although this was not confirmed by reconstitution. These data demonstrate the requirement for divalent cations during RSV infection and may suggest the involvement of a C-type lectin(s) in RSV infection of primary human DCs.
Fig 1.
Dose-dependent inhibition of RSV infection and maturation of primary human DCs. rgRSV or isolated mDCs or pDCs (not shown) were treated with increasing concentrations of EDTA. Virus and cells were then mixed and incubated overnight. The following morning, the cells were stained and examined by flow cytometry for infection as evidenced by GFP expression (A) or CD83, CD86, and CD209 expression (B). The data represent the degree of infection and maturation marker expression relative to untreated (no EDTA) cells for mock- and RSV-infected mDCs (MOI = 3). n was equal to 4 unique donors for all EDTA concentrations except 50 mM, where 11 unique donors were included. The error bars indicate standard deviations.
RSV G binds DC-SIGN and L-SIGN.
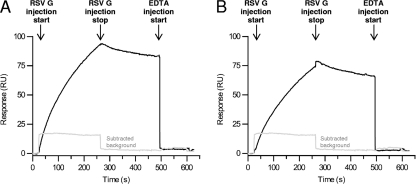
Algorithms have predicted the RSV G glycoprotein may bind L-SIGN (52). Therefore, RSV G binding to DC- and L-SIGN was examined by surface plasmon resonance. Purified RSV G tends to oligomerize in the presence of calcium, preventing accurate determination of the rate constants. Qualitative binding experiments, rather than kinetic experiments, were therefore performed. RSV G, at approximately 35 μM, bound immobilized DC-SIGN and L-SIGN in the presence of 10 mM CaCl2 (Fig. 2). Binding to either DC-SIGN or L-SIGN was abrogated in the presence of 50 mM EDTA, indicating that the binding is dependent on divalent cations (presumably calcium, as magnesium was not added to the BIAcore binding buffer).
Fig 2.
Purified RSV G glycoprotein binds to DC-SIGN (CD209) and L-SIGN (DC-SIGN/R; CD299). Biacore chips were coated with 6,000 response units of DC-SIGN–Fc (A) or L-SIGN–Fc (DC-SIGN/R) (B), and purified RSV G was allowed to flow over the chip. Changes in relative intensity were monitored. The black lines are the background-subtracted responses for RSV G binding to immobilized DC-SIGN (A) and DC-SIGN/R (B). The addition of 50 mM EDTA causes bound RSV G to dissociate from the lectins, as indicated by the return to baseline. The gray lines are the responses for RSV G passing over a reference surface (activated and blocked). It resembles a delta function due to a difference in the bulk refractive index between the running buffer and the RSV G-containing buffer.
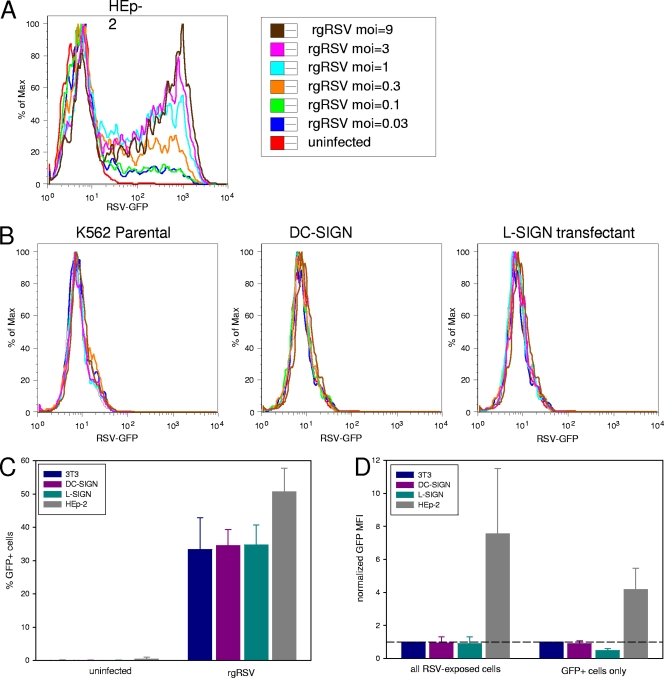
Expression of DC-SIGN or L-SIGN does not increase susceptibility to RSV infection.
K562 cells (Fig. 3) and Raji cells (data not shown) are not permissive to RSV infection. Transfectants of either cell line stably expressing DC-SIGN or L-SIGN did not become susceptible to RSV infection (Fig. 3A). However, this resistance to infection may reflect defects in steps of viral entry or replication other than binding of the virus particle. The 3T3 parental cell line (Fig. 3B) is semipermissive to RSV infection relative to HEp-2 cells (Fig. 3C). Therefore, stable DC-SIGN and L-SIGN 3T3 transfectants were examined. However, expression of DC-SIGN or L-SIGN did not increase the rate of RSV infection as assessed by GFP expression (Fig. 3C and D). Infection rates of 3T3 cells (parental or DC/L-SIGN transfectants) at lower multiplicities of infection did not increase with longer incubation (up to 72 h), nor did infection result in syncytium formation, suggesting blocks in later steps of viral replication, assembly, and/or spread (data not shown).
Fig 3.
Expression of DC-SIGN (CD209) or L-SIGN (CD299) does not increase RSV infection rates. (A) HEp-2 cells are permissive for RSV (MOI = 3). (B) Nonpermissive K562 or Raji cells (not shown) stably transfected with DC-SIGN or L-SIGN were infected with increasing doses of rgRSV. (C and D) Stable transfectants of semipermissive 3T3 cells were not more susceptible to RSV infection, as evaluated by the percentage of infected cells (C) or the expression of GFP (D). Infection was evaluated by flow cytometry 24 h postinfection. The dotted line in panel B represents normalization at 1, where GFP MF1 of DC/L-SIGN transfectants or HEp-2 cells equals the GFP MF1 of 3T3 parental cells. The error bars indicate standard deviations.
Neutralization of DC/L-SIGN does not reduce RSV infection of primary human mDCs or pDCs but does alter DC maturation and cytokine production.
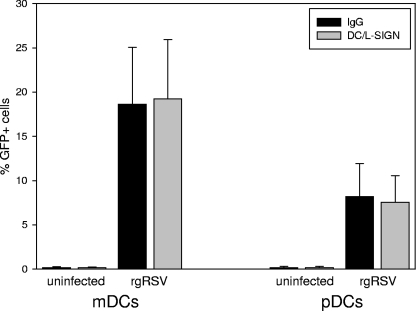
To determine if interactions between RSV and DC/L-SIGN were critical for infection of primary human DCs, isolated mDCs and pDCs were incubated with neutralizing antibodies to DC-SIGN and L-SIGN (10 μg/ml of each) that were demonstrated to neutralize binding of ICAM-3, the natural ligand for DC/L-SIGN. The cells were then exposed to rgRSV. Infection was evaluated by GFP expression at 24 h postinfection. Neutralization of DC-SIGN and L-SIGN did not significantly alter the rates of mDC or pDC infection in any donor tested (Fig. 4).
Fig 4.
DC-SIGN (CD209)- and L-SIGN (CD299)-specific antibodies do not interfere with RSV infection of mDCs or pDCs. Primary mDCs or pDCs were incubated with neutralizing antibodies to DC-SIGN and L-SIGN for 1 h at 37°C and then exposed to rgRSV (MOI = 1). Infection was evaluated by flow cytometry 24 h postinfection. The error bars indicate standard deviations.
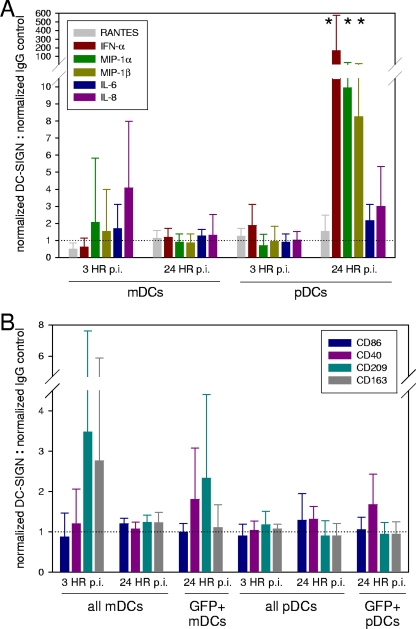
Exposure of human DCs to RSV induces maturation as measured by increased expression of CD80, CD83, and CD86 (12, 23, 29, 46) and activates DCs to produce an array of cytokines and chemokines (12, 23, 29, 46). While infection rates were not altered, anti-DC/L-SIGN treatment resulted in significant changes in cytokine and chemokine production in RSV-exposed pDCs (Fig. 5A). Neutralization of DC/L-SIGN significantly increased alpha interferon (IFN-α), MIP-1α, and MIP-1β production stimulated by 24-h exposure to rgRSV. Although the differences did not attain statistical significance, IL-6 and IL-8 production at 24 h postinfection and IFN-α levels at 3 h postinfection were increased in pDCs. Similarly, marked but nonsignificant changes were seen in mDCs at 3 h postinfection with anti-DC/L-SIGN-treated mDCs producing less RANTES and greater levels of IL-6 (169%) and IL-8 (407%) than IgG-treated mDCs (Fig. 5A).
Fig 5.
Neutralization of DC-SIGN (CD209) or L-SIGN (CD299) alters DC activation. (A and B) Primary mDCs or pDCs were incubated with neutralizing antibodies to DC-SIGN and L-SIGN for 1 h at 37°C and then exposed to rgRSV (MOI = 1). At 24 h postinfection, DC activation was compared by examination of cytokine production in the culture supernatants by ELISA (A) and by analyses of DC maturation by flow cytometry (B). The data represent means and standard deviations of the fold increase when RSV-exposed samples were normalized to mock-infected samples from the same donor, and then the responses in anti-DC/L-SIGN-treated samples were normalized to isotype control-treated samples for each individual donor. The dotted lines represent normalization at 1, where responses in anti-DC/L-SIGN-treated cells equal responses in control IgG-treated cells. n = 5 separate experiments with 5 unique donors. *, significant increases relative to samples treated with control IgG.
As with cytokine production, neutralization of DC/L-SIGN resulted in modest changes in expression of DC maturation markers (Fig. 5B). Although the differences did not achieve statistical significance (P > 0.05), anti-DC/L-SIGN-treated samples tended to have increased expression of canonical DC maturation markers that were 20 to 30% greater than expression in isotype control-treated samples (Fig. 5B). This was particularly apparent in mDCs at 3 h postinfection and in infected (i.e., GFP+) mDCs at 24 h after infection, when maturation in DC/L-SIGN-neutralized samples was 348% and 233% greater, respectively, than in IgG-treated samples based on CD209 levels. Similarly, anti-DC/L-SIGN treatment increased CD40 expression in infected mDCs and pDCs and in all pDCs at 3 h p.i., when CD40 levels were 181%, 132%, and 168% greater, respectively, than in isotype control-treated samples (Fig. 5B).
Neutralization of DC/L-SIGN decreases RSV G-induced ERK1/2 phosphorylation.
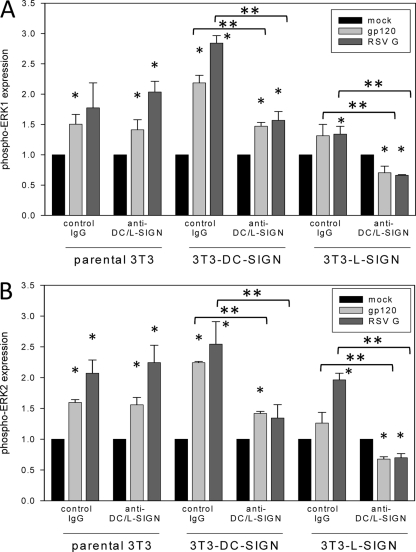
Ligand binding to or antibody cross-linking of DC/L-SIGN has been shown to result in phosphorylation of multiple cellular kinase pathways, including ERK1/2, p38/mitogen-activated protein kinase (MAPK), Akt, and c-Raf (5, 7, 38, 48). Similarly, RSV phosphorylates ERK1/2 during infection of epithelial cells (49). As moderate changes were observed in DC maturation and cytokine and chemokine production following DC/L-SIGN neutralization and RSV exposure, we examined the impact of RSV G on signaling events in 3T3 parent cells and 3T3–DC-SIGN and 3T3–L-SIGN transfectants. The results were compared to stimulation with HIV-1 gp120, which has been previously shown to induce ERK1 phosphorylation in DC-SIGN-transfected 3T3 cells (48). We therefore used HIV-1 gp120 as a positive control and confirmed that it induces ERK1 phosphorylation in 3T3–DC-SIGN cells (Fig. 6A) and demonstrated phosphorylation of ERK1 in 3T3–L-SIGN cells (Fig. 6A) and of ERK2 in 3T3 cells transfected with DC- or L-SIGN (Fig. 6B). Upon incubation of parental 3T3 cells with 10 μg purified RSV G, phosphorylation of ERK1/2 was observed, with markedly greater increases in pERK2 than in pERK1 (Fig. 6). When 3T3 cells were treated with 1 μg RSV G, very low levels of pERK2 were consistently observed, and pERK1 was observed in some samples. These levels were noticeably less than those seen in cells treated with 1 μg HIV-1 gp120, particularly in pERK1 expression (data not shown). Furthermore, pERK1/2 levels did not appreciably increase in 3T3 cells transfected with DC- or L-SIGN following treatment with 1 μg RSV G. Therefore, in order to consistently induce sufficient pERK1/2 levels to ensure reliable image analyses, we performed all subsequent experiments with 10 μg purified RSV G. In DC/L-SIGN transfectants, RSV G induced ERK1 and ERK2 (Fig. 6). Levels of ERK1/2 phosphorylation were lower in 3T3–L-SIGN cells than in 3T3–DC-SIGN transfectants, reflecting the somewhat lower binding affinity observed in the surface plasmon resonance assay (Fig. 2). When DC/L-SIGN were neutralized, levels of phosphorylated ERK1 and ERK2 were reduced in cells treated with HIV-1 gp120 or RSV G (Fig. 6). Notably, DC/L-SIGN neutralization did not inhibit kinase phosphorylation in 3T3 parental cells and only partially reduced phosphorylation in DC-SIGN transfectants, further supporting the roles of other cellular proteins in RSV infection and RSV G binding in 3T3 cells.
Fig 6.
Neutralization of DC- and L-SIGN reduces ERK1 and ERK2 signaling induced by RSV G. Parental 3T3 cells and 3T3–DC-SIGN and 3T3–L-SIGN transfectants were incubated with neutralizing DC/L-SIGN antibodies for 1 h at 37°C and then exposed to 10 μg purified RSV G or HIV gp120 for 30 min. The cells were then lysed, and Western blots were performed. Densitometry readings for phosphorylated ERK1/p-p44 (A) and ERK2/p-p42 (B) were normalized to α-tubulin levels (as a loading control), and then the ratio of phospho-ERK1/2 levels in HIV-1 gp120- and RSV G-treated samples to those in mock-treated samples was calculated. The levels of phospho-Akt, pan-Akt, and pan-ERK1/2 were also examined (data not shown). The data shown are the averages and standard deviations for 3 independent experiments. *, statistically different (P < 0.045) relative to the mock-treated sample for the same cell line and antibody treatment; **, statistically different (P < 0.03) compared to isotype control IgG and anti-DC/L-SIGN-treated cells for the same stimulus and cell line.
DISCUSSION
RSV infection and interaction with dendritic cells results in activation and maturation events that play important roles in establishing virus-specific immunity (12, 23, 29). Early events during the initial immune response may determine the quality and durability of host immunity and influence susceptibility to reinfection. In our prior work, we showed a dependence on C-type lectins for RSV infection and maturation of DCs. Here, we asked how 2 selected C-type lectins, DC-SIGN and L-SIGN, interact with RSV virions and the RSV G glycoprotein. We found that RSV G can interact with both DC-SIGN and L-SIGN but that interaction is not required for virus entry. However, the RSV G interaction with DC-SIGN results in intracellular signaling and phosphorylation of ERK-1 and ERK-2. Neutralization of DC-SIGN or L-SIGN in RSV-exposed mDCs and pDCs increases both DC maturation and cytokine/chemokine production, suggesting a potential role for RSV G interactions with DC/L-SIGN in modulation of the early immune response to RSV infection.
DC-SIGN and L-SIGN are involved in the binding, uptake, and dissemination of a number of pathogens (2, 10, 11, 18, 19, 21, 28, 32, 36, 38, 45, 53, 56). Subsequently, DC-SIGN engagement can initiate both MAPK and phosphatidylinositol 3-kinase (PI3K)/mTOR signaling with phosphorylation of ERK1/2 and Akt (5, 7, 38, 48). ERK1/2 signaling is involved in infection, replication, and host responses to a number of pathogens (19), particularly viruses (41, 48, 49, 61). PI3K and mTOR have well-defined roles in controlling activation of adaptive immune responses but have now also been shown to be critical in regulation of innate immunity (58). Thus, DC/L-SIGN-mediated signaling events may regulate pathogen-specific immunity (16, 17, 19, 21, 38, 54, 60). While it was initially perceived as an activator of host immunity, there are now data that suggest interaction with DC-SIGN can also modulate or suppress immune responses. For example, binding of HIV-1 gp120 to DC-SIGN induces IL-10 production by moDCs in direct correlation with ERK1 phosphorylation (48). Likewise, mycobacteria or their cell wall component ManLAM can activate moDCs through DC-SIGN, but ManLAM inhibits moDC activation by whole bacteria or by lipopolysaccharide (LPS), reducing CD86 and major histocompatibility complex (MHC) class II expression (19). Thus, alternative outcomes may result from interactions between invading pathogens and pattern recognition receptors, depending upon the combination of factors in the microenvironment (e.g., spatial and kinetic), demonstrating the intricacy and balance between induction and regulation of host immunity.
While our data demonstrate that RSV G binds DC- and L-SIGN and induces both DC/L-SIGN-dependent and -independent phosphorylation of ERK1 and ERK2, this interaction is not required for infection. However, interactions between RSV G and DC/L-SIGN may complement and facilitate binding of the virus to other cellular proteins by colocalization of DC/L-SIGN with the RSV receptor. A portion of cellular DC-SIGN is located in lipid-rich microdomains in the cell membrane (5). This may potentially result in colocalization of DC-SIGN with the RSV receptor(s), as we have demonstrated RSV infection requires intact lipid microdomains (37). The importance of lipid rafts in viral entry and assembly has also been demonstrated for HIV-1 (6, 40), Ebola virus (15), dengue virus (50), and hepatitis C virus (1), viruses known to utilize DC-SIGN as a PRR during infection (2, 10, 18, 21, 36, 45, 53, 56). Furthermore, as inhibition of RSV binding to DC-SIGN in mDCs and pDCs resulted in increased DC maturation and cytokine production as early as 3 h postexposure, these data demonstrate that interactions between RSV glycoproteins and DC/L-SIGN on the surfaces of dendritic cells suppress some aspects of DC activation. The inability of DC/L-SIGN neutralization to inhibit RSV infection of primary human DCs and the relatively modest effects of neutralization on the expression of DC maturation markers suggest that RSV G and F have additional interactions that contribute to DC activation. It is likely that other C-type lectins expressed by DCs are involved based on our prior finding that DC activation requires divalent cations (29). The data presented here indicate a role for DC/L-SIGN-mediated events in modulating DC functions through interactions with RSV G present on virions or secreted from infected cells. The interactions between RSV G and DC/L-SIGN may contribute to the failure of RSV infection to induce sustained protective immunity by altering DC function.
ACKNOWLEDGMENTS
We thank the volunteers who donated blood as a source for the primary mDCs and pDCs. We also acknowledge Mark Peeples for the gift of rgRSV, Peter Kwong for use of the BIACore instrument and for manuscript review, Theodore Pierson for sharing the K562 and Raji cell DC/L-SIGN transfectants, and Mary Marovich for the 3T3 and 3T3-DC-SIGN cell lines, in addition to their insightful discussions on DC-SIGN and viruses.
Footnotes
Published ahead of print 16 November 2011
REFERENCES
- 1. Aizaki H, et al. 2008. Critical role of virion-associated cholesterol and sphingolipid in hepatitis C virus infection. J. Virol. 82: 5715–5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alvarez CP, Lasala F, Carrillo J, Muniz O, Delgado R. 2002. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J. Virol. 76: 6841–6844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barr FE, Pedigo H, Johnson TR, Shepherd VL. 2000. Surfactant protein-A enhances uptake of respiratory synctial virus by monocytes and U937 macrophages. Am. J. Respir. Cell Mol. Biol. 23: 586–592 [DOI] [PubMed] [Google Scholar]
- 4. Bont L, et al. 2002. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr. Res. 52: 363–367 [DOI] [PubMed] [Google Scholar]
- 5. Caparros E, et al. 2006. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood 107: 3950–3958 [DOI] [PubMed] [Google Scholar]
- 6. Carter GC, et al. 2009. HIV entry in macrophages is dependent on intact lipid rafts. Virology 386: 192–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen QL, et al. 2010. Activation of p38 MAPK pathway by hepatitis C virus E2 in cells transiently expressing DC-SIGN. Cell Biochem. Biophys. 56: 49–58 [DOI] [PubMed] [Google Scholar]
- 8. Chi B, et al. 2006. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. J. Virol. 80: 5032–5040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collins PL, Graham BS. 2008. Viral and host factors in human respiratory syncytial virus pathogenesis. J. Virol. 82: 2040–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cormier EG, et al. 2004. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus. Proc. Natl. Acad. Sci. U. S. A. 101: 14067–14072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis CW, et al. 2006. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol. 80: 1290–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Graaff PM, et al. 2005. Respiratory syncytial virus infection of monocyte-derived dendritic cells decreases their capacity to activate CD4 T cells. J. Immunol. 175: 5904–5911 [DOI] [PubMed] [Google Scholar]
- 13. Feldman SA, Audet S, Beeler JA. 2000. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J. Virol. 74: 6442–6447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feldman SA, Hendry RM, Beeler JA. 1999. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J. Virol. 73: 6610–6617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Freitas MS, et al. 2011. Measuring the strength of interaction between the Ebola fusion peptide and lipid rafts: implications for membrane fusion and virus infection. PLoS One 6: e15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geijtenbeek TBH, den Dunnen J, Gringhuis SI. 2009. Pathogen recognition by DC-SIGN shapes adaptive immunity. Future Microbiol. 4: 879–890 [DOI] [PubMed] [Google Scholar]
- 17. Geijtenbeek TBH, Gringhuis SI. 2009. Signalling through C-type lectin receptors: shaping immune responses. Nat. Rev. Immunol. 9: 465–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Geijtenbeek TBH, et al. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100: 587–597 [DOI] [PubMed] [Google Scholar]
- 19. Geijtenbeek TBH, et al. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197: 7–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghildyal R, et al. 1999. Surfactant protein A binds to the fusion glycoprotein of respiratory syncytial virus and neutralizes virion infectivity. J. Infect. Dis. 180: 2009–2013 [DOI] [PubMed] [Google Scholar]
- 21. Gringhuis SI, et al. 2009. Carbohydrate-specific signaling through the DC-SIGN signalsome tailors immunity to Mycobacterium tuberculosis, HIV-1, and Helicobacter pylori. Nat. Immunol. 10: 1081–1088 [DOI] [PubMed] [Google Scholar]
- 22. Gringhuis SI, et al. 2010. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat. Immunol. 11: 419–426 [DOI] [PubMed] [Google Scholar]
- 23. Guerrero-Plata A, et al. 2006. Differential response of dendritic cells to human metapneumovirus and respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 34: 320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hallak LK, Collins PL, Knudson W, Peeples ME. 2000. Iuronic acid-containing glycosaminoglycans on target cells are required for efficient respiratory syncytial virus infection. Virology 271: 264–275 [DOI] [PubMed] [Google Scholar]
- 25. Hickling TP, et al. 2000. Lung surfactant protein A provides a route of entry for respiratory syncytial virus into host cells. Viral Immunol. 13: 125–135 [DOI] [PubMed] [Google Scholar]
- 26. Hobson L, Everard ML. 2008. Persistence of respiratory syncytial virus in human dendritic cells and influence of nitric oxide. Clin. Exp. Immunol. 151: 359–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hornung V, et al. 2004. Replication-dependent potent IFN-α induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J. Immunol. 173: 5935–5943 [DOI] [PubMed] [Google Scholar]
- 28. Jeffers SA, et al. 2004. CD209L (L-SIGN) is a receptor for severe acute respiratory syndrome coronavirus. Proc. Natl. Acad. Sci. U. S. A. 101: 15748–15753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson TR, Johnson CN, Corbett KS, Edwards GC, Graham BS. 2011. Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus. PLoS One 6: e16458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jones A, Morton I, Hobson L, Evans GS, Everard ML. 2006. Differentiation and immune function of human dendritic cells following infection by respiratory syncytial virus. Clin. Exp. Immunol. 143: 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kurt-Jones EA, et al. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1: 398–401 [DOI] [PubMed] [Google Scholar]
- 32. Lambrecht BN, Hammad H. 2009. Biology of lung dendritic cells at the origin of asthma. Immunity 31: 412–424 [DOI] [PubMed] [Google Scholar]
- 33. LeVine AM, et al. 2004. Surfactant protein-D enhances phagocytosis and pulmonary clearance of respiratory syncytial virus. Am. J. Respir. Cell Mol. Biol. 31: 193–199 [DOI] [PubMed] [Google Scholar]
- 34. Liu CF, Rivere M, Huang HJ, Puzo G, Wang JY. 2010. Surfactant protein D inhibits mite-induced alveolar macrophage and dendritic cell activations through TLR signalling and DC-SIGN expression. Clin. Exp. Allergy 40: 111–122 [DOI] [PubMed] [Google Scholar]
- 35. Looney RJ, Falsey AR, Walsh EE, Campbell D. 2002. Effect of aging on cytokine production in response to respiratory syncytial virus infection. J. Infect. Dis. 185: 682–685 [DOI] [PubMed] [Google Scholar]
- 36. Lozach PY, et al. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J. Biol. Chem. 280: 23698–23708 [DOI] [PubMed] [Google Scholar]
- 37. McCurdy LH, Graham BS. 2003. Role of plasma membrane lipid microdomains in respiratory syncytial virus filament formation. J. Virol. 77: 1747–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mittal R, Bulgheresi S, Emami C, Prasadaro NV. 2009. Enterobacter sakazakii targets DC-SIGN to induce immunosuppressive responses in dendritic cells by modulating MAPKs. J. Immunol. 183: 6588–6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murphy BR, et al. 1986. Serum and nasal-wash immunoglobulin G and A antibody response of infants and children to respiratory syncytial virus F and G glycoproteins following primary infection. J. Clin. Microbiol. 23: 1009–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ono A. 2010. Relationships between plasma membrane microdomains and HIV-1 assembly. Biol. Cell 102: 335–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pleschka S. 2008. RNA viruses and the mitogenic Raf/MEK/ERK signal transduction cascade. Biol. Chem. 389: 1273–1282 [DOI] [PubMed] [Google Scholar]
- 42. Preston FM, Beier PL, Pope JH. 1992. Infectious respiratory syncytial virus (RSV) effectively inhibits the proliferative T cell response to inactivated RSV in vitro. J. Infect. Dis. 165: 819–825 [DOI] [PubMed] [Google Scholar]
- 43. Roberts N. 1982. Different effects of influenza virus, respiratory syncytial virus, and Sendai virus on human lymphocytes and macrophages. Infect. Immun. 35: 1142–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roberts SR, Lichtenstein DL, Ball LA, Wertz GW. 1994. The membrane-associated and secreted forms of the respiratory syncytial virus attachment glycoprotein G are synthesized from alternative initiation codons. J. Virol. 68: 4538–4546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sakuntabhai A, et al. 2005. A variant in the CD209 promoter is associated with severity of dengue disease. Nat. Genet. 37: 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schlender J, et al. 2005. Inhibition of Toll-like receptor 7- and 9-mediated alpha/beta interferon production in human plasmacytoid dendritic cells by respiratory syncytial virus and measles virus. J. Virol. 79: 5507–5515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shahrabadi MS, Lee PWK. 1988. Calcium requirement for syncytium formation in HEp-2 cells by respiratory syncytial virus. J. Clin. Microbiol. 26: 139–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shan M, et al. 2007. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 3: 1637–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sharma NR, et al. 2010. Reciprocal regulation of AKT and MAP kinase dictates virus-host cell fusion. J. Virol. 84: 4366–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Silva BM, et al. 2011. The dengue virus nonstructural protein 1 (NS1) increases NF- k B transcriptional activity in HepG2 cells. Arch. Virol. 156: 1275–1279 [DOI] [PubMed] [Google Scholar]
- 51. Singleton R, Etchart N, Hou S, Hyland L. 2003. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus infection in mice correlates with ineffective nasal antibody responses. J. Virol. 77: 11303–11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Snyder GA, Colonna M, Sun PD. 2005. The structure of DC-SIGNR with a portion of its repeat domain lends insights to modeling of the receptor tetramer. J. Mol. Biol. 347: 979–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Snyder GA, et al. 2005. Characterization of DC-SIGN/R interaction with human immunodeficiency virus type 1 gp120 and ICAM molecules favors the receptor's role as an antigen-capturing rather than an adhesion receptor. J. Virol. 79: 4589–4598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Svajger U, Anderluh M, Jeras M, Obermajer N. 2010. C-type lectin DC-SIGN: an adhesion, signaling and antigen-uptake molecule that guides dendritic cells in immunity. Cell. Signal. 22: 1397–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Swedan S, Musiyenko A, Barik S. 2009. Respiratory syncytial virus nonstructural proteins decrease levels of multiple members of the cellular interferon pathways. J. Virol. 83: 9682–9693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tassaneetrithep B, et al. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197: 823–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tripp RA, et al. 2001. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 2: 732–738 [DOI] [PubMed] [Google Scholar]
- 58. Weichhart T, Saemann MD. 2008. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann. Rheum. Dis. 67 (Suppl. III): iii70–iii74 [DOI] [PubMed] [Google Scholar]
- 59. Welliver RC, Kaul TN, Sun M, Ogra PL. 1984. Defective regulation of immune responses in respiratory syncytial virus infection. J. Immunol. 133: 1925–1930 [PubMed] [Google Scholar]
- 60. Zeituni AE, Jotwani R, Carrion J, Cutler CW. 2009. Targeting of DC-SIGN on human dendritic cells by a minor fimbriated Porphyromonas gingivalis strains elicits a distinct effector T cell response. J. Immunol. 183: 5694–5704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zimmermann N, et al. 2000. Murine eotaxin-2: a constitutive eosinophil chemokine induced by allergen challenge and IL-4 overexpression. J. Immunol. 165: 5839–5846 [DOI] [PubMed] [Google Scholar]