Abstract
To determine the influence of asymptomatic genital viral infections on the cellular components of semen and blood, we evaluated the associations between the numbers and activation statuses of CD4+ and CD8+ T lymphocytes in both compartments and the seminal levels of cytomegalovirus (CMV), herpes simplex virus (HSV), and human immunodeficiency virus 1 (HIV). Paired blood and semen samples were collected from 36 HIV-infected antiretroviral-naïve individuals and from 40 HIV-uninfected participants. We performed multiparameter flow cytometry analysis (CD45, CD45RA, CD3, CD4, CD8, and CD38) of seminal and blood cellular components and measured HIV RNA and CMV and HSV DNA levels in seminal and blood plasma by real-time PCR. Compared to HIV-uninfected participants, in the seminal compartment HIV-infected participants had higher levels of CMV (P < 0.05), higher numbers of total CD3+ (P < 0.01) and CD8+ subset (P < 0.01) T lymphocytes, and higher CD4+ and CD8+ T lymphocyte activation (RA-CD38+) (P < 0.01). Seminal CMV levels positively correlated with absolute numbers of CD4+ and CD8+ T cells in semen (P < 0.05) and with the activation status of CD4+ T cells in semen and in blood (P < 0.01). HIV levels in semen (P < 0.05) and blood (P < 0.01) were positively associated with T-cell activation in blood. Activation of CD8+ T cells in blood remained an independent predictor of HIV levels in semen in multivariate analysis. The virologic milieu in the male genital tract strongly influences the recruitment and activation of immune cells in semen and may also modulate T-cell immune activation in blood. These factors likely influence replication dynamics, sexual transmission risk, and disease outcomes for all three viruses.
INTRODUCTION
Human immunodeficiency virus 1 (HIV) RNA viral loads in blood (31, 59) and semen (7, 13) of HIV-infected individuals correlate with the risk of sexual transmission (15, 28, 44). Although HIV RNA levels in blood roughly correlate with levels in seminal plasma (13, 39, 56, 69, 72), local genital factors, particularly concomitant sexually transmitted infections (STI), can increase HIV shedding in semen (38, 39, 61). Common bacterial STI can also increase the number of immune cells in the genital tract (9, 52), and elevated counts of white blood cells in semen are associated with higher seminal HIV shedding (3, 4, 69, 80). Since HIV principally infects and replicates in CD4+ T lymphocytes, monocytes, and macrophages (16, 30), an accumulation of these cells in semen is likely to increase the risk of sexual HIV transmission (40, 79).
HIV is not the only virus that replicates in the genital tract and is sexually transmitted. Herpes simplex virus 1 and 2 (HSV-1 and -2) and cytomegalovirus (CMV) are sexually transmitted and are extremely prevalent worldwide. All three viruses often infect the same host and likely influence each other's dynamics and replication. For HSV-2, among HIV-infected people, the seroprevalence is ∼70 to 90% (50, 76), and seminal shedding of HSV is associated with higher HIV RNA genital levels (8, 53, 64). Also, HSV-2 seropositivity of the source partners is associated with HIV transmission among men who have sex with men (MSM) (13); however, the use of acyclovir for chronic HSV infection among HIV-infected individuals does not reduce HIV transmission to their partners (14). The seroprevalence of CMV among HIV-infected men is even higher at 95 to 100% (20, 60), and CMV is associated with HIV disease progression in both treated and untreated individuals (18, 21, 24, 25, 37, 58, 70, 75). A possible mechanism for this accelerated disease progression may be CMV enhancement of HIV replication, especially in the male genital tract, where CMV levels positively correlate with HIV levels (17, 66, 67, 69). Moreover, asymptomatic CMV coinfection is associated with higher T-cell immune activation (32, 36, 48, 71), which is linked to blunted CD4 cell recovery during antiretroviral therapy and to premature mortality (29, 35, 36).
To further understand the role that chronic viral infections of the male genital tract play in the immune dynamics of an HIV-infected individual, we measured viral levels of CMV, HSV, and HIV in relation to the numbers, phenotypes, and immune activation status of T lymphocytes in semen and blood from 36 HIV-infected antiretroviral-naïve men. We then examined the relationships between levels of these three viruses and the activation state of T-lymphocyte subsets in blood and semen. These results were then compared to those obtained from control groups of 27 HIV-uninfected MSM and 13 HIV-uninfected men who have sex with women (MSW).
MATERIALS AND METHODS
Participants, samples, and clinical laboratory tests.
Thirty-six recently HIV-infected antiretroviral-naïve participants from the San Diego Primary Infection Cohort (34, 51) and 40 HIV-uninfected subjects (27 MSM and 13 MSW) were included in this study. A total of 69 paired blood and seminal cell samples were collected from the HIV-infected participants (median of 1 time point per subject; range, 1 to 5). Additionally, single-time-point samples were collected from 40 HIV-uninfected participants. Blood plasma and peripheral blood mononuclear cells (PBMC) samples were separated as previously described (12), aliquoted, frozen, and stored at −80°C and −150°C, respectively. Semen was collected by masturbation without lubricant after 48 h of abstinence.
Time between infection and collection of specimens was estimated based on the participant's estimated duration of infection (EDI) (27, 34, 51) and the date of sample collection. Viral transport medium (2 ml of RPMI 1640 with 2 mMol l = glutamine and 10% fetal bovine serum (FBS), with the addition of 100 U/ml penicillin 100 μl/ml of streptomycin, and 200 U/ml of nystatin) was added to seminal samples at collection. Seminal plasma was separated from seminal cells by centrifugation at 700 × g for 12 min within 4 h of collection and stored at −80°C and −150°C, as previously described (12, 68). Neisseria gonorrhoeae and Chlamydia trachomatis infections were assessed in urine samples collected at baseline (LabCorp). Also at baseline, syphilis infection was screened by rapid plasma reagin titers in blood plasma and confirmed by treponema-specific antibody testing.
In blood, CD4+ and CD8+ T-lymphocyte subsets were measured by flow cytometry (LabCorp) and HIV RNA was quantified (Amplicor HIV Monitor test; Roche Molecular Systems, Inc.). Clinical data were collected, including baseline demographics, symptoms and resolution of STI, and standard laboratory values.
HIV subtype was determined using HIV pol sequence data generated by Viroseq 2.0 (Applied Biosystems) using SCUEAL (http://www.datamonkey.org/) (42).
These studies were conducted with appropriate subject consent and were approved by the Human Research Protections Program at the University of California, San Diego, CA. Signed written informed consent was provided by all study participants and/or their legal guardians.
RNA extraction from seminal plasma and HIV RNA quantification.
HIV RNA levels were measured in seminal plasma by first concentrating HIV RNA from 500 μl of seminal plasma by high-speed centrifugation (23,500 × g at 4°C for 1 h) after 1:1 dilution with phosphate-buffered saline (PBS). Concentrated RNA was then extracted using the High Pure viral RNA kit (Roche, Basel, Switzerland) according to the manufacturer's protocol. Using extracted RNA, HIV cDNA was generated using the SuperScript III first-strand synthesis kit (Invitrogen, CA) according to the manufacturers' protocol with specific primer mf302 (2). HIV RNA in seminal plasma was quantified by real-time PCR in an ABI 7900HT thermocycler (Applied Biosystems, CA) with 0.005 μM ROX dye (Invitrogen) as a passive reference. A total reaction volume of 50 μl was added to each well consisting of 5 μl of cDNA template, TaqMan Environmental Mastermix 2.0 (Applied Biosystems, CA), PCR primers mf302 and mf299 (1 μM each) (2), and probe mf348 (0.3 μM) (2). The PCR conditions were 2 min at 50°C, 10 min at 95°C, and 60 cycles of 15 s at 95°C and 60 s at 60°C. HIV RNA quantification standard was obtained from the DAIDS Virology Quality Assurance (VQA) Program (82).
DNA extraction from seminal plasma, and CMV and HSV-1/2 viral load quantification.
Viral DNA was extracted from 200 μl of seminal and blood plasma using a QIAamp DNA minikit (Qiagen, CA) per the manufacturer's protocol. EDTA (50 mM) was added to seminal plasma to inhibit DNase activity. HSV and CMV viral loads in semen were measured by real-time PCR in an ABI 7900HT thermocycler (Applied Biosystems, CA) with 0.005 μM ROX as a passive reference. A total reaction volume of 50 μl was added to each well consisting of 10 μl DNA extract, TaqMan Environmental Mastermix 2.0 (Applied Biosystems, CA), PCR primers (1 μM each) CMV-F (AGGTCTTCAAGGAACTCAGCAAGA), CMV-R (CGGCAATCGGTTTGTTGTAAA) HSV-1/2-F (ACCGCCGAACTGAGCAGAC), and HSV-1/2-R (TGAGCTTGTAATACACCGTCAGGT) and probes (0.3 μM) CMV-P (6-carboxyfluorescein [FAM]-AACCCGTCAGCCATTCTCTCGGC-BHQ-1) and HSV-1/2-P (FAM-CGCGTACACCAACAAGCGCCTG-BHQ-1). The PCR conditions were 2 min at 50°C, 10 min at 95°C, and 60 cycles of 15 s at 95°C and 60 s at 60°C. CMV and HSV quantification standards were obtained using plasmid preparations with known concentrations.
Multiparameter flow cytometry analysis (FACS).
One-third of the seminal cell sample (divided from total ejaculate volume) and 1 million paired PBMC from each included time point were analyzed by flow cytometry on a dual-laser, 6-color Becton Dickinson fluorescence-activated cell sorter (FACS) Canto using Diva (6.1) or FlowJo (9.0) software. Cells were washed once in RPMI (with 10% FBS) and stained for 30 min in the dark at 4°C with mouse monoclonal anti-human antibodies: 10 μl (15 μl for semen) of CD45-peridinin chlorophyll protein (PerCP)-Cy5.5 (clone 2D1), CD45RA-phycoerythrin (PE) (clone HI100), and CD4-fluorescein isothiocyanate (FITC) (Leu 3a/3b multiclone) and 5 μl (7.5 μl for semen) of CD38-PE-Cy7 (clone HB7), CD3-allophycocyanin (APC) (clone SK7) and CD8-APC-Cy7 (clone SK1) (BD Biosciences, CA). After staining, samples were washed twice in Dulbecco's phosphate-buffered saline (PBS-A) and fixed in 1% formaldehyde solution (Polysciences, Inc.).
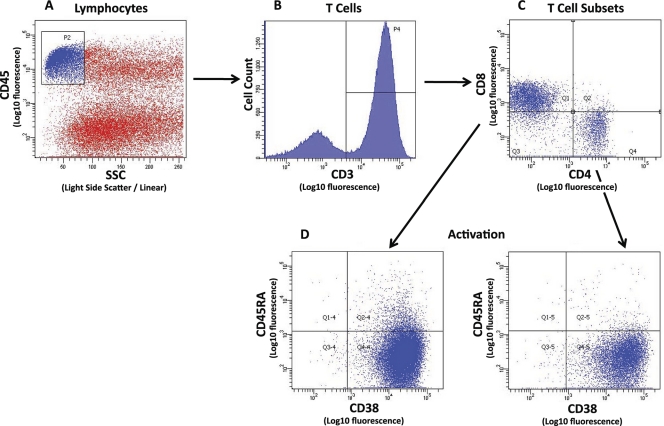
The initial analysis region was set on forward (FSC) versus side (SSC) light scatter to eliminate the majority of individual and clustered sperm. T lymphocytes were identified by sequential gating on the CD45+-bright, low-SSC subset that was CD3+ (Fig. 1). The CD3+ CD4+ and CD3+ CD8+ cell subsets were analyzed for activation status based on CD45RA and CD38 expression (CD45RA− CD38+).
Fig 1.
Example of flow cytometry gating strategy for semen sample from an HIV- and CMV-coinfected participant. The initial analysis region used CD45 staining intensity versus side light scatter (SSC) to identify the CD45+-bright, low-SSC population of lymphocytes among the sperm cells (A). Sequential gating was used to select the total CD3+ T-cell population (B) and the CD3+ CD4+ and CD3+ CD8+ cell subsets (C). Activation status of the mature CD4+ and CD8+ cells (CD45RA−) was based on CD38 expression (D).
Statistics.
Statistical analyses were performed using Graph-Pad Prism 5.0 software (GraphPad Software, Inc., San Diego, CA) and SAS (version 9.2, SAS Institute, Cary, NC). Comparisons between HIV-infected and uninfected groups were evaluated with the Mann-Whitney test (78) using median values from the longitudinal data for each participant in the HIV-infected group with repeated measurements. For our primary analysis, we analyzed correlations between the log-transformed viral loads (in blood and semen) and the flow cytometry data using nonparametric univariate (Spearman) correlation analysis. Stepwise multiple linear regressions were performed using an entry criterion of P < 0.10 and a retention criterion of P < 0.05. For patients with repeated measurements, only the first available time point was included in this part of the analysis.
The absolute numbers of T-lymphocyte subsets in each analyzed aliquot of semen were calculated by multiplying the percentage of each subset, measured by FACS analysis, by the absolute number of CD45+ CD3+ (total T lymphocytes) events, measured in the entire volume of analyzed seminal sample. The stained volume of seminal cells corresponded to one-third of the cells present in the original ejaculate (see staining protocol above). Samples, in which no viral nucleic acid was detected by PCR were assigned a nominal value of half the detection limit of the PCR assay (20 copies per ml for HIV and 50 copies per ml for CMV and HSV) for statistical purposes.
RESULTS
Study participants.
A total of 69 paired blood and seminal cell samples were collected from 36 HIV-infected participants (median, 1 time point per subject; range, 1 to 5). HIV-infected participants were all men infected with HIV-1 subtype B virus who reported sex with other men as their HIV risk factor. They were predominantly white (81%), with a median age of 42 years (Table 1). For 13 patients with longitudinal measurements, samples were collected over a median follow-up of 167 days (range, 7 to 1,150 days). At baseline, their median CD4 count was 540 cells/ml (range, 259 to 1,374 cells/ml), their median estimated duration of infection (EDI) was 138 days (range, 31 to 418 days), and their blood plasma HIV levels ranged between 3.0 and 6.6 HIV RNA log10 copies/ml (median, 4.9 HIV RNA log10 copies/ml). All HIV-infected participants had positive CMV serology, and 40% had positive serology for HSV-2. One participant had positive syphilis screening tests at baseline, and another participant had a positive syphilis test at the time his third sample was taken. Both patients were treated for these infections. Of note, both of these individuals with syphilis had undetectable CMV and HSV DNA levels in all of their semen samples.
Table 1.
Patient demographics at baselinea
| Parameter | Result for patient group: |
||
|---|---|---|---|
| HIV+ |
HIV− | ||
| MSW | MSM | ||
| No. of participants in study | 36 | 13 | 27 |
| No. (%) of male patients | 36 (100) | 13 (100) | 27 (100) |
| Median age, yr (range) | 42 (31–73) | 30 (22–36) | 37 (26–67) |
| No. with MSM HIV risk factor | 36 (100) | 0 (0) | 27 (100) |
| No. Caucasian/Hispanic | 29 (81) | 12 (92) | 20 (74) |
| No. antiretroviral naive | 13 (100) | ||
| No. with HIV-1 subtype B | 13 (100) | ||
| Median EDI, days (range) | 138 (31–418) | ||
| Median CD4+ cell count/ml (range) | 540 (259–1374) | ||
| Median log10 HIV RNA copies (range) | 4.9 (3.0–6.6) | ||
MSM, men who have sex with men; MSW, men who have sex with women; EDI, estimated duration of infection at baseline.
HIV-uninfected participants (n = 40) were healthy men without any reported relevant medical conditions. Twenty-seven of them (68%) reported sex with other men. Baseline screening for bacterial STI turned out positive for three subjects in the MSM group (two cases of syphilis and one Chlamydia infection, with the patients treated for these infections); serology for CMV was positive in 50% of the MSW and 80% of the MSM, while only three subjects had positive HSV-2 serology (one MSW and 2 MSM). Paired semen and PBMC samples were collected from these participants at a single time point. Additional characteristics of the study participants are summarized in Table 1.
Viral levels and lymphocyte subsets in blood and semen among HIV-infected and uninfected participants.
(i) Viral levels.
Out of 36 HIV-infected participants, 17 presented at least one semen sample positive for detectable CMV DNA by RT-qPCR. Among the 69 total seminal samples collected from these participants, 26 had detectable levels of CMV DNA, 8 had detectable HSV DNA, and 57 had detectable HIV RNA. In the samples with detectable values, the median viral loads were 4.8 log10 copies of CMV DNA/ml, 2.2 log10 copies of HSV DNA/ml, and 3.0 log10 copies of HIV RNA/ml. Of note, three patients with HSV-positive semen by RT-qPCR had negative HSV-2 serology, but positive HSV-1 serology. Among the 69 total blood samples analyzed, HIV RNA was detectable at levels of >3.0 log10 copies/ml in each sample (median, 4.7 log10 copies/ml; range, 3.0 to 6.6 log10 copies/ml), while none had detectable levels of CMV DNA (see Table S1 in the supplemental material); HSV DNA levels in blood were not measured.
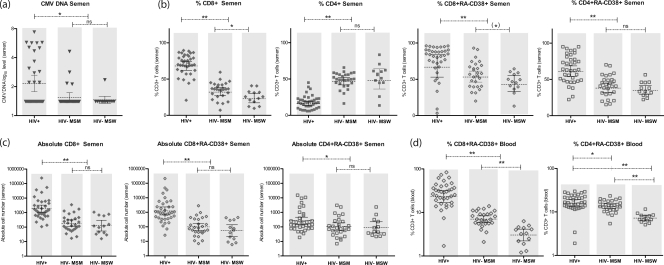
Significantly higher levels of CMV DNA in semen were measured in HIV-infected participants, compared to both HIV-uninfected MSM (P = 0.01) and MSW (P = 0.02) (Fig. 2a), and this remained true even when the comparison was conservatively restricted to the 22 CMV-seropositive, HIV-negative MSM participants (P = 0.03), while for HSV, no differences were observed among the three groups.
Fig 2.
Comparisons of seminal CMV DNA levels and T lymphocytes in semen and blood between HIV-infected and uninfected participants. (a) CMV DNA (log10 copies/ml) in seminal plasma (gray triangles) compared between HIV-infected men (n = 36), HIV-uninfected men who have sex with men (MSM) (n = 27), and HIV-uninfected men who have sex with women (MSW) (n = 13) (Mann-Whitney test). The lower limit of detection for CMV DNA is 50 copies/ml. Error bars show geometric means and their 95% confidence intervals. ∗, P < 0.05. ns, not significant (P > 0.1). (b) Proportions (within CD3+ T cells) of CD8+ T cells (full diamonds), CD4+ T cells (full squares), activated (RA-CD38+) CD8+ T cells (empty diamonds), and activated (RA-CD38+) CD4+ T cells (empty squares) in semen compared between HIV-infected (n = 36), HIV-uninfected MSM (n = 27), and HIV-uninfected MSW (n = 13) (Mann-Whitney test). Error bars show geometrical means and their 95% confidence intervals. ∗, P < 0.05; ∗∗, P < 0.01; (∗), P = 0.08. ns, not significant (P > 0.1). (c) Absolute numbers of CD8+ T cells (full diamonds), activated (RA-CD38+) CD8+ T cells (empty diamonds), and activated (RA-CD38+) CD4+ T cells (empty squares) in semen compared between HIV-infected men (n = 36), HIV-uninfected MSM (n = 27), and HIV-uninfected MSW (n = 13) (Mann-Whitney test). Absolute CD4 cell counts were not significantly different between HIV+ and HIV− individuals (data not shown). Error bars show geometrical means and their 95% confidence intervals. ∗, P < 0.05; ∗∗, P < 0.01. ns, not significant (P > 0.1). (d) Proportions (within CD3+ T cells) of activated (RA-CD38+) CD8+ T cells (empty diamonds) and activated (RA-CD38+) CD4+ T cells (empty squares) in blood compared between HIV-infected men (n = 36), HIV-uninfected MSM (n = 27), and HIV-uninfected MSW (n = 13) (Mann-Whitney test). Error bars show geometrical means and their 95% confidence intervals. ∗, P < 0.05; ∗∗, P < 0.01.
(ii) Lymphocytes.
Among HIV-infected participants, a median of 1,758 CD3+ T lymphocytes were detected in the ejaculate aliquots (range, 67 to 271,649 T cells). Of these T cells, 70% were CD8+ and 18% were CD4+. Compared to blood, the semen contained higher proportions of CD8+ T cells (70% versus 54%) and lower percentages of CD4+ T cells (18% versus 32%) (see Table S1 in the supplemental material).
For HIV-uninfected participants, medians of 395 (MSM) and 383 (MSW) CD3+ T lymphocytes were measured in the ejaculate aliquots (range, 47 to 11,430 cells). Within the HIV-negative participants, MSM had a significantly higher proportion of seminal CD8+ (36% versus 26%; P < 0.01), a trend toward higher CD8 T-cell activation (82% versus 59%; P = 0.08), and significantly higher activation levels of both CD8 (7% versus 3%; P < 0.01) and CD4 (14% versus 7%; P < 0.01) T cells in blood than MSW (Fig. 2).
In semen, HIV-infected participants had significantly more total CD3+ T lymphocytes (P < 0.01), total CD8+ T cells (P < 0.01), activated (RA-CD38+) CD8+ T cells (P < 0.01), and activated (RA-CD38+) CD4+ T cells (P < 0.05) than HIV-uninfected MSM and MSW. While the overall percentage of seminal CD4+ T lymphocytes was lower in the HIV-infected participants (P < 0.01), the proportion (P < 0.01) and absolute number (P < 0.05) of activated CD4+ cells (RA-CD38+) were higher than those of the HIV-uninfected groups. There was, however, no difference in the absolute numbers of seminal CD4+ T cells between the three groups (median, 266 for HIV+ versus 131 [MSM] and 194 [MSW] CD4+ T cells). Of note, all differences remained equally significant also when the HIV-negative control group was restricted to MSM seropositive for CMV (n = 22) and after excluding the 3 subjects with diagnosed STI.
A similar pattern of T-lymphocyte subset distribution was observed in the blood of HIV-infected participants compared to both HIV-uninfected groups, with an increase in the total number of CD3+ (P < 0.05) and CD8+ T cells (P < 0.01) and an increase in the activated proportion of both CD8+ (P < 0.01) and CD4+ T-cell subsets (P < 0.05). As expected, the CD4+ T-cell fraction in blood was significantly lower (P < 0.01) among the HIV-infected participants.
Associations between viral levels and lymphocyte subsets.
(i) Overall.
Similar to previous studies (13, 39, 56, 69, 72), we found a positive trend between HIV RNA levels in blood and semen (P = 0.07), while no correlations were found between seminal HIV and CMV levels (P = 0.35) or between seminal HSV and CMV levels (P = 0.39).
Activation levels (RA-CD38+) of CD4+ and CD8+ subsets in blood were highly correlated with each other (P < 0.001) and with activation of CD8+ T cells in semen (P < 0.01). Similarly, activation levels of CD4+ and CD8+ subsets in semen were highly correlated with each other (P < 0.001). We found no association between absolute numbers of CD4+ and CD8+ T cells between the two compartments (P > 0.25).
(ii) Seminal CMV.
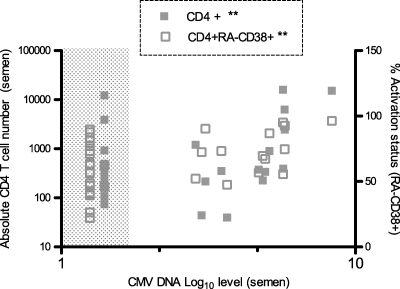
In univariate analysis, higher seminal CMV DNA levels strongly correlated with higher absolute numbers of CD8+ (P = 0.02) and CD4+ (P = 0.02) T lymphocytes in semen and with a higher proportion of activated (RA-CD38+) CD4+ T cells (P = 0.01) (Fig. 3) in semen. CMV DNA levels in semen were also positively correlated with the CD4+ T-cell activation state in blood (P < 0.01). Seminal CMV levels remained a predictor of increased CD4+ activation in blood in multivariate analysis and were independent of HIV RNA levels in blood (P < 0.01, r = 0.5).
Fig 3.
Correlative analyses between levels of CMV in semen and absolute numbers and activation statuses of seminal CD4+ T-cell subsets. Shown are the results of correlation analysis between CMV DNA (log10 copies/ml seminal plasma) and absolute numbers of CD4+ T cells (full squares) and activated (RA-CD38+) CD4+ T cells (empty squares) using nonparametric Spearman correlation analysis. The lower limit of detection is 50 copies/ml. ∗∗, P = 0.01.
(iii) Seminal HIV.
In univariate analysis, HIV RNA levels in both semen (P = 0.02) and blood (P < 0.01) correlated with the CD8+ activation status in blood.
Seminal HIV levels were not associated with any of the parameters in semen (i.e., CD4+ and CD8+ T cells or CMV or HSV levels). After including all other measured variables as covariates in our model, the only independent predictor for higher HIV RNA levels in semen was an increased proportion of activated CD8+ T cells in blood (P = 0.02, r = 0.40).
(iv) Seminal HSV.
In univariate analysis, higher HSV levels in semen correlated with lower absolute numbers of total CD3+ T cells (P = 0.03) and CD8+ cells (P < 0.01) in blood. There was no correlation between HSV DNA levels in semen and the absolute numbers, proportions, subsets, or activation statuses of T cells in semen. The statistical power of these analyses was limited, however, by the low prevalence of HSV DNA in the collected semen samples (11% among HIV-infected participants). The study was thus insufficiently powered for multivariate analysis of predictors of HSV DNA.
(v) Blood HIV.
As expected, higher HIV RNA levels in blood correlated with greater proportions of activated CD8+ (P < 0.001) and CD4+ (P < 0.05) T lymphocytes in blood. In both univariate and multivariate analyses, there was no correlation between HIV levels in blood and any of the measured cellular or viral parameters in semen (i.e., CMV and HSV DNA levels and lymphocyte numbers and activation).
DISCUSSION
The inability to reliably predict genital shedding of HIV and other sexually transmitted viruses represents a public health problem. Understanding the viral and immunologic dynamics in the genital tract and characterizing the factors that increase the risk of sexual transmission could provide important information for the development of effective prevention strategies. In this study, we investigated the interactions between CMV, HSV, and HIV replication in the male genital tract and the associated lymphocytic changes, locally in semen and systemically in blood.
Similar to previous studies (20, 60), HIV-infected participants in our cohort had higher levels of CMV in semen than HIV-uninfected controls. Unlike historical reports, however, our HIV-infected participants had a lower proportion of semen samples with detectable CMV DNA: 38% versus 56 to 66% (22, 66). Most likely, this is a consequence of the higher average CD4 cell count found in our study participants (43, 47, 66), who are also likely more representative of the current HIV-infected population in the United States (34, 51). As might be expected, CMV DNA was undetectable in all of our HIV-infected participants in blood plasma, despite frequent detection of very high levels of CMV replication in semen (median of 4.8 log10 copies CMV DNA/ml seminal plasma among positive samples). Such compartmentalized replication of CMV in the genital tract, first described in 1972 (45) and subsequently confirmed by others (11, 46, 60), probably has consequences for CMV transmission (11, 43), impacts localized immune responses, and interacts with other sexually transmitted viruses, like HIV-1 (66).
Our investigation of localized immune reactions showed that HIV-infected participants had higher numbers of seminal CD3+ and CD8+ T lymphocytes and higher levels of activated CD4+ and CD8+ lymphocyte subsets compared to HIV-uninfected groups. HIV-negative MSM had higher proportion of seminal CD8+ T cells, higher seminal CD8+ T-cell activation, and significantly higher activation levels of both CD8+ and CD4+ T cells in blood than HIV-negative MSW (Fig. 2). As reported by others (41, 81), the higher immune activation seen in blood among MSM controls may be secondary to increased antigen exposure. This is consistent with what is reflected also in the higher seroprevalence of viral infections and STI in our MSM control group, but further evaluation is needed.
As previously described (19, 57), our HIV-infected participants had fewer CD4+ T cells in semen than the HIV-uninfected group, which is consistent with findings at other mucosal sites (10, 54). In the male genital tract, low numbers of CD4+ T cells, which are the primary target cells for HIV replication, may explain the inefficient rate of transmission seen for HIV compared to other STI (62, 74).
In contrast to our findings, a prior study (57) reported significantly lower concentrations of total leukocytes, including CD4+ and CD8+ T-cell subsets and activated T lymphocytes in semen of HIV-infected men compared to HIV-uninfected individuals. Unlike our study, the prior study population mostly consisted of chronically infected patients with advanced HIV disease. In fact, when a subanalysis of the same study was performed to include only subjects with high peripheral CD4+ cell counts (>500/mm3), a reduction in CD4+ T cells but not CD8+ T cells was the only difference between the HIV-infected and uninfected groups. This prior study also did not assess the cellular subset changes in relation to viral levels in the genital tract, which may have revealed additional mechanisms.
Although a recent study from our group suggested that virus transmitted during sexual exposure between MSM originated from seminal plasma (12), data from other studies support the hypothesis that HIV-infected cells, deposited in the genital tract or rectal lumen during sexual intercourse, shelter and transport virus to susceptible cells within or below the mucosal epithelium during infection of a new host (reviewed in reference 5). It is, therefore, possible that an accumulation of infected CD4+ T cells in semen together with enhanced cellular HIV transcription and replication in activated T lymphocytes (6, 17, 26, 32, 65, 67, 83) could increase the risk of sexual transmission of HIV. Moreover, persistent immune activation of lymphocytes in semen and in blood may impact HIV disease progression and overall clinical outcome. To evaluate these hypotheses, we examined the correlations between the levels of CMV, HSV, and HIV and the absolute numbers and activation statuses of CD4+ and CD8+ T lymphocytes in semen and blood.
In multivariate analysis, seminal HIV levels were not associated with any of the measured parameters in semen (i.e., CD4+ and CD8+ T cells and CMV or HSV levels), and the only independent predictor for higher HIV RNA levels in semen was an increased proportion of activated CD8+ T cells in blood (P = 0.02). On the other hand, higher seminal levels of CMV correlated with increased number and activation status of T lymphocytes in semen and were an independent predictor of increased CD4+ T-cell activation in blood (P < 0.01).
The last observation is supported by previous findings that CMV replication in the male genital tract contributes to systemic immune activation (32, 36, 48, 71).
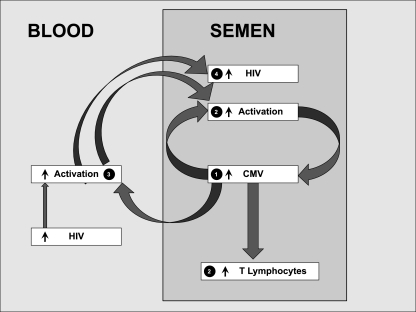
Taken together, these results suggest that CMV, much more than HIV, influences immune cell dynamics within the male genital tract and support a model in which localized CMV replication recruits T lymphocytes to the male genital tract and induces activation in these lymphocytes (Fig. 4).
Fig 4.
Theoretical model showing interactions between CMV, T lymphocytes, and HIV in blood and semen. CMV replication in semen (part 1) drives T-cell recruitment and cellular activation, directly in semen (part 2) and indirectly through T-cell activation in blood (part 3). These data implicate local replication of CMV in the male genital tract as a contributor to HIV sexual transmission and HIV disease progression associated with systemic immune activation (part 4).
Since chronic persistent immune activation negatively impacts HIV disease progression (18, 21, 24, 25, 37, 58, 70, 75) and immune activation in response to CMV may be responsible for accelerated immunosenescence (1, 23, 33, 49, 55, 63, 73, 77), CMV replication localized to the genital tract is likely a major factor in HIV disease progression. Alternatively, seminal CMV replication may be a proxy for increased CMV shedding in other tissues that were not sampled in this study.
Whether or not suppression of this localized CMV replication influences HIV-related disease progression or transmission is an open question.
Although these data are intriguing, there are a number of limitations to the current study. First, because this was a retrospective, observational study, we cannot establish a true causal relationship between the detected correlations. For example, it is possible that an untracked variable caused the observed increases in T-cell numbers and activation, which then triggered a reactivation of HIV, CMV, or both. However, consistent with our study results, a recent randomized study (36) found a significant decrease of immune activation in the blood of HIV-infected individuals following treatment with valganciclovir, suggesting that CMV replication is likely a significant contributor to T-cell activation in the blood. A second limitation is that screening for STI was performed at baseline and only repeated after 3 months for some subjects. Unrecognized bacterial STI could, therefore, confound our results and might explain why some subjects had elevated seminal lymphocyte counts despite undetectable levels of any of the three measured viruses. We believe this scenario is unlikely, given that none of the subjects reported symptoms consistent with a bacterial STI. A more likely possibility is that another highly prevalent genital tract viral coinfection that was not evaluated, such as Epstein-Barr virus (EBV) or human herpesviruses (HHVs), caused the observed immunologic modulation. Future studies will need to be more comprehensive in evaluating the semen for all potential viral coinfections. Third, this study is limited by a relatively small sample size, especially for participants with HSV infection. However, the driver of genital tract and systemic immune activation at the population level is likely to be the more prevalent virus, i.e., CMV in populations with relatively low HSV prevalence.
To our knowledge, this is the first report describing in detail the interactions between CMV, HSV, HIV, and T-lymphocyte subsets and activation status in semen. Although our study was relatively small, both univariate and mutivariate analyses support the hypothesis that CMV replication in the genital tract drives lymphocyte recruitment and activation in both semen and blood. However, the virologic and immunologic relationships that exist in different anatomic compartments are likely too complex to fit into a single mechanistic model in which “CMV replication in the male genital tract equals systemic lymphocyte activation.” Despite its limitations, the present study provides some important insights with regard to (i) the interaction between CMV and HIV in the seminal compartment, which is likely to be important in the biology of sexual transmission of both viruses and (ii) the interactions between CMV replication in the genital tract and localized and systemic inflammation, which probably impact both HIV transmission and disease progression.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all participants in the San Diego Primary Infection Cohort and the uninfected subject volunteers; the CFAR Flow Cytometry Core, especially Judy Nordberg and Neal Sekiya for excellent and essential support; the CFAR Genomics Core for RT-qPCR quantification procedures; the AVRC team, especially Paula Potter and DeeDee Pacheco for extraordinary patient care and efficient recruitment; Nancy Keating, Joe Prioriello, Lily Tracy, and Shahrzad Rostami for extraordinary technical support; and Nadir Weibel, Stephen Espitia, and Winston Tilghman for support and inspiring discussions. We also thank and commemorate our dear friend and outstanding research colleague Marek Fischer for all of his contributions and support for our research over many years. Primers and probes for CMV and HSV as well as the plasmid quantification standards were kindly provided by Fred Lakeman.
S.G. participated in the study design, performed the laboratory experiments, participated in the data analyses for this study, and wrote the primary version of the manuscript. M.C.S. performed statistical analysis and revised the manuscript. S.E.R. helped in assessment of RT-PCR protocols and revised the manuscript. M.V.V. performed the laboratory experiments. S.J.L. and D.M.S. enrolled participants. D.D.R., S.J.L., C.A.S., and D.M.S. designed the present study, participated in data analysis, and revised the manuscript. All authors read and approved the final manuscript.
S.G. does not have any commercial or other associations that might pose a conflict of interest. D.D.R. has served as a consultant for Theraclone Sciences, Myriad Genetics, Bristol-Myers Squibb, Gilead Sciences, Merck & Co, Monogram Biosciences, Biota, Chimerix, Gen-Probe, and Idenix Pharmaceuticals. D.M.S. has received grant support from ViiV Pharmaceuticals and consultant fees from Gen-Probe.
This work was supported by the James Pendleton Charitable Trust; National Institutes of Health (NIH) awards AI69432, AI043638, MH62512, MH083552, AI077304, AI36214, AI047745, AI74621, GM093939, AI080353, and AI306214 (CFAR); Swiss National Science Foundation grant PBZHP3-125533; and the Gustav and Ruth Jacob Foundation (Switzerland). The HIV RNA quantification standard was obtained through the NIH AIDS Research and Reference Reagent Program, DAIDS, NIAID, and the HIV VQA RNA quantification standard from the DAIDS Virology Quality Assurance (VQA) Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 23 November 2011
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Almanzar G, et al. 2005. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J. Virol. 79: 3675–3683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Althaus CF, Gianella S, et al. 2010. Rational design of HIV-1 fluorescent hydrolysis probes considering phylogenetic variation and probe performance. J. Virol. Methods 165: 151–160 [DOI] [PubMed] [Google Scholar]
- 3. Anderson BL, et al. 2008. Genital tract leukocytes and shedding of genital HIV type 1 RNA. Clin. Infect. Dis. 47: 1216–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson DJ, et al. 1992. Effects of disease stage and zidovudine therapy on the detection of human immunodeficiency virus type 1 in semen. JAMA 267: 2769–2774 [PubMed] [Google Scholar]
- 5. Anderson DJ, et al. 2010. Targeting Trojan Horse leukocytes for HIV prevention. AIDS 24: 163–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson JA, et al. 2010. HIV-1 populations in semen arise through multiple mechanisms. PLoS Pathog. 6: e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baeten JM, et al. 2011. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci. Transl. Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baeten JM, et al. 2004. Vitamin A supplementation and genital shedding of herpes simplex virus among HIV-1-infected women: a randomized clinical trial. J. Infect. Dis. 189: 1466–1471 [DOI] [PubMed] [Google Scholar]
- 9. Barratt CL, Harrison PE, Robinson A, Kessopoulou E, Cooke ID. 1991. Seminal white blood cells in men with urethral tract infection. A monoclonal antibody study. Br. J. Urol. 68: 531–536 [DOI] [PubMed] [Google Scholar]
- 10. Brenchley JM, et al. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200: 749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bresson JL, et al. 2003. Risk of cytomegalovirus transmission by cryopreserved semen: a study of 635 semen samples from 231 donors. Hum. Reprod. 18: 1881–1886 [DOI] [PubMed] [Google Scholar]
- 12. Butler DM, et al. 2010. The origins of sexually transmitted HIV among men who have sex with men. Sci. Transl. Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Butler DM, et al. 2008. Herpes simplex virus 2 serostatus and viral loads of HIV-1 in blood and semen as risk factors for HIV transmission among men who have sex with men. AIDS 22: 1667–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Celum C, et al. 2010. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N. Engl. J. Med. 362: 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen MS, et al. 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. AIDSCAP Malawi Research Group. Lancet 349: 1868–1873 [DOI] [PubMed] [Google Scholar]
- 16. Dalgleish AG, et al. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312: 763–767 [DOI] [PubMed] [Google Scholar]
- 17. Davis MG, Kenney SC, Kamine J, Pagano JS, Huang ES. 1987. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc. Natl. Acad. Sci. U. S. A. 84: 8642–8646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deayton JR, et al. 2004. Importance of cytomegalovirus viraemia in risk of disease progression and death in HIV-infected patients receiving highly active antiretroviral therapy. Lancet 363: 2116–2121 [DOI] [PubMed] [Google Scholar]
- 19. Denny TN, et al. 1995. Evaluation of T-lymphocyte subsets present in semen and peripheral blood of healthy donors: a report from the heterosexual transmission study. Cytometry 20: 349–355 [DOI] [PubMed] [Google Scholar]
- 20. Detels R, et al. 1994. Persistent cytomegalovirus infection of semen increases risk of AIDS. J. Infect. Dis. 169: 766–768 [DOI] [PubMed] [Google Scholar]
- 21. Detels R, et al. 1987. Predictors of clinical AIDS in young homosexual men in a high-risk area. Int. J. Epidemiol. 16: 271–276 [DOI] [PubMed] [Google Scholar]
- 22. Diamond C, et al. 2000. Comparison of assays to detect cytomegalovirus shedding in the semen of HIV-infected men. J. Virol. Methods 90: 185–191 [DOI] [PubMed] [Google Scholar]
- 23. Effros RB, et al. 2008. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin. Infect. Dis. 47: 542–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El Amari EB, et al. 2011. Clinical relevance of cytomegalovirus viraemia. HIV Med. 12: 394–402 [DOI] [PubMed] [Google Scholar]
- 25. Emery VC, et al. 1999. Quantitative effects of valacyclovir on the replication of cytomegalovirus (CMV) in persons with advanced human immunodeficiency virus disease: baseline CMV load dictates time to disease and survival. The AIDS Clinical Trials Group 204/Glaxo Wellcome 123-014 International CMV Prophylaxis Study Group. J. Infect. Dis. 180: 695–701 [DOI] [PubMed] [Google Scholar]
- 26. Fauci AS. 1988. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science 239: 617–622 [DOI] [PubMed] [Google Scholar]
- 27. Fiebig EW, et al. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17: 1871–1879 [DOI] [PubMed] [Google Scholar]
- 28. Galvin SR, Cohen MS. 2004. The role of sexually transmitted diseases in HIV transmission. Nat. Rev. Microbiol. 2: 33–42 [DOI] [PubMed] [Google Scholar]
- 29. Gandhi RT, et al. 2006. Effect of baseline- and treatment-related factors on immunologic recovery after initiation of antiretroviral therapy in HIV-1-positive subjects: results from ACTG 384. J. Acquir. Immune Defic. Syndr. 42: 426–434 [DOI] [PubMed] [Google Scholar]
- 30. Gartner S, et al. 1986. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 233: 215–219 [DOI] [PubMed] [Google Scholar]
- 31. Gray RH, et al. 2001. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 357: 1149–1153 [DOI] [PubMed] [Google Scholar]
- 32. Haas A, et al. 2010. HIV-1 replication activates CD4+ T cells with specificities for persistent herpes viruses. EMBO Mol. Med. 2: 231–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hadrup SR, et al. 2006. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol. 176: 2645–2653 [DOI] [PubMed] [Google Scholar]
- 34. Hecht FM, et al. 2006. A multicenter observational study of the potential benefits of initiating combination antiretroviral therapy during acute HIV infection. J. Infect. Dis. 194: 725–733 [DOI] [PubMed] [Google Scholar]
- 35. Hunt PW, et al. 2003. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 187: 1534–1543 [DOI] [PubMed] [Google Scholar]
- 36. Hunt PW, et al. 2011. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J. Infect. Dis. 203: 1474–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jabs DA, et al. 2005. Risk factors for mortality in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology 112: 771–779 [DOI] [PubMed] [Google Scholar]
- 38. Johnson LF, Lewis DA. 2008. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex. Transm. Dis. 35: 946–959 [DOI] [PubMed] [Google Scholar]
- 39. Kalichman SC, Di Berto G, Eaton L. 2008. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex. Transm. Dis. 35: 55–60 [DOI] [PubMed] [Google Scholar]
- 40. Kaul R, et al. 2008. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J. Reprod. Immunol. 77: 32–40 [DOI] [PubMed] [Google Scholar]
- 41. Killian MS, et al. 2004. Persistent alterations in the T-cell repertoires of HIV-1-infected and at-risk uninfected men. AIDS 18: 161–170 [DOI] [PubMed] [Google Scholar]
- 42. Kosakovsky Pond SL, et al. 2009. An evolutionary model-based algorithm for accurate phylogenetic breakpoint mapping and subtype prediction in HIV-1. PLoS Comput. Biol. 5: e1000581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krieger JN, et al. 1995. Seminal shedding of human immunodeficiency virus type 1 and human cytomegalovirus: evidence for different immunologic controls. J. Infect. Dis. 171: 1018–1022 [DOI] [PubMed] [Google Scholar]
- 44. Laga M, et al. 1993. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7: 95–102 [DOI] [PubMed] [Google Scholar]
- 45. Lang DJ, Kummer JF. 1972. Demonstration of cytomegalovirus in semen. N. Engl. J. Med. 287: 756–758 [DOI] [PubMed] [Google Scholar]
- 46. Lange M, Klein EB, Kornfield H, Cooper LZ, Grieco MH. 1984. Cytomegalovirus isolation from healthy homosexual men. JAMA 252: 1908–1910 [PubMed] [Google Scholar]
- 47. Leach CT, et al. 1993. The relationship between T-cell levels and CMV infection in asymptomatic HIV-1 antibody-positive homosexual men. J. Acquir. Immune Defic. Syndr. 6: 407–413 [PubMed] [Google Scholar]
- 48. Lenkei R, Andersson B. 1995. High correlations of anti-CMV titers with lymphocyte activation status and CD57 antibody-binding capacity as estimated with three-color, quantitative flow cytometry in blood donors. Clin. Immunol. Immunopathol. 77: 131–138 [DOI] [PubMed] [Google Scholar]
- 49. Limaye AP, et al. 2008. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 300: 413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lingappa JR, et al. 2010. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet 375: 824–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Little SJ, et al. 2008. Persistence of transmitted drug resistance among subjects with primary human immunodeficiency virus infection. J. Virol. 82: 5510–5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lomas DA, Natin D, Stockley RA, Shahmanesh M. 1993. Chemotactic activity of urethral secretions in men with urethritis and the effect of treatment. J. Infect. Dis. 167: 233–236 [DOI] [PubMed] [Google Scholar]
- 53. Mbopi-Keou FX, et al. 2000. Interactions between herpes simplex virus type 2 and human immunodeficiency virus type 1 infection in African women: opportunities for intervention. J. Infect. Dis. 182: 1090–1096 [DOI] [PubMed] [Google Scholar]
- 54. Mehandru S, et al. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200: 761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ouyang Q, et al. 2003. Large numbers of dysfunctional CD8+ T lymphocytes bearing receptors for a single dominant CMV epitope in the very old. J. Clin. Immunol. 23: 247–257 [DOI] [PubMed] [Google Scholar]
- 56. Pinto-Neto LF, et al. 2002. Lack of correlation between seminal and plasma HIV-1 viral loads is associated with CD4 T cell depletion in therapy-naive HIV-1+ patients. Mem. Inst. Oswaldo Cruz 97: 563–567 [DOI] [PubMed] [Google Scholar]
- 57. Politch JA, Mayer KH, Anderson DJ. 2009. Depletion of CD4+ T cells in semen during HIV infection and their restoration following antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 50: 283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Polk BF, et al. 1987. Predictors of the acquired immunodeficiency syndrome developing in a cohort of seropositive homosexual men. N. Engl. J. Med. 316: 61–66 [DOI] [PubMed] [Google Scholar]
- 59. Quinn TC, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342: 921. [DOI] [PubMed] [Google Scholar]
- 60. Rinaldo CR, Jr, Kingsley LA, Ho M, Armstrong JA, Zhou SY. 1992. Enhanced shedding of cytomegalovirus in semen of human immunodeficiency virus-seropositive homosexual men. J. Clin. Microbiol. 30: 1148–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rottingen JA, Cameron DW, Garnett GP. 2001. A systematic review of the epidemiologic interactions between classic sexually transmitted diseases and HIV: how much really is known? Sex. Transm. Dis. 28: 579–597 [DOI] [PubMed] [Google Scholar]
- 62. Royce RA, Sena A, Cates W, Cohen MS. 1997. Sexual transmission of HIV. N. Engl. J. Med. 336: 1072. [DOI] [PubMed] [Google Scholar]
- 63. Sauce D, et al. 2009. Evidence of premature immune aging in patients thymectomized during early childhood. J. Clin. Invest. 119: 3070–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. 2002. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J. Infect. Dis. 186: 1718–1725 [DOI] [PubMed] [Google Scholar]
- 65. Sheth PM, et al. 2005. HIV-specific CD8R lymphocytes in semen are not associated with reduced HIV shedding. J. Immunol. 175: 4789–4796 [DOI] [PubMed] [Google Scholar]
- 66. Sheth PM, et al. 2006. Disproportionately high semen shedding of HIV is associated with compartmentalized cytomegalovirus reactivation. J. Infect. Dis. 193: 45–48 [DOI] [PubMed] [Google Scholar]
- 67. Skolnik PR, Kosloff BR, Hirsch MS. 1988. Bidirectional interactions between human immunodeficiency virus type 1 and cytomegalovirus. J. Infect. Dis. 157: 508–514 [DOI] [PubMed] [Google Scholar]
- 68. Smith DM, et al. 2004. The prostate as a reservoir for HIV-1. AIDS 18: 1600. [DOI] [PubMed] [Google Scholar]
- 69. Speck CE, et al. 1999. Risk factors for HIV-1 shedding in semen. Am. J. Epidemiol. 150: 622–631 [DOI] [PubMed] [Google Scholar]
- 70. Spector SA, Wong R, Hsia K, Pilcher M, Stempien MJ. 1998. Plasma cytomegalovirus (CMV) DNA load predicts CMV disease and survival in AIDS patients. J. Clin. Invest. 101: 497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sylwester AW, et al. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202: 673–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vernazza PL, et al. 1997. Quantification of HIV in semen: correlation with antiviral treatment and immune status. AIDS 11: 987. [PubMed] [Google Scholar]
- 73. Vescovini R, et al. 2010. Intense antiextracellular adaptive immune response to human cytomegalovirus in very old subjects with impaired health and cognitive and functional status. J. Immunol. 184: 3242–3249 [DOI] [PubMed] [Google Scholar]
- 74. Wawer MJ, et al. 2005. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J. Infect. Dis. 191: 1403–1409 [DOI] [PubMed] [Google Scholar]
- 75. Webster A, et al. 1989. Cytomegalovirus infection and progression towards AIDS in haemophiliacs with human immunodeficiency virus infection. Lancet ii: 63–66 [DOI] [PubMed] [Google Scholar]
- 76. Weiss H. 2004. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes 11 (Suppl 1): 24A–35A [PubMed] [Google Scholar]
- 77. Wikby A, et al. 2002. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp. Gerontol. 37: 445–453 [DOI] [PubMed] [Google Scholar]
- 78. Wilcoxon F. 1945. Individual comparisons by ranking methods. Biometrics Bull. 1: 80–83 [Google Scholar]
- 79. Wolff H, Anderson DJ. 1988. Male genital tract inflammation associated with increased numbers of potential human immunodeficiency virus host cells in semen. Andrologia 20: 404–410 [PubMed] [Google Scholar]
- 80. Xu C, et al. 1997. Factors associated with increased levels of human immunodeficiency virus type 1 DNA in semen. J. Infect. Dis. 176: 941–947 [DOI] [PubMed] [Google Scholar]
- 81. Yang OO, et al. 2002. Immunologic profile of highly exposed yet HIV type 1-seronegative men. AIDS Res. Hum. Retroviruses 18: 1051–1065 [DOI] [PubMed] [Google Scholar]
- 82. Yen-Lieberman B, et al. 1996. Evaluation of a quality assurance program for quantitation of human immunodeficiency virus type 1 RNA in plasma by the AIDS Clinical Trials Group virology laboratories. J. Clin. Microbiol. 34: 2695–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zagury D, et al. 1986. Long-term cultures of HTLV-III-infected T cells: a model of cytopathology of T-cell depletion in AIDS. Science 231: 850–853 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.