Abstract
Background
We previously reported that intravenous scopolamine administration produced rapid and robust antidepressant effects in a sample consisting of both unipolar and bipolar depressives. The present study aimed to replicate this finding in an independent sample limited to unipolar depressives.
Methods
Outpatients with major depressive disorder (MDD) (n=23; 22 were included in analyses) participated in a double-blind, placebo-controlled, cross-over trial. Subjects were randomized into either a P/S or S/P sequence [P=block of three placebo sessions; S= block of three scopolamine sessions (4.0 μg/kg i.v.)]. Sessions occurred 3-to-5 days apart, such that time spent in each block lasted 1½-2 weeks and the interval between blocks was 3 to 5 days. The Montgomery-Asberg Depression Rating Scale (MADRS) served as the primary outcome measure.
Results
Following the initial block the group receiving scopolamine first (S/P) showed a 32 percent reduction in MADRS scores (p<0.001) which exceeded the corresponding change of 6.5 percent under placebo (P/S) (p=0.009), confirming the a priori hypothesis. Improvement was significant at the first evaluation that followed scopolamine administration (p=0.011). In block 2 the P/S group showed a 53 percent reduction in MADRS scores (p=0.001) following scopolamine versus placebo, while the reduction seen in S/P subjects who received scopolamine during block 1 persisted as they received placebo during block 2. Scopolamine induced drowsiness, blurred vision, dry mouth, light-headedness and reduced blood pressure, which were sufficiently well-tolerated that no subject dropped out due to side effects.
Conclusions
These results replicate previous finding that scopolamine produces a rapid and robust antidepressant response.
Keywords: antimuscarinic, anticholinergic
The need to develop improved antidepressant treatments that more quickly and effectively treat major depression remains critical(1). We reported previously the results of a clinical trial conducted at the NIMH showing that the muscarinic cholinergic receptor antagonist, scopolamine, exerted antidepressant effects in depressed patients (total n=18) with either major depressive disorder (MDD; n=9) or bipolar disorder (n=9)(2). In this double-blind, placebo-controlled, cross-over trial, subjects underwent multiple sessions in which they received i.v. infusions of placebo or scopolamine (4 μg/kg i.v.). Individuals were randomized into either a P/S or S/P sequence, in which the former received placebo followed by scopolamine and the latter received scopolamine followed by placebo. The P/S group showed no significant improvement during the placebo series, but significant reductions in ratings of depression and anxiety severity following the administration of scopolamine as compared to placebo. The S/P group also showed significant reductions in depression and anxiety ratings following scopolamine and these effects persisted throughout the subsequent placebo series, well beyond the expected duration of scopolamine’s direct action at muscarinic receptors. Moreover, in both the P/S and S/P subgroups, improvement was significant at the first evaluation that followed scopolamine administration (i.e., 3 to 4 days following the initial administration), suggesting that the antidepressant responses to scopolamine was relatively rapid.
In the current study we sought to replicate the finding that scopolamine exerts antidepressant effects in an independent subject sample. Since the original sample consisted of both unipolar and bipolar cases, we recognized the need to replicate the findings independently in each mood disorder. The current study thus limited recruitment to MDD subjects.
METHODS
Participants
Volunteers between 18 and 45 years of age evaluated at the NIMH outpatient clinic were assessed for eligibility if they were non-smokers and met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV)(3) criteria for recurrent MDD, based upon an unstructured interview conducted by a psychiatrist and the Structured Clinical Interview for DSM-IV. Exclusion criteria included exposure to psychotropic drugs or other medications likely to affect cholinergic function within 3 weeks (8 weeks for fluoxetine), serious risk of suicide, delusions or hallucinations, lifetime history of substance dependence or substance abuse within one year, medical or neurological disorders, narrow angle glaucoma, hypersensitivity to anticholinergic agents, hepatic dysfunction, electrolyte disturbance, HIV or hepatitis viral infection, or weight >125 kg. Pregnant or nursing females also were excluded. Subjects provided written informed consent as approved by the NIMH IRB.
Study Design
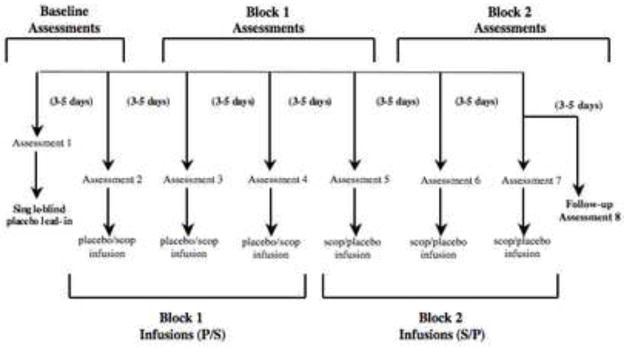
During each of seven sessions, subjects received a 15 minute intravenous infusion of either a placebo saline solution or scopolamine (4.0 μg/kg). A single-blind, lead-in session was used in which all subjects received a placebo infusion. As psychiatric assessments were obtained prior to infusions, the lead-in placebo in session 1 allowed for a second baseline assessment to be obtained immediately prior to the session 2 infusion. Subsequently, individuals were randomized into either a P/S or S/P double-blind, placebo-controlled, cross-over design whereby P constituted a block of 3 placebo sessions and S a block of 3 scopolamine sessions (figure 1). A follow-up evaluation provided the final assessment following session 7 (i.e., “assessment 8”). Randomization sequences were determined by the NIH outpatient pharmacy and assigned by subject number at consenting. Sessions were scheduled 3–5 days apart.
Figure 1.
Study blocked experimental design reflecting infusion series and assessment sessions for each of the two randomized patient groups. P/S reflects the infusion series of placebo followed by scopolamine; S/P indicated scopolamine followed by placebo.
Sample size was determined using power calculations involving data obtained from our initial study, where we observed a group difference in MADRS scores at the end of study block 1 of 17.4 points. If we predicted an improvement of one-half the magnitude of that seen in the earlier study and the same group variance, a sample size of eleven per group provided power of 80 percent for alpha = 0.05.
Assessment
Prior to each infusion, depression severity was rated using the Montgomery-Asberg Depression Rating Scale (MADRS)(4), anxiety symptoms were rated using the Hamilton Anxiety Rating Scale (HARS)(5), the development of hypomanic symptoms was assessed using the Young Mania Rating Scale (YMRS)(6), and the Clinical Global Impressions (CGI)(4) scale was applied as a global assessment of illness severity. To evaluate within session changes in mood, visual analog scales (VAS-components included happy, sad, drowsy, irritated, alert, anxious and restless) were administered at baseline and 20, 60, 120 and 150 minutes following initiation of the infusion, and the Profile of Mood State (POMS)(7) was administered at baseline, 20, 60 and 150 minutes post-infusion. Blood pressure and heart rate were measured at baseline, at 15 min intervals for 60 min following the infusion start, and at 30 min intervals for the remainder of the session using a Dynamap Vital Signs Monitor (Critikon Inc., Tampa, FL).
Outcome measures
The antidepressant response was evaluated by assessing changes in MADRS scores. Using conventional criteria(8) patients were characterized as achieving: full response (≥50% reduction in MADRS score from baseline), partial response (<50% but ≥25% reduction) or nonresponse (<25% reduction). Patients achieving remission (post-treatment MADRS score≤10) also were identified. Secondary outcome measures included the HARS, CGI, VAS and POMS. The VAS and POMS scores were assessed by comparing the mean ratings for each time-point across the drug or placebo sessions.
Data analysis
A group (P/S versus S/P) by assessments repeated measures ANOVA was performed to evaluate overall group differences in the MADRS. To provide a balanced design, MADRS data were separated into a baseline block (assessments 1 and 2), the first and last measures of block 1 (assessments 3 and 5), and block 2 (assessments 6 and 8). The a priori hypothesis that scopolamine would exert antidepressant effects relative to placebo was tested primarily using the group-by-block ANOVA. The a priori hypothesis that the antidepressant effect of scopolamine is rapid was tested using the ANOVA limited to the results for the first assessment that followed the first exposure to scopolamine versus the corresponding change under placebo. Between and within group t-tests were used in planned comparisons to identify where significant effects occurred in the presence of significant overall ANOVA’s. Post hoc tests were performed to assess the significance of changes in the secondary outcome measures (HARS, CGI-I, VAS, POMS). All p-values reported are two-tailed.
RESULTS
Subjects
The passage of subjects through the phases of this clinical trial is detailed in Figure S1 (see Supplement 1). Of 42 eligible patients, 19 were assessed for eligibility but were excluded for not meeting entrance criteria (n=6) or declining to participate (n=13), so 23 were randomized into the study (figure 2). One subject dropped out after randomization but prior to session 1, so this subject did not contribute any data to the analysis. Twenty-one subjects completed the trial as intended and another subject dropped out after session 6 due to non-response; this subject’s data were included in the analysis based upon last observation carried forward. Thus a total of 22 patients received the intended treatment and were included in all analyses, 11 of whom were randomized into the P/S group and 11 into the S/P group. In three cases who completed all 7 infusions the follow-up evaluations could not be obtained for the assessment following session seven (i.e., assessment 8), so analyses were performed using the last observation (from session 7) carried forward (LOCF). The S/P and P/S groups did not differ in MADRS or HARS scores at baseline (F=0.055, p=0.82).
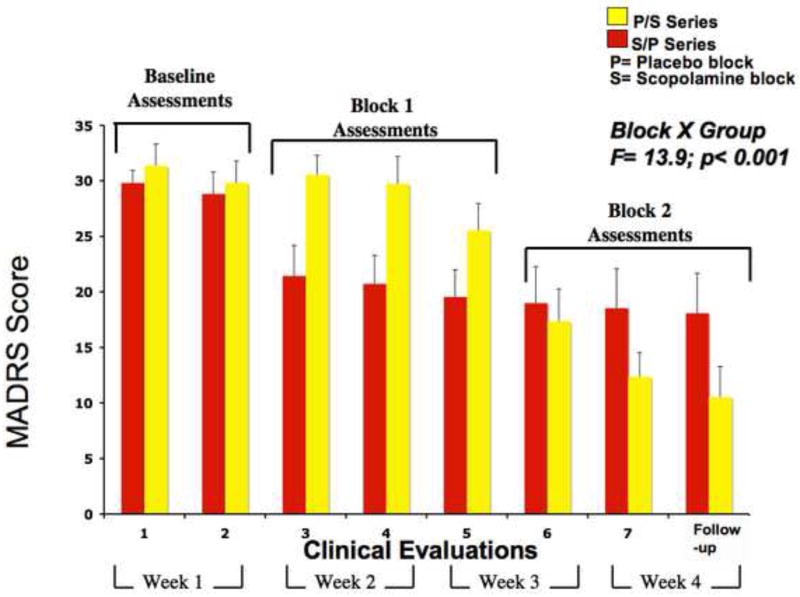
Figure 2.
Mean MADRS scores for the P/S group (yellow bars) and the S/P group (red bars) across eight assessments. P= the placebo sessions and includes a block of 3 assessments of placebo infusions; S= the scopolamine sessions and includes a block of 3 assessments of scopolamine infusions. Two baseline, three block 1 and three block 2 assessments are identified in each panel. Error bars show standard error of the mean. The p-value reflects a significant block by group interaction.
Adverse and Side Effects
Scopolamine was well-tolerated and no medically serious adverse events were encountered. Side effects reported under scopolamine (S) and placebo (P) conditions are listed in table 2. Heart rate, systolic BP and diastolic BP decreased following scopolamine infusion relative to placebo infusion (p<0.05; Figures S3, S4, and S5 in Supplement 1), although no subject developed symptoms of hypotension or evidence of cardiovascular insufficiency. No subject developed hypomania during the study. Moreover, the mean YMRS score decreased (F=9.6; p>0.006) between baseline (mean=2.1±0.91) and study end (1.2±1.0).
Table 2.
Side effects reported under scopolamine and placebo conditions presented as number of cases.
| Side Effect | Placebo (n of 22) | Drug (n of 22) |
|---|---|---|
| Drowsiness | 13 | 17 |
| Dry Mouth | 3 | 18 |
| Blurred Vision | 4 | 16 |
| Lightheadedness | 4 | 15 |
| Dizziness | 3 | 9 |
| Hypotension- (no intervention) | 0 | 1 |
| Nausea | 0 | 0 |
| Headache | 0 | 1 |
| Nervousness | 1 | 1 |
| Diplopia | 0 | 0 |
| Palpitations | 3 | 0 |
| Derealization | 0 | 0 |
| Mental Clouding | 0 | 0 |
| Irritability | 0 | 0 |
| Restlessness | 0 | 0 |
| Euphoria | 0 | 0 |
| Vertigo | 0 | 1 |
Primary Outcome Indices
The mean MADRS scores for the two groups across the eight evaluations appear in figure 2. Repeated measures ANOVA showed a group-by-assessment interaction (F=8.36, p<0.001). The 3-way ANOVA (group-by-study block-by-assessment) also was significant (F=14.0, p<0.001). For the difference between baseline and study block 1, the group-by-block interaction was significant (F=8.32, p=0.009). This effect was attributable to the reduction in MADRS scores in the S/P group (F=22.4, p=0.001) being greater than the corresponding reduction in the P/S group (F=5.18, p=0.046; i.e., placebo effect). This difference between groups showed an effect size of 1.38 (Cohen’s d: CI= −2.22 – 3.51) and reached significance by the first evaluation in study block 1 (t=2.79, p=0.011).
Between experimental blocks 1 and 2 the change in MADRS scores also differed between groups (F=15.8, p=0.001; Cohen’s d=2.27: −1.28 to 6.52). This effect was attributable to a reduction in MADRS scores in the P/S group between blocks 1 and 2 (F=48.0, p<0.001) while the MADRS scores in the S/P group did not change in block 2 versus block 1 (F=0.733; p=0.41), indicating that the antidepressant effect observed in this group in block 1 persisted as they received placebo in block 2. For the group that received scopolamine second the difference between the final evaluation in block 1 and the first evaluation in block 2 (i.e., the first post-scopolamine assessment) was significant (t=3.98, p=0.003). Within each scopolamine block, the reduction in MADRS scores in the final assessment relative to the first was significant (t=2.52; p=0.020; for S/P and P/S subjects combined) showing further reduction in symptom severity following repeated scopolamine administrations.
By study end 14 of the 22 (64%) subjects achieved a full response and 11/22 (50%) experienced remission (based upon attaining a MADRS score<10]) (Table 3). Further post-hoc assessments of the antidepressant effects of scopolamine appear in Supplement 1.
Table 3.
Outcome indices for patients treated with scopolamine (n=22).
| Baseline Block | Block 1 | Block 2 | |
|---|---|---|---|
| S/P group (n=11) | |||
| Full Response (> 50%) | 0 | 4 | 5 |
| Partial Response (25–49%) | 2 | 3 | 1 |
| Non-response (< 25%) | 9 | 4 | 5 |
|
| |||
| P/S group (n=11) | |||
| Full Response | 0 | 1 | 9 |
| Partial Response | 0 | 3 | 1 |
| Non-response | 11 | 7 | 1 |
Each entry reflects the number of participants from the identified group showing the described effect. When the two groups were combined, by study end 14 of the 22 (64%) subjects achieved a full response (11 of whom experienced remission based upon attaining a MADRS score≤10). Of these, one subject attained full response and remission under placebo, and this response persisted during the subsequent scopolamine sessions. The remaining subjects achieved full response and/or remission under scopolamine.
Abbreviations: P/S – subjects randomized to receive placebo in study block 1 and scopolamine in block 2; S/P – subjects randomized to receive scopolamine in block 1 and placebo in block 2
Secondary Outcome Measures
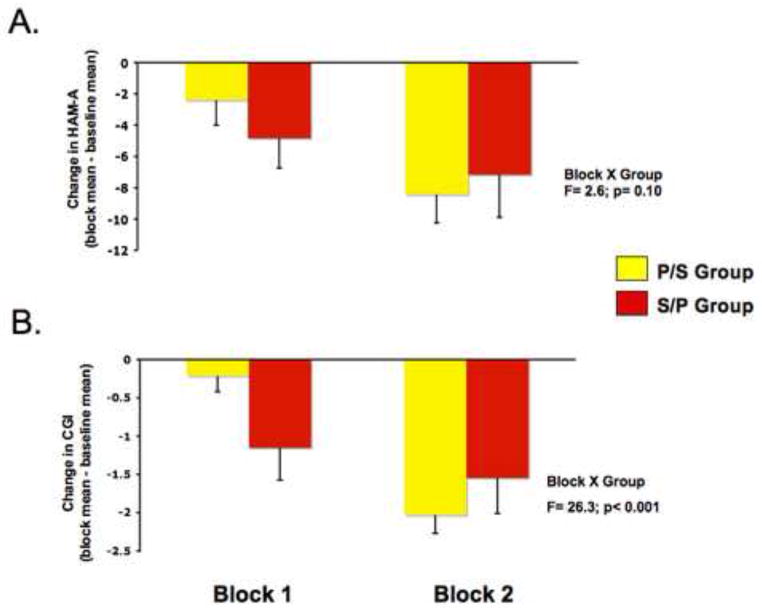
The CGI-I (from session 2 through follow-up) showed a group by assessment interaction (F=5.49, p=0.003), while the corresponding interaction for HARS was not significant (p> 0.20). Considering change from baseline, the block by group interaction was significant for the CGI-I (F= 26.3, P, 0.001; figure 3A), while this interaction for the HARS trended towards significance (F=2.6; p=0.10; figure 3B).
Figure 3.
Mean changes in (A) the Hamilton Anxiety Rating Scale (HARS) and (B) the Clinical Global Impressions-Improvement scores (CGI-I) between Study Block 1 versus the Baseline Block (left bar) and between Study Block 2 versus Baseline (right bar). (A) The HARS data showed a nonsignificant trend in the block by group interaction (F=2.6; p=0.10). To accommodate the variation in mean baseline HARS scores between the S/P and P/S groups (20±9 and 18±8, respectively) the effect of scopolamine on anxiety ratings was evaluated within each group separately. In the P/S group, a block-by-assessment analysis indicated differences among study blocks (F=10.4, p=0.005). Anxiety scores in the P/S group were lower in study block 2 as compared to baseline (F=23.0, p=0.001), this effect was significant with the first assessment in block 2 (t=2.7, p=0.022). The difference between baseline and experimental block 1 was not significant (F=2.6, p=0.14). In the S/P group, the block-by-assessment analysis showed a nonsignificant trend toward differing among blocks (F=3.8, p<0.063). In this group the HARS scores evaluated in block 1 were lower than baseline (F=7.17 p=0.023) and the scores in block 2 were lower than baseline (F=8.03, p=0.018) but did not differ from the scores obtained in block 1 (F=1.79, p=0.21), indicating the antianxiety effect persisted as this group received placebo in block 2. (B) The CGI-I data showed a block-by-group interaction (F= 26.3, P, 0.001). The change in CGI scores in the S/P group was greater than the change in the P/S group during block 1 (F=6.61; p=0.018). No group difference was observed in ratings from study block 2 evaluations (F=1.54; p=0.23) suggesting that the magnitude of clinical improvement did not differ after both groups received scopolamine.
The VAS and POMS ratings indicated that no acute, within-session changes in emotion ratings occurred during scopolamine relative to placebo sessions (see Supplement 1). Of the VAS ratings the drug-by-time interaction was not significant for happiness, sadness, anxiety, irritation, restlessness or alertness (p>0.15), but was significant for drowsiness (F=7.2; p=0.002). On the POMS the drug-by-time interaction was not significant for the depression (p=0.35), anger (p=0.66) or tension factors (p=0.32). However, the drug-by-time interaction on the POMS vigor factor was significant (F=7.8, p=0.003), as this factor decreased at 20 and 60 min after the start of the scopolamine infusion and then returned to baseline levels by session-end.
DISCUSSION
Scopolamine (4.0 μg/kg, i.v.) showed antidepressant efficacy relative to placebo in unipolar depressives, replicating the results we obtained previously in an independent sample of depressed patients that included both unipolar and bipolar patients(2). Our study used a cross-over design, so the improvement observed independently in the two treatment groups provided additional, within-study replications of the antidepressant effect. Between the baseline block and experimental block 1 the S/P group showed a greater reduction in MADRS scores under scopolamine than under the baseline placebo, and this reduction exceeded that seen concomitantly in the P/S group while they received placebo. In addition, subjects randomized to the P/S schedule showed a reduction in MADRS scores during experimental block 2 versus block 1 as they transitioned from placebo to scopolamine.
As in our initial study, the rapidity of the antidepressant response was evidenced by the improvement seen in the evaluation that followed the first scopolamine administration, 3 to 5 days after the first treatment. In both treatment groups, the initial post-scopolamine MADRS ratings were lower than those obtained during the previous session. Moreover, the reduction in MADRS scores seen in the S/P group after their first scopolamine exposure exceeded the corresponding reduction observed in the P/S group under placebo. Each session’s assessment evaluated symptoms experienced since the previous visit so this finding indicated that the antidepressant effects occurred within 3 to 5 days, and treatment-responders generally reported improvement in symptoms by the morning following the first infusion. This timeframe compares favorably to the 3 to 4 weeks typically required for conventional treatments to become effective.
Other findings from the current study that replicated those of our previous study merit comment. First, subjects showed further improvement across the scopolamine block, suggesting that repeated administrations provided additional benefit. Second, in individuals who received scopolamine during block 1, the improvement seen during drug administration persisted as they received placebo during block 2, indicating the antidepressant effects persisted at least 12 to 16 days after the final scopolamine administration. This carry-over effect was confirmed by demonstrating that depression ratings did not differ between the S/P and P/S groups in the final study block, when both groups showed improvement relative to the pre-treatment baseline.
The demonstration that an antimuscarinic agent produces potent antidepressant effects extends evidence linking muscarinic receptor function to the pathophysiology of mood disorders (9–18) although the precise mechanism underlying scopolamine’s antidepressant action remains unclear. The persistence of scopolamine’s antidepressant effect for weeks after its expected clearance from plasma (elimination t1/2=2 to 4 hours) suggests a mechanism beyond the direct pharmacological actions on muscarinic receptors. Moreover, the delay in the onset of the antidepressant response until well after the resolution of anticholinergic side effects appears compatible with an effect on transcription of “late-response” genes or synaptic plasticity, rather than a direct action on muscarinic receptors(19).
One effect scopolamine shares with other somatic antidepressant treatments involves the modulation of N-methyl-D-aspartate receptor (NMDAR) function. Blocking muscarinic receptors via scopolamine administration reduces mRNA concentrations for NMDAR types 1A and 2A in the rat brain in vivo(20) and protects hippocampal neurons from glutamate-mediated neurotoxicity in vitro(21). Chronic administration of antidepressant drugs from various classes and repeated electroconvulsive shock reduce cortical NMDAR function(22–24), and treatments associated with a rapid onset of antidepressant effects either exert direct NMDAR antagonist effects (ketamine)(22, 25) or induce NMDR internalization (sleep-deprivation)(26, 27). Taken together with evidence that abnormal glutamatergic transmission is involved in the pathophysiology of depression, these data suggest the hypothesis that scopolamine’s effect on NMDAR function plays a role in its antidepressant action.
Another possible mechanism that merits consideration is scopolamine’s paradoxical effect of enhancing parasympathetic autonomic outflow when administered in the low dose range that encompasses the doses used here(28). Reductions in heart rate and blood pressure during scopolamine administration like those we observed (see Supplement 1) have been reported previously at comparable doses(29, 30) and putatively reflect central effects on autonomic function(28). While it remains unclear whether the effect of scopolamine (at 4.0 ug/kg iv) on parasympathetic activity plays any role in the antidepressant response, it is noteworthy that the pathophysiology of depression is associated with a reduction in the parasympathetic-to-sympathetic balance(31). The scopolamine effect of enhancing parasympathetic tone may thus reverse this pathological state, analogous to the effect of some neurostimulation approaches that produce antidepressant effects and also enhance the parasympathetic-to-sympathetic ratio(32).
Patient acceptance of the adverse effects was good, as no subject dropped out due to a drug side-effect. This favorable tolerability was attributable partly to the transient nature of the side effects and the ~bi-weekly, as opposed to daily, dosing schedule. Thus, while scopolamine administration acutely produced sedation, visual blurring, dry mouth, light headedness/dizziness and small reductions in heart rate and blood pressure (which were clinically non-significant in our subjects) these side-effects were relatively transient. For example, blurred vision and light-headedness lasted 1 to 2 hours while sedation typically lasted 2 ½ to 3 hours, and ranged to as long as 5 hours. Nevertheless, subjects spent the days between infusions without side-effect s and did not develop adverse reactions commonly associated with daily antimuscarinic administration, such as urinary retention or constipation. Finally, while the POMS data indicated that patients experienced a transient increase in subjective confusion during the two hour period following scopolamine infusion, no subject developed delirium, psychosis or overt confusion, and scopolamine’s effects on performance on selective attention were neither generalized nor unidirectional(33). The transient nature of the side effects also aided the preservation of the double-blind since the primary outcome measure (MADRS) was obtained at the beginning of each session when subjects were side-effect free (i.e., before they received the infusion for that day).
Another design feature that mitigated the likelihood of un-blinding by side effects was that the placebo challenge, which involved i.v. infusion while sitting in a reclining hospital chair or bed, commonly produced side effects similar to those expected under scopolamine. For example, 13 of the 22 subjects reported experiencing sedation under placebo. Most of these subjects also spontaneously reported believing that they had received the study drug while actually having received only placebo. In these cases, the experience of subsequently receiving scopolamine may have compromised their blind during study arm 2 (i.e., by contrast to previous sessions), but this would not have influenced their ratings obtained during the placebo sessions in study arm 1. Moreover, subjects were unaware that the 3 scopolamine sessions would occur consecutively in a block, reducing the likelihood that experiencing side effects during the previous session would bias the ratings obtained at the beginning of the subsequent session.
The post hoc item-by-item analysis of the MADRS indicated that the reductions in total MADRS scores were attributable to improvements in most symptom domains assessed. There was no evidence of a euphoric effect under scopolamine and the within-session VAS and POMS assessments revealed no acute positive effects of scopolamine on mood (see Supplement 1). In contrast, the POMS and VAS scales suggested that subjects showed subtle but statistically significant improvements in mood across the 2 ½ hour sessions during the placebo sessions which did not occur during the scopolamine sessions (see Supplement 1).
Fifty-percent (n=11) of the subjects experienced remission, which occurred under scopolamine in 10 subjects and under placebo in one. Similarly, in our original study 56% of subjects remitted under scopolamine(2). These results compare favorably to the 10 to 20% placebo-adjusted remission rates obtained using selective serotonin reuptake inhibitors(34).
The effect sizes of the difference between scopolamine and placebo in this study (d=1.2 and 1.7 for blocks 1 and 2, respectively) also compare favorably to those typically observed in antidepressant treatment studies, which range from 0.5 to 1.1 in moderately and severely depressed cases, respectively (35). The subjects studied herein manifested depression severity in the moderate-to-severe range, as reflected by their relatively high mean MADRS scores (table 1). Although the effect sizes obtained herein were numerically smaller than those seen in our original study, the Cohen’s d values nevertheless fell within the confidence intervals of those from our original study. The clinical significance of this antidepressant response was further reflected by the robust change in the mean CGI score (figure 3).
Table 1.
Patient characteristics at baseline
| Placebo/Scopolamine Group (n=11) (mean ± SD) |
Scopolamine/Placebo Group (n=11) (mean ± SD) |
|
|---|---|---|
| Mean age ± SD | 30 ± 7.0 | 33 ± 7.1 |
| Gender | 7F/4M | 5F/6M |
| Mean MADRS ± SD | 31 ± 6.5 | 30 ± 3.7 |
| Mean HAM-A ± SD | 18 ± 8.2 | 20 ± 9.2 |
| Chronic illness | 8/11 | 5/11 |
| Comorbid anxiety | 3/11 | 5/11 |
| Unresponsive to treatment | 3/11 | 3/11 |
In the S/P group (N=11; 5F; 4 African American, 6 Caucasian, 1 Hispanic; mean age=33±7.1 years), 5/11 patients were chronically ill (current episode duration>2 years), 5/11 had a comorbid anxiety disorder and 3/11 were unresponsive to previous treatment. In the P/S group (N= 11; 7F; 4 African American, 6 Caucasian, 1 Hispanic; mean age= 30±7.0), 8/11 patients were chronically ill, 3/11 had a comorbid anxiety disorder, and 3/11 were unresponsive to previous treatment, based upon the response to the most recent therapeutic trial of a conventional antidepressant agent. In total, based upon their history of having either nonresponse to previous treatment, chronicity or a comorbid anxiety disorder(38–41), 16/22 patients had a poor prognosis for response to treatment, including 7 in the S/P group and 9 in the P/S group.
Abbreviations: MADRS – Montgomery-Asberg Depression Rating Scale; HARS – Hamilton Anxiety Rating Scale
Several aspects of the sample selection limit the generalizability of these results. First, the sample was small. Second, elderly and pediatric subjects, bipolar depressives and current nicotine users were excluded, so the current results may not generalize to such cases. As in our original study, smokers were excluded because we were uncertain whether functional interactions between the muscarinic and nicotinic cholinergic receptor systems might influence the antidepressant effect of scopolamine. Due to sample size limitations we did not address sex effects on scopolamine’s antidepressant efficacy, although such effects have been reported for conventional antidepressant drugs. Finally, while these data are significant and replicate our previous results, these findings still await independent replication.
Our results hold promise that scopolamine treatment offers rapid, robust relief of symptoms to individuals suffering from depression. We previously proposed that the powerful effects we report with scopolamine may have been missed in previous studies using this agent in depressed patients because these studies used lower effective doses(13, 15) or assessed clinical effects only acutely (120 min)(36). For example, small but statistically significant antidepressant effects were observed the day following the administration of scopolamine 0.4 mg i.m.(13), which would have a bioavailability similar to that of about 2 ug/kg i.v.(37). Nevertheless the finding that scopolamine at or near the doses we exerts antidepressant effects awaits replication by an independent laboratory. Finally, determination of the optimal route and schedule of administration for out-patient treatment and the maintenance of scopolamine’s antidepressant efficacy during long-term use require further study.
Supplementary Material
Acknowledgments
We thank Ashish Khanna, Mark Opal, Summer Peck and Elana Hoffman for technical support, Michele Drevets and Joan Williams for patient recruitment and evaluation, David Luckenbaugh for statistical advice, and Paul Carlson, Alan Mallinger, Carlos Zarate, Meena Vythilingam and the 5SW Day Hospital nursing staff for medical support. This research was supported by the NIH NIMH-DIRP.
Footnotes
Clinical trials registration name “The Antidepressant Efficacy of the Anticholinergic Scopolamine”; NCT00369915; www.clinicaltrials.gov
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pacher P, Kecskemeti V. Trends in the development of new antidepressants. Is there a light at the end of the tunnel? Curr Med Chem. 2004;11:925–943. doi: 10.2174/0929867043455594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diagnostic and Statistical Manual of Mental Disorders, IV. American Psychiatric Association; 2000. [Google Scholar]
- 4.Khan A, Khan SR, Shankles EB, Polissar NL. Relative sensitivity of the Montgomery-Asberg Depression Rating Scale, the Hamilton Depression rating scale and the Clinical Global Impressions rating scale in antidepressant clinical trials. Int Clin Psychopharmacol. 2002;17:281–285. doi: 10.1097/00004850-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 6.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 7.McNair DM, Lorr M, Droppleman LF. EITS Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- 8.Nierenberg AA, DeCecco LM. Definitions of antidepressant treatment response, remission, nonresponse, partial response, and other relevant outcomes: a focus on treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl16):5–9. [PubMed] [Google Scholar]
- 9.Berger M, Riemann D, Hochli D, Spiegel R. The cholinergic rapid eye movement sleep induction test with RS-86. State or trait marker of depression? Arch Gen Psychiatry. 1989;46:421–428. doi: 10.1001/archpsyc.1989.01810050035006. [DOI] [PubMed] [Google Scholar]
- 10.Browne RG. Effects of antidepressants and anticholinergics in a mouse “behavioral despair” test. Eur J Pharmacol. 1979;58:331–334. doi: 10.1016/0014-2999(79)90483-7. [DOI] [PubMed] [Google Scholar]
- 11.Comings DE, Wu S, Rostamkhani M, McGue M, Iacono WG, MacMurray JP. Association of the muscarinic cholinergic 2 receptor (CHRM2) gene with major depression in women. American Journal of Medical Genetics. 2002;114:527–529. doi: 10.1002/ajmg.10406. [DOI] [PubMed] [Google Scholar]
- 12.Dilsaver SC. Pathophysiology of “cholinoceptor supersensitivity” in affective disorders. Biological Psychiatry. 1986;21:813–829. doi: 10.1016/0006-3223(86)90246-5. [DOI] [PubMed] [Google Scholar]
- 13.Gillin JC, Sutton L, Ruiz C, Darko D, Golshan S, Risch SC, et al. The effects of scopolamine on sleep and mood in depressed patients with a history of alcoholism and a normal comparison group. Biological Psychiatry. 1991;30:157–169. doi: 10.1016/0006-3223(91)90170-q. [DOI] [PubMed] [Google Scholar]
- 14.Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- 15.Janowsky DS, Overstreet DH. The role of acetylcholine mechanisms in mood disorders. New York: Raven Press; 1995. [Google Scholar]
- 16.Riemann D, Hohagen F, Krieger S, Gann H, Muller WE, Olbrich R, et al. Cholinergic REM induction test: muscarinic supersensitivity underlies polysomnographic findings in both depression and schizophrenia. J Psychiatr Res. 1994;28:195–210. doi: 10.1016/0022-3956(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 17.Risch SC, Kalin NH, Janowsky DS. Cholinergic challenges in affective illness: behavioral and neuroendocrine correlates. Journal of Clinical Psychopharmacology. 1981;1:186–192. doi: 10.1097/00004714-198107000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Wang JC, Hinrichs AL, Stock H, Budde J, Allen R, Bertelsen S, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum Mol Genet. 2004;13:1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- 19.Nestler EJHSE. Regulation of Gene Expression. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology The Fifth Generation of Progress. Philadelphia: Lippincott Williams & Wilkins; 2002. pp. 217–228. [Google Scholar]
- 20.Liu HF, Zhou WH, Xie XH, Cao JL, Gu J, Yang GD. Muscarinic receptors modulate the mRNA expression of NMDA receptors in brainstem and the release of glutamate in periaqueductal grey during morphine withdrawal in rats. Sheng Li Xue Bao. 2004;56:95–100. [PubMed] [Google Scholar]
- 21.Rami A, Ausmeir F, Winckler J, Krieglstein J. Differential effects of scopolamine on neuronal survival in ischemia and glutamate neurotoxicity: relationships to the excessive vulnerability of the dorsoseptal hippocampus. J Chem Neuroanat. 1997;13:201–208. doi: 10.1016/s0891-0618(97)00044-6. [DOI] [PubMed] [Google Scholar]
- 22.Krystal JH, Sanacora G, Blumberg H, Anand A, Charney DS, Marek G, et al. Glutamate and GABA systems as targets for novel antidepressant and mood-stabilizing treatments. Mol Psychiatry. 2002;7(Suppl 1):S71–80. doi: 10.1038/sj.mp.4001021. [DOI] [PubMed] [Google Scholar]
- 23.Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann N Y Acad Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- 24.Skolnick P, Layer RT, Popik P, Nowak G, Paul IA, Trullas R. Adaptation of N-methyl-D-aspartate (NMDA) receptors following antidepressant treatment: implications for the pharmacotherapy of depression. Pharmacopsychiatry. 1996;29:23–26. doi: 10.1055/s-2007-979537. [DOI] [PubMed] [Google Scholar]
- 25.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 26.Chen C, Hardy M, Zhang J, Lahoste GJ, Bazan NG. Altered NMDA receptor trafficking contributes to sleep deprivation-induced hippocampal synaptic and cognitive impairments. Biochem Biophys Res Commun. 2006;340:435–440. doi: 10.1016/j.bbrc.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 27.McDermott CM, Hardy MN, Bazan NG, Magee JC. Sleep deprivation-induced alterations in excitatory synaptic transmission in the CA1 region of the rat hippocampus. J Physiol. 2006;570:553–565. doi: 10.1113/jphysiol.2005.093781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raeder EA, Stys A, Cohen RJ. Effect of low-dose scopolamine on autonomic control of the heart. Ann Noninvasive Electrocardiol. 1997;2:236–241. doi: 10.1111/j.1542-474x.1997.tb00332.x. [DOI] [PubMed] [Google Scholar]
- 29.Sunderland T, Tariot PN, Cohen RM, Weingartner H, Mueller EA, 3rd, Murphy DL. Anticholinergic sensitivity in patients with dementia of the Alzheimer type and age-matched controls. A dose-response study. Arch Gen Psychiatry. 1987;44:418–426. doi: 10.1001/archpsyc.1987.01800170032006. [DOI] [PubMed] [Google Scholar]
- 30.Vitiello B, Martin A, Hill J, Mack C, Molchan S, Martinez R, et al. Cognitive and behavioral effects of cholinergic, dopaminergic, and serotonergic blockade in humans. Neuropsychopharmacology. 1997;16:15–24. doi: 10.1016/S0893-133X(96)00134-0. [DOI] [PubMed] [Google Scholar]
- 31.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67(Suppl 1):S29–33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 32.Udupa K, Sathyaprabha TN, Thirthalli J, Kishore KR, Raju TR, Gangadhar BN. Modulation of cardiac autonomic functions in patients with major depression treated with repetitive transcranial magnetic stimulation. J Affect Disord. 2007;104:231–236. doi: 10.1016/j.jad.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology. 2008;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newhouse PA, Sunderland T, Tariot PN, Weingartner H, Thompson K, Mellow AM, et al. The effects of acute scopolamine in geriatric depression. Archives of General Psychiatry. 1988;45:906–912. doi: 10.1001/archpsyc.1988.01800340028004. [DOI] [PubMed] [Google Scholar]
- 37.Ebert U, Grossmann M, Oertel R, Gramatte T, Kirch W. Pharmacokinetic-pharmacodynamic modeling of the electroencephalogram effects of scopolamine in healthy volunteers. J Clin Pharmacol. 2001;41:51–60. doi: 10.1177/00912700122009836. [DOI] [PubMed] [Google Scholar]
- 38.Bagby RM, Ryder AG, Cristi C. Psychosocial and clinical predictors of response to pharmacotherapy for depression. J Psychiatry Neurosci. 2002;27:250–257. [PMC free article] [PubMed] [Google Scholar]
- 39.Kupfer DJ, Spiker DG. Refractory depression: prediction of non-response by clinical indicators. J Clin Psychiatry. 1981;42:307–312. [PubMed] [Google Scholar]
- 40.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- 41.Klein DN, Schatzberg AF, McCullough JP, Dowling F, Goodman D, Howland RH, et al. Age of onset in chronic major depression: relation to demographic and clinical variables, family history, and treatment response. J Affect Disord. 1999;55:149–157. doi: 10.1016/s0165-0327(99)00020-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.