Abstract
Most US insurance companies require patients to participate in a medically supervised weight loss regimen prior to bariatric surgery. However, the utility of this requirement has not been documented. Data was collected from 94 bariatric surgery patients who were required, and 59 patients who were not required, by their insurance company to participate in a presurgical weight loss regimen. Weight change in the required group, as well as group differences in weight change, was examined from 3 and 6 months presurgery to 1 week presurgery, and from 1 week presurgery to 3 months postsurgery. Weight change presurgery was then used to predict weight loss postsurgery. In the 6 months prior to surgery, required patients gained 3.7 kg ± 5.9 (s.d.) (P < 0.0005), which did not differ from nonrequired patients. From surgery to 3 months postsurgery, required patients lost 23.6 ± 8 kg (P < 0.0005), also without differing from nonrequired patients. Patients who gained more weight prior to surgery, lost more weight postsurgery (P = 0.001), while controlling for initial weight. Findings suggest that the common weight loss regimen requirements of US insurance carriers were ineffective in producing presurgical weight loss in this sample. Most patients (>70%) in this sample gained weight prior to surgery, potentially taking advantage of final opportunities to overindulge in preferred foods. Required patients fared no better in terms of weight change postsurgically and, surprisingly, presurgical weight gain predicted better postsurgical weight loss outcome. Several potential explanations for this finding are offered.
INTRODUCTION
Clinically severe obesity is associated with numerous medical comorbidities (1) and is increasing in prevalence (2). Estimates of the yearly cost for the treatment and management of comorbidities associated with obesity have surpassed $100 billion in the US alone, representing 6% of direct health-care expenses (3). Currently, bariatric surgery represents the only treatment for clinically severely obese individuals that demonstrates long-term effectiveness (4,5). The National Institutes of Health Consensus Development Conference panel (1991) (6) recommended that bariatric surgery be considered for well-informed and motivated individuals who have BMI >40 kg/m2, or >35 kg/m2 with associated medical comorbidities (e.g., diabetes, hypertension), and acceptable operative risks. According to the American Society for Metabolic and Bariatric Surgery, 16,200 obesity surgeries were performed in 1992, which increased markedly to 140,640 in 2004, and to 177,000 in 2006 (7).
Presurgical weight loss requirements
The high cost of bariatric surgery ($20,000–$25,000) (8) makes self-pay untenable for most individuals, forcing candidates to rely on 3rd party payment (9). Most US insurance carriers cover the cost of obesity surgery but require candidates to participate in a 6-month, physician-supervised, weight loss regimen (9). However, neither actual weight loss, nor any specific diet/exercise prescription is required. Physicians are expected to monitor the patient’s diet, exercise, and weight each month. At the end of this 6-month period, the physicians must submit a letter to the 3rd party payer stating that the patient participated in a weight loss regimen, was unable to sustain significant (unspecified) weight loss using conventional methods, does not have any endocrine or metabolically correctable problem, and is recommended for surgery.
Although no study to date has evaluated the effectiveness of 3rd party payer requirements alone, several studies have examined various preoperative weight loss regimens (4,10–15). These range from entirely unstructured regimens (13,14) to well-structured programs carrying requirements beyond those imposed by insurance companies (4,11,12,15), and range in length from 2 to 52 weeks (4,11). Most (4,10–14), but not all (15) of these studies report modest weight loss prior to surgery in the majority of patients participating in presurgical weight loss programs. However, these findings are based on patients who either agreed to participate in a clinical trial or were required by the medical center to participate in a weight loss program, thereby limiting the generalizability of these findings with respect to the requirements of most insurance carriers utilized by bariatric patients in the US.
Relationship between changes in weight pre- and postsurgery
The effect of preoperative weight loss on postoperative outcomes remains inconclusive. Studies have reported a positive relationship between pre- and postoperative weight loss (14,16,17), no relationship (12,18,19), and two studies suggest that a presurgical weight loss regimen requirement may be detrimental to postsurgical weight loss outcome (15,20) Thus, there remains a need for data on the effectiveness of presurgical weight loss regimens required by US insurance carriers, and further investigation into the relationship between pre- and postsurgical weight change.
The primary aims of this study were to evaluate the effectiveness of the presurgical weight loss regimen requirement of most US insurance companies, as well as to determine whether presurgical weight change predicted short-term postsurgical weight loss outcome, in a sample of surgical patients carrying insurance that required completion of a presurgical weight loss regimen. In addition, this study was designed to compare group differences between individuals required, and not required, to participate in a preoperative weight loss regimen in both pre- and postsurgical weight change. The grouping variable was the presence or absence of the insurance requirement, regardless of treatment modality, which varied between individuals. Finally, the relationship between pre- and postsurgical weight change was examined across groups (in all patients).
METHODS AND PROCEDURES
Participants
Data were collected on 153 (125 women, 28 men) clinically severely obese individuals ranging in age from 18 to 65 (40 ± 11 (s.d.)) years, who received laparoscopic bariatric surgery (112 Roux-en-Y gastric bypass, 41 adjustable gastric banding) at the Center for Weight Loss Surgery, at St Luke’s Roosevelt Hospital Center in New York City, between January 2005 and April 2007. One week prior to surgery, BMI ranged from 36.9 to 86.8 (48.7 ± 7.8). Body weight and BMI at each assessment point are reported in Table 1. The ethnic breakdown of this sample was 49% Hispanic, 34% African American, 15% white, 1% other, and 1% missing. All patients met the criteria proposed by the National Institutes of Health Consensus Panel in 1991 (6).
Table 1.
Body weight and BMI at each assessment point
| Group | 6 Months presurgery | 3 Months presurgery | 1 Week presurgery | 3 Months postsurgery |
|---|---|---|---|---|
| Required | ||||
| Body weight (kg) | 129.4 ± 24.6 | 134.2 ± 25.9 | 132.9 ± 25.3 | 111.3 ± 26.0 |
| BMI (kg/m2) | 47.2 ± 7.4 | 49.2 ± 8.2 | 48.5 ± 7.7 | 40.3 ± 8.1 |
| n | 86 | 59 | 94 | 36 |
| Nonrequired | ||||
| Body weight (kg) | 129.5 ± 25.9 | 127.9 ± 19.3 | 132.7 ± 23.5 | 109.7 ± 20.4 |
| BMI (kg/m2) | 49.5 ± 11.8 | 47.2 ± 5.3 | 48.9 ± 8.1 | 40.1± 5.8 |
| n | 14 | 32 | 59 | 51 |
| Total | ||||
| Body weight (kg) | 129.4 ± 24.7 | 132.0 ± 23.9 | 132.8 ± 24.5 | 110.4 ± 22.7 |
| BMI (kg/m2) | 47.5 ± 8.1 | 48.5 ± 7.4 | 48.7 ± 7.8 | 40.2 ± 6.8 |
| n | 100 | 91 | 153 | 87 |
Data presented as mean ± s.d. Required and nonrequired groups did not differ in body weight or BMI at any time point (all P s > 0.25).
Required group
Ninety-four patients (78 women, 16 men) were mandated by their insurance carrier to participate in a 6-month, physician-supervised weight loss regimen preoperatively. Per their insurance companies, patients were required to have the supervising physician submit a record of the monthly body weight measurements but were not required to adhere to any specific dietary program or demonstrate actual weight loss. The degree of structure received by patients during this period was determined by the physician, and ranged from no dietary recommendations or reading materials to highly structured or commercial weight loss program prescriptions.
Nonrequired group
Fifty-nine patients (47 women, 12 men) were not required to participate in any weight loss regimen preoperatively, either by their insurance carriers or the Center for Weight Loss Surgery. Nonrequired patients (as are required patients) are informally encouraged to lose some weight prior to surgery by the surgeon during two routine presurgical office visits.
Design
Retrospective analyses of patient data were conducted utilizing a surgeon database and medical charts maintained at the Center for Weight Loss Surgery. Body weight was recorded at four time points: 6 months presurgery, 3 months presurgery, 1 week presurgery, and 3 months postsurgery. Patient records must have reflected weight measurements for at least two of these assessment points to be included in analyses. Patients received either Roux-en-Y gastric bypass (70 required, 42 nonrequired) or adjustable gastric banding (24 required, 17 nonrequired). Patients with significantly enlarged livers (sagittal diameter ≥20 cm), and patients requiring open (performed only as needed due to complications with laparoscopic procedure) or revision surgeries, were excluded. Approval for this study was granted by the St Luke’s Roosevelt Institutional Review Board.
Statistical analyses
Analyses were performed from 6 months presurgery to 1 week presurgery, 3 months presurgery to 1 week presurgery, and from 1 week presurgery to 3 months postsurgery in order to first identify changes in required patients, then to compare changes between required and nonrequired patients. Three months Pre to 1 week presurgery data were analyzed in order to confirm 6 months presurgery to 1 week presurgery weight change results, as the availability of 6 months presurgery body weight data were limited in nonrequired patients. Repeated-measure ANOVAs were first used to examine changes in body weight and percent (%) of initial body weight (body weight at the earliest time point included in each set of analyses) in individuals required to participate in the presurgical weight loss regimen.
In-between group (required vs. nonrequired) analyses, univariate ANOVAs (for continuous variables) and χ2-tests (for dichotomous variables) were first used to identify any differences in age, sex, surgeon, type of surgery, body weight, or BMI between groups at 6 months presurgery, 3 months presurgery, and 1 week presurgery. Mixed model ANOVAs were then used to examine group differences in changes in body weight, and % of initial body weight, with time as the within subjects factor and group as the between subjects factor. Type of surgery was entered as a covariate in analyses of postsurgical outcome, given expected differences in weight loss outcomes between Roux-en-Y gastric bypass and adjustable gastric banding (21). All analyses were repeated controlling for age, sex, and surgeon. Post hoc χ2-tests were used to compare the number of individuals who gained, vs. lost, weight presurgically between groups and ANCOVAs (both with and without relevant control variables) were used to determine whether there was any difference in postoperative weight loss between individuals who gained vs. lost weight preoperatively. Finally, linear regression analyses were used to test whether postsurgical changes in body weight could be predicted by weight change from 6 months presurgery, or 3 months presurgery, to 1 week presurgery. Regression analyses were also repeated controlling for initial body weight, age, sex, and surgeon.
RESULTS
Weight change from 6 months presurgery to 1 week presurgery
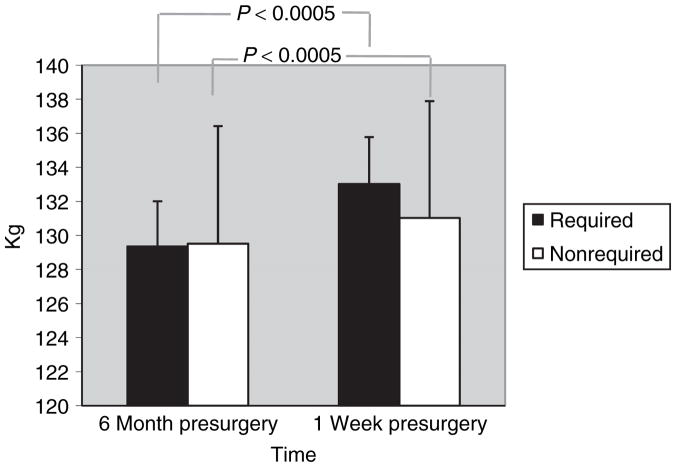
The required group gained 3.7 ± 5.9 kg (F(1,85) = 33.1, P < 0.0005) and 2.9 ± 4.4 % of initial body weight (F(1,85) = 37.1, P < 0.0005). In all patients, there was a 3.4 ± 5.8 kg increase in body weight (F(1,98) = 9.6, P = 0.003), which did not differ between groups (non significant (NS), P = 0.20). Body weight in each group are shown in Figure 1. Similarly, in all patients, there was a 2.7 ± 4.4 % increase in initial body weight (F(1,98) = 11.3, P = 0.001), which did not differ between groups (NS, P = 0.21). At 6 months presurgery, required and nonrequired groups did not differ in age, sex, surgeon, type of surgery, body weight, or BMI (NS, all Ps > 0.3), and no change in results was found when controlling for age, sex, and surgeon. Of 100 (86 required; 14 nonrequired) participants for which 6 months presurgery and 1 week presurgery weights were available, 22% (22% required; 21% nonrequired) lost weight and 78% (78% required; 79% nonrequired) gained weight, which did not differ between groups (NS, P = 0.99). Changes in body weight are reported in Table 2.
Figure 1.
Body weight at 6 months presurgery and 1 week presurgery. From 6 months presurgery to 1 week presurgery, there was a 3.4 ± 5.8 kg increase in body weight (P = 0.003). Although the required group gained more than twice the weight (3.6 ± 5.9 kg) than did the nonrequired group (1.5 ± 5.1 kg), this difference failed to reach significance (NS, P = 0.20).
Table 2.
Changes in body weight and percent of initial body weight during various intervals
| Group | 6 Months presurgery | 3 Months presurgery | 3 Months postsurgery |
|---|---|---|---|
| Required | |||
| Body weight (kg) | 3.7 ± 5.9** | 1.6 ± 4.9* | −25.8 ± 7.3** |
| % Initial weight | 2.9 ± 4.4** | 1.1 ± 3.2** | −19.0 ± 4.1** |
| n | 86 | 59 | 36 |
| Nonrequired | |||
| Body weight (kg) | 1.5 ± 5.1 | 1.9 ± 3.6** | −21.9 ± 8.0** |
| % Initial weight | 1.3 ± 3.8 | 1.6 ± 2.6** | −16.7 ± 5.3** |
| n | 14 | 32 | 51 |
| Total | |||
| Body weight (kg) | 3.4 ± 5.8** | 1.7 ± 4.5** | −23.6 ± 7.9** |
| % Initial weight | 2.7 ± 4.4** | 1.3 ±3.0** | −17.6 ± 5.0** |
| n | 100 | 91 | 87 |
Data presented as mean ± s.d.
Significant change during interval at P < 0.05.
Significant change during interval at P < 0.01. Change in weight, or % initial weight, did not differ between groups across any measurement point (all Ps ≥ 0.2).
Weight change from 3 months presurgery to 1 week presurgery
The required group gained 1.6 ± 4.9 kg (F(1,58) = 5.9, P = 0.018) and 1.1 ± 3.2% of initial body weight (F(1,58) = 7.2, P = 0.01). In all patients, there was a 1.7 ± 4.5 kg increase in body weight (F(1,89) = 12.2, P = 0.001), which did not differ between group (NS, P = 0.73). Similarly, in all patients, there was a 1.3 ± 3.0% increase in initial body weight (F(1,89) = 16.2, P < 0.0005), which did not differ between groups (NS, P = 0.52). At 3 months presurgery, groups did not differ in age, sex, surgeon, type of surgery, body weight or BMI (all Ps > 0.2). When controlling for age, sex, and surgeon, there was no change in percentage weight change results; however, changes in body weight became only marginally significant (F(1,89) = 3.5, P = 0.066). Of 91 (59 required; 32 nonrequired) participants for which 3 months presurgery and 1 week presurgery weights were available, 30% (35% required; 22% nonrequired) lost weight and 70% (66% required; 78% nonrequired) gained weight, which did not differ between groups (NS, P = 0.25; see Table 2).
Weight change from 1 week presurgery to 3 months postsurgery
The required group lost 25.8 ± 7.3 kg and 19.0 ± 4.1 % of initial (1 week presurgery) body weight (F(1,35) = 440.4, P < 0.0005 and F(1,35) = 758.8, P < 0.0005, respectively). In all patients, there was a 23.6 ± 7.9 kg decrease in body weight (F(1,85) = 236.6, P < 0.0005), which did not differ between groups (NS, P = 0.39). Similarly, across groups, there was a 17.6 ± 5.0 % decrease in initial body weight (F(1,85) = 329.7, P < 0.0005), which did not differ between groups (NS, P = 0.56). At 1 week presurgery, groups did not differ in age, sex, surgeon, type of surgery, body weight or BMI (NS, all Ps > 0.5), and no change in results was found when controlling for age, sex, and surgeon (see Table 2). Individuals who gained weight preoperatively did not differ from individuals who lost weight preoperatively in age, sex, type of surgery, or weight or BMI at 6 months presurgery, 3 months presurgery, 1 week presurgery, or 3 months postsurgery (all Ps > 0.1). In addition, no difference was found in postsurgical weight change (absolute or % of initial) with or without controlling for age, sex, surgeon, type of surgery, body weight, or BMI (NS, all Ps > 0.55).
Postsurgical weight loss outcome in relation to presurgical weight change
In the required group, changes in body weight from 6 months presurgery, and 3 months presurgery, to 1 week presurgery inversely predicted changes in body weight from 1 week presurgery to 3 months postsurgery (t(31) = −3.9, P = 0.001 and t(21) = −3.6, P = 0.002, respectively), indicating that required individuals gaining more weight presurgically lost more weight from 1 week presurgery to 3 months postsurgery. Similarly, across groups, changes in body weight from 6 months presurgery, and 3 months presurgery, to 1 week presurgery inversely predicted changes in body weight from 1 week presurgery to 3 months postsurgery (t(41) = −4.1, P < 0.0005 and t(49) = −2.8, P = 0.008, respectively), indicating that across all participants, those who gained more weight presurgically lost more weight at 3 months postsurgery. Controlling for initial body weight, age, sex, and surgeon did not affect results.
DISCUSSION
Presurgical weight loss regimens and weight change
With an increasing number of bariatric procedures being performed (7), more attention is being paid to presurgical requirements of surgery centers and, particularly, of insurance companies (13). The American Society for Metabolic and Bariatric Surgery has not yet made recommendations regarding preoperative weight loss, potentially due to conflicting and insufficient data (5). The only available randomized clinical trial demonstrates the efficacy of a presurgical weight loss (10% of initial) requirement in reducing body weight both pre- and postsurgery (13); however, the generalizability of these findings is limited in that actual weight loss is typically not required for surgery or insurance coverage of surgery. Two studies (4,14) have evaluated the effectiveness of surgery center imposed weight loss regimens, demonstrating weight gain in some patients, but moderate (5–10% excess) weight loss in most. Conversely, two additional studies (15,22) have shown weight gain in the majority of patients prior to surgery. However, the first of these (15) fails to specify whether the presurgical weight gain was significant and neither differentiates between required and nonrequired patients. The present study is the first to investigate the effectiveness of the presurgical weight loss regimen requirements held by the majority of US insurance carriers (9).
Results from this study suggest that 3rd party payer presurgical requirements, which often do not specify any particular mode(s) of treatment, may be ineffective in promoting weight loss prior to surgery. This finding may be partially explained by patient knowledge that coverage for surgery is not contingent upon actual weight loss. Patients in this study gained an average of 3.4 kg in the 6 months prior to surgery, without differing between individuals required and not required to participate in a weight loss regimen. This rate of weight gain far surpasses the 0.5–1 kg per year average weight gain currently reported in the US, even within an obese population (23).
The significant increase in body weight in the months preceding bariatric surgery suggests that patients may have repeatedly consumed large quantities of food with the knowledge they would no longer be able to do so following surgery. Research on eating behavior prior to anticipated caloric deprivation suggests that increases in body weight may be more representative of weight change prior to a weight loss diet or surgery in the free-living environment, dubbed the “last supper” phenomenon (24). Further, the presurgical weight loss regimen requirement itself was not related to short-term weight loss outcome following surgery in this sample. Presurgical weight change, however, was related to postsurgical weight loss outcome.
Relationship between weight change pre- and postsurgery
The majority of investigations of the association between pre- and postsurgical weight change show no relationship (12,18,19,25–27), including one (12) reporting no differences in weight loss outcome between presurgery dieters (losing an average of 17 kg) and nondieters (no significant weight loss) across a 4-year follow-up. However, other investigations have found a positive relationship (i.e., increased weight loss presurgery related to increased weight loss postsurgery (14,17)), a positive relationship under certain circumstances but no relationship under other circumstances (e.g., positive relationship when presurgery weight loss exceeded 10% EBW, but no relationship with a presurgery weight loss of 5–10% EBW (4,13,28,29)), and an inverse relationship (20). In addition, one study (15) demonstrated that patients with a stringent presurgical weight loss program requirement lost less weight (%EBW) at 1 year as compared to patiens without this requirement, despite the fact that required patients did not lose weight preoperatively. Findings in this study indicate no difference in postsurgical weight loss between patients who lost vs. gained weight preoperatively and, in all patients, an inverse relationship between weight change pre- and postobesity surgery. Irrespective of initial starting weight or presurgical weight loss regimen requirement, individuals gaining the most weight presurgically lost the most weight postsurgery in this sample.
Although speculative, we can suggest several potential explanations for this finding. If individuals seek bariatric surgery at, or close to, their highest lifetime weight (30), overeating in the months prior to surgery may elevate body weight beyond its biological set point (31). Accordingly, these patients may, in addition to surgery, experience an increased physiological drive to decrease body weight (31). Diet fatigue may also have influenced results. At 3 months postsurgery, individuals who lost weight prior to surgery had been restricting their intake for 6–9 months (vs. only 3 months in individuals gaining weight presurgically) and may have been less likely to comply with postsurgical dietary prescriptions. In addition, up to 50% of individuals seeking bariatric surgery report some form of binge eating (32). Such individuals may gain more weight prior to surgery, and lose more weight following surgery, due to the presence and absence (respectively) of objective binge episodes (33). Finally, a small number of patients admitted to intentionally avoiding weight loss prior to surgery for fear that they might be denied for surgery by their insurance company if they demonstrated the ability to lose weight through traditional (nonsurgical) methods.
Presurgical weight loss regimen requirement
The impetus for requiring a presurgical weight loss regimen remains a point of debate among researchers and clinicians. On the one hand, this requirement may confirm the inability of surgical candidates to lose weight by conventional treatment methods (30). On the other hand, this requirement may test the candidate’s motivation (13), with some weight loss potentially demonstrating the individual’s ability to adhere to diet prescriptions considered essential for successful outcome following surgery (14). Websites of the largest insurance carriers state that the requirement is imposed in order to “minimize potential morbidity and mortality” (34), “improve surgical outcomes” (35) and “reduce the potential for postsurgical complications” (35). Although some evidence suggests that preoperative weight loss may result in shorter operative times and fewer complications (4,14), data in this study suggest that presurgical weight loss may be related to less postsurgical weight loss. What remains clear is that further research is needed in this area in order to replicate findings in this study and suggest a definitive course of action in terms of presurgical requirements for bariatric patients.
The limitations of the study include the unavailability of data on socio-economic status, medical complications, or comorbidities (physical or psychological). Postsurgical weight loss data beyond 3 months was insufficient for analyses; however, several studies have reported comparable degrees of weight loss (absolute and % initial) at much longer postsurgical intervals (36,37). Although retrospective, one of the advantages of the design of this study is the external validity achieved through the evaluation of real-world practice. Consistent with Jamal et al. (15) and de Witt Hamer & Tuinebreijer (22), results in this study may better represent actual weight changes seen prior to surgery than those reported in manipulated trials. Thus, the strength of this report lies in its generalizability, being based on the most commonly utilized pre bariatric surgery requirements in the US today. Findings from this study at one large hospital center suggest that the 6-month presurgical physician-supervised weight loss regimen requirement held by most insurance companies in the US may be ineffective in reducing body weight prior to surgery, and raise questions about the perceived postsurgical benefits of presurgical weight loss. Further studies with a similar degree of external validity from other surgical centers are needed.
Acknowledgments
We thank Katie Bauer, Heidi Kujac, and Keely Elgethun for their assistance with data collection and reference formatting.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
References
- 1.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119:655–660. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Bond DS, Evans RK, DeMaria EJ, et al. A conceptual application of health behavior theory in the design and implementation of a successful surgical weight loss program. Obes Surg. 2004;14:849–856. doi: 10.1381/0960892041590917. [DOI] [PubMed] [Google Scholar]
- 4.Still CD, Benotti P, Wood GC, et al. Outcomes of preoperative weight loss in high-risk patients undergoing gastric bypass surgery. Arch Surg. 2007;142:994–8. doi: 10.1001/archsurg.142.10.994. discussion 999. [DOI] [PubMed] [Google Scholar]
- 5.Buchwald H, Williams SE. Bariatric surgery worldwide 2003. Obes Surg. 2004;14:1157–1164. doi: 10.1381/0960892042387057. [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health. Gastrointestinal surgery for severe obesity consensus statement. NIH Consensus Development Conference. 1991;9:1–20. [PubMed] [Google Scholar]
- 7.American Society for Metabolic and Bariatric Surgery. [Accessed 10 March 2008.];Laparoscopic adjustable gastric banding. 2007 doi: 10.1016/j.soard.2005.02.018. < http://www.asbs.org/html/patients/banding.html>. [DOI] [PubMed]
- 8.National Institutes of Health. Bariatric surgery for obesity. NIH Publication. 2008;08(4006):1–6. [Google Scholar]
- 9.Frezza EE. Six steps to fast-track insurance approval for bariatric surgery. Obes Surg. 2006;16:659–663. doi: 10.1381/096089206776945129. [DOI] [PubMed] [Google Scholar]
- 10.Liu RC, Sabnis AA, Forsyth C, Chand B. The effects of acute preoperative weight loss on laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2005;15:1396–1402. doi: 10.1381/096089205774859155. [DOI] [PubMed] [Google Scholar]
- 11.Fris RJ. Preoperative low energy diet diminishes liver size. Obes Surg. 2004;14:1165–1170. doi: 10.1381/0960892042386977. [DOI] [PubMed] [Google Scholar]
- 12.Martin LF, Tan TL, Holmes PA, et al. Can morbidly obese patients safely lose weight preoperatively? Am J Surg. 1995;169:245–253. doi: 10.1016/s0002-9610(99)80145-7. [DOI] [PubMed] [Google Scholar]
- 13.Alami RS, Morton JM, Schuster R, et al. Is there a benefit to preoperative weight loss in gastric bypass patients? A prospective randomized trial. Surg Obes Relat Dis. 2007;3:141–5. doi: 10.1016/j.soard.2006.11.006. discussion 145. [DOI] [PubMed] [Google Scholar]
- 14.Alvarado R, Alami RS, Hsu G, et al. The impact of preoperative weight loss in patients undergoing laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2005;15:1282–1286. doi: 10.1381/096089205774512429. [DOI] [PubMed] [Google Scholar]
- 15.Jamal MK, DeMaria EJ, Johnson JM, et al. Insurance-mandated preoperative dietary counseling does not improve outcome and increases dropout rates in patients considering gastric bypass surgery for morbid obesity. Surg Obes Relat Dis. 2006;2:122–127. doi: 10.1016/j.soard.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Alger-Mayer S, Polimeni JM, Malone M. Preoperative weight loss as a predictor of long-term success following Roux-en-Y gastric bypass. Obes Surg. 2008;18:772–775. doi: 10.1007/s11695-008-9482-2. [DOI] [PubMed] [Google Scholar]
- 17.Ali MR, Baucom-Pro S, Broderick-Villa GA, et al. Weight loss before gastric bypass: feasibility and effect on postoperative weight loss and weight loss maintenance. Surg Obes Relat Dis. 2007;3:515–520. doi: 10.1016/j.soard.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Taylor EL, Chiasson PM, Perey BJ. Predicting Bariatric Surgical Outcomes: Does Preoperative Weight Gain Correlate with Lesser Postoperative Weight Loss? Obes Surg. 1995;5:375–377. doi: 10.1381/096089295765557421. [DOI] [PubMed] [Google Scholar]
- 19.Carlin AM, O’Connor EA, Genaw JA, Kawar S. Preoperative weight loss is not a predictor of postoperative weight loss after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2008;4:481–485. doi: 10.1016/j.soard.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Riess KP, Baker MT, Lambert PJ, Mathiason MA, Kothari SN. Effect of preoperative weight loss on laproscopic gastic bypass outcomes. Surg Obes Relat Dis. 2008;4:704–708. doi: 10.1016/j.soard.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–4231. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
- 22.de Witt Hamer PC, Tuinebreijer WE. Preoperative weight gain in bariatric surgery. Obes Surg. 1998;8:300–301. doi: 10.1381/096089298765554511. [DOI] [PubMed] [Google Scholar]
- 23.Brown WJ, Williams L, Ford JH, Ball K, Dobson AJ. Identifying the energy gap: magnitude and determinants of 5-year weight gain in midage women. Obes Res. 2005;13:1431–1441. doi: 10.1038/oby.2005.173. [DOI] [PubMed] [Google Scholar]
- 24.Eldredge KL, Agras WS, Arnow B. The last supper: emotional determinants of pretreatment weight fluctuation in obese binge eaters. Int J Eat Disord. 1994;16:83–88. doi: 10.1002/1098-108x(199407)16:1<83::aid-eat2260160109>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 25.Jantz EJ, Larson CJ, Mathison MA, Kallies KJ, Kothari SN. Number of weight loss attempts and maximum weight loss before Roux-en-Y laproscopic gastric bypass surgery are not predictive of postoperative weight loss. Surg Obes Relat Dis. 2009;5:208–211. doi: 10.1016/j.soard.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Fujioka K, Yan E, Wang He-Jing, Li Z. Evaluating preoperative weight loss, binge eating disorder, and sexual abuse history on Rouz-en-Y gastric bypass outcome. Surg Obes Relat Dis. 2008;4:137–143. doi: 10.1016/j.soard.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Harnisch MC, Portenier DD, Pryor AD. Preoperative gain does not predict failure of weight loss or co-morbidity resolution of laparoscopic Roux-en-Y gastric bypass for morbid obesity. Surg Obes Relat Dis. 2008;4:445–450. doi: 10.1016/j.soard.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Mrad BA, Stoklossa CJ, Birch DW. Does preoperative weight loss predict success following surgery for morbid obesity? Am J Surg. 2008;195:570–3. doi: 10.1016/j.amjsurg.2007.12.043. discussion 573. [DOI] [PubMed] [Google Scholar]
- 29.Solomon H, Liu GY, Alami R, Morton J, Curet MJ. Benefits to patients choosing preoperative weight loss in gastric bypass surgery: new results of a randomized trial. J Am Coll Surg. 2009;208:241–245. doi: 10.1016/j.jamcollsurg.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 30.Gibbons LM, Sarwer DB, Crerand CE, et al. Previous weight loss experiences of bariatric surgery candidates: how much have patients dieted prior to surgery? Surg Obes Relat Dis. 2006;2:159–164. doi: 10.1016/j.soard.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Nisbett RE. Hunger, obesity, and the ventromedial hypothalamus. Psychol Rev. 1972;79:433–453. doi: 10.1037/h0033519. [DOI] [PubMed] [Google Scholar]
- 32.Bocchieri-Ricciardi LE, Chen EY, Munoz D, et al. Pre-surgery binge eating status: effect on eating behavior and weight outcome after gastric bypass. Obes Surg. 2006;16:1198–1204. doi: 10.1381/096089206778392194. [DOI] [PubMed] [Google Scholar]
- 33.Kalarchian MA, Wilson GT, Brolin RE, Bradley L. Effects of bariatric surgery on binge eating and related psychopathology. Eat Weight Disord. 1999;4:1–5. doi: 10.1007/BF03376581. [DOI] [PubMed] [Google Scholar]
- 34.Empire Blue Cross Blue Shield. Surgery for Clinically Severe Obesity. Medical Policy. Available online at: http://www.empireblue.com/provider/noapplication/f2/s5/t9/pw_ad080419.pdf.
- 35.Aetna. Clinical Policy Bulletin: Obesity Surgery. Available at http://www.aetna.com/cpb/medical/data/100_199/0157.html.
- 36.Sjostrom L, Lindroos AK, Peltonen M, et al. Lifestyle, Diabetes, and Cardiovascural Risk Factors 10y after Bariatric Surgery. New Eng J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 37.Torgerson JS, Sjostrom L. The Sweedish Obese Subjects (SOS) study-rationale and results. Int J Obes. 2001;25:S2–S4. doi: 10.1038/sj.ijo.0801687. [DOI] [PubMed] [Google Scholar]