Abstract
Purpose of review
The present review develops a framework from which to understand the role of the cholinergic system in healthy cognition and in cognitive dysfunction. Traditionally, the cholinergic system has been thought to have direct influence on cognitive processes such as working memory and attention. Although the influence of cholinergic function on stimulus processing has been long appreciated, the notion that cholinergic effects on stimulus processing is the mechanism by which acetylcholine influences cognitive processes has only more recently been considered.
Recent findings
Literature supporting the hypothesis that cholinergic modulation influences cognitive functions through stimulus processing mechanisms has been growing for over a decade. Recent conceptualizations of the developing literature have argued for a new interpretation to an old and developing literature.
Summary
The argument that cholinergic function modulates cognitive processes by direct effects on basic stimulus processing extends to cognitive dysfunction in neuropathological conditions including dementia and mood disorders. Memory and attention deficits observed in these and other conditions can be understood by evaluating the impact of cholinergic dysfunction on stimulus processing, rather than on the cognitive function in general.
Keywords: acetylcholine, cognition, stimulus processing
Introduction
Acetylcholine was the first neurotransmitter identified in the human body and, thus, was the first to undergo major study. The culmination of decades of work point primarily to the role of acetylcholine in stimulus processing and memory/attention, and current interests include efforts to understand how the dysfunction of this system contributes to conditions and syndromes that implicate these basic cognitive functions. The goal of this review is to offer a cognitive framework based on our understanding of basic cholinergic function from which to better understand cholinergic dysfunction and cognition in human illness.
Acetylcholine and cognition
Cells that constitute the cholinergic neurotransmitter system originate primarily in the basal forebrain. These cells provide extensive projections throughout the cortex and, thus, by design have widespread effects on information processing. Cholinergic neuromodulation is known to influence multiple cognitive processes, including memory and attention [1,2]. In rodents and in nonhuman primates, lesions of the cholinergic nucleus basalis of Meynert (nBM) [3] result in impaired performance on learning and memory tasks. Blockade of the cholinergic muscarinic receptors by the antagonist scopolamine results in impaired performance on learning and memory tasks [4,5,6••,7•,8] and on tasks of attention [1,9], whereas enhancing cholinergic function improves memory and attention [10]. In healthy humans, scopolamine produces a transitory impairment of a wide range of memory and attention functions [11–14]. In contrast, several drugs that enhance cholinergic neuromodulation improve performance on short-term memory tasks both in animals and in humans [13,15–17] and can reverse the memory deficits created by nBM lesions [3]. Historically, the literature has identified acetylcholine as related to cognition and cognitive processing. A closer look at the literature, however, argues that cholinergic effects on cognitive functions, such as working memory and attention, occur specifically as a result of direct effects on stimulus processing mechanisms.
Stimulus processing
The literature is rich with evidence of the involvement of the cholinergic system in memory and attention mechanisms [1,18–24]. Researchers have hypothesized that attentional processes are mediated through cholinergic mechanisms that facilitate the processing of sensory information [13,24,25] and evidence exists to support this idea [13,26,27].
In general, the cholinergic neurons of the basal forebrain that project throughout neocortex are thought to enhance signal-to-noise ratios (S/N) for neural sensory processing [24,28,29]. Early studies by Sillito and Kemp [27] and Sato et al. [28] demonstrated that the direct application of acetylcholine to cat visual cortex increased the selectivity of the cells, response to stimulus orientation, by enhancing response selectivity and increasing response magnitude, consistent with the hypothesis that acetylcholine modulates S/N. Similarly, Buzsaki [30] showed that cholinergic input to hippocampus is inhibitory, suggesting that acetylcholine may enhance S/N in hippocampus by reducing the response to noise. More recently, comprehensive animal work by Sarter et al. [24] and Hasselmo and Sarter [31] provides convincing evidence that the role of the cholinergic system in sensory information processing that leads to stimulus or cue detection, via S/N modulation, is central to cognitive functioning.
Human studies using functional brain imaging techniques designed to evaluate the effects of cholinergic modulation on cognitive functions such as working memory and attention have identified changes in neural activity that are consistent with modulation of stimulus processing [13,32]. Direct modulation of S/N may constitute the neural mechanism through which the cholinergic system may establish the relative strengths of the neural representations of stimuli. For example, a functional imaging study [26] demonstrated that enhanced cholinergic activity selectively increased neural responses to task-relevant stimuli (i.e. signal) with reduced or no change in neural responses to task-irrelevant stimuli (i.e. noise), consistent with a selective enhancement for target stimuli via S/N processing. These findings showing stimulus-specific effects of cholinergic modulation are consistent with the hypothesis that cholinergic activity influences cognitive processes by influences on stimulus processing mechanisms.
Working memory
Working memory denotes a cognitive process that temporarily maintains an active representation of information for further processing or recall [33,34]. The cholinergic system strongly modulates working memory, whereby enhancing cholinergic activity improves working memory [35–38] and blocking normal cholinergic function impairs working memory performance [5,8,39]. Functional brain imaging studies have facilitated the understanding of neural mechanisms that underlie cholinergic effects on cognitive function [6••,9–15].
Functional brain imaging studies have demonstrated that increases in cholinergic activity preferentially enhance neural responses [26,40,41] and blocking cholinergic function reduces neural responses selectively during stimulus task encoding processes [42,43]. In a series of studies with PET and functional magnetic resonance imaging (fMRI), enhancing cholinergic function with an anticholinesterase modulated neural response to a working memory task across brain regions [17,26,36–38] but the only regions consistently found to show an increase in neural activity included visual processing areas, consistent with the idea that cholinergic function directly influences stimulus processing given the visual nature of the stimuli. Moreover, an fMRI study that evaluated each working memory component (i.e. encoding, maintenance, recognition) demonstrated increases in neural response selectively to task-relevant stimuli in ventral visual cortical regions, particularly during stimulus encoding [26]. Others also have reported selective effects during the encoding phase of working memory [32,40,41], a finding that is consistent with the hypothesis that improvement in working memory following cholinergic enhancement is mediated by influences on stimulus processing. Early on, these findings were considered paradoxical [36] in that enhanced working memory function was expected to be associated with enhancement of classic working memory regions in prefrontal cortex. The absence of enhanced prefrontal cortical function as demonstrated with functional imaging, together with the isolated enhancement of function in stimulus processing regions, pointed to a cholinergically mediated modulation of basic stimulus processing mechanisms in the context of a working memory task.
Selective attention
Selective attention constitutes the ability to discriminate significant or relevant stimuli from irrelevant stimuli (i.e. noise) and to process information preferentially [44–47]. The presentation of multiple stimuli simultaneously produces a competition for neural representation [44,48]. Single-unit recording studies [44,49,50] and functional brain imaging studies [48,51,52] have demonstrated that the processing of a visual stimulus is influenced by the presence of other, unattended visual stimuli. Two mechanisms that each contribute to the biasing of attention, including ‘bottom-up’ and ‘top-down’ processes [44,53], are thought to resolve the competition among stimuli. Bottom-up stimulus-based mechanisms refer to neural processing that is biased toward stimuli with inherently salient or meaningful features (i.e. stimuli that retain sensory salience, or that hold biological relevance) [54–57]. Top-down mechanisms refer to knowledge-based processes in which attention is oriented intentionally, resulting in the enhancement of neural representations of relevant, goal-directed stimuli [58,59]. The interaction of bottom-up and top-down mechanisms [58,60,61] produces a biased neural representation of the stimuli. Both bottom-up and top-down processing mechanisms are mediated through the cholinergic system and, thus, selective attention reflects the combined influence of cholinergic processing effects via these mechanisms (reviewed by [24]).
In a behavioral study of selective attention, in which two stimuli were presented simultaneously (face and house) and, thus, competed for representation, the cholinergic system was both enhanced using physostigmine and blocked using scopolamine [13]. Participants were instructed to attend to one stimulus component (face or house) and perform a matching task while ignoring the other stimlus. The expectation was that a processing bias toward one stimulus type over another would be reflected in performance measures and would be altered by cholinergic manipulation. For example, a face holds more innate salience and will be easier to attend to (while ignoring the house) and harder to ignore (when attending to the house). The results showed that the effects on performance measures were selective to the attention/target stimulus condition both during cholinergic enhancement and impairment, indicating that the effects of cholinergic modulation are stimulus-dependent. Specifically, enhancing cholinergic activity resulted in a selective reduction in reaction time when attending to houses (thus reducing the baseline bias toward faces) and impairing cholinergic activity increased reaction time selectively when attending to faces (also reducing the bias toward faces). The findings are consistent with the hypothesis that the behavioral outcome reflects the resolution of stimulus interactions via top-down and bottom-up mechanisms, and that the effects are driven by stimulus properties that contribute to the resolution of this competition.
Cognitive and cholinergic dysfunction
The extent to which cognitive impairment is explained by cholinergic dysfunction in neurological and psychiatric conditions remains empirical. As dysregulation of any single neurotransmitter system results in cascading effects throughout other transmitter systems, attributing behavioral deficits to single transmitter systems is challenging. Nonetheless, several neurological and psychiatric conditions that have hallmark neurocognitive features and implicate the cholinergic system will be discussed.
Acetylcholine and aging
Aging is associated with anatomical, chemical, and functional changes in the brain [62,63], the most prominent of which are alterations in the cholinergic system. These changes include decreases in basal forebrain cholinergic neurons, cholinergic receptors, and afferent projections to cortex [64–66]. Although their significance has been questioned [63,67], these age-associated changes have led to the cholinergic hypothesis of aging, which suggests cholinergic alterations contribute to the deficits in working memory, attention, and other cognitive functions observed during aging [25,62,68,69•]. The pharmacological potentiation of cholinergic neurotransmission improves performance on cognitive tasks in the elderly [37,38,70], and chronic treatment with drugs that enhance the cholinergic function is used to ameliorate cognitive dysfunction in the elderly [71,72].
The study of working memory in aging [73,74] indicates that the elderly recruit brain regions (including prefrontal cortical regions) that are not recruited in younger individuals performing the same working memory task, a finding that is thought to reflect compensatory mechanisms that accompany aging [75]. In a simple delayed-match-to-sample working memory task used in groups of young and healthy elderly individuals, cholinergic enhancement reduced activity in the prefrontal regions that had been selectively recruited during working memory in each age group. Notably, visual cortical areas were the only brain regions to show increases in activity during cholinergic enhancement and working memory in both groups, and this increase was larger in older participants [37]. In a similar working memory study with a group of young and a group of older healthy participants, task delay was modulated to increase task difficulty. Again we observed reduced prefrontal activity across levels of task difficulty during cholinergic enhancement, and visual processing areas showed increases in activity [38]. Moreover, elderly participants recruited more extended visual processing areas than did younger participants, but both groups showed increased neural activity exclusively in visual processing areas. Together, these findings suggest that even in the presence of changes in the cholinergic system and compensatory functional changes in aging, cholinergically mediated improvements in working memory performance appear to be associated with augmentation of stimulus processing mechanisms.
A recent paper evaluating aging and memory postulates a specific neural mechanism responsible for memory impairment. Specifically, young and older adults showed reduced activity in brain regions important for encoding when encoding was unsuccessful, but older individuals also showed increased activity in regions that likely mediate distraction. This argument is consistent with changes in S/N processing, with an increase in noise overwhelming the signal, and thus fits well with the hypothesis that cognitive dysfunction in aging is associated with changes in stimulus-based processing mechanisms.
Alzheimer’s disease
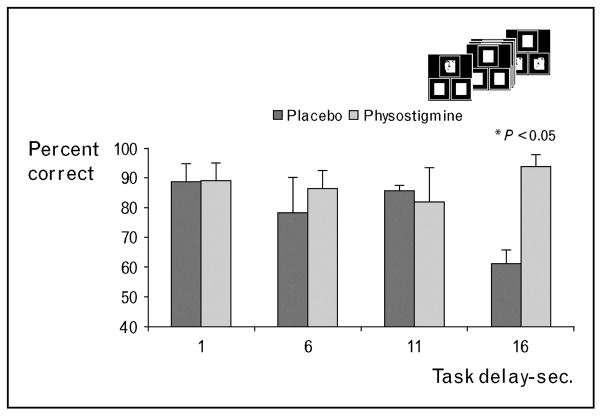
Alzheimer’s disease implicates many neurotransmitter systems and includes widespread cortical damage, but the cholinergic system is the most prominently implicated system. Modulation of cholinergic activity improves cognitive impairments [76•] and affects task-specific neural activity in Alzheimer’s disease [77]. Although cognitive function is impaired across multiple domains, working memory shows deficits early in the disease process [78]. In another working memory study, a task that included the variation in task difficulty was administered to patients with early Alzheimer’s disease to evaluate the impact of enhancing cholinergic function on task performance. In a group of five patients, significant improvement in performance accuracy was observed in the longer working memory delay conditions under cholinergic enhancement as compared to placebo (Fig. 1; unpublished finding), with accuracy increasing from approximately 62% during the longest working memory delay under placebo, to approximately 95% under physostigmine. On the basis of the results reported from both healthy young and healthy older participants discussed above, we can argue that the improvement in task performance in patients suffering from Alzheimer’s disease likely results from the enhancement of stimulus processing in early visual areas, and by extension the working memory impairment may be due at least partially to deficits in stimulus processing mechanisms rather than in the cognitive aspects of working memory per se.
Figure 1. The effect of enhancing cholinergic activity on performance during a working memory task in patients with Alzheimer’s disease is shown.
The impairment in performance associated with longer task delays under placebo (dark gray bars) shows a significant improvement following physostigmine (light gray bars). The graph reflects the group mean ± SE for each of the four working memory delay conditions. A small inset shows the working memory (WM) task; the delay was manipulated by the number of presentations of the blank three-square array resulting in 1–s (no array), 6, 11, or 16-s delays.
Cholinergic system and mood disorder
Multiple neurotransmitter systems also are implicated in depressive disorders including the dopaminergic, serotonergic, noradrenergic, and cholinergic systems. The cholinergic system has been found to be hypersensitive in depression, whereby depressed patients show exaggerated neuroendocrine and pupillary responses to cholinergic agents [79–81] and experience forms of sleep disturbance (decreases latency to REM and increased REM density) that are consistent with increased cholinergic muscarinic sensitivity [82,83]. Janowsky et al. [84–86] reported that in manic bipolar patients, increasing cholinergic activity induced depressive symptoms, and in major depressive disorder (MDD), increasing cholinergic activity worsened symptoms of depression [87–89]. The role of the cholinergic system in mood disorders has been highlighted more recently through the demonstration that blocking cholinergic muscarinic activity with scopolamine produces rapid antidepressant effects [90•,91].
Behavioral and cognitive features of depression are associated primarily with the processing of affective information. A consistently reported finding is a mood congruent processing bias in depressed individuals, which is defined as a tendency to show a bias for processing negative information as compared to positive or neutral information [92,93,94••,95]. Results of memory studies show that MDD patients recall more negatively toned material than positively toned information [96–99]. In the context of attention paradigms [100–102], the influence of mood congruent processing is demonstrated by the finding that depression-related words produce more interference on emotional stroop tasks than do happy or neutral words. Similarly, Murphy et al. [93] showed in an affective attention-shifting task that depressed individuals are slower in responding to the presentation of happy word as compared to sad word targets, and that their ability to shift attention from happy to sad or sad to happy targets is impaired.
The mood congruent processing bias observed in MDD readily can be characterized within the framework of the cholinergic system and stimulus processing mechanisms. The biased processing of negative or sad information is consistent with an overactive cholinergic system in depression resulting in the over-representation of negative information. This framework would hypothesize that competition among competing stimuli in the environment engages the cholinergic system, and the overactive system alters the bias preferentially toward negative stimuli in MDD. A functional brain imaging study that used a selective attention task with images of emotional faces and houses observed processing biases between emotional faces in visual processing areas that were opposite to each other in healthy controls and patients with MDD [103], a finding that would be predicted by this hypothesis. The effect of cholinergic modulation on these baseline differences will be informative.
Conclusion
The cholinergic neurotransmitter system traditionally has been linked with cognitive functions including attention and memory. Evidence concerning the direct effects of cholinergic function on stimulus processing, together with findings from cognitive studies that characterize stimulus processing effects within the context of cognitive functions, leads to the hypothesis that the cholinergic system retains the role of supporting and modulating the processing of task-related stimuli in the context of cognitive functions. This concept carries forward to pathological conditions that both implicate cholinergic activity and retain hallmark cognitive features that can be explained by changes in cholinergic influence on stimulus processing.
Key points.
The cholinergic system likely influences cognitive functions via stimulus processing mechanisms.
Some cognitive changes observed during healthy aging can be understood through cholinergic influences on stimulus processing.
Reduced cholinergic function as seen in Alzheimer’s disease, and increased cholinergic function as seen in unipolar and bipolar depression may alter stimulus processing mechanisms to produce the patterns of cognitive deficit observed in these illnesses.
Acknowledgments
The author would like to thank the many colleagues who have collaborated on various aspects of work that is reflected in this review, with particular thanks to Drs James Haxby, Pietro Pietrini, and Emiliano Ricciardi. The author would also like to thank Drs Emiliano Ricciardi and Allison Nugent for comments on the article. The work presented here associated with the author was supported by the intramural research programs of the NIA/National Institutes of Health and the National Institute of Mental Health/NIH. A use-patent application for the use of scopolamine as an antidepressant agent has been filed by the NIMH/NIH.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 411).
- 1.Klinkenberg I, Sambeth A, Blokland A. Acetylcholine and attention. Behav Brain Res. 2010;221:430–442. doi: 10.1016/j.bbr.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Graef S, Schonknecht P, Sabri O, Hegerl U. Cholinergic receptor subtypes and their role in cognition, emotion, and vigilance control: an overview of preclinical and clinical findings. Psychopharmacology (Berl) 2011;215:205–229. doi: 10.1007/s00213-010-2153-8. [DOI] [PubMed] [Google Scholar]
- 3.Murray CL, Fibiger HC. Learning and memory deficits after lesions of the nucleus basalis magnocellularis: reversal by physostigmine. Neuroscience. 1985;14:1025–1032. doi: 10.1016/0306-4522(85)90273-8. [DOI] [PubMed] [Google Scholar]
- 4.Sitaram N, Weingartner H, Gillin JC. Human serial learning: enhancement with arecholine and choline impairment with scopolamine. Science. 1978;201:274–276. doi: 10.1126/science.351808. [DOI] [PubMed] [Google Scholar]
- 5.Rusted JM, Warburton DM. The effects of scopolamine on working memory in healthy young volunteers. Psychopharmacology (Berl) 1988;96:145–152. doi: 10.1007/BF00177553. [DOI] [PubMed] [Google Scholar]
- 6••.Barak S, Weiner I. Differential role of muscarinic transmission within the entorhinal cortex and basolateral amygdala in the processing of irrelevant stimuli. Neuropsychopharmacology. 2010;35:1073–1082. doi: 10.1038/npp.2009.210. Through an assessment of the cholinergic system and cognition, this study highlights selective effects of scopolamine based on the relevance of stimuli to task performance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Blake MG, Boccia MM, Krawczyk MC, Baratti CM. Scopolamine prevents retrograde memory interference between two different learning tasks. Physiol Behav. 2011;102:332–337. doi: 10.1016/j.physbeh.2010.11.026. This study demonstrates the specificity of cholinergic impairment on acquisition as compared to reconsolidation within a memory task, a finding that is specific to modulation of stimulus processing in the context of a memory paradigm. [DOI] [PubMed] [Google Scholar]
- 8.Klinkenberg I, Blokland A. A comparison of scopolamine and biperiden as a rodent model for cholinergic cognitive impairment. Psychopharmacology (Berl) 2011;215:549–566. doi: 10.1007/s00213-011-2171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wesnes K, Warburton DM. Effects of scopolamine and nicotine on human rapid information processing performance. Psychopharmacology (Berl) 1984;82:147–150. doi: 10.1007/BF00427761. [DOI] [PubMed] [Google Scholar]
- 10.Terry AV, Jr, Buccafusco JJ, Herman EJ, et al. The prototypical ranitidine analog JWS-USC-75-IX improves information processing and cognitive function in animal models. J Pharmacol Exp Ther. 2011;336:751–766. doi: 10.1124/jpet.110.175422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drachman DA, Leavitt J. Human memory and the cholinergic system. A relationship to aging? Arch Neurol. 1974;30:113–121. doi: 10.1001/archneur.1974.00490320001001. [DOI] [PubMed] [Google Scholar]
- 12.Sunderland T, Tariot PN, Weingartner H, et al. Pharmacologic modelling of Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:599–610. doi: 10.1016/0278-5846(86)90030-8. [DOI] [PubMed] [Google Scholar]
- 13.Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology. 2008;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thienel R, Kellermann T, Schall U, et al. Muscarinic antagonist effects on executive control of attention. Int J Neuropsychopharmacol. 2009;12:1307–1317. doi: 10.1017/S146114570999068X. [DOI] [PubMed] [Google Scholar]
- 15.Bartus RT, Dean RL, 3rd, Sherman KA, et al. Profound effects of combining choline and piracetam on memory enhancement and cholinergic function in aged rats. Neurobiol Aging. 1981;2:105–111. doi: 10.1016/0197-4580(81)90007-5. [DOI] [PubMed] [Google Scholar]
- 16.Peterson C, Gibson GE. Amelioration of age-related neurochemical and behavioral deficits by 3,4-diaminopyridine. Neurobiol Aging. 1983;4:25–30. doi: 10.1016/0197-4580(83)90050-7. [DOI] [PubMed] [Google Scholar]
- 17.Furey ML, Pietrini P, Alexander GE, et al. Cholinergic enhancement improves performance on working memory by modulating the functional activity in distinct brain regions: a positron emission tomography regional cerebral blood flow study in healthy humans. Brain Res Bull. 2000;51:213–218. doi: 10.1016/s0361-9230(99)00219-1. [DOI] [PubMed] [Google Scholar]
- 18.Himmelheber AM, Sarter M, Bruno JP. The effects of manipulations of attentional demand on cortical acetylcholine release. Brain Res Cogn Brain Res. 2001;12:353–370. doi: 10.1016/s0926-6410(01)00064-7. [DOI] [PubMed] [Google Scholar]
- 19.Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- 20.Yu AJ, Dayan P. Acetylcholine in cortical inference. Neural Netw. 2002;15 (4–6):719–730. doi: 10.1016/s0893-6080(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 21.Himmelheber AM, Sarter M, Bruno JP. Increases in cortical acetylcholine release during sustained attention performance in rats. Brain Res Cogn Brain Res. 2000;9:313–325. doi: 10.1016/s0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 22.Sarter M, Bruno JP. Cortical cholinergic inputs mediating arousal, attentional processing and dreaming: differential afferent regulation of the basal forebrain by telencephalic and brainstem afferents. Neuroscience. 2000;95:933–952. doi: 10.1016/s0306-4522(99)00487-x. [DOI] [PubMed] [Google Scholar]
- 23.Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem. 2003;80:245–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 24.Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Brain Res Rev. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 26.Furey ML, Pietrini P, Haxby JV. Cholinergic enhancement and increased selectivity of perceptual processing during working memory. Science. 2000;290:2315–2319. doi: 10.1126/science.290.5500.2315. [DOI] [PubMed] [Google Scholar]
- 27.Sillito AM, Kemp JA. Cholinergic modulation of the functional organization of the cat visual cortex. Brain Res. 1983;289 (1–2):143–155. doi: 10.1016/0006-8993(83)90015-x. [DOI] [PubMed] [Google Scholar]
- 28.Sato H, Hata Y, Masui H, Tsumoto T. A functional role of cholinergic innervation to neurons in the cat visual cortex. J Neurophysiol. 1987;58:765–780. doi: 10.1152/jn.1987.58.4.765. [DOI] [PubMed] [Google Scholar]
- 29.Murphy PC, Sillito AM. Cholinergic enhancement of direction selectivity in the visual cortex of the cat. Neuroscience. 1991;40:13–20. doi: 10.1016/0306-4522(91)90170-s. [DOI] [PubMed] [Google Scholar]
- 30.Buzsaki G. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 31.Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bentley P, Driver J, Dolan RJ. Modulation of fusiform cortex activity by cholinesterase inhibition predicts effects on subsequent memory. Brain. 2009;132:2356–2371. doi: 10.1093/brain/awp176. [DOI] [PubMed] [Google Scholar]
- 33.Baddeley A. Working memory and language: an overview. J Commun Disord. 2003;36:189–208. doi: 10.1016/s0021-9924(03)00019-4. [DOI] [PubMed] [Google Scholar]
- 34.Baddeley A, Logie R, Bressi S, et al. Dementia and working memory. Q J Exp Psychol A. 1986;38:603–618. doi: 10.1080/14640748608401616. [DOI] [PubMed] [Google Scholar]
- 35.Glasky AJ, Melchior CL, Pirzadeh B, et al. Effect of AIT-082, a purine analog, on working memory in normal and aged mice. Pharmacol Biochem Behav. 1994;47:325–329. doi: 10.1016/0091-3057(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 36.Furey ML, Pietrini P, Haxby JV, et al. Cholinergic stimulation alters performance and task-specific regional cerebral blood flow during working memory. Proc Natl Acad Sci U S A. 1997;94:6512–6516. doi: 10.1073/pnas.94.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freo U, Ricciardi E, Pietrini P, et al. Pharmacological modulation of prefrontal cortical activity during a working memory task in young and older humans: a PET study with physostigmine. Am J Psychiatry. 2005;162:2061–2070. doi: 10.1176/appi.ajp.162.11.2061. [DOI] [PubMed] [Google Scholar]
- 38.Ricciardi E, Pietrini P, Schapiro MB, et al. Cholinergic modulation of visual working memory during aging: a parametric PET study. Brain Res Bull. 2009;79:322–332. doi: 10.1016/j.brainresbull.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dawson GR, Iversen SD. The effects of novel cholinesterase inhibitors and selective muscarinic receptor agonists in tests of reference and working memory. Behav Brain Res. 1993;57:143–153. doi: 10.1016/0166-4328(93)90130-i. [DOI] [PubMed] [Google Scholar]
- 40.Bentley P, Husain M, Dolan RJ. Effects of cholinergic enhancement on visual stimulation, spatial attention, and spatial working memory. Neuron. 2004;41:969–982. doi: 10.1016/s0896-6273(04)00145-x. [DOI] [PubMed] [Google Scholar]
- 41.Kukolja J, Thiel CM, Fink GR. Cholinergic stimulation enhances neural activity associated with encoding but reduces neural activity associated with retrieval in humans. J Neurosci. 2009;29:8119–8128. doi: 10.1523/JNEUROSCI.0203-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schon K, Atri A, Hasselmo ME, et al. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci. 2005;25:9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosier A, Cornette L, Orban GA. Scopolamine-induced impairment of delayed recognition of abstract visual shapes. Neuropsychobiology. 1998;37:98–103. doi: 10.1159/000026486. [DOI] [PubMed] [Google Scholar]
- 44.Desimone R. Visual attention mediated by biased competition in extrastriate visual cortex. Philos Trans R Soc Lond B Biol Sci. 1998;353:1245–1255. doi: 10.1098/rstb.1998.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 46.Kastner S, Pinsk MA. Visual attention as a multilevel selection process. Cogn Affect Behav Neurosci. 2004;4:483–500. doi: 10.3758/cabn.4.4.483. [DOI] [PubMed] [Google Scholar]
- 47.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 48.Kastner S, De Weerd P, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds JH, Chelazzi L, Desimone R. Competitive mechanisms subserve attention in macaque areas V2 and V4. J Neurosci. 1999;19:1736–1753. doi: 10.1523/JNEUROSCI.19-05-01736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chelazzi L, Duncan J, Miller EK, Desimone R. Responses of neurons in inferior temporal cortex during memory-guided visual search. J Neurophysiol. 1998;80:2918–2940. doi: 10.1152/jn.1998.80.6.2918. [DOI] [PubMed] [Google Scholar]
- 51.Beck DM, Kastner S. Stimulus context modulates competition in human extrastriate cortex. Nat Neurosci. 2005;8:1110–1116. doi: 10.1038/nn1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deco G, Rolls ET. Neurodynamics of biased competition and cooperation for attention: a model with spiking neurons. J Neurophysiol. 2005;94:295–313. doi: 10.1152/jn.01095.2004. [DOI] [PubMed] [Google Scholar]
- 53.Duncan J. Converging levels of analysis in the cognitive neuroscience of visual attention. Philos Trans R Soc Lond B Biol Sci. 1998;353:1307–1317. doi: 10.1098/rstb.1998.0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friesen CK, Ristic J, Kingstone A. Attentional effects of counterpredictive gaze and arrow cues. J Exp Psychol Hum Percept Perform. 2004;30:319–329. doi: 10.1037/0096-1523.30.2.319. [DOI] [PubMed] [Google Scholar]
- 55.Ohman A. The role of the amygdala in human fear: automatic detection of threat. Psychoneuroendocrinology. 2005;30:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 56.Parkhurst D, Law K, Niebur E. Modeling the role of salience in the allocation of overt visual attention. Vision Res. 2002;42:107–123. doi: 10.1016/s0042-6989(01)00250-4. [DOI] [PubMed] [Google Scholar]
- 57.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 58.Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- 59.Connor CE, Egeth HE, Yantis S. Visual attention: bottom-up versus top-down. Curr Biol. 2004;14:R850–R852. doi: 10.1016/j.cub.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 60.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 61.Reynolds JH, Desimone R. Interacting roles of attention and visual salience in V4. Neuron. 2003;37:853–863. doi: 10.1016/s0896-6273(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 62.Creasey H, Rapoport SI. The aging human brain. Ann Neurol. 1985;17:2–10. doi: 10.1002/ana.410170103. [DOI] [PubMed] [Google Scholar]
- 63.Davis KL, Mohs RC, Marin D, et al. Cholinergic markers in elderly patients with early signs of Alzheimer disease. JAMA. 1999;281:1401–1406. doi: 10.1001/jama.281.15.1401. [DOI] [PubMed] [Google Scholar]
- 64.Altavista MC, Rossi P, Bentivoglio AR, et al. Aging is associated with a diffuse impairment of forebrain cholinergic neurons. Brain Res. 1990;508:51–59. doi: 10.1016/0006-8993(90)91116-x. [DOI] [PubMed] [Google Scholar]
- 65.Gibson GE, Peterson C, Jenden DJ. Brain acetylcholine synthesis declines with senescence. Science. 1981;213:674–676. doi: 10.1126/science.7256270. [DOI] [PubMed] [Google Scholar]
- 66.Mesulam M. The cholinergic lesion of Alzheimer’s disease: pivotal factor or side show? Learn Mem. 2004;11:43–49. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- 67.DeKosky ST, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- 68.Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2010;221:555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- 69•.Dumas JA, Newhouse PA. The cholinergic hypothesis of cognitive aging revisited again: cholinergic functional compensation. Pharmacol Biochem Behav. 2011 doi: 10.1016/j.pbb.2011.02.022. [Epub ahead of print]. This study reviews current literature to reconsider the cholinergic hypothesis in aging, including a discussion of studies that utilize cholinergic agents that produce task-specific effects on behavior and brain activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greenwood PM. The frontal aging hypothesis evaluated. J Int Neuropsychol Soc. 2000;6:705–726. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- 71.Wallace TL, Ballard TM, Pouzet B, et al. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav. 2011 doi: 10.1016/j.pbb.2011.03.022. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 72.Kobayashi S, Iwamoto SM, Kon K, et al. Acetyl-L-carnitine improves aged brain function. Geriatr Gerontol Int. 2010;10 (Suppl 1):S99–S106. doi: 10.1111/j.1447-0594.2010.00595.x. [DOI] [PubMed] [Google Scholar]
- 73.Grady CL, McIntosh AR, Bookstein F, et al. Age-related changes in regional cerebral blood flow during working memory for faces. Neuroimage. 1998;8:409–425. doi: 10.1006/nimg.1998.0376. [DOI] [PubMed] [Google Scholar]
- 74.Grady CL, Yu H, Alain C. Age-related differences in brain activity underlying working memory for spatial and nonspatial auditory information. Cereb Cortex. 2008;18:189–199. doi: 10.1093/cercor/bhm045. [DOI] [PubMed] [Google Scholar]
- 75.Grady CL, Craik FI. Changes in memory processing with age. Curr Opin Neurobiol. 2000;10:224–231. doi: 10.1016/s0959-4388(00)00073-8. [DOI] [PubMed] [Google Scholar]
- 76•.Shioda N, Yamamoto Y, Han F, et al. Neurochemical mechanisms of a novel Alzheimer’s disease therapeutics on improvement of cognition and depressive behavior. Yakugaku Zasshi. 2011;131:505–511. doi: 10.1248/yakushi.131.505. This study reports results of a new cholinergic agent as a cognitive enhancer. The authors report restoration of cognitive deficits and improvement in depressive behaviors induced by lesion in rodents using a new cholinergic agent that increases cholinergic release. The authors propose that this agent can potentially improve cognitive and depressive symptoms observed in Alzheimer’s disease. [DOI] [PubMed] [Google Scholar]
- 77.Bentley P, Driver J, Dolan RJ. Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer’s disease and health. Brain. 2008;131 (Pt 2):409–424. doi: 10.1093/brain/awm299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Britton GB, Rao KS. Cognitive aging and early diagnosis challenges in Alzheimer’s disease. J Alzheimers Dis. 2011;24:153–159. doi: 10.3233/JAD-2011-110239. [DOI] [PubMed] [Google Scholar]
- 79.Dilsaver SC. Pathophysiology of ‘cholinoceptor supersensitivity’ in affective disorders. Biol Psychiatry. 1986;21 (8–9):813–829. doi: 10.1016/0006-3223(86)90246-5. [DOI] [PubMed] [Google Scholar]
- 80.Janowsky DS, Overstreet DH. The role of acetylcholine mechanisms in mood disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. New York: Raven Press; 1995. [Google Scholar]
- 81.Rubin RT, O’Toole SM, Rhodes ME, et al. Hypothalamo-pituitary-adrenal cortical responses to low-dose physostigmine and arginine vasopressin administration: sex differences between major depressives and matched control subjects. Psychiatry Res. 1999;89:1–20. doi: 10.1016/s0165-1781(99)00085-2. [DOI] [PubMed] [Google Scholar]
- 82.Coble P, Foster FG, Kupfer DJ. Electroencephalographic sleep diagnosis of primary depression. Arch Gen Psychiatry. 1976;33:1124–1127. doi: 10.1001/archpsyc.1976.01770090114012. [DOI] [PubMed] [Google Scholar]
- 83.Kupfer DJ. REM latency: a psychobiologic marker for primary depressive disease. Biol Psychiatry. 1976;11:159–174. [PubMed] [Google Scholar]
- 84.Janowsky EC, Risch C, Janowsky DS. Effects of anesthesia on patients taking psychotropic drugs. J Clin Psychopharmacol. 1981;1:14–20. doi: 10.1097/00004714-198101000-00004. [DOI] [PubMed] [Google Scholar]
- 85.Janowsky DS, el-Yousef MK, Davis JM. Acetylcholine and depression. Psychosom Med. 1974;36:248–257. doi: 10.1097/00006842-197405000-00008. [DOI] [PubMed] [Google Scholar]
- 86.Janowsky DS, el-Yousef MK, Davis JM, et al. Cholinergic reversal of manic symptoms. Lancet. 1972;1:1236–1237. doi: 10.1016/s0140-6736(72)90956-7. [DOI] [PubMed] [Google Scholar]
- 87.Davis KL, Berger PA, Hollister LE, Defraites E. Physostigmine in mania. Arch Gen Psychiatry. 1978;35:119–122. doi: 10.1001/archpsyc.1978.01770250121012. [DOI] [PubMed] [Google Scholar]
- 88.Risch SC, Kalin NH, Janowsky DS. Cholinergic challenges in affective illness: behavioral and neuroendocrine correlates. J Clin Psychopharmacol. 1981;1:186–192. doi: 10.1097/00004714-198107000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Nurnberger JI, Jr, Jimerson DC, Simmons-Alling S, et al. Behavioral, physiological, and neuroendocrine responses to arecoline in normal twins and ‘well state’ bipolar patients. Psychiatry Res. 1983;9:191–200. doi: 10.1016/0165-1781(83)90043-4. [DOI] [PubMed] [Google Scholar]
- 90•.Drevets WC, Furey ML. Replication of scopolamine’s antidepressant efficacy in major depressive disorder: a randomized, placebo-controlled clinical trial. Biol Psychiatry. 2010;67:432–438. doi: 10.1016/j.biopsych.2009.11.021. This study demonstrates rapid and robust antidepressant effects in patients with MDD, further demonstrating the role of cholinergic dysfunction in mood disorders. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Furey ML, Drevets WC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry. 2006;63:1121–1129. doi: 10.1001/archpsyc.63.10.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. Selective attention to emotional stimuli in a verbal go/no-go task: an fMRI study. NeuroReport. 2000;11:1739–1744. doi: 10.1097/00001756-200006050-00028. [DOI] [PubMed] [Google Scholar]
- 93.Murphy FC, Sahakian BJ, Rubinsztein JS, et al. Emotional bias and inhibitory control processes in mania and depression. Psychol Med. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 94••.Victor TA, Furey ML, Fromm SJ, et al. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. This study demonstrates the presence of a negative processing bias at the level of neural activity in amygdala under conditions of preconscious processing. Patients with depression showed negative processing biases in a face-masked task in which negative emotion in a face stimulus produced larger responses than positive emotion, despite the fact that participants were unaware of the stimulus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koster EH, De Raedt R, Leyman L, De Lissnyder E. Mood-congruent attention and memory bias in dysphoria: exploring the coherence among information-processing biases. Behav Res Ther. 2010;48:219–225. doi: 10.1016/j.brat.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 96.Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- 97.Clark DM, Teasdale JD. Diurnal variation in clinical depression and accessibility of memories of positive and negative experiences. J Abnorm Psychol. 1982;91:87–95. doi: 10.1037//0021-843x.91.2.87. [DOI] [PubMed] [Google Scholar]
- 98.Bradley BP, Mogg K, Williams R. Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behav Res Ther. 1995;33:755–770. doi: 10.1016/0005-7967(95)00029-w. [DOI] [PubMed] [Google Scholar]
- 99.Bradley BP, Mogg K, Millar N. Implicit memory bias in clinical and nonclinical depression. Behav Res Ther. 1996;34 (11–12):865–879. doi: 10.1016/s0005-7967(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 100.Segal ZV, Gemar M, Truchon C, et al. A priming methodology for studying self-representation in major depressive disorder. J Abnorm Psychol. 1995;104:205–213. doi: 10.1037//0021-843x.104.1.205. [DOI] [PubMed] [Google Scholar]
- 101.Gotlib IH, Cane DB. Construct accessibility and clinical depression: a longitudinal investigation. J Abnorm Psychol. 1987;96:199–204. doi: 10.1037//0021-843x.96.3.199. [DOI] [PubMed] [Google Scholar]
- 102.Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: the role of awareness. Br J Clin Psychol. 1995;34 (Pt 1):17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- 103.Furey ML, Hoffman EM, Drevets WC. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neurosciences; 2009. Emotional processing biases during selective attention in visual processing areas in healthy and patients with depression. Available online. [Google Scholar]