Abstract
Substance abuse typically begins in adolescence; therefore, the impact of alcohol during this critical time in brain development is of particular importance. Epidemiological data indicate that excessive alcohol consumption is prevalent among adolescents and may have lasting neurobehavioral consequences. Loss of cholinergic input to the forebrain has been demonstrated following fetal alcohol exposure and in adults with Wernicke-Korsakoff syndrome. In the present study, immunohistochemistry for choline acetyltransferase (ChAT) was determined to assess forebrain cholinergic neurons (Ch1–4), and behavioral changes following periadolescent alcohol exposure. Wistar rats were exposed to intermittent ethanol vapor (14 hrs on/10 hrs off/day) for 35 days from PD 22-PD 57 (average blood alcohol concentration (BAC): 163 mg%). Rats were withdrawn from vapor and assessed for locomotor activity, startle response, conflict behavior in the open field, and immobility in the forced swim test, as adults. Rats were then sacrificed at day 71/72 and perfused for histochemical analyses. Ethanol vapor exposed rats displayed: increased locomotor activity 8 hrs after the termination of vapor delivery for that 24 hr period at day 10 and day 20 of alcohol vapor exposure, significant reductions in the amplitude of their responses to prepulse stimuli during the startle paradigm at 24 hrs withdrawal, and at two weeks following withdrawal, less anxiety-like and/or more “disinhibitory” behavior in the open field conflict, and more immobility in the forced swim test. Quantitative analyses of ChAT immunoreactivity revealed a significant reduction in cell counts in the Ch1–2 and Ch3–4 regions of the basal forebrain in ethanol vapor exposed rats. This reduction in cell counts was significantly correlated with less anxiety-like and/or more “disinhibitory” behavior in the open field conflict test. These studies demonstrate that behavioral measures of arousal, affective state, disinhibitory behavior and ChAT+IR, are all significantly impacted by periadolescent ethanol exposure and withdrawal in Wistar rats.
Keywords: Adolescent, alcohol exposure, ChAT, forced swim test, open field conflict, startle
1.0 Introduction
Adolescence is a transition period between childhood and adulthood, that is defined both biologically and behaviorally, that has been suggested to encompasses the entire second decade of life (10–20 yrs) (Spear, 2000). It has been suggested that during this time period social and emotional fluency is acquired as well as the ability to function independently (Dahl and Spear, 2004). While there has been a tendency to define adolescence by endocrine events such as puberty, it actually involves changes in a number of organ systems, including the brain that may occur in a separate time frame from endocrine events associated with puberty (Spear and Varlinskaya, 2010). During this developmental period alterations in neurobiological organization and behavior are seen that have been notably conserved during evolution with a number of similarities seen across mammalian species. In the rat it has been suggested that the periadolescent period may as a conservative estimate span postnatal days 28–42 (Spear and Brake, 1983; Varlinskaya et al., 1999; Ojeda and Skinner, 2006). However depending on gender and the measures used to define adolescence, early harbingers of adolescence may be seen as early as P22 in females, and it may last until P55 or so in males (Spear, 2000).
Adolescence is a critical stage of brain development when humans are initially exposed to a number of potentially toxic external stimuli such as ethanol and other drugs of abuse (Johnston, 1995; Clark et al., 2008; Squeglia et al., 2009a). Given that the brain continues to develop before and throughout the adolescent period into early adulthood (Markus and Petit, 1987; Sowell et al., 1999a,b), ethanol exposure during that time period may have unique deleterious consequences including changes in disinhibitory, cognitive, and affectively driven behaviors. Several studies in humans have provided data showing that early alcohol exposure is associated with behavioral deficits as measured by MRI scans and psychological testing (McQueeny et al., 2009; Squeglia et al., 2009b; Hanson et al., 2011; Schweinsburg et al., 2011). However, is still not clear whether all such deficits are the result of alcohol usage or represent pre-existing conditions (Nagel et al., 2005). The use of animal models of adolescent and young adult alcohol exposure allows for the control necessary to evaluate the effects of alcohol on the developing brain and separate such effects from genetic background.
Alcohol exposure during the adolescent period in rodents has been demonstrated to produce some effects that differ from adults (Jain and Balhara, 2010; Spear and Varlinskaya, 2010). Attenuated sensitivity to the acute effects of alcohol has been demonstrated in adolescent rats as compared to adults in measures of: sedation (Moy et al., 1998; Silveri and Spear, 1998; Draski et al., 2001; Pian et al., 2008a), motor impairment (White et al., 2002a,b) and electrophysiological responses (Pian et al., 2008b). However, adolescent rats appear to be more sensitive to ethanol induced impairments in spatial learning and inhibition of long-term potentiation than adults (Swartzwelder et al., 1995; Rajendran and Spear, 2004). Some measures of both short and long term withdrawal from ethanol have also been demonstrated to differ between adolescents and adults. Electroencephalographic (EEG) signs of increased arousal and behavioral signs of hypoactivity during early withdrawal have been found in adolescent rats as compared to adults (Slawecki and Roth, 2004; Slawecki et al., 2006). Additionally, it has been demonstrated that adolescents show an attenuated sensitivity to acute withdrawal related anxiogenesis in the elevated plus maze (Doremus-Fitzwater and Spear, 2007). Enhanced prepulse inhibition of the startle response has also been demonstrated to occur in rats exposed to alcohol during adolescence as compared to comparable exposure during adulthood, at 6 days following cessation of ethanol exposure (Slawecki and Ehlers, 2005). Fewer studies have compared the longer-term consequences of alcohol exposure in adolescents and adults. However, preliminary studies suggest that alcohol exposure during adolescence appears to cause: increased vulnerability to ethanol-induced spatial memory impairments (White and Swartzwelder, 2005), reductions in the P3 component of the event-related potential in hippocampus (Slawecki et al., 2001) and changes in the frequency of slow waves during sleep (Criado et al., 2008).
Adolescent brain development also involves dramatic changes in a number of brain neurochemical systems including dopaminergic, cholinergic, and serotonergic innervation of forebrain systems (Kalsbeek et al., 1988; Kostovic, 1990; Gould et al., 1991; Rosenberg and Lewis, 1994; Giedd, 2004; Giedd et al., 2008). All of these systems are potentially influenced by alcohol exposure during development. Chronic ethanol exposure in adults that leads to amnesia associated with Wernik's encephalopathy and Korsakoff's psychosis is known to cause a dramatic reduction in neurons in the nucleus basalis (Ch4) in the basal forebrain (Arendt et al., 1983; Cullen et al., 1997). These findings of reduced cholinergic tone in alcoholic dementia are not unlike the brain pathology seen in the basal forebrain in Alzheimer's disease, which may account for the difficulty that has occurred in establishing alcoholic dementia as a distinct disorder (Lishman, 1986). A loss of muscarinic cholinergic receptors from the temporal cortex of alcohol abusers, who had histologically normal brains and no signs of significant atrophy and/or dementia, has also been reported (Freund and Ballinger, 1989). This suggests that cholinergic loss may precede the development of significant alcohol encephalopathy in adulthood. Whether alcohol exposure during early adolescence, in humans, might cause specific loss of cholinergic signaling is not known. However, a recent study in mice has demonstrated that alcohol exposure, that mimicked binge drinking during adolescence, causes reduced volumes in the olfactory bulb and basal forebrain in magnetic resonance imaging (MRI) scans as well as fewer basal forebrain cholinergic neurons in immunohistochemical (IHC) analyses (Coleman et al., 2011).
The present study was designed to extend the study of Coleman et al. (2011) to rats and to test whether adolescent alcohol vapor exposure, at a high/moderate level (< 200 mg%) produced specific and persistent (weeks) effects on IHC measures of cholinergic neurons in the basal forebrain. Additionally, behavioral measures of: affect (anxiety and depression), arousal (prepulse inhibition of the startle) and disinhibition that have been demonstrated previously to be sensitive to periadolescent alcohol vapor exposure (Slawecki et al., 2003, 2004; Slawecki and Ehlers, 2005; Pian et al. 2008b), were also tested and correlations made to the IHC analyses.
2.0 Experimental procedures
2.1 Subjects
Male Wistar rats who were received at postnatal day (PD) 23 (n = 42; 36 juveniles, 6 dams, Charles River, USA) were used in this study. The adolescent animals (PD 23) were housed 3 per cage respectively in standard cages for the duration of the experiment. Animals were kept in a light/dark (12 hrs light/12 hrs dark, lights on at 06:00 a.m.) and temperature-controlled environment. Food and water were available ad libitum throughout the experiment, except where noted. All experimental protocols were approved by the Institutional Animal Care and Use Committee at The Scripps Research Institute and were consistent with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996).
2.2 Ethanol vapor exposure
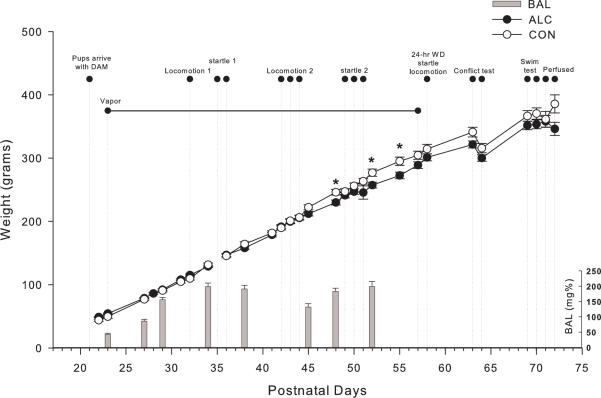
Ethanol vapor exposure has been shown to reliably allow for the titration of blood alcohol concentrations (BACs) that are sufficient for inducing ethanol physical dependence (Roberts et al., 1996, 2000). The ethanol vapor inhalation procedure and the chambers used in this study were previously described (Rogers et al., 1979; Slawecki et al., 2001; Slawecki, 2002; O'Dell et al., 2004; Funk et al., 2006; Gilpin et al., 2008; Zahr et al., 2011). Ethanol vapor chambers were calibrated to produce high to moderate BACs between 150–225 mg/dL. In brief, adolescent (n = 36) were randomly divided into two groups each (ethanol-exposed group, n = 24; control group, n = 12). Ethanol-exposed rats were housed in sealed chambers, which were infused with vaporized 95% ethanol from 6 p.m. to 8 a.m. For the remaining of the 10 hrs of the day, ethanol vapor was not infused into the chambers. This pattern of daily ethanol exposure does not mimic the typical pattern of ethanol drinking in human adolescents who are more likely to experience intermittent binge drinking. However, adolescence in the human may span a 10 year period whereas in the rat periadolescence is condenced into a period of 35 days. At the start of the ethanol exposure, adolescent rats were 23 days old and the exposure continued until they were 58 days old. This exposure period, although not directly translatable to humans, was selected to ensure that the animals were exposed during the entire rat's extended periadolescent period (Spear, 2000). Age-matched controls were handled identically to ethanol-exposed rats. Food and water were always available. Blood samples were collected from the tip of the tail approximately 8 times during the 5 week exposure period in order to assess BACs (target: 150 to 200 mg/dl). Control animals also had blood removed from the tail at the same time points. BACs were determined in the alcohol exposed animals using the Analox micro-statGM7 (Analox Instr. Ltd., Lunenberg, MA). Following the 5 week exposure animals were tranferred to standard vivarium cages for the duration of the experiment. Figure 1 shows graphical representation of the timing of the experimental protocol.
Figure 1.
Graphical representation of the timing of the experimental protocol. Blood alcohol levels (BACs) were determined 8 times during the 5 week ethanol vapor exposure. Ethanol-exposed (n=24) and control rats (n=12) showed parallel increases in body weight. Rats exposed to ethanol showed reductions in body weights at ages PD 48, PD 52 and PD55, compared to control rats. Ethanol-exposed and control rats showed similar body weights during all behavioral tests and at sacrifice. * indicates P< 0.05 for significant difference from control rats. Error bars = S.E.M (Body weights) and S.D. (BACs).
2.3 Locomotor activity
Locomotor activity has been demonstrated to be a sensitive measure of alcohol vapor exposure levels and withdrawal (Ehlers and Chaplin, 1987; Slawecki et al., 2005). In the current study locomotor activity was measured at 3 time points, at PD 32, 10 days after ethanol exposure, at PD 42–44 at 20 days following ethanol exposure and at PD 58, 35 days following ethanol exposure. At PD 32 and 42 the animals' locomotion was measured 8 hrs after the vapor was terminated for that 24 hr period and at PD 58 locomotion was measured 24 hrs following the final withdrawal of alcohol vapor. Locomotion was measured in individual wire cages (20cm × 25 cm × 36 cm). Each cage was equipped with two infrared photocell beams placed 2 cm above the floor. Activity was initially quantified by totaling photocell beam interruptions which were recored on electromechanical counters for 5 minute epochs over the entire test session.
2.4 Acoustic startle response/prepulse inhibition
Acoustic startle response (ASR) and prepulse inhibition (PPI) has been previously demonstrated to be sensitive to adolescent alcohol adminitration (Slawecki and Ehlers, 2005; Pian et al., 2008b). In the present study ASR/PPI was assessed at 3 different time points both during and after ethanol exposure on the same days as the locomotor measurements immediately following the locomotor sessions, (e.g. PD 35–36, PD 49–51, PD 58). Acoustic startle responses were measured in SR LAB Startle chambers (San Diego Instruments, San Diego, CA). A speaker mounted in the ceiling of the chamber produced background noise and the acoustic stimuli. Within each test chamber, a single Plexiglas cylinder (9 cm diameter, 16 cm length) was housed. A piezoelectric accelerometer (San Diego Instruments, San Diego, CA) mounted on the bottom of each cylinder detected movement and transduced this movement into a voltage signal. The voltage signal was collected and analyzed using software developed for the laboratory by Dr. James Havstad. This software also controlled the timing and generation of the auditory stimuli. After the recording was started, each session contained 45 trials and consisted of randomly presented pulse trails (120 dB auditory pulse burst for 40 msec) or prepulse + pulse trials (120 dB auditory pulse burst with preceded 100 msec by a 85 dB auditory prepulse burst for 20 msec duration). The Plexiglas cylinders were cleansed with alcohol and water between each test. The outcome variables assessed included: ASR and pre-pulse magnitude on prepulse + pulse trials and ASR magnitude on pulse trials. The order of assessment on the test day was counterbalanced across treatment groups to minimize any potential influence of time of day during testing.
2.5 Modified open field conflict
Assessment of anxiety-like behavior/ disinhibition was accomplished in the modified open field at PD 64, 6 days following final withdrawal from ethanol vapor. This procedure has been demonstrated previously in our lab to be highly sensitive to periadolescent drug exposure (Slawecki et al., 2003). The test apparatus was constructed from a standard 32 gallon trash can. A single 5 g food pellet was fixed in place at the center of the apparatus prior to each test. The apparatus was illuminated by a single white light (50 lux) located 3.5–4 feet above the floor of the apparatus. Twenty-four hours prior to the test, all subjects were food deprived. To start each test, a rat was placed in the center of the apparatus. Since the animals have been food deprived they would be motivated to approach and eat the food pellet, however, the presence of a bright light shining on the food pellet will also produce a reluctance to approach the food, thus producing a behavioral conflict situation. Increased contact with the food by treated rats suggests disinihibited behavior and/or less “anxiety-like” behavior as compared to controls. Rats were given 5 minutes to freely explore the apparatus. The number of food contacts, the time of contact with food and the amount of food eaten were recorded during each test. The average time spent in contact with food during each approach was also assessed (i.e., total food contact time/number of food approaches). At the conclusion of the test, the rat was returned to its home cage. The apparatus was cleaned with alcohol and water prior to assessing the next subject. Tests were run between 9 a.m. and 12 p.m. On the test day, an individual selected ethanol and control rats to be run in an alternating fashion. A separate individual, who was blind to treatment group, scored behavior in the modified open field (Slawecki et al., 2003).
2.6 Forced swim test
Immobility in the forced swim test (FST) has been demonstrated to be enhanced in animals that experience periadolescent alcohol vapor exposure (Slawecki et al., 2004) as well as adults exposed to alcohol vapors (Walker et al., 2010). In the present study animals were tested in the force swim test at PD 69–70, 11–12 days following termination of ethanol exposure. The FST apparatus was a white plastic tub (diameter = 34 cm, height = 66 cm). The tub was filled to a level of 48 cm with 24±2° C water. Illumination at the surface of the water was approximately 60 lux. One day prior to the initial acute withdrawal test, rats were placed in the apparatus for 10 minutes but behavior was not recorded. On the day of the test, behavior in the apparatus was assessed during a 5 minute test session. Each test session was videotaped and later analyzed by two researchers that were blind to the exposure conditions. The behaviors that were measured in the 5 minute FST consisted of latency to immobility once being placed in the apparatus, swim time, immobility time and defecation. Immobility time was defined as a lack of active swimming and floating/ and or sinking. Inter-rater reliability was very high, with less than 10% deviation between scorers on all parameters that were evaluated.
2.7 Perfusion, brain tissue preparation and immunohistochemistry and image analyses
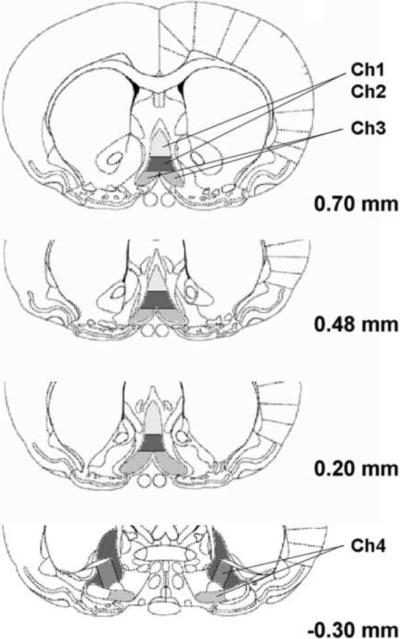
Rats were sacrificed on postnatal day 71 and 72. They were first anesthetized with pentobarbital (100 mg/kg, intraperitoneal) and then killed by perfusion as described previously (Crews et al., 2004). The animals were perfused transcardially with 0.1 M phosphate buffered saline (PBS, pH 7.4), followed by 4% paraformaldehyde in PBS. Brains were removed and postfixed in 4% paraformaldehyde (PFA) after perfusion for 24 hours at 4°C, and then were transferred to PBS solution until they were sectioned. The brains were sliced coronally on a vibratome into 40 μm thick sections and stored in cryoprotectant at −20°C until use. Every twelfth section was used for immunohistochemistry. Free-floating sections were incubated in mouse anti-ChAT monoclonal antibody (1:200, Millipore MAB 305, Temecula, CA) for 2 hrs at room temperature and then for 16 hrs at 4°C. Sections were rinsed in PBS, and incubated with biotinylated secondary anti-mouse antibody for 1 hr. Subsequently, avidin-biotin complex (Vector ABC kit, Vector Laboratories) was applied for 1 hr at room temperature, and ChAT positive neurons were visualized using nickel-enhanced diaminobenzidine (DAB) reaction. The number of positive neurons was quantified by a modified stereological procedure for labeled cells within a specified region of interest (Crews et. al., 2004). Bioquant Nova Advanced Image Analysis (R&M Biometric, Nashville, TN) was used for image capture and analysis. Images were captured by using an Olympus BX50 Microscope and Sony DXC-390 video camera linked to a computer. ChAT+IR neurons were counted within the region of interest and expressed as cells per square millimeter (mm). Ch1 and Ch2 are contained in the medial septal nucleus (MS) and the nucleus of vertical limb of the diagonal band (VDB) respectively. Ch3 is mostly in the lateral portion of the horizontal limb nucleus of the diagonal band, and Ch4 is the nucleus basalis, and also parts of the diagonal band nuclei. For Ch1 and Ch2 sectors, coronal sections were from bregma 0.7 to 0.2 mm; for Ch3 and Ch4, from 0.7 to −0.40 mm. Both left and right hemisphere of an individual brain subregion of each animal was counted, and the average value was used.
2.8 Data Analysis
Statistical analyses were performed by using SPSS (SPSS, Inc., Chicago, IL). Analysis of variance (ANOVA) was used to determine the effects of chronic ethanol exposure on body weight and BACs. Independent ANOVAs (1- way or 2-way) were also used to assess the effects of ethanol exposure on locomotion, startle, and open field conflict data. Mann-Whitney U for continuous variables (due to non normality of the distribution of the data points) and Chi-Square for discrete variables were used to analyze behavior in the swim test. ANOVA was used to evaluate ChAT+IR. Spearman correlations were used to determine significance between the behavioral and measures of ChAT+IR neuronal density. Significance was taken at p<0.05.
3.0 Results
3.1 Body weight and BACs
As seen in figure 1, all rats gained weight over the course of the experiment. Rats grew in both groups from about 50 g at PD 22 to about 360 g being 373.6(± 9.4) and 352.1 (± 6.8) for control and ethanol at PD 72 respectively. Ethanol vapor exposed rats showed parallel increases in weight gain to controls, although ethanol vapor rats had slightly reduced body weights at 3 of the 27 weightings, e.g. at ages PD 48, PD 52 and PD 55 (p<0.05) as seen in figure 1. Both groups had similar body weights during all behavioral tests and at sacrifice. Blood alcohol was measured 8 times during the 5 week vapor exposure period as graphically represented in figure 1. Mean blood alcohol levels (±S.D.) over the 5 week period were 162.8 (± 7.85) mg/dL. These blood alcohol levels are consistent with this protocol being a model of adolescent binge drinking.
3.2 Locomotor behavior
Locomotor behavior was assessed during the alcohol exposure period at 2 time points, PD 32, 10 days into vapor exposure and PD 42–44, 20 days into vapor exposure, 8 hrs after the termination of vapor delivery for that 24 hr period. Locomotor activity tended to decrease in controls, consistent with a decline in activity during maturation within adolescence. Ethanol treated animals were almost twice as active, as assessed by the number of beam breaks, at both 10 days of vapor treatment and 20 days of vapor treatment. ANOVA with repeated measures revealed that in the first locomotor measurement session, at 10 days into vapor exposure, ethanol exposed animals exhibited significantly more locomotor activity than air exposed controls (group effect: F=17.9, df=1,35, p<0.0001) as seen in figure 2. As also seen in figure 2 enhanced locomotor activity was also found in ethanol vapor treated animals in the second test session that occurred 20 days following alcohol exposure (group effect: F=9.28, df=1,35, p<0.004). However at 24 hrs after the final withdrawal of ethanol vapor, ethanol exposed animals did not differ from controls in their locomotor activity levels.
Figure 2.
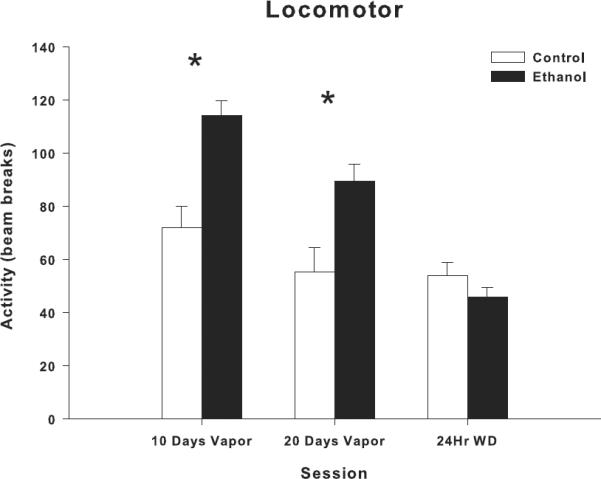
Effects of adolescent ethanol vapor exposure on locomotor behavior. Ethanol-exposed rats (n=24) showed a significant increase in locomotor activity at both PD 32 (10 days of vapor exposure) and PD 42–44 (20 days of vapor exposure), compared to controls (n=12). Ethanol-exposed and control rats showed no difference in locomotor activity levels 24 hrs after the final withdrawal from ethanol vapor exposure. Locomotor behavior is expressed as activity obtained by quantifying beam breaks. * indicates P< 0.05 for significant difference from control rats. Error bars=S.E.M.
3.3 Startle and Pre-pulse inhibition of the startle
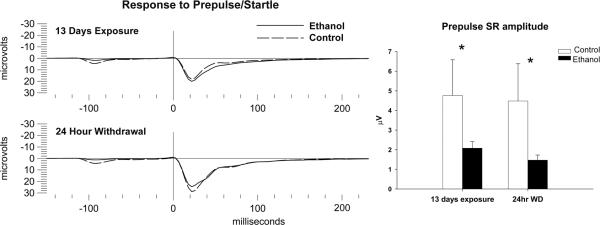
ASR and PPI were assessed at 3 different time points during and after the ethanol exposure period on PD 35–36, PD 49–51, and 24 hrs after final withdrawal from ethanol vapor at PD58. One way ANOVA revealed that there were no significant differences in the response to the pulse tone of the startle response between ethanol exposed animals and controls at any of the 3 time points. Additionally there were no significantly different values for the response of the pulse tone following presentation of the prepulse between ethanol and control animals at any of the 3 time points. However, response to the prepulse alone, 100 msec prior to the pulse tone, was found to be significantly diminished in ethanol-exposed animals as compared to controls at the P35, 13 day time point (F=3.94, df=1,35, p<0.05) and at 24 hrs following withdrawal (F=4.79, df=1,35, p<0.05) as seen in figure 3.
Figure 3.
Effects of adolescent ethanol vapor exposure on the Acoustic Startle Response (ASR) and Prepulse Inhibition (PPI). (Left) Representative grand averages of the Prepulse/Startle response from ethanol-exposed (n=24) and control (n=12) groups at PD 35 (13 days of vapor exposure) and PD 58 (24 hrs after the final withdrawal from ethanol vapor exposure). (Right) Ethanol vapor exposure significantly reduced the amplitude of the startle response to the prepulse tone during both PD 35 and PD 58 time points, compared to controls. Control group = Dashed lines, Ethanol-exposed group = continuous lines. * indicates P< 0.05 for significant difference from control rats. Error bars= S.E.M.
3.4 Behavior in the modified open field conflict test
Behavior in the modified open field was assessed at PD 64, 7 days after ethanol exposure ended. Although on the test day, there were no differences in body weight between ethanol and control rats, ethanol exposed rats approached the food 50% more (F = 9.37, df=1,35, p<0.004, Fig 4B), and ate almost twice as much food as controls (F = 6.15, df=1,35, p<.018, Fig 4A). They also spent significantly more time in contact with food (F= 6.12, df=1,35, p<0.018, Fig 4C). Spending more time in contact with food in the open field conflict test suggests that the ethanol exposed animals may be displaying more disinhibitory behaviors or less “anxiety-like” behaviors as compared to control animals.
Figure 4.
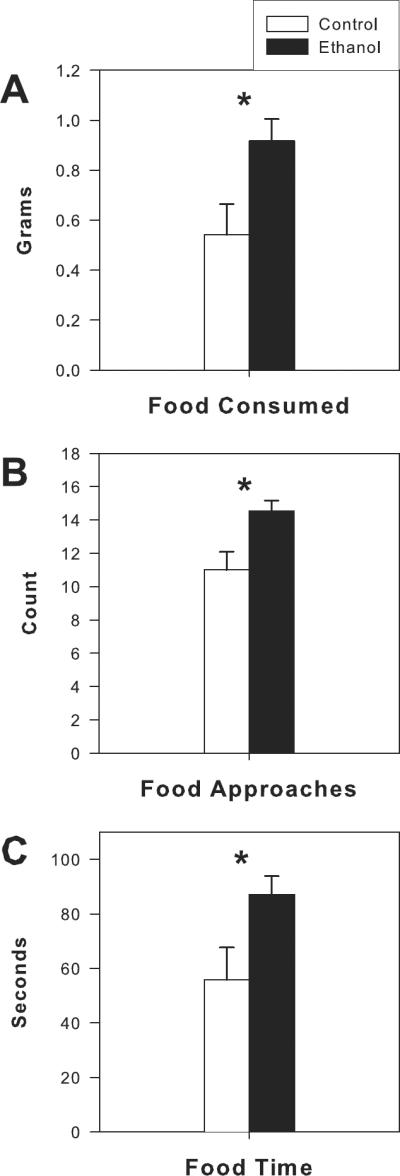
Effects of adolescent ethanol vapor exposure on the modified open field conflict test. Ethanol-exposed (n=24) rats showed a significant increase in food consumption (A), food approaches (B) and time in contact with food (C) at PD 64 (7 days after the final withdrawal from ethanol vapor exposure). * indicates P< 0.05 for significant difference from control rats (n=12). Error bars= S.E.M.
3.5 Behavior in the Forced Swim test
Behavior in the forced swim test was assessed at PD 69–70, 12 days after ethanol exposure ended. On the test day, there were no differences in body weight between ethanol and control rats. Air-exposed controls did not differ from the vapor-exposed animals on: latency to immobility, or amount of time spent swimming in the test. However Mann-Whitney U revealed that ethanol exposed animals had significantly more episodes of immobility and/or number of sinkings that controls (ethanol exposed=9.0 ± 1.9, controls=3.42 ± 1.37; U=84.5, p<0.045) and also were significantly more likely to defecate during the test session (ethanol exposed=23/24, control=8/12; Chi-Square: 5.69, p<0.034). Thus, the ethanol vapor-exposed animals displayed behavioral signs indicative of “increased stress” also interpreted as an increased “depressive-like” state in the forced swim test.
3.6 Immunohistochemistry (IHC) for choline acetyltransferase (ChAT)
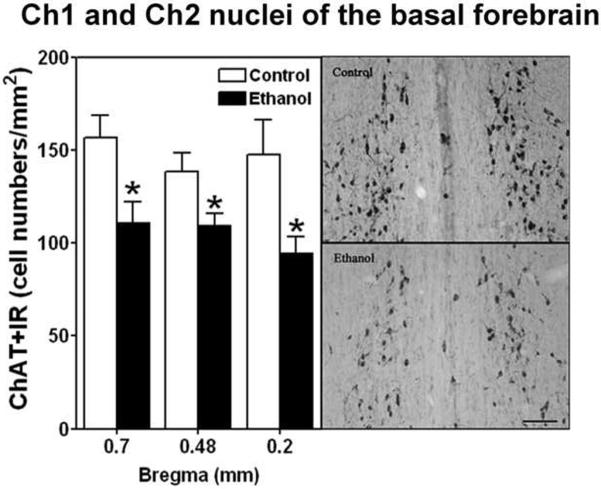
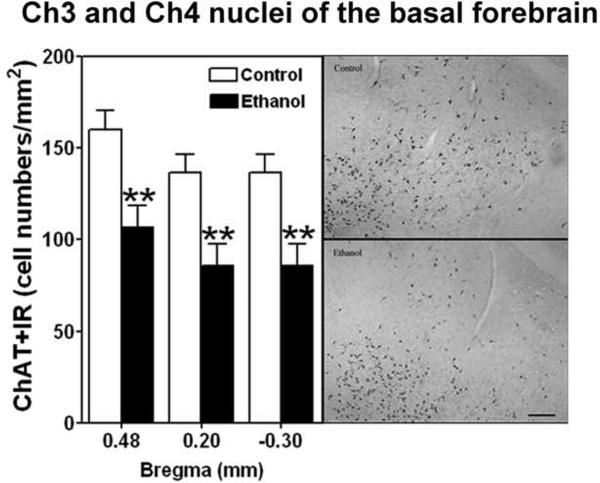
IHC for ChAT was used to assess the density of cholinergic neurons in the four major cholinergic nuclei in the basal forebrain (Ch1–4) on day 71–72 14 to 15 days following termination of 35 days of ethanol vapor exposure. The Ch 1,2 and Ch 3,4 sectors were combined for analyses. Figure 5 gives a representative photomicrograph of the location of the four regions. Figure 6 displays the cell density of ChAT+IR in the ethanol vapor exposed and control rats and demonstrates that vapor exposed rats have significantly decreased cell density in the Ch1, Ch2 sectors at the 0.70 mm (F=9.7, df=1,10, p<0.01), 0.48mm (F=6.86, df=1,10, p<0.026) and 0.2 mm (F=8.79, df=1,7, p<0.02) from bregma locations. The density of ChAT+IR neurons was also significantly decreased in the Ch3, Ch4 sector regions at 0.48 mm (F=10.60, df=1,9, p<0.01), 0.02 mm (F=23.88, df=1,9, p<0.001) and −0.30 mm (F=8.6, df=1,11, p<0.013) from bregma locations as seen in Figure 7. This reduction in ChAT+IR cell counts was significantly correlated with the increase in disinhibitory behavior (increased time spent in contact with food) in the open field conflict test in Ch1,2 at the 0.2 mm from bregma position (Spearmans rho= 0.75, p<0.02), and at Ch3,4 at the 0.48 mm (r=0.78, p<0.004), 0.20 mm (r=0.78, p<0.004) and −0.30 mm (r=0.56, p<0.046) from bregma position.
Figure 5.
Representative photomicrograph of the locations of the four major cholinergic nuclei (Ch1-Ch4) in the basal forebrain. The Ch1 and Ch2 nuclei are contained in the medial septal nucleus and the vertical limb nucleus of the diagonal band, respectively. However, there are no definite boundaries between Ch1 and Ch2 nuclei. The Ch3 sector is contained within the horizontal limb nucleus of the diagonal band, and Ch4 is within the nucleus basalis, and also in parts of the diagonal band, and these nuclei were combined for count. The darkened area at −0.30 mm from bregma was measured as Ch3 and part of Ch4 nuclei. The numbers on the right of each slice indicate distance (mm) from bregma.
Figure 6.
Left side, Adolescent intermittent ethanol (AIE) exposure decreases cholinergic neurons (ChAT+IR) in the Ch1 and Ch2 nuclei of the basal forebrain of adult rats. The cell density of ChAT+IR is significantly decreased in the Ch1 and Ch2 sectors at 0.70 (ethanol n=6, controls n=4) 0.48 (ethanol n=5, control n=4) and 0.20 (ethanol n=4, controls n=3) mm from bregma after ethanol treatment. * indicates P< 0.05 for significant difference from control rats. Error bars= S.E.M. Right side, Representative photomicrography ChAT+IR neurons in the Ch1 and Ch2 nuclei from control and ethanol (bregma from 0.7 to 0.20 mm) are shown on the right side. Scale bar=50 μm.
Figure 7.
Left side, Adolescent intermittent ethanol (AIE) exposure decreases cholinergic neurons (ChAT+IR) in the Ch3 and Ch4 nuclei of the basal forebrain of adult rats. The cell density of ChAT+IR is significantly decreased in the Ch3 and Ch4 nuclei at 0.48 (ethanol n=5, controls n=3), 0.20 (ethanol n=5, controls n=3) and −0.30 (ethanol n=6, controls n=4) mm from bregma after ethanol treatment. ** indicates P< 0.01 for significant difference from control rats. Error bars= S.E.M. Right side, Representative photomicrography ChAT+IR neurons in the Ch3 and Ch4 nuclei from control and ethanol are shown on the right side. Scale bar=100 μm
4.0 Discussion
In the present study, rats were exposed to ethanol vapors during the periadolescent period in order to examine ethanol's effects on cholinergic neurons in the basal forebrain and correlated behavioral changes. Ethanol vapor exposed rats displayed: increased locomotor activity 8 hrs after the termination of vapor delivery for that 24 hr period at day 10 and day 20 of alcohol vapor exposure, significant reductions in the amplitude of their responses to prepulse stimuli during the startle paradigm at 24 hrs withdrawal, and at 2 weeks following withdrawal, less anxiety-like and/or more “disinhibitory” behavior in the open field conflict, and more immobility in the forced swim test. Quantitative analyses of ChAT immunoreactivity (+IR) revealed a significant reduction in cell counts in the Ch1–2 and Ch3–4 regions of the basal forebrain in ethanol vapor exposed rats. This reduction in cell counts was significantly and selectively correlated with less anxiety-like and/or more “disinhibitory” behavior in the open field conflict test. These studies demonstrate that behavioral measures of arousal and affective state, and ChAT+IR, are all significantly impacted by chronic adolescence ethanol exposure and withdrawal in Wistar rats, and further suggest that adolescent ethanol induced loss of ChAT could underlie persistent changes in adult disinhibitory behaviors.
Consistent with our previous studies (Slawecki and Ehlers, 2002), alcohol administration via vapor produced a transient lag in weight gain during the exposure period. In the present study, this weight reduction was only present for a short period (PD-48–55) during the end of vapor exposure and was not significant after vapor was terminated. The increase in motor activity, seen 8 hrs after the termination of vapor delivery for that 24 hr period, at day 10 and day 20 of alcohol vapor exposure, is also consistent with previous studies in adolescents (Slawecki et al., 2005) and adults (Ehlers and Chaplin, 1987) following ethanol vapor exposure and acute withdrawal. By monitoring motor activity during ethanol exposure, it was also demonstrated that ethanol reached physiologically relevant levels able to induce signs of early withdrawal.
The ASR is a neurobehavioral measure that is known to be affected by chronic ethanol exposure. The ASR is decreased after ethanol administration/ consumption in rodents and humans (Pohorecky et al., 1976; Rassnick et al., 1992; Grillon et al., 2000; Hutchison et al., 2003). During the early phases of ethanol withdrawal, ASR is increased (Pohorecky et al., 1976; Macey et al., 1996; Krystal et al., 1997; Chester et al., 2004). It has been suggested the enhanced ASR during the early phases of withdrawal from drugs is an index of increased anxiety (Harris and Gewirtz, 2004). As such, assessment of the ASR can provide an index of persistent anxiety-like behavior after adolescent ethanol exposure. Prepulse inhibition (PPI) is a measure that is derived from the ASR. It measures the ability of low-intensity acoustic stimuli presented just before the startle eliciting stimulus to reduce the magnitude of the ASR. It is considered to be an index of sensorimotor gating (Koch and Schnitzler, 1997; Swerdlow et al., 2001). As such, selective alterations in PPI after adolescent ethanol exposure could influence subsequent cognitive function. It has been reported that acute ethanol administration or consumption of ethanol reduces PPI (Jones et al., 2000; Hutchison et al., 2003). In addition, decreased PPI has been reported during the acute phase of ethanol withdrawal in rats treated as adults (Rassnick et al., 1992). In the present study, a reduced behavioral response to the PPI tone was observed, in rats exposed to alcohol vapor during adolescence, at 24 hrs following withdrawal. These data suggest adolescent alcohol treatment reduces sensorimotor gating during alcohol withdrawal.
Adolescent alcohol exposure was also found to produce more long lasting effects on measures derived from the open field test. In the standard open field, decreased time and/or entries into the center squares have been suggested to serve as indices of enhanced anxiety whereas; increased time spent in the center of the open field can indicate disinhibitory behavior (Sarbadhikari et al., 1996; Blokland et al., 2002; Bowman et al., 2002; Yilmazer-Hanke et al., 2002). In the modified open field, an anxiety-like profile is characterized by decreased time spent in contact with food, decreased approaches to food and decreased food eaten whereas the opposite responses would indicate more disinhibitory behavior (Britton and Britton, 1981; Britton et al., 1982; Rex et al., 1998). A disinhibitory profile of behaviors was observed in rats exposed to alcohol vapors during adolescence. Food contact time, in the modified open-field test, is not influenced by overall activity levels suggesting that these behaviors are not likely related to simply an increase in overall activity levels and/or increased level of arousal. In fact, it has been previously demonstrated that long term alcohol drinking (6 months) and 3 weeks of withdrawal produce reductions in measures of “anxiety” in the: open field, the plus maze, and in a punished drinking paradigm (Blokland et al., 1992). Therefore, increases in the average amount of time spent in contact with food during each approach in the modified open-field test in rats exposed to ethanol vapors during periadolescence suggests an increased “motivation” to enter the center of the open field, and/or less fear of open spaces or a combination of the two. Such behaviors may be a reflection of increased motivation to eat, perhaps driven by a greater hunger drive in ethanol exposed animals, resulting in more disinhibitory behavior. However, taken together, it seems reasonable to hypothesize that one of the protracted neurobehavioral effects of adolescent ethanol exposure may include disinhibition. Further assessment of disinhibitory behaviors using additional operant paradigms such as tasks requiring withholding an action to receive a reward (Flagel et al., 2010), will strengthen this hypothesis.
In the present study, we confirmed our previous findings that ethanol vapor exposed animals show differential behavior in the Forced swim test (FST) when compared with air-exposed controls after multiple weeks of withdrawal (Slawecki et al., 2004; Walker et al., 2010). Specifically, in the present study more immobility/sinking was seen following 2 weeks of withdrawal between air- and vapor-exposed animals. Furthermore, during protracted withdrawal, not only was differential immobility seen but vapor exposed animals also displayed more defecation during the test suggesting they may have been more “stressed” by the procedure. Thus, indices of depressive-like behavior changed for the ethanol vapor exposed group in a manner consistent with increased “depression”. The present data also lends support to clinical data showing that a proportion of individuals diagnosed with comorbid depression and alcohol dependence have a substance-induced disorder (Schuckit et al., 1997; Hasin and Grant, 2002). In clinical studies, it has been shown that major depressive symptoms generally last for 2 to 4 weeks after abstinence is initiated (Brown and Schuckit, 1988). However, individuals with symptoms of clinical depression after 1 month of abstinence also had a significantly greater incidence of withdrawal symptoms (Brown and Schuckit, 1988), suggesting that they may have had a greater severity of ethanol dependence before abstinence. Although the present study only tested for depressive-like behavior at two weeks abstinence, in a previous study depressive-like behavior was observed in adult rats after ethanol exposure for up to 8 weeks into abstinence (Walker et al., 2010) suggesting that substance-induced depression may potentially be long lasting. Taken together these studies suggest that ethanol exposure during adolescence can lead to increases in depressive-like behavior well into protracted abstinence.
Molecular and cellular adaptations to drug exposure are believed to lead to persistent changes in transcription, translation, synaptic morphology and function that are extremely long-lived and are analogous to the plastic processes that underlie learning and memory (Nestler, 2001; Ron and Jurd, 2005). In the present study, long-lasting changes in ChAT+IR were found after chronic ethanol exposure during adolescence in the basal forebrain in areas Ch1,2 and Ch 3,4. These data are consistent with previous studies using gene array methodology that found decreases in the expression of many cholinergic-specific genes including ChAT as well as all 5 subtypes of the muscarinic cholinergic receptors in young adult mice following adolescent binge alcohol treatment (Coleman et al., 2011). In those studies, reduced forebrain histologic areas and cholinergic neuron density were found using IHC in ethanol treated mice as compared to controls (Coleman et al., 2011). These findings are also consistent with previous studies in adult rats where prolonged chronic alcohol treatment has been shown to produce cholinergic hypoactivity in hippocampal and basal forebrain cholinergic structures (Arendt et al., 1988a,b, 1989, 1995; Hodges et al., 1991; Floyd et al., 1997; Savage et al., 2000; Cadete-Leite et al., 2003).
The basal forebrain, through widespread projections to cerebral cortex, plays an important role in the regulation of cortical processes and behavioral states such as sleep, learning, and memory (Everitt and Robbins, 1997; Sarter et al., 2003; Weinberger, 2003; Jones, 2004). Impairments in working and reference memory on the radial arm maze task seen following chronic ethanol treatment in adult rats (Hodges et al., 1991) and alterations in reversal learning seen in young adult mice after adolescent alcohol (Coleman et al., 2011) are congruent with reduced ChAT activity in the basal forebrain. Adolescent vapor treatment in rats has also been demonstrated to disrupt adult sleep and electrophysiology consistent with altered cholinergic systems (Ehlers and Criado, 2010). The “cholinergic deficit” in the reversal of maze performance produced by chronic ethanol exposure also appears to be reversed by cholinergic agonists and/or transplantation of ACh-rich fetal tissue (Arendt et al., 1989; Hodges et al., 1991). This has led some authors to suggest that the forebrain cholinergic system may be an important therapeutic target for the treatment of cognitive deficits associated with ethanol exposure (Vetreno et al., 2011).
In humans, chronic ethanol exposure that leads to amnesia associated with Wernike's encephalopathy and Korsakoff's psychosis (WKS) is also known to be associated with a dramatic reduction in neurons in the nucleus basalis (Ch4) in the basal forebrain (Arendt et al., 1983; Cullen et al., 1997). Animal models of WKS have been developed and reduced levels of AChE have been found in the cortex and hippocampus (Nakagawasai et al., 2000; Pires et al., 2001, 2005; Savage et al., 2007; Roland and Savage, 2009) and the forebrain (Zhao et al., 2008) in those models. These reductions in cholinergic tone have also been associated with deficits in passive avoidance and in the forced swim test (Nakagawasai et al., 2000, 2001), as well as deficits in memory on the Morris water maze (Pires et al., 2005). Some of the deficits seen in the WKS animal model can also be partially reversed by increasing hippocampal acetylcholine levels (Roland et al., 2008) or administering acetylecholinesterase inhibitors (Roland et al., 2010).
Loss of muscarinic cholinergic receptors from the temporal cortex of alcohol abusers with histologically normal brains in the absence of significant atrophy/and or dementia has also been reported (Freund and Ballinger, 1989). This suggests that cholinergic loss may precede the development of significant alcohol encephalopathy in adulthood. It has been suggested that mental dysfunction associated with alcohol-induced degeneration of the cholinergic pathway of the ascending activation system may cause a “syndrome of partial cholinergic deafferentation of the cortical mantle” (Arendt, 1994). Human studies using functional MRI (fMRI) to follow basal forebrain activation during cognitive tasks find alcoholic patients do not show normal basal forebrain activation (De Rosa et al., 2004). Our studies in animal models are consistent with the hypothesis that alcohol exposure during adolescence might also cause a selective loss of cholinergic signaling that, over time, may lead to significant cognitive deficits.
In the present study reductions in ChAT+IR were specifically found to be correlated with measures of behavioral disinhibition (food time, food approach) in the open field conflict test. Why the loss of cholinergic tone was found to be selectively associated with behavioral disinhibition in the present study is not known. Nicotine has been shown to produce disinhibitory behavior in the rats after subchronic peripheral nicotinic acetylchline receptor blockage (Ericson et al., 2000). It has also been suggested that response disinhibition in the variable-interval differential reinforcement of low rate responding and stop signal tasks are related in a systematic manner to nicotinic-acetylcholine receptor activation (Kirshenbaum et al., 2011). Thus it is possible that the loss of cholinergic tone produced by adolescent alcohol exposure seen in the present study could result in a “sensitization” of nicotinic receptors that promote the expression of disinhibitory behaviors under the conditions of stress/ food restriction such as those that occur in the modified open field conflict test.
Several authors have posited that acute and chronic effects of alcohol may cause toxic effects on developing brain systems that may result in an increase in affective, impulsive and or disinhibitory behaviors, which may in turn may facilitate further alcohol use (Crews and Boettiger, 2009; de Wit, 2009; White et al., 2011). Our data support the hypothesis that adolescent alcohol exposure can have significant effects on brain and behavior in an animal model where control of alcohol exposure can help delineate environmental effects from genetic background. However, the model of alcohol exposure used in the present study, 14 hours of daily vapor exposure, does not mimic the typical pattern of ethanol drinking in human adolescents who are more likely to experience intermittent binge drinking at weekly or monthly intervals. However, adolescence in the human may span a 10 year period whereas in the rat periadolescence is condenced into a period of 35 days. This exposure period, although not directly translatable to humans was selected to ensure that the animals were exposed during the entire extended periadolescent period (Spear, 2000). However, additional studies will be necessary to determine whether shorter intermittent periods of exposure produce similar effects, and to test whether such effects are persistent or represent a more transitory developmental phenomenon.
5.0 Conclusions
Our data suggest that rats exposed to daily ethanol vapor for 5 weeks over the adolescent period display: increased locomotor activity 8 hrs after the termination of vapor delivery for that 24 hr period at day 10 and day 20 of alcohol vapor exposure, significant reductions in the amplitude of their responses to prepulse stimuli during the startle paradigm at 24 hrs withdrawal, and at two weeks following withdrawal, less anxiety-like and/or more “disinhibitory” behavior in the open field conflict, and more immobility in the forced swim test. Quantitative analyses of ChAT immunoreactivity revealed a significant reduction in cell counts in the Ch1–2 and Ch3–4 regions of the basal forebrain in ethanol vapor exposed rats. This reduction in cell counts was significantly correlated with less anxiety-like and/or more “disinhibitory” behavior in the open field conflict test. These studies demonstrate that behavioral measures of arousal, affective state, disinhibitory behavior and ChAT+IR, are all significantly impacted by chronic adolescence ethanol exposure and withdrawal in Wistar rats.
Highlights
Adolescence ethanol exposure produces disinhibitory behavior at 2 weeks withdrawal
Adolescence ethanol exposure produces depressive-like behavior at 2 weeks withdrawal
Adolescent ethanol exposure reduced cholinergic neurons in basal forebrain
Reduced cholinergic neurons were correlated with more disinhibitory behavior
Acknowledgements
This study was supported in part by the NADIA Initiative Project of the NIH National Institute on Alcoholism and Alcohol Abuse grants AA019969 (CLE) and AA020022, AA020023 and AA020024 (FTC) and the UNC Bowles Center for Alcohol Studies. The authors thank Greta Berg for her assistance in data collection and Shirley Sanchez for editing the manuscript.
Abbreviations
- a.m.
ante meridiem
- ANOVA
analysis of variance
- ASR
Acoustic startle response
- BACs
blood alcohol concentrations
- C
degree Celsius
- ChAT
choline acetyltransferase
- Ch1-4
forebrain cholinergic neurons
- cm
centimeter
- dB
decibels
- FST
Force swim test
- g
grams
- hr
hours
- IHC
immunohistochemical
- IR
immunoreactivity
- mg/dl
milligrams per deciliter
- mg/kg
grams per kilogram
- mg%
milligrams percent
- msec
milliseconds
- mm
millimeters
- MS
Medial Septum
- NIH
National Institutes of Health
- p.m.
post meridiem
- PBS
phosphate buffered saline
- PD
postnatal day
- PPI
Prepulse inhibition
- VDB
nucleus of the vertical limb of the diagonal band
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arendt T, Bigl V, Arendt A, Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer's disease, paralysis agitans and Korsakoff's Disease. Acta Neuropathol. 1983;61:101–108. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- Arendt T, Henning D, Gray JA, Marchbanks R. Loss of neurons in the rat basal forebrain cholinergic projection system after prolonged intake of ethanol. Brain Res Bull. 1988a;21:563–569. doi: 10.1016/0361-9230(88)90193-1. [DOI] [PubMed] [Google Scholar]
- Arendt T, Allen Y, Sinden J, Schugens MM, Marchbanks RM, Lantos PL, Gray JA. Cholinergic-rich brain transplants reverse alcohol-induced memory deficits. Nature. 1988b;332:448–450. doi: 10.1038/332448a0. [DOI] [PubMed] [Google Scholar]
- Arendt T, Allen Y, Marchbanks RM, Schugens MM, Sinden J, Lantos PL, Gray JA. Cholinergic system and memory in the rat: effects of chronic ethanol, embryonic basal forebrain brain transplants and excitotoxic lesions of cholinergic basal forebrain projection system. Neuroscience. 1989;33:435–462. doi: 10.1016/0306-4522(89)90397-7. [DOI] [PubMed] [Google Scholar]
- Arendt T. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol. J Neural Transm Suppl. 1994;44:173–187. doi: 10.1007/978-3-7091-9350-1_13. [DOI] [PubMed] [Google Scholar]
- Arendt T, Bruckner MK, Pagliusi S, Krell T. Degeneration of rat cholinergic basal forebrain neurons and reactive changes in nerve growth factor expression after chronic neurotoxic injury--I. Degeneration and plastic response of basal forebrain neurons. Neuroscience. 1995;65:633–645. doi: 10.1016/0306-4522(94)00526-b. [DOI] [PubMed] [Google Scholar]
- Blokland A, Prickaerts J, Raaijmakers W. Reduced level of anxiety in adult Lewis rats after chronic ethanol consumption. Physiol Behav. 1992;51:245–248. doi: 10.1016/0031-9384(92)90137-q. [DOI] [PubMed] [Google Scholar]
- Blokland A, Lieben C, Deutz NE. Anxiogenic and depressive-like effects, but no cognitive deficits, after repeated moderate tryptophan depletion in the rat. J Psychopharmacol. 2002;16:39–49. doi: 10.1177/026988110201600112. [DOI] [PubMed] [Google Scholar]
- Bowman RE, Ferguson D, Luine VN. Effects of chronic restraint stress and estradiol on open field activity, spatial memory, and monoaminergic neurotransmitters in ovariectomized rats. Neuroscience. 2002;113:401–410. doi: 10.1016/s0306-4522(02)00156-2. [DOI] [PubMed] [Google Scholar]
- Britton DR, Britton KT. A sensitive open field measure of anxiolytic drug activity. Pharmacol Biochem Behav. 1981;15:577–582. doi: 10.1016/0091-3057(81)90212-4. [DOI] [PubMed] [Google Scholar]
- Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- Brown SA, Schuckit MA. Changes in depression among abstinent alcoholics. J Stud Alcohol. 1988;49:412–417. doi: 10.15288/jsa.1988.49.412. [DOI] [PubMed] [Google Scholar]
- Cadete-Leite A, Pereira PA, Madeira MD, Paula-Barbosa MM. Nerve growth factor prevents cell death and induces hypertrophy of basal forebrain cholinergic neurons in rats withdrawn from prolonged ethanol intake. Neuroscience. 2003;119:1055–1069. doi: 10.1016/s0306-4522(03)00205-7. [DOI] [PubMed] [Google Scholar]
- Chester JA, Blose AM, Froehlich JC. Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcohol Clin Exp Res. 2004;28:677–687. doi: 10.1097/01.alc.0000125345.19665.09. [DOI] [PubMed] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcohol Clin Exp Res. 2008;32:375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Coleman LG, Jr., He J, Lee J, Styner M, Crews FT. Adolescent binge drinking alters adult brain neurotransmitter gene expression, behavior, brain regional volumes, and neurochemistry in mice. Alcohol Clin Exp Res. 2011;35:671–688. doi: 10.1111/j.1530-0277.2010.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Wills DN, Walker BM, Ehlers CL. Effects of adolescent ethanol exposure on sleep in adult rats. Alcohol. 2008;42:631–639. doi: 10.1016/j.alcohol.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KM, Halliday GM, Caine D, Kril JJ. The nucleus basalis (Ch4) in the alcoholic Wernicke-Korsakoff syndrome: reduced cell number in both amnesic and non-amnesic patients. J Neurol Neurosurg Psychiatry. 1997;63:315–320. doi: 10.1136/jnnp.63.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Spear LP. Adolescent brain development: Vulnerabilities and opportunities. The New York Academy of Sciences; New York, N.Y: 2004. [DOI] [PubMed] [Google Scholar]
- De Rosa E, Desmond JE, Anderson AK, Pfefferbaum A, Sullivan EV. The human basal forebrain integrates the old and the new. Neuron. 2004;41:825–837. doi: 10.1016/s0896-6273(04)00080-7. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Spear LP. Developmental differences in acute ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2007;31:1516–1527. doi: 10.1111/j.1530-0277.2007.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draski LJ, Bice PJ, Deitrich RA. Developmental alterations of ethanol sensitivity in selectively bred high and low alcohol sensitive rats. Pharmacol Biochem Behav. 2001;70:387–396. doi: 10.1016/s0091-3057(01)00621-9. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Chaplin RI. Chronic ethanol exposure potentiates the locomotor-activating effects of corticotropin-releasing factor (CRF) in rats. Regul Pept. 1987;19:345–353. doi: 10.1016/0167-0115(87)90176-5. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Criado JR. Adolescent ethanol exposure: does it produce long-lasting electrophysiological effects? Alcohol. 2010;44:27–37. doi: 10.1016/j.alcohol.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Olausson P, Engel JA, Soderpalm B. Nicotine induces disinhibitory behavior in the rat after subchronic peripheral nicotinic acetylcholine receptor blockade. Eur J Pharmacol. 2000;397:103–111. doi: 10.1016/s0014-2999(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, Phillips PE, Akil H. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd EA, Young-Seigler AC, Ford BD, Reasor JD, Moore EL, Townsel JG, Rucker HK. Chronic ethanol ingestion produces cholinergic hypofunction in rat brain. Alcohol. 1997;14:93–98. doi: 10.1016/s0741-8329(97)86147-2. [DOI] [PubMed] [Google Scholar]
- Freund G, Ballinger WE., Jr. Loss of muscarinic cholinergic receptors from the temporal cortex of alcohol abusers. Metab Brain Dis. 1989;4:121–141. doi: 10.1007/BF00999390. [DOI] [PubMed] [Google Scholar]
- Funk CK, O'Dell LE, Crawford EF, Koob GF. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006;26:11324–11332. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lenroot RK, Shaw P, Lalonde F, Celano M, White S, Tossell J, Addington A, Gogtay N. Trajectories of anatomic brain development as a phenotype. Novartis Found Symp. 2008;289:101–112. doi: 10.1002/9780470751251.ch9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci Chapter 9:Unit. 2008 doi: 10.1002/0471142301.ns0929s44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolf NJ, Butcher LL. Postnatal development of cholinergic neurons in the rat: I. Forebrain. Brain Res Bull. 1991;27:767–789. doi: 10.1016/0361-9230(91)90209-3. [DOI] [PubMed] [Google Scholar]
- Grillon C, Sinha R, Ameli R, O'Malley SS. Effects of alcohol on baseline startle and prepulse inhibition in young men at risk for alcoholism and/or anxiety disorders. J Stud Alcohol. 2000;61:46–54. doi: 10.15288/jsa.2000.61.46. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. J Child Adolesc Subst Abuse. 2011;20:135–154. doi: 10.1080/1067828X.2011.555272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC. Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacology (Berl) 2004;171:140–147. doi: 10.1007/s00213-003-1573-0. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Grant BF. Major depression in 6050 former drinkers: association with past alcohol dependence. Arch Gen Psychiatry. 2002;59:794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- Hodges H, Allen Y, Sinden J, Mitchell SN, Arendt T, Lantos PL, Gray JA. The effects of cholinergic drugs and cholinergic-rich foetal neural transplants on alcohol-induced deficits in radial maze performance in rats. Behav Brain Res. 1991;43:7–28. doi: 10.1016/s0166-4328(05)80048-8. [DOI] [PubMed] [Google Scholar]
- Hutchison KE, McGeary J, Wooden A, Blumenthal T, Ito T. Startle magnitude and prepulse inhibition: effects of alcohol and attention. Psychopharmacology (Berl) 2003;167:235–241. doi: 10.1007/s00213-002-1332-7. [DOI] [PubMed] [Google Scholar]
- Jain R, Balhara YP. Impact of alcohol and substance abuse on adolescent brain: a preclinical perspective. Indian J Physiol Pharmacol. 2010;54:213–234. [PubMed] [Google Scholar]
- Johnston MV. Neurotransmitters and vulnerability of the developing brain. Brain Dev. 1995;17:301–306. doi: 10.1016/0387-7604(95)00079-q. [DOI] [PubMed] [Google Scholar]
- Jones AE, McBride WJ, Murphy JM, Lumeng L, Li T, Shekhar A, McKinzie DL. Effects of ethanol on startle responding in alcohol-preferring and -non-preferring rats. Pharmacol Biochem Behav. 2000;67:313–318. doi: 10.1016/s0091-3057(00)00363-4. [DOI] [PubMed] [Google Scholar]
- Jones BE. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res. 2004;145:157–169. doi: 10.1016/S0079-6123(03)45011-5. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HB. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AP, Jackson ER, Brown SJ, Fuchs JR, Miltner BC, Doughty AH. Nicotine-induced impulsive action: sensitization and attenuation by mecamylamine. Behav Pharmacol. 2011;22:207–221. doi: 10.1097/FBP.0b013e328345ca1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats--circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Kostovic I. Structural and histochemical reorganization of the human prefrontal cortex during perinatal and postnatal life. Prog Brain Res. 1990;85:223–239. doi: 10.1016/s0079-6123(08)62682-5. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Grillon C, Cooney N, Casal L, Morgan CA, III, Southwick SM, Davis M, Charney DS. Evidence of acoustic startle hyperreflexia in recently detoxified early onset male alcoholics: modulation by yohimbine and mchlorophenylpiperazine (mCPP) Psychopharmacology (Berl) 1997;131:207–215. doi: 10.1007/s002130050285. [DOI] [PubMed] [Google Scholar]
- Lishman WA. Alcoholic dementia: a hypothesis. Lancet. 1986;1:1184–1186. doi: 10.1016/s0140-6736(86)91162-1. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Petit TL. Neocortical synaptogenesis, aging, and behavior: lifespan development in the motor-sensory system of the rat. Exp Neurol. 1987;96:262–278. doi: 10.1016/0014-4886(87)90045-8. [DOI] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Duncan GE, Knapp DJ, Breese GR. Sensitivity to ethanol across development in rats: comparison to [3H]zolpidem binding. Alcohol Clin Exp Res. 1998;22:1485–1492. [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawasai O, Tadano T, Hozumi S, Tan-no K, Niijima F, Kisara K. Immunohistochemical estimation of brain choline acetyltransferase and somatostatin related to the impairment of avoidance learning induced by thiamine deficiency. Brain Res Bull. 2000;52:189–196. doi: 10.1016/s0361-9230(00)00248-3. [DOI] [PubMed] [Google Scholar]
- Nakagawasai O, Tadano T, Hozumi S, Taniguchi R, Tan-no K, Esashi A, Niijima F, Kisara K. Characteristics of depressive behavior induced by feeding thiamine-deficient diet in mice. Life Sci. 2001;69:1181–1191. doi: 10.1016/s0024-3205(01)01206-1. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, Skinner MK. Puberty in the rat. In: Knobil E, Neill JD, editors. Knobil and Neill's physiology of reproduction. Elsevier; Amsterdam: 2006. pp. 2061–2126. [Google Scholar]
- Pian JP, Criado JR, Ehlers CL. Differential effects of acute alcohol on prepulse inhibition and event-related potentials in adolescent and adult Wistar rats. Alcohol Clin Exp Res. 2008b;32:2062–2073. doi: 10.1111/j.1530-0277.2008.00794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pian JP, Criado JR, Walker BM, Ehlers CL. Differential effects of acute alcohol on EEG and sedative responses in adolescent and adult Wistar rats. Brain Res. 2008a;1194:28–36. doi: 10.1016/j.brainres.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires RG, Pereira SR, Pittella JE, Franco GC, Ferreira CL, Fernandes PA, Ribeiro AM. The contribution of mild thiamine deficiency and ethanol consumption to central cholinergic parameter dysfunction and rats' open-field performance impairment. Pharmacol Biochem Behav. 2001;70:227–235. doi: 10.1016/s0091-3057(01)00593-7. [DOI] [PubMed] [Google Scholar]
- Pires RG, Pereira SR, Oliveira-Silva IF, Franco GC, Ribeiro AM. Cholinergic parameters and the retrieval of learned and re-learned spatial information: a study using a model of Wernicke-Korsakoff Syndrome. Behav Brain Res. 2005;162:11–21. doi: 10.1016/j.bbr.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA, Cagan M, Brick J, Jaffe SL. The startle response in rats: effect of ethanol. Pharmacol Biochem Behav. 1976;4:311–316. doi: 10.1016/0091-3057(76)90247-1. [DOI] [PubMed] [Google Scholar]
- Rajendran P, Spear LP. The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. Ann N Y Acad Sci. 2004;1021:441–444. doi: 10.1196/annals.1308.060. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Koob GF, Geyer MA. Responding to acoustic startle during chronic ethanol intoxication and withdrawal. Psychopharmacology (Berl) 1992;106:351–358. doi: 10.1007/BF02245417. [DOI] [PubMed] [Google Scholar]
- Rex A, Voigt JP, Voits M, Fink H. Pharmacological evaluation of a modified open-field test sensitive to anxiolytic drugs. Pharmacol Biochem Behav. 1998;59:677–683. doi: 10.1016/s0091-3057(97)00461-9. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Cole M, Koob GF. Intra-amygdala muscimol decreases operant ethanol self- administration in dependent rats. Alcohol Clin Exp Res. 1996;20:1289–1298. doi: 10.1111/j.1530-0277.1996.tb01125.x. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rogers J, Wiener SG, Bloom FE. Long-term ethanol administration methods for rats: advantages of inhalation over intubation or liquid diets. Behav Neural Biol. 1979;27:466–486. doi: 10.1016/s0163-1047(79)92061-2. [DOI] [PubMed] [Google Scholar]
- Roland JJ, Mark K, Vetreno RP, Savage LM. Increasing hippocampal acetylcholine levels enhance behavioral performance in an animal model of diencephalic amnesia. Brain Res. 2008;1234:116–127. doi: 10.1016/j.brainres.2008.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Savage LM. The role of cholinergic and GABAergic medial septal/diagonal band cell populations in the emergence of diencephalic amnesia. Neuroscience. 2009;160:32–41. doi: 10.1016/j.neuroscience.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland JJ, Levinson M, Vetreno RP, Savage LM. Differential effects of systemic and intraseptal administration of the acetylcholinesterase inhibitor tacrine on the recovery of spatial behavior in an animal model of diencephalic amnesia. Eur J Pharmacol. 2010;629:31–39. doi: 10.1016/j.ejphar.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Jurd R. The “ups and downs” of signaling cascades in addiction. Sci STKE. 2005;2005:re14. doi: 10.1126/stke.3092005re14. [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Lewis DA. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biol Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Sarbadhikari SN, Dey S, Ray AK. Chronic exercise alters EEG power spectra in an animal model of depression. Indian J Physiol Pharmacol. 1996;40:47–57. [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Givens B. Attentional functions of cortical cholinergic inputs: what does it mean for learning and memory? Neurobiol Learn Mem. 2003;80:245–256. doi: 10.1016/s1074-7427(03)00070-4. [DOI] [PubMed] [Google Scholar]
- Savage LM, Candon PM, Hohmann HL. Alcohol-induced brain pathology and behavioral dysfunction: using an animal model to examine sex differences. Alcohol Clin Exp Res. 2000;24:465–475. [PubMed] [Google Scholar]
- Savage LM, Roland J, Klintsova A. Selective septohippocampal - but not forebrain amygdalar - cholinergic dysfunction in diencephalic amnesia. Brain Res. 2007;1139:210–219. doi: 10.1016/j.brainres.2006.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL. Comparison of induced and independent major depressive disorders in 2,945 alcoholics. Am J Psychiatry. 1997;154:948–957. doi: 10.1176/ajp.154.7.948. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22:670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128:63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res. 2002;26:246–254. [PubMed] [Google Scholar]
- Slawecki CJ, Ehlers CL. Lasting effects of adolescent nicotine exposure on the electroencephalogram, event related potentials, and locomotor activity in the rat. Brain Res Dev Brain Res. 2002;138:15–25. doi: 10.1016/s0165-3806(02)00455-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Grahame NJ, Roth J, Katner SN, Ehlers CL. EEG and ERP profiles in the high alcohol preferring (HAP) and low alcohol preferring (LAP) mice: relationship to ethanol preference. Brain Res. 2003;961:243–254. doi: 10.1016/s0006-8993(02)03959-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Ann N Y Acad Sci. 2004;1021:448–452. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J. Comparison of the onset of hypoactivity and anxiety-like behavior during alcohol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2004;28:598–607. doi: 10.1097/01.alc.0000122767.69206.1b. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Antagonism of neuropeptide YY1 receptors does not inhibit ethanol's effects on cortical EEG and ERPs in Wistar rats. J Stud Alcohol. 2005;66:559–566. doi: 10.15288/jsa.2005.66.559. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Ehlers CL. Enhanced prepulse inhibition following adolescent ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res. 2005;29:1829–1836. doi: 10.1097/01.alc.0000183024.47167.27. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Roth J, Gilder DA. Neurobehavioral profiles during the acute phase of ethanol withdrawal in adolescent and adult Sprague-Dawley rats. Behav Brain Res. 2006;170:41–51. doi: 10.1016/j.bbr.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage. 1999a;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999b;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16:83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol. 2010;52:236–243. doi: 10.1002/dev.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009a;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009b;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res. 1995;19:1480–1485. doi: 10.1111/j.1530-0277.1995.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 2001;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP, Spear NE. Social behavior and social motivation in adolescent rats: role of housing conditions and partner's activity. Physiol Behav. 1999;67:475–482. doi: 10.1016/s0031-9384(98)00285-6. [DOI] [PubMed] [Google Scholar]
- Vetreno RP, Hall JM, Savage LM. Alcohol-related amnesia and dementia: Animal models have revealed the contributions of different etiological factors on neuropathology, neurochemical dysfunction and cognitive impairment. Neurobiol Learn Mem. 2011 Jan 21; doi: 10.1016/j.nlm.2011.01.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Drimmer DA, Walker JL, Liu T, Mathe AA, Ehlers CL. Effects of prolonged ethanol vapor exposure on forced swim behavior, and neuropeptide Y and corticotropin-releasing factor levels in rat brains. Alcohol. 2010;44:487–493. doi: 10.1016/j.alcohol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiol Learn Mem. 2003;80:268–284. doi: 10.1016/s1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002a;73:673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- White AM, Bae JG, Truesdale MC, Ahmad S, Wilson WA, Swartzwelder HS. Chronic-intermittent ethanol exposure during adolescence prevents normal developmental changes in sensitivity to ethanol-induced motor impairments. Alcohol Clin Exp Res. 2002b;26:960–968. doi: 10.1097/01.ALC.0000021334.47130.F9. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults. Recent Dev Alcohol. 2005;17:161–176. doi: 10.1007/0-306-48626-1_8. [DOI] [PubMed] [Google Scholar]
- White HR, Marmorstein NR, Crews FT, Bates ME, Mun EY, Loeber R. Associations between heavy drinking and changes in impulsive behavior among adolescent boys. Alcohol Clin Exp Res. 2011;35:295–303. doi: 10.1111/j.1530-0277.2010.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmazer-Hanke DM, Faber-Zuschratter H, Linke R, Schwegler H. Contribution of amygdala neurons containing peptides and calcium-binding proteins to fear-potentiated startle and exploration-related anxiety in inbred Roman high- and low-avoidance rats. Eur J Neurosci. 2002;15:1206–1218. doi: 10.1046/j.1460-9568.2002.01945.x. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Bell RL, Ringham HN, Sullivan EV, Witzmann FA, Pfefferbaum A. Ethanol-induced changes in the expression of proteins related to neurotransmission and metabolism in different regions of the rat brain. Pharmacol Biochem Behav. 2011;99:428–436. doi: 10.1016/j.pbb.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Zhong C, Wang Y, Zhao Y, Gong N, Zhou G, Xu T, Hong Z. Impaired hippocampal neurogenesis is involved in cognitive dysfunction induced by thiamine deficiency at early pre-pathological lesion stage. Neurobiol Dis. 2008;29:176–185. doi: 10.1016/j.nbd.2007.08.014. [DOI] [PubMed] [Google Scholar]