Abstract
Orthopaedic gene therapy has been the topic of considerable research for two decades. The preclinical data are impressive and many orthopaedic conditions are well suited to genetic therapies. But there have been few clinical trials and no FDA-approved product exists. This paper examines why this is so. The reasons are multifactorial. Clinical translation is expensive and difficult to fund by traditional academic routes. Because gene therapy is viewed as unsafe and risky, it does not attract major funding from the pharmaceutical industry. Start-up companies are burdened by the complex intellectual property environment and difficulties in dealing with the technology transfer offices of major universities. Successful translation requires close interactions between scientists, clinicians and experts in regulatory and compliance issues. It is difficult to create such a favourable translational environment. Other promising fields of biological therapy have contemplated similar frustrations approximately 20 years after their founding, so there seem to be more general constraints on translation that are difficult to define. Gene therapy has noted some major clinical successes in recent years, and a sense of optimism is returning to the field. We hope that orthopaedic applications will benefit collaterally from this upswing and move expeditiously into advanced clinical trials.
Introduction
Life as an orthopaedic gene therapy researcher can be embarrassing at times. After 20 years of promises, there is no FDA-approved product and few clinical trials, only one of which, for rheumatoid arthritis (RA), completed Phase II [Evans, 2010; Mease et al., 2010]. And even this milestone was marred by the death of one of the subjects in the study, albeit unrelated to the gene transfer [Evans et al., 2008; Frank et al., 2009]. Given that the science behind orthopaedic gene therapy is generally sound, and the pre-clinical data impressive, why is its clinical realization so slow? The lack of clinical trials is all the more puzzling because many orthopaedic conditions are well suited for gene therapies[Evans, 2004].The present article examines this matter.
Gene therapy basics
Conceptually, gene therapy is simple. By compensating for individual mutations, genes can be used medicinally to treat Mendelian disorders. For non-Mendelian disorders gene transfer can serve to provide therapeutic gene products, both RNA and protein, to the patient.
Gene transfer requires the use of vectors that deliver the therapeutic genes, or more usually their cDNAs, to the target cells in ways that ensure the appropriate level and duration of transgene expression. Viral vectors are particularly efficient in this regard. Commonly used viral vectors include those derived from adenoviruses, adeno-associated viruses (AAV), oncoretroviruses, lentiviruses and herpes simplex viruses. Non-viral vectors are less efficient, but have advantages that include lower cost and easier production. They also raise less safety issues. Non-viral vectors are often simple plasmids that can be combined with carriers, such as lipsosomes, polymers or other materials, to enhance uptake and expression. Transfection may be improved by physical means, such as electroporation and sonication.
Vectors may be introduced directly into the patient (in vivo delivery) or introduced indirectly via cells that are genetically modified outside the body and then implanted, injected or infused (ex vivo delivery) The choice of vector and its mode of application depend upon the indication; orthopaedic examples are given below.
In certain settings it is necessary to regulate the level and duration of transgene expression. This can be achieved by the judicial use of promoters and other regulatory mechanisms, or take advantage of the biology of the target cell or the host’s response to the vector.
Orthopaedic applications of gene therapy
It is possible to define four areas of orthopaedics where gene therapy might be clinically useful [Evans et al., 2005a]: Mendelian disorders; tumors; arthritis and other joint diseases; tissue repair and regenerative medicine (TERM). Although there has been some pre-clinical progress in addressing genetic diseases such as osteogenesis imperfecta and the orthopaedic sequelae of lysosomal storage disorders, as well as in confronting certain orthopaedic tumors, most research has focused on arthritis and TERM [Evans et al., 2009].
Arthritis
Arthritis was the first indication to be investigated, taking advantage of the opportunities offered by the ability to inject directly into individual diseased joints [Bandara et al., 1992]. Because the joint is a discrete cavity, and intra-articular injection is generally unproblematic, it lends itself to local gene transfer. It is lined by naked synovium, which facilitates in vivo gene delivery because any intra-articularly injected vectors have little choice but to engage synovial cells. Genetically modified synovial cells then express the transgene and deliver secreted transgene products into the joint. When ex vivo gene transfer is used, the injected cells adhere to the joint lining to deliver the gene product. Either way, gene transfer converts the joint into a local “factory” that synthesizes its own medicine.
This capability confers important advantages. No other delivery method can achieve the sustained, intra-articular accumulation of proteins or other such macromolecules. This partly reflects the fact that materials exit joints very rapidly via the lymphatics and repeated, frequent, intra-articular injection is not feasible or safe. Macromolecules can be introduced systemically, but they are inefficiently delivered to joints in this fashion. The restricted transfer of large proteins from the circulation to the joint following systemic delivery is well illustrated by the lysosomal storage disorder mucopolysaccharidosis type VI. This disease, caused by lack of N-acetyl galactosamine-4-sulfatase, affects multiple organs including the joints, which undergo degenerative changes. Systemic delivery of the recombinant protein successfully treats all organs apart from the joint, because of the inefficiencies noted above. However, in animal models, intra-articular delivery of a cDNA encoding this protein normalizes joint function [Byers et al., 2009]. A similar phenomenon has been noted in the treatment of hemarthritis in mice with hemophilia [Sun et al., 2008].
Local, intra-articular gene delivery has the additional advantage of minimizing side-effects in non-target organs. Indeed, experimental studies in laboratory animals have been unable to detect circulating transgene product following gene delivery to joints unless intra-articular transgene expression is exceptionally high [Gouze et al., 2003].
A large number of pre-clinical studies, reviewed by [Ghivizzani et al., 2008] and a small number of clinical studies, described later, confirm the validity of this approach to therapy. These studies have successfully used a variety of different vectors, transgenes, and both in vivo and ex vivo delivery in animal models of RA and osteoarthritis (OA).
Tissue Engineering and Regenerative Medicine
Tissues of orthopaedic interest are frequently injured as a result of sporting activities, combat, motor vehicle accidents and so forth. Their ability to repair ranges from very high, as in bone, to very low, as in cartilage. For a number of reasons, TERM seems an excellent application of orthopaedic gene therapy. In many cases, we have a good understanding of the repair processes that we are trying to promote, and promising candidate reparative transgenes have been identified and cloned. Moreover, it is likely that transgene expression will not need to be prolonged or closely regulated. Indeed, it is likely that existing technology can already provide the necessary levels and duration of expression. Adenovirus vectors, for example, with commonly used, constitutive promoters such as the cytomegalovirus immediate-early promoter, typically provide 1-2 weeks of high transgene expression in vivo, followed by 1-4 weeks of declining expression in laboratory animals [Baltzer et al., 1999; Gouze et al., 2007]. By 6 weeks, the host immune system and cell turnover usually combine to eliminate viral expression [Gouze et al., 2007]. This could well be an ideal expression profile for the purpose of healing an organ such as bone.
Bone healing has been a focus area for orthopaedic gene therapy research because its biology is well understood, it is remarkably responsible to gene transfer, and there is a pressing clinical need for better ways to heal bone [Carofino and Lieberman, 2008; Evans, 2010]. Its responsiveness to gene transfer reflects the native ability of bone to heal exceptionally well – indeed it is one of the few organs in the body that can spontaneously heal without scarring. A large number of pre-clinical studies in rodents and rabbits, using a variety of vectors, transgenes and strategies, have confirmed the utility of gene transfer as an agent of bone healing. However, relatively few such studies have used the large animal models, such as goat or sheep, that are necessary before human studies can be contemplated, and this represents one bottleneck for clinical translation [Evans, 2010].
Cartilage lies at the other extreme of the regeneration spectrum. Unlike bone, it has almost no ability to repair itself. Because of this, there is no natural, biological template to follow when designing gene therapy repair strategies. Most literature on the subject describes combinations of growth factor genes, scaffolds, and chondrocytes or chondroprogenitor cells. The data suggest mixed success [Steinert et al., 2008].
Tendons and ligaments lie somewhere in between these two extremes. Tendons and extra-articular ligaments are able to heal spontaneously, but the regenerate is of inferior biology and mechanical strength, and thus liable to re-rupture. In contrast, intra-articular ligaments, notably the anterior cruciate ligament of the knee, are unable to heal and its rupture frequently leads to OA. Gene therapy is being explored as a means of improving the repair of ligaments and tendons, and some promising data have emerged from studies in rodents [Hildebrand et al., 2004].
Clinical trials
Clinical trials in arthritis have been reviewed recently [Evans et al. 2011].There have been 4 published human trials for the gene therapy of RA (Table 1). Two of these used an ex vivo strategy using a retrovirus to deliver the interleukin-1 receptor antagonist (IL-1Ra) cDNA to metacarpophalangeal joints that were about to undergo sialistic joint replacement surgery or synovectomy. The data from these two studies [Evans et al., 2005b; Wehling et al., 2009] suggest that the procedure is safe, feasible and leads to the intra-articular expression of a biologically active gene product. One subject reported remarkable clinical improvement [Wehling et al., 2009]. Despite these promising findings, it was not possible to obtain additional funding for the project, partly a consequence of the introduction of infliximab, etanercept and other powerful anti-rheumatics.
Table 1. Published clinical trials in the gene therapy of rheumatoid arthritis.
Adapted from Evans et al., 2011
| Transgene | Method of delivery | Phase | Institution or sponsor (principal investigator(s)) |
NIH OBA protocol number |
Number of subjects |
Reference |
|---|---|---|---|---|---|---|
| IL-1Ra | Retrovirus, Ex Vivo | I | University of Pittsburgh (Evans and Robbins) |
9406-074 | 9 | Evans et al., 2005 |
| IL-1Ra | Retrovirus, Ex Vivo | I | University of Düsseldorf, Germany (Wehling) |
NA | 2 | Wehling et al., 2009 |
| Etanercept | AAV, In Vivo | I | Targeted Genetics (Mease) |
0307-588* | 15 | Mease et al., 2009 |
| Etanercept | AAV, In Vivo | I/II | Targeted Genetics (Mease) |
0503-705** | 127 | Mease et al., 2010 |
IL-1Ra: Interleukin-1 receptor antagonist
AAV: Adeno-associated virus
OBA: Office of biotechnology activities
NA: Not applicable
Included one subject with ankylosing spondylitis
Included subjects with ankylosing spondylitis and psoriatic arthritis
The other two published studies used the intraarticular injection of recombinant AAV encoding etanercept [Mease et al., 2009; Mease et al., 2010]. The phase II trial was complicated by the death of a subject receiving the highest dose of vector, leading to the study’s suspension while the authorities examined the circumstances surrounding this fatality. Eventually, the trial was allowed to continue with minor modifications to the protocol, suggesting that gene transfer was not held accountable for the death [Evans et al., 2008; Frank et al., 2009]. Although there was a trend towards clinical improvement, the differences were not statistically significant due, in part, to the trial design [Mease et al., 2010]. It is not known whether there will be further trials of this compound in arthritis.
OA may be a better target for gene therapy than RA. Unlike the case with RA, there is no reliably effective treatment for OA and it is incurable. Many patients progress to total joint replacement. Moreover, OA affects a restricted number of joints and, unlike RA, has no important extra-articular or systemic sequelae. Furthermore, 27 million Americans have OA, a number that is predicted to rise to 67 million by 2030 [Lawrence et al., 2008].
The one approach that has entered clinical trials for OA relies on the use of an allogeneic line of human chondrocytes that have been transduced with a retrovirus expressing transforming growth factor-β1 (TGF-β1) [Lee et al., 2005]. These aneuploid cells are irradiated to prevent division, and thus the possibility of malignancy, before injection into the knee joints of subjects with advanced OA. Two Phase I trials have confirmed safety, and phase II trials are underway in Korea and the US (Table 2). As an alternative strategy, our group is developing a protocol using AAV to deliver IL-1Ra to joints with OA. This is presently the subject of pre-IND discussions with the FDA.
Table 2. Clinical trials in the gene therapy of osteoarthritis.
| Transgene | Vector Ex/In Vivo |
Phase | PI, Institution or Sponsor |
OBA Protocol Number |
Status | Number of subjects |
|---|---|---|---|---|---|---|
| TGF-β1 | Retrovirus Ex Vivo |
I | Ha, Kolon Life Sciences, Korea |
NA | Closed | 12 |
| TGF-β1 | Retrovirus Ex Vivo |
I | Mont TissueGene Inc |
0307-594 | Closed | 9 |
| TGF-β1 | Retrovirus Ex Vivo |
IIa | Ha, Kolon Life Sciences, Korea |
NA | Recruitment Complete |
28 |
| TGF-β1 | Retrovirus Ex Vivo |
II | Mont TissueGene Inc |
0912-1016 | Open | 100 |
OBA : Office of Biotechnology Activities
N = number of subjects in study
NA = Not Applicable
There has been one additional human trial in orthopaedics. This targeted aseptic loosening, an iatrogenic condition where a prosthetic joint loosens. During this process, the bone around the prosthesis is resorbed and the space fills with fibrous tissue, sometimes referred to as a pseudosynovium. Such joints are normally revised surgically and a new prosthesis is inserted. Because this is an expensive operation and not all patients are good candidates for such major surgery, there is interest in developing non-surgical methods for re-stabilizing the loosened joint. De Poorter et al. [de Poorter et al., 2008] adopted a gene transfer approach in which recombinant adenovirus carrying the nitrate reductase gene was injected intra-articularly. The vector tracked to the peri-prosthetic pseudosynovium where the transgene was expressed. A prodrug CB1954 was then administered. Nitrate reductase converted this prodrug to a cytotoxic product that killed cells locally within the pseudosynovium. After this tissue was ablated in this fashion, liquid bone cement was introduced into the space, which, once solidified, re-stabilized the prostheses. Twelve subjects were treated in this fashion, with evidence of reduced pain and increased walking distance. Despite these promising data, it has not been possible to attract additional funding for the protocol and no new trials are planned in the near future (Huizinga, personal communication).
Constraints to clinical translation
As the foregoing synopsis indicates, approaches to orthopaedic gene therapy rest on a solid conceptual and scientific footing and are supported by a wealth of pre-clinical, and some clinical, data. So why is there no product? The reasons are multifactorial.
To begin with, the field of gene therapy as a whole has a bad name. It is seen as risky, unsafe and, until recently, unable to deliver. The safety issue is particularly pertinent for orthopaedic applications, as most of these are not lethal and therefore the risk: benefit ratio is skewed. An examination of the data suggests that the reputation of gene therapy as being dangerous is unfounded. There have been over 1700 clinical trials worldwide (http://www.wiley.com/legacy/wileychi/genmed/clinical/), involving tens of thousands of individuals. Yet the number of fatalities associated with gene transfer can be counted on one hand (Table 3). The problem is that each of the few adverse events is seized on by the media and becomes expanded into a major roadblock for the rest of the gene therapy field. Thus an adverse event in a gene therapy study at a distant institution can block an unrelated protocol elsewhere.
Table 3. Published deaths of subjects in human gene therapy trials.
Adapted from Evans et al., 2008
| Year | Disease target | Vector | Comment | Death related to gene transfer? |
Ref |
|---|---|---|---|---|---|
| 1999 | Ornithine transcarbamylase deficiency |
Adenovirus | Patient died within 4 days from cytokine storm |
Yes | Raper et al.,2003 |
| 2002 | X-linked severe combined immunodeficiency disease |
Retrovirus | Leukemia developed, linked to insertion of retrovirus adjacent to oncogene |
Yes | Hacein-Bey et al., 2003 |
| 2006 | X-linked chronic granulomatous disease |
Retrovirus | Loss of transgene expression led to death from underlying disease |
No | Anon., 2006 |
| 2007 | Rheumatoid arthritis | AAV | Histoplasmosis and retroperitoneal aneurysm |
No | Frank et al., 2009 |
AAV: Adeno-associated virus
The perception of risk makes industry, especially large pharmaceutical companies, reluctant to invest in orthopaedic gene therapy. This presents a major obstacle to progress, because its clinical translation is extremely expensive. From personal experience, we can vouch for the difficulties of trying to bring gene therapy into the clinic using standard academic funding sources. The present precarious condition of major western economies does not auger well for any improvement in this state of affairs any time soon.
Related, but separate, issues constraining the enthusiasm of pharmaceutical companies include the perceived long time-lines and the dubious return on investment. This is not only a problem for gene therapy, as demonstrated by the increasing enthusiasm for the “repurposing” of existing drugs rather than developing new ones through traditional pipelines [Lussier and Chen, 2011]. Small, start-up biotechnology companies have frequently been the engine for driving novel and slightly unorthodox, therapies. Such companies, however, often reply on venture capital to fund their activities, and venture capital is now difficult to obtain.
The regulatory barriers to human gene therapy have also become increasingly burdensome. When we took our first gene therapy protocol for arthritis to the authorities in the 1990s [Evans et al., 1996], it was dealt with expeditiously and resulted in a safe, patient-friendly, successful study [Evans et al., 2005b]. We are now trying to introduce a similar study using a different, and theoretically safer, vector. This has run into all sorts of expensive, time-consuming, regulatory headwinds.
In addition to financial and regulatory constraints, there is the more general problem of research translation as a whole. A major part of this, at least in the orthopaedic arena, falls into the psychosocial category. As described in more detail by [Evans, 2011], successful translation of orthopaedic indications requires orthopaedic surgeons in the laboratory and PhDs in the operating room. This is very difficult to achieve when surgeons have an imperative to generate clinical dollars for the institution, and sometimes themselves, while many PhDs lack secure positions and are required to generate a steady stream of grants to pay for their own salaries and those of the people in their laboratories. Once the obvious pre-clinical experiments have been accomplished and published, progress towards the clinic slows dramatically and the cost escalates considerably (figure 1). A large amount of the investigator’s effort goes towards paperwork (not the scientists’ favorite activity), ticking boxes (ditto) and dealing with regulatory issues (ditto again). These activities do not lead to a wealth of papers in high-impact journals, and they are difficult to fund – two black marks for tenure and promotion. If promising laboratory findings are to advance into the clinic, there needs to be a supportive environment for activities associated with research translation, which supports not only the science but also the process. This will require funding and an enlightened institutional mindset.
Figure 1. Rate of progress and cost as gene therapy studies progress from in vitro experiments to pre-clinical animal studies and then translate into clinical trials.
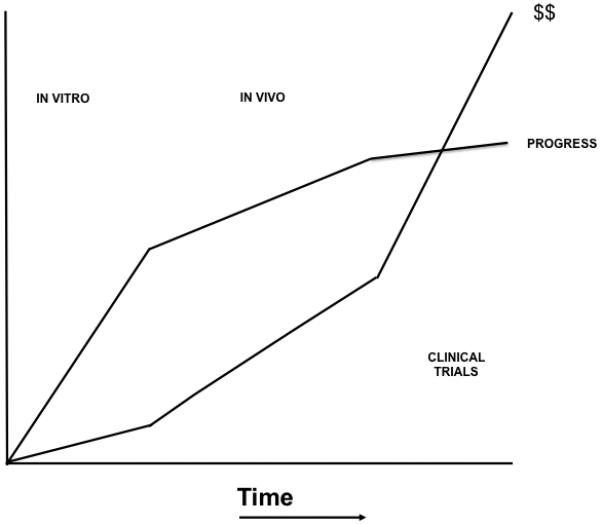
Modified from Evans, 2008
The extensive intellectual property (IP) landscape of the gene therapy field in regard to methods, vectors, therapeutic genes and applications also is an impediment to commercialization. The majority of the groundbreaking gene therapy research is being done in Universities and Research Institutions with aggressive Offices of Technology Management responsible for filing and managing patent portfolios. Thus it has become exceedingly difficult for small biotechnology companies to acquire the needed complex and expensive IP to allow the raising of funds to support clinical studies. Indeed, in a recent survey, working with technology transfer offices was cited as a major hurdle issue for startup companies [Johnson et al., 2011] This also limits the desire of big pharmaceutical companies to commit to developing gene therapies, either through support of small gene therapy companies or by acquiring IP directly.
There may also be additional factors at work. It is interesting how every new field of this type seems to go through the same cycles of initial, irrational enthusiasm, excessive expectations, disillusionment, and introspection leading to the sense of over-promising and under-delivering. This is true of tissue engineering [Nerem, 2006], stem cells [Wilson, 2009] and gene therapy, among others, and seems to occur about 20 years after the initial discoveries and breakthroughs; genomics has recently been called to question on this score after only ten [Evans et al., 2011].
Perhaps there is something in the nature of biologically based medicines that requires time. Even Elias Zerhouni, former Director of NIH, has recently commented that bench to bedside research is more difficult than he thought (Wall Street Journal, May 2011) and is quoted as saying: “at the end of the day, there’s a gap in translation”. Given that he is now head of R&D for a large pharmaceutical company, this suggests that more than size and money are needed for efficient clinical translation.
Conclusions and Perspectives
Orthopaedics provides a wealth of opportunities for gene therapy – indeed it has been described as “gene therapy’s dark horse” [Evans, 2004]. If successful, certain applications, such as OA and bone healing, would improve the clinical management of tens of millions of patients and expand the scope of gene therapy from the treatment of a small number of individuals with rare genetic diseases, to the treatment of large segments of the population with everyday disorders. The barriers to achieving this are financial, regulatory, sociological, the absence of an effective translational environment and, possibly, the lack of a reliable road map detailing how to get there.
It is not clear how these barriers can be easily overcome. One source of optimism, however, is the increasingly impressive record of clinical success for gene therapy. Recent years have seen the first hard evidence of genuine cures and there is the sense that the entire field is on an upswing [Anon. 2009; Sheridan, 2011]. In the past such optimism has proved transient, largely as a result of a few adverse events. This time, the gains seem more substantial, but time will tell.
Although the clinical successes so far have been limited to small number of patients with rare genetic conditions, it is to be hoped that the improved perception of gene therapy as a whole will enable its orthopaedic applications to benefit collaterally, leading to greater and more sustained investment. If this can be turned into evidence of success, even in a few patients, it is likely that the enthusiasm the orthopaedic community for novelty will supply the needed momentum for further development.
Acknowledgements
The author’s research in this area has been funded by NIH grants R01 AR43623, R21 AR049606, R01 AR048566, R01 AR057422, R01 AR051085, X01 NS066865 and by Orthogen
We thank TWJ Huizinga, MD for reviewing an earlier draft of this manuscript.
References
- Anon Gene therapy deserves a fresh chance. Nature. 2009;461:1173. doi: 10.1038/4611173a. [DOI] [PubMed] [Google Scholar]
- Baltzer AW, Lattermann C, Whalen JD, Braunstein S, Robbins PD, Evans CH. A gene therapy approach to accelerating bone healing. Evaluation of gene expression in a New Zealand white rabbit model. Knee Surg Sports Traumatol Arthrosc. 1999;7:197–202. doi: 10.1007/s001670050147. [DOI] [PubMed] [Google Scholar]
- Bandara G, Robbins PD, Georgescu HI, Mueller GM, Glorioso JC, Evans CH. Gene transfer to synoviocytes: prospects for gene treatment of arthritis. DNA Cell Biol. 1992;11:227–31. doi: 10.1089/dna.1992.11.227. [DOI] [PubMed] [Google Scholar]
- Byers S, Rothe M, Lalic J, Koldej R, Anson DS. Lentiviral-mediated correction of MPS VI cells and gene transfer to joint tissues. Mol Genet Metab. 2009;97:102–8. doi: 10.1016/j.ymgme.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Carofino BC, Lieberman JR. Gene therapy applications for fracture-healing. J Bone Joint Surg Am 90 Suppl. 2008;1:99–110. doi: 10.2106/JBJS.G.01546. [DOI] [PubMed] [Google Scholar]
- de Poorter JJ, Hoeben RC, Hogendoorn S, Mautner V, Ellis J, Obermann WR, Huizinga TW, Nelissen RG. Gene therapy and cement injection for restabilization of loosened hip prostheses. Hum Gene Ther. 2008;19:83–95. doi: 10.1089/hum.2007.111. [DOI] [PubMed] [Google Scholar]
- Evans C. Arthritis gene therapy trials reach phase II. J Rheumatol. 2010;37:683–5. doi: 10.3899/jrheum.100055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. Gene therapy for the regeneration of bone. Injury. 2011;42:599–604. doi: 10.1016/j.injury.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH. Barriers to the Clinical Translation of Orthopedic Tissue Engineering. Tissue Eng Part B Rev. 2011 doi: 10.1089/ten.teb.2011.0228. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH. Gene therapy for bone healing. Expert Rev Mol Med. 2010;12:e18. doi: 10.1017/S1462399410001493. doi:10.1017/S1462399410001493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH. Orthopaedics: gene therapy’s dark horse. Gene Ther. 2004;11:343. doi: 10.1038/sj.gt.3302212. [DOI] [PubMed] [Google Scholar]
- Evans CH, Ghivizzani SC, Herndon JH, Robbins PD. Gene therapy for the treatment of musculoskeletal diseases. J Am Acad Orthop Surg. 2005a;13:230–42. doi: 10.5435/00124635-200507000-00003. [DOI] [PubMed] [Google Scholar]
- Evans CH, Ghivizzani SC, Robbins PD. Getting arthritis gene therapy into the clinic. Nat Rev Rheumatol. 2011;7:244–9. doi: 10.1038/nrrheum.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH, Ghivizzani SC, Robbins PD. Arthritis gene therapy’s first death. Arthritis Res Ther. 2008;10:110. doi: 10.1186/ar2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH, Ghivizzani SC, Robbins PD. Orthopedic gene therapy in 2008. Mol Ther. 2009;17:231–44. doi: 10.1038/mt.2008.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CH, Robbins PD, Ghivizzani SC, Herndon JH, Kang R, Bahnson AB, Barranger JA, Elders EM, Gay S, Tomaino MM, Wasko MC, Watkins SC, Whiteside TL, Glorioso JC, Lotze MT, Wright TM. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum Gene Ther. 1996;7:1261–80. doi: 10.1089/hum.1996.7.10-1261. [DOI] [PubMed] [Google Scholar]
- Evans CH, Robbins PD, Ghivizzani SC, Wasko MC, Tomaino MM, Kang R, Muzzonigro TA, Vogt M, Elder EM, Whiteside TL, Watkins SC, Herndon JH. Gene transfer to human joints: progress toward a gene therapy of arthritis. Proc Natl Acad Sci U S A. 2005b;102:8698–703. doi: 10.1073/pnas.0502854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JP, Meslin EM, Marteau TM, Caulfield T. Genomics. Deflating the genomic bubble. Science. 2011;331:861–2. doi: 10.1126/science.1198039. [DOI] [PubMed] [Google Scholar]
- Frank KM, Hogarth DK, Miller JL, Mandal S, Mease PJ, Samulski RJ, Weisgerber GA, Hart J. Investigation of the cause of death in a gene-therapy trial. N Engl J Med. 2009;361:161–9. doi: 10.1056/NEJMoa0801066. [DOI] [PubMed] [Google Scholar]
- Ghivizzani SC, Gouze E, Gouze JN, Kay JD, Bush ML, Watson RS, Levings PP, Nickerson DM, Colahan PT, Robbins PD, Evans CH. Perspectives on the use of gene therapy for chronic joint diseases. Curr Gene Ther. 2008;8:273–86. doi: 10.2174/156652308785160638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouze E, Gouze JN, Palmer GD, Pilapil C, Evans CH, Ghivizzani SC. Transgene persistence and cell turnover in the diarthrodial joint: implications for gene therapy of chronic joint diseases. Mol Ther. 2007;15:1114–20. doi: 10.1038/sj.mt.6300151. [DOI] [PubMed] [Google Scholar]
- Gouze E, Pawliuk R, Gouze JN, Pilapil C, Fleet C, Palmer GD, Evans CH, Leboulch P, Ghivizzani SC. Lentiviral-mediated gene delivery to synovium: potent intra-articular expression with amplification by inflammation. Mol Ther. 2003;7:460–6. doi: 10.1016/s1525-0016(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Hildebrand KA, Frank CB, Hart DA. Gene intervention in ligament and tendon: current status, challenges, future directions. Gene Ther. 2004;11:368–78. doi: 10.1038/sj.gt.3302198. [DOI] [PubMed] [Google Scholar]
- Johnson PC, Bertram TA, Tawil B, Hellman KB. Hurdles in tissue engineering/regenerative medicine product commercialization: a survey of North American academia and industry. Tissue Eng Part A. 2011;17:5–15. doi: 10.1089/ten.TEA.2010.0411. [DOI] [PubMed] [Google Scholar]
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DK, Choi KB, Oh IS, Song SU, Hwang S, Lim CL, Hyun JP, Lee HY, Chi GF, Yi Y, Yip V, Kim J, Lee EB, Noh MJ, Lee KH. Continuous transforming growth factor beta1 secretion by cell-mediated gene therapy maintains chondrocyte redifferentiation. Tissue Eng. 2005;11:310–8. doi: 10.1089/ten.2005.11.310. [DOI] [PubMed] [Google Scholar]
- Lussier YA, Chen JL. The emergence of genome-based drug repositioning. Sci Transl Med. 2011;3:96ps35. doi: 10.1126/scitranslmed.3001512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mease PJ, Hobbs K, Chalmers A, El-Gabalawy H, Bookman A, Keystone E, Furst DE, Anklesaria P, Heald AE. Local delivery of a recombinant adenoassociated vector containing a tumour necrosis factor alpha antagonist gene in inflammatory arthritis: a phase 1 dose-escalation safety and tolerability study. Ann Rheum Dis. 2009;68:1247–54. doi: 10.1136/ard.2008.089375. [DOI] [PubMed] [Google Scholar]
- Mease PJ, Wei N, Fudman EJ, Kivitz AJ, Schechtman J, Trapp RG, Hobbs KF, Greenwald M, Hou A, Bookbinder SA, Graham GE, Wiesenhutter CW, Willis L, Ruderman EM, Forstot JZ, Maricic MJ, Dao KH, Pritchard CH, Fiske DN, Burch FX, Prupas HM, Anklesaria P, Heald AE. Safety, tolerability, and clinical outcomes after intraarticular injection of a recombinant adeno-associated vector containing a tumor necrosis factor antagonist gene: results of a phase 1/2 Study. J Rheumatol. 2010;37:692–703. doi: 10.3899/jrheum.090817. [DOI] [PubMed] [Google Scholar]
- Nerem RM. Tissue engineering: the hope, the hype, and the future. Tissue Eng. 2006;12:1143–50. doi: 10.1089/ten.2006.12.1143. [DOI] [PubMed] [Google Scholar]
- Sheridan C. Gene therapy finds its niche. Nat Biotechnol. 2011;29:121–8. doi: 10.1038/nbt.1769. [DOI] [PubMed] [Google Scholar]
- Steinert AF, Noth U, Tuan RS. Concepts in gene therapy for cartilage repair. Injury 39 Suppl. 2008;1:S97–113. doi: 10.1016/j.injury.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Hakobyan N, Valentino LA, Feldman BL, Samulski RJ, Monahan PE. Intraarticular factor IX protein or gene replacement protects against development of hemophilic synovitis in the absence of circulating factor IX. Blood. 2008;112:4532, 41. doi: 10.1182/blood-2008-01-131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling P, Reinecke J, Baltzer AW, Granrath M, Schulitz KP, Schultz C, Krauspe R, Whiteside TW, Elder E, Ghivizzani SC, Robbins PD, Evans CH. Clinical responses to gene therapy in joints of two subjects with rheumatoid arthritis. Hum Gene Ther. 2009;20:97–101. doi: 10.1089/hum.2008.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM. Medicine. A history lesson for stem cells. Science. 2009;324:727–8. doi: 10.1126/science.1174935. [DOI] [PubMed] [Google Scholar]


