Abstract
Background
Both Aicardi-Goutières syndrome, a Mendelian mimic of congenital infection, and the autoimmune disease systemic lupus erythematosus can result from mutations in the gene encoding the enzyme Trex1. In mice, the absence of Trex1 causes severe myocarditis. The enzyme is thought to degrade endogenous retroelements, thus linking them to autoimmune disease. However, inhibition of reverse transcription by the inhibitor zidovudine (AZT) did not ameliorate the disease, weakening the link to retroelements.
Findings
Here, we show that two other FDA-approved drugs that inhibit reverse transcriptase can ameliorate the myocarditis in Trex1-null mouse.
Conclusions
The result suggests that retroelements contribute to this hereditary form of autoimmunity, and that treatment with retroelement inhibitors might ameliorate Aicardi-Goutières syndrome in humans.
Keywords: Aicardi-Goutières syndrome, myocarditis, Trex1, reverse transcriptase inhibitors
Findings
Aicardi-Goutières syndrome (AGS) [1] is a genetically-determined encephalopathy with remarkable phenotypic overlap with the sequelae of congenital infection. Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the production of autoantibodies that target nucleic acids and their associated proteins. Like AGS [2], SLE is associated with a perturbation of type I interferon metabolism [3]. Both AGS [4], and a cutaneous subtype of SLE called familial chilblain lupus [5,6], can result from mutations in TREX1. Furthermore, mutations in TREX1 represent the single most common cause of monogenic SLE identified to date [7].
Trex1 is a ubiquitous DNA 3' exonuclease [8] that can degrade retroelements (retroviruses and retrotransposons) [9-11]. In Trex1-deficient mice, single-stranded DNA [12] derived from retroelement cDNA [9] accumulates in the cytoplasm of cells in the heart and is thought to trigger the sterile inflammatory myocarditis [13]. On the basis that unrestricted retroelements may cause, or at least contribute to, the disease [9], it was reasoned that it ought to be possible to treat or prevent disease with anti-retroviral agents. However, treatment of the mice with the reverse transcription inhibitor azidothymidine (AZT) did not rescue the mice from lethality [9]. It was argued that the absence of Trex1 may unleash hundreds of diverse reverse transcriptases encoded by the mouse genome, some of them being AZT resistant [9]. As a single agent, AZT also may leave some retroelements out of its range of activity. Finally, although it leads to premature termination of cDNA synthesis, AZT has only little effect on the synthesis of short reverse transcription intermediates, including those of spliced retroelement products [14,15]. The interrupted or slowed reverse transcription may create persistent exposure to cytoplasmic DNA products that elicit an antiviral innate immune response [16] coordinated by activation of type I IFNs (the so-called IFN-stimulatory DNA response [17]). Along this line, raltegravir, a drug that inhibits retroviral integrase and thus increases the concentration of cDNA in the cell, also exacerbates autoimmune disease [10].
In Trex1 deficient mice, the inflammation of the heart muscle takes an aggressive course, with mice starting to die after 4 weeks of age (Figure 1). We sought to prevent the autoimmune disease with anti-retroviral drugs other than AZT. Keeping in mind that a single drug may leave some retroelements out of its range of activity, we decided to use a combination of drugs that inhibit reverse transcriptase. Because nucleoside reverse transcription inhibitors also inhibit human LINE-1 retrotransposition [18], we assumed that a Truvada/Viramune combination (both FDA-approved drugs) would inhibit both classes of retroelements--retroviruses and retrotransposons. Truvada is a fixed-dose combination tablet containing emtricitabine and tenofovir disoproxil fumarate [19]. Emtricitabine is a synthetic nucleoside analog of cytidine. Tenofovir disoproxil fumarate is converted in vivo to tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5'-monophosphate. Viramune (nevirapine) [20] blocks the reproduction of retrovirus earlier in its cycle than Truvada. It binds directly to reverse transcriptase and blocks the RNA-dependent and DNA-dependent DNA polymerase activities by disrupting the enzyme's catalytic site. Viramune does not compete with template or nucleoside triphosphates, or inhibit the cellular DNA polymerases tested so far [21].
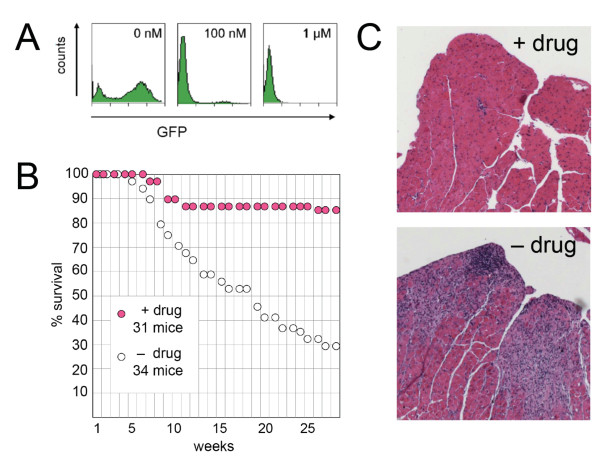
Figure 1.
Effect of reverse transcriptase inhibitors on survival of Trex1-deficient mice. A) Inhibition of MLV cDNA synthesis by Truvada/Viramune. Flow cytometry graphs displaying GFP intensity generated by provirus: y-axis, cell number; x-axis, fluorescence intensity on a logarithmic scale. An MLV-based vector encoding GFP was added to NIH/3T3 cell cultures with 0, 100 nM, or 1 μM. B) Survival curves showing the effect of Truvada/Viramune (+ drug; magenta circles) on Trex1-deficient mice [13] obtained from D. Stetson [9]. The drugs were given from conception via the drinking water as a solution of 3 × 10-4 M nevirapine, 1.6 × 10-4 M emtricitabine and 9.4 × 10-5 M tenofovir. Log rank test for the drug effect, p = 0.000014. C) Hematoxylin-eosin stained sections of the left heart ventricle of treated (+ drug) and non-treated (- drug) mice killed at 9 and 7 months of age, respectively. Sections from three mice were examined in each category.
We first determined that the combination of Truvada and Viramune is effective against MLV. Using flow cytometry, we titrated the drug concentration for its ability to inhibit expression of green fluorescence protein encoded by MLV provirus upon infection; the EC50 was well below 100 nM (Figure 1A). When fed to Trex1-deficient mice at a dose comparable to that given to patients with HIV, the drugs substantially reduced mortality (Figure 1B). On sections of the heart from 9-month old treated mice, there were some mild patchy inflammatory infiltrates with little myocyte injury; but the difference to the marked inflammatory infiltrates with myocyte necrosis and dropout in 7-month old non-treated mice (at 9 months all untreated mice were dead) was striking (Figure 1C).
Almost half of the human genome consists of retroelements, many of them active. There are several ways that retroelements might trigger an autoimmune response, including (i) sensing of retroelement RNA and cDNA, (ii) generation of mimetopes through error-prone reverse transcription of mRNA encoding retroelement proteins, and (iii) insertional mutagenesis. We showed here that a hereditary autoimmune inflammation in the mouse that is likely caused by accumulation of retroelement cDNA can be treated with reverse transcriptase inhibitors. Other autoimmune diseases might be amenable to different interventions of retroelement activities.
Abbreviations
AZT: zidovudine; AGS: Aicardi-Goutières syndrome; IFN: interferon; MLV: murine leukemia virus; SLE: systemic lupus erythematosus.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
GBE, DE, and MW planned the study; GBE carried out the experiments; and MW wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Gabriele B Beck-Engeser, Email: Gabriele.Beck-Engeser@ucsf.edu.
Dan Eilat, Email: eilatd@cc.huji.ac.il.
Matthias Wabl, Email: mutator@ucsf.edu.
Acknowledgements
We thank Dan Stetson for the Trex1-deficient mice; Jean Olson for the micrographs; Cliff Wang and Jay Lalezari for suggestions; and Mary McKenney for editing the manuscript. Supported by grants from the NIH (R01AI041570) and the Lupus Research Institute to MW; and from the United States - Israel Binational Foundation (BSF) to MW and DE.
References
- Aicardi J, Goutieres F. Systemic lupus erythematosus or Aicardi-Goutieres syndrome? Neuropediatrics. 2000;31:113. doi: 10.1055/s-2000-7533. [DOI] [PubMed] [Google Scholar]
- Lebon P, Badoual J, Ponsot G, Goutieres F, Hemeury-Cukier F, Aicardi J. Intrathecal synthesis of interferon-alpha in infants with progressive familial encephalopathy. J Neurol Sci. 1988;84:201–208. doi: 10.1016/0022-510X(88)90125-6. [DOI] [PubMed] [Google Scholar]
- Pascual V, Banchereau J, Palucka AK. The central role of dendritic cells and interferon-alpha in SLE. Curr Opin Rheumatol. 2003;15:548–556. doi: 10.1097/00002281-200309000-00005. [DOI] [PubMed] [Google Scholar]
- Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, Black DN, van Bokhoven H, Brunner HG, Hamel BC, Corry PC, Cowan FM, Frints SG, Klepper J, Livingston JH, Lynch SA, Massey RF, Meritet JF, Michaud JL, Ponsot G, Voit T, Lebon P, Bonthron DT, Jackson AP, Barnes DE, Lindahl T. Mutations in the gene encoding the 3'-5' DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet. 2006;38:917–920. doi: 10.1038/ng1845. [DOI] [PubMed] [Google Scholar]
- Rice G, Newman WG, Dean J, Patrick T, Parmar R, Flintoff K, Robins P, Harvey S, Hollis T, O'Hara A, Herrick AL, Bowden AP, Perrino FW, Lindahl T, Barnes DE, Crow YJ. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutieres syndrome. Am J Hum Genet. 2007;80:811–815. doi: 10.1086/513443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Kirsch MA, Chowdhury D, Harvey S, Gong M, Senenko L, Engel K, Pfeiffer C, Hollis T, Gahr M, Perrino FW, Lieberman J, Hubner N. A mutation in TREX1 that impairs susceptibility to granzyme A-mediated cell death underlies familial chilblain lupus. J Mol Med. 2007;85:531–537. doi: 10.1007/s00109-007-0199-9. [DOI] [PubMed] [Google Scholar]
- Lee-Kirsch MA, Gong M, Chowdhury D, Senenko L, Engel K, Lee YA, de Silva U, Bailey SL, Witte T, Vyse TJ, Kere J, Pfeiffer C, Harvey S, Wong A, Koskenmies S, Hummel O, Rohde K, Schmidt RE, Dominiczak AF, Gahr M, Hollis T, Perrino FW, Lieberman J, Hübner N. Mutations in the gene encoding the 3'-5' DNA exonuclease TREX1 are associated with systemic lupus erythematosus. Nat Genet. 2007;39:1065–1067. doi: 10.1038/ng2091. [DOI] [PubMed] [Google Scholar]
- Lindahl T, Barnes DE, Yang YG, Robins P. Biochemical properties of mammalian TREX1 and its association with DNA replication and inherited inflammatory disease. Biochem Soc Trans. 2009;37:535–538. doi: 10.1042/BST0370535. [DOI] [PubMed] [Google Scholar]
- Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. doi: 10.1016/j.cell.2008.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck-Engeser GB, Eilat D, Harrer T, Jack HM, Wabl M. Early onset of autoimmune disease by the retroviral integrase inhibitor raltegravir. Proc Natl Acad Sci USA. 2009;106:20865–20870. doi: 10.1073/pnas.0908074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N, Regalado-Magdos AD, Stiggelbout B, Lee-Kirsch MA, Lieberman J. The cytosolic exonuclease TREX1 inhibits the innate immune response to human immunodeficiency virus type 1. Nat Immunol. 2010;11:1005–1013. doi: 10.1038/ni.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YG, Lindahl T, Barnes DE. Trex1 exonuclease degrades ssDNA to prevent chronic checkpoint activation and autoimmune disease. Cell. 2007;131:873–886. doi: 10.1016/j.cell.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Morita M, Stamp G, Robins P, Dulic A, Rosewell I, Hrivnak G, Daly G, Lindahl T, Barnes DE. Gene-targeted mice lacking the Trex1 (DNase III) 3'--> 5' DNA exonuclease develop inflammatory myocarditis. Mol Cell Biol. 2004;24:6719–6727. doi: 10.1128/MCB.24.15.6719-6727.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan Y, Liang C, Inouye P, Wainberg MA. Enhanced impairment of chain elongation by inhibitors of HIV reverse transcriptase in cell-free reactions yielding longer DNA products. Nucleic Acids Res. 1998;26:5692–5698. doi: 10.1093/nar/26.24.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houzet L, Morichaud Z, Mougel M. Fully-spliced HIV-1 RNAs are reverse transcribed with similar efficiencies as the genomic RNA in virions and cells, but more efficiently in AZT-treated cells. Retrovirology. 2007;4:30. doi: 10.1186/1742-4690-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Jones RB, Garrison KE, Wong JC, Duan EH, Nixon DF, Ostrowski MA. Nucleoside Analogue Reverse Transcriptase Inhibitors Differentially Inhibit Human LINE-1 Retrotransposition. PLoS ONE. 2008;3:e1547. doi: 10.1371/journal.pone.0001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzard BG. Use of tenofovir disoproxil fumarate and emtricitabine combination in HIV-infected patients. Expert Opin Pharmacother. 2006;7:793–802. doi: 10.1517/14656566.7.6.793. [DOI] [PubMed] [Google Scholar]
- Merluzzi VJ, Hargrave KD, Labadia M, Grozinger K, Skoog M, Wu JC, Shih CK, Eckner K, Hattox S, Adams J, Rosenthal AS, Faanes R, Eckner RJ, Koup RA, Sullivan JL. Inhibition of HIV-1 replication by a nonnucleoside reverse transcriptase inhibitor. Science. 1990;250:1411–1413. doi: 10.1126/science.1701568. [DOI] [PubMed] [Google Scholar]
- http://us.viramune.com/hcp/viramune/general-information.jsp