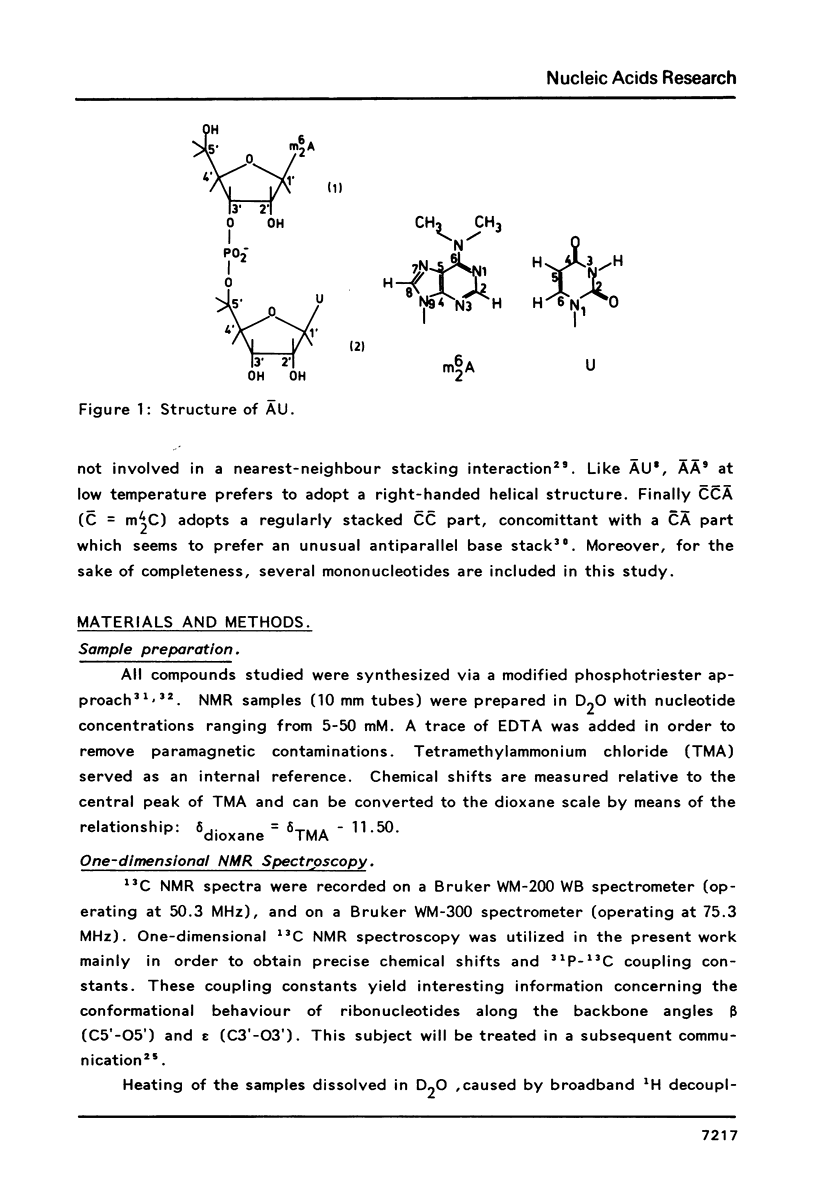
Abstract
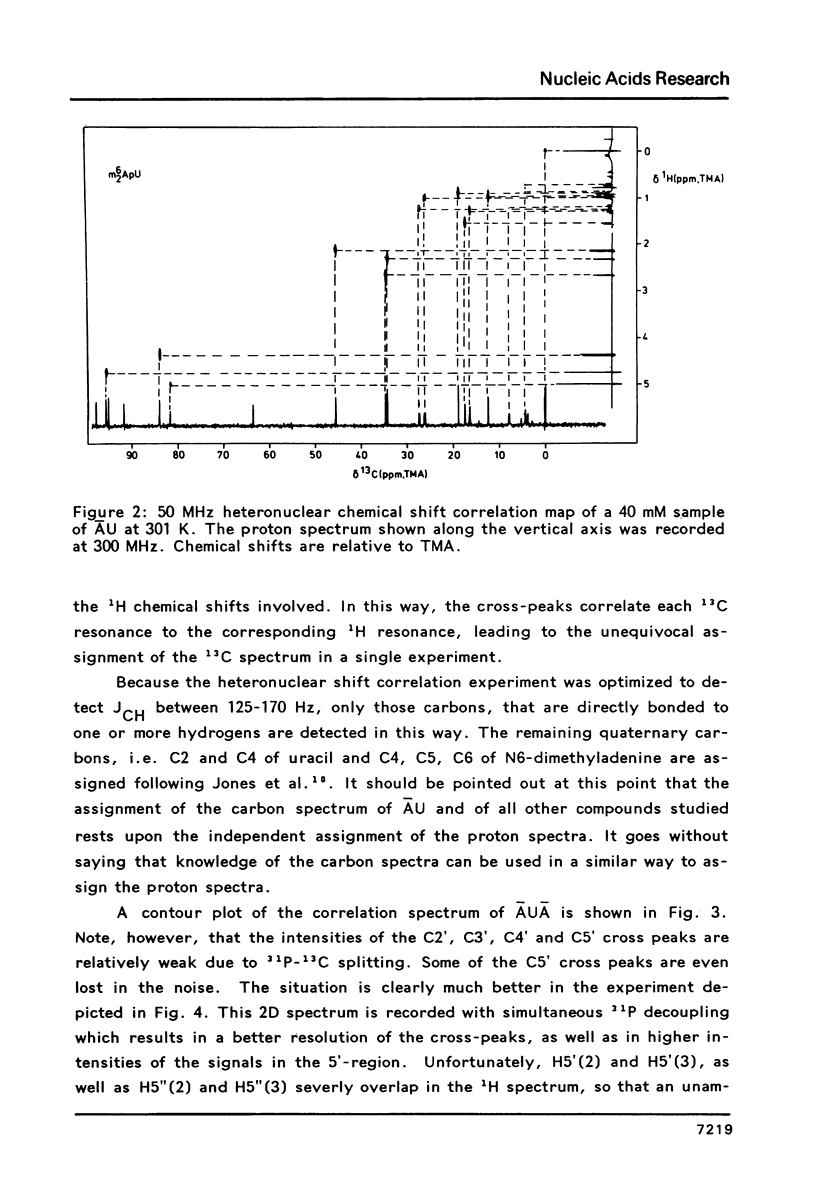
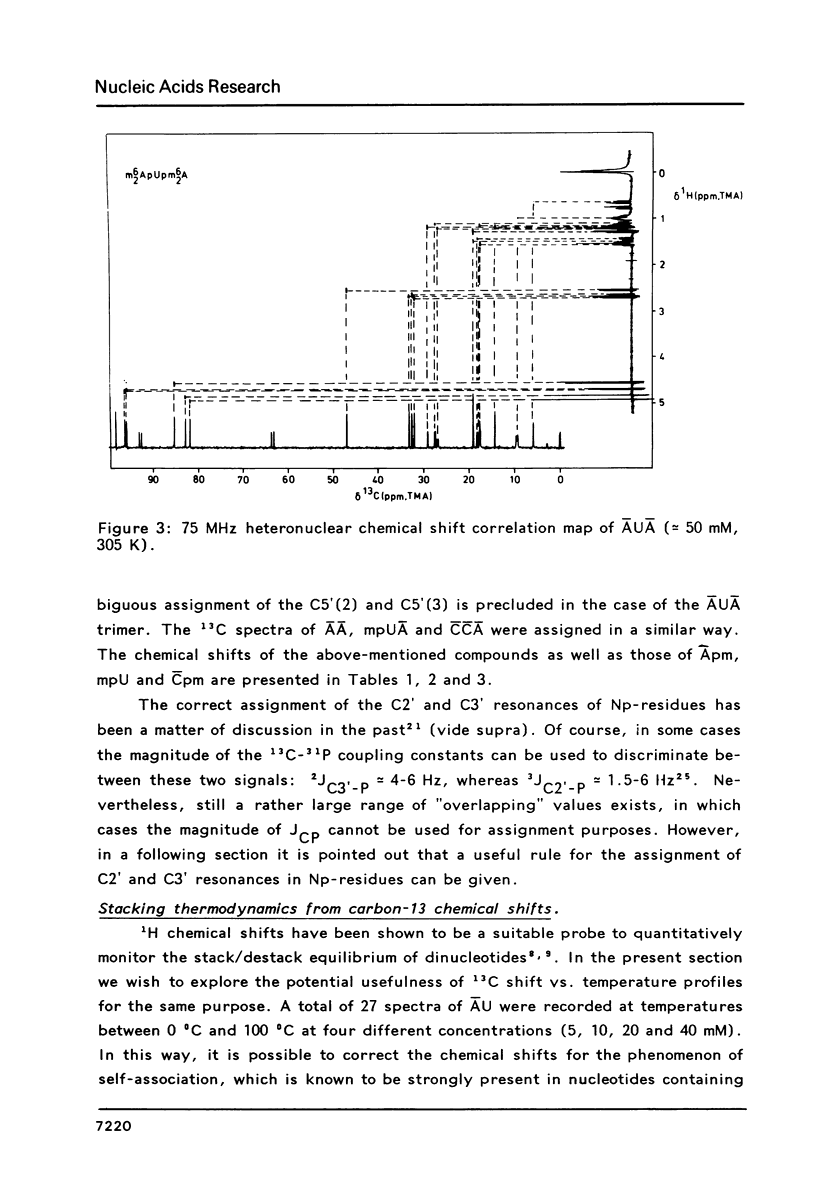
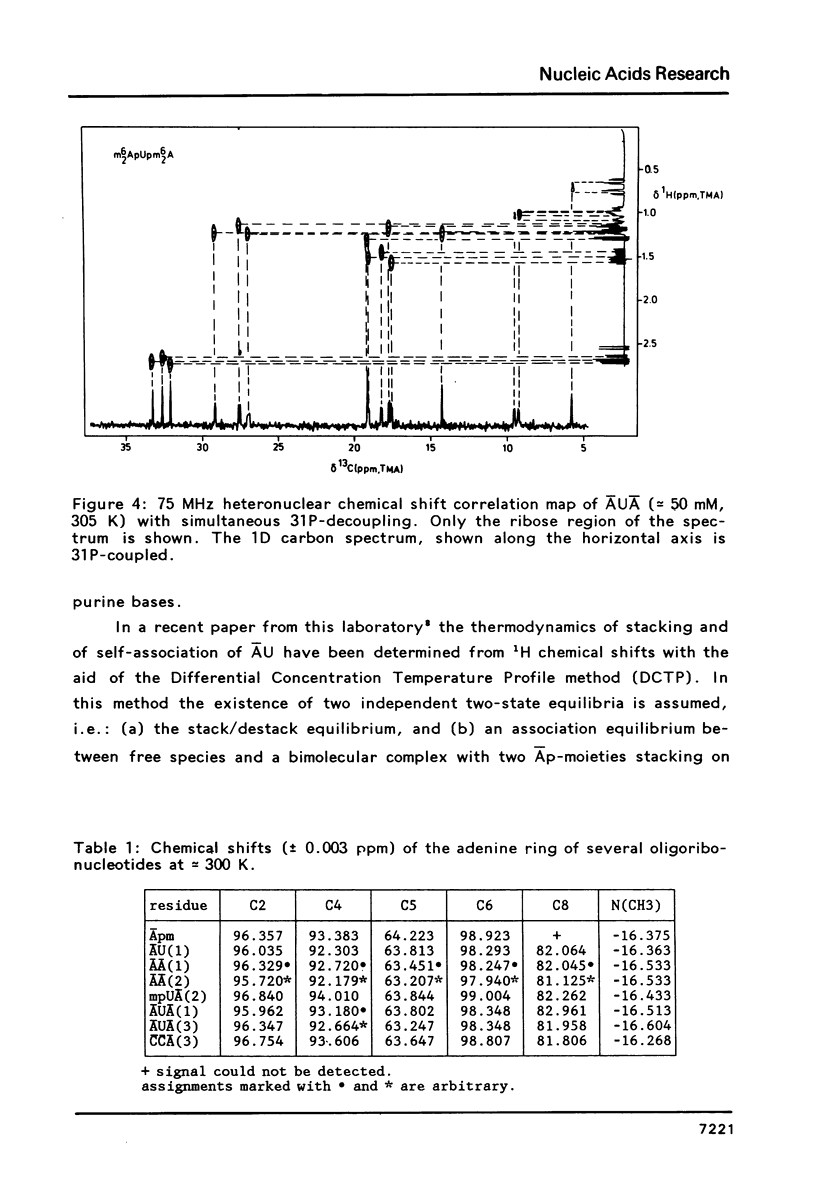
The assignment of the non-quaternary 13C resonances by means of two-dimensional heteronuclear chemical shift correlation spectroscopy is presented for several oligoribonucleotides: The dimers m6(2)AU, m6(2)Am6(2)A and mpUm6(2)A and the trimers m6(2)AUm6(2)A and m4(2)Cm4(2)Cm6(2)A. The temperature and concentration dependency of the 13C chemical shifts are studied with emphasis on the behaviour of the dimer m6(2)AU. The present study shows that in the 5-50 mM range the concentration-dependent chemical shift changes of the ribose carbons are negligible compared to chemical shift changes due to intramolecular events. All compounds studied show a surprising correlation between the chemical shifts of the carbon atoms of the ribose ring and the sugar conformational equilibrium as expressed by the percentage N or S conformer. Thus the chemical shift data can be used to obtain the thermodynamical parameters of the two-state N/S equilibrium. Parameters deduced for m6(2)AU are Tm = 306 K and delta S = -25 cal mol-1 K-1, which values are in satisfactory agreement with results obtained earlier from 1H NMR and from Circular Dichroism.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderfer J. L., Ts'o P. O. Conformational properties of the furanose phosphate backbone in nucleic acids. A carbon-13 nuclear magnetic resonance study. Biochemistry. 1977 May 31;16(11):2410–2416. doi: 10.1021/bi00630a016. [DOI] [PubMed] [Google Scholar]
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. A new description using the concept of pseudorotation. J Am Chem Soc. 1972 Nov 15;94(23):8205–8212. doi: 10.1021/ja00778a043. [DOI] [PubMed] [Google Scholar]
- Altona C., Sundaralingam M. Conformational analysis of the sugar ring in nucleosides and nucleotides. Improved method for the interpretation of proton magnetic resonance coupling constants. J Am Chem Soc. 1973 Apr 4;95(7):2333–2344. doi: 10.1021/ja00788a038. [DOI] [PubMed] [Google Scholar]
- Dorman D. E., Roberts J. D. Nuclear magnetic resonance spectroscopy: 13C spectra of some common nucleotides. Proc Natl Acad Sci U S A. 1970 Jan;65(1):19–26. doi: 10.1073/pnas.65.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman D. E., Roberts J. D. Nuclear magnetic resonance spectroscopy: 13C spectra of some common nucleotides. Proc Natl Acad Sci U S A. 1970 Jan;65(1):19–26. doi: 10.1073/pnas.65.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartel A. J., Lankhorst P. P., Altona C. Thermodynamics of stacking and of self-association of the dinucleoside monophosphate m2(6)A-U from proton NMR chemical shifts: differential concentration temperature profile method. Eur J Biochem. 1982 Dec 15;129(2):343–357. doi: 10.1111/j.1432-1033.1982.tb07057.x. [DOI] [PubMed] [Google Scholar]
- Lapper R. D., Smith I. C. A 13 C and 1 H nuclear magnetic resonance study of the conformations of 2',3'-cyclic nucleotides. J Am Chem Soc. 1973 May 2;95(9):2878–2880. doi: 10.1021/ja00790a024. [DOI] [PubMed] [Google Scholar]
- Mantsch H. H., Smith I. C. Fourier-transformed 13 C NMR spectra of polyuridylic acid, uridine, and related nucleotides--the use of 31 POC 13 C couplings for conformational analysis. Biochem Biophys Res Commun. 1972 Jan 31;46(2):808–815. doi: 10.1016/s0006-291x(72)80213-4. [DOI] [PubMed] [Google Scholar]
- Mellema J. R., Haasnoot C. A., van der Marel G. A., Wille G., van Boeckel C. A., van Boom J. H., Altona C. Proton NMR studies on the covalently linked RNA-DNA hybrid r(GCG)d(TATACGC). Assignment of proton resonances by application of the nuclear Overhauser effect. Nucleic Acids Res. 1983 Aug 25;11(16):5717–5738. doi: 10.1093/nar/11.16.5717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsthoorn C. S., Doornbos J., de Leeuw H. P., Altona C. Influence of the 2'-hydroxyl group and of 6-N-methylation on the conformation of adenine dinucleoside monophosphates in solution. A nuclear magnetic resonance and circular dichroism study. Eur J Biochem. 1982 Jul;125(2):367–382. doi: 10.1111/j.1432-1033.1982.tb06693.x. [DOI] [PubMed] [Google Scholar]
- Olsthoorn C. S., Haasnoot C. A., Altona C. Circular dichroism studies of 6-N-methylated adenylyladenosine and adenylyluridine and their parent compounds. Thermodynamics of stacking. Eur J Biochem. 1980 May;106(1):85–95. doi: 10.1111/j.1432-1033.1980.tb05999.x. [DOI] [PubMed] [Google Scholar]
- Schleich T., Cross B. P., Smith I. C. A conformational study of adenylyl-(3',5')-adenosine and adenylyl-(2',5')-adenosine in aqueous solution by carbon-13 magnetic resonance spectroscopy. Nucleic Acids Res. 1976 Feb;3(2):355–370. doi: 10.1093/nar/3.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]



