Abstract
Pre-eclampsia, an acute complication of human pregnancy, is associated within complete physiological modification of decidual spiral arteries. This is thought to promote oxidative stress from perfusion/reperfusion of the placenta and to restrict placental and fetal growth. Alymphoid (genotype Rag2−/−/Il2rg−/−) mice, sufficient in dendritic and myeloid cell functions, lack spiral arterial modification with individual spiral arteries having ~1.7x the vascular resistance and 0.66x the blood velocity of +/+ mice. Their placentae are measurably hypoxic yet neither placental growth nor fetal survival is impaired and gestational hypertension is not seen. Thus, lymphocytes rather than vascular adaptations appear to be the pivotal contributors to the clinical complications of pre-eclampsia.
Keywords: Fecundity, Immune System, Mean Arterial Pressure, Placental Growth, Vascular Remodeling
1. INTRODUCTION
Pre-eclampsia is an acute, urgent care complication commonly seen during the second half of human pregnancy1;2. Pre-eclampsia is characterized by the combined sudden onset of de novo hypertension and renal impairment, seen as proteinuria. The most common histopathology associated with this syndrome is incomplete modification of the endometrial spiral arteries3–6. The physiological process of gestational spiral arterial modification normally turns spiral arteries from high pressure, contractile conduits into dilated, low pressure structures that are unresponsive to vasoactive signals. In humans, the process of spiral arterial modification is generally attributed to migration of trophoblast cells into the walls of the spiral arteries and to the retrograde migration of trophoblast over the endothelial surface which it transiently replaces. In these locations, trophoblast cells deposit large amounts of extracellular matrix known as fibrinoid that further reduces arterial wall elasticity2;6. Elevation of circulating anti-angiogenic factors and antibodies that engage the angiotensin II receptor (AGTR) 1 are also implicated in the deviation of pregnancies towards pre-eclampsia7–11. AGTR1 is a component of the renal renin-angiotensin system (RAS), a key long-term blood pressure regulatory system. Others have linked patterns between mothers and fetuses in the inheritance of genes regulating immune functions in gains in risk for pre-eclampsia12;13.
Our interest in pre-eclampsia arose from histological studies of implantation sites in various strains of immune deficient mice. Our studies were directed towards characterization of the functional immune cell subsets at mouse implantation sites and identification of the lineage of unique, transient lymphocytes of the deciduas basal is that accumulated in the first half of gestation. At that time, these cells were called “granulated metrial gland” or “GMG” cells14. We identified two mouse strains, tgε2615 and common cytokine receptor chain gamma null (formerly γc−/−; now Il2rg−/−)16, both genetically deficient in NK cells and T cells, as being deficient in the transient granulated lymphocyte population of the pregnant mouse uterus17. Unexpectedly, in both strains, we found very thick-walled coiled vessels crossing the decidua basal is that were not seen in implantation sites from normal mice (Figure 1). Subsequent studies revealed that decidual tissue in NK-T-mice was edematous and not fully mature18 and that engraftment of NK cells (without co-engraftment of T cells or B cells), fully restored normal implantation site histology, including thinning of the walls of the coiled arteries and their dilation19. Thus, mice do not require uterine (u)NK cells for successful pregnancy and deliver healthy pups in the absence of spiral arterial modification.
Figure 1.
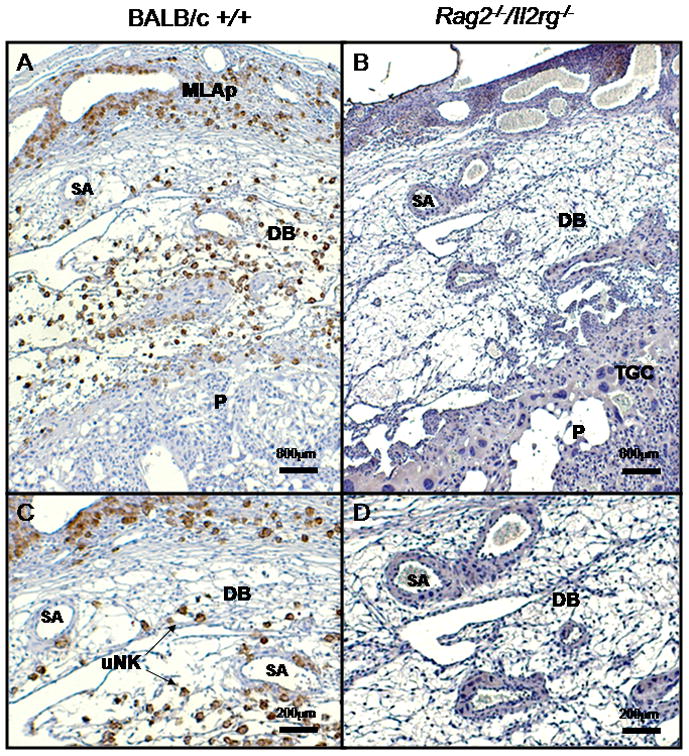
Photomicrograph of mids aggital sections of placenta and deciduas basalis of (A) +/+ and (B) Rag2 −/−/Il2rg−/− on gd12. Mesometrial aspect is to the top of each image. Panels (C, D) show at higher magnification, the typically observed differences in the decidua basalis between (A) modified and (B) unmodified spiral arteries, respectively. The differences in wall thicknesses and lumen diameters can be quantified morphometrically. From a series of comparisons between these genotypes on the BALB/c background, it was estimated that vascular resistance is 1.7x greater in an unmodified artery at gd1231. Stained using DBA lectin42. DB, decidua basalis; MLAp, mesometrial lymphoid aggregate of pregnancy P, placenta; SA, spiral artery; TGC, trophoblast giant cells; uNK + arrows, indicates several of the very abundant DBA+ uNK cell population. Bars indicate magnifications.
Further studies revealed that the major NK cell-derived cytokine interferon gamma (Ifng) was central to the process that we called mouse spiral arterial modification20. Ifng, widely known as a pro-inflammatory cytokine, has complex pleiotrophic effects on many cell types and has been estimated to alter the expression of 0.5% of allgenes in mice under physiological and pathological conditions21.
A number of theories have been advanced to explain the pathogenesis of pre-eclampsia6;9;22–25. Often included as a key feature in these models are defects in endothelial cell function, angiogenesis and/or spiral arterial modification (Figure 2). These defects are associated with renal, hepatic and central nervous system signs clinically and are thought to lead to notched Doppler pulses in the uterine arteries during diastole and to high pulsatility indices (> 1.45)26, intermittent flow to the intervillous space, placental hypoxia, oxidative-reperfusion stress27, coagulation28 and exaggerated inflammation of the systemic endothelium22. These complex interactions are also considered to restrict placental growth and thus birth weight in many pre-eclamptic pregnancies6;9 and to aggregate long term cardiovascular risk factors of mothers and children experiencing pre-eclamptic gestations29;30. Here, we summarize a series of murine experiments designed to separate the primary from the secondary outcomes that result from deficient spiral arterial modification during pregnancy. Our studies compare gestations between mice that lack all lymphocytes (Rag2−/−/Il2rg−/−(formerly Rag2−/−/γc−/−)) on BALB/c (current work) or C57Bl/6 (previously published work) backgrounds and their congenic normal (genotype +/+) controls. The immune deficient mice used in this study are fully competent in the responses of macrophages, dendritic cells and all myeloid lineage cells such as neutrophils. The studies of pregnant mice reviewed here utilize histological and immunohistological assessments of placental growth, hypoxia and cellular composition, micro-ultrasound, blood pressure evaluation by chronic radiotelemetric recording, reproductive outcomes and post-partum growth to build the argument that deficits in spiral arterial remodeling per se mildly alter perfusion of the placenta but alone are of little consequence. This leads to the hypothesis that circulating and/or tissue-based lymphocytes are central to the generation of the adverse, gestational outcomes seen in women with impaired spiral arterial remodeling.
Figure 2.
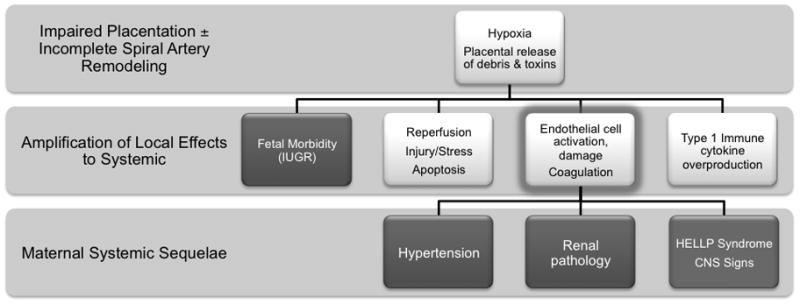
A model for the pathogenesis of pre-eclampsia in which endothelial cell dysfunction (ie activation, insufficient availability of viability and growth factor molecules, pro-coagulant state etc) is seen as the pivotal systemic event.
2. HISTOLOGICAL COMPARISONS BETWEEN IMPLANTATION SITES WITH AND WITHOUT SPIRAL ARTERIAL MODIFICATION
As shown in Figure 1, there is an easily recognized difference between spiral arterial structure at mid-pregnancy (term ~19 days) in histological sections of implantation sites of mice with and without lymphocytes. When the wall to lumen ratios are calculated for both genotypes on a BALB/c background at gestation day (gd) 12 using perfusion-fixed specimens, the Rag2−/−/Il2rg−/− ratio is almost twice that seen in +/+ (0.39±0.02 versus 0.21±0.009; P< 0.001)31. The Rag2−/−/Il2rg−/− value does not change significantly between gd 8 and 12, indicating vessel stability. Calculations indicate that resistance in Rag2−/−/Il2rg−/− spiral arteries should be ~1.7 fold greater than in BALB/c+/+. We undertook a time course study from gd10 to gd18 in Rag2−/−/Il2rg−/− and BALB/c+/+ mice using micro-ultrasound to establish whether there are differences in blood flow in the uterine and spiral arteries.
3. DOPPLER MICRO-ULTRASOUND COMPARISONS BETWEEN IMPLANTATION SITES WITH AND WITHOUT SPIRAL ARTERIAL MODIFICATION
The Doppler wave forms detected in the uterine arteries of BALB/c+/+ changed dynamically over pregnancy as is reported for random bred+/+ mice32. At gd2 when morula stage embryos are present in the oviducts, peak systolic velocity was ~2 cm/sand end diastolic flow was just below 1 cm/s. After spiral arterial modification, peak uterine artery systolic velocity was ~4 cm/s, a two-fold increase that was stable over the rest of pregnancy. End diastolic flow at gd12 was just over 2 cm/sand later in pregnancy reached 5 cm/s. Preliminary examination of tracings from Rag2−/−/Il2rg−/− uterine arteries found a major difference to these control strain values after gd10. Peak velocity rose to reach ~9 cm/s by gd14 and then declined (Zhang and Croy, unpublished).
Spiral arterial Doppler wave forms were also compared between BALB/c+/+ and Rag2−/−/Il2rg−/− on gd 10–1833. Mean velocities were similar in both genotypes over the interval of study. The pulsatility and resistance indices of the spiral arteries became statistically greater in the unmodified Rag2−/−/Il2rg−/− spiral arteries by gd14 and remained elevated to gd18 (Zhang and Croy, unpublished).
4. ASSESSMENT OF PLACENTAL AND FETAL HYPOXIA AND FETAL SURVIVAL BETWEEN IMPLANTATION SITES WITH AND WITHOUT SPIRAL ARTERIAL MODIFICATION
It is possible to assess hypoxia histologically in animal tissue by infusion of Hypoxyprobe-1™ (Millipore) shortly before euthanasia and applying immunohistochemical reagents to detect metabolized adducts of the probe. We compared implantation sites of Rag2−/−/Il2rg−/− and BALB/c+/+ mice that were given Hypoxyprobe-1™ 60 min prior to euthanasia on gd12, a time point following spiral arterial modification in the +/+ mice. Placentae in both mice were reactive with the probe, particularly the trophoblast giant cells. Reactivity was somewhat stronger in the placentae of Rag2−/−/Il2rg−/− than of +/+. Rag2−/−/Il2rg−/− and +/+ fetal tissues were unreactive at gd12. Studies of later gestational times are in progress.
We hypothesized that even mild placental hypoxia from mid-gestation would have consequences for the viability of Rag2−/−/Il2rg−/− fetuses. Therefore, pregnant mice were euthanized and fetal survival rates were addressed. As shown in Figure 3A, when 3–4 pregnancies were examined for each strain a teach time point studied (gd7–18), both strains appeared to lose concept uses during the second half of pregnancy. However, in this small sample, Rag2−/−/Il2rg−/− tended overall to have more implantation sites that were viable than +/+. At peripartum gd18, the viability of fetuses was similar for both genotypes. Thus, the mild hypoxic stress of mid-pregnancy placental giant cells that accompanies lack of spiral arterial modification does not promote fetal death.
Figure 3.
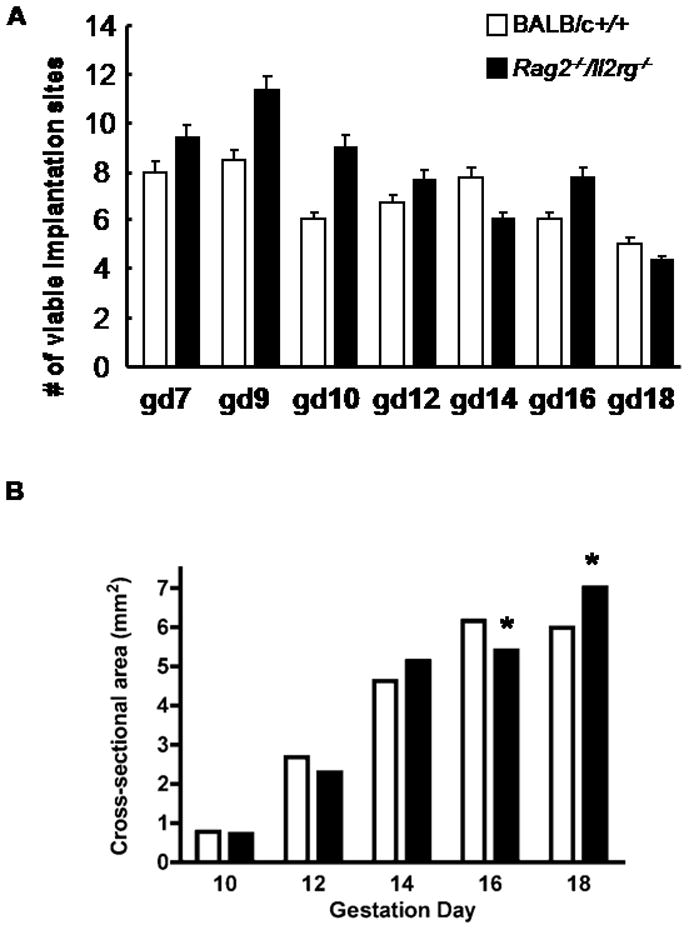
(A) Data are shown for mean ± SEM of viable fetuses in BALB/c+/+ (open bars) and Rag2 −/−/Il2rg−/− (solid bars) mice mated and euthanized within our colony. Either 3 or 4 litters were studied per histogram bar. Many more litters would need to be sacrificed to obtain an accurate estimation of fetal success for these genotypes. These preliminary data show however that viability of Rag2 −/−/Il2rg−/− is not disadvantaged compared with +/+. (B) Data are shown for mean± SEM cross sectional areas of placentae. Errors are too small for visualization of the error bars. A total of 135 tissues sections were scanned for each mean. At gd10, 12 and 14, there were no significant differences in placenta size between the genotypes. At gd 16, +/+ placentae were significantly larger (6.16mm2 ± 0.17 versus 5.4 mm2 ± 0.14 P<0.001) and there was no subsequent placental growth (+/+ placental size at gd18 was 5.99 mm2 ± 0.29). Rag2 −/−/Il2rg−/− placentae continued to grow and had a peripartum gd18 mean size of 7.02 mm2 ± 0.26. This was statistically larger (P<0.01) than in the +/+ females31.
5. ASSESSMENT OF PLACENTAL SIZE AND VASCULARIZATION BETWEEN IMPLANTATION SITES WITH AND WITHOUT SPIRAL ARTERIAL MODIFICATION
Intrauterine growth restriction is commonly associated with pre-eclampsia6;8. We asked whether the failure to modify spiral arteries at mid-gestation leads to placental growth restriction. Mid-saggital histological sections stained with hematoxylin and eosin were quantified using 15 sections/placenta and three placentas from each of 3 mothers for each time point (i.e. 135 tissue sections). As shown in Figure 3B, placentas from Rag2−/−/Il2rg−/− mice were equivalent in surface area to those from +/+ at gd10, the first day after spiral arterial modification in +/+ mice. Over the next 4 days, placental growth was equivalent in the two genotypes but by gd16 the placentas from Rag2−/−/Il2rg−/− were smaller than those from +/+ mice. The placentas in BALB/c +/+ mice did not grow between gd16 and gd18. This is not unexpected as others studying normal CD1 mice by micro-ultrasound report that no growth occurs in +/+ mouse placenta after gd1432. In contrast to BALB/c +/+ placentas, Rag2−/−/Il2rg−/− placentas continued to grow after gd16 and were statistically larger on the last day of pregnancy (P<0.05).
6. ASSESSMENT OF POST-NATAL GROWTH BETWEEN MICE GESTATED IN IMPLANTATION SITES WITH AND WITHOUT SPIRAL ARTERIAL MODIFICATION
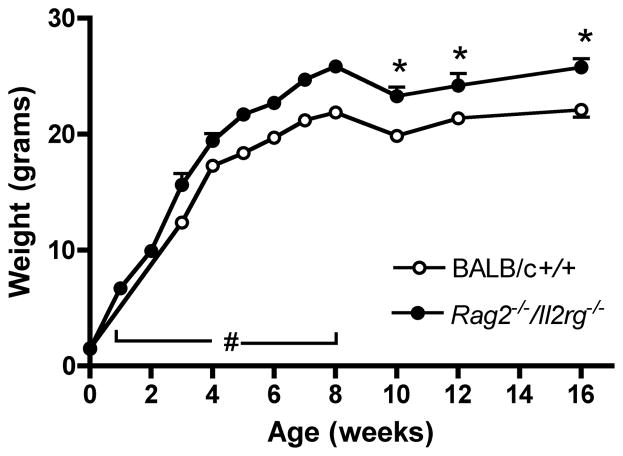
To address whether absence of spiral arterial modification has an effect on post-partum growth, we weighed female Rag2−/−/Il2rg−/− between birth and 16 weeks of age (Figure 4). We do not maintain BALB/c+/+ breeding stocks but purchase +/+ females at 8 weeks of age. Thus, comparisons from birth to week 8 are based on published information34;35 and <www.jax.org>. BALB/c +/+ weights at weeks 10–16, that were statistically less than age-matched weights of Rag2−/−/Il2rg−/−, are measurements on animals in our care. At 10 weeks of age, female Rag2−/−/Il2rg−/− mice were on average 3.2 g heavier that BALB/c mice (P<0.001), a finding that had implications for our anaesthetic protocol and overall post-surgical success for surgical implantation of radiotransmitters in BALB/c control mice (see below). Thus, the continued late gestational growth of the placenta in Rag2−/−/Il2rg−/− mice appears to promote larger than normal offspring. This finding contrasts with the IUGR-associated phenotype seen in many human pre-eclamptic gestations and suggests that IUGR must be attributed to processes that are not linked with or are secondary to a lack of spiral arterial modification.
Figure 4.
Postnatal growth (mean weight ±SEM) of BALB/c +/+ (○) and Rag2 −/−/Il2rg−/− (●) from birth to 16 wks of age. All Rag2 −/−/Il2rg−/− data are from our Queen’s University laboratory with 6–9 animals weighed/time point. BALB/c+/+ data are from mixed sources. BALB/c birth weights were published by Pal et al., (n=9)34 and Buzas et al., (n=11)35. Data for BALB/c growth to 8 wks of age (#) are from the Jackson Laboratory Mouse Phenome Database (n=40; www.jax.org). Data for BALB/c+/+ growth after 8 wks is from our Queen’s University laboratory. Because husbandry and diet may influence weight measurements, statistics are not presented for measures made before wk 10, which was 2 wk after receipt and conditioning of BALB/c +/+ at our colony. For both genotypes, data collected before weaning (<4 wk), include both sexes. Wk 4–16 data are for females only. *P<0.01 compared with BALB/c+/+ females.
7. IS THERE PROTEINURIA OR RENAL HISTOPATHOLOGY IN DAMS EXPERIENCING PREGNANCIES WITHOUT SPIRAL ARTERIAL MODIFICATION?
Renal function was assessed over pregnancy in Rag2−/−/Il2rg−/− and +/+ using an ELISA for urinary albumin (corrected with creatinine; Exocell, Philadelphia PA). No significant changes in protein excretion were detected over pregnancy for either genotype. Protein and creatinine excretion were stable and comparable to virgin animals of each genotype (Burke and Croy, unpublished data). We also examined pregnant Rag2−/−/Il2rg−/− mice for gross and microscopic signs of renal and hepatic pathology. None were found (Burke, Zhang and Croy, unpublished data). Indeed, Rag2−/−/Il2rg−/− dams can be maintained in excellent health and routinely deliver 6–8 healthy litters (Zhang, Bilinski and Croy, unpublished data). No CNS disturbances have been seen in our C57Bl/6 Rag2−/−/Il2rg−/− or BALB/c Rag2−/−/Il2rg−/− pregnant or non-pregnant animals during the 15 years we have held this genotype.
8. DO DAMS EXPERIENCING PREGNANCIES WITHOUT SPIRAL ARTERIAL MODIFICATION BECOME HYPERTENSIVE DURING LATE GESTATION?
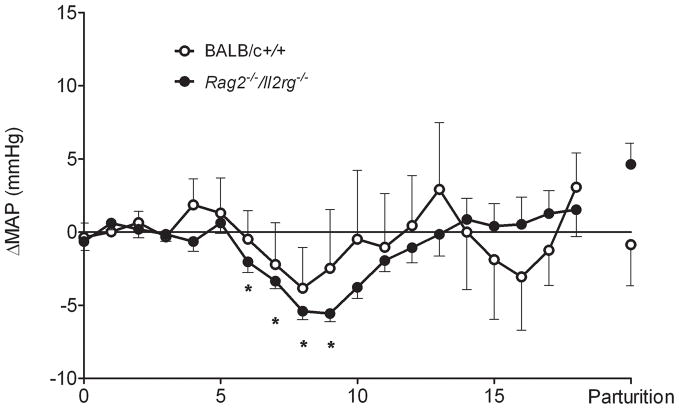
To address whether spiral arterial modification prevents gestational hypertension, radiotransmitters (PA-C10, DSI St. Paul, MN) were surgically implanted into ten week old Rag2−/−/Il2rg−/− and BALB/c mice to record hemodynamic properties from the aortic arch. Ten days of recovery were given before baseline recordings commenced. Both strains have arterial pressures that change in response to dietary salt manipulation31. Using 12 or 24 hr means of arterial pressure (MAP), a five phase pattern of gestational MAP was defined for BALB/c mice (Figure 5). Unexpectedly, a similar pattern was present in Rag2−/−/Il2rg−/− (Figure 5). The pattern was coincident with significant events occurring in mouse placental development. Over the pre-implantation period from gd0 (day copulation plug detected and post-mating recording began) to gd3, there was no change (Δ) in MAP from that recorded in the post-salt challenge baseline. Between gd4 and gd9, ΔMAP declined. This is the interval of post-implantation/pre-placental development in mice associated with ectoplacental conetrophoblast. After gd9, ΔMAP rose. The increase in ΔMAP was more rapid between gd10 and gd14 than after gd14. The gd10–14 interval follows fusion of the allantois and chorion and initiation of placental circulation. In this phase, growth of both the fetus and placenta continue. The fourth stage appears to be when fetal growth continues but placental growth does not. The final stage is peripartum. The status of spiral arterial modification had no direct effect on the regulation of gestational blood pressure.
Figure 5.
Change (Δ) in 24 hr mean arterial pressures (MAP) across pregnancy in BALB/c +/+ (○; n=5) and Rag2 −/−/Il2rg−/− (●; n=9) for females delivering live born offspring. Because no changes from a 4 day pre-conception baseline were found during pre-implantation gestation in these mice or C57Bl/6J, gd0–3 mean 24 hr MAP was used for subsequent daily 24hr mean statistical comparisons31. Both strains showed a decline in ΔMAP shortly after implantation that continued until the time of spiral arterial modification and opening of the placental circulation (~gd9–10). The decline was statistically significant from the pre-implantation baseline for Rag2 −/−/Il2rg−/−. A gain in ΔMAP followed and baseline values were achieved a few days later. These remained relatively stable, without hypertension until parturition (P). A pattern of late gestational hypertensive (not shown) was seen in two Rag2 −/−/Il2rg−/− females that gave birth to litters of stillborn pups31.
Of note, two other pregnant Rag2−/−/Il2rg−/− females had severe, late gestational hypertension. These were the only two females in the study to give birth to litters comprised of stillborn pups. This raises the question of whether the concept us provides unique hemodynamic control during pregnancy. Some of our radiotransmitter-implanted females mated but failed to conceive and were endocrinologically pseudopregnant. There was no ΔMAP in these animals following mating which indicates that the hormonal milieu does not dynamically regulate gestational MAP31. We have yet to establish whether maternal regulation is possible through differentiation of decidual tissue in the absence of a conceptus but these studies are ongoing.
9. ARE THERE POST-PARTUM HEMODYNAMIC DIFFERENCES BETWEEN DAMS EXPERIENCING PREGNANCIES WITH OR WITHOUT SPIRAL ARTERIAL MODIFICATION?
Post-partum sequelae to pre-eclampsia include elevated risk for hypertension29;30. We therefore collected and compared pre-partum and post-partum hemodynamic data from Rag2−/−/Il2rg−/− to determine whether absence of spiral arterial modification during pregnancy might elevate post-partum MAP. Twenty-four hour MAP were compared from pre-partum baselines (n=16), early pregnancy (gd 0–3; n=9) and 10–14 days post-partum (n=9). There were no differences between these three time points, indicating that no apparent systemic circulatory alterations result from pregnancies lacking spiral arterial modification, at least in the first few weeks after parturition (Burke, Barrette, Adams and Croy, unpublished data).
10. MECHANISMS THAT MAYBE KEY TO UNK CELL ACTIONS ON SPIRAL ARTERIES
The molecules Ephrin B2 (Efnb2) and EphB4 (Ephb4) are used to discriminate arterial from venous endothelia. We examined the expression of these markers in mouse implantation sites36. At gd 8 in both Rag2−/−/Il2rg−/− and +/+ mice, spiral arteries are typical Efnb2+Ephb4-arteries and histologically similar. By +/+ gd10, these vessels express much less detectable Efnb2 and are beginning to express Ephb4. By gd12, +/+ spiral arteries have lost Efnb2 expression and weakly express Ephb4. In contrast, Efnb2 is expressed by spiral arteries of Rag2−/−/Il2rg−/− between gd6 and gd12, suggesting retention of arterial functions. These functions would include vasoreactivity and higher vascular resistance but might also include expression of lymphocyte recruitment molecules. Between gd6 and gd10, uNK cells in +/+ mice became strongly Efnb2+, which may account for their localization with arterial rather than venous structures. In Rag2−/−/Il2rg−/−, mesometrial myometrium became strongly positive for Efnb2. This region was non reactive with anti-Efnb2 in normal mice at mid-pregnancy.
T cells of humans and mice and human NK cells are reported to express all components of RAS, including angiotensin receptors Agtr1 and Agtr237–40. Because of the close association between uNK cells and spiral arteries in mouse implantation sites, we postulated that uNK cells would be equipped with Agtr1 and Agrt2 and that this would contribute to gestational blood pressure regulation. Using dual staining immunohistological techniques to identify uNK cells and localize one or other receptor, we found that many (~40%) but not all gd10 mouse uNK cells express Agtr2. Agtr1 is also expressed but by a smaller proportion of uNK cells (Hatta K, Carter A, Zhen A, Leno-Duran E, Ruiz-Ruiz C, Olivares EG, Tse Y, Pang SC and Croy BA, manuscript submitted). However, it is not yet clear if any uNK cell co-expresses these receptors or expresses other components of the renin angiotensin system Thus, despite our findings using radiotelemetry to examine blood pressure in pregnant, alymphoid Rag2−/−/Il2rg−/− mice, it remains possible that deviations in uNK, NK and/or T cell functions in pregnant +/+ mice may contribute directly to dysregulation of MAP.
11. CONCLUSIONS
The studies presented here report in detail our current findings on outcomes from impairment of gestational spiral arterial modification in mice. We estimated a 1.7-fold higher resistance to flow in individual spiral arteries of alymphoid mice compared with controls from histological specimens. Decreased spiral arterial flow was not confirmed in Rag2−/−/Il2rg−/− by micro-ultrasound at mid-gestational though from mid to late gestation these vessels had elevated pulsatility and resistance indices. The upstream (towards the heart) uterine arteries in Rag2−/−/Il2rg−/− had elevated flow compared with +/+ uterine arteries from gd10 and this may account for the relatively mild placental hypoxia we found in Rag2−/−/Il2rg−/− at gd12. Despite the vascular abnormalities in Rag2−/−/Il2rg−/− implantation sites, fetal viability was maintained and post-natal growth was promoted rather than impaired. These data call into question the mechanisms that have been proposed for the association between spiral arterial modification and human intrauterine growth restriction. Trophoblast growth was not restricted by failure of remodeling of the spiral arteries. Rather trophoblast appeared to compensate for reduced perfusion by continued growth into the peripartum interval. Thus, in mice, it is unlikely that uterine lymphocytes uniquely provide any essential growth factors to trophoblast and it is more probable that their primary actions are on maternal tissue.
The data presented here differ from those we reported for the very first NK cell deficient strain, tgε2641. The tgε26 strain was developed as a T cell deficient strain and bred with phenotype selection for the most severe T cell deficit. At the time we received breeding stock (1994), small litters (mean ~3) and significant fetal death characterized this strain. As previously reported, tgε26 lost this reproductive phenotype after 3 years by applying breeding selection pressure on large litter size. Our studies with that strain were terminated and transferred to superior genotypes as they became available. The alymphoid BALB/c Rag2−/−/Il2rg−/−, as summarized above, is our most thoroughly investigated strain. We have not found significant differences between these mice and C57BL/6 Rag2−/−/Il2rg−/−, used in many of our previous publications.
The immune deficit in Rag2−/−/Il2rg−/− is restricted to lymphocytes. Thus, antigen uptake and presentation pathways are intact as are chemokine and cytokine production by macrophages, dendritic cells and myeloid cells. This implies that consequences that lead from placental hypoxia to fetal growth restriction or death must depend upon lymphocyte functions. Figure 6 presents a “lymphocentric” model for the pathogenesis of pre-eclampsia based upon the data summarized here and suggests that clinical interventions for pre-eclampsia might be beneficially designed by consideration of alymphocyte-based pathology. In Figure 7, known pathways of lymphocyte function that could contribute to pregnancy complications such as intrauterine growth restriction and pre-eclampsia are summarized. It will be important to identify the subsets of lymphocytes critical in promotion of gestational complications to advance these hypotheses.
Figure 6.
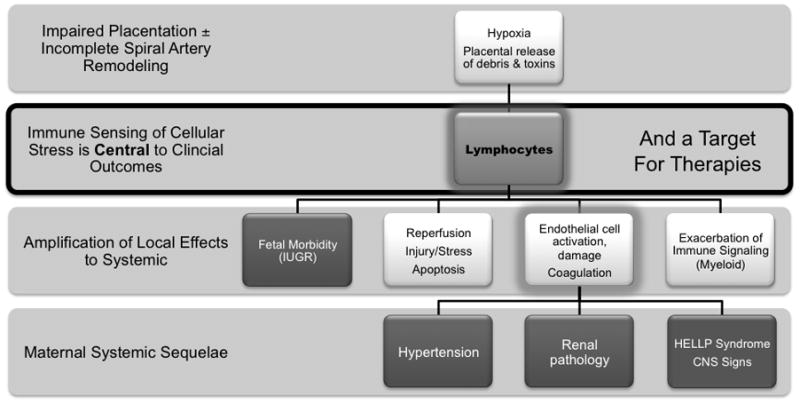
A model for the pathogenesis of pre-eclampsia in which lymphocyte dysfunction triggers the pregnancy complications that arise from absence of spiral arterial modification. Although immune activation has been documented by others as a component of the pre-eclampsia syndrome, our studies suggest that it is exclusively the changes in lymphocytes and not those in antigen presenting or myeloid-lineage immune cells such as macrophages, neutrophils and mast cells that are central to pathogenesis.
Figure 7.
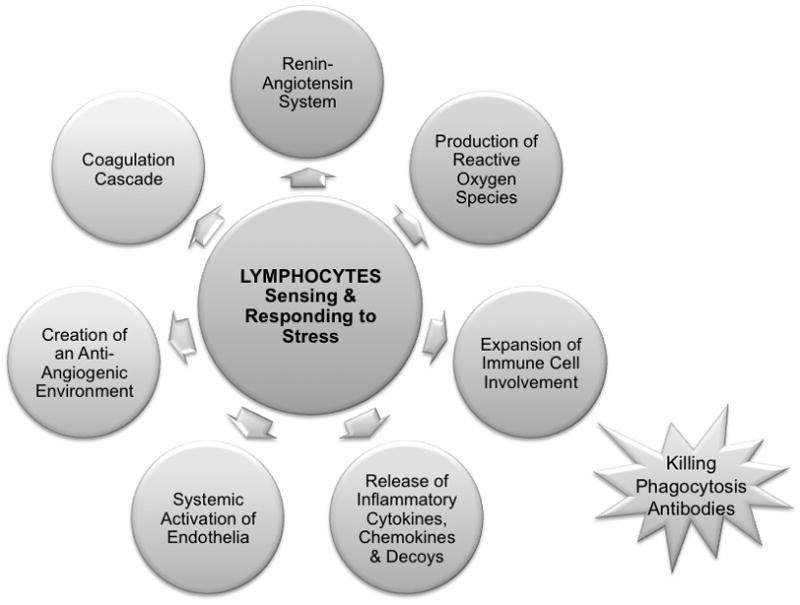
A model for pathways that lymphocytes may utilize to drive pregnancy complications including intrauterine growth restriction and fetal death.
Phenotypic characterization of pregnancies in Rag2−/−/Il2rg−/− mice provides a strong, novel platform for further studies on the pathogenesis of pre-eclampsia. This strain has consistent histological evidence for failure of gestational spiral arterial modification but retains normal placenta growth, fetal survival and hemodynamic function. When these features are quantitatively assayed in combination with chronic radiotelemetry and micro-ultrasound, the actions and dosages of test molecules or combinations of molecules on each feature can be assessed independently. This should provide an in vivo model in which step wise incremental reconstitution of elements suspected to contribute to the pathogenesis of pre-eclampsia can be tested. For example, osmotic pump infusions of s-Vegfr1, Tnf, STBMs, uric acid or antibodies activating Agtr1 could be tested over wide dose ranges and in combinations. Because Rag2−/−/Il2rg−/− mice readily accept xenografts, they could also be constructed to assess the functions of specific human lymphocyte subsets within implantation sites.
Acknowledgments
Support for these studies was provided by NSERC, CIHR, and the Canada Research Chairs Program (awarded to B.A.C.). Trainee support came from a CIHR fellowship awarded to S.D.B. and a CAPES fellowship awarded to J.B.. We appreciate helpful discussions with Michael Bilinski and Ester Leno-Durán and the dedication of the animal care staff members who support our mice. We acknowledge and thank Dr. Christopher Redman for his interest and encouragement of our studies in mice and wish him the best in his future activities.
Footnotes
Invited Contribution from ISSHP Meeting Celebrating the Retirement of Dr. C.W.G. Redman
Reference List
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Etiologiy and Clinical Practice. Cambridge, UK: Cambridge University Press; 2007. Pre-eclampsia. [Google Scholar]
- 3.Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta. 1980;1:3–19. doi: 10.1016/s0143-4004(80)80012-9. [DOI] [PubMed] [Google Scholar]
- 4.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Redline RW, Patterson P. Pre-eclampsia is associated with an excess of proliferative immature intermediate trophoblast. Hum Pathol. 1995;26:594–600. doi: 10.1016/0046-8177(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 7.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu Rev Med. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 10.Herse F, Verlohren S, Wenzel K, et al. Prevalence of agonistic autoantibodies against the angiotensin II type 1 receptor and soluble fms-like tyrosine kinase 1 in a gestational age-matched case study. Hypertension. 2009;53:393–398. doi: 10.1161/HYPERTENSIONAHA.108.124115. [DOI] [PubMed] [Google Scholar]
- 11.Herse F, Staff AC, Hering L, et al. AT1-receptor autoantibodies and uteroplacental RAS in pregnancy and pre-eclampsia. J Mol Med. 2008;86:697–703. doi: 10.1007/s00109-008-0332-4. [DOI] [PubMed] [Google Scholar]
- 12.Hiby SE, Walker JJ, O’shaughnessy KM, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Founds SA, Conley YP, Lyons-Weiler JF, et al. Altered global gene expression in first trimester placentas of women destined to develop preeclampsia. Placenta. 2009;30:15–24. doi: 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peel S. Granulated metrial gland cells. Adv Anat Embryol Cell Biol. 1989;115:1–112. doi: 10.1007/978-3-642-74170-8. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Biron C, She J, et al. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3E gene. Proc Natl Acad Sci USA. 1994;91:9402–9406. doi: 10.1073/pnas.91.20.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci USA. 1995;92:377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guimond MJ, Wang B, Fujita J, Terhorst C, Croy BA. Pregnancy-associated uterine granulated metrial gland cells in mutant and transgenic mice. Am J Reprod Immunol. 1996;35:501–509. doi: 10.1111/j.1600-0897.1996.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 18.Greenwood JD, Minhas K, di Santo JP, et al. Ultrastructural studies of implantation sites from mice deficient in uterine natural killer cells. Placenta. 2000;21:693–702. doi: 10.1053/plac.2000.0556. [DOI] [PubMed] [Google Scholar]
- 19.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187:217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashkar AA, di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy SP, Tayade C, Ashkar AA, et al. Interferon gamma in successful pregnancies. Biol Reprod. 2009;80:848–859. doi: 10.1095/biolreprod.108.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Germain SJ, Sacks GP, Sooranna SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. J Immunol. 2007;178:5949–5956. doi: 10.4049/jimmunol.178.9.5949. [DOI] [PubMed] [Google Scholar]
- 23.Redman CW, Sargent IL. Microparticles and immunomodulation in pregnancy and pre-eclampsia. J Reprod Immunol. 2007;76:61–67. doi: 10.1016/j.jri.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Gammill HS, Roberts JM. Emerging concepts in preeclampsia investigation. Front Biosci. 2007;12:2403–2411. doi: 10.2741/2242. [DOI] [PubMed] [Google Scholar]
- 25.Bainbridge SA, Roberts JM, von Versen-Hoynck F, et al. Uric acid attenuates trophoblast invasion and integration into endothelial cell monolayers. Am J Physiol Cell Physiol. 2009;297:C440–C450. doi: 10.1152/ajpcell.00593.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hershkovitz R, de SM, Kingdom J. Mid-trimester placentation assessment in high-risk pregnancies using maternal serum screening and uterine artery Doppler. Hypertens Pregnancy. 2005;24:273–280. doi: 10.1080/10641950500280995. [DOI] [PubMed] [Google Scholar]
- 27.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Thachil J, Toh CH. Disseminated intravascular coagulation in obstetric disorders and its acute haematological management. Blood Rev. 2009;23:167–176. doi: 10.1016/j.blre.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Roberts JM, Gammill H. Pre-eclampsia and cardiovascular disease in later life. Lancet. 2005;366:961–962. doi: 10.1016/S0140-6736(05)67349-7. [DOI] [PubMed] [Google Scholar]
- 30.Smith GN, Walker MC, Liu A, et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol. 2009;200:58e1–e8. doi: 10.1016/j.ajog.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 31.Burke SD, Barrette VF, Bianco J, et al. Spiral arterial remodeling is not essential for normal blood pressure regulation in pregnant mice. Hypertension. 2010:55. doi: 10.1161/HYPERTENSIONAHA.109.144253. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mu J, Slevin JC, Qu D, McCormick S, Adamson SL. In vivo quantification of embryonic and placental growth during gestation in mice using micro-ultrasound. Reprod Biol Endocrinol. 2008;6:34. doi: 10.1186/1477-7827-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J, Croy BA. Using ultrasonography to define fetal-maternal relationships: moving from humans to mice. Comp Med. 2009;59:527–533. [PMC free article] [PubMed] [Google Scholar]
- 34.Pal S, Peterson EM, de la Maza LM. A murine model for the study of Chlamydia trachomatis genital infections during pregnancy. Infect Immun. 1999;67:2607–2610. doi: 10.1128/iai.67.5.2607-2610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buzas EI, Hollo K, Rubliczky L, et al. Effect of pregnancy on proteoglycan-induced progressive polyarthritis in BALB/c mice: remission of disease activity. Clin Exp Immunol. 1993;94:252–260. doi: 10.1111/j.1365-2249.1993.tb03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J, Dong H, Wang B, Zhu S, Croy BA. Dynamic changes occur in patterns of endometrial EFNB2/EPHB4 expression during the period of spiral arterial modification in mice. Biol Reprod. 2008;79:450–458. doi: 10.1095/biolreprod.108.067975. [DOI] [PubMed] [Google Scholar]
- 37.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoch NE, Guzik TJ, Chen W, et al. Regulation of T-cell function by endogenously produced angiotensin II. Am J Physiol Regul Integr Comp Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jurewicz M, McDermott DH, Sechler JM, et al. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18:1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 40.Madhur MS, Lob HE, McCann LA, et al. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2009 doi: 10.1161/HYPERTENSIONAHA.109.145094. Epub 12/28/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guimond M, Wang B, Croy BA. Immune competence involving the natural killer cell lineage promotes placental growth. Placenta. 1999;20:441–450. doi: 10.1053/plac.1999.0398. [DOI] [PubMed] [Google Scholar]
- 42.Paffaro VA, Jr, Bizinotto MC, Joazeiro PP, Yamada AT. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta. 2003;24:479–488. doi: 10.1053/plac.2002.0919. [DOI] [PubMed] [Google Scholar]