“Because DNA sequencing technology is inherently simpler and more scalable than protein analytical technology, and because the finiteness of genomes invited a spirit of rapid conquest, the notion of genome sequencing has displaced that of protein databases in the minds of most molecular biologists for the last decade…As proteomics now takes center stage in molecular biology, it is appropriate to take stock of progress to date and consider the major strategic objectives that can be achieved during the next stages of its evolution.”
Norman G. Anderson et al. 2001 [1]
1. TOWARDS POST-PROTEOMIC BIOLOGY
A decade after the insightful analysis by Anderson et al. on a strategic future agenda for post-proteomic biology [1], we still have limited knowledge of protein products of the human genes discovered through the Human Genome Project (HGP). The abundance, distribution, sub-cellular localization, post-translational characteristics, interaction networks, and function of the human proteome are in need of further in-depth research before we can transition to proteomics based diagnostics in personalized medicine. Despite the reality of these remaining informational gaps in our collective understanding of the human proteome, there are tremendous efforts and strides currently being undertaken towards their attainment. The Human Proteome Project (HPP), which was officially launched in September 2010 under the leadership of the Human Proteome Organization (HUPO) at its 9th Annual World Congress in Sydney, Australia, serves as a prime example of such an initiative [2]. HPP strives to map and characterize human proteins in their biological context by deploying an experimental strategy centered upon protein antibody capture, mass spectrometry and bioinformatics tools [2]. The anticipated deliverables from this international federated effort have prime importance for the field of personalized medicine [3]. Proteomics fundamentally requires sensitive and specific diagnostic capabilities, such as those being developed under the global vision of the HPP, in order to integrate information from multiple levels of the biological hierarchy. It is reasonable to envision that such a collective amalgamation of protein-targeted scientific discovery and developmental knowledge will help form a firm foundation towards a theory of biological variation in regards to personalized medicine [4–7]. The tenets of this argument, in which personalized medicine initiatives would benefit from a greater understanding of the human proteome and its biological variability, are directly applicable to the emergent field of pharmacoproteomics.
Pharmacoproteomics represents the use of high-throughput proteomic technologies in basic and clinical pharmacology with a view to novel drug target discovery, drug metabolism and transport as well as drug efficacy and toxicity. All of these domains of inquiry are directly relevant to personalized medicine as they help explain the mechanisms of person-to-person and population variability in pharmacokinetics and pharmacodynamics. The practice of personalized medicine is transforming with current attempts to creatively mine such large post-genomics proteomics datasets with the tools of data intensive science [8, 9].
As we steer towards post-proteomic biology, the applications of pharmacoproteomics are rapidly expanding well beyond developed countries and being launched de novo in developing countries and resource-limited settings. When viewing such geographical areas through the specific lens of their current respective proteomic and pharmacoproteomic initiatives and capabilities, it is surprising that India, despite recent extensive investments in health research, has not been thoroughly examined in this context.
In this editorial analysis, we review and “map” the growing fields of proteomics and pharmacoproteomics in India and the attendant promise of protein microarrays for the development of novel diagnostics in resource-limited settings. We also point to the increasing global realization that the social and economic benefits of novel biotechnologies such as proteomics and pharmacoproteomics, especially in resource-limited settings, are not automatic (i.e., “they do not flow inevitably from the marriage of biology and technology” [10]). In this regard, we drew from the experience of genomics and other “-omics” based large scale biology research funding agencies to devise and suggest ways forward for the “socio-technical” integration and creation of knowledge-based innovations that are attuned to the societal norms, contextually sensitive and thus, socially robust and sustainable. Finally, we suggest that the progress being made and the lessons learned in India might usefully inform pharmacoproteomics applications in low and middle income countries (LMICs) in South Asia and the Asia-Pacific region.
2. INDIA: AN OVERVIEW OF THE RISE OF PROTEOMICS AND PHARMACOPROTEOMICS
It is interesting to note that India could not play a crucial role in genome sequencing projects in a manner that is commensurate to the extensive scientific investments that have been made in the country. Learning from the past lessons in high-throughput biology, India quickly realized, however, the importance of proteomics research as a pivotal complement of functional genomics. Indeed, in 2005 Dr. Abdul Kalam, the former President of India, aptly observed that “India has the potential to tap research opportunities in proteomics and biochips to help understand the biological processes and treat diseases. This is possible even though the country has missed the opportunity to partner in the Human Genome Project” [11]. India’s rapidly growing bioeconomy and policy innovations by the Department of Science and Technology, the Department of Biotechnology and other government funding agencies have helped further shape the large scale clinical proteomics research infrastructure in India.
Researchers in India have been active contributors to proteomics and pharmacoproteomics with a keen eye to personalized medicine and knowledge-based innovations in the region. Cancer proteomics for the identification of biomarkers and mechanistic insights into the complex biology of cancer is one of the leading areas. For example, works at the Indian Institute of Science, Bangalore; and the Centre for Cellular and Molecular Biology, Hyderabad, have advanced the knowledge of glioma proteomics variation. Tissue proteomic analysis of patients with astrocytomas revealed molecular alteration of cytoskeleton intermediate filament proteins and heat shock proteins [12]. Serum proteomic analysis of glioma samples found Haptoglobin-α-2 as one of the potential biomarkers in glioblastoma multiforme (GBM) [13].
Decisions to focus on disease-based therapies often arise out of a specific socio-cultural context. For example, gingivo-buccal complex (GBC) tumors are quite common in Indian population due to tobacco chewing. Comparative proteomic analysis of GBC tissue carried out at the Advanced Center for Treatment, Research and Education in Cancer, Mumbai, has provided mechanistic insight for differentially modulated proteins involved in oral cancer [14]. Retinoblastoma pathogenesis was investigated by the Vision Research Foundation, Sankara Nethralaya group at Chennai. This proteomic analysis identified 27 candidate proteins as potential biomarkers for prognosis and therapy [15]. Research groups from the Central Drug Research Institute, and the Central Institute of Medicinal and Aromatic Plants, Lucknow, are actively working on pharmacoproteomics. A recent investigation on a human epithelial colorectal adenocarcinoma cell line suggested the modulation of tubulin polymerization by L-menthol and inhibition of cell proliferation [16]. A study on the effect of ormeloxifen in chronic myeloid leukemia cells K562 found an association with induction of G0-G1 growth arrest and ERK mediated apoptosis [17]. Collectively, these cancer focused, protein-based research investigations provide a concise and broad overview of the types of studies currently being undertaken within India.
Further to these reports, in a proteomics guided effort towards discovery and clinical correlations, the Institute of Bioinformatics (IOB) and the Centre for Cellular and Molecular Platforms (C-CAMP) in Bangalore are extensively engaged with the mass spectrometry based proteomics applications. Some examples related to quantitative proteomics such as Isobaric Tags for Relative and Absolute Quantitation (iTRAQ) and Stable Isotope Labeling by Amino Acids in Cell Culture (SILAC) include those in pancreatic cancer, hepatocellular carcinoma, esophageal squamous cell carcinoma secretome [18] and a urinary proteome map for the diagnosis of pathological conditions [19].
Despite the rise of chronic non-communicable diseases in many developing countries [20], infectious diseases remain as one of the leading priority public health needs of India. Infectious disease burden in India is significant and stands to benefit from post-genomics new technologies such as proteomics. Researchers at the International Center for Genetic Engineering and Biotechnology, New Delhi, have studied the molecular interactions between plasmodium and erythrocytes and found that merozoite surface protein-1 complex is responsible for the invasion of parasite into the RBC [21]. Patient-derived malarial parasites were investigated using mass spectrometry based approach by the Indian Institute of Science Group; this study identified potential new molecular targets for malaria diagnostics and therapeutics [22]. Researchers from the Central Drug Research Institute, Lucknow, used proteomic approach to identify immunostimulatory proteins from soluble antigens of Leishmania donovani [23]. The National Institute for Research in Reproductive Health, Mumbai, has performed a proteomic and transcriptomic analysis of polyene antifungal drug amphotericin B against Aspergillus fumigates which provided mechanistic insight for the novel drug targets involved in cell stress, ergosterol synthesis, cell-wall maintenance and transport proteins [24].
The proteomic-based investigations in India are not only limited to cancer biology and infectious diseases but also have been pursued for chronic non-communicable diseases. A case in point is diabetes mellitus, which is another major public health problem in India. New biosampling strategies for biomarker development including the use of saliva as a source of noninvasive biomarkers are also being pursued [25]. A study by Singh et al. examined the protein expression in IB3-1 cystic fibrosis bronchial epithelial cells and identified proteins that were differentially expressed in response to treatment of these cells with 4-phenylbutyrate, a drug used to treat the urea cycle disorders [26].
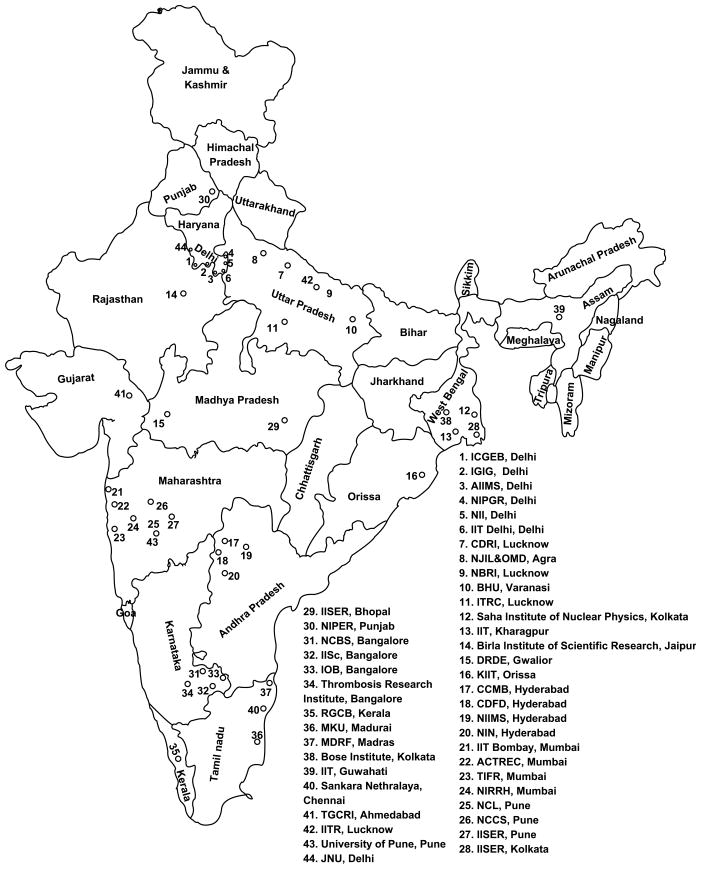
While it is not possible to provide the full details of all proteomics and pharmacoproteomics related research in India within the limits of this concise editorial, we provide a “map” of the related research groups in India in the hopes that this can usefully inform future collective work in pharmacoproteomics nationally and internationally as global personalized medicine efforts accelerate (Table 1 and Figure 1).
Table 1.
Institutes Involved in Proteomics Research in India
| Institute/Center | Area of Research Focus |
|---|---|
| All India Institute of Medical Sciences (AIIMS), New Delhi (www.aiims.edu) | Clinical proteomics for biomarker discovery |
| Bose Institute, Kolkata (www.boseinst.ernet.in) | Stress physiology, genomics & Proteomics |
| Central Drug Research Institute (CDRI), Lucknow (www.cdriindia.org) | New drug targets for Leishmania by proteomics, Mechanism of anti-cancer drugs |
| Centre for Cellular and Molecular Biology (CCMB), Hyderabad (www.ccmb.res.in) | Secretome of glioma cell lines, Human sperm and endometrium proteome, Cerebellum proteome in rat, Neuroproteomics on Zebrafish |
| Centre for DNA Fingerprinting and Diagnostics (CDFD), Hyderabad (www.cdfd.org.in) | Genomic and proteomic profiling |
| Indian Council of Agricultural Research (ICAR), New Delhi (www.icar.org.in) | Lens proteome |
| Indian Institute of Science (IISc), Bangalore (www.iisc.ernet.in) | Pathogenic proteomics P. falciparum, vivax, Trypanosoma evansi, Biomarker for glioblastoma, Stress proteome study on mycobacterium smegmatis |
| Indian Institute of Technology (IIT) Bombay, Mumbai (www.iitb.ac.in) | Clinical proteomics and bacterial proteomics |
| Indian Institute of Technology (IIT) Guwahati (www.iitg.ac.in) | Functional proteomics for carbohydrate enzymes |
| Indian Institute of Technology (IIT), Kharagpur (www.iitkgp.ernet.in) | Clinical proteomics and metabolomics |
| Indian Institute of Technology (IITD), New Delhi (www.iitd.ac.in) | Proteomics and drug design |
| Indian Institute of Toxicology Research (IITR), Lucknow (www.iitrindia.org) | Biomarker discovery in neurodegenerative disease |
| Industrial Toxicology Research Centre (ITRC), Lucknow (www.itrcindia.org) | Mechanism of Parkinson’s disease by proteomics & genomics |
| Institute of Bioinformatics (IOB), Bangalore (www.ibioinformatics.org) | Human urinary proteome, neuroproteome, proteome of various diseases and organisms |
| Institute of Genomics and Integrative Biology (IGIB), New Delhi (www.igib.res.in) | Proteomic approach for aberrant metabolite levels in diseases, anthrax toxin on mouse macrophages, Immunoproteomic approach for novel allergens |
| Institute of Molecular Medicine, New Delhi (www.immindia.org) | Biomarker detection for infectious diseases and bacterial proteomics |
| International Centre for Genetic Engineering and Biotechnology (ICGEB), New Delhi (www.icgeb.org) | Biomarker detection for hepatitis E, Secretory proteome of P. falciparum & Urine proteomics |
| Jawaharlal Nehru University (JNU), New Delhi (www.jnu.ac.in) | Group A Streptococcus proteome analysis, Biomarker detection in drug resistant Leishmania donovani |
| Madarai Kamaraj University (MKU), Madurai (www.mkuniversity.org) | Genomic & proteomic analysis of M. leprae and tear proteomics |
| National Centre for Biological Sciences (NCBS), Bangalore (www.ncbs.res.in) | Lipidomics and proteomics |
| National Centre for Cell Science (NCCS), Pune (www.nccs.res.in) | Cancer proteomics |
| National Chemical Laboratory (NCL), Pune (www.ncl-india.org) | Proteomic study of anti-diabetic drugs in rat |
| National Institute for Research in Reproductive Health (NIRRH), Mumbai (www.nirrh.res.in) | Human follicular fluid proteome in PCOS, Functional sperm proteomics, Endometrium proteomics |
| Rajiv Gandhi Center for Biotechnology (RGCB), Kerala (rgcb.res.in) | Biomarker discovery in breast cancer and reproductive disorders |
| Saha Institute of Nuclear Physics (SINP), Kolkata (www.saha.ac.in) | Proteomic analysis of blood related disorders |
| Sankara Nethralaya, Chennai (www.sankaranethralaya.org) | Primary retinoblastoma tumor proteome, Tear proteomics and metabolomics, Toxicoproteomics |
| Tata Institute of Fundamental Research (TIFR), Mumbai (www.tifr.res.in) | Proteomics and genomics |
| The Advanced Centre for Treatment, Research and Education in Cancer (ACTREC), Mumbai (www.actrec.gov.in) | Global profiling of oral cancer by proteomics and immunoproteomics |
| The Gujarat Cancer & Research Institute (TGCRI), Ahmadabad (www.cancerindia.org) | Cancer proteomics |
| Vellore Institute of Technology (VIT), VIT (www.vit.ac.in) | Separation proteomics |
Figure 1.
A map of the research institutions engaged in basic and clinical pharmacoproteomics research in India.
3. PROTEIN MICROARRAYS: A NEW FRONTIER FOR POST-PROTEOMIC BIOLOGY AND PERSONALIZED MEDICINE
Protein microarrays allow the study of the complex proteome and are necessary to understand the structure, function and dynamics of proteins as well as for better insights on physiology of living systems. The low sample consumption, high-throughput, sensitivity and ease of assays are among the many advantages of the protein microarrays. MacBeath and Schreiber have first demonstrated the proof-of-concept of protein microarrays in 2000 by immobilizing purified proteins on a chemically modified glass slide [27]. Since then, high-throughput protein purification systems have made it possible to purify and print thousands of proteins. However, in response to several technical limitations associated with this technology, such as tedious protein expression and purification, poor stability, shelf life and purity of proteins, cell-free based protein microarray approaches, including the Nucleic Acid Programmable Protein Arrays, are emerging as alternative analytical platforms [28]. These protein microarrays have been used for various applications including biomarker detection, immunogenicity studies, drug action, substrate identification and protein interaction (Figure 2) [28].
Figure 2. Continuum from genomic to proteomic microarray platforms and detection techniques.

(a) The traditional genomic microarrays for gene expression studies print oligos or cDNA on substrates, (b) Protein microarrays are generated by immobilizing purified proteins on glass slide, (c) Nucleic Acid Programmable Protein Arrays, a novel cell-free based protein microarray platform where cDNA clones are immobilized to generate protein microarray by using in vitro transcription and translation system, (d) Antibody microarray is another advanced platform which is generated by immobilizing antibodies, (e) “Label-based detection” is traditional method used in microarray for signal detection by using radio-labeled, fluorescence, chemiluminiscence, (f) “Label-free detection” techniques such as surface Plasmon resonance and nanotube have been successfully integrated with microarrays.
It should be noted that although several research groups in India have experience with the genomic microarrays, protein microarray technologies remain underutilized within the subcontinent. Most proteomics research in India has largely used gel-based two-dimensional electrophoresis to target the protein of interest during sample preparation and mass spectrometry for its respective analysis. The current research efforts at the Indian Institute of Technology Bombay are attempting to address this deficiency and the need to advance label-free protein detection systems [28]. Such initiatives might offer the way forward in this field. Researchers attempting to drive advancement in novel proteomic platforms should be aware of the substantial technical and developmental challenges currently facing translational proteomic research, the tenets of which were recently reviewed in the context of cancer biomarkers [29] and personalized medicine [30] initiatives.
4. PHARMACOPROTEOMICS IN ASIA/OCEANIA
In Asia/Oceania region, several research groups are actively involved in pharmacoproteomics and its sister field, pharmacogenomics. For example, the Korean Pharmacogenomics Research Network has laid a strong research infrastructure led by Kyung-Eop Min at the Seoul National University [31]. This network focuses on pharmacogenomics of drug action, metabolism and membrane transporters, in particular, for drugs against pulmonary and psychiatric illness with a view to develop quantitative predictive algorithms towards personalized pharmacotherapy. After eight years of phase I research (2003–2011) during which a knowledge database and a genomic biobank were established, research is now moving into the second phase (2011–2015) with an emphasis being placed on translation and practical applications of personalized medicine. These efforts are supported by the Korean Ministry of Health and Welfare [32] and importantly, often involve integrated utilization of genomics and proteomics methodologies. For example, Park et al. at the Soonchunhyang University Hospital in Bucheon have investigated bronchial asthma using both proteomics and pharmacogenomics approaches [33]. Pharmacoproteomics was also intensively utilized in Asia/Oceania to identify differentially expressed proteins in response to therapeutic interventions for diabetes mellitus [34]. Using protein microarray and a pharmacoproteomics approach, the inhibitory mechanism of P11 (HSDVHK) on tumor-induced angiogenesis was shown recently [35]. P11 was also identified as a novel anti-angiogenic agent that inhibits bFGF-induced HUVEC proliferation via MEK/ERK inhibition as well as p53-mediated apoptosis in tumorigenesis [35]. In a similar research approach, Ha et al. have developed a novel on-chip assay using protein arrays for quantitative and rapid analysis of blood coagulation factor XIII (FXIII) activity in human plasma [36]. With this new device, authors provided molecular insights into roles of FXIII in the human disease in the era of personalized medicine [36].
In other countries such as Australia, Kavallaris et al. reported evidence for a role of the actin cytoskeleton in intrinsic and acquired in vivo anti-microtubule drug resistance in childhood leukemia using proteomic analysis [37]. In Hong Kong, Chan et al. at the Chinese University of Hong Kong, reported pharmacoproteomics study of cetuximab in nasopharyngeal carcinoma where they identified both clinical responders to therapy and potential new areas of drug development [38]. In China, Liao et al. at University of South China, Hunan, identified the anti-apoptotic effects of probucol with respect to vascular smooth muscle cell physiology [39]. Kondo et al. at the National Cancer Center Research Institute in Japan reported development of a putative biomarker, Secernin-1, for synovial sarcoma using pharmacoproteomics approaches [40]. Lastly, Chowbay et al. at National Cancer Centre in Singapore showed the consequence of different dose of warfarin anti-coagulant treatment using iTRAQ-coupled LC-MS/MS to analyze plasma protein profiles of patients [41]. They found significantly up-regulated level of transthyretin precursor in patients receiving low dose of warfarin, which was absent in those on high dose of warfarin therapy. Although the collective pharmacoproteomics and genomics research within this region is still within its early stages, much like other global regions, it is anticipated that current and future research investments will accelerate the pace of research in the Asia/Oceania [42], as evidenced by the above examples from Korea, China, Singapore, Japan and other countries in the region.
5. FROM PHARMACOPROTEOMICS TO KNOWLEDGE-BASED INNOVATIONS
The concept of “knowledge society” has been in existence for more than two decades [43], reflecting the predominance of scientific knowledge in everyday life. Indeed, knowledge-based innovations and the emerging technologies that enable them (e.g., proteomics) are considered and promoted as key drivers for the prosperity and well-being of nations, particularly by government leaders [11]. Public “upstream engagement” at the design stage of new technologies, or midstream modulation of technology trajectory by integration of attendant anticipated or actual societal impacts are gaining attention in policy responses to uncertainties posed by emerging technologies and innovations [44–46]. Moreover, we are now entering a period of knowledge convergence across the “-omics” sciences (e.g., genomics, metagenomics, proteomics) where social sciences and humanities research (SSH) will play a significant role in the development of new tools, treatments and applications that are socially robust and sustainable [5, 7, 10, 46]. Increasingly, research funders in the domain of large-scale biology research are actively requesting SSH research as an integral part of science and biotechnology R&D. More than a study of “social impacts” of new technologies or addressing issues such as public concerns, SSH research and public engagement can usefully influence the conception, design and direction of science and technology trajectory in personalized medicine [47, 48]. A recent report from Genome British Columbia (BC) Genomics Society and Ethics Advisory Committee (GSEAC) subcommittee on ‘Pathways to Integration’ provides useful ways forward for the creation of socially robust knowledge within large-scale interdisciplinary “-omics” research [10].
The Genome BC paper emphasizes that SSH research has the potential to contribute to research in a variety of ways, including the production of shared cross-disciplinary understandings, which may have implications for enhanced communication within research communities and future uses of the research. Genome BC supported projects have also endeavored to build stakeholder interests into research outcomes such as industry guidelines in the case of mining and bioremediation (Acid Rock Drainage); and co-designed practices for the governance of biobanks (BC BioLibrary Project) [10]. Integrated research practices are still evolving and there are opportunities for SSH researchers to contribute to emerging outcomes in proteomics through developing an understanding of end user, future user and stakeholder needs, which can then be integrated into research outcomes, perhaps most effectively during the process of technology design as well as technology translation. For example, if emerging post-genomics diagnostic technologies (e.g., proteomics or nanotechniques) include user and stakeholder interests in design decisions, then this could lead to socially relevant applications that are “‘subject to multiple accountabilities” [49, 50]. We can imagine how including SSH-produced qualitative data, such as patient narratives in the development of new diagnostic “-omics” tools may help to balance purely technology-based expert assessments with tacit and situated knowledge that arises out of grounded experiences of the end-users of technoscience innovations [46]. Lehoux has recently underscored for the CPPM readership that personalized medicine scientists practice their profession in the post-genomics era in an “open and complex social and political environment, called by sociologists of science “Mode 2”, wherein the context of application, e.g., the milieu likely to adopt, transform and apply scientific findings and its stakeholders play a pivotal role” [48]. Furthermore, Nowotny et al. emphasize that “mode 2 knowledge production” should be based in part on the “refinement of research methods” and SSH researchers can certainly help with these efforts [49]. Research which incorporates a range of social and cultural expertise would surely increase the likelihood to produce socially robust knowledge products such as proteomics diagnostics for personalized medicine [49].
As new data intensive “-omics” biotechnologies and R&D strategies (e.g., pharmacoproteomics) are being developed in LMICs such as India, social and cultural dimensions should be included in assessment, foresight or “anticipatory governance” processes [46, 51, 52]. Again, SSH researchers can play a key role in helping to shape new developments through shedding light on policy cultures and community practices (e.g., traditional clinical diagnostics vs. emerging “-omics” diagnostics in developing countries). Interdisciplinary collaborations provide unique opportunities to include non-scientific expertise, proactively assess future users, and help stakeholders to share risks and benefits.
Finally, insofar as public engagement for novel technologies (e.g., proteomics/pharmacoproteomics) is concerned, the goal of such exercise should not be about pacifying public “resistance” or making the public(s) “accept” an emerging technology. By framing public responses to science and innovation as “resistance” or “acceptance”, the scientific enterprise in the 20th century has been quick to (incorrectly) bring to the fore the “public knowledge deficit thesis”, an idea that has been contested and rejected for a long time in the social studies of science and technology field [46–48]. Instead, beyond a simplistic dichotomy of public acceptance or resistance to emerging technologies, the scientific enterprise ought to reflect “upstream” on ways in which scientific priorities and questions are framed by experts without citizen participation, and how this one-sided practice might lead to greater uncertainties by bracketing out, for example, the end-users of innovations or the political determinant of health [46, 53–55].
CONCLUSIONS AND OUTLOOK
Current research in the field of personalized medicine would benefit from a deeper knowledge of proteomics biotechnologies and the ways in which they are being applied in Asia-Pacific where there is extensive research investments in “-omics” technologies and personalized medicine [42]. The government of India has taken important steps to support proteome research initiatives through the Department of Biotechnology; the Department of Science and Technology; the Ministry of Human Resources and Development, and other funding agencies. Proteomics infrastructure is being set up at various research institutions in India. These funding agencies support collaborative network of basic researchers and clinicians involved in pharmacoproteomics. Funding agencies in India through partnerships, for example, with the Wellcome Trust alliance, are offering several fellowships to young Indian researchers returning from abroad. Promotion of research activities in the Indian Institute of Technology (IIT), opening of the Indian Institute of Science Education and Research (IISER), and the Centre for Cellular and Molecular Platforms, Bangalore, collectively attest to the initiatives to advance state of the art technology and post-genomics data intensive large scale biology research in India. Additionally, the Ministry of Human Resources and Development has taken initiative to promote e-learning in India; a virtual proteomics laboratory based on simulations has been initiated at the Indian Institute of Technology Bombay to facilitate the distance learning and investigation. To provide a forum for the interaction of proteomic researchers in India, Protein Society of India was formed in 2009. An Asia Oceania Human Proteome Organization Proteomic Congress was held in 2010, which highlighted the contributions of Indian researchers in proteomics and pharmacoproteomics. However, these biotechnology developmental efforts would be well served by keeping in mind the needs of both public health and the emergent field of public health genomics [56]. In addition, it is very important not to forget the significant technical and sociological challenges of translating pharmacoproteomics into functional protocols or therapies for personalized medicine and knowledge-based innovations.
Scientists often respond to socio-cultural trends, needs and concerns, such as a desire to focus on a treatment area in response to a specific public health issue. Through integrated research efforts social scientists and humanists can help to guide scientific inquiry, inform research decisions, shape technical outcomes through including qualitative data in technical applications and achieve shared understandings of future uses, users and stakeholders in personalized medicine [10, 46, 48, 57]. The integrated research funding model used by Genome Canada and Genome BC has yielded some practical applications from SSH researchers and the model is still evolving; other funding organizations such as those in India can certainly profit from paying attention to include SSH researchers on interdisciplinary teams. With such appropriately targeted research funding integrated with SSH, the extant investments in post-genomics biology in India might also in the future attract Indian scholars, trained abroad, to return to the country and perhaps even help reverse the “brain drain” and promote “brain gain”.
It is hoped that the progress being made in pharmacoproteomics and large-scale data intensive biology research within India, South Asia and the Asia-Pacific region will be aided by the presented discussion in this editorial analysis, and perhaps might also help inform health and innovation policy and governance of future proteomics-based personalized medicine initiatives in LMICs.
Acknowledgments
The work, findings and concepts presented herein were developed with support from the following research grants to the authors: the Ministry of Human Resources and Development (MHRD) grant 10MHRD005 to SS; the Korean Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea grant A030003 to YKP; a FRSQ Investigator Salary Award for science-in-society research in personalized medicine and -Omics biotechnologies, and the Canadian Institutes of Health Research operating research grant (CIHR) #84620 to VO. This editorial manuscript has been peer reviewed by the CPPM editorial board.
ABBREVIATIONS
- GBC
Gingivo-Buccal Complex
- GBM
Glioblastoma Multiforme
- HGP
Human Genome Project
- HPP
Human Proteome Project
- HUPO
Human Proteome Organization
- IISER
Indian Institute of Science Education and Research
- IIT
Indian Institute of Technology
- LMICs
Low and Middle Income Countries
- NAPPA
Nucleic Acid Programmable Protein Arrays
- SILAC
Stable Isotope Labeling by Amino Acids in Cell Culture
- SSH
Social Sciences and Humanities Research
- iTRAQ
Isobaric Tags for Relative and Absolute Quantitation
Footnotes
CONFLICT OF INTERESTS
None declared/applicable.
References
- 1.Anderson NG, Matheson A, Anderson NL. Back to the future: the human protein index (HPI) and the agenda for post-proteomic biology. Proteomics. 2001;1:3–12. doi: 10.1002/1615-9861(200101)1:1<3::AID-PROT3>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 2.Legrain P, Aebersold R, Archakov A, et al. The human proteome project: Current state and future direction. Mol Cell Proteomics. 2011 Apr 29; doi: 10.1074/mcp.O111.009993. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Paik YK, Kim H, Lee EY, et al. Overview and introduction to clinical proteomics. Methods Mol Biol. 2008;428:1–31. doi: 10.1007/978-1-59745-117-8_1. [DOI] [PubMed] [Google Scholar]
- 4.Kalow W, Ozdemir V, Tang BK, et al. The science of pharmacological variability: an essay. Clin Pharmacol Ther. 1999;66(5):445–7. doi: 10.1016/S0009-9236(99)70006-8. [DOI] [PubMed] [Google Scholar]
- 5.Ozdemir V, Suarez-Kurtz G, Stenne R, et al. Risk assessment and communication tools for genotype associations with multifactorial phenotypes: the concept of “edge effect” and cultivating an ethical bridge between omics innovations and society. OMICS. 2009;13(1):43–61. doi: 10.1089/omi.2009.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armengaud J. Proteogenomics and systems biology: quest for the ultimate missing parts. Expert Rev Proteomics. 2010;7(1):65–77. doi: 10.1586/epr.09.104. [DOI] [PubMed] [Google Scholar]
- 7.Ozdemir V, Armengaud J, Dubé L, et al. Nutriproteomics and proteogenomics: cultivating two novel hybrid fields of personalized medicine with added societal value. [[Accessed June 27, 2011].];Curr Pharmacogenomics Person Med. 2010 8(4):240–44. doi: 10.2174/187569210793368230. Available from: http://www.benthamscience.com/openaccessplus.php?JCode=CPPM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Science Foundation. Office of Cyberinfrastructure. Advisory Committee for Cyberinfrastucture (ACCI) Task Force Reports. 2011. [[Accessed June 27, 2011].]. Available from: http://www.nsf.gov/od/oci/taskforces/
- 9.Kolker E, Higdon R, Welch D, et al. SPIRE: Systematic protein investigative research environment. J Proteomics. 2011 May 13; doi: 10.1016/j.jprot.2011.05.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Ommer R, Wynne B, Downey R, et al. Pathways to Integration. Vancouver: Genome British Columbia GSEAC Subcommittee on Pathways to Integration; 2011. [Accessed on June 27, 2011]. Available from: www.genomebc.ca/index.php/download_file/view/611/910/ [Google Scholar]
- 11.The Hindu, Business Line. Focus on Gene Chips and Proteomics, Says Kalam. Aug 06, 2005. [Accessed on June 27, 2011]. Available from: http://www.hinduonnet.com/thehindu/thscrip/print.pl?file=2005080601180300.htm&date=2005/08/06/&prd=bl&.
- 12.Chumbalkar VC, Subhashini C, Dhople VM, et al. Differential protein expression in human gliomas and molecular insights. Proteomics. 2005;5:1167–77. doi: 10.1002/pmic.200401202. [DOI] [PubMed] [Google Scholar]
- 13.Kumar DM, Thota B, Shinde SV, et al. Proteomic identi cation of Haptoglobin α2 as a glioblastoma serum biomarker: implications in cancer cell migration and tumor growth. J Proteome Res. 2010;9:5557–67. doi: 10.1021/pr1001737. [DOI] [PubMed] [Google Scholar]
- 14.Govekar RB, D’Cruz AK, Pathak KA, et al. Proteomic profiling of cancer of the gingivo-buccal complex: Identification of new differentially expressed markers. Proteomics Clin Appl. 2009;3:1451–62. doi: 10.1002/prca.200900023. [DOI] [PubMed] [Google Scholar]
- 15.Mallikarjuna K, Sundaram CS, Sharma Y, et al. Comparative proteomic analysis of differentially expressed proteins in primary retinoblastoma tumors. Proteomics Clin Appl. 2010;4(4):449–63. doi: 10.1002/prca.200900069. [DOI] [PubMed] [Google Scholar]
- 16.Faridi U, Sisodia BS, Shukla AK, et al. Proteomics indicates modulation of tubulin polymerization by L-menthol inhibiting human epithelial colorectal adenocarcinoma cell proliferation. Proteomics. 2011;11(10):2115–9. doi: 10.1002/pmic.201000691. [DOI] [PubMed] [Google Scholar]
- 17.Pal P, Kanaujiya JK, Lochab S, et al. 2-D gel electrophoresis-based proteomic analysis reveals that ormeloxifen induces G0-G1 growth arrest and ERK-mediated apoptosis in chronic myeloid leukemia cells K562. Proteomics. 2011;11(8):1517–29. doi: 10.1002/pmic.201000720. [DOI] [PubMed] [Google Scholar]
- 18.Kashyap MK, Harsha HC, Renuse S, et al. SILAC-based quantitative proteomic approach to identify potential biomarkers from the esophageal squamous cell carcinoma secretome. Cancer Biol Ther. 2010;10(8):796–810. doi: 10.4161/cbt.10.8.12914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marimuthu A, O’Meally RN, Chaerkady R, et al. A comprehensive map of the human urinary proteome. J Proteome Res. 2011;10(6):2734–43. doi: 10.1021/pr2003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daar AS. Chronic non-communicable diseases as a threat for all: recent global initiatives. Sultan Qaboos Univ Med J. 2010;10(3):306–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Ranjan R, Chugh M, Kumar S, et al. Proteome analysis reveals a large merozoite surface protein-1 associated complex on the Plasmodium falciparum merozoite surface. J Proteome Res. 2011;10(2):680–91. doi: 10.1021/pr100875y. [DOI] [PubMed] [Google Scholar]
- 22.Acharya P, Pallavi R, Chandran S, et al. A glimpse into the clinical proteome of human malaria parasites Plasmodium falciparum and Plasmodium vivax. Proteomics Clin Appl. 2009;3:1314–25. doi: 10.1002/prca.200900090. [DOI] [PubMed] [Google Scholar]
- 23.Gupta SK, Sisodia BS, Sinha S, et al. Proteomic approach for identification and characterization of novel immunostimulatory proteins from soluble antigens of Leishmania donovani promastigotes. Proteomics. 2007;7(5):816–23. doi: 10.1002/pmic.200600725. [DOI] [PubMed] [Google Scholar]
- 24.Gautam P, Shankar J, Madan T, et al. Proteomic and transcriptomic analysis of Aspergillus fumigatus on exposure to amphotericin B. Antimicrob Agents Chemother. 2008;52:4220–7. doi: 10.1128/AAC.01431-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao PV, Reddy AP, Lu X, et al. Proteomic Identi cation of salivary biomarkers of Type-2 Diabetes. J Proteome Res. 2009;8:239–45. doi: 10.1021/pr8003776. [DOI] [PubMed] [Google Scholar]
- 26.Singh OV, Vij N, Mogayzel PJ, Jr, et al. Pharmacoproteomics of 4-phenylbutyrate-treated IB3-1 cystic fibrosis bronchial epithelial cells. J Proteome Res. 2006;5(3):562–71. doi: 10.1021/pr050319o. [DOI] [PubMed] [Google Scholar]
- 27.MacBeath G, Schreiber SL. Printing proteins as microarrays for high-throughput function determination. Science. 2000;289(5485):1760–3. doi: 10.1126/science.289.5485.1760. [DOI] [PubMed] [Google Scholar]
- 28.Chandra H, Reddy PJ, Srivastava S. Protein microarrays and novel detection platforms. Expert Rev Proteomics. 2011;8(1):61–79. doi: 10.1586/epr.10.99. [DOI] [PubMed] [Google Scholar]
- 29.Makawita S, Diamandis EP. The bottleneck in the cancer biomarker pipeline and protein quantification through mass spectrometry-based approaches: current strategies for candidate verification. Clin Chem. 2010;56:212–22. doi: 10.1373/clinchem.2009.127019. [DOI] [PubMed] [Google Scholar]
- 30.Rahbar A, Rivers R, Boja E, et al. Realizing individual medicine: the road to translating proteomics from the laboratory to the clinic. Personal Med. 2011;8:45–57. doi: 10.2217/pme.10.76. [DOI] [PubMed] [Google Scholar]
- 31.Korean Pharmacogenomics Research Network. [[Accessed on June 27, 2011].]. Available from: http://www.pharmacogenomics.or.kr/english/introduction.php.
- 32.The Knowledge Base for Korean Pharmacogenomics Research Network. [[Accessed on June 27, 2011].]. Available from: http://kpgrn.snubi.org/index.html.
- 33.Park CS, Rhim T. Application of proteomics in asthma research. Expert Rev Proteomics. 2011;8:221–30. doi: 10.1586/epr.11.4. [DOI] [PubMed] [Google Scholar]
- 34.Chung HK, Chae JS, Hyun YJ, et al. Influence of adiponectin gene polymorphisms on adiponectin level and insulin resistance index in response to dietary intervention in overweight-obese patients with impaired fasting glucose or newly diagnosed type 2 diabetes. Diabetes Care. 2009;32:552–8. doi: 10.2337/dc08-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bang JY, Kim EY, Kang DK, et al. Pharmaco-proteomic analysis of a novel cell-permeable peptide inhibitor of tumor-induced angiogenesis. Mol Cell Proteomics. 2011 May 10; doi: 10.1074/mcp.M110.005264. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon MH, Kong DH, Jung SH, et al. Rapid determination of blood coagulation factor XIII activity using protein arrays for serodiagnosis of human plasma. Anal Chem. 2011;83:2317–23. doi: 10.1021/ac1032275. [DOI] [PubMed] [Google Scholar]
- 37.Verrills NM, Liem NL, Liaw TY, et al. Proteomic analysis reveals a novel role for the actin cytoskeleton in vincristine resistant childhood leukemia-an in vivo study. Proteomics. 2006;6:1681–94. doi: 10.1002/pmic.200500417. [DOI] [PubMed] [Google Scholar]
- 38.Sung FL, Pang RT, Ma BB, et al. Pharmacoproteomics study of cetuximab in nasopharyngeal carcinoma. J Proteome Res. 2006;5:3260–7. doi: 10.1021/pr050452g. [DOI] [PubMed] [Google Scholar]
- 39.Su B, Yang YB, Tuo QH, et al. Anti-apoptotic effects of probucol are associated with down regulation of Daxx expression in THP-1 macrophage. Cardiovasc Drugs Ther. 2007;21:37–45. doi: 10.1007/s10557-007-6002-x. [DOI] [PubMed] [Google Scholar]
- 40.Suehara Y, Tochigi N, Kubota D, et al. Secernin-1 as a novel prognostic biomarker candidate of synovial sarcoma revealed by proteomics. J Proteomics. 2011;74:829–42. doi: 10.1016/j.jprot.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 41.Saminathan R, Bai J, Sadrolodabaee L, et al. VKORC1 pharmacogenetics and pharmacoproteomics in patients on warfarin anticoagulant therapy: transthyretin precursor as a potential biomarker. PLoS One. 2010;5(12):e15064. doi: 10.1371/journal.pone.0015064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozdemir V, Muljono DH, Pang T, et al. Asia-Pacific Health 2020 and genomics without borders: co-production of knowledge by science and society partnership for global personalized medicine. [[Accessed June 27, 2011].];Curr Pharmacogenomics Person Med. 2011 9(1):1–5. doi: 10.2174/187569211794728841. Available from: http://www.benthamscience.com/cppm/openaccessarticles/cppm9-1/001AF.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Böhme G, Stehr N. The Knowledge Society. Reidel; Dordrecht: 1986. [Google Scholar]
- 44.Fisher E, Mahajan RL, Mitcham C. Midstream modulation of technology: governance from within. Bull Science Technol Soc. 2006;26:485–96. [Google Scholar]
- 45.Guston DH. Innovation policy: not just a jumbo shrimp. Nature. 2008;454(7207):940–1. doi: 10.1038/454940a. [DOI] [PubMed] [Google Scholar]
- 46.Ozdemir V, Knoppers BM. From government to anticipatory governance: responding to challenges set by emerging technologies and innovation. Discussion Paper for the Study on Governance for Health in the 21st Century, Global Health Program, The Graduate Institute of International and Development Studies; Geneva: Switzerland. February 18, 2011. [Google Scholar]
- 47.Wynne B. Public engagement as a means of restoring public trust in science -- hitting the notes, but missing the music? Community Genet. 2006;9(3):211–20. doi: 10.1159/000092659. [DOI] [PubMed] [Google Scholar]
- 48.Lehoux P. Moving beyond our mutual ignorance. Or, how would engaging the public benefit the personalized medicine community? [[Accessed June 27, 2011].];Curr Pharmacogenomics Person Med. 2011 9(2):76–9. Available from: http://www.benthamscience.com/openaccessplus.php?JCode=CPPM. [Google Scholar]
- 49.Nowotny H, Scott P, Gibbons M. Introduction: Mode 2 revisited: The new production of knowledge. Minerva. 2003;41(3):179–94. [Google Scholar]
- 50.Oudshoorn N, Pinch T. How Users Matter: The Co-Construction of Users and Technology (Inside Technology) Boston: MIT Press; 2003. [Google Scholar]
- 51.Barben D, Fisher E, Selin C, et al. Anticipatory governance of nanotechnology: Foresight, engagement and integration. In: Hackett EJ, Amsterdamska O, Lynch M, Wajcman J, editors. The Handbook of Science and Technology Studies. Cambridge, MA: MIT Press; 2008. pp. 979–1000. [Google Scholar]
- 52.Ozdemir V, Faraj SA, Knoppers BM. Steering vaccinomics innovations with anticipatory governance and participatory foresight. OMICS. 2011 doi: 10.1089/omi.2011.0087. (in press) [DOI] [PubMed] [Google Scholar]
- 53.Kickbusch I. Tackling the political determinants of global health. BMJ. 2005;331(7511):246–7. doi: 10.1136/bmj.331.7511.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kickbusch I. The Leavell lecture—the end of public health as we know it: constructing global public health in the 21st century. Public Health. 2004;188(7):463–9. [Google Scholar]
- 55.Kamal SM, Warnich L, Ferguson LR, et al. Forward look: tenth anniversary of the human genome sequence and 21st century postgenomics global health — a close up on Africa and women’s health. [[Accessed June 27, 2011].];Curr Pharmacogenomics Person Med. 2011 9(3) doi: 10.2174/187569211796957511. (in press). Available from: http://www.benthamscience.com/openaccessplus.php?JCode=CPPM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khoury MJ, Muin J. Khoury discusses the future of public health genomics and why it matters for personalized medicine and global health. [[Accessed June 27, 2011].];Curr Pharmacogenomics Person Med. 2009 7(3):158–63. Available from: http://www.benthamscience.com/cppm/openaccessarticles/cppm7-3/003AF.pdf. [Google Scholar]
- 57.Corrigan OP. Personalized medicine in a consumer age. Curr Pharmacogenomics Person Med. 2011;9(3) (in press) [Google Scholar]