Summary
Totipotent cells in early embryos are progenitors of all stem cells and are capable of developing into a whole organism, including extraembryonic tissues such as placenta. Pluripotent cells in the inner cell mass (ICM) are the descendants of totipotent cells and can differentiate into any cell type of a body except extraembryonic tissues. The ability to contribute to chimeric animals upon reintroduction into host embryos is the key feature of murine totipotent and pluripotent cells. Here, we demonstrate that rhesus monkey embryonic stem cells (ESCs) and isolated ICMs fail to incorporate into host embryos and develop into chimeras. However, chimeric offspring were produced following aggregation of totipotent cells of the 4-cell embryos. These results provide insights into the species-specific nature of primate embryos and suggest that a chimera assay using pluripotent cells may not be feasible.
INTRODUCTION
ESCs are the in vitro counterparts of pluripotent cells residing in the ICM of blastocysts (Evans and Kaufman, 1981; Martin, 1981; Thomson et al., 1998; Thomson et al., 1995). While natural pluripotent cells in the developing embryo exist transiently, ESCs can be maintained in vitro indefinitely providing an unlimited source of undifferentiated cells. When reintroduced into blastocysts, mouse ESCs engraft into the ICM and participate, in concert with host embryonic cells, in the development of chimeric fetuses and offspring (Bradley et al., 1984). Furthermore, in ICM-deficient, tetraploid host embryos, injected mouse ESCs can rescue the embryo proper resulting in exclusively ESC-derived offspring (Nagy et al., 1990). This unique feature of ESCs has been greatly exploited in the creation of knock-out mice and studies of mammalian gene function (Capecchi, 1989).
The first chimera studies of Tarkowski (Tarkowski, 1961) and Mintz (Mintz, 1962) independently demonstrated that two or more cleaving mouse embryos when aggregated together could produce a single chimeric mouse of normal size. The organs and tissues of such animals consist of a mixture of genetically divergent cells derived from the parental embryos. A modified technique was developed by Gardner (Gardner, 1968), whereby cells injected into blastocysts were incorporated into the host ICM to form chimeras. A variety of donor cell types support mouse chimera production including ICM (Gardner, 1968), teratocarcinoma cells (Mintz and Illmensee, 1975), ESCs (Bradley et al., 1984), embryonic germ cells (Matsui et al., 1992) as well as pluripotent cells experimentally generated by somatic cell nuclear transfer (SCNT) (Wakayama et al., 2001) or direct reprogramming (iPS cells) (Okita et al., 2007). Chimeric animals have also been produced in several other mammals including rats (Mayer and Fritz, 1974), rabbits (Gardner and Munro, 1974), sheep (Tucker et al., 1974) and cattle (Brem et al., 1984). Moreover, live chimeras have been produced by aggregating preimplantation embryos of different species (Fehilly et al., 1984). The ability of mouse cultured pluripotent cells, including those derived experimentally, to contribute to chimeric tissues of the embryo proper after introduction into preimplantation host embryos has become an ultimate test for pluripotency. However, such a stringent chimera-based pluripotency assay has not been developed for primates, in large part, due to the limited availability of animals and the lack of relevant technological and genotyping expertise.
RESULTS
Potential of monkey ESCs to form chimeras
We initially evaluated the ability of rhesus monkey ESCs to contribute to chimeric fetuses upon injection into in vitro fertilization (IVF)-derived host blastocysts. To aid in the tracking of injected cells, we transduced ESCs with a lentiviral vector carrying GFP and selected pure populations of cells highly expressing the transgene. Approximately 20–30 disaggregated ESCs were injected into the host blastocyst and placed next to the ICM (Figure S1; Movie S1, ESC injection). To eliminate risks that ESC disaggregation may affect cell survival, some blastocysts were injected with mechanically dispersed cell clumps. To exclude the possibility that GFP-expressing ESCs may have compromised developmental potential, we also injected non-transgenic ESCs. We evaluated several previously characterized rhesus ESC lines including IVF-derived ORMES-22 (XX) and -23 (XY) as well as SCNT-derived CRES-2 (Byrne et al., 2007).
A total of 26 ESC-injected blastocysts was immediately transplanted into seven synchronized recipients. The details of this experiment including host embryo stage, ESC type and embryo transfer outcomes are presented in Table S1. Four females became pregnant - one carrying quadruplets and three carrying singletons. In addition, three recipients contained gestational sacs without fetuses. The overall pregnancy and implantation rates were 57% (4/7) per recipient animal and 27% (7/26) per embryos transferred, respectively. All pregnancies were terminated at mid-gestation and seven fetuses were recovered by caesarean section. Multiple tissues and organs from each fetus were analyzed for the presence of ESC progeny by: i) microsatellite parentage analysis of genomic DNA employing 41 short tandem repeats (STR); ii) mitochondrial (mt)DNA parentage analysis using restriction fragment length polymorphism (RFLP); and iii) direct GFP fluorescence analysis followed by GFP-specific PCR. None of these assays showed a contribution of ESCs in analyzed fetuses (Table 1).
Table 1.
Summary of in vivo chimera studies with rhesus monkey ESCs and embryos
| Tested cells | Host embryo stage | Total offspring generated | Developed to separate embryo | Developed to embryo proper chimera |
|---|---|---|---|---|
| ORMES-22 | Blastocyst | 4 | No | No |
| ORMES-23 | Blastocyst | 2 | No | No |
| CRES-2 GFP | Blastocyst | 1 | No | No |
| Whole ICM | Blastocyst | 3 | Yes | Restricteda (hematopoietic) |
| 4-cell embryo | 4-cell embryo | 10 | No | Yes |
| ORMES-22 GFP | 4-cell embryo | 1 | No | No |
Chimerism was restricted to livers, spleens and placentas.
ORMES-22 (XX) and -23 (XY) are IVF-derived rhesus monkey embryonic stem cell lines. CRES-2 (XY) is SCNT-derived ESC line(Byrne et al., 2007)
(See also Tables S1 through S6, Figures S1 through S6, and Movie S1)
Potency determination in monkey ICMs
The failure to generate chimeras may indicate either limited developmental potential of primate ESCs or inability of host blastocysts to incorporate foreign embryonic cells. To address these questions, we tested if non-cultured pluripotent cells residing in the ICM can incorporate into host embryos and form chimeras. Our attempts to enzymatically disaggregate monkey ICMs into single cells prior to injections resulted in poor survival and cell death. Therefore, whole ICMs were immunosurgically isolated from IVF-produced expanded blastocysts and immediately injected into host blastocysts from unrelated monkeys (Figure S1; Movie S1, ICM injection). A total of 44 ICM-injected blastocysts was generated and transferred into 11 recipients (Table S1). Three females were confirmed pregnant based on blood progesterone profiles. However, one recipient carried a single gestational sac without a viable fetus. The second recipient (recipient #10) carried two sacs; one empty while the second contained two fetuses separated by a thin membrane, indicative of a mono-chorionic di-amniotic twin pregnancy (Figure 1A and B). The third recipient (recipient #18) carried a single gestational sac with one fetus. The pregnancy and implantation rates with ICM-injected blastocysts were 18% (2/11) and 7% (3/44, respectively (Table S1). All three fetuses were recovered by caesarean section as described above and analyzed for the contribution of transplanted ICMs.
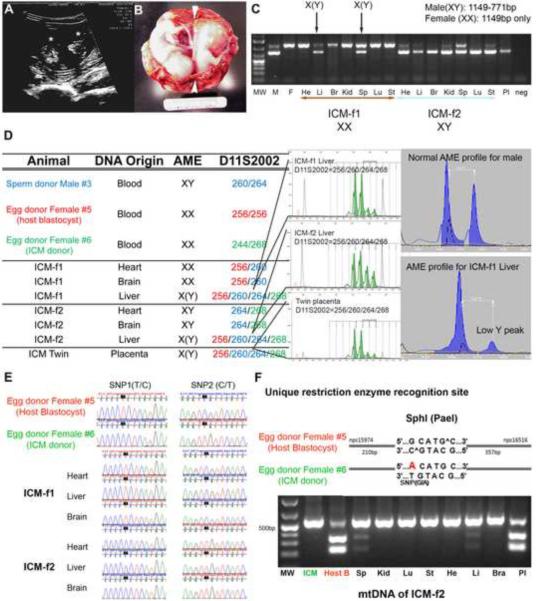
Figure 1.
Mono-chorionic twin fetuses produced by injection of an ICM into a blastocyst
A, Ultrasonography image of a twin pregnancy at 30 days of gestation. Asterisks depict individual fetuses. B, Morphological analysis of fetuses recovered on day 51 of gestation. Note that while two fetuses share single placenta, a thin septum (arrowheads) separates each fetal cavity indicating mono-chorionic but di-amniotic pregnancy. C, PCR amplification of ZFX and ZFY regions produced two DNA fragments (male and female). Detection of 771bp fragment in liver and spleen samples of ICM-f1 indicates presence of male cells in female organs. D, Analysis of D11S2002 and AME microsatellite loci detected the presence of 3 different alleles in livers and a placenta of fetuses. E, Chromatogram of the rhesus mtDNA DHV1 region showing informative SNPs. Fetus ICM-f1 originated from a host blastocyst while ICM-f2 developed from the injected ICM. F, mtDNA RFLP analysis. G allele in mtDNA of the host blastocyst egg donor female #5 is recognized and digested by SphI (PaeI) while an A allele in the injected ICM egg donor female #6 precludes restriction. MtDNA haplotype of egg donor female #5 was detected in the liver and spleen of the ICM-f2 fetus derived from the ICM egg donor female #6.
Abbreviations in Fig. 1C: MW, M, F, He, Li, Br, Kid, Sp, Lu, St, Pl and neg indicate Molecular Weght, Male, Female. Heart, Liver, Brain, Kidney, Spleen, Lung, Stomach, Placenta and negative control, respectively. (See also Table S2 for detailed STR data)
Naturally occurring, mono-chorionic twin pregnancies usually carry monozygotic, genetically-identical fetuses due to spontaneous duplication of the embryo during early stages of development (Cunningham and Williams, 2005). Unexpectedly, our initial morphological examinations of the mono-chorionic twin pregnancy revealed that the fetuses were of different genders (ICM-f1 - female and ICM-f2 - male). Moreover, detailed STR analysis demonstrated that one fetus originated from the host blastocyst, whereas the second fetus was derived from the injected ICM (Figure 1 and Table S2). Interestingly, we detected chimerism in the livers and spleens of both fetuses, but not in other organs and tissues. For example, analysis of the STR locus for D11S2002 in livers indicated the presence of three different alleles representing both the host blastocyst and injected ICM (Figure 1). In addition, the gender-specific STR marker (AME) confirmed the presence of male cells within the liver of the female fetus ICM-f1. To further validate these results, we analyzed gender using PCR-based size differences in the amplicons of the X- and Y-linked zinc finger protein genes (ZFX and ZFY) (Mitalipov et al., 2007) and confirmed chimerism in livers and spleens. We also genotyped fetal mtDNAs based on the G/A single nucleotide polymorphism (SNP) within the rhDHV1 region (Tachibana et al., 2009) and the ability of the SphI (PaeI) enzyme to digest the G but not the A allele. MtDNA analysis confirmed that ICM-f2 originated from transplanted ICM and chimerism in livers and spleens (Figure 1). The placental sample containing a mixture of several extraembryonic membranes (including chorion and amnion) was contributed by both the host embryo and injected ICM.
The fetus associated with the singleton pregnancy (ICM-f3) was male and originated solely from the injected ICM, whereas the placental (trophectoderm) component was female and mainly contributed by the host blastocyst (Figure 2 and Table S2).
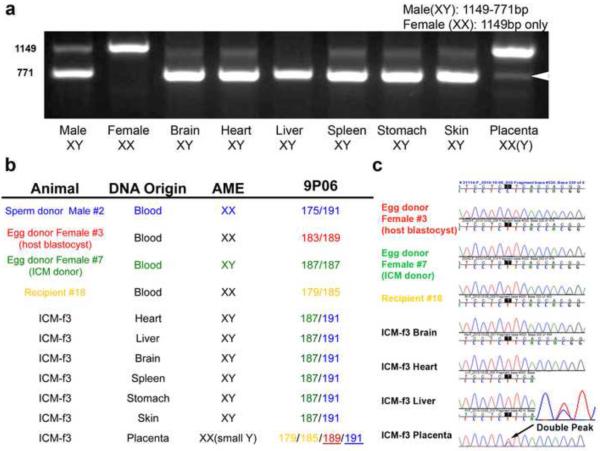
Figure 2.
Parentage analysis of offspring derived from ICM
A. Gender-specific PCR analysis demonstrating normal profiles for rhesus male (2 fragments of 1149bp and 771pb size) and female (one 1149bp fragment) DNA samples. Analysis of tissues and organs from ICM-f3 fetus produced by ICM injection demonstrated that the fetus is male while the placenta is female. In addition, the placenta showed a faint 771bp fragment (arrowhead) indicating presence of male cells at low levels. B. Microsatellite genotyping within the STR locus 9P06 clearly demonstrating that the fetus originated from the injected ICM whereas placenta was mainly from the host blastocyst. AME STR locus showed a limited presence of male cells in the female placenta possibly indicating amniotic contribution from the transplanted ICM. C. Chromatogram of mtDNA DHV1 region demonstrating informative SNPs that can distinguish mitochondrial contribution in tissues. An mtDNA profile of the ICM-f3 fetus matched to the transplanted ICM, while the placental mitochondrial genome was mixture of the host blastocyst and ICM-f3. Limited mtDNA contribution from the fetus was also evident in the placental tissues. (See also Table S2 for detailed STR data)
These results demonstrate that, in contrary to the mouse and some other species, monkey blastocysts do not readily incorporate ESCs or foreign ICMs and form embryo proper chimeras. However, transplanted ICMs were capable of forming separate viable fetuses while sharing the trophectodermal compartment of the host embryo. The chimerism detected exclusively in livers and spleens of twin fetuses could result from the exchange of blood and hematopoietic progenitors through placental perfusions. We also observed contribution of transplanted ICMs to extraembryonic membranes. This is an expected outcome based on the evidence that mouse ICMs contribute to several vital extraembryonic tissues including amnion and extraembryonic mesoderm of the chorion (Nagy et al., 1990).
Production of monkey chimeras by aggregation of 4-cell embryos
We reasoned that the totipotent blastomeres of cleaving embryos should be capable of incorporating foreign blastomeres and forming chimeras. We focused on the 4-cell stage based on the evidence that an isolated single blastomere from this stage embryo can implant and develop into a viable rhesus offspring (Chan et al., 2000). To investigate this, we initially attempted to generate chimeric embryos by replacing two blastomeres in the 4-cell stage embryo with two blastomeres isolated from different developmentally comparable embryo (Figure S1). We generated 29 chimeric 4-cell embryos of which 19 reached the blastocyst stage. Analysis revealed that only 10 of these blastocysts contained total cell counts similar to non-manipulated controls indicative of successful aggregation (Table S3). However, remaining embryos either failed to aggregate and formed two separate blastocysts or developed into a single blastocyst with significantly reduced cell numbers indicating that either transplanted or host blastomeres arrested and failed to contribute to chimeric blastocysts (Figure S2). We also observed blastocysts with two distinct cavities or two ICMs within a single trophectoderm vesicle (Figure S2). Although we did not incorporate markers that would distinguish between donor and host blastomeres, this phenomenon was not seen in control intact embryos, or in prior studies of preimplantation embryogenesis (Wolf et al., 2004). Nevertheless, these observations motivated us to carry out further studies whereby we aggregated together whole embryos in order to increase the yield of chimeric blastocysts and eventually offspring.
We reasoned that aggregating of three or more whole cleaving embryos together would allow better contact between blastomeres and ensure that at least two of these embryos would develop to blastocysts and contribute to chimeric ICMs (Figure S1). We created a total of 29 aggregates using between three to six individual 4-cell stage embryos and cultured to the blastocyst stage (Table S4). Remarkably, all 29 aggregates developed to blastocysts and cell count analysis suggested that 26 blastocysts (90%) consisted of at least twice the normal cell counts indicating successful aggregation (Table S5 and Figure S3). To corroborate these observations we injected GFP-RNA construct into parental oocytes and generated GFP expressing cleaving embryos. GFP signal was always confined to the RNA injected oocytes and daughter blastomeres but was not found in aggregated blastomeres from non-injected embryos (Figure S3). We aggregated GFP tagged embryos with non-injected controls and confirmed successful aggregation of parental embryos into a singe blastocyst. Finally, we selected 14 chimeric blastocysts consisting of high cell counts and transplanted these into five recipient females (Table S1). All five recipients became pregnant including two with singletons, two with twins and one female carrying quadruplet. The pregnancy and implantation rates with chimeric blastocysts were 100% (5/5) and 71% (10/14), respectively. These remarkably high pregnancy outcomes were not seen among other treatments in this study, or in our prior studies (Wolf et al., 2004). On average, pregnancy and implantation rates with non-manipulated rhesus embryos do not exceed 36% and 17%, respectively (Wolf et al., 2004). High pregnancy and implantation results observed with chimeric blastocysts suggest that higher cell numbers in embryos are critical for pregnancy initiation.
Three pregnancies with chimeric embryos were terminated and seven fetuses recovered for genetic analysis. Remarkably, all fetuses were of normal size and had no obvious defects or congenital abnormalities. As expected, STR analysis confirmed that all seven fetuses were indeed chimeras (Table S6). Moreover, chimerism was found in all sampled organs and tissues of all fetuses. Some chimeras displayed up to 5 different alleles within informative individual STR loci indicating that at least 3 separate genotypes (embryos) contributed to chimeric tissues (Figure S4, Table S6). Further, AME marker allows determining the gender of fetuses and three chimeras (EA-f1, 5 and 7) were identified as sex chimeras indicating that contributing parental embryos were of different gender. Since several chimeras were generated by aggregating embryos from two unrelated females, we also used mtDNA genotyping to confirm presence of two different mtDNA haplotypes in offspring (Figures S4 and S5).
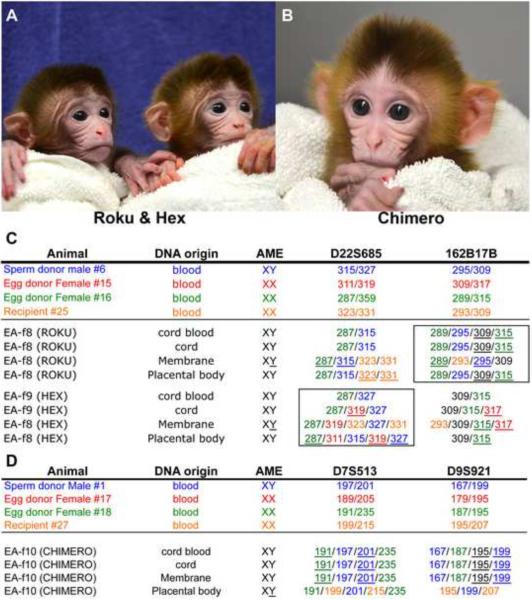
The remaining two recipients (#25 and #27) were allowed to carry pregnancies to term and delivered healthy twin (named Roku and Hex) and singleton (Chimero) infants, respectively (Figure 3). All three infants were phenotypic males with no obvious congenital abnormalities. We genotyped cord blood, cord (representing yolk sac and allantois), fetal membrane (representing amnion, chorion and decidua) and placental (chorio-amniotic placenta) samples and confirmed that all 3 infants were indeed chimeras (Figure 3). To our knowledge, these infants are the world's first primate chimeras. Although all three offspring were phenotypic males we reasoned that these infants could be also sex chimeras due to high probability that some contributing embryos were genetically females (XX). To investigate this, we carried out detailed cytogenetic analyses of blood by G-banding and fluorescence in situ hybridization (FISH). Indeed, results confirmed that blood samples from Roku contained both male and female cells. Molecular cytogenetic studies revealed the presence of two signals for the rhesus monkey X chromosome in approximately 4% of analyzed cells, while remaining cells showed one signal for the X chromosome and one signal for the Y chromosome (Figure S6).
Figure 3.
Chimeric infants generated by whole embryo aggregation
A and B, Live chimeric offspring (Roku, Hex indicating 6 in Japanese and Greek, and Chimero) each produced by aggregating of six individual embryos. The pictures were taken at 7 days after birth. C and D, Genetic analysis of blood and extraembryonic tissues based on microsatellite examination demonstrating presence of more than two alleles for each locus. (See also Tables S4, S6, and Figure S6)
Lineage segregation in primate blastocysts
We next revisited the question why monkey blastocysts are unable to incorporate transplanted ICMs or ESCs and form embryo proper chimeras. Mouse chimeras with ESCs are routinely generated using E3.5 blastocysts as host embryos. However, ability to form chimeras is sharply declined when more advanced stage host blastocysts are used (Ohta et al., 2008). While underlying mechanisms remain unclear, differentiation of host ICMs into epiblast (EPI) and extraembryonic progenitors is believed to restrict homing of injected ESCs into an ICM (Ohta et al., 2008). For example, in peri-implantation mouse blastocysts (E4.5) the ICM differentiates into two restricted lineages, EPI and primitive endoderm (PE) (Chazaud et al., 2006; Morrisey et al., 1998). Developmental studies indicated that while the ICM of E3.5 mouse blastocysts can contribute to all tissues except those of trophectodermal origin, chimeric contributions of EPI or PE cells are restricted to their own lineages (Gardner, 1982, 1984; Gardner and Rossant, 1979). We reasoned that such differentiation and segregation may already be initiated in the ICM of monkey blastocysts, thus restricting their chimeric potential. To investigate this possibility, we immunolabeled whole monkey blastocysts or isolated ICMs with markers for EPI (NANOG) and PE (GATA-6). The results indicated that blastocysts indeed contain a layer of GATA-6 positive cells overlaying NANOG-positive EPI cells within ICMs (Figure S7). Even early monkey blastocysts contained spatially segregated GATA-6 positive cells within ICMs. Thus, it is reasonable to speculate that primate ICMs in preimplantation blastocysts consist of at least two lineage restricted cell types resulting in limited ability to incorporate foreign pluripotent cells.
ESC integration into 4-cell embryos
Based on our results suggesting that monkey cleaving 4-cell embryos are capable of incorporating foreign embryonic cells and form chimeras, we reasoned that injection of ESCs into 4-cell embryos might support generation of embryo proper chimeras. GFP expressing monkey ESCs were injected under the zona pellucida and placed between blastomeres of 4-cell embryos and resulting aggregates cultured to the blastocyst stage. We examined blastocysts for aggregation with ESCs based on GFP expression and transferred six GFP-positive embryos into two recipients (Figure 4 and Table S1). One became pregnant carrying a singleton fetus that was recovered a midgestation for the analysis. However, again, we found no contribution of ESCs in tested tissues and organs.
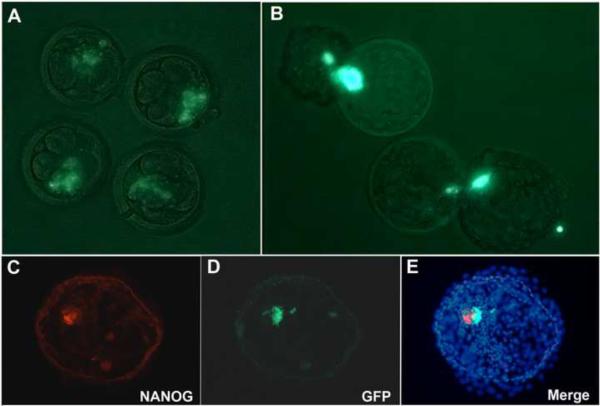
Figure 4.
Detection of ESCs in blastocysts developed from 4-cell embryos injected with GFP positive ESCs
A, GFP expressing ESCs were injected into 4-cell embryos and placed between blastomeres. B, Blastocysts with GFP embedded cells. C–E, Immune staining for NANOG demonstrated that GFP expressing ESCs within an ICM were NANOG negative indicating premature differentiation. Original magnifications: A – E; ×200
Since ESCs are pluripotent and are not developmentally equivalent to host totipotent blastomeres of the 4-cell embryo, we reasoned that injected ESCs may prematurely differentiate prior to blastocyst formation thus precluding their contribution to the ICM. To test this assumption, we injected GFP expressing ESCs into 4-cell embryos and analyzed resulting blastocysts by immunocytochemistry. Based on GFP expression, majority of blastocysts contained embedded ESCs within TE or ICM (Figure 4). In some blastocysts, ESCs detached from host embryos and formed free-floating embryoid bodies. We selected and labeled blastocysts expressing GFP specifically in the ICM area with NANOG and subsequent analysis indicated that GFP expressing ESC cells are NANOG negative while remaining host embryo ICM cells strongly express NANOG. Thus, these results support the notion that while cleaving host embryos can incorporate ESCs, the environment does not support undifferentiated growth of ESCs. During 4–5 days window that is required for injected embryos to reach the blastocyst stage, aggregated ESCs undergo differentiation and lose pluripotency. This phenomenon is likely precludes contribution of ESCs to the fetal tissues and organs.
DISCUSSION
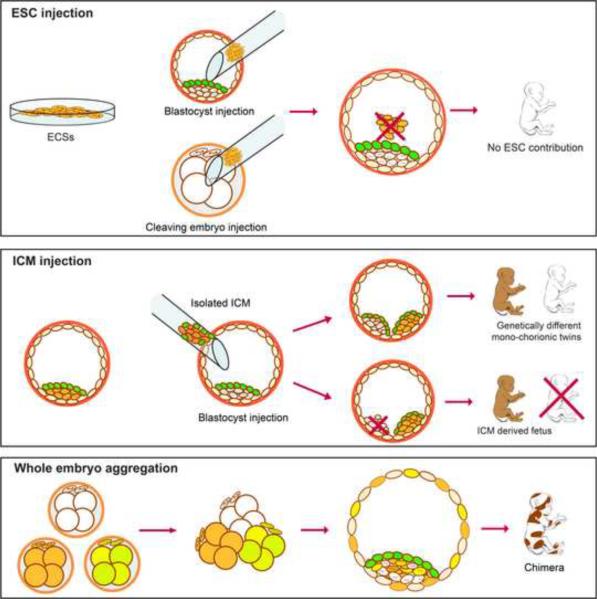
Based on the comprehensive analysis of the developmental potency in vivo, we demonstrate that monkey blastocysts do not readily aggregate with transplanted ICMs or ESCs and form embryo proper chimeras (Figure 5). Our results suggest that cells within monkey ICMs, even in early preimplantation blastocysts, are not homogeneous. We detected two cell types within monkey ICMs where a cluster of NANOG positive EPI cells is covered by GATA-6 positive PE cells. Mouse studies have shown that segregation of ICMs into EPI and PE becomes apparent in mouse peri-implantation stage blastocysts (E4.5) (Cockburn and Rossant). Mouse chimeras are routinely generated using E3.5 host blastocysts, where the ICM is not visibly differentiated into EPI and PE fates yet. However, differentiation and lineage commitment in later stage blastocysts significantly inhibits integration of injected ESCs into EPI and formation of embryo proper chimeras (Ohta et al., 2008). It is possible that, segregation of ICMs seen in monkey blastocysts may have diminished their ability to incorporate foreign cells and development of embryo proper chimeras.
Figure 5.
Summary of chimera studies with monkey embryos and embryonic cells.
Rhesus monkey ESCs as well as isolated ICMs, blastomeres or whole embryos were tested for their ability to incorporate into host embryos and generate chimeric offspring. Established ESCs and freshly isolated ICMs failed to produce chimeras when injected into host blastocysts. However, ICMs developed into separate fetuses with placental support from the host embryo. Aggregating of several 4-cell embryos efficiently produced live chimeric offspring. (See also Figure S7 and Movie S1)
Remarkably, transplanted monkey ICMs developed into viable fetuses with a TE support from a host blastocyst. This demonstrates an extraordinary developmental potential of primate ICMs compared to that of established ESCs.
Mouse studies showed that in addition to the embryo proper, the EPI gives rise to several extraembryonic derivatives including amniotic ectoderm, extraembryonic mesoderm of the amnion, allantois, visceral yolk sac and chorion (Gardner, 1983; Mackay and West, 2005). The PE contributes to the endoderm layer of the visceral yolk sac and the parietal endoderm associated with Reichert's membrane. The TE part underlying the ICM, known as the polar trophectoderm, forms the chorionic ectoderm and placental trophoblast, while the TE part surrounding the blastocyst cavity, known as the mural trophectoderm, contributes to the primary trophoblast giant cells (Mackay and West, 2005). Thus, while the embryo proper develops exclusively from the ICM, both the ICM and TE contribute to the extraembryonic lineages. In this study, we did not incorporate easily traceable markers that would allow examination of what specific extraembryonic cells and layers were contributed by the host embryo or developed from the transplanted ICMs. However, DNA analysis of mixed extraembryonic samples containing presumably chorion and amnion revealed that both host embryo and injected ICMs contributed to these tissues.
Chimerism in the body of ICM twins produced in this study was limited to organs rich in blood, suggesting that placental vascular anastomoses and blood mixture could be involved rather than true chimerism in solid tissues. Blood chimerism has been reported in human twin pregnancies (van Dijk et al., 1996). Interestingly, in marmosets and other callitrichid primates, even fraternal twins exchange blood through chorionic fusions and up to 95% of pregnancies result in the birth of hematopoietic chimeras (Gengozian et al., 1964). As mentioned above, naturally occurring human mono-chorionic twins are predominantly monozygotic, but rare cases of mono-chorionic dizygotic twin pregnancies have been reported in association with clinical IVF procedures (Quintero et al., 2003; Souter et al., 2003). Mono-chorionic pregnancies carry increased risk of abortions or premature birth due to aberrant vascularizations leading to twin-to-twin transfusion syndrome and/or vascular disruptions. In the case of our monkey twin fetuses, we did not find any apparent malformations or abnormalities, and examination of the placenta showed no obvious defects. Our experimental approach for generating offspring from transplanted ICMs may represent an important nonhuman primate model for studying the development of genetically different fetuses in a mono-chorionic environment.
In contrast to ICMs, monkey ESCs did not form completely stem cell-derived fetuses, most likely due to inability of host embryos to support with vital extraembryonic tissues. Comparative studies between mouse ICMs and ESCs suggested that the latter is restricted to the embryo proper and extraembryonic mesoderm lineages while ICMs contribute to embryo proper and a wide range of extraembryonic lineages (Beddington and Robertson, 1989; Tam and Rossant, 2003). Particularly, it is well known that mouse ESCs are not capable of contributing to the PE lineage that gives rise to yolk sac, a critical structure for normal fetal development (Beddington and Robertson, 1989; Rossant, 2007). Chimera studies indicated that this lineage is preserved in isolated mouse ICMs and when transplanted into host embryo, ICMs can contribute to the PE (Tam and Rossant, 2003). Interestingly, while mouse ICMs injected into host 2N blastocysts form chimeras, their ability to develop into separate fetuses has not been reported.
Mouse ESCs can develop into whole stem cell-derived offspring when aggregated with tetraploid (4N) host embryos (Nagy et al., 1990; Nagy et al., 1993). Chimera studies with 4N-2N embryos have shown that tetraploid cells can contribute to functional trophectoderm and PE lineages but not to the EPI (James et al., 1995). Thus, in mouse ESC-4N chimeras, two embryonic cell types complement each other, where ESCs form embryo proper while 4N embryos develop into extraembryonic lineages. It is important to note that tetraploid complementation is considered to be the most stringent but extremely inefficient pluripotency test for mouse ESCs. While the majority of mouse ESC lines do contribute to conventional chimeras, only selected lines are able to produce whole ESC-derived live offspring after aggregation with tetraploid host embryos (Nagy et al., 1990; Nagy et al., 1993).
We also demonstrate here that totipotent, cleaving monkey embryos cannot serve as a host for testing pluripotency of ESCs. Unlike mouse, where 2-8-cell stage embryos engraft ESCs and form chimeras (Wood et al., 1993), cleaving primate embryos do not seem provide a niche for undifferentiated maintenance of ESCs until the host ICM is formed. While we did not test directly in this study, it is likely that ICMs injected into 4-cell embryos would also prematurely differentiate. Based on these results, it is reasonable to speculate that chimera assay is not feasible pluripotency assay using approaches employed here.
The ability of mouse pluripotent cells to generate chimeras or completely ESC-derived offspring incited ethical concerns that human ESCs and iPS cells could be used to clone humans or create chimeras (Lanza, 2007). However, based on our study, these concerns seem to be unattainable or more challenging, at least when using monkey cells and embryos.
On the other hand, although monkey ESCs did not contribute to fetuses in our study, we cannot rule out their broad pluripotency and ability to differentiate to many if not all cell types of the embryo proper. As we previously described, monkey ESCs can form teratomas in vivo or differentiate in vitro into a broad range of cell and tissue types representing all three germ layers (Mitalipov et al., 2006). It will be important to develop additional in vivo assays defining potency of primate ESCs and determining their potential in regenerative medicine. In addition, further studies with other human or monkey experimental pluripotent stem cells may be warranted.
On a related issue, monkey and human ESCs are considered to be more similar to mouse epiblast stem cells (EpiSCs) derived from post-implantation stage embryos than to mouse ESCs (Brons et al., 2007; Rossant, 2008; Tesar et al., 2007). Notably, while mouse EpiSCs can differentiate into teratomas, they display limited capacity to form chimeras. Therefore, failure of monkey ESCs to contribute to chimeras may be due to their epiblast stem cell-like nature. Interestingly, recent studies have shown that mouse EpiSCs can be converted to more potent “naive” ESC state capable of forming chimeras (Guo et al., 2009). It has been speculated that human and monkey ESCs can be also directed towards similar naïve state (Hanna et al., 2010). If such cells become available, our chimera assay would be critical for ultimate testing of their potential.
We demonstrate here, for the first time, that aggregating of 3 or more cleaving monkey embryos results in chimeric offspring with extensive contribution to the embryo proper and extraembryonic lineages. In fetuses, chimerism was present in all tissues and organs including gonads suggesting a broad developmental potential of parental blastomeres. Moreover, since we aggregated up to six individual embryos per chimera, we observed that both female (XX) and male (XY) parental embryos contributed to a chimera. Gender ratio in mouse chimeras produced by injection of male ESCs is skewed towards males due to conversion of some sex (XY-XX) chimeras into phenotypic males (Delhaise et al., 1993). All three live infants in our study were phenotypically males suggesting that presence of XY cells may also converted chimeras to males. Based on the cytogenetic analysis of blood, sex chimerism was confirmed in one infant. It would be critical to investigate further chimerism in various tissues including gonads and reproductive capacity in these animals.
Remarkable in vivo developmental rates were obtained when several 4-cell embryos were aggregated together. It would be interesting to investigate if such “enhanced” host embryos can improve chimera outcomes with ESCs. Another potential use of this chimera assay is to enhance or complement development of cloned monkey embryos. We previously demonstrated that monkey SCNT embryos are capable of developing efficiently into ESCs (Byrne et al., 2007; Sparman et al., 2009). However, their in vivo potential after transfer into recipients remains limited. We have been able to establish several early pregnancies, but none of them progressed to term (Sparman et al., 2010). Aggregation chimeras of SCNT embryos with fertilized counterparts would be extremely useful to determine if developmental failure of SCNT embryos is due to inefficient reprogramming into specific extraembryonic or embryo proper lineages.
Currently, there is little known about human and nonhuman primate embryo development and lineage specification and how closely the mouse development reflects primates. Our study presents a first glimpse at the similarities and differences between mouse and primate preimplantation embryo development and offers an important experimental model to investigate lineage commitment and interactions.
EXPERIMENTAL PROCEDURES
All animal procedures were approved by the Institutional Animal Care and Use Committee at the ONPRC/OHSU.
ESC culture and preparation for injections
ESCs were cultured on a feeder layer consisting of mouse embryonic fibroblasts (mEFs) in DMEM/F12 medium with high glucose but without sodium pyruvate and supplemented with 1% nonessential amino acids, 2 mM l-glutamine, 0.1 mM β-mercaptoethanol and 15% FBS at 37°C, 3% CO2, 5% O2 and 92% N2. Established rhesus monkey ESCs were fully characterized, and tested by in vitro and in vivo differentiation as previously described(Byrne et al., 2007; Mitalipov et al., 2006). To generate GFP expressing cells, ESCs were transduced with replication deficient lentiviral vector carrying a GFP reporter gene downstream of the pSin-EF2-Puromycin sequence (Addgene, Inc). Twenty-four hours after transduction, ESCs were split onto new puromycin-resistant mEFs feeders and cultured in a medium with 2 μg/ml puromycin for 2–3 days. Culture medium was changed daily and ESC colonies were typically split every 5–7 days by manual dissociation and replating collected clumps onto fresh mEFs.
For disaggregation prior to injections, ESCs were treated with non-enzymatic TRYPLE (Invitrogen), dissociated by pipetting into single cells and re-suspended with TH3 medium. Alternatively, ESC colonies were manually picked up and mechanically dispersed to smaller clumps before injections.
Injection of ESCs
Embryos were generated by ICSI as described above. Host embryos at the 4-cell or blastocyst stages were transferred to a 30 μl manipulation droplet of TH3 placed on the center of glass bottom manipulation dish (http://www.wpiinc.com) covered with paraffin oil (Zander IVF). ESCs were placed into a separate 5 μl droplets next to the manipulation drop containing host embryos. A host blastocyst was held with holding pipette with an ICM positioned at 12 o'clock (see Suppl. Movie). Approximately 20–30 disaggregated ESCs or clumps of cells were drawn into an injection pipette. A single laser pulse (www.hamiltonthorne.com) was fired to ablate the zona pellucida and underlying trophectodermal layer and an injection pipette was immediately pierced through the hole. Next, ESCs were expelled and placed close to the host ICM (see Movie S1). Injected blastocysts were immediately transferred into oviducts of synchronized recipient females. For 4-cell stage host embryos, ESCs were injected between blastomeres and embryos were cultured to the blastocyst stage. When GFP expressing ESCs were used, GFP expression was regularly monitored using an epifluorescent microscope. Only blastocysts with visible GFP expressing cells were transferred into recipients.
Injection of ICMs
Intact ICMs were isolated by immunosurgery. In brief, the zona pellucida from each blastocyst was removed by brief (10 sec) treatment with 0.5% protease (pronase, Sigma, P-8811). Blastocysts were then incubated in Anti Monkey Whole Serum (Sigma, M-0403) for 30 min at 37 °C, washed three times with TH3 and transferred into Guinea pig complement (Sigma, S-1639) for 30 min. Blastocysts were gently pipetted with a small bore pipette to disperse lysed trophectodermal cells and isolated ICMs were washed and immediately injected into host blastocysts as described above (See Movie S1).
Blastomere replacement
Four-cell stage (D2) embryos were generated by ICSI from unrelated females and treated with 0.5% pronase to remove zona pellucidae. Embryos were incubated in Ca- and Mg-free medium (Invitrogen) for 15 min and transferred to 30 μl manipulation drop of TH3 medium containing 10 μg/ml cytochalasin B. Embryos were further incubated at 37°C for additional 10–15 min before manipulation. Blastomeres from an 4-cell embryo were split in half using injection pipette (45–50 outer diameter, polished) and each half (2 blastomeres) was transferred inside of two separate empty zonae pellucida. Then, another half set of blastomeres from an unrelated female were added under each zona. Aggregated embryos were cultured to the blastocyst stage and transferred into oviducts of synchronized recipients.
Whole embryo aggregation
Four-cell stage (D2) embryos were treated with 0.5% pronase to remove zona pellucida. Three to six zona-free embryos were aggregated together by mechanically pushing against each other. Aggregates were transferred into 30 μl culture drop containing HECM-9 medium and cultured individually at 37°C in 6% CO2, 5% O2, and 89% N2.
Embryo Transfers
Beginning 8 days after menses during a spontaneous menstrual cycle, blood samples were collected daily from the saphenous vein of rhesus females for estradiol level analysis by radioimmunoassay. The day after the serum estradiol peak was considered the day of ovulation (Day 0). This peak occurred on average on day 11 post-menses, with a range from 8 to 17 days. Two to 6 days after the estradiol peak, embryos were transferred surgically into the oviduct ipsilateral to the ovary bearing the ovulatory stigma a described previously(Wolf et al., 2004).
Fetal recovery and sample collection
Fetuses were recovered by cesarean section at mid-gestation and fetuses were euthanized with trans-umbilical cord injection of pentobarbital. Autopsies were conducted to collect tissues from each organ separately.
Parentage analysis
DNA was extracted from blood or tissues using commercial kits (Gentra). Short tandem repeat (STR) microsatellite parentage analysis was conducted by the Veterinary Genetics Laboratory at University of California, Davis as described previously (Byrne et al., 2007; Tachibana et al., 2009). In brief, six multiplexed PCR reactions were set up for the amplification of 39 markers representing 25 autosomal loci and 14 autosomal, MHC-linked loci. On the basis of the published rhesus macaque linkage map (Rogers et al., 2006), these markers are distributed in 19 chromosomes. Two of the markers included in the panel, MFGT21 and MFGT22 (Domingo-Roura et al., 1997), were developed from Macaca fuscata and do not have chromosome assignment.
For gender determination, X- and Y-linked zinc finger protein genes (ZFX and ZFY) were amplified as previously described (Mitalipov et al., 2007). MtDNA analysis was performed as previously described (Tachibana et al., 2009). In brief, the rhesus macaque mitochondrial D-loop hypervariable region 1 (RhDHV1) sequence was amplified using forward (5'-CCAACACCCAAAGCTGGCATTCTA-3') and reverse (5'-ATGGCCCTGAGGTAAGAACCAGAT-3') primers. PCR analysis was performed using PCR super mix high-fidelity DNA polymerase (Invitrogen) containing 0.5 μM of each primer (final volume 50 μl). Reaction conditions were initial denaturation at 94° C for 5 min; 35 cycles of denaturation at 94° C for 30 s, annealing at 60° C for 30 s, extension at 68° C for 90 s and a final extension at 68° C for 3 min, generating 547 bp of sequence covering the RhDHV1 region. PCR products were sequenced and informative SNPs encompassing Macaca mulatta mtDNA nucleotide positions 15974–16516 (GenBank NC_005943) were identified using Sequencher v. 4.7 software (GeneCodes). For Restriction Fragment Length Polymorphism (RFLP) analysis, PCR products were amplified as described above. Unique restriction digestion sites were identified with Sequencher v. 4.7. Restriction enzymes were from Fermentas. For reactions, quantity of PCR products was adjusted to 500ng and digested by appropriate enzymes. Samples were analyzed using 3% agarose gel.
For PCR-based detection of ESCs progeny carrying GFP, eGFP sequence was amplified using forward (5'-GCACAAGCTGGAGTACAACTACAACAGC-3') and reverse (5'-TCACGAACTCCAGCAGGACCAT-3') primers as previously described(Sasaki et al., 2009). PCR reaction was performed as follows: initial denaturation at 94° C for 5 min; 35 cycles of denaturation at 94° C for 30 s, annealing at 58° C for 30 s, extension at 68° C for 90 s and final extension at 68° C for 3 min.
Supplementary Material
Highlights
-
●
Primate ESCs injected into host embryos did not contribute to chimeras
-
●
Transplanted ICMs did not incorporate into host ICMs, but formed separate fetuses
-
●
Aggregation of 3–6 cleaving embryos formed a single chimeric infant
-
●
Cleaving embryos cannot serve as a host for testing ESC pluripotency
Acknowledgements
The authors would like to acknowledge the Assisted Reproductive Technology & Embryonic Stem Cell Core, Division of Animal Resources, Surgery Team, Endocrine Technology Core, and Molecular & Cellular Biology Core at the Oregon National Primate Research Center for providing expertise and services that contributed to this project. We are grateful to Dr. Warren Sanger from Human Cytogenetic Laboratory, Nebraska Medical Center for proving karyotyping services. We are obliged to Hathaitip Sritanaudomchai, Keith Masterson, Lisa Clepper, Joy Woodward, Maidina Touhetahuntila and Erin Wolff for their technical support, Joel Ito for help with illustrative materials and Dr. Mary Herbert for providing a GFP-RNA vector. We are indebted to Drs. Richard Stouffer and Don Wolf for consulting, helpful discussions and critical reading of the manuscript.
This study was supported by start up funds from Oregon National Primate Research Center and grants from the National Institutes of Health HD057121, HD059946, HD063276, HD047675, HD018185 and RR000163.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial affiliation that may be perceived as biasing this work.
References
- Beddington RS, Robertson EJ. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–737. doi: 10.1242/dev.105.4.733. [DOI] [PubMed] [Google Scholar]
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Brem G, Tenhumberg H, Krausslich H. Chimerism in cattle through microsurgical aggregation of morulae. Theriogenology. 1984;22:609–613. doi: 10.1016/0093-691x(84)90061-x. [DOI] [PubMed] [Google Scholar]
- Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Pedersen DA, Clepper LL, Nelson M, Sanger WG, Gokhale S, Wolf DP, Mitalipov SM. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497–502. doi: 10.1038/nature06357. [DOI] [PubMed] [Google Scholar]
- Capecchi MR. Altering the genome by homologous recombination. Science. 1989;244:1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chan AW, Dominko T, Luetjens CM, Neuber E, Martinovich C, Hewitson L, Simerly CR, Schatten GP. Clonal propagation of primate offspring by embryo splitting. Science. 2000;287:317–319. doi: 10.1126/science.287.5451.317. [DOI] [PubMed] [Google Scholar]
- Chazaud C, Yamanaka Y, Pawson T, Rossant J. Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell. 2006;10:615–624. doi: 10.1016/j.devcel.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Cockburn K, Rossant J. Making the blastocyst: lessons from the mouse. J Clin Invest. 120:995–1003. doi: 10.1172/JCI41229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham FG, Williams JW. Williams obstetrics. 22nd edn McGraw-Hill Professional; New York: 2005. [Google Scholar]
- Delhaise F, Zhao X, Bralion V, Dessy F, Georges M. Quantitative estimation of chimerism in mice using microsatellite markers. Molecular reproduction and development. 1993;34:127–132. doi: 10.1002/mrd.1080340203. [DOI] [PubMed] [Google Scholar]
- Domingo-Roura X, Lopez-Giraldez T, Shinohara M, Takenaka O. Hypervariable microsatellite loci in the Japanese macaque (Macaca fuscata) conserved in related species. Am J Primatol. 1997;43:357–360. doi: 10.1002/(SICI)1098-2345(1997)43:4<357::AID-AJP7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fehilly CB, Willadsen SM, Tucker EM. Interspecific chimaerism between sheep and goat. Nature. 1984;307:634–636. doi: 10.1038/307634a0. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Mouse chimeras obtained by the injection of cells into the blastocyst. Nature. 1968;220:596–597. doi: 10.1038/220596a0. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Investigation of cell lineage and differentiation in the extraembryonic endoderm of the mouse embryo. J Embryol Exp Morphol. 1982;68:175–198. [PubMed] [Google Scholar]
- Gardner RL. Origin and differentiation of extraembryonic tissues in the mouse. International review of experimental pathology. 1983;24:63–133. [PubMed] [Google Scholar]
- Gardner RL. An in situ cell marker for clonal analysis of development of the extraembryonic endoderm in the mouse. J Embryol Exp Morphol. 1984;80:251–288. [PubMed] [Google Scholar]
- Gardner RL, Munro AJ. Successful construction of chimaeric rabbit. Nature. 1974;250:146–147. doi: 10.1038/250146a0. [DOI] [PubMed] [Google Scholar]
- Gardner RL, Rossant J. Investigation of the fate of 4–5 day post-coitum mouse inner cell mass cells by blastocyst injection. J Embryol Exp Morphol. 1979;52:141–152. [PubMed] [Google Scholar]
- Gengozian N, Batson JS, Eide P. Hematologic and Cytogenetic Evidence for Hematopoietic Chimerism in the Marmoset, Tamarinus Nigricollis. Cytogenetics. 1964;10:384–393. doi: 10.1159/000129828. [DOI] [PubMed] [Google Scholar]
- Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RM, Klerkx AH, Keighren M, Flockhart JH, West JD. Restricted distribution of tetraploid cells in mouse tetraploid<==>diploid chimaeras. Dev Biol. 1995;167:213–226. doi: 10.1006/dbio.1995.1018. [DOI] [PubMed] [Google Scholar]
- Lanza R. Stem cell breakthrough: don't forget ethics. Science. 2007;318:1865. doi: 10.1126/science.318.5858.1865a. [DOI] [PubMed] [Google Scholar]
- Mackay GE, West JD. Fate of tetraploid cells in 4n<-->2n chimeric mouse blastocysts. Mech Dev. 2005;122:1266–1281. doi: 10.1016/j.mod.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y, Zsebo K, Hogan BL. Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell. 1992;70:841–847. doi: 10.1016/0092-8674(92)90317-6. [DOI] [PubMed] [Google Scholar]
- Mayer JF, Jr., Fritz HI. The culture of preimplanta rat embryos and the production of allophenic rats. J Reprod Fertil. 1974;39:1–9. doi: 10.1530/jrf.0.0390001. [DOI] [PubMed] [Google Scholar]
- Mintz B. Experimental Study of the Developing Mammalian Egg: Removal of the Zona Pellucida. Science. 1962;138:594–595. doi: 10.1126/science.138.3540.594. [DOI] [PubMed] [Google Scholar]
- Mintz B, Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975;72:3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitalipov S, Kuo HC, Byrne J, Clepper L, Meisner L, Johnson J, Zeier R, Wolf D. Isolation and characterization of novel rhesus monkey embryonic stem cell lines. Stem Cells. 2006;24:2177–2186. doi: 10.1634/stemcells.2006-0125. [DOI] [PubMed] [Google Scholar]
- Mitalipov SM, Zhou Q, Byrne JA, Ji WZ, Norgren RB, Wolf DP. Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Human reproduction (Oxford, England) 2007;22:2232–2242. doi: 10.1093/humrep/dem136. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Tang Z, Sigrist K, Lu MM, Jiang F, Ip HS, Parmacek MS. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Gocza E, Diaz EM, Prideaux VR, Ivanyi E, Markkula M, Rossant J. Embryonic stem cells alone are able to support fetal development in the mouse. Development. 1990;110:815–821. doi: 10.1242/dev.110.3.815. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Sakaide Y, Wakayama T. Generation of mice derived from embryonic stem cells using blastocysts of different developmental ages. Reproduction. 2008;136:581–587. doi: 10.1530/REP-08-0184. [DOI] [PubMed] [Google Scholar]
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- Quintero RA, Mueller OT, Martinez JM, Arroyo J, Gilbert-Barness E, Hilbelink D, Papenhausen P, Sutcliffe M. Twin-twin transfusion syndrome in a dizygotic monochorionic-diamniotic twin pregnancy. J Matern Fetal Neonatal Med. 2003;14:279–281. doi: 10.1080/jmf.14.4.279.281. [DOI] [PubMed] [Google Scholar]
- Rogers J, Garcia R, Shelledy W, Kaplan J, Arya A, Johnson Z, Bergstrom M, Novakowski L, Nair P, Vinson A, et al. An initial genetic linkage map of the rhesus macaque (Macaca mulatta) genome using human microsatellite loci. Genomics. 2006;87:30–38. doi: 10.1016/j.ygeno.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Rossant J. Stem cells and lineage development in the mammalian blastocyst. Reprod Fertil Dev. 2007;19:111–118. doi: 10.1071/rd06125. [DOI] [PubMed] [Google Scholar]
- Rossant J. Stem cells and early lineage development. Cell. 2008;132:527–531. doi: 10.1016/j.cell.2008.01.039. [DOI] [PubMed] [Google Scholar]
- Sasaki E, Suemizu H, Shimada A, Hanazawa K, Oiwa R, Kamioka M, Tomioka I, Sotomaru Y, Hirakawa R, Eto T, et al. Generation of transgenic non-human primates with germline transmission. Nature. 2009;459:523–527. doi: 10.1038/nature08090. [DOI] [PubMed] [Google Scholar]
- Souter VL, Kapur RP, Nyholt DR, Skogerboe K, Myerson D, Ton CC, Opheim KE, Easterling TR, Shields LE, Montgomery GW, et al. A report of dizygous monochorionic twins. N Engl J Med. 2003;349:154–158. doi: 10.1056/NEJMoa030050. [DOI] [PubMed] [Google Scholar]
- Sparman M, Dighe V, Sritanaudomchai H, Ma H, Ramsey C, Pedersen D, Clepper L, Nighot P, Wolf D, Hennebold J, et al. Epigenetic reprogramming by somatic cell nuclear transfer in primates. Stem Cells. 2009;27:1255–1264. doi: 10.1002/stem.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparman ML, Tachibana M, Mitalipov SM. Cloning of non-human primates: the road “less traveled by”. The International journal of developmental biology. 2010;54:1671–1678. doi: 10.1387/ijdb.103196ms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana M, Sparman M, Sritanaudomchai H, Ma H, Clepper L, Woodward J, Li Y, Ramsey C, Kolotushkina O, Mitalipov S. Mitochondrial gene replacement in primate offspring and embryonic stem cells. Nature. 2009;461:367–372. doi: 10.1038/nature08368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam PP, Rossant J. Mouse embryonic chimeras: tools for studying mammalian development. Development. 2003;130:6155–6163. doi: 10.1242/dev.00893. [DOI] [PubMed] [Google Scholar]
- Tarkowski AK. Mouse chimaeras developed from fused eggs. Nature. 1961;190:857–860. doi: 10.1038/190857a0. [DOI] [PubMed] [Google Scholar]
- Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci U S A. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker EM, Moor RM, Rowson LE. Tetraparental sheep chimaeras induced by blastomere transplantation. Changes in blood type with age. Immunology. 1974;26:613–621. [PMC free article] [PubMed] [Google Scholar]
- van Dijk BA, Boomsma DI, de Man AJ. Blood group chimerism in human multiple births is not rare. Am J Med Genet. 1996;61:264–268. doi: 10.1002/(SICI)1096-8628(19960122)61:3<264::AID-AJMG11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Wakayama T, Tabar V, Rodriguez I, Perry AC, Studer L, Mombaerts P. Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science. 2001;292:740–743. doi: 10.1126/science.1059399. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Thormahlen S, Ramsey C, Yeoman RR, Fanton J, Mitalipov S. Use of assisted reproductive technologies in the propagation of rhesus macaque offspring. Biol Reprod. 2004;71:486–493. doi: 10.1095/biolreprod.103.025932. [DOI] [PubMed] [Google Scholar]
- Wood SA, Pascoe WS, Schmidt C, Kemler R, Evans MJ, Allen ND. Simple and efficient production of embryonic stem cell-embryo chimeras by coculture. Proc Natl Acad Sci U S A. 1993;90:4582–4585. doi: 10.1073/pnas.90.10.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.