Abstract
Mast cells (MCs) are tissue resident immune cells that participate in a variety of allergic and other inflammatory conditions. In most tissues, MCs are found in close proximity to nerve endings of primary afferent neurons that signal pain (i.e. nociceptors). Activation of MCs causes the release of a plethora of mediators that can activate these nociceptors and promote pain. Although MCs are ubiquitous, conditions associated with systemic MC activation give rise primarily to two major types of pain, headache and visceral pain. In this study we therefore examined the extent to which systemic MC degranulation induced by intraperitoneal administration of the MC secretagogue compound 48/80 activates pain pathways that originate in different parts of the body and studied whether this action can lead to development of behavioral pain hypersensitivity. Using c-fos expression as a marker of central nervous system neural activation, we found that intraperitoneal administration of 48/80 leads to the activation of dorsal horn neurons at two specific levels of the spinal cord; one responsible for processing cranial pain, at the medullary/C2 level, and one that processes pelvic visceral pain, at the caudal lumbar/rostral sacral level (L6-S2). Using behavioral sensory testing, we found that this nociceptive activation is associated with development of widespread tactile pain hypersensitivity within and outside the body regions corresponding to the activated spinal levels. Our data provide a neural basis for understanding the primacy of headache and visceral pain in conditions that involve systemic MC degranulation. Our data further suggest that MC activation may lead to widespread tactile pain hypersensitivity.
Keywords: Mast cell, pain, dorsal horn, fos, dura, viscera, headache, visceral pain, allodynia
1. Introduction
Mast cells (MCs) are immune cells that play an important role in allergy and anaphylactic response. In recent years, clinical and preclinical evidence has accumulated to support a cardinal role for MCs in a host of other inflammatory and functional painful disorders including inflammatory/irritable bowl disease (Klooker et al., 2010; Wood, 2011), bladder pain syndrome/interstitial cystitis (Theoharides et al., 1995) and migraine headache (Levy, 2009). MCs respond to immunological (e.g. IgE) and non-immunological stimuli (e.g. physical stimuli, neuropeptides, chemokines, stress hormones). Upon their activation by these stimuli, MCs secrete a plethora of proinflammatory algogenic mediators, primarily through the process of degranulation (Rao and Brown, 2008). These secreted factors in turn can activate chemo-sensitive primary afferent nociceptive neurons with receptive fields that terminate in the vicinity of the activated MCs (Ahluwalia et al., 1998; Barbara et al., 2007; Greene et al., 1988; Levy et al., 2007). Theoretically, release of algogenic mediators from activated MCs should promote pain from any tissue where MCs are localized near the peripheral terminals of chemosensitive nociceptive afferents. Nonetheless, in conditions associated with systemic MC degranulation, such as MC activation syndrome and systemic mastocytosis, visceral pain and headache are the prominent pains (Ashina and Ashina, 2005; Hamilton et al., 2011; Horan and Austen, 1991). While other types of pain have been reported, particularly in mastocytosis (Hermine et al., 2008) the higher prevalence of headache and visceral pain related to MC diseases remains poorly understood and may be, at least in part, due to relatively increased neuroimmune interactions between MCs and afferents innervating visceral organs (as the source of visceral pain) and the meninges (as the source of the headache).
Upon inflammation, persistent activation of nociceptors that innervate visceral organs as well as the meninges can lead to long-term changes in the responsiveness of the second-order dorsal horn neurons upon which they terminate, a phenomenon termed central sensitization. Because these central nociceptive neurons also receive convergent input from cutaneous afferents, this central sensitization can be manifested as pain hypersensitivity in tissues or body regions that are somatotopically distinct from those becoming inflamed. For example, persistent activation of colon nociceptive afferents can be manifested as referred tactile cutaneous hypersensitivity in the lumbosacral region (Verne et al., 2001), a process likely to occur as a result of viscerosomatic convergence in the lumbosacral dorsal horn. Similarly, the central sensitization that develops after inflammatory-related activation of meningeal nociceptors leads to cutaneous hypersensitivity in the ophthalmic dermatome of the trigeminal nerve (Burstein et al., 2000), due to the sensitization of dorsal horn neurons that receive overlapping input (meningeal-ophthalmo-somatic convergence).
We have shown previously that meningeal MC activation can lead to persistent excitation of meningeal nociceptive afferents (Levy et al., 2007). In the current study, our first aim was to expand our understanding about the potential nociceptive targets of MC degranulation by examining, using a marker of dorsal horn neural activation (c-fos expression), whether MC degranulation can also lead to activation of pain pathways that originate in other body regions. Because neuroimmune interaction between MCs and nociceptive afferents may lead to central sensitization, our second aim was to determine whether MC degranulation promotes cutaneous tactile pain hypersensitivity. Our results suggest that MC activation (1) leads to activation of pain pathways that originate in trigeminal/upper cervical and lumbosacral-innervated organs, and (2) is associated with development of widespread skin tactile pain hypersensitivity both within and outside these body regions.
2. Materials and Methods
2.1. Animals
All experiments were carried out using adult male Sprague-Dawley rats (250–350g, Taconic). Different cohorts of animals were used for each of the studies described below. Only one pharmacological treatment was applied in each group of animals. Experiments were approved by the Institutional Animal Care and Use Committee of the Beth Israel Deaconess Medical Center, the standing committee on animals of the Harvard Medical School and were conducted following the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain
2.2. Drug administration and MC staining
All drugs were administered while animals were fully awake and lightly restrained. Systemic MC degranulation was evoked using a single i.p. administration of a sub-anaphylactic dose of the MC secretagogue compound 48/80 (2 mg/kg, in sterile saline, i.p., Sigma) (Levy et al., 2007). To examine the effect of the MC stabilizing agent sodium cromoglycate (SCG) on 48/80-evoked MC degranulation, animals were injected first with SCG (10 mg/kg i.p) and 30 min later with 48/80 as above (Levy et al., 2007). In initial experiments, we did not observe any effects upon administration of SCG alone. In vehicle control studies, animals were injected i.p with 0.9% saline. For quantitative histological assessment of MC degranulation, animals were deeply anesthetized 1 hr after 48/80/vehicle injections and perfused through the ascending aorta with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde solution, and the dura, bladder and medial dorsal hairy skin of the hindpaw were removed. To visualize dura and bladder MCs, tissues were stained as a wholemount preparation for 1 min with an acidified toluidine blue solution (TB, pH 2.4) (Levy et al., 2007). For evaluation of skin MC degranulation, skin pieces were cryoprotected in 30% sucrose/PBS solution overnight, then frozen in optimum cutting medium (OCT) and cut on a cryostat at 15–20 μm. The sections were thawed onto slides, air-dried and stained as above with a TB solution. MC degranulation was evaluated in a blinded manner under X200 magnification in 10 random fields. MCs were considered as degranulated if there was an extensive dispersion of more than 15 extruded vesicles localized near the cell, or an extensive loss of granule staining, giving the cell a “ghostly” look.
2.3. C-Fos immunohistochemistry
Two and a half hours after drug treatments animals were deeply anesthetized with sodium pentobarbital and perfused through the ascending aorta with PBS followed by paraformaldehyde solution as above. A cervical craniotomy and laminectomy were performed to expose the brainstem and spinal cord, which were excised as one piece caudal to the obex. The brainstem and spinal cord were then divided into ~1 cm portions, cryoprotected overnight in 30% sucrose/PBS and embedded in (OCT) compound. Transverse 40 μm cryostat sections were collected and processed as free-floating for fos immunohistochemistry. Sections were incubated overnight at 4°C with a polyclonal rabbit antibody to c-fos (Ab-2, 1:20,000, Calbiochem, San Diego, California). For immuno-detection, the ABC method was used followed by DAB and 0.02% Nickel sulfate to enhance staining (Levy et al., 2007). Antibody specificity was tested by omitting the primary antibody. C-fos immunoreactive dorsal horn cell nuclei were visualized using a standard upright microscope and counted in every 5th section under X200 magnification. Cell counts were pooled from the following medullary/spinal levels, Vc-C2, C3-C4, C5-C8, T1-T4, T5-T9, T10-L3, L4-L5, L6-S2, and S3-S5. Given that there was no difference between the left and right sides, data was averaged from both sides. Data is expressed as mean number of labeled cell nuclei counted.
2.4. Behavioral sensory testing
To test whether MC degranulation leads to cutaneous tactile pain hypersensitivity, animals were placed in a non-restraining holding apparatus that consisted of a 7.5×24.5 cm plexiglass tube (Oshinsky and Gomonchareonsiri, 2007). Several small holes (2 mm diameter) were made along the top and sides of the tube to allow for tactile stimulation at the various locations. Animals were tested once at baseline (30–60 minutes prior to drug injections) and then every hour over 4 hours after drug administration. After each of the hourly testings, animals were returned to their home cage. Prior to baseline testing the rats were allowed to acclimatize to the holding apparatus for at least 30 min. In addition, animals were further habituated to the holding apparatus for 10 min before each of the hourly testings. To determine whether animals developed an aversive response to light touch (i.e. dynamic or brush-evoked allodynia), the area tested was stroked using a small soft paintbrush (2 mm tip size). To examine whether animals developed static (punctuate) tactile pain hypersensitivity, the skin was stimulated with different von Frey (VF) filaments (18011 Semmes-Weinstein Anesthesiometer Kit) that exert forces of 1.2, 1.9, 3.6, 5.5, 8.5, 11.75 and 15 grams. In each testing session, the areas tested were (in that order), (1) a pericranial (cephalic) region, which included the midline area just above the eyes and about 1 cm posterior; (2) an area around the base of the tail (i.e. within the lumbosacral region) (Bon et al., 2003); (3) the mid-dorsal part of the left hind-paw. In preliminary studies we did not observe a difference between the responses to stimulation of the right and left hind paws. The development of tactile pain hypersensitivity was evaluated by employing four behavioral elements adapted from the work of Vos et al. (Vos et al., 1994) as follows (see table 1). (1) Detection: the rat turned its head towards the VF filament and the latter was explored (usually by sniffing); (2) Withdrawal: the rat turned its head away or pulled it briskly from the VF filament, had a brisk hind paw withdrawal or moved away from it; (3) Escape/attack: the rat turned its body briskly in the restrainer or attacked (biting and grabbing movements) the VF filament; (4) Face/body grooming: the rat displayed at least 3 uninterrupted series of grooming strokes directed to the stimulated area. Starting with the lowest forces, each VF hair was applied 3 times (intra-trial interval 10 sec) and the behavior that was elicited at least twice was recorded. In each testing session all the VF filaments were tested. For statistical analysis, the score recorded was based on the most aversive behavior noted. The force that elicited a withdrawal response was considered as the pain threshold (Edelmayer et al., 2009). To evaluate pain behavior in addition to changes in threshold, for each rat, at each time point, a cumulative response score was determined by combining the individual scores (0–4, table 1) for each one of the VF filaments tested.
Table 1.
Behavioral response scoring system
| Response category | Observed response elements | ||||
|---|---|---|---|---|---|
| Detection | Withdrawal | Escape/attack | grooming | Score | |
| No response | - | - | - | - | 0 |
| Nonaversive response | 1 | - | - | - | 1 |
| Mild aversive response | - | 1 | - | - | 2 |
| Strong aversive response | - | - | 1 | - | 3 |
| Prolonged aversive behavior | - | - | - | 1 | 4 |
2.5. Statistical analysis
Statistical analyses were conducted using Statview (SAS institute). Data are presented as means ± S.E.M. The level of MC degranulation was analyzed using the Kruskal-Wallis analysis of ranks. Changes in c-fos expression were analyzed using 2-way ANOVA with one factor being the treatment and the other being the brainstem/spinal segment. Post hoc analyses for differences between treatments were conducted using Tukey/Kramer test. Changes in VF thresholds and cumulative pain scores in response to treatments were analyzed initially by using non-parametric Friedman test and applying it to all data points. When this test was significant, post hoc Wilcoxon Signed rank test was applied to determine the increase in pain behavior onset latency followed by Bonferroni correction to account for multiple testing. p < 0.05 was considered significant for all initial tests.
3. Results
3.1. Effect of intraperitoneal administration of 48/80 on dural, skin and bladder MCs
Intraperitoneal administration of 48/80 evoked a prominent MC degranulation in all the tissues tested (all p<0.05, 48/80 vs. Vehicle). The level of MC degranulation was comparable between the dura (58±12%), hind-paw skin (69±6%) and bladder (71±13%) (See, table 2). Prior administration of the MC stabilizer SCG blocked the MC degranulation in all the tissues tested (p < 0.05, SCG+48/80 vs 48/80). The level of MC degranulation following SCG+48/80 treatment was not statistically different than following treatment with vehicle in all the tissues (p > 0.05, SCG+48/80 vs. vehicle)
Table 2.
Effects of intraperitoneal administration of vehicle (saline), 48/80 and 48/80 following sodium cromoglycate (SCG+48/80) on the level of mast cells degranulation within the intracranial dura mater, bladder and hind paw skin.
| Degranulation level (%) | |||
|---|---|---|---|
| Treatment | Dura mater (n=4) | Hind paw skin (n=4) | Bladder (n=5) |
| Vehicle | 16±7 | 12±3 | 18±5 |
| 48/80 | 58±12*# | 69±6*# | 71±13*# |
| SCG+48/80 | 23±6 | 28±11 | 31±9 |
p < 0.05, Kruskal-Wallis test, 48/80 vs. vehicle,
p < 0.05 48/80 vs. SCG+48/80.
3.2 Dorsal horn c-fos expression following intraperitoneal administration of 48/80
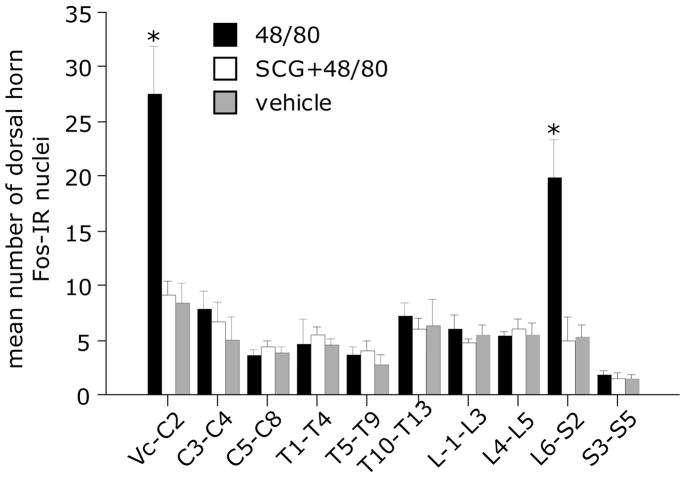
Dorsal horn c-fos expression is considered a marker of neural activation, including nociceptor-induced activation in spinal dorsal horn neurons. However, in spite of the presumed ability of MC degranulation to cause widespread nociceptor activation, intraperitoneal administration of 48/80 produced an increase in fos expression in dorsal horn neurons only at two highly restricted rostrocaudal levels: at the medullary/C2 level, and the caudal lumbar/rostral sacral level (L6-S2). As Fig 1 depicts, the rostrocaudal distribution of fos immunoreactivity in the dorsal horn following 48/80 shows no change throughout most of the length of the spinal cord, except for two large, sharp peaks of labeling at each of these two levels. Overall, two-way ANOVA revealed significant effects of treatment (F 2,9 = 8.11, p < 0.01), spinal cord level (F 2,9 = 22.63, p < 0.001) and interaction (F2,18 = 9.69, p < 0.001). Tukey/Kramer post hoc tests indicated significant increases in the number of dorsal horn fos-labeled nuclei in Vc-C2 and L6-S2 segments following 48/80 compared to vehicle and SCG+48/80 treatments (both p < 0.01, Fig 2).
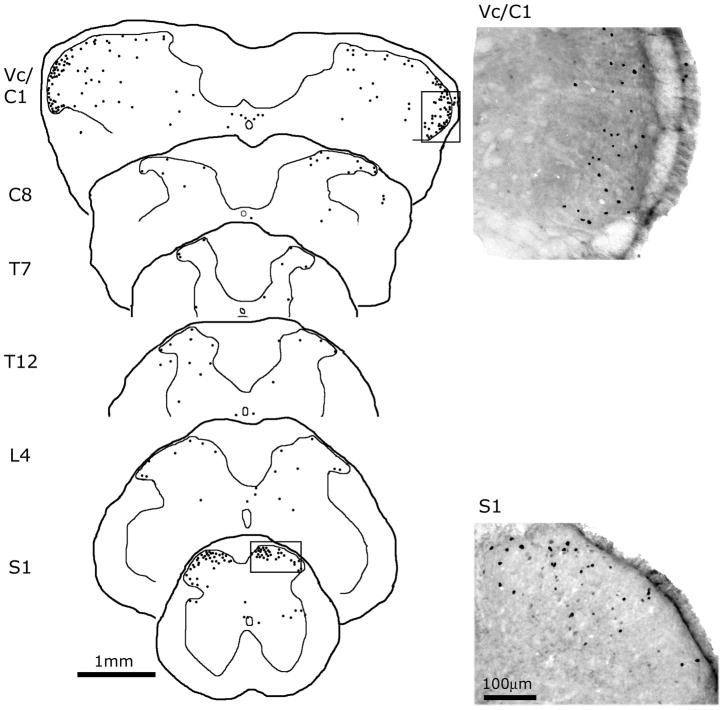
Figure 1.
Medullary and spinal c-fos expression following intraperitoneal administration of 48/80. On the left panel are camera lucida reconstructions of the anatomical locations of fos-IR nuclei at the medullary/cervical (Vc/C1,) and spinal cord levels following MC degranulation with 48/80. Each drawn section plots the location of fos-IR cells from 3 consecutive, alternate, 40 μm sections. Note the high of level fos-IR in the medullary/C1 and sacral dorsal horn levels. On the right are representative high magnification photomicrographs demonstrating c-fos IR in the medullary dorsal horn and S1 spinal segment from animals treated with 48/80 (areas correspond to the boxes on the camera lucida views on the left).
Figure 2.
Histogram comparing the number (mean ± SEM) of dorsal horn fos-IR cells throughout the length of the medullary and spinal dorsal horn from animals receiving 48/80 (n=7), vehicle (saline, n=5) and SCG 30 minutes prior to 48/80 (n=5). * p < 0.05 Tukey/Kramer post-hoc tests 48/80 vs. saline or SCG+48/80.
3.3 Development of cutaneous tactile pain hypersensitivity following intraperitoneal administration of 48/80
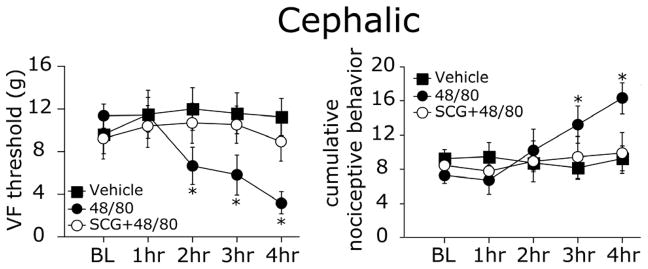
To test for possible emergence of dynamic (brush-evoked) allodynia we evaluated whether animals display a nociceptive/aversive response to lightly brushing the skin after 48/80 treatment. Following MC degranulation, none of the animals displayed aversive responses to light brushing of the skin, in any of the tested regions. To determine whether MC degranulation can elicit static (punctuate) tactile pain hypersensitivity, we tested the responses to tactile stimulation with VF monofilaments. In contrast to the lack of nociceptive responses to brushing, MC degranulation evoked a widespread decrease in VF threshold (punctate allodynia) and increase in overall behavioral pain scores. VF thresholds at the pericranial (cephalic) region showed a significant decrease over time (p < 0.05, Fig 3 left panel). Post hoc analysis revealed a significant decrease already at 2 hours post 48/80 administration (p < 0.01, 48/80 vs. baseline). At that site, cumulative pain responses showed a similar increase over time, which were also delayed and became statistically significant at 2 hours post 48/80 treatment (p < 0.01, 48/80 vs. baseline, Fig 3 right panel). Following vehicle administration there were no changes in pericranial VF thresholds (p = 0.94) or cumulative pain scores (p = 0.29). In animals were SCG was administered prior to 48/80, there was neither a decrease in VF thresholds (p = 0.35) nor an increase in cumulative pain behavior scores (p = 0.11), indicating that SCG abrogated the effect of 48/80 on both indices of pain behavior.
Figure 3.
MC degranulation - evoked cephalic tactile pain hypersensitivity. Withdrawal thresholds (left panel) and cumulative nociceptive pain scores (right panel) to VF stimulation of the scalp skin of 48/80 (n=9), vehicle (n=7) and SCG+48/80 (n=7) treated animals. Note the decrease in VF threshold at 2–4 hrs and increased pain scores at 3–4 hrs after MC degranulation with 48/80. * p < 0.01 Wilcoxon Signed Rank test.
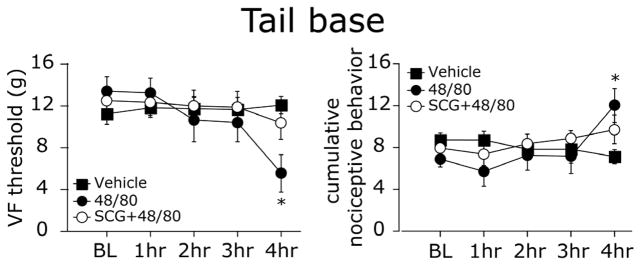
Using VF stimulation of the lumbosacral region, at the tail base, we observed a significant decrease in VF thresholds over time that became statistically significant at 4 hours after 48/80 treatment (p < 0.01 vs. baseline, Fig 4 left panel). Similarly, cumulative pain behavior scores also increased with a delay and were significantly increased at 3 hours (p < 0.01 vs. baseline, Fig 4 right panel). There was neither a decrease in VF thresholds (p = 0.38), nor an increase in cumulative pain behavior (p = 0.224) over time in the animals treated with vehicle. SCG administration prior to 48/80 also inhibited both indices of pain behavior (p = 0.265 for VF thresholds and p = 0.37 for cumulative scores).
Figure 4.
MC degranulation - evoked tactile pain hypersensitivity at the lumbosacral dermatomal region (tail base). Withdrawal thresholds (left panel) and cumulative nociceptive pain scores (right panel) to VF stimulation at the tail base of 48/80 (n=9), vehicle (n=7) and SCG+48/80 (n=7) treated animals. Note the delayed decrease in VF threshold and increase in pain scores 4 hrs after MC degranulation with 48/80. * p < 0.01, Wilcoxon Signed Rank test.
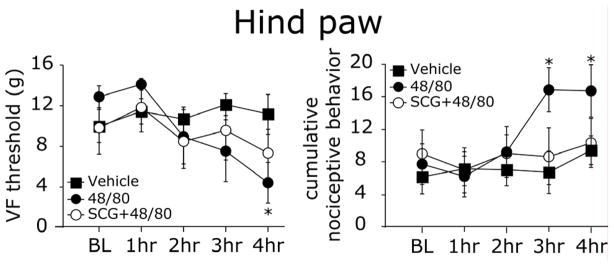
At the hind paw, there was also a delayed development of tactile hypersensitivity characterized by a decrease in VF thresholds and increased cumulative pain scores. Both of these indices became significant at 4 hours (both p < 0.01 vs. baseline, Fig 5). Vehicle treatment neither affected hind paw VF thresholds (p = 0.35), nor cumulative pain scores (p = 0.12). Administration of SCG prior to 48/80 blocked the decrease in VF thresholds (p = 0.12) as well as the increase in cumulative pain scores (p = 0.29).
Figure 5.
MC degranulation - evoked hind paw tactile pain hypersensitivity. Withdrawal thresholds (left panel) and cumulative nociceptive pain scores (right panel) to VF stimulation of the hind paw of 48/80 (n=9), vehicle (n=7) and SCG+48/80 (n=7) treated animals. Note the delayed decrease in VF threshold at 4 hrs and increase in pain scores 3–4 hrs after MC degranulation with 48/80. * p < 0.01, Wilcoxon Signed Rank test.
4. Discussion
A major finding of this study was that intraperitoneal administration of the MC degranulating agent 48/80, at a sub-anaphylactic dose, provides a unique nociceptive stimulus that selectively activates dorsal horn neurons that receive input from trigeminal/upper cervical and lower lumbar/upper sacral-innervated areas. The lack of 48/80-evoked fos labeling in dorsal horn segments that receive their major input from somatic nociceptors such as the lumbar and cervical enlargements strongly suggests that MC degranulation does not activate somatic nociceptors (i.e. originating in the skin, muscle or joints). Although our finding cannot conclusively pinpoint the tissues that are responsible for the dorsal horn fos labeling, we propose that MC degranulation provides a nociceptive stimulus that is selective for a specific subset of tissues, which are represented anatomically by the two very narrow levels along the trigeminal/spinal cord dorsal horn where fos labeling was observed. We suggest that the major tissues that are likely to contribute to this selective fos expression are the intracranial dura and the pelvic visceral organs, in particular the bladder and colon.
We have shown previously that dural MC degranulation as well as various MC constituents including serotonin, histamine, prostanoids and tryptase (via the activation of protease-activated receptor 2) can activate trigeminal nociceptors that innervate the intracranial dura mater (Zhang et al., 2007; Zhang and Levy, 2008). The rostral peak of fos observed after MC degranulation (at Vc/C2) has a similar transverse and rostrocaudal distribution to that produced by dural stimulation (Strassman et al., 1994). Furthermore, this rostral peak of fos labeling is greatly reduced in animals that had received a prior surgical procedure to locally degranulate the dural MC population and thus reduce the dural effects of the subsequent administration of the degranulating agent (Levy et al., 2007). While MC degranulation might potentially lead to the activation of other trigeminal-innervated tissues, the fos distribution we observed was primarily restricted to the areas that receives ophthalmic input (i.e. ventrolateral region), and so cannot be explained by activation of nociceptive afferents that innervate for example intraoral tissues such as the tongue (Strassman et al., 1994; Strassman and Vos, 1993). Activation of ophthalmic innervated areas such as the cornea or nasal mucosa afferents is also unlikely to account for the fos distribution we observed because corneal and mucosal stimulation-evoked fos does not extend so far caudally as that following MC degranulation (Anton et al., 1991; McCulloch and Panneton, 1997; Strassman and Vos, 1993). Taken together, this evidence suggests that the intracranial dura mater is the primary trigeminal tissue that can account for the rostral peak of fos distribution we observed following MC degranulation.
Visceral afferents can also be excited by MC mediators (Barbara et al., 2007) and MC degranulation has been implicated in mediating visceral pain hypersensitivity (Anton et al., 2001; Coelho et al., 1998; Ohashi et al., 2008). Importantly, noxious stimulation of afferents innervating the urinary bladder (Birder and de Groat, 1992) as well as the proximal (Martinez et al., 1998) and distal colon (Lanteri-Minet et al., 1993) leads to increased c-fos expression in the dorsal horn primarily at the lumbosacral level. Our finding of 48/80-evoked increased c-fos expression in this dorsal horn region is therefore consistent with the activation of visceral afferents that innervate the bladder and colon by MC degranulation.
MC-related mediators, including histamine, 5-HT (Davis et al., 1993) and TNF-α (Sorkin et al., 1997) have been shown to excite somatic nociceptors in animal models. It was thus surprising that despite the extensive MC degranulation seen in the skin following 48/80 treatment there was no increase in c-fos expression in dorsal horn segments that receive their major input from somatic nociceptors. What might account for this discrepancy? One possible explanation is that deep trigeminal (e.g. meningeal nociceptors) and visceral afferents (i.e. bladder/colon nociceptors) have higher chemosensitivity than cutaneous nociceptors and that the level of MC-related activation of somatic nociceptors is not sufficient to activate dorsal horn neurons. Of note is a study showing that 48/80 – evoked cutaneous MC degranulation in humans does not lead to overt pain (Drummond, 2004). Such a differential responsiveness of somatic and visceral nociceptive afferents to MC mediators might explain the higher prevalence of headache and visceral pain in conditions that are associated with systemic MC degranulation. Our data thus supports the view that the mechanisms underlying somatic and visceral pain are different (Cervero and Laird, 1999). Our findings are also congruent with the notion that trigeminal nociceptive afferents that innervate deep tissues, such as the meninges, have similar nociceptive properties as visceral nociceptive afferents and that headache and visceral pain share similar nociceptive mechanisms. Taken together, while MC activation may play an important role in mediating headache and visceral pain, their contribution to somatic pain may be less relevant as evidenced by both clinical work as well as the current study.
Activation of primary afferent nociceptors that innervate deep tissues, such as visceral organs and the meninges, can lead to the development of referred cutaneous hypersensitivity (Bon et al., 1996; Burstein et al., 1998). The development of skin hypersensitivity in response to visceral and intracranial pathologies has been attributed to the activation and ensuing central sensitization of second-order dorsal horn neurons that receive convergent input from afferents that innervate deep and somatic tissues. In agreement with the neuroimmune interaction between dural MCs and meningeal nociceptors and the resultant activation of trigeminal dorsal horn neurons to which meningeal nociceptors project (Levy et al., 2007), we observed in this study development of cephalic tactile hypersensitivity. This behavioral response developed with a delay that resembles the time course of the trigeminal central sensitization after noxious stimulation of meningeal nociceptors (Burstein et al., 1998) and the emergence of cephalic pain hypersensitivity in humans during a migraine attack (Burstein et al., 2000). The magnitude of decrease in threshold following intraperitoneal administration of 48/80 was larger than previously described following local administration of inflammatory mediators to the cranial meninges (Edelmayer et al., 2009), which could be explained by the much more widespread action throughout the dura mater of the systemically administered agent.
Similar to the effect of dural MC degranulation on trigeminal pain processing, we propose that activation of lumbosacral dorsal horn neurons by local neuroimmune excitatory interaction between pelvic visceral nociceptors and the adjacent MCs was responsible for the cutaneous tactile pain hypersensitivity we observed at the tail base; an area innervated by afferents that also terminate on dorsal horn neurons at the lumbosacral level. It is noteworthy that delayed pain hypersensitivity at the tail base was also seen in a model of bladder inflammation (Bon et al., 1996). Whether activation of bladder and/or other visceral afferents by local MC degranulation contributed to the development of cutaneous tactile pain hypersensitivity over the lumbosacral area will require further testing.
In the current study we also observed delayed development of tactile pain hypersensitivity at the hind paw. This pain behavior developed despite the lack of dorsal horn neuronal activation in the lumbar region, the area that receives major nociceptive input from the hind paw. Given the lack of lumbar dorsal horn neural activation and, therefore, the likelihood that central sensitization did not develop in these dorsal horn neurons, what might be the mechanism that mediated the hind paw tactile hypersensitivity? One possibility is a delayed sensitization (increased responsiveness) of mechanosensitive skin nociceptors by MC mediators. We have shown recently that a number of MC mediators, in addition to exciting meningeal nociceptors, can also promote an increase in their mechanosensitivity (Zhang et al., 2007; Zhang et al., 2010; Zhang and Levy, 2008). However such immune mediator-evoked nociceptor sensitization, as seen in our previous studies as well as in other studies that specifically tested hind paw skin nociceptors (Martin et al., 1988), develops much more rapidly (30–60 min) than the tactile hypersensitivity we noted after 48/80 treatment (3–4 hrs). In addition, behavioral pain hypersensitivity that arises after sensitization of skin nociceptors is also relatively fast (within 30–60 min) (Taiwo and Levine, 1992). Taken together, it is likely that other mechanisms played a role in mediating the hind paw hypersensitivity.
Activation of meningeal nociceptors can lead to delayed cutaneous tactile pain hypersensitivity in the hind paws (Edelmayer et al., 2009). The development of such extra-cephalic pain hypersensitivity has been attributed to sensitization of 3rd order thalamic nociceptive neurons that receive convergent input from the cranial meninges and extra-cephalic skin including the hind paw (Burstein et al., 2010). An additional explanation has been provided by Edelmayer et al. (Edelmayer et al., 2009) who suggested the involvement of yet another supraspinal mechanism, activation of pain-facilitating processes in the rostral ventromedial medulla (RVM). The mechanisms that mediate the development of extra-segmental pain hypersensitivity in response to meningeal nociception are unlikely to be unique given that bladder (Jaggar et al., 1999) as well as colon inflammation (Cameron et al., 2008; Zhou et al., 2008), both of which activate lumbosacral dorsal horn neurons, also give rise to a delayed hind paw pain hypersensitivity. Finally, the alternative hypothesis may also be considered that the extended cutaneous pain hypersensitivity we observed is mediated, at least in part, through the action of MC degranulation on the CNS, including breaching of the blood brain barrier (Esposito et al., 2001) and the activation of resident CNS immune cells, such as microglia.
In summary, our data suggests that MC degranulation is a unique nociceptive stimulus that likely excites selectively deep tissue nociceptors that originate in the intracranial meninges and visceral organs and that this nociceptive activation leads to development of tactile pain hypersensitivity that is mediated by spinal and supraspinal nociceptive processes and potentially through MC degranulation - evoked CNS neuroinflammation. Activation of intracranial and visceral afferents following MC degranulation may explain why conditions associated with MC degranulation give rise primarily to headache and visceral pain.
Highlights.
The study provides a neural basis for understanding the primacy of headache and visceral pain in conditions that involve systemic MC degranulation.
Acknowledgments
Supported by NIH grants NS046502, NS061116 to D.L. and NS032534 to A.M.S. and the National Headache Foundation. We would like to thank Dr. Moshe Jakubowski for providing help with the statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahluwalia A, Giuliani S, Scotland R, Maggi CA. Ovalbumin-induced neurogenic inflammation in the bladder of sensitized rats. British journal of pharmacology. 1998;124:190–196. doi: 10.1038/sj.bjp.0701793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton F, Herdegen T, Peppel P, Leah JD. c-FOS-like immunoreactivity in rat brainstem neurons following noxious chemical stimulation of the nasal mucosa. Neuroscience. 1991;41:629–641. doi: 10.1016/0306-4522(91)90355-r. [DOI] [PubMed] [Google Scholar]
- Anton PM, Theodorou V, Fioramonti J, Bueno L. Chronic low-level administration of diquat increases the nociceptive response to gastric distension in rats: role of mast cells and tachykinin receptor activation. Pain. 2001;92:219–227. doi: 10.1016/s0304-3959(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Ashina S, Ashina M. Headache in systemic mastocytosis: a case report with pathophysiological considerations. Cephalalgia. 2005;25:314–316. doi: 10.1111/j.1468-2982.2004.00852.x. [DOI] [PubMed] [Google Scholar]
- Barbara G, Wang B, Stanghellini V, de Giorgio R, Cremon C, Di Nardo G, Trevisani M, Campi B, Geppetti P, Tonini M, Bunnett NW, Grundy D, Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Birder LA, de Groat WC. Increased c-fos expression in spinal neurons after irritation of the lower urinary tract in the rat. J Neurosci. 1992;12:4878–4889. doi: 10.1523/JNEUROSCI.12-12-04878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bon K, Lanteri-Minet M, de Pommery J, Michiels JF, Menetrey D. Cyclophosphamide cystitis as a model of visceral pain in rats. A survey of hindbrain structures involved in visceroception and nociception using the expression of c-Fos and Krox-24 proteins. Experimental brain research. Experimentelle Hirnforschung. 1996;108:404–416. doi: 10.1007/BF00227263. [DOI] [PubMed] [Google Scholar]
- Bon K, Lichtensteiger CA, Wilson SG, Mogil JS. Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: species and strain differences. J Urol. 2003;170:1008–1012. doi: 10.1097/01.ju.0000079766.49550.94. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, Becerra L, Borsook D. Thalamic sensitization transforms localized pain into widespread allodynia. Annals of neurology. 2010;68:81–91. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. Journal of neurophysiology. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Annals of neurology. 2000;47:614–624. [PubMed] [Google Scholar]
- Cameron DM, Brennan TJ, Gebhart GF. Hind paw incision in the rat produces long-lasting colon hypersensitivity. J Pain. 2008;9:246–253. doi: 10.1016/j.jpain.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F, Laird JM. Visceral pain. Lancet. 1999;353:2145–2148. doi: 10.1016/S0140-6736(99)01306-9. [DOI] [PubMed] [Google Scholar]
- Coelho AM, Fioramonti J, Bueno L. Mast cell degranulation induces delayed rectal allodynia in rats: role of histamine and 5-HT. Dig Dis Sci. 1998;43:727–737. doi: 10.1023/a:1018853728251. [DOI] [PubMed] [Google Scholar]
- Davis KD, Meyer RA, Campbell JN. Chemosensitivity and sensitization of nociceptive afferents that innervate the hairy skin of monkey. Journal of neurophysiology. 1993;69:1071–1081. doi: 10.1152/jn.1993.69.4.1071. [DOI] [PubMed] [Google Scholar]
- Drummond PD. The effect of cutaneous mast cell degranulation on sensitivity to heat. Inflamm Res. 2004;53:309–315. doi: 10.1007/s00011-004-1263-3. [DOI] [PubMed] [Google Scholar]
- Edelmayer RM, Vanderah TW, Majuta L, Zhang ET, Fioravanti B, De Felice M, Chichorro JG, Ossipov MH, King T, Lai J, Kori SH, Nelsen AC, Cannon KE, Heinricher MM, Porreca F. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Annals of neurology. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito P, Gheorghe D, Kandere K, Pang X, Connolly R, Jacobson S, Theoharides TC. Acute stress increases permeability of the blood-brain-barrier through activation of brain mast cells. Brain research. 2001;888:117–127. doi: 10.1016/s0006-8993(00)03026-2. [DOI] [PubMed] [Google Scholar]
- Greene R, Fowler J, MacGlashan D, Jr, Weinreich D. IgE-challenged human lung mast cells excite vagal sensory neurons in vitro. J Appl Physiol. 1988;64:2249–2253. doi: 10.1152/jappl.1988.64.5.2249. [DOI] [PubMed] [Google Scholar]
- Hamilton MJ, Hornick JL, Akin C, Castells MC, Greenberger NJ. Mast cell activation syndrome: A newly recognized disorder with systemic clinical manifestations. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.04.037. [DOI] [PubMed] [Google Scholar]
- Hermine O, Lortholary O, Leventhal PS, Catteau A, Soppelsa F, Baude C, Cohen-Akenine A, Palmerini F, Hanssens K, Yang Y, Sobol H, Fraytag S, Ghez D, Suarez F, Barete S, Casassus P, Sans B, Arock M, Kinet JP, Dubreuil P, Moussy A. Case-control cohort study of patients’ perceptions of disability in mastocytosis. PloS one. 2008;3:e2266. doi: 10.1371/journal.pone.0002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan RF, Austen KF. Systemic mastocytosis: retrospective review of a decade’s clinical experience at the Brigham and Women’s Hospital. J Invest Dermatol. 1991;96:5S–13S. discussion 13S–14S, 60S–65S. [PubMed] [Google Scholar]
- Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83:442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- Klooker TK, Braak B, Koopman KE, Welting O, Wouters MM, van der Heide S, Schemann M, Bischoff SC, van den Wijngaard RM, Boeckxstaens GE. The mast cell stabiliser ketotifen decreases visceral hypersensitivity and improves intestinal symptoms in patients with irritable bowel syndrome. Gut. 2010;59:1213–1221. doi: 10.1136/gut.2010.213108. [DOI] [PubMed] [Google Scholar]
- Lanteri-Minet M, Isnardon P, de Pommery J, Menetrey D. Spinal and hindbrain structures involved in visceroception and visceronociception as revealed by the expression of Fos, Jun and Krox-24 proteins. Neuroscience. 1993;55:737–753. doi: 10.1016/0306-4522(93)90439-m. [DOI] [PubMed] [Google Scholar]
- Levy D. Migraine pain, meningeal inflammation, and mast cells. Current pain and headache reports. 2009;13:237–240. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin HA, Basbaum AI, Goetzl EJ, Levine JD. Leukotriene B4 decreases the mechanical and thermal thresholds of C- fiber nociceptors in the hairy skin of the rat. Journal of neurophysiology. 1988;60:438–445. doi: 10.1152/jn.1988.60.2.438. [DOI] [PubMed] [Google Scholar]
- Martinez V, Wang L, Mayer E, Tache Y. Proximal colon distention increases Fos expression in the lumbosacral spinal cord and activates sacral parasympathetic NADPHd-positive neurons in rats. The Journal of comparative neurology. 1998;390:311–321. [PubMed] [Google Scholar]
- McCulloch PF, Panneton WM. Fos immunohistochemical determination of brainstem neuronal activation in the muskrat after nasal stimulation. Neuroscience. 1997;78:913–925. doi: 10.1016/s0306-4522(96)00633-1. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Sato Y, Kawai M, Kurebayashi Y. Abolishment of TNBS-induced visceral hypersensitivity in mast cell deficient rats. Life sciences. 2008;82:419–423. doi: 10.1016/j.lfs.2007.11.027. [DOI] [PubMed] [Google Scholar]
- Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao KN, Brown MA. Mast cells: multifaceted immune cells with diverse roles in health and disease. Annals of the New York Academy of Sciences. 2008;1143:83–104. doi: 10.1196/annals.1443.023. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255–262. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- Strassman AM, Mineta Y, Vos BP. Distribution of fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J Neurosci. 1994;14:3725–3735. doi: 10.1523/JNEUROSCI.14-06-03725.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman AM, Vos BP. Somatotopic and laminar organization of fos-like immunoreactivity in the medullary and upper cervical dorsal horn induced by noxious facial stimulation in the rat. The Journal of comparative neurology. 1993;331:495–516. doi: 10.1002/cne.903310406. [DOI] [PubMed] [Google Scholar]
- Taiwo YO, Levine JD. Serotonin is a directly-acting hyperalgesic agent in the rat. Neuroscience. 1992;48:485–490. doi: 10.1016/0306-4522(92)90508-y. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Sant GR, el-Mansoury M, Letourneau R, Ucci AA, Jr, Meares EM., Jr Activation of bladder mast cells in interstitial cystitis: a light and electron microscopic study. J Urol. 1995;153:629–636. doi: 10.1097/00005392-199503000-00021. [DOI] [PubMed] [Google Scholar]
- Verne GN, Robinson ME, Price DD. Hypersensitivity to visceral and cutaneous pain in the irritable bowel syndrome. Pain. 2001;93:7–14. doi: 10.1016/S0304-3959(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JD. Visceral Pain: Spinal Afferents, Enteric Mast Cells, Enteric Nervous System and Stress. Curr Pharm Des. 2011 doi: 10.2174/138161211796196918. [DOI] [PubMed] [Google Scholar]
- Zhang X, Strassman AM, Burstein R, Levy D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322:806–812. doi: 10.1124/jpet.107.123745. [DOI] [PubMed] [Google Scholar]
- Zhang XC, Kainz V, Burstein R, Levy D. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain. 2010;152:140–149. doi: 10.1016/j.pain.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XC, Levy D. Modulation of meningeal nociceptors mechanosensitivity by peripheral proteinase-activated receptor-2: the role of mast cells. Cephalalgia. 2008;28:276–284. doi: 10.1111/j.1468-2982.2007.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Price DD, Caudle RM, Verne GN. Visceral and somatic hypersensitivity in a subset of rats following TNBS-induced colitis. Pain. 2008;134:9–15. doi: 10.1016/j.pain.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]