Abstract
Adiponectin is a cardioprotective adipokine derived predominantly from visceral fat. We recently demonstrated that exogenous adiponectin induces vascular smooth muscle cell (VSMC) differentiation via repression of mTORC1 and FoxO4. Here we report for the first time that VSMC express and secrete adiponectin, which acts in an autocrine and paracrine manner to regulate VSMC contractile phenotype. Adiponectin was found to be expressed in human coronary artery and mouse aortic VSMC. Importantly, siRNA knock-down of endogenous adiponectin in VSMC significantly reduced the expression of VSMC contractile proteins. Contractile protein deficiency was also observed in primary VSMC isolated from Adiponectin-/- mice. This deficiency could be rescued by culturing Adiponectin-/- VSMC in conditioned media from wild type (WT) VSMC. Moreover, the paracrine effect of VSMC-derived adiponectin was confirmed as adiponectin neutralizing antibody blocked the rescue. Overexpressed adiponectin also exerted paracrine effects on neighboring untransfected VSMC, which was also blocked by adiponectin neutralizing antibody. Interestingly, adiponectin expression was inducible by the PPARγ agonist rosiglitazone. Our data support an important role for VSMC-derived adiponectin in maintaining VSMC contractile phenotype, contributing to critical cardioprotective functions in the vascular wall.
Keywords: Adiponectin, vascular smooth muscle, contractile proteins, contractile phenotype, autocrine, paracrine
1. Introduction
While most adipokines are expressed in direct proportion to visceral fat mass and exert deleterious effects on the cardiovascular system [1], adiponectin, in stark contrast, is a cardioprotective adipokine whose expression inversely correlates with fat mass [2]. It is now well-established that reduced plasma adiponectin concentrations are associated with coronary artery diseases in humans [3-5]. Adiponectin has anti-inflammatory[6], anti-diabetic[7], anti-atherogenic[ 8], anti-hypertensive[9], and insulin-sensitizing properties[10-12] [13]. Emerging data suggest that adiponectin may be a key molecule that links obesity and cardiovascular disease, as adiponectin-knockout mice exhibit diet-induced insulin resistance [14], increased neointimal formation and thrombus formation post-injury [15, 16], higher blood pressure [17] and increased myocardial infarct (MI) size [18]. Furthermore, hypoadiponectinemia in humans is a significant predictor of endothelial dysfunction [19], hypertension, coronary artery disease, acute coronary syndrome, myocardial infarction and ischemic cardiovascular disease, independent of BMI, insulin resistance index and dyslipidemia [13].
Adiponectin is the most abundant transcript in adipocytes [20] [21]. This transcript encodes a 244 amino acid (~30 kDa) monomer [21], but adipocytes also secrete higher order oligomeric forms that are stable in serum. Plasma adiponectin exists in predominantly trimeric, hexameric and high molecular weight (HMW) complexes containing 12-18 monomers [22], and circulates at levels ranging from 0.5 to 30 μg/mL [23], which is about 1000-fold higher than the concentrations of most other hormones. Two adiponectin receptors, AdipoR1 and AdipoR2 have been identified [24]. AdipoR1 is believed to be the most important form mediating the effects of adiponectin in skeletal muscle, while AdipoR2 may be the predominant receptor in liver [23]. In addition, T-cadherin is reported to be another adiponectin receptor expressed in vascular endothelial cells, smooth muscle cells [25], and cardiac myocytes [26].
VSMC in vivo do not terminally differentiate, but retain remarkable plasticity. VSMC are subject to reversible phenotypic modulations in response to local environmental cues [27-29] which contributes to pathological conditions such as intimal hyperplasia, atherosclerosis and hypertension. Differentiated VSMC exhibit a quiescent and contractile phenotype, while activated VSMC switch to a synthetic and proliferative phenotype that is essential for formation of a restenotic lesion. However, these two processes are separable and not mutually exclusive. Differentiated VSMC are characterized by spindle-shaped morphology, increased contractile protein expression such as smooth muscle myosin heavy chain (SM-MHC) and SM α-actin, increased calcium binding protein expression including calponin and h-caldesmon, and decreased protein synthesis, especially extracellular matrix synthesis [30]. We recently reported that HMW or trimeric adiponectin induces VSMC differentiation via AMPK activation, and mTORC1 and FoxO4 inhibition [31]. Previously, adiponectin was reported to inhibit VSMC migration and proliferation by direct binding to platelet-derived growth factor-BB (PDGF-BB) and inhibiting growth factor-stimulated ERK signal [2]. In addition, adiponectin is the major adipokine in periadventitial fat which protects against neointimal formation after angioplasty [32], and low adiponectin levels in patients after coronary stenting predict late in-stent restenosis [33, 34].
It was originally thought that adiponectin is exclusively expressed within white adipose tissue [35]. However, several recent publications suggested that adiponectin is also expressed in cardiomyocytes, skeletal muscle, bone-forming cells, placenta, liver, and pituitary cells [36-42]. Whether vascular smooth muscle expresses and secretes functional adiponectin is not known. Given the emerging new paradigm that smooth muscle plays many important metabolic roles by synthesizing and secreting interleukins, chemokines and peptide growth factors in addition to its contractile function [43], we hypothesized that VSMC may also secrete adiponectin. Here, we demonstrate that VSMC express and secrete functional adiponectin, which acts in an autocrine and paracrine manner to regulate vascular smooth muscle phenotype.
2. Materials and Methods
2.1 Materials
Rosiglitazone was purchased from Enzo Life Sciences Inc. (Farmingdale, NY), Brefeldin A was from MP Biomedicals (Solon, OH).
2.2 Cell culture
Transgelin-Cre driver mice (Tg(Tagln-cre)/1Her/J, Jackson Laboratory #004746) were bred with mT/mG mice (B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J, Jackson Laboratory #007676) to generate homozygous mice hereafter referred to as “SM-GFP”. 6-8 week old, female C57Bl6, Adiponectin-/- or SM-GFP mice were used for aortic smooth muscle cell isolation using a standard enzymatic digestion technique. Briefly, aortas were isolated and incubated for 10 min at 37°C in HBSS containing 315 U/mL collagenase type 2 (Invitrogen) and 1.25 U/mL elastase (Sigma), penicillin-streptomycin and amphotericin B. The adventitia was then dissected away and the aorta incubated for 1h at 37°C in enzymatic solution. The cell suspension was centrifuged and re-suspended in DMEM with 20% fetal bovine serum, L-glutamine, penicillin-streptomycin and amphotericin B. VSMC at passage 3-6 were grown in 0.1% gelatin-coated culture slides chambers (BD Falcon) and used for experiments in serum-free media for the duration of drug treatments. Where indicated, conditioned media collected from WT VSMC after 24h culture was used to treat Adiponectin-/- VSMC. In these experiments, all the controls were grown in DMEM with 20% fetal bovine serum, L-glutamine, penicillin-streptomycin and amphotericin B to mimic the same conditions as WT conditioned media.
Human coronary artery VSMC (hCASMC) were purchased from Cascade Biologics (Portland, OR) and cultured in M199 media with 10% fetal bovine serum (FBS) as previously described [27-29].
2.3 Transfection of siRNA and plasmids
Transient transfection of small interfering RNA (siRNA) or plasmid were performed in hCASMC via Nucleofector (Lonza Walkersville Inc, MD) as previously published [44]. For gene knockdown, 1 to 1.5 million cells were cultured in 2.5% FBS and transfected with 0.5 to 2.5 μg siRNA for 24 hours. Human adiponectin (SMARTpool) and nonsilencing siRNA (siCONTROL) were purchased from Dharmacon (Lafayette, CO).
Human adiponectin plasmid was provided courtesy of Dr. Kenneth Walsh. For gene overexpression, 1 million hCASMC were transfected with 0.5 to 2 μg plasmid and cultured in 10% FBS for 24 hours, then cultured in 2.5% FBS for another 24 hours before harvesting.
2.4 RT-PCR
Total RNA from hCASMC was isolated using the Qiagen RNeasy kit with DNase I as described [27, 29]. RNA (500 ng) was reverse transcribed using ImProm-II™ Reverse Transcriptase system (Promega) and oligo dT primer. Primer sets (Supplemental Material) were utilized to specifically amplify human SM-MHC (Myh11), calponin (Cnn), GATA6 (GATA6), myocardin (Myocd), adiponectin (AdipoQ) and pyruvate dehydrogenase (Pdhb). PCR was performed using 20 pmol each primer, 0.04 mM dNTPs, Red taq (Sigma), and TaqStart antibody (Clontech) for 32 cycles: 94°C for 30 s, 51~60°C for 45 s, and 72°C for 1 min. PCR products were resolved on 1 to 2% agarose gels with GelStar (Lonza, Switzerland) and quantitated using a Typhoon Scanner (Molecular Dynamics).
2.5 Real time RT-PCR
RNA was isolated from mouse or human VSMC as in 2.4. Aortas were isolated from wild-type mice and subjected to enzyme digestion to remove adventitia as in 2.2, soleus muscle was also isolated from wild-type mice, and RNA was isolated from both digested aortas and soleus muscle using Qiagen miRNeasy Mini Kit. Two-step RT-PCR was carried out using the iScript cDNA synthesis kit and QPCR EvaGreen Mix with 500 ng of total cDNA in a real-time PCR detection system (all Bio-Rad Laboratories). Commercially available primer sequences for adiponectin (Qiagen) and selected contractile or smooth muscle-specific genes (Integrated DNA Technologies) were used. Data were analyzed using the ΔΔCt method with the Pdhb gene serving as reference.
2.6 Western blotting
hCASMC,were lysed in lysis buffer (Cell Signalling) and a protease phosphatase inhibitor cocktail (Roche). Equal amounts of protein (20-30 μg) per lane were separated by 7.5% or 10% SDS polyacrylamide gel electrophoresis (SDS-PAGE), or 40-60 μg protein per lane was separated by 4-20% gradient gel in non-denaturing and non-reducing conditions, transferred to nitrocellulose membrane, and immunoblotted overnight using primary antibodies against human adiponectin (BioVision, Mountain View, California), h-caldesmon, calponin, SM α-actin (Sigma, St. Louis, MO), SM2-MHC (Seikagaku America, Cape Cod, MA), β-tubulin (Santa Cruz Biotechnology, CA), phospho-Thr 172 AMPK, AMPK-α, phospho-Thr56 eEF2, phospho-Ser 79 ACC, phospho-Thr 389 p70S6K1, and phospho-Ser 473 Akt (Cell Signaling, Boston, MA), HRP-conjugated secondary antibody and detected as above. Protein expression was quantitated and normalized to β-tubulin using ImageJ.
2.7 Confocal microscopy
After treatments, mouse VSMC were fixed with 4% paraformaldehyde. Slides were then washed with Tris-Tween buffer, permeabilized for 10 min in 0.1% triton-X, blocked for 1h (5% BSA) and incubated overnight (4°C) with the following primary antibodies: adiponectin (0.2 mg/mL) (R&D Systems, Minneapolis, MN), calnexin (1/50), NogoB (1/4000) (Imgenex, San Diego, CA), caldesmon (1/250) (Abcam), adiponectin neutralizing antibody (2 mg/mL) (R&D Systems). The next day, slides were incubated with Alexa Fluor®594 Phalloidin (1/250) for F-actin, and Alexa Fluor®488 chicken anti-goat or anti-rabbit or Alexa Fluor®594 chicken anti-rabbit secondary antibodies (Invitrogen), washed and mounted in DAPI anti-fading agent (VectaShield) for nuclei visualization and analyzed using an Ultra View VoX High Speed Confocal microscope (Perkin & Elmer, Waltham, MA).
hCASMC were transfected with plasmids and cultured on glass coverslips. Cells were fixed in 1:1 methanol/acetone, blocked with 3% BSA in TBS-T, and incubated with primary antibodies to adiponectin, MHC and calponin in TBS-T with 1% BSA at 4°C overnight. Cells were then incubated with AlexaFluor568-conjugated secondary antibodies (Molecular Probes Inc., Eugene, OR) in TBS-T with 1% BSA for one hour, and DAPI staining was performed before mounting slides. The slides were analyzed as above.
2.8 TCA Precipitation
In order to detect secreted oligomeric adiponectin, media was harvested, any cells removed by centrifugation, and the media was precipitated using 0.15% Sodium Deoxycholate and 10% tricholoroacetic acid (TCA) and centrifuged at high speed, then pellet was washed using ice cold acetone and centrifuged again at high speed, air dried and subjected to non-reducing western blotting analysis.
2.9 Statistics
All statistical analyses (Student’s t-tests) were carried out using the STATA or GraphPad PRISM programs, with significance at a minimum of p<0.05.
3. Results
3.1 Adiponectin is expressed in VSMC and co-localizes with the endoplasmic reticulum
We recently discovered that adiponectin plays an important role in regulating VSMC phenotype [44]. In light of recent evidence that VSMC are highly active metabolic cells that secrete many regulatory hormones and bioactive lipids [43], we aimed to determine whether VSMC synthesize adiponectin. We detected adiponectin cDNA by RT-PCR in the mouse aorta (isolated medial layer) and in skeletal muscle (Fig. 1A), and in cultured human coronary artery VSMC (hCASMC) (data not shown). Western blot analysis of hCASMC lysate or TCA-precipitated media revealed that these cells express and secrete adiponectin oligomers, including dimer, trimer, hexamer, and the most potent [44] HMW form (Fig. 1B). hCAMSC transfected with adiponectin plasmid also express and secrete these oligomers (Fig. 1B, 1C). Immunostaining of VSMC isolated from the aortas of WT and Adiponectin-/- mice confirmed expression of endogenous adiponectin in WT VSMC which was notably localized to the endoplasmic reticulum (ER) after brefeldin A treatment (to inhibit transport of proteins from the ER) and by co-localization with the ER marker calnexin (Fig. 2A middle row). In the absence of brefeldin A, the native ER architecture is visible and adiponectin colocalization was also detected with NogoB, a resident ER protein [45] (Fig 2A bottom panels).
Figure 1. Adiponectin is expressed in and secreted from VSMC.
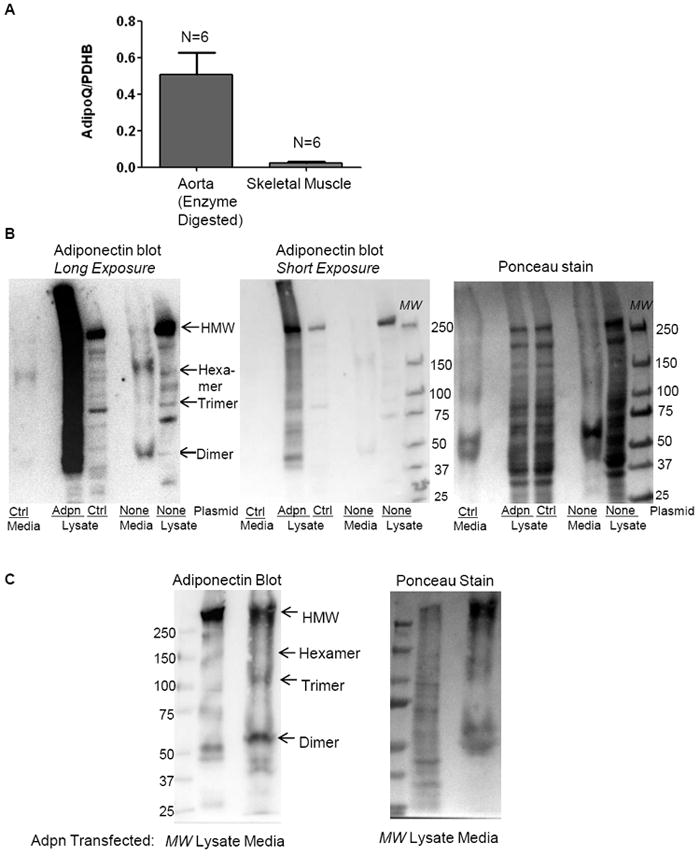
(A) Adiponectin gene expression is detected in wild-type mouse aorta and soleus muscle. Mouse aortas were subjected to brief enzymatic digestion to facilitate complete adventitia removal prior to RNA isolation. (B-C) Adiponectin oligomers are detected in both non-transfected (B) and adiponectin plasmid-transfected hCASMC (B-C) lysates and media. Lysates and serum-free cell culture media (TCA precipitated) from non-transfected hCASMC (B), as well as from hCASMC transfected with pcDNA3 (Ctrl) (B) or adiponectin plasmid (B-C) for 48h were subjected to non-denaturing and non-reducing western blotting analysis for adiponectin oligomers expression. In (B), long and short ECL exposures, as well as Ponceau staining (loading control) of the same blot are shown. 60 μg protein per lane was loaded for the non-transfected hCASMC lysates, and 40 ug protein per lane was loaded for the pcDNA3 (Ctrl) (B) or adiponectin plasmid (B-C) transfected lysates. Media from two 10 cm dishes of non-transfected hCASMC, as well as from two 6 cm dishes of pcDNA3 (Ctrl) (B) or adiponectin plasmid (B-C) transfected hCASMC were collected and subjected to TCA precipitation.
Figure 2. Adiponectin is synthesized in VSMC.
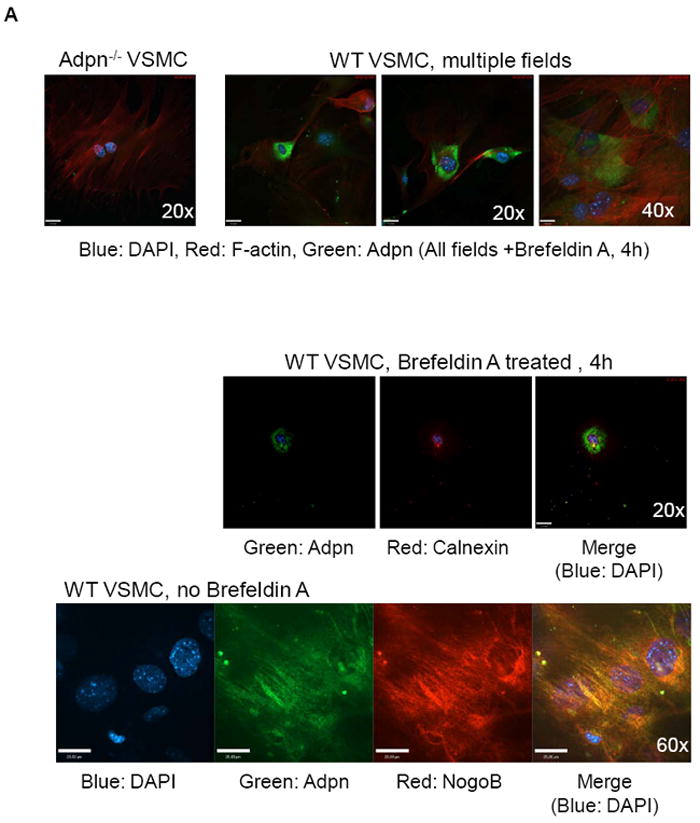
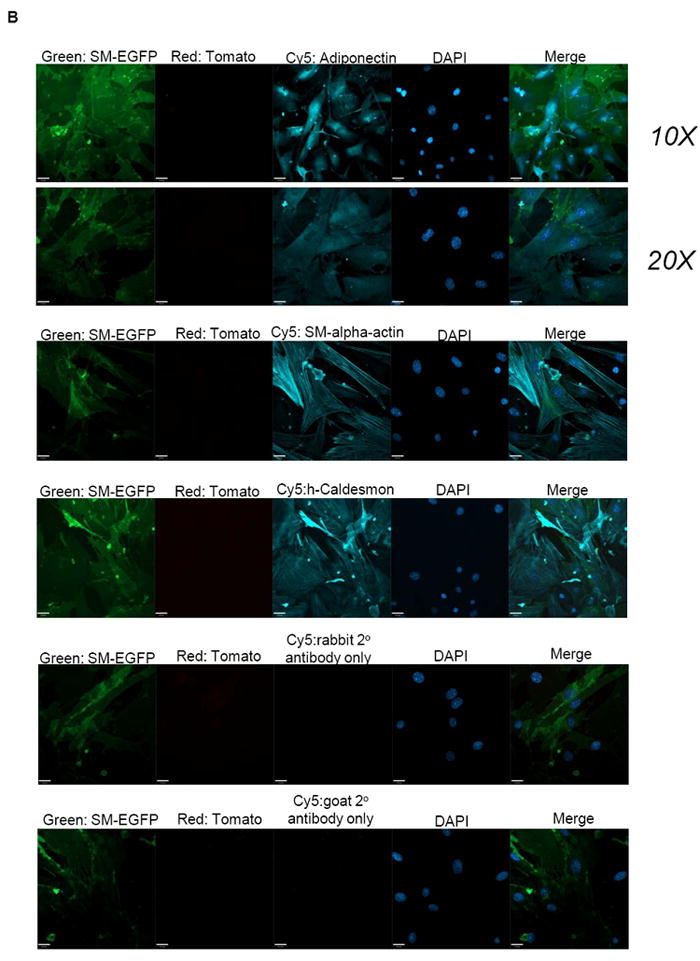
(A) Upper panel: VSMC from WT and adiponectin knock-out mice were treated with 100 ng/mL brefeldin A for 4 hours to arrest transport from ER to golgi to facilitiate visualization of ER proteins. Confocal microscopy was performed to show adiponectin (green), F-actin (red) and nuclei (DAPI, blue) staining, and multiple WT fields are shown. Middle panel: WT mouse VSMC treated with 100 ng/mL brefeldin A for 4 hours were stained for adiponectin (green), Calnexin (an ER marker, red), and DAPI (blue). Bottom panel: WT mouse VSMC (without brefeldin A treatment) were stained for adiponectin (green), NogoB (an ER protein, red), and DAPI (blue). The merged image (co-localization) is at right. (B) VSMC isolated from SM-GFP mice were stained for adiponectin, SM-alpha-actin, h-Caldesmon (blue, Cy5 secondary antibody), or fluorescent secondary antibodies only, and subjected to confocal microscopy analysis. Transgelin-lineage VSMC are green, non-SMC are red (tomato). Nuclei are stained with DAPI. No tomato red staining was detected.
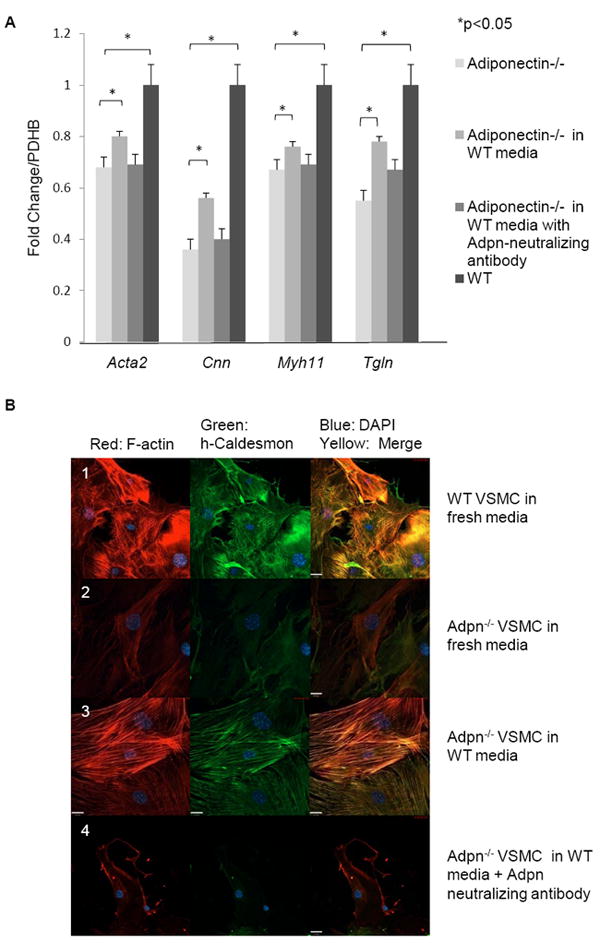
Because adipocytes secrete copious amounts of adiponectin [21], it remained possible that contaminating adventitial cells may be a source of adiponectin detected in our VSMC cultures. To address this possibility, we cultured aortic VSMC from SM-GFP mice (see methods). In this strain, smooth muscle cells are marked by membrane-targeted EGFP. Activation of the transgelin promoter in SMC drives Cre recombinase, triggering a recombination event which leads to expression of the floxed EGFP, while non-smooth muscle cells remain marked by a red fluorescent (membrane-targeted tdTomato) signal. This lineage tracing approach allowed us to definitively demonstrate that adiponectin is expressed in the ER of vascular smooth muscle (EGFP positive) cells (Fig. 2B, 3A). All the EGFP positive cells expressed adiponectin and also stained positive for SM-α-actin and h-caldesmon (Fig 2B).
Figure 3. Rosiglitazone upregulates adiponectin expression in VSMC.
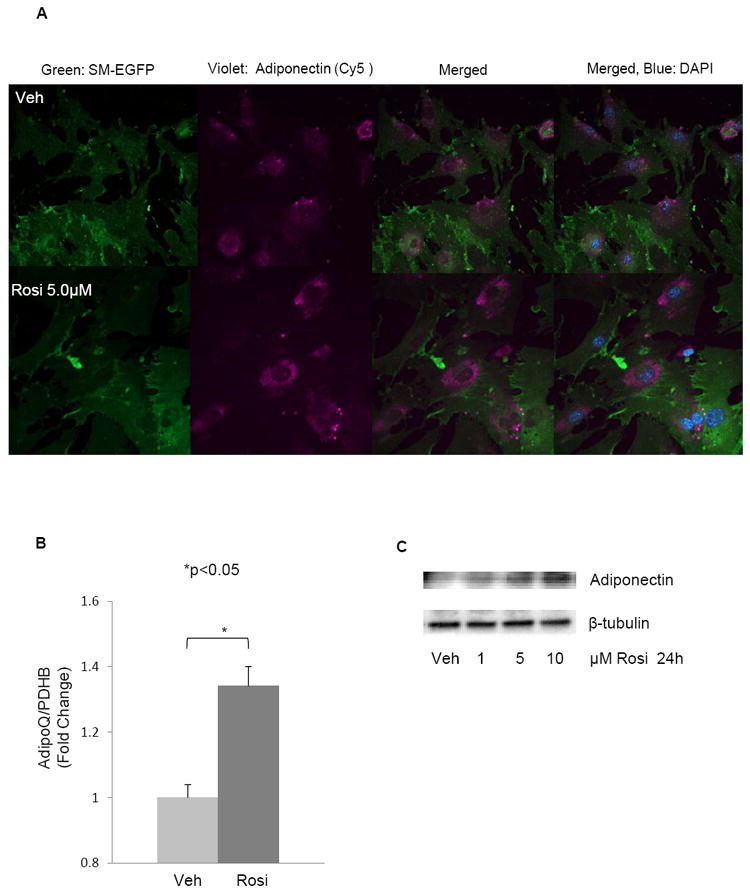
(A) Confocal immunofluorescence analysis shows that adiponectin expression is upregulated in 5 μM rosiglitazone treated VSMC isolated from SM-GFP mice as compared with vehicle treated cells. Transgelin-lineage (SM-EGFP) (green), adiponectin (violet, Cy5 secondary antibody) and merged image without and with DAPI are shown, respectively. All cells were treated with brefeldin A as in Fig. 2A. No tomato red staining was detected (not shown). (B) Adiponectin mRNA levels in WT mouse VSMC treated with 5 μM Rosiglitazone or vehicle for 24 hours were analyzed by real time RT-PCR. Data are means plus standard error of 3 independent experiments. *p <0.05. (C) hCASMC were cultured in 2.5%FBS media overnight and then treated with the indicated doses of rosiglitazone for 24 hours prior to western blot analysis (denaturing conditions) for adiponectin (monomeric) and β-tubulin expression. Figure is representative of two independent experiments.
3.2 PPARγ agonist, rosiglitazone, induces adiponectin in cultured VSMC
As PPARγ agonists are known to upregulate adiponectin in adipocytes [46], we next determined the effect of rosiglitazone on VSMC. The immunostaining revealed that 24 hour treatment with 5μM rosiglitazone, a selective PPARγ ligand, upregulates adiponectin synthesis in VSMC from SM-GFP mice (Fig. 3A-B). Rosiglitazone induced a significant increase in adiponectin gene expression in WT mouse VSMC (Fig. 3B). We confirmed adiponectin protein induction with a rosiglitazone over a dose response (1-10 μM) in hCASMC (Fig. 3C).
3.3 Endogenous adiponectin expressed by VSMC is necessary to maintain basal contractile protein expression
To address the functional role of VSMC-expressed adiponectin, we used siRNA to knock-down endogenous adiponectin in cultured hCASMC and analyzed contractile protein expression. Adiponectin knockdown in hCASMC was associated with decreased expression of the mRNAs encoding contractile-associated proteins SM-α-actin, calponin, transgelin, and myosin heavy chain (MHC), as well as the pro-differentiation master regulatory transcriptional co-activator myocardin (Fig. 4A). Adiponectin knock-down inhibited h-caldesmon protein expression in a dose-dependent manner (Fig. 4B), and significantly inhibited expression of h-caldesmon and SM-α-actin protein (Fig. 4C-D). Adiponectin knockdown also inhibited MHC expression, which was rescued by treatment with recombinant HMW adiponectin (Fig. 4E).
Figure 4. Endogenous adiponectin regulates contractile protein expression in hCASMC.
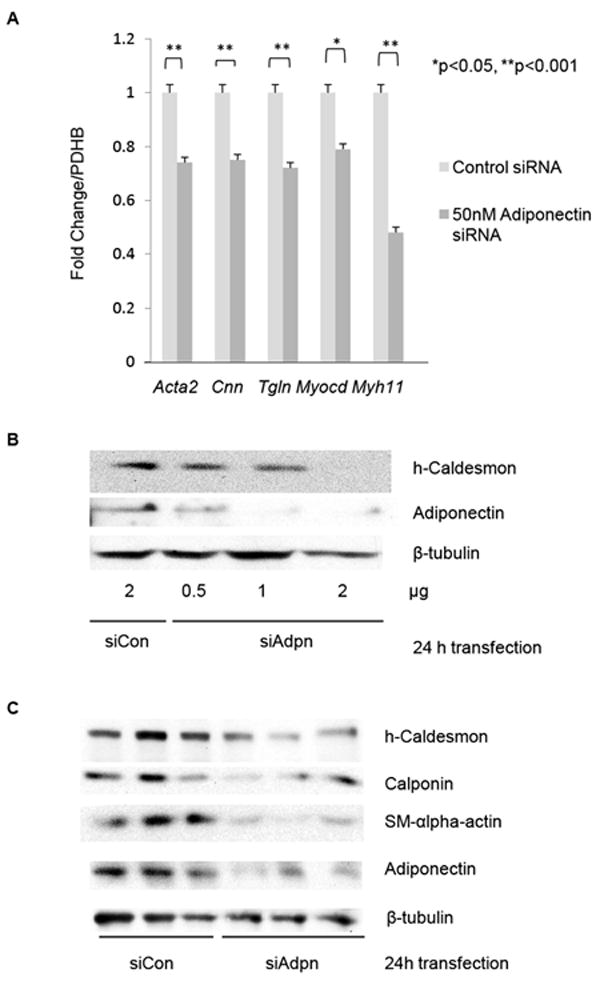
(A) hCASMC were cultured in M199 with 2.5% FBS for 24h prior to transfection with control or adiponectin siRNA for another 24h and harvested for real time RT-PCR with indicated primers. Data are means plus standard error of a minimum of 3 independent experiments. *p <0.05, **p <0.001 relative to control siRNA transfected samples. (B-D) hCASMC were cultured in M199 with 2.5% FBS for 24h prior to transfection with control or adiponectin siRNA for another 24h, and subjected to western blotting analysis for the indicated contractile proteins. (D) shows quantitations of 4-5 independent experiments. p values are indicated (Paired t-test). (E) hCASMC were transfected with siRNA as in (D) for 24h, and HMW enriched recombinant adiponectin (5 μg/ml) was added to the indicated sample for 4h prior to western analysis as above. Densitometric quantitation of MHC normalized to β-tubulin is shown above the blot.
We also evaluated the levels of contractile-associated protein gene and protein expression in VSMC isolated from WT and Adiponectin-/- mice. Real time RT-PCR data revealed that Adiponectin-/- VSMC have significantly lower levels of SM-α-actin (Acta2), Calponin (Cnn), Myosin Heavy Chain (Myh11) and Transgelin (Tgln) mRNA than WT VSMC (Fig. 4A). Reduced expression of h-caldesmon protein in Adiponectin-/- VSMC versus WT is also apparent by confocal immunofluorescence microscopy in Figure 4B (rows 1 and 2). These findings demonstrate that VSMC adiponectin expression regulates baseline levels of contractile protein expression.
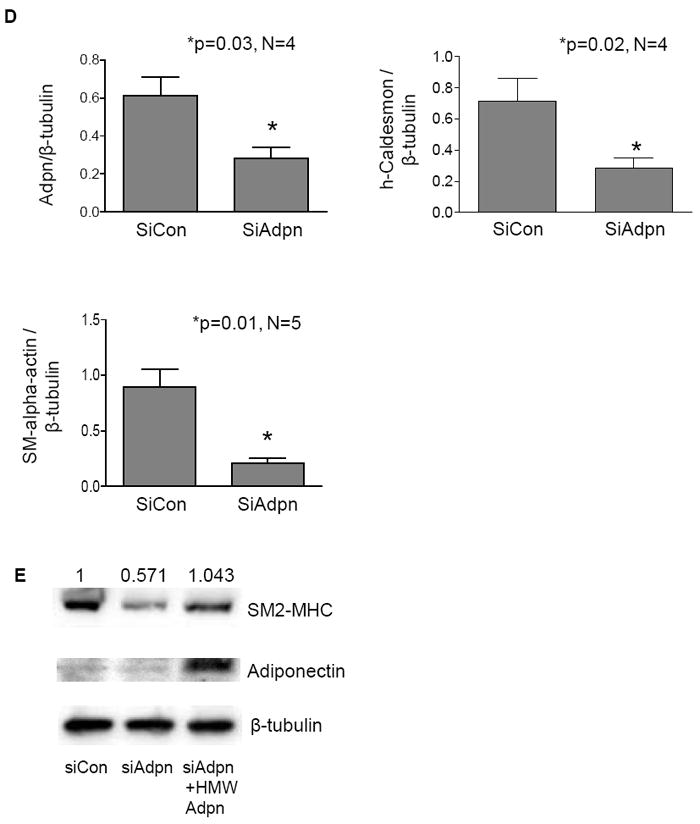
3.4 Adiponectin secreted by WT VSMC induces contractile and smooth muscle-specific gene expression in Adiponectin-/- VSMC
In order to test the hypothesis that adiponectin secreted from WT VSMC can act in a paracrine manner, we collected the culture media from these cells to determine whether this could rescue the reduced levels of contractile-associated protein expression in Adiponectin-/- VSMC. As can be seen in Figure 5A, Adiponectin-/- VSMC cultured for 24h in conditioned media from WT VSMC exhibited a significant increase in the levels of Acta2, Cnn, Myh11 and Tgln gene expression, although these levels do not rise to those seen in WT VSMC. The addition of an adiponectin neutralizing antibody [47] blocked the WT CM-induced increase in contractile-associated gene expression (Fig. 5A), demonstrating the specificity of this effect. This result was also observed at the protein level (Fig. 5B). Similar to hCASMC (in Fig 1B), multiple active adiponectin oligomers, including trimer and HMW, were detected in the media secreted by WT mouse VSMC (data not shown).
Figure 5. Adiponectin secreted by WT primary mouse VSMC restores contractile protein expression in Adiponectin-/- VSMC.
(A) Smooth Muscle alpha-actin (Acta2), Calponin (Cnn), Myosin Heavy Chain (Myh11) and Transgelin (Tgln) mRNA levels in WT VSMC, Adiponectin-/- VSMC, Adiponectin-/- VSMC treated for 24h with media secreted from WT cells, and Adiponectin-/- VSMC treated for 24h with media secreted from WT cells and adiponectin neutralizing antibody (2 mg/mL). Data are means plus SE of a minimum of 3 independent experiments. *P <0.05. (B) Immunofluorescent confocal images of VSMC subjected to the same treatments as in (A) and stained for F-actin (red, left column), h-Caldesmon (green, middle column), DAPI (blue), and composite image (right column).
3.5 Adiponectin overexpression in VSMC promotes AMPK-dependent differentiation marker expression
To verify the functional effect of VSMC-secreted adiponectin, we transfected hCASMC with an adiponectin expression plasmid and assessed the effect on VSMC phenotype. We found that adiponectin overexpression in VSMC is associated with induced contractile protein mRNA and protein expression (Fig. 6A-B), as well as induction of mRNA encoding the pro-differentiation transcription factors GATA6 and myocardin (Fig. 6A). This suggests that VSMC transfected with adiponectin cDNA express and secrete functional adiponectin protein in order to induce differentiation. We have confirmed expression of HMW and other adiponectin oligomers in lysates and media supernatants of transfected hCASMC in Fig. 1B-C. We have recently demonstrated that recombinant HMW adiponectin promotes VSMC differentiation through a signaling pathway involving AMPK activation and subsequent mTORC1 inhibition and Akt activation [44]. Adiponectin overexpression was also sufficient to activate AMPK in hCASMC, as measured by increased phosphorylation of AMPK-α subunit (Thr172) and confirmed by increased phosphorylation of the AMPK substrates acetyl-CoA carboxylase (ACC) (Ser79) and eukaryotic translation elongation factor 2 (eEF2) (Thr56) (Fig. 6B). Adiponectin overexpression also inhibited mTORC1 signaling as evidenced by the reduced phosphorylation of the mTORC1 substrate S6K1 (Thr389), as well as the enhanced phosphorylation of Akt at Ser 473 (Fig. 6B). As with recombinant adiponectin, AMPK activity is similarly necessary for adiponectin overexpression-induced VSMC differentiation, as the specific AMPK inhibitor compound C inhibited both the adiponectin-induced AMPK phosphorylation and contractile protein expression (data not shown).
Figure 6. Adiponectin overexpression in hCASMC induces differentiation marker expression via AMPK signaling.
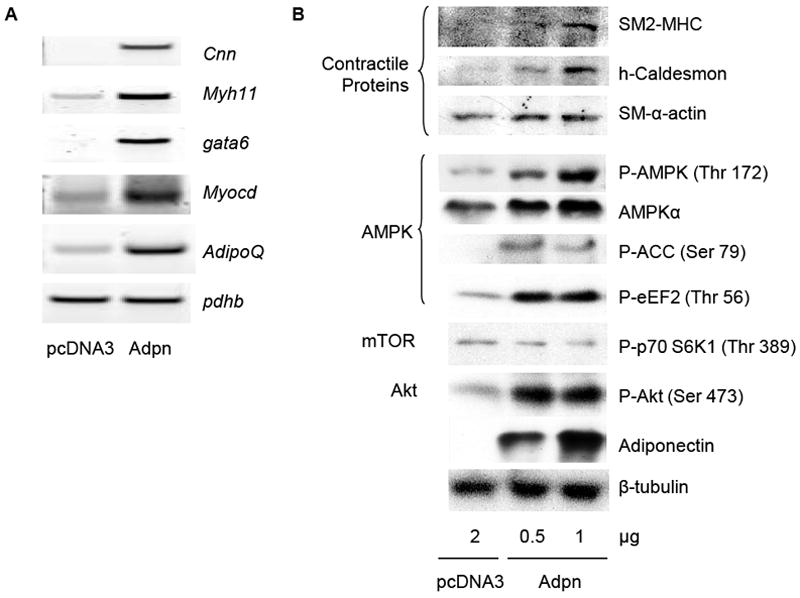
(A) hCASMC transfected with 2 μg pcDNA3 or adiponectin plasmid for 48h were subjected to RT-PCR analysis for contractile protein and pro-differentiation transcription factor mRNA expression as indicated. Pdhb serves as a housekeeping control gene. (B) hCASMC were transfected as in (A) with the indicated plasmids and harvested for western blotting analysis with the indicated antibodies.
Transfection experiments further demonstrated the ability of adiponectin to act in a paracrine manner. We transfected hCASMC with adiponectin at relatively low transfection efficiency, such that only one to two cells per field overexpressed sufficient adiponectin to detect by immunohistochemistry using a low concentration of primary antibody. Notably, contractile protein expression was highly upregulated, not only in the transfected cells, but also in the neighboring VSMC, suggesting that the transfected cells secrete adiponectin that acts in a paracrine manner. This effect was not observed upon transfection of a control empty vector (Fig. 7A-B) and specifically required secretion of adiponectin, as an adiponectin neutralizing antibody blocked the contractile protein upregulation (Fig. 7B, low power shown in Supplemental Fig. 1)
Figure 7. Overexpression of adiponectin in hCASMC induces contractile protein expression via autocrine and paracrine actions.
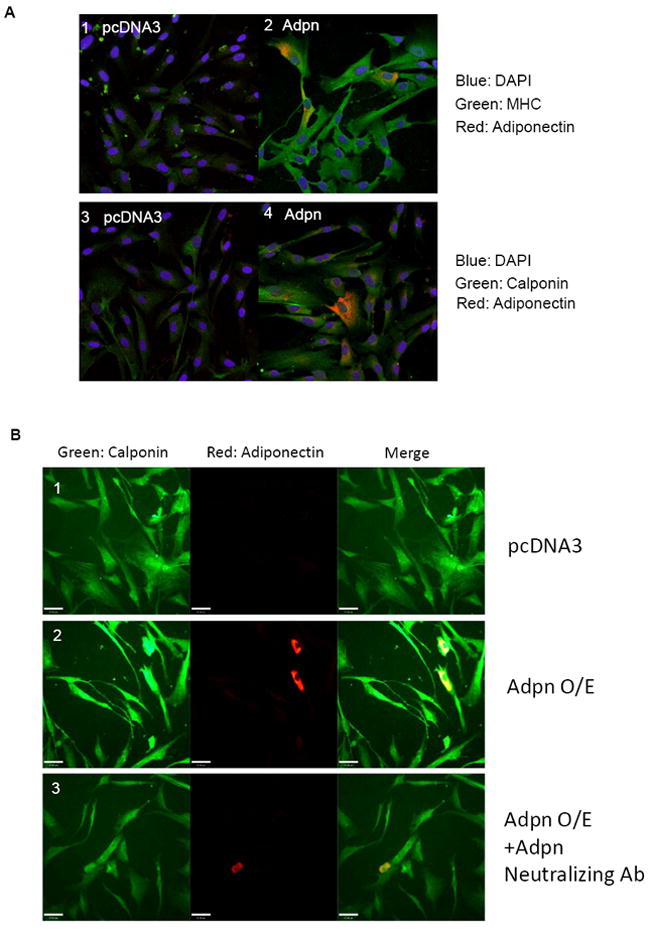
(A) hCASMC were transfected with 2 μg pcDNA3 (panel 1,3) or adiponectin (panel 2, 4) plasmid and cultured on coverslips for 48h prior to immunohistochemistry analysis using anti-adiponectin and anti-MHC or anti-calponin primary antibodies, and AlexaFluor568/488-conjugated secondary antibodies. Contractile protein signals are green, adiponectin signal is red. Merge is shown in yellow. (B) A replicate experiment performed as above and immunostained for adiponectin and calponin, with the addition of adiponectin neutralizing antibody (2 μg/ml) in the bottom panels.
4. Discussion
Using multiple carefully controlled approaches, we demonstrate for the first time that adiponectin is expressed and secreted by VSMC. Notably, we find that adiponectin is required for expression of contractile proteins in VSMC. While most studies of the cardioprotective effects of adiponectin in both humans and mouse models have largely correlated these effects with circulating concentrations [48], several studies suggest that locally produced adiponectin also contributes to the cardioprotective effect of this hormone. Although adiponectin was originally thought to be exclusively secreted by adipose tissue, our study, in addition to a growing body of evidence, reveals that other cells types can express and secrete adiponectin and that this local signaling has functional significance [36-42, 49].
Adipocytes abundantly synthesize and secrete adiponectin which circulates at high levels in the plasma [13] [50]. Even amid this background of circulating adiponectin, recent evidence describes important physiologic roles for locally-synthesized adiponectin. Our findings that VSMC-derived adiponectin regulates contractile phenotype support an emerging new paradigm that adiponectin is an important local mediator of myocyte function. It was recently demonstrated that cardiomyocytes secrete adiponectin [51] and that this locally-derived adiponectin is protective against ischemia-reperfusion injury in vitro [52] and adrenergic-induced hypertrophy in cultured cardiomyocytes [53]. Skeletal muscle also expresses adiponectin which is modulated by diet [54] and has been shown to influence muscle phenotype and function [37]. Interestingly, we detected greater adiponectin mRNA levels in aortic smooth muscle than in skeletal muscle. In addition to myocyte effects, adiponectin secreted from bone-forming cells acts in autocrine/paracrine manner to promote osteogenesis [55].
While we have previously demonstrated that the addition of recombinant adiponectin to cultured VSMC (synthetic phenotype) increases contractile protein expression[44], it was unexpected that the endogenous adiponectin produced by VSMC would be essential for the basal contractile protein expression. Our studies in which adiponectin was knocked down or overexpressed suggest that it may serve as a key regulator of contractile phenotype by regulating expression of pro-differentiation transcription factors expression including myocardin and GATA-6. Using several different approaches, we demonstrate that adiponectin can function to regulate VSMC phenotype in an autocrine or paracrine manner. Most notably, conditioned media from wild type VSMC was able to rescue the reduced contractile protein expression in adiponectin-/- VSMC. The overexpression approach demonstrates that this secreted adiponectin activates the same AMPK-mTORC1-Akt signaling pathway employed by recombinant HMW adiponectin [44]. Contractile proteins were upregulated not only in VSMC overexpressing adiponectin, but also in non-transfected neighboring cells, and this effect was blocked by a neutralizing antibody. The effect of adiponectin knockdown in VSMC cultures could be rescued by recombinant adiponectin. Collectively, these studies suggest that adiponectin produced by VSMC may help to maintain contractile phenotype by acting on neighboring cells.
The PPARγ agonist rosiglitazone is a potent inducer of adiponectin synthesis and secretion in other tissues including adipocytes [46], cardiomyocytes [53, 56] and myocytes [49, 57]. We report that VSMC adiponectin synthesis is also stimulated by rosiglitazone. Interestingly, adiponectin treatment [58] or PPARγ activation by rosiglitazone [59] have independently been shown to inhibit VSMC proliferation and migration. PPARγ has also been implicated in regulating VSMC contractile phenotype in models of rosiglitazone-sensitive spontaneous hypertension [60]. Our study suggests that the effects of rosiglitazone on VSMC may be mediated, at least in part, by adiponectin induction. This would be in accordance with studies of adiponectin knockout mice which reveal that the major insulin-sensitizing [61] and cardioprotective [62] effects of anti-diabetic thiazolidinedione drugs are mediated by induction of adiponectin.
Our data and the work of others suggest a new paradigm in which there may be distinct functions for local versus systemic adiponectin. One might speculate that the adipocyte-derived adiponectin that circulates at high levels may serve the function of a traditional adipokine, i.e. to relay information to the peripheral tissues regarding levels of energy stored in the visceral adipose depot and to adjust peripheral insulin sensitivity accordingly. Conversely, there might be different roles for adiponectin synthesized and acting locally in muscular tissues. These differential roles may be mediated, at least in part, by differential expression of oligomers and receptors. Whether peripheral sources of adiponectin are similarly regulated in response to fat storage remains to be determined, but there is some evidence in skeletal muscle that obesity alters the oligomeric profile of locally synthesized adiponectin [37], and that high fat diet, together with PPARγ activation, induces adiponectin in the heart [53]. Distinct roles for local versus systemic adiponectin might be analogous to other hormones such as catecholamines, wherein low level local catecholamine release at synapses is essential for neurotransmission where receptors are concentrated at the postsynaptic membrane, while in contrast, adrenal release of high levels of catecholamines into the circulation is required to coordinate the whole body fight or flight response [63].
In summary, our findings indicate that VSMC can synthesize and secrete adiponectin, which promotes the maintenance of the differentiated contractile phenotype in VSMC. The case for autocrine and paracrine actions of locally-derived adiponectin in smooth, skeletal, and cardiac myocytes as well as in other tissues is growing. These studies suggest that the construction of tissue-specific adiponectin knockout mouse models is warranted in order to provide insights into the relative contributions of local and systemic depots of this adipokine.
Supplementary Material
Acknowledgments
We thank Dr. Kenneth Walsh for adiponectin plasmid and advice. We thank Drs. Daniel Greif and Zsolt Kasza for helpful discussions and Xiaoyue Hu for technical assistance.
Sources of Funding This work is supported by NIH R01 RHL091013A (KAM) a FAMRI Clinical Innovator Award (KAM), an NIH NHLBI R01 (JH), and funding from Yale Vascular Biology and Therapeutics (JY).
Abbreviations
- VSMC
vascular smooth muscle cell(s)
- hCASMC
human coronary artery smooth muscle cell(s)
- ER
endoplasmic reticulum
- Adpn
adiponectin
- Rosi
Rosiglitazone
Footnotes
Disclosures: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007 Apr 1;74(1):11–8. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okamoto Y, A Y, Nishida M, Muraguchi M, Ouchi N, Takahashi M, Igura, lnui Y, Kihara S, N T, Yamashita s, Miyagawa j, Funahashi T, Matsuzawa Y. An Adipocyte-Derived Plasma Protein, Adiponectin, Adheres to Injured Vascular Walls. Horm Metab Res. 2000;32:47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- 3.Dzielinska Z, Januszewicz A, Wiecek A, Demkow M, Mkowiecka-Ciesla M, Prejbisz A, et al. Decreased plasma concentration of a novel anti-inflammatory protein--adiponectin--in hypertensive men with coronary artery disease. Thromb Res. 2003 Jun 15;110(5-6):365–9. doi: 10.1016/j.thromres.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Yaturu S, Bridges JF, Subba Reddy DR. Decreased levels of plasma adiponectin in prediabetes, Type 2 diabetes and coronary artery disease. Med Sci Monit. 2006 Jan;12(1):CR17–20. [PubMed] [Google Scholar]
- 5.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003 Jan 1;23(1):85–9. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 6.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000 September 1;96(5):1723–32. [PubMed] [Google Scholar]
- 7.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, et al. Circulating Concentrations of the Adipocyte Protein Adiponectin Are Decreased in Parallel With Reduced Insulin Sensitivity During the Progression to Type 2 Diabetes in Rhesus Monkeys. Diabetes. 2001 May 1;50(5):1126–33. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 8.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, et al. Globular Adiponectin Protected ob/ob Mice from Diabetes and ApoE-deficient Mice from Atherosclerosis. Journal of Biological Chemistry. 2003 January 24;278(4):2461–8. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin Stimulates Production of Nitric Oxide in Vascular Endothelial Cells. Journal of Biological Chemistry. 2003 November 7;278(45):45021–6. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–6. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 11.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7(8):947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 12.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8(7):731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 13.Matsuzawa Y. Establishment of a concept of visceral fat syndrome and discovery of adiponectin. Proc Jpn Acad Ser B Phys Biol Sci. 2010;86(2):131–41. doi: 10.2183/pjab.86.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002 Jul;8(7):731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002 Oct 4;277(40):37487–91. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 16.Kato H, Kashiwagi H, Shiraga M, Tadokoro S, Kamae T, Ujiie H, et al. Adiponectin acts as an endogenous antithrombotic factor. Arterioscler Thromb Vasc Biol. 2006 Jan;26(1):224–30. doi: 10.1161/01.ATV.0000194076.84568.81. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, et al. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006 Jun;47(6):1108–16. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 18.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005 Oct;11(10):1096–103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okui H, Hamasaki S, Ishida S, Kataoka T, Orihara K, Fukudome T, et al. Adiponectin is a better predictor of endothelial function of the coronary artery than HOMA-R, body mass index, immunoreactive insulin, or triglycerides. Int J Cardiol. 2008 May 7;126(1):53–61. doi: 10.1016/j.ijcard.2007.03.116. [DOI] [PubMed] [Google Scholar]
- 20.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes. Journal of Biological Chemistry. 1995 November 10;270(45):26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 21.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996 Apr 16;221(2):286–9. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Lam KS, Yau MH, Xu A. Post-translational modifications of adiponectin: mechanisms and functional implications. Biochem J. 2008 Feb 1;409(3):623–33. doi: 10.1042/BJ20071492. [DOI] [PubMed] [Google Scholar]
- 23.Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009 May;30(5):234–9. doi: 10.1016/j.tips.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423(6941):762–9. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 25.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci U S A. 2004 Jul 13;101(28):10308–13. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010 Dec 1;120(12):4342–52. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin KA, Rzucidlo EM, Merenick BL, Fingar DC, Brown DJ, Wagner RJ, et al. The mTOR/p70 S6K1 pathway regulates vascular smooth muscle cell differentiation. Am J Physiol Cell Physiol. 2004 Mar;286(3):C507–17. doi: 10.1152/ajpcell.00201.2003. [DOI] [PubMed] [Google Scholar]
- 28.Martin KA, Merenick BL, Ding M, Fetalvero KM, Rzucidlo EM, Kozul CD, et al. Rapamycin promotes vascular smooth muscle cell differentiation through insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt2 feedback signaling. J Biol Chem. 2007 Dec 7;282(49):36112–20. doi: 10.1074/jbc.M703914200. [DOI] [PubMed] [Google Scholar]
- 29.Fetalvero KM, Shyu M, Nomikos AP, Chiu YF, Wagner RJ, Powell RJ, et al. The prostacyclin receptor induces human vascular smooth muscle cell differentiation via the protein kinase A pathway. Am J Physiol Heart Circ Physiol. 2006 Apr;290(4):H1337–46. doi: 10.1152/ajpheart.00936.2005. [DOI] [PubMed] [Google Scholar]
- 30.Banga A, Bodles AM, Rasouli N, Ranganathan G, Kern PA, Owens RJ. Calcium is involved in formation of high molecular weight adiponectin. Metab Syndr Relat Disord. 2008 Summer;6(2):103–11. doi: 10.1089/met.2007.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ding M, Xie Y, Wagner RJ, Jin Y, Carrao AC, Liu LS, et al. Adiponectin induces vascular smooth muscle cell differentiation via repression of mammalian target of rapamycin complex 1 and FoxO4. Arterioscler Thromb Vasc Biol. 2011 Jun;31(6):1403–10. doi: 10.1161/ATVBAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, et al. Endovascular Injury Induces Rapid Phenotypic Changes in Perivascular Adipose Tissue. Arterioscler Thromb Vasc Biol. 2010 August 1;30(8):1576–82. doi: 10.1161/ATVBAHA.110.207175. [DOI] [PubMed] [Google Scholar]
- 33.Kitta Y, Takano H, Nakamura T, Kodama Y, Umetani K, Fujioka D, et al. Low adiponectin levels predict late in-stent restenosis after bare metal stenting in native coronary arteries. Int J Cardiol. 2008 Dec 17;131(1):78–82. doi: 10.1016/j.ijcard.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Moldoveanu E, Mut-Vitcu B, Tanaseanu GR, Marta DS, Manea G, Kosaka T, et al. Low basal levels of circulating adiponectin in patients undergoing coronary stenting predict in-stent restenosis, independently of basal levels of inflammatory markers: lipoprotein associated phospholipase A2, and myeloperoxidase. Clin Biochem. 2008 Dec;41(18):1429–3. doi: 10.1016/j.clinbiochem.2008.09.109. [DOI] [PubMed] [Google Scholar]
- 35.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995 Nov 10;270(45):26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 36.Pineiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett. 2005 Sep 26;579(23):5163–9. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 37.Krause MP, Liu Y, Vu V, Chan L, Xu A, Riddell MC, et al. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. American Journal of Physiology - Cell Physiology. 2008 July 1;295(1):C203–C12. doi: 10.1152/ajpcell.00030.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, et al. Adiponectin and its receptors are expressed in bone-forming cells. Bone. 2004 Oct;35(4):842–9. doi: 10.1016/j.bone.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 39.Caminos JE, Nogueiras R, Gallego R, Bravo S, Tovar S, Garcia-Caballero T, et al. Expression and regulation of adiponectin and receptor in human and rat placenta. J Clin Endocrinol Metab. 2005 Jul;90(7):4276–86. doi: 10.1210/jc.2004-0930. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, et al. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007 Jan 1;148:401–10. doi: 10.1210/en.2006-1019. [DOI] [PubMed] [Google Scholar]
- 41.Yoda-Murakami M, Taniguchi M, Takahashi K, Kawamata S, Saito K, Choi-Miura NH, et al. Change in expression of GBP28/adiponectin in carbon tetrachloride-administrated mouse liver. Biochem Biophys Res Commun. 2001 Jul 13;285(2):372–7. doi: 10.1006/bbrc.2001.5134. [DOI] [PubMed] [Google Scholar]
- 42.Maresh JG, Shohet RV. In vivo endothelial gene regulation in diabetes. Cardiovasc Diabetol. 2008;7:8. doi: 10.1186/1475-2840-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer CA, Salinthone S, Baker KJ, Gerthoffer WT. Synthesis of immune modulators by smooth muscles. Bioessays. 2004 Jun;26(6):646–55. doi: 10.1002/bies.20041. [DOI] [PubMed] [Google Scholar]
- 44.Ding M, Xie Y, Wagner RJ, Jin Y, Carrao AC, Liu L, et al. Adiponectin Induces Vascular Smooth Muscle Cell Differentiation via repression of mTORC1 and FoxO4. ATVB. 2011 doi: 10.1161/ATVBAHA.110.216804. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, et al. A new role for Nogo as a regulator of vascular remodeling. Nat Med. 2004 Apr;10(4):382–8. doi: 10.1038/nm1020. [DOI] [PubMed] [Google Scholar]
- 46.Iwaki M, Matsuda M, Maeda N, Funahashi T, Matsuzawa Y, Makishima M, et al. Induction of Adiponectin, a Fat-Derived Antidiabetic and Antiatherogenic Factor, by Nuclear Receptors. Diabetes. 2003 July 1;52(7):1655–63. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 47.Rasouli N, Yao-Borengasser A, Varma V, Spencer HJ, McGehee RE, Jr, Peterson CA, et al. Association of Scavenger Receptors in Adipose Tissue With Insulin Resistance in Nondiabetic Humans. Arterioscler Thromb Vasc Biol. 2009 September 1;29(9):1328–35. doi: 10.1161/ATVBAHA.109.186957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldstein BJ, Scalia RG, Ma XL. Protective vascular and myocardial effects of adiponectin. Nat Clin Pract Cardiovasc Med. 2009 Jan;6(1):27–35. doi: 10.1038/ncpcardio1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Amin RH, Mathews ST, Camp HS, Ding L, Leff T. Selective activation of PPAR{gamma} in skeletal muscle induces endogenous production of adiponectin and protects mice from diet-induced insulin resistance. Am J Physiol Endocrinol Metab. 2010 January 1;298(1):E28–37. doi: 10.1152/ajpendo.00446.2009. [DOI] [PubMed] [Google Scholar]
- 50.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and biophysical research communications. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 51.Piñeiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, et al. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS letters. 2005;579(23):5163–9. doi: 10.1016/j.febslet.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Lau WB, Gao E, Tao L, Yuan Y, Li R, et al. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2010 March 1;298(3):E663–70. doi: 10.1152/ajpendo.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amin RH, Mathews ST, Alli A, Leff T. Endogenously produced adiponectin protects cardiomyocytes from hypertrophy by a PPARγ-dependent autocrine mechanism. American Journal of Physiology - Heart and Circulatory Physiology. 2010 September 1;299(3):H690–H8. doi: 10.1152/ajpheart.01032.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y, Chewchuk S, Lavigne C, Brule S, Pilon G, Houde V, et al. Functional significance of skeletal muscle adiponectin production, changes in animal models of obesity and diabetes, and regulation by rosiglitazone treatment. Am J Physiol Endocrinol Metab. 2009 Sep;297(3):E657–64. doi: 10.1152/ajpendo.00186.2009. [DOI] [PubMed] [Google Scholar]
- 55.Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006 Sep 1;99(1):196–208. doi: 10.1002/jcb.20890. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Lau WB, Gao E, Tao L, Yuan Y, Li R, et al. Cardiomyocyte-derived adiponectin is biologically active in protecting against myocardial ischemia-reperfusion injury. Am J Physiol Endocrinol Metab. 2010 Mar;298(3):E663–70. doi: 10.1152/ajpendo.00663.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouedraogo R, Wu X, Xu S-Q, Fuchsel L, Motoshima H, Mahadev K, et al. Adiponectin Suppression of High-Glucose–Induced Reactive Oxygen Species in Vascular Endothelial Cells. Diabetes. 2006 June;55(6):1840–6. doi: 10.2337/db05-1174. [DOI] [PubMed] [Google Scholar]
- 58.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002 Jun 18;105(24):2893–8. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 59.Law RE, Goetze S, Xi XP, Jackson S, Kawano Y, Demer L, et al. Expression and function of PPARgamma in rat and human vascular smooth muscle cells. Circulation. 2000 Mar 21;101(11):1311–8. doi: 10.1161/01.cir.101.11.1311. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, Xie P, Wang J, Yang Q, Fang C, Zhou S, et al. Impaired Peroxisome Proliferator-activated Receptor-γ Contributes to Phenotypic Modulation of Vascular Smooth Muscle Cells during Hypertension. Journal of Biological Chemistry. 2010 April 30;285(18):13666–77. doi: 10.1074/jbc.M109.087718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006 Feb 3;281(5):2654–60. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 62.Li P, Shibata R, Unno K, Shimano M, Furukawa M, Ohashi T, et al. Evidence for the importance of adiponectin in the cardioprotective effects of pioglitazone. Hypertension. 2010 Jan;55(1):69–75. doi: 10.1161/HYPERTENSIONAHA.109.141655. [DOI] [PubMed] [Google Scholar]
- 63.Thomas GD. Neural control of the circulation. Adv Physiol Educ. 2011 Mar;35(1):28–32. doi: 10.1152/advan.00114.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


