Summary
The Daam family of proteins consists of Daam1 and Daam2. While Daam1 participates in non-canonical Wnt signaling during gastrulation, Daam2 function remains completely uncharacterized. Here we describe the role of Daam2 in canonical Wnt signal transduction during spinal cord development. Loss-of-function studies revealed that Daam2 is required for dorsal progenitor identities and canonical Wnt signaling. These phenotypes are rescued by β-catenin, demonstrating that Daam2 functions in dorsal patterning through the canonical Wnt pathway. Complementary gain-of-function studies demonstrate that Daam2 amplifies Wnt signaling by potentiating ligand activation. Biochemical examination found that Daam2 association with Dvl3 is required for Wnt activity and dorsal patterning. Moreover, Daam2 stabilizes Dvl3/Axin2 binding, resulting in enhanced intracellular assembly of Dvl3/Axin2 complexes. These studies demonstrate that Daam2 modulates the formation of Wnt receptor complexes, revealing new insight into the functional diversity of Daam proteins and how canonical Wnt signaling contributes to pattern formation in the developing spinal cord.
Introduction
The generation of cellular diversity in the developing central nervous system (CNS) relies on spatially distinct signaling centers that release morphogenic signals. Cellular integration of these extrinsic morphogenic cues results in the activation of a transcriptional network that drives changes in gene expression and ultimately determines cell fate. The embryonic spinal cord serves as an excellent paradigm for this model of neural development, as the molecular nature of the morphogens and their associated transcriptional programs have been well established.
In the dorsal spinal cord, members of the bone morphogenic protein (BMP) and Wnt families are secreted from the dorsal ectoderm and the dorsal roof plate signaling centers, respectively, and play critical roles in the generation of dorsal neuron populations through the action of their respective transcriptional effectors, SMADs and β-catenin/Tcf (Kim et al., 2000; Liu and Niswander, 2005; Lupo et al., 2006). The signal transduction pathways that integrate BMP and Wnt signals consist of multiple molecular components whose response must be coordinated in order to elicit the appropriate signal interpretation. In particular, Wnt signal integration remains enigmatic due to the complexity of the signal transduction mechanism, which includes numerous, dynamic molecular components, mediating both canonical and non-canonical pathways (Wodarz and Nusse, 1998). A key step in canonical Wnt signal transduction is the phosphorylation of LRP mediated by GS3Kβ, which requires the formation of a complex between Dishevelled (Dvl) and Axin proteins that bridges the Frizzled (Fzd) and LRP receptors (Bilic et al., 2007; MacDonald et al., 2009; Zeng et al., 2008). While Dvl/Axin interaction is crucial for Wnt signaling, studies have revealed that they have relatively low binding affinity, thus aggregation of Fzd/LRP/Dvl/Axin complexes into signalsome structures is thought to facilitate amplification and maintenance of Wnt signaling (Bilic et al., 2007; Schwarz-Romond et al., 2007; Wong et al., 2003). While this model provides a framework for understanding the Dvl/Axin relationship in the context of canonical Wnt signaling, there is evidence that other proteins are associated with this complex and can potentiate Dvl/Axin mediated Wnt signaling as well (Chen, 2006; Ding et al., 2008). Thus, a more complete understanding of the dynamics and constituents of the Dvl/Axin complex is necessary to fully understand the processes that control canonical Wnt signaling.
Dvl and Axin have been shown to interact with many proteins, which has implicated their function in a wide range of cellular processes, though only a small sub-set are associated with their role in canonical Wnt signaling (Wallingford and Habas, 2005). Among these proteins, Daam1 has been shown to interact with Dvl to control cell polarity and movement via non-canonical Wnt signaling during Xenopus gastrulation (Habas et al., 2001). The Daam family of proteins consists of Daam1 and Daam2 and share conserved formin-homology (FH) and GBD domains, which are suggestive of a role in cell motility (Alberts, 2001; Kida et al., 2004). While the function of Daam1 has been extensively studied during gastrulation, Daam2 function remains undefined (Habas et al.,2001). In a previous study Daam1 and Daam2 were found to have complementary and non-overlapping expression patterns in the developing spinal cord, with Daam1 demonstrating expression in the mantle regions and Daam2 in ventricular zone (VZ) populations (Kida et al., 2004). While the expression of Daam genes has been associated with both neuronal and progenitor populations in the embryonic spinal cord, their roles during spinal cord development remain uncharacterized.
In our effort to identify genes that contribute to CNS development we performed microarray analysis on embryonic spinal cord and confirmed that Daam2 is expressed in VZ populations throughout early embryogenesis. Subsequent loss-of-function studies revealed that Daam2 is required for dorsal progenitor identities and canonical Wnt signaling. These phenotypes are rescued by β-catenin, which directly links Daam2 function in dorsal patterning to the canonical Wnt pathway. Gain-of-function studies demonstrated that Daam2 amplifies Wnt signaling via potentiation of ligand activation in vivo. Next, we investigated the mechanistic basis for its role in the Wnt pathway and found that Daam2 associates with Dvl3 via the DIX domain through its GBD domain and that this interaction is required for Wnt-activity and functions on a biochemical level to stabilize Dvl3/Axin2 binding. These studies identify Daam2 as a critical modulator of canonical Wnt signal integration during pattern formation in the developing spinal cord.
Results
Daam2 is expressed in progenitor populations of the embryonic spinal cord
Gene expression profiling of VZ populations during early spinal cord development identified Daam2 as specifically expressed in early progenitor populations. To verify this, we performed in situ hybridization on embryonic chick spinal cord from E2-E5 and confirmed that Daam2 is expressed in VZ populations during this developmental interval (Figure 1A-1D). To validate its expression in progenitor populations we performed immunolabeling with antibodies to Daam2. This revealed that Daam2 is expressed exclusively in dorsal populations at E2 and throughout the dorsal/ventral extent of the VZ at subsequent stages (Figure 1E-1P). To confirm the domain-specific expression of Daam2 at E2, we next performed co-immunolableing with progenitor markers that demarcate the dorsal/ventral axis, Pax7, Pax6, and Nkx2.2. This analysis revealed that Daam2 is co-expressed with Pax7 and Pax6, but not the ventral-most marker, Nkx2.2, at E2 (Figure 1E, 1I and 1M ). Analysis at subsequent developmental stages found that Daam2 is co-expressed with both dorsal and ventral markers in the VZ (Figure 1F-1P).
Figure 1. Daam2 is expressed in the VZ of the developing spinal cord.
(A–D) In situ hybridization analysis of Daam2 in the chick spinal cord from E2-E5. (E–P) Co-localization of Daam2 with dorsal and ventral VZ markers. (E–I) Daam2 is co-expressed with the dorsal marker Pax7. (I–L) Daam2 is co-expressed with the dorsal-medial marker Pax6. (M–P) Daam2 is not co-expressed with the ventral maker Nkx2.2 at E2 (M), but demonstrates co-expression at subsequent stages of development (N–P).
Daam2 is necessary for the expression of dorsal progenitor markers
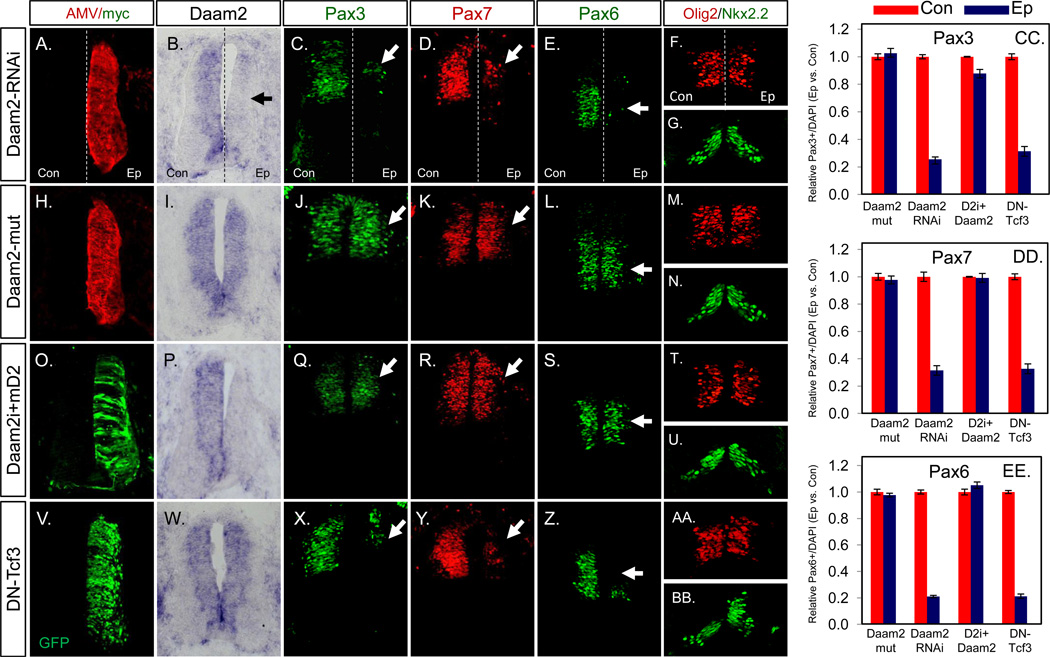
The observation that Daam2 is expressed in VZ populations during the early stages of embryonic spinal cord development raised the question of whether it is playing a role in patterning or maintenance of progenitor populations. To determine the role of Daam2 during early spinal cord development we performed knockdown experiments utilizing an RCAS-shRNAi system we have previously employed (Deneen et al., 2006). The effective knockdown of endogenous Daam2 was verified by in situ hybridization and immunostaining (Figure 2B, arrow; see also Figure S1M available online). To determine the effect of Daam2 knockdown on spinal cord development we assessed expression of progenitor markers along the dorsal-ventral axis at E4. We found that knockdown of Daam2 results in a significant reduction of dorsal progenitor makers, Pax3 and Pax7, and the dorsal-medial marker Pax6 (Figure 2C-2E and 2CC-2EE), but not ventral markers Olig2 and Nkx2.2 (Figure 2F and 2G). This loss of dorsal progenitor markers resulted in the concomitant decrease in the generation of dorsal neuron populations, but not ventral neuron populations (Figure S1D–S1F) (Jessell, 2000). Lastly, reduction of dorsal markers in the absence of Daam2 is not the result of cell death, as there is no increase in active caspase 3 staining or gross reduction in cell numbers in these regions (Figure S1B) (Villa et al., 1997). Moreover, these populations maintained Sox2 expression, indicating retention of progenitor status (Figure S1C) (Graham et al., 2003). Analysis of Daam2 knockdown at E3 revealed a similar loss of dorsal, but not ventral markers; we did not witness a loss of dorsal neuron populations at this stage, likely due to the timing of Daam2-shRNAi introduction and analysis (Figure S2).
Figure 2. RNAi knockdown of Daam2 causes loss of dorsal VZ markers.
(A–G) Expression of the RCAS-cDaam2 shRNAi construct results in the knockdown of endogenous Daam2 (B) and loss of dorsal markers Pax3, Pax7, and Pax6 (C-E; arrows), but not ventral markers Olig2 and Nkx2.2 (F,G). (H–N) Mutant version of cDaam2-shRNAi does not effect endogenous Daam2 expression (I) or the expression of dorsal markers (J-L; arrows). (O–U) Rescue of the cDaam2-shRNAi phenotypes by murine Daam2. Ectopic expression of mDaam2 (O) restores dorsal marker expression (Q-S; arrows). “Daam2i” denotes Daam2-shRNAi. (V-BB) Overexpression of pCIG/DN-Tcf3 results in loss of dorsal marker expression. (CC-DD) Corresponding quantification of the phenotypes demonstrated in the images. Quantification was performed by comparing the number of DAPI+ cells expressing a given marker between the control side (con) and the electroporated side (Ep) of the neural tube. Values presented for each manipulation are derived from at least 6 different embryos, 10 sections per embryo. Note that images for each manipulation are derived from an adjacent series of sections. Immunostaining of AMV in A,H reflects expression of the RCAS retrovirus used to deliver the cDaam2-shRNAi. Error bars indicate standard error of mean (SEM). Please also see Figure S1 and S2.
To assess the specificity of the Daam2 knockdown phenotype, we generated a mutant version of the Daam2-shRNAi containing five nucleotide substitutions, which had no effect on expression of endogenous Daam2 or dorsal markers Pax3, Pax7, or Pax6, indicating that the loss of dorsal markers is specific to the wild-type Daam2-shRNAi (Figure 2H-2N). Next we sought to confirm that the effects of the Daam2-shRNAi are the result of a loss of Daam2. To this end we performed a “rescue” experiment by co-electroporating the Daam2-shRNAi with the mouse Daam2 coding sequence, which is sufficiently divergent so as not to anneal with, and silenced by, the cDaam2-shRNAi. A myctag was included to report expression of the exogenous mDaam2 protein (Figure 2O and 2P) and we found that expression of mDaam2 is sufficient to fully restore the expression of dorsal markers in the absence of endogenous cDaam2 (Figure 2Q-2S and 2CC-2EE). Collectively, these experiments indicate that knockdown of Daam2 is responsible for the loss of Pax3, Pax7, and Pax6 caused by the cDaam2-shRNAi.
Daam2 expression is required for canonical Wnt signaling
The BMP- and Wnt-signaling pathways control expression of dorsal progenitor markers, thus the phenotypes manifest in the absence of Daam2 resembled what one might expect if BMP- or Wnt-signaling is compromised (Alvarez-Medina et al., 2008; Liem et al., 1995; Megason and McMahon, 2002). Given that members of the Daam-family have been shown to interact with components of the Wnt signaling pathway and participate in non-canonical Wnt signaling during gastrulation, we reasoned that Daam2 could be participating in the canonical Wnt signaling pathway during early spinal cord development. As a first step in testing this hypothesis, we compared the Daam2-shRNAi phenotype with the expression of a dominant-negative version of Tcf3 (dnTcf3), which has been shown to mimic a loss of canonical Wnt signaling in the embryonic spinal cord (Alvarez-Medina et al., 2008). These studies revealed that overexpression of dnTcf3 resembles knockdown of Daam2, where there is a significant reduction in Pax3, Pax7, and Pax6 expression (Figure 2V-2Z and 2CC-2EE). That overexpression of dnTcf3 phenocopies the loss of Daam2 expression raises the possibility that Daam2 may be required for canonical Wnt signaling during spinal cord development.
To determine whether knockdown of Daam2 directly affects canonical Wnt signaling we co-electroporated the Daam2-shRNAi along with a TOPGAL reporter, where the LacZ has been replaced with a nuclear RFP reporter (TOP-nRFP), into the chick spinal cord (DasGupta and Fuchs, 1999; Lassiter et al., 2007). Consistent with previous reports, our TOP-nRFP reporter was only active in dorsal regions of the spinal cord, indicating that these regions are sites of highest-level Wnt signaling (Figure 3A-3C). As demonstrated in Figure 3D-3F and 3Q, knockdown of Daam2 resulted in an 80% reduction in TOP-nRFP activity in these regions, whereas the mutant Daam2-shRNAi did not affect reporter activity (Figure 3H-3J and 3Q). Furthermore, the effects of the Daam2-shRNAi on the reporter are rescued by mDaam2 (Figure 3L-3N and 3Q), indicating that the loss of Wnt activity is the result of Daam2 knockdown. Importantly, this loss of Wnt activity is not secondary to a loss of key members of the Wnt pathway, as the expression of Wnt3a, β-catenin, and Tcf4 are not affected by the loss of Daam2 (Figure S3). Moreover, analysis of BMP-ligands (BMP4) and key transcriptional targets of the BMP-pathway in the dorsal spinal cord (Lmx) revealed that they are not affected by the loss of Daam2 (Figure 3G and 3K; Figure S3) (Chizhikov et al., 2004). Taken together, these data indicate that Daam2 is required for canonical Wnt signaling.
Figure 3. Knockdown of Daam2 causes loss of canonical Wnt activity.
(A–C) TOP-nRFP reporter demonstrates activity in the dorsal Pax7 domain (A), but not in the medial Pax6 domain (B) or ventral Nkx2.2 domain (C) at E4 in the chick spinal cord. (D–J) Knockdown of Daam2 via RCAS-cDaam2 shRNAi (F) results in decreased TOP-nRFP reporter activity (E; arrow) compared to the mutant Daam2-shRNAi (I,J; arrow). (L–N) TOP-nRFP activity is restored by ectopic expression of mDaam2 (M; arrow). “D2i” denotes Daam2-shRNAi. (O–P) Overexpression of DN-Tcf3 results in loss of TOP-nRFP activity. (G,K) Expression Lmx, a key target of the BMP pathway in the dorsal spinal cord, is not affected by Daam2 knockdown or by the mutant Daam2-shRNAi. (Q) Quantification of reporter activity. Fluorescent intensity values for nRFP and the GFP standardization were acquired and the normalized TOP-nRFP value, relative to GFP, is presented in Q. Quantification for each manipulation is derived from at least 8 different embryos, 10 sections per embryo. (D,H,L,O) GFP control images. Images are from an adjacent series of sections. “Ep” denotes electroporated side of neural tube, “Con” control side. Error bars indicate SEM. Please also see Figure S3.
β-catenin rescues loss of dorsal markers in absence of Daam2
The foregoing results correlate the loss of dorsal markers in the absence of Daam2 with a loss of Wnt activity. These observations raise the question of whether the loss of Wnt signaling in the absence of Daam2 is directly responsible for the loss of dorsal progenitor markers. To investigate this possibility we sought to rescue the Daam2-shRNAi with a constitutively activated form of β-catenin (CA-βcat) (Tetsu and McCormick, 1999). To this end we co-electorporated the combination of Daam2-shRNAi, Flag/CA-βcat, and the TOP-nRFP reporter into the chick spinal cord, and assessed the expression of Pax3, Pax7, Pax6, and the TOP-nRFP reporter. Here we found that ectopic expression of CA-βcat is sufficient to restore expression of Pax3, Pax7, and Pax6 in the absence of Daam2 (Figure 4F-4I, 4P and 4Q). Importantly, rescue of dorsal progenitor markers by CA-βcat is correlated with the restoration of canonical Wnt signaling in the dorsal regions of the spinal cord (Figure 4J and 4R), indicating that rescue of dorsal markers is the result of restored canonical Wnt signaling. These data demonstrate that the loss of canonical Wnt signaling is responsible for the loss of dorsal markers in the absence of Daam2.
Figure 4. β-catenin rescues loss of dorsal markers in the absence of Daam2.
(A–E) Knockdown of Daam2 results in loss of dorsal markers and Wnt-activity; shown to facilitate comparison. (F–J) Ectopic expression of CA-βcatenin (F) in the absence of Daam2 (G) restores expression of dorsal markers and Wnt-activity (H-J; arrows). (K–O) Ectopic expression of Wnt1/3a (K) does not restore dorsal marker expression or Wnt-activity (M-O; arrows) in the absence of Daam2 (L). (P–R) Corresponding quantification of the phenotypes demonstrated in the images. Quantification in P,Q was performed by comparing the number of DAPI+ cells expressing a given marker between the control side (con) and the electroporated side (Ep) of the neural tube. (R) Fluorescent intensity values for nRFP and the GFP standardization were acquired and the normalized TOP-nRFP value, relative to GFP. Quantification for each manipulation is derived from at least 6 different embryos, 10 sections per embryo. Images are from an adjacent series of sections. In F–R, “D2i” denotes Daam2-shRNAi. “Ep” denotes electroporated side of neural tube, “Con” control side. Error bars indicate SEM.
Our observation that CA-βcat is sufficient to rescue the phenotypes manifest in the absence of Daam2 indicate that Daam2 functions upstream of β-catenin in the canonical Wnt pathway. We next sought to determine where in the Wnt pathway Daam2 is acting. Given the complexities of Wnt signaling, we sought to address this question by determining whether overexpression of Wnt ligands can rescue the loss of Daam2. It has been previously established that combined overexpression of Wnt1 and Wnt3a results in ventral expansion of Wnt activity in the chick spinal cord (Alvarez-Medina et al., 2008). We confirmed these observations (Figure 5G and 5O) and used this paradigm to model Wnt ligand activation of canonical Wnt signaling in the spinal cord. To determine whether Wnt ligand activation is sufficient to rescue the loss of Wnt signaling in the absence of Daam2, we co-electroporated Wnt1/3a, Daam2-shRNAi, and the TOP-nRFP reporter into the chick spinal cord. Strikingly, activation of Wnt signaling via Wnt1/3a is not sufficient to restore dorsal progenitor markers or Wnt activity in the absence of Daam2 (Figure 4K-4O). Collectively, these data establish a role for Daam2 in the canonical Wnt pathway, downstream of Wnt ligands and upstream of β-catenin, and implicate it as a critical mediator of Wnt signaling in the embryonic spinal cord.
Figure 5. Daam2 potentiates ligand mediated, canonical Wnt activity.
(A–H) Ectopic expression of transgenes; (A–B) empty vector and Daam2, (C–D) Wnt1 and Wnt1/Daam2, (E–F) Wnt3a and Wnt3a/Daam2, and (G–H) Wnt1/3a and Wnta1/3a–Daam2. (I–P) TOP-nRFP activity, ventral expansion of Wnt-activity in presence of Daam2 is denoted by arrow in L, N, and P. (Q–X) Co-expression of ventral marker Nkx2.2 and TOP-nRFP. (Y-FF) In situ hybridization analysis of cyclin D1 expression. (GG) Quantification of the number of cells co-expressing Nkx2.2 and the TOP-nRFP reporter. (HH-II) Single-cell quantification from dissociated spinal cord of the relative number of cells expressing TOP-nRFP (HH) and TOP-nRFP/Nkx2.2 (II). See Figure S4 for Pax6/TOP-nRFP images and quantification. Quantification for each manipulation is derived from at least 6 different embryos, 10 sections per embryo. Images are from an adjacent series of sections. “Ep” denotes electroporated side of neural tube, “Con” control side. Error bars indicate SEM. Please also see Figure S4.
Daam2 potentiates canonical Wnt signaling
Next we sought to determine whether overexpression of Daam2 is sufficient to initiate Wnt signaling. In these studies we electroporated myc-tagged mouse Daam2 and the TOP-nRFP reporter into the chick spinal cord, harvested embryos at E4, and assessed the expression of progenitor markers and reporter activity. Titration of TOP-nRFP reporter sensitivity in vivo was used to determine the optimal amount of reporter to include in our electroporation experiments (Figure S4HH; see Experimental Procedures). These studies revealed that overexpression of Daam2 did not affect the expression of dorsal markers or TOP-nRFP reporter activity, indicating that it is not sufficient to initiate Wnt signaling (Figure 5J and 5R; Figure S4L and S4V).
Our epistasis tests placed Daam2 function downstream of Wnt1 and Wnt3a (Figure 4K-4O), therefore we next examined whether Daam2 can potentiate Wnt-signaling in the presence of these canonical Wnt ligands. Here we co-electroporated myc-Daam2, TOP-nRFP reporter, and HA-tagged versions of Wnt1, Wnt3a, or a combination of Wnt1 and Wnt3a (Wnt1/3a). Enhanced Wnt signaling was assessed by quantifying the number of TOP-nRFP-expressing cells in medial and ventral domains that normally do not demonstrate reporter activity at E4 (Pax6, Nkx2.2; see Figure 3A-3C). This form of analysis was possible because normal patterning is not disrupted in these experiments (Figure S4). Co-expression of Daam2 with Wnt1 resulted in the ventral expansion of reporter activity, compared to the Daam2-only or the Wnt1-only controls (Figure 5Q-5T and 5GG; Figure S4). Additionally, co-expression of Daam2 with Wnt3a or Wnt1/3a resulted in enhanced reporter activity in the ventral most-domain, Nkx2.2, compared to Wnt3a- or Wnt1/3a-alone (Figure 5U-5X and 5GG; Figure S4). We supplemented these in situ studies with single-cell analysis of dissociated spinal cord on a subset of these experiments and found that combined Daam2 and Wnt1 expression resulted in an 80% increase in the number of TOP-nRFP-expressing cells and a four- and three- fold increase in the number of Nkx2.2- and Pax6- TOP-nRFP-expressing cells, respectively (Figure 5HH-5II; Figure S4). Further analysis of known targets of the canonical Wnt-pathway, cyclin D, revealed that their expression is similarly increased in the presence of combined Daam2 and Wnt1 and/or Wnt3a (Figure 5Y-5FF; Figure S4) (Megason and McMahon, 2002; Alvarez-Medina et al., 2008). Lastly, we co-electroporated Daam2 and the TOP-nRFP reporter with Wnt4, which primarily activates non-canonical pathways, and did not observe an obvious effect on TOP-nRFP activity, indicating that Daam2 cannot non-specifically activate canonical Wnt signaling in response to inappropriate Wnts (Figure 5GG; Figure S4) (Lyuksyutova et al., 2003; Chang et al., 2007). This suggests that Daam2 is likely to act downstream of Wnt receptor complexes, and may contribute to the maintenance or amplification, but not initiation, of Wnt activity.
Daam2 physically associates with Dvl3 via the DIX domain
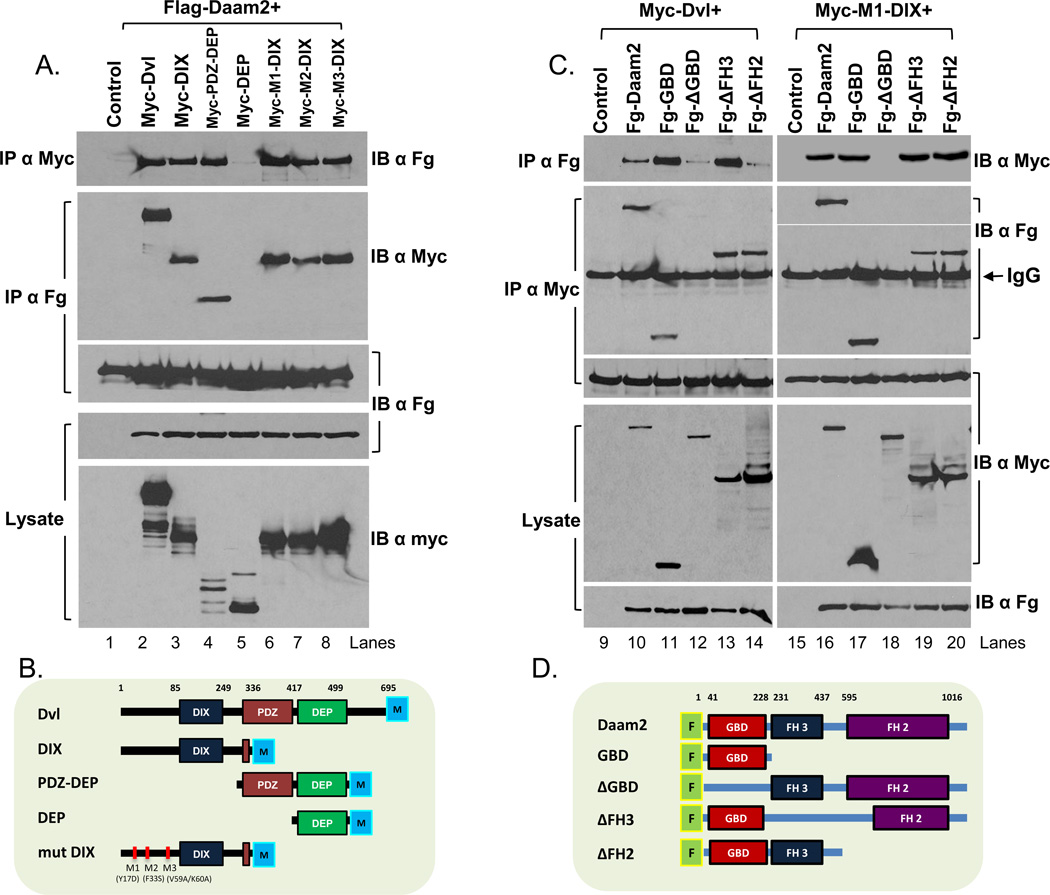
Our gene manipulation studies indicate that Daam2 plays a critical role in canonical Wnt signaling, yet do not address its molecular relationships with proteins associated with Wnt signal transduction. Previously it was shown that Daam1 directly interacts with Dishevelled (Dvl) during Xenopus gastrulation and given the key role of Dvl in canonical Wnt signaling, we reasoned that the effects of Daam2 manipulation are due in part to a direct relationship with Dvl (Habas et al., 2001). Previous studies revealed that Dvl3 is expressed in dorsal regions of the developing chick spinal cord during early embyrogenesis, therefore we next determined whether Daam2 can physically associate with Dvl3 (Gray et al., 2009). To this end, we co-transfected HEK 293 cells with tagged versions of Dvl3 and Daam2. Extracts from these cells were immunoprecipitated (IP) with antibodies to the Dvl3 tag and immunoblotted with antibodies to the Daam2 tag, and vice versa. The results of these IP-Westerns indicate that Daam2 and Dvl3 physically associate (Figure 6A-lane2 and 6C-lane10). Given that the DIX domain of Dvl is necessary for canonical Wnt-signaling, we next examined whether Daam2 is capable of associating with the DIX domain of Dvl3 (Kishida et al., 1999; Schwarz-Romond et al., 2007; Zeng et al., 2008). We performed co-transfection of HEK 293 cells with tagged versions of the DIX domain and Daam2, performed IP-Westerns with the appropriate antibodies, and found that Daam2 is capable of associating with the DIX domain of Dvl (Figure 6A-lane3 and 6B). One consideration when interpreting these results is that the polymerization activity of the DIX domain can result in spurious precipitations. Therefore to confirm the association between DIX and Daam2, we tested the ability of Daam2 to immunoprecipitate with polymerization-defective DIX mutants (Figure 6B) (Wong et al., 2003; Schwarz-Romond et al., 2007). We generated and tested three polymerization defective DIX point mutants for association with Daam2 using the methods described above and found that all three of these DIX mutants are capable of associating with Daam2 (Figure 6A-lanes6-8).
Figure 6. Daam2-GBD domain associates with Dvl-DIX domain.
(A) Immunoblot (IB) of IP extracts from 293 cells expressing Flag-Daam2 and various Myc-Dvl3 mutants (B) Diagram depicting various Dvl mutant used in co-IP studies. Co-IP of Daam2/Dvl and Daam2/DIX is demonstrated in lanes 2 and 3. Daam2 co-IP with DIX polymerization mutants is demonstrated in lanes 6–8. Control (Lane1) is a transfection with Flag-Daam2 and IP with αMyc. (C) Immunoblot (IB) of IP extracts from 293 cells expressing Myc-Dvl or Myc-M1-DIX and various Flag-Daam2 mutants. (D) Diagram depicting various Daam2 mutants used in co-IP studies. Daam2-GBD association with Dvl is demonstrated by co-IP in lane 11 and requirement for Daam2-GBD for co-IP with Dvl is demonstrated in lane 12. Control (lane9,15) is transfection with Myc-Dvl or Myc-DIX and IP with αFlag.
Next we sought to identify the Daam2 domain that associates with Dvl-DIX. To this end we generated a series of Daam2 mutants (Figure 6D) and tested their ability to immunoprecipitate with Dvl3. These studies revealed that the GBD-domain of Daam2 is required for its association with Dvl3 (Figure 6C-lanes 12-14) and that the GBD-domain alone is capable of associating with Dvl3 (Figure 6C-lane 11). Next we tested the ability of Daam2-∆GBD to associate with DIX-polymerization mutants and found that it is not capable of associating with Dvl-DIX domain (Figure 6C-lane18; Figure S5). Consistent with these results, the Daam2-∆FH2 and Daam2-∆FH3 mutants, which contain the GBD-domain, and the Daam2-GBD domain alone are capable of binding the DIX-domain (Figure 6C-lane 17,19-20; Figure S5). In sum, these biochemical studies establish that Daam2 can interact with Dvl3 through an association between the Dvl-DIX and the Daam2-GBD domains.
Role of Daam2 in Wnt signaling is dependent upon DIX domain of Dvl3
Having established a biochemical link between Daam2 and Dvl3, we next investigated their functional relationship in the embryonic chick spinal cord. In other systems excess levels of Dvl can induce canonical Wnt activity, we therefore reasoned that Daam2 functions to potentiate Dvl activation of Wnt activity (Liu et al., 2001; Rothbacher et al., 2000; Wehrli et al., 2000). In these studies we co-electroporated Dvl3, Daam2, and the TOP-nRFP reporter and used Wnt activity in ventral and medial domains (Nkx2.2 and Pax6) as our index of Wnt induction. We found that expression of Daam2 increases Dvl3-mediated ventral expansion of Wnt-activity (Figure 7G-7I, 7M-7O and 7S; Figure S6C). Given the importance of the Dvl-DIX domain in canonical Wnt-signaling and that our biochemical studies indicate Daam2 associates with the DIX domain via its GBD domain, we next tested whether Daam2 potentiation of Dvl-mediated Wnt induction is dependent upon these associations. First we co-electroporated a Dvl3 mutant that lacks the DIX domain (Dvl3-∆DIX), Daam2, and TOP-nRFP reporter and found that Daam2 is not capable of inducing ventral Wnt activity in the presence of the Dvl3-∆DIX (Figure 7J, 7P and 7S). Next we performed the converse experiment by electroporating Dvl3, Daam2-∆6GBD, and the TOP-nRFP and found that Daam2-∆GBD is not able to induce ventral Wnt-induction activity (Figure 7L, 7R vs 7I, 7O; 7S; Figure S6).
Figure 7. Daam2 function is reliant upon its association with Dvl3.
(A–R) Daam2 potentiates Dvl-activation of Wnt activity via their GBD and DIX domains. (A–F) Ectopic expression of transgenes; (G–L) TOP-nRFP activity, ventral expansion of Wnt activity is denoted by arrows. (M–R) Co-expression of ventral marker Nkx2.2 and TOP-nRFP. (S) Quantification of the number of cells co-expressing Nkx2.2 and the TOP-nRFP reporter. (T-AA) Expression of Dvl3 rescues dorsal markers and Wnt-activity in the absence of Daam2, while expression of Dvl3-∆DIX mutant does not. (BB-II) M1-Dvl3 and Daam2-DGBD do not rescue dorsal markers and Wnt-activity in the absence of Daam2. (JJ-KK) Quantification of the phenotypes described in T-II. Corresponding quantification of the phenotypes demonstrated in the images. Quantification in JJ was performed by comparing the number of DAPI+ cells expressing a given marker between the control side and the electroporated side of the neural tube. In II, the TOP-nRFP value for each condition was normalized to the GFP control and compared across samples. In T-KK, “D2i” denotes Daam2-shRNAi. “Ep” denotes electroporated side of neural tube, “Con” control side. Error bars indicate SEM. Please also see Figure S6.
Because Dvl3 can induce Wnt activity on its own and has a direct association with Daam2, we next assessed whether it can rescue the Daam2-shRNAi phenotype. Here we co-electroporated the Daam2-shRNAi, TOP-nRFP-reporter and either Dvl3 or Dvl3-∆DIX and assessed expression of dorsal progenitor markers and Wnt-activity. These studies revealed that Dvl3 can rescue the Daam2-shRNAi phenotype (Figure 7V, 7W, 7JJ and 7KK; Figure S6) and does so via the DIX domain, as the Dvl3-ADIX did not restore dorsal marker expression or Wnt activity (Figure 7Z, 7AA, 7JJ and 7KK; Figure S6). These studies suggest that Daam2 function is linked to the DIX domain, therefore we next examined whether Daam2-∆GBD, which does not bind DIX (Figure 6C), is capable of restoring canonical Wnt-signaling in the absence of Daam2. Electroporation of Daam2-shRNAi with Daam2-∆GBD and the TOP-nRFP reporter did not rescue dorsal marker expression or Wnt activity (Figure 7HH, 7II, 7JJ and 7KK; Figure S6). Taken together, our structure/function analysis demonstrates that the role of Daam2 in canonical Wnt signaling is linked to the biochemical association between its GBD domain and Dvl-DIX.
The ability of Dvl to enhance Wnt-signaling is dependent upon the polymerization activity of its DIX domain; therefore we next tested whether Daam2 function is linked to Dvl polymerization. To examine this relationship we generated a mutant version of Dvl3 (M1-Dvl3) that lacks polymerization activity and found that Daam2 is not capable of potentiating Wnt-signaling in the presence of M1-Dvl3 (Figure 7K, 7Q and 7S). Moreover, the converse experiments that examine the ability of M1-Dvl3 to restore Wnt-activity in the absence of Daam2, revealed that it is not able to rescue dorsal markers or Wnt-signaling in the absence of Daam2 (Figure 7DD, 7EE, 7JJ and 7KK; Figure S6). These data indicate that Daam2 function in canonical Wnt-signaling requires the polymerization activity of Dvl3 and further support our findings that link Daam2 function to the Dvl-DIX domain.
Daam2 stabilizes Dvl3/Axin2 binding
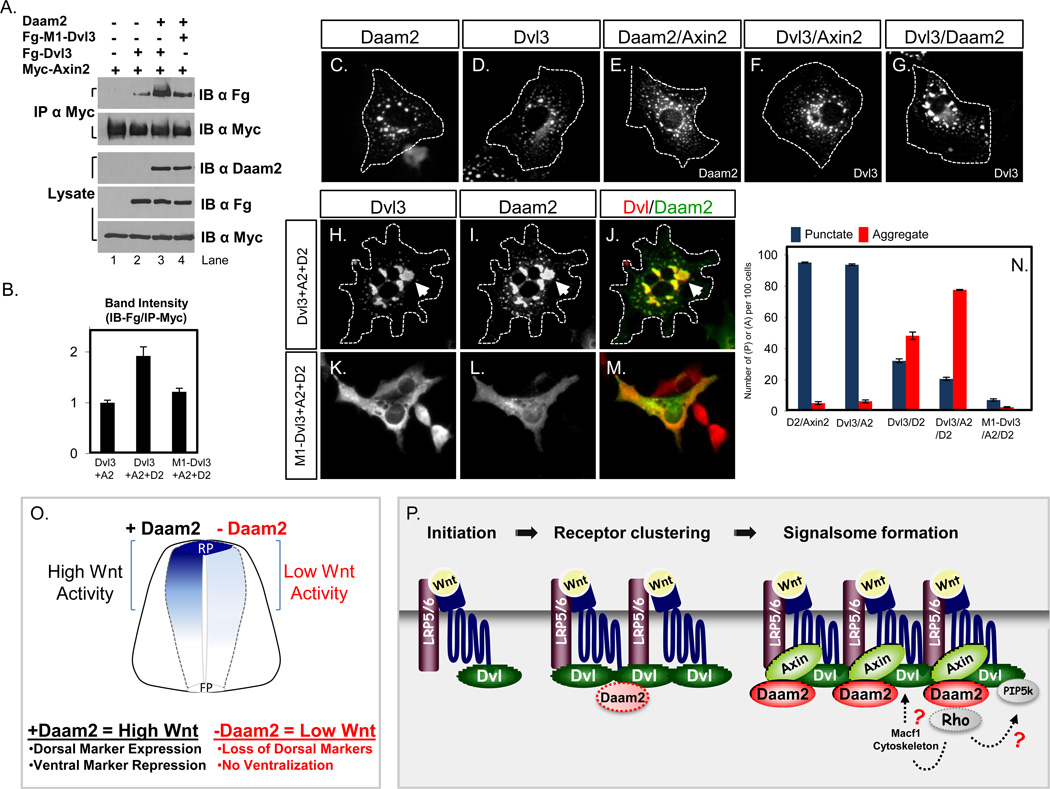
The ability of Dvl proteins to promote canonical Wnt signaling is dependent upon their interaction with Axin2, which is mediated via the DIX domain (Kishida et al., 1999; Zeng et al., 2008). Several reports indicate clustering of Dvl/Axin complexes, via the polymerization of Dvl-DIX, into signalsomes promotes Wnt signaling and have postulated that this mechanism can compensate for the low-binding affinity between Axin and Dvl. (Bilic et al., 2007; MacDonald et al., 2009; Schwarz-Romond, 2007) Our studies indicate excess Dvl3 can rescue Daam2-shRNAi phenotypes in a DIX-domain dependent manner, suggesting that aggregation of Dvl3 can mimic the role of Daam2 in canonical Wnt signaling.Given these observations we reasoned that under normal physiological conditions, without excess Dvl3, Daam2 facilitates canonical Wnt signaling by stabilizing Dvl/Axin binding. To test this hypothesis, we first examined whether Daam2 can associate with Axin2 and other components of the signalsome complex. These studies revealed that Daam2 is capable of associating with Axin2 and Fzd1, but not LRP6 (Figure S5).
Next, we tested whether Daam2 can stabilize Dvl3 and Axin2 binding by measuring the extent of Dvl3/Axin2 co-IP with- and without- Daam2. In these studies we co-transfected HEK 293 cells with Dvl3/Axin2 or with Daam2/Dvl3/Axin2, performed IP with the tags to Axin2 (α-myc) and performed immunoblotting with the tag to Dvl3 (α-Flag). As indicated in Figure 8A-8B, the amount of Dvl3 that co-IPs with Axin2 is increased in the presence of Daam2 (lane 2 vs lane 3), indicating that Daam2 enhances Dvl3/Axin2 binding, supporting our hypothesis that Daam2 stabilizes the Dvl/Axin interaction. Moreover, consistent with our functional studies, stabilization by Daam2 requires the polymerization activity of Dvl, as Daam2 does not enhance M1-Dvl3/Axin interaction (lane 3 vs lane 4). As a first step in examining this phenomenon in vivo, we took advantage of previous observations demonstrating that Dvl and Axin2 form intracellular protein assemblies when overexpressed in COS-7 cells (Schwarz-Romond, 2007). These assemblies have been correlated with stabilized Dvl/Axin complexes (Bilic et al., 2007; Schwarz-Romond, 2007). In these studies we expressed Dvl3 and Axin2 with- and without-Daam2 in COS-7 cells and assessed the extent of protein assembly by examining the formation of intracellular punctate structures containing ectopic Dvl3 or Axin2 protein. Consistent with previous studies, our analysis revealed that expression of Dvl3/Axin2 resulted in the formation of discrete punctate structures, as did Daam2 (Figure 8C-8G and 8N). Combined expression of Daam2/Dvl3/Axin2, however, resulted in the formation of large, aggregated intracellular structures that reflect enhanced clustering of punctate protein assemblies (Figure 8H-8J and 8N; arrows). Consistent with our other studies combined expression with M1-Dvl3 did not result in aggregation (Figure 8K-8N). Collectively, these data demonstrate that Daam2 promotes intracellular Dvl3/Axin2 protein assembly and coupled with our IP studies, indicate that Daam2 enhances Dvl3/Axin2 interaction.
Figure 8. Daam2 enhances Dvl3/Axin2 binding and intracellular assembly.
(A) IP extracts from 293 cells expressing tagged versions Daam2, Dvl3, M1-Dvl3 or Axin2. Enhanced Dvl3/Axin2 co-IP in the presence is Daam2 is demonstrated by comparing the αFlag-IB band in Lane 2 and Lane3. (B) Quantification of band intensity in indicated lanes was performed using Image J. The normalized IB-Flag/IB-Myc values are represented in the graph. (C–M) Expression of Daam2 (C), Dvl3 (D), Axin2/Daam2 (E), Axin2/Dvl3 (F) results in intracellular punctate staining; Daam2/Dvl3 results in some aggregation (G). (H-J; arrows) Co-expression of Daam2 with Dvl3 and Axin2 results in aggregation of protein assemblies. (K–M) Co-expression of Daam2 with M1-Dvl3/Axin2 does not produce aggregated protein assemblies or punctate staining. Dashed lines denote the cell membrane. (N) Quantification of the relative number of cells per condition demonstrating intracellular punctate (P) or aggregrated (A) phenotype. For each condition 100 cells were counted and the experiments were performed three times in duplicate. Error bars indicate SEM. Please also see supplemental figure S6. (O) Summary of Daam2 knockdown phenotypes in the dorsal embryonic spinal cord. Wnt activity is depicted by blue shading in VZ; while knockdown of Daam2 results in a complete loss of TOP-nRFP activity, the lack of ventralized phenotypes in dorsal regions, suggests that there is residual, low level Wnt activity present and is reflected by the light blue shading in the VZ (see discussion). (P) Depiction of the role of Daam2 in signalsome formation and its molecular interactions with key components of the Wnt receptor complex. The dashed Daam2 image reflects a possible role in receptor clustering (see discussion). Our studies indicate that the Daam2-GBD domain mediates its role in Wnt-receptor complex formation, thus implicating Rho-GTPases and its associated biology in this process. This is reflected in the dashed lines and the speculative relationships with PIP5K and Macf1. For clarity, β-catenin, GS3K, and other proteins have been omitted.
Discussion
Here we found that Daam2 plays a key role in dorsal pattern formation and established that this role in patterning is directly linked to its requirement for canonical Wnt signaling. Subsequent analysis revealed that Daam2 functions to amplify Wnt signaling, by potentiating Wnt ligand induced activity. These findings implicate Daam2 as a key intracellular modulator of Wnt signal transduction during embryonic spinal cord development. Structure/function studies revealed that Daam2 binds the DIX domain of Dvl3 via its GBD domain and that its role in Wnt signaling is mediated by this relationship. Biochemical examination into the nature of these interactions found that Daam2 increases Dvl3/Axin2 binding and intracellular clustering, supporting a model whereby it promotes canonical Wnt signaling through stabilization of Dvl/Axin complexes (Figure 8P).
Daam2 plays a key role in spinal cord development
Our gene manipulation studies in the embryonic chick spinal cord indicate that Daam2 expression is necessary for dorsal identities. Subsequent analysis revealed that the loss of dorsal markers is directly linked to canonical Wnt signaling, as Wnt activity is drastically reduced in the absence of Daam2. Moreover, we found that CA-βcat rescues dorsal identities and loss of Wnt activity in the absence of Daam2, indicating that the requirement of Daam2 for dorsal identities is mediated via canonical Wnt signaling. Collectively, these data implicate Daam2 as a critical factor in specification of dorsal populations and canonical Wnt signaling in the developing spinal cord.
Overexpression studies indicate that Daam2 alone is not sufficient to activate Wnt activity or expand dorsal identities into ventral regions, however when combined with Wnt1 or Wnt3a, results in increased Wnt activation. This observation indicates that Daam2 is not capable of initiating Wnt signaling on its own, but rather potentiates the existing signal, suggesting that it plays a role in the maintenance or amplification of signal transduction. This notion is supported by our epistasis analysis, where Daam2-knockdown phenotypes are not rescued by Wnt ligands, but are rescued by CA-βcat, placing Daam2 function downstream of the ligand/receptor complex and upstream of the transcriptional component of the canonical Wnt pathway.
Our studies on Daam2 indicate that it functions as a key accessory protein that modulates the activity of the core, conserved components of the Wnt-receptor complex and its associated signal transduction processes. Recently, studies in Xenopus and during mouse gastrulation have identified caprin2 and MACF1, respectively, as accessory proteins that similarly modulate the activity of Wnt signaling by directly influencing the dynamics and constituency of the signalsome complex (Chen, 2006; Ding et al., 2008). It will be important to determine whether these proteins play tissue- or species-specific roles in Wnt signaling or if a functional relationship exists between Daam2, Macf1, and caprin2. Globally, these findings suggest that for Wnt signaling there are tissue-or species-specific modifiers that profoundly influence the core signal transduction mechanism, implying the presence of another layer of regulatory complexity that can ultimately alter signal interpretation and cellular output.
Daam2 and Wnts: New insight into role of Wnt signaling during pattern formation
In the course of analyzing our Daam2-knockdown phenotype, we noticed that while dorsal markers are lost, these regions do not become “ventralized” and concomitantly express ventral markers. One possible explanation is that while there is a clear reduction in canonical Wnt activity in the absence of Daam2, there could be residual Wnt activity that is below the threshold detectable by the TOP-nRFP-reporter and not sufficient to maintain dorsal identities, but is still capable of repressing ventral identities. Wnt regulation of Gli3 has been linked to repression of ventral identities in the dorsal spinal (Alvarez-Medina et al., 2008).Analysis of Gli3-expression in the absence of Daam2 revealed that while its expression is significantly reduced (Figure S3), it is still present in dorsal regions and is likely responsible for repression of ventral identities. Moreover, that the expression of Wnt ligands and the transcriptional effectors of the Wnt pathway are not affected by a loss of Daam2 also supports the possibility that low-levels of Wnt activity are likely present (Figure S3). Collectively, these data suggest a concentration-dependent uncoupling of ventral repression and dorsal induction by Wnt signaling, where low-levels of Wnt signaling are sufficient to repress ventral identities, while high-levels of Wnt activity are necessary to maintain dorsal identities (Figure 8O). An analogous relationship for Shh has been established in the ventral spinal cord, where very low-levels of Shh are sufficient to repress dorsal identities, and higher-levels of Shh are necessary for induction of ventral identities (Briscoe, 2000; Ericson et al., 1997).
Canonical Wnt signaling has been linked to progenitor cell proliferation and, more recently, pattern formation in the developing spinal cord, though its precise role in patterning remains unclear (Alvarez-Medina et al., 2008; Dickinson, 1994; Megason and McMahon, 2002; Muroyama et al., 2002). Studies that have targeted transcriptional effectors using dominant negative approaches resulted in repression of dorsal identities coupled with ventralized phenotypes (Alvarez-Medina et al., 2008). While these studies have provided important insight into the role of Wnt signaling in pattern formation, potential ectopic effects of dominant negative transcription factors confound them. In contrast, deletion of both Wnt1 and Wnt3a ligands resulted in a specific loss of dorsal neuron populations without expansion of ventral neuron populations or general effects on patterning (Ikeya et al., 1997; Muroyama et al., 2002). Whereas our studies, which modulate the expression of a protein involved in the transduction of Wnt signaling, resulted in a partial recapitulation of the dominant negative phenotypes (ie. loss of dorsal identities). The differences in phenotypes witnessed by these contrasting approaches illustrates the importance of using finely-tuned methods to dissect the various roles of Wnt signaling in CNS development, as our studies on Daam2 suggest, like other morphogens, that dorsal and ventral populations have a graded response to Wnt signals. Given the link between morphogen concentration, patterning, and cell fate, it will be important to rigorously dissect how different concentrations of canonical Wnt ligands or manipulation of the core signal transduction mechanisms influence spinal cord patterning and proliferation.
Daam2 stabilizes Wnt receptor signalsome structures
Assembly of signalsome structures involves clustering of Dvl/receptor complexes, which subsequently results in recruitment of Axin, phosphorylation of LRP, and activation of Wnt signaling (Figure 8P) (Bilic et al., 2007; MacDonald et al., 2009). The DIX domain of Dvl and Axin plays a central role in the assembly of signalsome complexes, as it is necessary for Dvl polymerization that results in clustering and mediates the interaction between Dvl and Axin that drives Wnt signal transduction and signalsome formation (Bilic et al., 2007; Schwarz-Romond et al., 2007; Zeng et al., 2008). Our findings with Daam2 point to a model where its interactions with Dvl3 and Axin2 serve to sustain signalsome integrity and concomitant Wnt activity. Another possibility to consider is that Daam2 also contributes to Dvl/receptor clustering, prior to Axin recruitment to the signalsome structure. Our observations that Dvl3 rescues Wnt signaling in the absence of Daam2 and Daam2 can promote intracellular Dvl3 protein assembly (Figure 8G and 8N) support such a role. However, our in vivo studies demonstrating that Daam2 function requires the polymerization activity of Dvl3 and that it is not sufficient to initiate Wnt activity, indicate it likely functions through an existing signalsome structure and therefore contributes to Wnt signaling through stabilization or maintenance of the signalsome (Figure 8P).
Our structure/function analysis revealed that the Daam2-GBD domain mediates Daam2 interaction with Dvl-DIX and function in canonical Wnt signaling. The GBD domain functions to bind and modulate the activity of small Rho-GTPases, which ultimately influence the dynamics of the actin cytoskeleton. Together, these observations implicate Rho-GTPases and actin cytoskeleton in the maintenance of Wnt-receptor complexes through Daam2-GBD and Dvl3-DIX association. Recently Macf1, a gene associated with actin cytoskeleton remodeling, was shown to participate in Wnt-receptor complex formation, though no direct association with Rho-GTPases was demonstrated (Chen, et al. 2006). Alternatively, while Rho-GTPases have not previously been associated with Wnt-receptor complex stability, they have been linked to the transport and membrane localization of PI4K and PIP5K, which function together to promote PIP2 production and Wnt-receptor clustering (MacDonald et al., 2009; Schlessinger et al., 2009). Interestingly, PIP5K can associate with Dvl, therefore it is possible that Daam2 functions to facilitate this interaction via Rho-GTPases and thus contributes to signalsome maintenance through recruitment of PIP5K to Dvl (Wei et al., 2002; Weernink et al., 2004; Yang et al., 2004; Schlessinger et al., 2009). It will be important to discern whether Daam2 functions through Rho-GTPases and the actin cytoskeleton or indirectly via recruitment of PIP5K to influence the stability Wnt-receptor complexes and signalsomes (Figure 8P).
Functional diversity within the Daam-family
Previous studies have demonstrated that Daam1 functions specifically in non-canonical Wnt signaling and does not contribute to canonical Wnt signaling (Habas et al., 2001). That Daam1 and Daam2 share ∼60% identity and conserved functional domains, suggests that they may have similar functions (Kida et al., 2004). Unexpectedly, we found that during spinal cord development, Daam2 plays a role in canonical Wnt signaling and does so through a direct interaction with the DIX domain of Dvl (Dvl-DIX), a key domain in canonical Wnt signal transduction. This is in contrast to Daam1, which does not interact with Dvl-DIX, suggesting that the molecular basis for this functional difference between Daam1 and Daam2 is the ability to bind the Dvl-DIX domain.
Our studies on Daam2 were performed in the embryonic chick spinal cord, in contrast to previous studies on Daam1, which was functionally analyzed during Xenopus gastrulation. Given these tissue and species differences, we cannot formally rule out the possibility that Daam1 may contribute to canonical Wnt signaling in the embryonic spinal cord. This possibility, however, is unlikely because Daam1 and Daam2 have non-overlapping expression patterns in the embryonic spinal cord, which suggests they have distinct roles, and there is no evidence of canonical Wnt activity in the mantle regions of spinal cord that express Daam1. Lastly, our studies do not address the role of Daam2 in non-canonical Wnt signaling. However, our Daam2 overexpression studies did not enhance the migration of neuronal progenitor populations (data not shown), suggesting that it does not affect the PCP and migration pathways linked to non-canonical Wnt signaling, though a more comprehensive study is necessary to fully examine this potential relationship.
Experimental Procedures
In Ovo Electroporation
Daam2 shRNAi and cDNA constructs were cloned into avian retrovirus RCAS(B) vector, pCS2+, pCIG, or pcDNA expression vectors. The constructs were electroporated unilaterally into the neural tube of stage-11 to stage-13 chick embryos as described (Deneen. et al. 2006). Embryos were collected 48 hrs later.
In vivo Top-nRFP reporter assay
To characterize the reporter sensitivity, chick embryos were electroporated as indicated (Figure S4) with different concentrations of reporter construct together with a GFP normalization control. Embryos were harvested after 48 hrs incubation in ovo and a GFP positive electroporated side was dissected and dissociate as a single cell level. The number of Top-nRFP reporter and GFP positive cell was counted from randomly selected 10 different areas (10 x magnifications) of each embryo. DAPI-positive cells from same regions were counted as controls. We generated titration curve to decipher the appropriate amount of reporter concentration for assay. All reporter assays were performed using at 0.5 ug/ul of Top-nRFP concentration. For the single cell analysis, the Pax6 or Nkx2.2 positive dissociated cells were detected by immunofluorescent staining. The quantification of the relative number of the Pax6 or Nkx2.2 and Top-nRFP double positive cell was performed by counting the relative number of DAPI-positive cells, and quantitative counting was measured by Image J software. The analysis of the intensity of the reporter fluorescent in chick sections was measured using the same setting (detector gain, amplifier offset/gain) under a constant power in every set of the experiments by AxioVision Rel 4.8 software. Images were obtained using Zeiss imager M2 microscope with ApoTome (Cal Zeiss).
In Situ Hybridization and Immunohistochemistry
Detection of mRNA and protein was performed on frozen chick embryos as described. Chick spinal cord was fixed in 4% paraformaldehyde, then cryoprotected with 20% sucrose for overnight. The following probes were used for in situ hybridization: cDaam2, cWnt3a, cβ-catenin, cTcf4, cGli3, BMP4. For the immunohistochemical analysis, the following antibodies were used; mouse anti-Pax7, anti-Pax3, anti-Pax6, anti-Nkx6.1, anti-Nkx2.2, anti-Isl1, anti-Lim1/2, anti-MNR2, anti-Lmx, rabbit anti-Daam2 (ABGENT), rabbit anti-active caspase3 (Chemicon), rabbit anti-Olig2 (gift of Dr. Ben Novitch), goat anti-Sox2 (Santa Cruz), mouse anti-Flag (Sigma), rat anti-HA (Sigma), rabbit anti-VSVG (Sigma) and rabbit anti-myc (Santa Cruz). Monoclonal mouse antibodies were obtained from the Developmental Studies Hybridoma Bank maintained at the University of Iowa.
Cell culture, Immunoprecipitaion and Western blotting
Human embryonic kidney cells (HEK293) and COS-7 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum. The cells were transfected with the expression constructs by calcium phosphate methods. The transfected cells were harvested after 2 days in a lysis buffer containing 100mM NaCl, 25mM Hepes pH 7.5, 5mM TritonX-100, 2mM KCl. The cell lysate was with incubated overnight with agarose bead-conjugated antibodies to myc, Flag and VSVG. The samples were subjected to SDS-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (Hybond-ECL, Amersham), and incubated with either anti-myc or Flag antibodies.The enhanced chemiluminescence reaction (Santa Cruz) was used to visualize the reaction.
Measurements and statistical analyses
All chick experiments analyzed were from 8 sections per at least 5–10 embryos each. The results described immunoprecipitaion and immunostaining were replicated in three independent experiments. All quantitative data are expressed as the mean±s.e.m.
Highlights.
Daam2 is required for dorsal progenitor identities in the developing spinal cord
Daam2 functions in dorsal patterning through the canonical Wnt pathway.
Daam2 stabilizes Dvl3/Axin2 binding and enhances assembly of Dvl3/Axin2 complexes
Supplementary Material
Acknowledgements
We would like to thank Terry Yamaguchi for the Daam2 cDNA, Andy Groves for the TOP-nRFP reporter, Zhen-Ge Luo for the Dvl constructs, Hoang Nguyen for the DN-Tcf3 construct, and Steve Fancy for the Axin2 cDNA. We appreciate the manuscript review by Andy Groves, Hoang Nguyen, and Margaret Goodell. This work was supported by funding from the V Foundation for Cancer Research (BD) and the National Institutes of Health R01-NS071153 (BD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
See Supplemental Experimental Procedures for construct and cloning details.
References
- Alberts AS. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J Biol Chem. 2001;276:2824–2830. doi: 10.1074/jbc.M006205200. [DOI] [PubMed] [Google Scholar]
- Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Marti E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development. 2008;135:237–247. doi: 10.1242/dev.012054. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science. 2007;316:1619–1622. doi: 10.1126/science.1137065. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. A Homeodomain Protein Cose Specifies Progentior Cell Identity and Neuronal Fate in the Ventral Neural Tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Chang J, Sonoyama W, Wang Z, Jin Q, Zhang C, Krebsbach PH, Giannobile W, Shi S, Wang CY. Noncanonical Wnt-4 signaling enhances bone regeneration of mesenchymal stem cells in craniofacial defects through activation of p38 MAPK. J Biol Chem. 2007;282:30938–30948. doi: 10.1074/jbc.M702391200. [DOI] [PubMed] [Google Scholar]
- Chen H-J, Lin C-M, Lin C-S, Olle RP, Leung CL, Liem R. The role of microtubule actin cross-linking factor 1 (MACF1) in the Wnt-signaling pathway. Genes &Dev. 2006;20:1933–1945. doi: 10.1101/gad.1411206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Control of roof plate formation by Lmx1a in the developing spinal cord. Development. 2004;131:2696–2705. doi: 10.1242/dev.01139. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. The Transcription Factor NFIA Controls the Onset of Gliogenesis in the Developing Spinal Cord. Neuron. 2006;52:953–968. doi: 10.1016/j.neuron.2006.11.019. [DOI] [PubMed] [Google Scholar]
- Dickinson M, Krumlauf R, McMahon AP. Evidence for a mitogenic effect of Wnt-1 in the developing mammalian central nervous system. Development. 1994;120:1453–1471. doi: 10.1242/dev.120.6.1453. [DOI] [PubMed] [Google Scholar]
- Ding Y, Xi Y, Chen T, Wang JY, Tao DL, Wu ZL, Li YP, Li C, Zeng R, Li L. Caprin-2 enhances canonical Wnt signaling through regulating LRP5/6 phosphorylation. J Cell Biol. 2008;182:865–872. doi: 10.1083/jcb.200803147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 Controls Progenitor Cell Identity and Neuronal Fate in Response to Graded Shh Signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, et al. Murine Dishevelled 3 Functions in Redundant Pathways with Dishevelled 1 and 2 in Normal Cardiac Outflow Tract, Cochlea, and Neural Tube Development. PLoS Genet. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham V, Khudyakov J, Ellis P, Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- Gray RS, Bayly RD, Green SA, Agarwala S, Lowe CJ, Wallingford JB. Diversification of the expression patterns and developmental functions of the dishevelled gene family during chordate evolution. Dev Dyn. 2009;238:2044–2057. doi: 10.1002/dvdy.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled Activation of Rho Regulates Vertebrate Gastrulation and Requires a Novel Formin Homology Protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Huangfu DAKV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Lee SMK, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Kida Y, Shiraishi T, Ogura T. Identification of chick and mouse Daam1 and Daam2 genes and their expression patterns in the central nervous system. Developmental Brain Research. 2004;153:143–150. doi: 10.1016/j.devbrainres.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–916. doi: 10.1038/35038097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korswagen HC. Canonical and non-canonical Wnt signaling pathways in Caenorhabditis elegans: variations on a common signaling theme. Bio Essays. 2002;24:801–810. doi: 10.1002/bies.10145. [DOI] [PubMed] [Google Scholar]
- Lassiter RNT, Dude CM, Reynolds SB, Winters NI, Baker CVH, Stark MR. Canonical Wnt signaling is required for ophthalmic trigeminal placode cell fate determination and maintenance. Developmental Biology. 2007;308:392–406. doi: 10.1016/j.ydbio.2007.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Shin SY, Chi C, Lee TH, Lee SJ. Formation and removal of alpha-synuclein aggregates in cells exposed to mitochondrial inhibitors. J. Bio Chem. 2002;277:5411–5417. doi: 10.1074/jbc.M105326200. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- Liu T, DeCostanzo AJ, Liu X, Wang H-y, Hallagan S, Moon RT, Malbon CC. G Protein Signaling from Activated Rat Frizzled-1 to the Œ≤-Catenin-Lef-Tcf Pathway. Science. 2001;292:1718–1722. doi: 10.1126/science.1060100. [DOI] [PubMed] [Google Scholar]
- Lupo G, Harris WA, Lewis KE. Mechanisms of ventral patterning in the vertebrate nervous system. Nat Rev Neurosci. 2006;7:103–114. doi: 10.1038/nrn1843. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–553. doi: 10.1101/gad.937102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery LA, Sutherland DJ. TGF-[beta] Family Signal Transduction in Drosophila Development: From Mad to Smads. Developmental Biology. 1999;210:251–268. doi: 10.1006/dbio.1999.9282. [DOI] [PubMed] [Google Scholar]
- Rothbacher U, Laurent MN, Deardorff MA, Klein PS, Cho KW, Fraser SE. Dishevelled phosphorylation, subcellular localization and multimerization regulate its role in early embryogenesis. EMBO J. 2000;19:1010–1022. doi: 10.1093/emboj/19.5.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger K, Hall A, Tolwinski N. Wnt signaling pathways meet Rho GTPases. Genes Dev. 2009;23:265–277. doi: 10.1101/gad.1760809. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol. 2007;14:484–492. doi: 10.1038/nsmb1247. [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Metcalfe C, Bienz M. Dynamic recruitment of axin by Dishevelled protein assemblies. J. Cell Science. 2007;120:2402–2412. doi: 10.1242/jcs.002956. [DOI] [PubMed] [Google Scholar]
- Smith JC. Hedgehog, the floor plate, and the zone of polarizing activity. Cell. 1994;76:193–196. doi: 10.1016/0092-8674(94)90325-5. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Villa P, Kaufmann SH, Earnshaw WC. Caspases and caspase inhibitors. Trends Biochem Sci. 1997;22:388–393. doi: 10.1016/s0968-0004(97)01107-9. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wang J, Hamblet NS, Mark S, Dickinson ME, Brinkman BC, Segil N, Fraser SE, Chen P, Wallingford JB, Wynshaw-Boris A. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development. 2006;133:1767–1778. doi: 10.1242/dev.02347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weernink PA, Meletiadis K, Hommeltenberg S, Hinz M, Ishihara H, Schmidt M, Jakobs KH. Activation of type I phosphatidylinositol 4-phosphate 5-kinase isoforms by the Rho GTPases, RhoA, Rac1, and Cdc42. J. Biol. Chem. 2004;279:7840–7849. doi: 10.1074/jbc.M312737200. [DOI] [PubMed] [Google Scholar]
- Wei YJ, Sun HQ, Yamamoto M, Wlodarski P, Kunii K, Martinez M, Barylko B, Albanesi JP, Yin HL. Type II phosphatidylinositol 4-kinase b is a cytosolic and peripheral membrane protein that is recruited to the plasmamembrane and activated by Rac-GTP. J. Biol Chem. 2002;277:46586–46593. doi: 10.1074/jbc.M206860200. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407:527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Wong HC, Bourdelas A, Krauss A, Lee HJ, Shao Y, Wu D, Mlodzik M, Shi DL, Zheng J. Direct binding of the PDZ domain of Dishevelled to a conserved internal sequence in the C-terminal region of Frizzled. Mol Cell. 2003;12:1251–1260. doi: 10.1016/s1097-2765(03)00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SA, Carpenter CL, Abrams CS. Rho and Rho-kinase mediate thrombin-induced phosphatidylinositol 4-phosphate 5-kinase trafficking in platelets. J. Biol Chem. 2004;279:42331–42336. doi: 10.1074/jbc.M404335200. [DOI] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, et al. Initiation of Wnt signaling: control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development. 2008;135:367–375. doi: 10.1242/dev.013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.