Abstract
Background
The aim of this study was to determine the effect of vagus nerve stimulation (VNS) on infarct size after transient and after permanent focal cerebral ischemia in rats and to test the hypothesis that VNS-induced neuroprotection is due to changes in cerebral blood flow.
Methods
Ischemia was produced by either temporary proximal middle cerebral artery occlusion (TMCAO) or permanent distal middle cerebral artery occlusion (PMCAO). Stimulating electrodes were implanted on the cervical part of the right vagus nerve, and electrical stimulation was initiated 30 minutes after the induction of ischemia and delivered for 30 seconds every 5 minutes for 1 hour. All the procedures were duplicated but no stimulus was delivered in control groups. Cerebral blood flow in the MCA territory was continuously monitored with laser speckle contrast imaging. A neurological evaluation was undertaken after 24 hours of ischemia, and animals were euthanized and neuronal damage evaluated.
Results
Ischemic lesion volume was smaller in VNS-treated animals in both the temporary and permanent ischemic groups (p<0.01). VNS-treated animals in TMCAO had better functional scores at 24 h as compared with control animals (p<0.01), but there were no statistically significant differences in the neurobehavioral scores in PMCAO (p=0.089). CBF changes in the MCA territory during ischemia did not differ between the VNS-treated animals and control animals in either group.
Conclusion
VNS offers neuroprotection against stroke in both temporary and permanent ischemia. Although the precise mechanism of this effect remains to be determined, alterations in cerebral blood flow do not appear to play a role. VNS could readily be translated to clinical practice.
Keywords: vagus nerve stimulation, cerebral ischemia, photothrombosis, neuroprotection
Introduction
Vagus nerve stimulation (VNS) was first used in 1988 to treat drug-resistant epilepsy. Since its introduction, clinical studies involving approximately 1000 patients with epilepsy have been completed 1 showing efficacy in treating epilepsy in both adults and children. VNS was approved in Europe in 1994 and in the United States and Canada in 1997 for treatment-resistant epilepsy, and a number of clinical studies 2–4 as well as studies in animal models of epilepsy 5–7 have demonstrated its benefit. After the success of VNS therapy in epilepsy, the technique has been applied to a wide variety of disorders, including depresssion, Alzheimer's disease, migraine, and multiple sclerosis 8–11. The results of these have been quite promising. There has even been some indication that VNS may be useful in the treament of eating disorders 12
The observation that VNS is beneficial in the treatment of epilepsy along with the similarity of the mechanisms of neuronal damage in epilepsy and cerebral ischemia 13 makes the study of VNS of interest as a potential treatment for stroke. Excitotoxicity plays a significant role in cerebral tissue damage in both cerebral ischemia and epilepsy 14–16, and strategies for reducing excitatory amino acids as treatment for cerebral ischemia have met with some success although clincial trials have been limited due to the neuropsychological adverse effects of most of the N-methyl-D-aspartate receptor antagonists 17. The general similarity between the cascade of deliterious events in epilepsy and cerebral ischemia has led to some pre-clinical investigations of VNS in global and focal brain ischemia. High current vagal nerve stimulation during transient forebrain ischemia in the gerbil reduces infarct volume 18, most likely due to the ability of VNS to attenuate extracellular glutamate that occurs during reperfusion, although in a follow-up study the VNS also attenuated the reactive hyperemia post reperfusion 19. If the vagus is stimulated intermittently during transient filament occlusion of the middle cerebral artery in the rat, infarct volume is reduced almost 50% and neurlogical deficits seen 24 hours after ischemia are attenuated 20. These previous studies involved transient ischemia, but since the vast majority of strokes are not eligible for tPA and since the median time for spontaneous reperfusion is days 21, 22, not just a few hours, it is important to examine VNS in a model of permanent ischemia more representative of that seen in humans. Additionally, the present study examined changes in cerebral blood flow during the period of ischemia as a possible mechanism of how VNS decreases the degree of the ischemic insult.
Materials and Methods
Surgical Preparation
Adult male Sprague-Dawley rats (260–330g, n=32) were anesthetized with isoflurane (4% for induction, 1.0–1.5% for maintainence) in a mixture of nitrous oxide and oxygen (7:3). Body temperature was monitored by a rectal probe and maintained at 37.5 ± 0.2°C with a heating pad (ATC1000, World Precision Instruments, Sarasota, FL). A polyethylene catheter (PE-50) was placed into the tail artery for the measurement of arterial blood pressure and for blood gas sampling and into the tail vein for infusion of drugs. Arterial blood pressure was monitored using a pressure transducer and recorded on a computer based recording system at 100 Hz (PowerLab, ADInstruments, Colorado Springs, CO). Laser speckle contrast imaging of CBF (see below) was done over a 6x6 mm area (centered 3 mm posterior and 4 mm lateral to the Bregma) uniformly thinned to translucency with a dental drill 23. The permanent (n=16) or temporary (n=16) middle cerebral artery occlusion procedures done on the animals are described below. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Temporary proximal middle cerebral artery occlusion (TMCAO)
Animals were prepared for temporary focal cerebral ischemia as described previously24, 25 but with slight modifications to permit optical imaging. Briefly, the right external and internal carotid arteries were exposed with the animal in a supine position and a 4.0 nylon monofilament suture (diameter = 0.39 mm) coated with silicone was inserted into the right common carotid artery and advanced about 9–10 mm into the right internal carotid artery. The animal was then turned prone and placed into the stereotaxic headholder; the scalp was reflected and the area for laser speckle contrast imaging thinned. In addition, at 1 mm anterior and 4 mm lateral to Bregma a small region of the skull was thinned and a laser Doppler flow probe (PeriFlux 4001; PeriMed) secured so as to monitor the changes in CBF while the filament was advanced into the internal carotid artery. The filament was gently advanced until perfusion as monitored from the LDF probe indicated adequate middle cerebral artery (MCA) occlusion by a sharp decrease in ipsilateral blood flow to 20–35% of baseline. If blood flow did not stay below 40% of baseline over the first 30 minutes of MCA occlusion, the animal was excluded from the dataset. After 120 min of occlusion, the suture was withdrawn to allow for cerebral reperfusion. All parameters were measured for 30 minutes after the suture was withdrawn at which time the wounds were closed, 4 mg/kg gentamicin given IM and the anesthetic discontinued.
Permanent distal middle cerebral artery occlusion (PMCAO)
Permanent focal cerebral ischemia was produced with photothrombotic occlusion of the distal middle cerebral artery 26. The right common carotid artery (CCA) was exposed by a ventral midline incision in the neck. The sternocleidomastoid muscle was retracted and a loose snare was placed around the artery. As with TMCAO, the rat was then placed in a stereotaxic head holder, and the laser speckle contrast area thinned. The zygomatic and squamosal bones were exposed by making a vertical incision midway between the right eye and the right ear and retracting the temporalis muscle. Using a high speed dental drill, a 4 mm burr hole was made just rostral to the anterior junction of the zygomatic and squamosal bones exposing the distal segment of the MCA. Erythosin B dye (17 mg/ml in saline, 40 mg/kg) (MP Biomedicals) was infused into the tail vein and the exposed MCA was irradiated with a diode laser (532 nm, 4 mW beam, LaserGlow Technologies, model LRS-0532-KFM-00030-03) focused on the artery through a spherical lens. Irradiation started at the end of the dye infusion and continued for 4–5 minutes. An orange fluorescence was immediately observed in the irradiated distal MCA segment under the operating microscope and a white thrombus formed approximately 4–5 minutes later within the fluorescent segment and gradually elongated distally to permanently occlude the MCA. Following the irradiation, the right common carotid artery was occluded permanently by tightening the snares. The temperature of the exposed brain was maintained at 37.5 ± 0.3°C with a small heat lamp connected to a thermocouple probe (Omega Engineering, HYP-2; 0.81 mm diameter) placed on the surface of the brain and covered with saline. Similar to the TMCAO study, 150 minutes after MCA occlusion, the wounds were closed, gentamicin given, and the anesthetic discontinued.
Vagus nerve electrode placement
During exposure of the right CCA, the right vagus nerve was isolated from the surrounding connective tissue, and a stimulating electrode based on the design of Smith et al. 27 was placed around the nerve and held in place by suturing it to the sternocleidomastoid muscle. This electrode was composed to two curved silver wires covered with polyethylene and held by a solid bar 1.5 mm apart. On the inside of the curve the wires were each exposed for 2 mm so as to come into contact with the exposed vagus nerve. Care was taken to insulate this surface from al tissue except the vagus nerve.
Laser speckle contrast imaging
For speckle imaging 23, 28, 29, a collimated laser diode (DL7140-201S, 785 nm, 80 mW; Thorlabs, Newton, NJ) driven by a commercial laser diode controller (LDC 500; Thorlabs, Newton, NJ) provided uniform illumination on the skull surface. Images over the right MCA territory were captured through a 60-mm lens (Schneider-Kreuznach, Apo-Componon 2.8/40, Germany) using a 12-bit, TEC cooled CCD camera (Uniq; Uniq Vision Inc.,) with a field of view of 5 × 5 mm. The aperature of the camera was adjusted so that the speckle size matched the pixel dimensions (9.9 × 9.9 μm). Images were acquired at 4 Hz with a camera exposure time of 16 ms. As described previously30, a sliding 7 × 7 pixel window was used to convert the intensity images from the camera into speckle contrast images. The speckle contrast is the standard deviation of intensity divided by the mean intensity in the sliding window, where the CBF is inversely proportional to the square of the speckle contrast 23, 28, 29. We note here that though the CBF is inversely proportional to the square of the speckle contrast, there is an intercept. When the flow is zero (i.e., the animal is dead), the inverse of the speckle contrast squared is not zero 31. Based on 6 animals, we estimated this biological zero to be 11% of the baseline image (i.e., average of first ten images collected), and subtracted it from all of the measured CBF images so that the measured CBF will be close to zero when the animal is dead.
CBF analysis
To improve the signal-to-noise ratio, fours speckle contrast images were averaged together providing a temporal resolution of 1 Hz for CBF. Changes in blood flow were determined relative to the average CBF over the first 10 seconds of data collection after correcting for biological zero 31. Although laser speckle contrast imaging produces an image of changes in blood flow throughout the field of view, for the purposes of quantitative analysis in this project blood flow in the core of the MCA territory was defined from a region of interest with a diameter of 0.3 mm with the greatest decrease of CBF during the first minute of occlusion. This region usually was located approximately 4–5 mm lateral and 3 mm posterior to Bregma. A region in the boundary of the MCA territory was obtained from an identical sized ROI placed at 2 mm lateral and 3 mm posterior to Bregma. All ROIs were chosen so as to avoid visible vessels30.
Experimental protocol
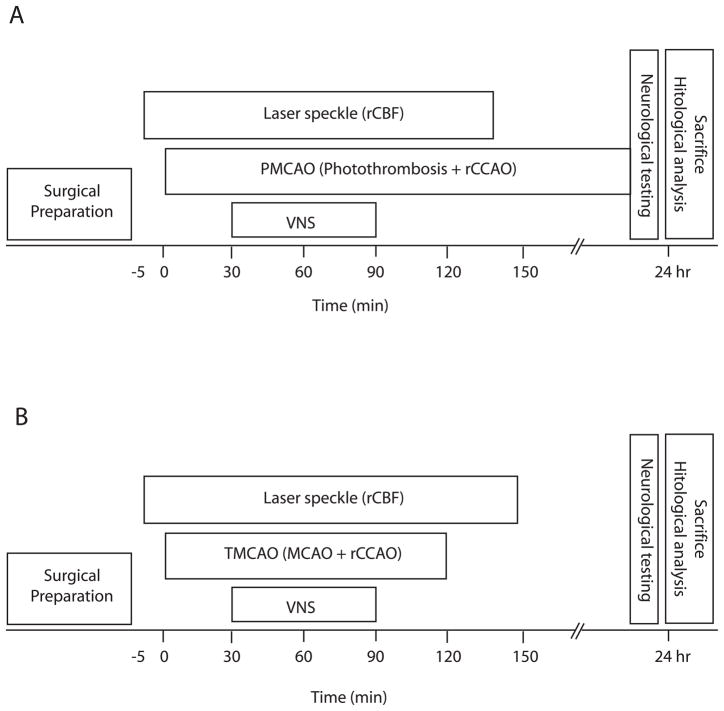
Four groups (n = 8 for each group) were studied, consisting of control and VNS treatment groups for both TMCAO and PMCAO (figure 1). In all groups, CBF was monitored five minutes before MCA occlusion to establish a baseline level and 150 minutes after MCA occlusion to observe effects from the VNS treatment. In the treatment groups, VNS started 30 minutes after MCA occlusion, and consisted of 30 second pulse trains (0.5 mA square pulses with width 0.3 msec and repetition rate of 20 Hz) delivered to the animal’s right vagus nerve every 5 minutes for a total period of 60 minutes using a stimulus isolator (Model A365, World Precision Instruments, Sarasota, Fl). These parameters are similar to those used clinically in the treatment of eplepsy and depression32, 33 and in animal studies in ischemia and brain trauma20, 27. The control groups were prepared exactly the same way, including the placement of the stimulating electrode on the vagus nerve, except that no stimulation was delivered (i.e. vagal nerve stimulator was off). The right vagus nerve was stimulated instead of the left primarily because it was more accessible to the surgeon and because the right vagus nerve was used in a previous VNS study in transient ischemia20. Studies in epilepsy show that both left and right VNS are effective treatment. In the TMCAO groups, the MCA was occluded for 120 minutes before reperfusion.
Figure 1.
Schematic of experimental protocol for the temporary middle cerebral artery occlusion (TMCAO) studies which involved filament occlusion along with right common carotid occlusion (rCCAO) (A) and the permanent middle cerebral artery occlusion (PMCAO) studies produced by photothrombosis along with rCCAO (B). Changes in regional cerebral blood flow (CBF) were monitored with laser speckle contrast imaging. Over the first 30 minutes following MCA occlusion, the vagal nerve was not stimulated, with vagal nerve stimulation (VNS) starting 30 minutes into ischemia and lasting for 60 minutes.
Neurological evaluation and infarct volume measurement
Twenty-four hours after MCA occlusion, a neurological evaluation to examine sensorimotor integration was performed using the criteria described by De Ryck 34. The testing included measurements of visual placing in both the forward and lateral directions, tactile placing of the dorsal and lateral paw surfaces, and proprioceptive placing, with scores ranging from 0 (no deficit) to 12 (maximum deficit).
Rats were sacrificed 24 hours after MCA occlusion by 100% CO2 breathing and the brain was removed from the skull and sectioned in the coronal plane at 1.5-mm intervals using a rodent brain matrix. Each brain slice was stained in a solution of 2% triphenyltetrazolium chloride (TTC) for 10 minutes. The resulting brain sections were photographed and the infarct volume was determined as described previously35 with corrections for edema.
Statistical analysis
Results are expressed as mean ± SEM. Physiological measurements (blood pressure (BP), heart rate (HR), and blood gases (pH, PCO2, PO2)), changes in CBF, and infarct volumes were analyzed by repeated measures ANOVA followed by a Student-Newman-Keuls test. Neurological scores were compared using repeated measures ANOVA followed by Mann–Whitney U-test. A p-value of <0.05 was considered statistically significant.
Results
There are two parts to the current study. The first study (TMCAO) examined the effect of vagus nerve stimulation in rats subjected to temporary cerebral ischemia using filament occlusion of the middle cerebral artery, while the second study (PMCAO) examined vagus nerve stimulation on permanent cerebral ischemia induced by photothrombosis of the distal middle cerebral artery.
Blood gas data
Blood gas parameters were within normal physiological range in all four groups of animals for the entire period that they were monitored. Furthermore, there were no statistical differences either between groups or within groups, as a result of stimulation (table 1).
Table 1.
|
TMCAO – Temporary MCA occlusion (intra-arterial filament) | |||
| Control | |||
| pH | PCO2 (mm Hg) | PO2 (mm Hg) | |
| Prior to VNS | 7.43 ± 0.020 | 40.6 ± 1.9 | 115 ± 10 |
| During VNS | 7.41 ± 0.016 | 44.4 ± 2.3 | 103 ± 7 |
| Vagus nerve stimulation | |||
| pH | PCO2 (mm Hg) | PO2 (mm Hg) | |
| Prior to VNS | 7.42 ± 0.023 | 41.9 ± 2.5 | 109 ± 4 |
| VNS | 7.41 ± 0.015 | 43.4 ± 2.1 | 100 ± 5 |
|
PMCAO – Permanent MCA occlusion (photothrombosis) | |||
| Control | |||
| pH | PCO2 (mm Hg) | PO2 (mm Hg) | |
| Prior to VNS | 7.43 ± 0.03 | 42.7 ± 3.1 | 111 ± 4 |
| VNS | 7.44 ± 0.02 | 41.7 ± 2.1 | 112 ± 5 |
| Vagus nerve stimulation | |||
| pH | PCO2 (mm Hg) | PO2 (mm Hg) | |
| Prior to VNS | 7.42 ± 0.01 | 44.1 ± 1.9 | 113 ± 5 |
| VNS | 7.44 ± 0.01 | 41.9 ± 1.5 | 114 ± 4 |
Blood pressure and heart rate data
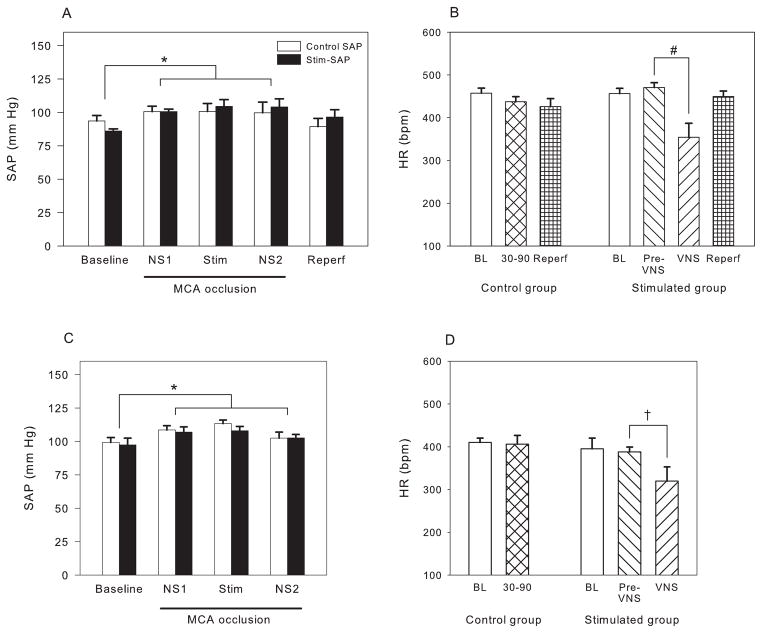
Blood pressure was measured continuously throughout the study and was quite stable. For the transient ischemic study we report only five epochs corresponding to periods of experimental manipulation: prior to MCAO (Baseline), following MCAO but prior to VNS (NS1), during VNS (Stim), following VNS but prior to reperfusion, and following reperfusion. Blood pressure in the baseline period prior to temporary MCA occlusion was 93.6 ± 4.1 mm Hg in the control group and 86.0 ± 1.6 mm Hg in the VNS stimulation group (NS). Following MCA occlusion, blood pressure increased to approximately 100–105 mm Hg (figure 2A), a statistically significant increase (p<0.01; two-way repeated measures ANOVA). Following reperfusion, blood pressure returned to the pre-ischemic level. VNS did not produce a mean change in blood pressure over the entire 60 minute period of stimulation (Stim), and there was a small but nonsignificant transient decrease in SAP (5.1 mm Hg) during the 30 seconds that the vagus nerve was being stimulated.
Figure 2.
Systemic arterial pressure (SAP) (A, C) and heart rate (HR) (B, D) for the TMCAO (A, B) and PMCAO (C, D) studies. For the first 30 minutes following MCA occlusion the vagus nerve was not stimulated (NS1), for the next 60 minutes the vagus nerve was stimulated (Stim); physiological parameters are shown for the 30 minutes after the end of stimulus (NS2). In panels B and D the heart rate is shown during the four minutes immediately preceding each pulse train (Pre-VNS) and for the 30 seconds of stimulation (VNS). Blood pressure increased equally with MCA occlusion, independent of VNS stimulation. Vagal nerve stimulation did produce a significant decrease in heart rate in both the transient and permanent studies. * p<0.05 in comparison to baseline; † p<0.02, # p<0.005 in comparison to no VNS stimulation. Data are shown as mean ± SEM. There were eight animals in each of the four groups (TMCAO control, TMCAO VNS, PMCAO control, PMCAO VNS).
In the photothrombosis study, blood pressure also showed an increase during MCA occlusion in both the control and stimulated groups (p<0.01) but there was no difference in SAP either prior to or during MCA occlusion between the two groups (figure 2C), except during the 30 sec stimulation epochs when SAP dropped by 9.6 mm Hg (NS).
Heart rate of the VNS group in the transient MCA occlusion study prior to stimulation (470 ± 12 BPM) was similar to that of the animals in the control group (457 ± 12 BPM) (figure 2B). However, during vagus nerve stimulation, HR decreased dramatically to 354 ± 32 (p<0.005), and then increased to the pre-stimulus level following the termination of each 30 second epoch of VNS. There was a similar decrease in HR in the photothrombosis study, with HR falling from 388 BPM to 320 BPM (p<0.05) (figure 2D).
Cerebral blood flow data
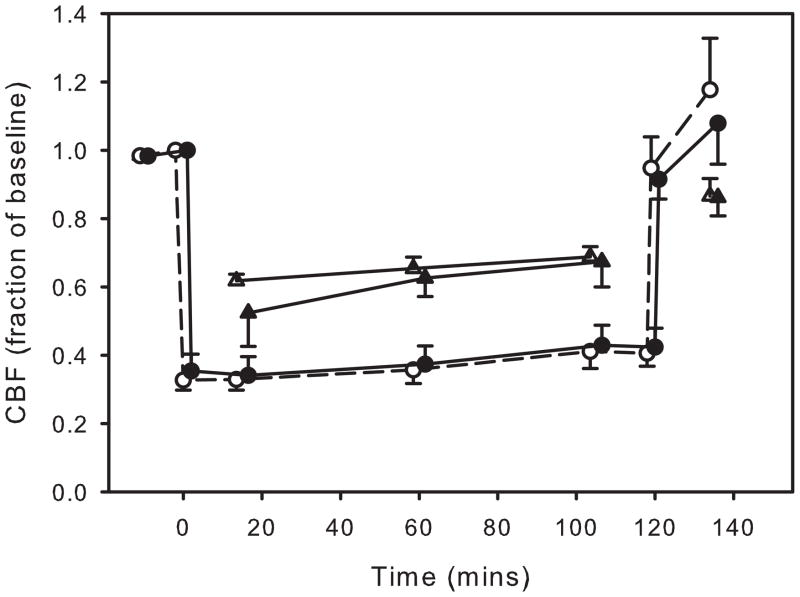
Cerebral blood flow was averaged across animals using ROIs both in the core of the MCA territory and ROIs from peri-ischemic regions. In the transient MCAO studies, CBF in the core of the MCA territory in the two groups fell to 30-35% of baseline after MCA occlusion in both the control and the VNS animals (as measured one minute after occlusion) and remained at this level until removal of the filament at which time it returned to the pre-ischemic level (figure 3). In the boundary zone CBF fell to about 60–70% of baseline with no difference between the control and stimulated animals (figure 4).
Figure 3.
Cerebral blood flow in the MCA territory (circles) and in the boundary zone (triangles) as determined from laser speckle contrast imaging during and following temporary MCA occlusion. Changes in flow are shown as a fraction of the baseline (pre-ischemia) flow. The open symbols are from the groups not receiving vagus nerve stimulation and the close symbols are from stimulated animals. VNS did not produce any change in blood flow. Data are shown as mean ± SEM. The symbols are slightly time-shifted so as to more easily visualize the data. There were eight animals in each group.
Figure 4.
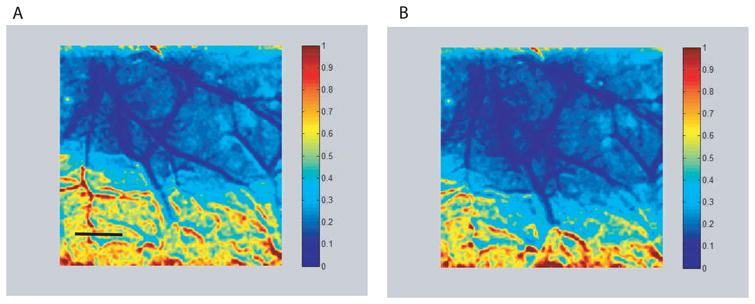
Speckle images showing changes in cerebral blood flow (CBF) with respect to blood flow just prior to filament occlusion of the MCA. The image on the left (A) was obtained following MCA occlusion but prior to start of vagus nerve stimulation, while the image on the right (B) was obtained during a 30 second train of stimulation pulses to the vagus nerve. There is a dramatic decrease in CBF in the MCA territory. Note the similarity of the two images with no apparent increase in CBF due to stimulation. The numbers on the color scale are fractions of the pre-occlusion CBF and the scale bar in panel A is 1 mm.
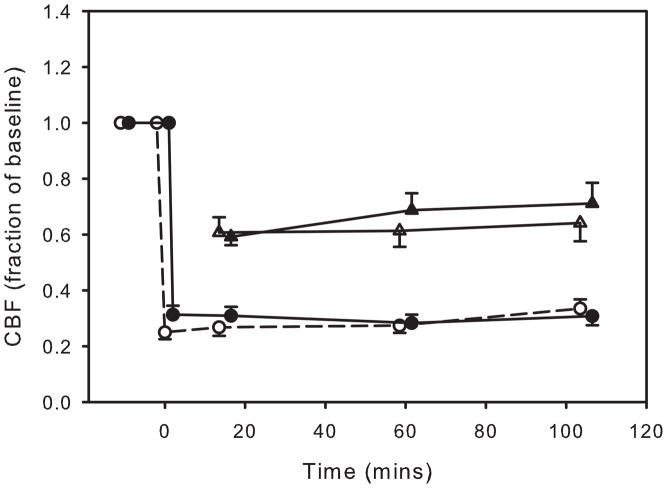
Changes in cerebral blood flow in the photothrombosis study were very similar to those in the transient MCAO studies (figure 5). Blood flow dropped to 24.5 ± 2.0% of baseline in the control group and 31.1 ± 2.9% in the stimulated animals during NS1 (prior to VNS) (NS). There was no difference in the degree of ischemia between the control group and the group receiving VNS. However, an ANOVA showed a slight, but statistically significant increase in blood flow in the control group over the period of blood flow monitoring to 34.2 ± 4.7% of baseline (p<0.05). In particular, VNS did not cause any change in CBF (p>0.2) between the groups during or following the period of stimulation. Similar to the TMCAO study, CBF in the boundary zone following photothrombosis decreased to 60-70% of baseline with no significant differences between the stimulated and nonstimulated groups.
Figure 5.
Cerebral blood flow in the MCA territory (circles) and in the peri-infarct territory (triangles) as determined from laser Doppler flowmetry (LDF) during and following permanent MCA occlusion. Changes in flow are shown as a fraction of the baseline (pre-ischemia) flow. The open symbols are from the groups not receiving vagus nerve stimulation and the close symbols are from stimulated animals. VNS did not produce any change in blood flow. Data are shown as mean ± SEM. There were eight animals in each group.
Infarct data
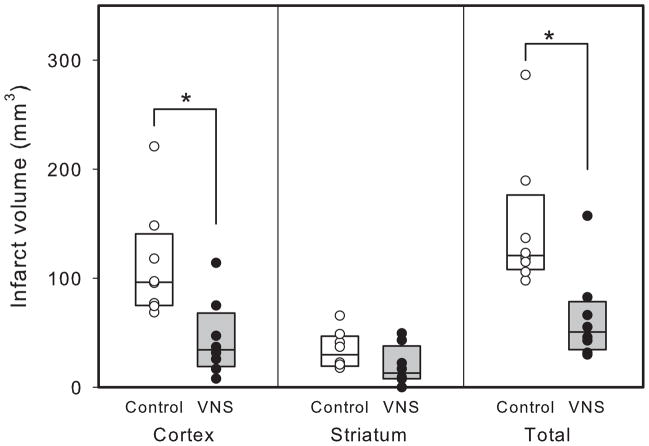
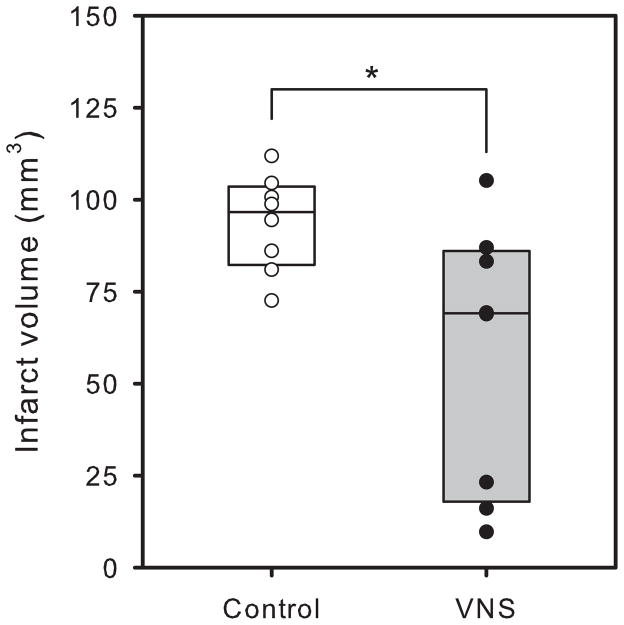
Animals receiving vagus nerve stimulation during temporary MCA occlusion showed smaller histological damage than animals not receiving VNS (figure 6). This decrease in damage was statistically significant for infarct size in the cortex (p<0.01) and in the total brain (64.0 ± 14.7 mm3, compared to 146.6 ± 22.3 mm3) (p<0.01), but not for the striatum (p=0.12). In the permanent MCA occlusion studies, there was also a decrease in infarct volume in the brains from animals receiving VNS (57.8 ± 12.8 mm3 vs. 93.8 ± 4.6mm3) (p<0.02) with the damage confined to the cerebral cortex (figure 7). There was no correlation between degree of ischemia and eventual infarct volume.
Figure 6.
Infarct volume in animals in the transient ischemia study with (closed circles) and without (open circles) vagus nerve stimulation (VNS) in the cerebral cortex (left panel), the striatum (center panel), and in the total brain (right panel). * p<0.005 with respect to unstimulated group. There were eight animals in each group.
Figure 7.
Infarct volume in animals in the permanent ischemia study with (closed circles) and without (open circles) vagus nerve stimulation (VNS). * p<0.02 with respect to unstimulated group. There were eight animals in each group.
Neurological data
The neurological score of the animal receiving VNS during transient MCA occlusion (5.5 ± 0.9) was significantly lower than those not being stimulated (9.8 ± 0.4) (p<0.001). In the photothrombosis study, however, although the score for the stimulated animals (4.0 ± 0.8) was less than the nonstimulated group (5.8 ± 0.5), the difference was not statistically significant (p=0.09).
Discussion
In the current study we found that vagus nerve stimulation can produce neuroprotection, leading to a 56.3% decrease in total infarct volume in transient MCA occlusion and a 38.4% decrease in permanent MCA occlusion. This is consistent with earlier reports suggesting a neuroprotective effect of VNS in global and focal models of transient cerebral ischemia 18–20. This is the first report, however, showing that VNS-induced neuroprotection extends to permanent cerebral ischemia. The second new finding of this study is that the decrease in infarct volume in both temporary and permanent ischemia is not due to changes in blood flow either during the period of ischemia or during the early period of reperfusion (in the case of transient ischemia).
The literature is replete with studies examining a variety of drug trials in both temporary and permanent ischemia. Many of these studies found that neuroprotection was similar in both models of permanent and transient cerebral ischemia 36–39, whereas in others, treatments found effective in transient ischemia did not carry over into permanent ischemia 24, 40–42. These differences were frequently related to the mechanism by which the treatment was able to reduce tissue damage. The observation that VNS was able to reduce infarct size in permanent cerebral ischemia strongly suggests that the mechanism of neuroprotection is not related to vagus nerve stimulation interfering with reperfusion injury 43, 44 but instead it in some way interferes with the deleterious ischemic cascade during the ischemic period.
Although the decrease in tissue damage was accompanied by a significant improvement in neurological score in the transient group, there was no statistically significant improvement as a result of VNS in the permanent ischemia studies. Although this may be due to the lack of sensitivity of the neurological test used to pick up behavioral differences accompanying salvage of cortical damage elicited by the vagus nerve stimulation in the photothrombosis model, it is most likely due to any neurological improvement being too small to reach statistical significance with the number of animals used in this study.
During the 30 sec stimulation epochs SAP dropped 5.1 mm Hg in TMCAO and 9.6 mm Hg in PMCAO. The decrease in SAP in the temporary MCA occlusion study was less than that observed by Ay et al. 20 who reported a 49.6 mm Hg drop in SAP using similar stimulation parameters but well within the range of other studies using comparable stimulation parameters. Similarly, the decrease in HR in the temporary MCAO study was less than that reported by Ay et al. 20, but well within reported studies in the literature. In the present transient cerebral ischemia study the decrease in infarct volume due to vagus nerve stimulation (56.4%) is very similar to that reported previously (50.9%)20 yet we report a smaller decrease in blood pressure and heart rate during stimulation. This suggests that SAP and HR are not of primary importance in the mechanism of neuroprotection.
Since the majority of clinical strokes are thrombotic in origin, we decided to use a photothrombotic model for the permanent ischemia studies. This model produces an arterial thrombus similar that that seen clinically and has been used to study a variety of neuroprotective strategies 45–47. Although the ischemic distribution area following photothrombosis does differ from that with MCA occlusion by placing a ligature on the MCA, by thermocoagulation, or by leaving a filament in the MCA lumen until sacrifice, the blood flow in the core remains at an approximately similar depressed value over at least the first 24 hours following photothrombosis 48, thereby making this a model of permanent cerebral ischemia.
The reduction in infarct volume seen in the temporary cerebral ischemia study (56%) was comparable to that obtained by Ay and associates 20 (48%) and the improvement in functional scores was almost identical. Changes in cerebral blood flow as measured with speckle contrast imaging in both the temporary MCA occlusion study (TMCAO) and in the permanent MCA occlusion study (TMCAO) were very similar in both the VNS and the control groups everywhere in the field of view which included both the core of the MCA territory in the cortex, and the peri-infarct zone. This strongly suggests that the neuroprotection seen with VNS is not due to any alterations in tissue perfusion elicited by the stimulation, but instead must be attributed to another mechanism. Although VNS has been shown to increase CBF in the thalamus of patients with partial epilepsy, no blood flow changes were observed in the cerebral cortex in that study 49, which was the only region of the brain monitored in the present study. However, in patients with depression, acute left VNS does increase CBF bilaterally in the temporal cortex and in the right parietal cortex 50. It is difficult to extend these findings to our rat study since in our study the right vagus nerve was stimulated, but a blood flow effect in only the contralateral parietal cortex would suggest that our right VNS did not alter CBF in the ischemic (right) territory. Examination of the speckle contrast images in both the transient and the permanent ischemia studies indicated that VNS was unable to augment CBF not only in the ischemic territory but also in the regions surrounding the ischemic core. This indicates that VNS is incapable of expanding the volume of tissue with blood flow above the critical threshold for damage.
Other proposed mechanisms that have been suggested for VNS-induced neuroprotection include its ability to attenuate excitatory amino acids 19, 51, increase GABA (an inhibitor amino acid) 52, attenuate inflammation 53, and reduce neuronal excitability 54 that accompanies ischemia 55. The similarity in neuroprotection in the transient and permanent ischemia studies does suggest that the mechanism of neuroprotection is not due to any ability to ameliorate reperfusion injury and that VNS can be used in both ischemia with early reperfusion and in permanent ischemia. Another potential mechanism for stimulus-induced neuroprotection involves the upregulation of neurotrophins. It has been shown that brain-derived neurotrophin factor (BDNF) expression, for example, increases after vagus nerve stimulation 56. BDNF also plays a neuroprotective role in focal cerebral ischemia 57, potentially acting through the high-affinity receptor tyrosine kinase58 or through BDNF-induced tissue type plasminogen activator secretion 59.These potential mechanisms need to be pursued with particular reference to cerebral ischemia in future studies.
One of the items of the STAIR recommendations for preclinical stroke drug development is to assess outcome measures with prolonged survival following promising results in acute studies60 in order to determine if the drug has simply slowed down the maturation of the damage61. Undertaking long-term follow-up is more challenging because of the difficulty in maintaining good physiological control and for this reason acute evaluation is frequently done first. It will be important to determine if the promising results obtained in this study extend to chronic neuroprotection.
In this study we have shown that vagus nerve stimulation during transient cerebral ischemia is neuroprotective - it reduces infarct volume and leads to a better functional recovery. This beneficial effect is not due to any alterations in cerebral blood flow by the vagal stimulation. Additionally, vagus nerve stimulation also reduces neuronal damage following permanent cerebral ischemia produced by photothrombosis of the middle cerebral artery and does so independent of any effect on cerebral blood flow. Although the functional score was lower (better), this did not reach statistical significance. The results of this study may have significant clinical implications for acute stroke treatment. Future studies are needed to specifically elucidate the timing duration, and stimulation parameters necessary to maximize this observed protective effect. Although implantable devices for VNS are FDA approved and used routinely in refractory epilepsy and treatment resistant depression, these devices are not ideal for acute use in stroke. Transcutaneous vagus nerve stimulators are currently available and have been shown to produce changes in cerebral hemodynamics as measured with functional magnetic resonance (fMRI) somewhat similar to those seen with implantable devices62. Should VNS prove beneficial in cerebral ischemia, the use of transcutaneous vagus nerve stimulation can be investigated.
Acknowledgments
This work was supported by NIH RO1 NS057400 and NS060653. The authors wish to thank Dr. Martin Reivich for many valuable discussions and helpful suggestions.
Footnotes
Financial Disclosures:
Dr. Zhenghui Sun, Mr. Wesley Baker, Dr. Teruyuki Hiraki and Dr. Joel H. Greenberg report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 2010;27:130–138. doi: 10.1097/WNP.0b013e3181d64d8a. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol. 2002;1:477–482. doi: 10.1016/s1474-4422(02)00220-x. [DOI] [PubMed] [Google Scholar]
- 3.Ramani R. Vagus nerve stimulation therapy for seizures. J Neurosurg Anesthesiol. 2008;20:29–35. doi: 10.1097/ANA.0b013e31815b7df1. [DOI] [PubMed] [Google Scholar]
- 4.Boon P, Raedt R, de HV, Wyckhuys T, Vonck K. Electrical stimulation for the treatment of epilepsy. Neurotherapeutics. 2009;6:218–227. doi: 10.1016/j.nurt.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dedeurwaerdere S, Gilby K, Vonck K, et al. Vagus nerve stimulation does not affect spatial memory in fast rats, but has both anti-convulsive and pro-convulsive effects on amygdala-kindled seizures. Neuroscience. 2006;140:1443–1451. doi: 10.1016/j.neuroscience.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Munana KR, Vitek SM, Tarver WB, et al. Use of vagal nerve stimulation as a treatment for refractory epilepsy in dogs. J Am Vet Med Assoc. 2002;221:977–983. doi: 10.2460/javma.2002.221.977. [DOI] [PubMed] [Google Scholar]
- 7.Sahin D, Ilbay G, Imal M, Bozdogan O, Ates N. Vagus nerve stimulation suppresses generalized seizure activity and seizure-triggered postictal cardiac rhythm changes in rats. Physiol Res. 2009;58:345–350. doi: 10.33549/physiolres.931344. [DOI] [PubMed] [Google Scholar]
- 8.Sjogren MJ, Hellstrom PT, Jonsson MA, et al. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer's disease: a pilot study. J Clin Psychiatry. 2002;63:972–980. doi: 10.4088/jcp.v63n1103. [DOI] [PubMed] [Google Scholar]
- 9.Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalalgia. 2005;25:82–86. doi: 10.1111/j.1468-2982.2005.00611.x. [DOI] [PubMed] [Google Scholar]
- 10.Nahas Z, Teneback C, Chae JH, et al. Serial vagus nerve stimulation functional MRI in treatment-resistant depression. Neuropsychopharmacology. 2007;32:1649–1660. doi: 10.1038/sj.npp.1301288. [DOI] [PubMed] [Google Scholar]
- 11.Marrosu F, Maleci A, Cocco E, et al. Vagal nerve stimulation improves cerebellar tremor and dysphagia in multiple sclerosis. Mult Scler. 2007;13:1200–1202. doi: 10.1177/1352458507078399. [DOI] [PubMed] [Google Scholar]
- 12.Faris PL, Hofbauer RD, Daughters R, et al. De-stabilization of the positive vago-vagal reflex in bulimia nervosa. Physiol Behav. 2008;94:136–153. doi: 10.1016/j.physbeh.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calabresi P, Cupini LM, Centonze D, Pisani F, Bernardi G. Antiepileptic drugs as a possible neuroprotective strategy in brain ischemia. Ann Neurol. 2003;53:693–702. doi: 10.1002/ana.10603. [DOI] [PubMed] [Google Scholar]
- 14.Meldrum BS. The role of glutamate in epilepsy and other CNS disorders. Neurology. 1994;44:S14–S23. [PubMed] [Google Scholar]
- 15.Bittigau P, Ikonomidou C. Glutamate in neurologic diseases. J Child Neurol. 1997;12:471–485. doi: 10.1177/088307389701200802. [DOI] [PubMed] [Google Scholar]
- 16.Hicks TP, Conti F. Amino acids as the source of considerable excitation in cerebral cortex. Can J Physiol Pharmacol. 1996;74:341–361. [PubMed] [Google Scholar]
- 17.Ginsberg MD. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masada T, Itano T, Fujisawa M, et al. Protective effect of vagus nerve stimulation on forebrain ischaemia in gerbil hippocampus. Neuroreport. 1996;7:446–448. doi: 10.1097/00001756-199601310-00017. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto O, Pang J, Sumitani K, et al. Mechanisms of the anti-ischemic effect of vagus nerve stimulation in the gerbil hippocampus. Neuroreport. 2003;14:1971–1974. doi: 10.1097/00001756-200310270-00018. [DOI] [PubMed] [Google Scholar]
- 20.Ay I, Lu J, Ay H, Sorensen AG. Vagus nerve stimulation reduces infarct size in rat focal cerebral ischemia. Neurosci Lett. 2009;459:147–151. doi: 10.1016/j.neulet.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Merino JG, Latour LL, An L, et al. Reperfusion half-life: a novel pharmacodynamic measure of thrombolytic activity. Stroke. 2008;39:2148–2150. doi: 10.1161/STROKEAHA.107.510818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorgensen HS, Sperling B, Nakayama H, Raaschou HO, Olsen TS. Spontaneous reperfusion of cerebral infarcts in patients with acute stroke. Incidence, time course, and clinical outcome in the Copenhagen Stroke Study. Arch Neurol. 1994;51:865–873. doi: 10.1001/archneur.1994.00540210037011. [DOI] [PubMed] [Google Scholar]
- 23.Durduran T, Burnett MG, Yu G, et al. Spatiotemporal quantification of cerebral blood flow during functional activation in rat somatosensory cortex using laser-speckle flowmetry. J Cereb Blood Flow Metab. 2004;24:518–525. doi: 10.1097/00004647-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Shimazu T, Inoue I, Araki N, et al. A peroxisome proliferator-activated receptor-γ agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005;36:353–359. doi: 10.1161/01.STR.0000152271.21943.a2. [DOI] [PubMed] [Google Scholar]
- 25.Luckl J, Keating J, Greenberg JH. Alpha-chloralose is a suitable anesthetic for chronic focal cerebral ischemia studies in the rat: A comparative study. Brain Res. 2008;1191:157–167. doi: 10.1016/j.brainres.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Markgraf CG, Kraydieh S, Prado R, et al. Comparative histopathologic consequences of photothrombotic occlusion of the distal middle cerebral artery in Sprague-Dawley and Wistar rats. Stroke. 1993;24:286–292. doi: 10.1161/01.str.24.2.286. [DOI] [PubMed] [Google Scholar]
- 27.Smith DC, Modglin AA, Roosevelt RW, et al. Electrical stimulation of the vagus nerve enhances cognitive and motor recovery following moderate fluid percussion injury in the rat. J Neurotrauma. 2005;22:1485–1502. doi: 10.1089/neu.2005.22.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briers JD. Laser Doppler, speckle and related techniques for blood perfusion mapping and imaging. Physiol Meas. 2001;22:R35–R66. doi: 10.1088/0967-3334/22/4/201. [DOI] [PubMed] [Google Scholar]
- 29.Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21:195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Luckl J, Baker W, Sun ZH, et al. The biological effect of contralateral forepaw stimulation in rat focal cerebral ischemia: a multispectral optical imaging study. Front Neuroenergetics. 2010:2. doi: 10.3389/fnene.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou C, Shimazu T, Durduran T, et al. Acute functional recovery of cerebral blood flow after forebrain ischemia in rat. J Cereb Blood Flow Metab. 2008;28:1275–1284. doi: 10.1038/jcbfm.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Binnie CD. Vagus nerve stimulation for epilepsy: a review. Seizure. 2000;9:161–169. doi: 10.1053/seiz.1999.0354. [DOI] [PubMed] [Google Scholar]
- 33.Sackeim HA, Rush AJ, George MS, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–728. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 34.De Ryck M, Van RJ, Borgers M, Wauquier A, Janssen PA. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke. 1989;20:1383–1390. doi: 10.1161/01.str.20.10.1383. [DOI] [PubMed] [Google Scholar]
- 35.Luckl J, Keating J, Greenberg JH. Alpha-chloralose is a suitable anesthetic for chronic focal cerebral ischemia studies in the rat: A comparative study. Brain Res. 2007;1191:157–167. doi: 10.1016/j.brainres.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abe T, Kunz A, Shimamura M, et al. The neuroprotective effect of prostaglandin E2 EP1 receptor inhibition has a wide therapeutic window, is sustained in time and is not sexually dimorphic. J Cereb Blood Flow Metab. 2009;29:66–72. doi: 10.1038/jcbfm.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell CA, Barone FC, Benham CD, et al. Characterisation of SB-221420-A - a neuronal Ca(2+) and Na(+) channel antagonist in experimental models of stroke. Eur J Pharmacol. 2000;401:419–428. doi: 10.1016/s0014-2999(00)00470-2. [DOI] [PubMed] [Google Scholar]
- 38.Johnson MP, McCarty DR, Velayo NL, et al. MDL 101,002, a free radical spin trap, is efficacious in permanent and transient focal ischemia models. Life Sci. 1998;63:241–253. doi: 10.1016/s0024-3205(98)00268-9. [DOI] [PubMed] [Google Scholar]
- 39.Nagel S, Papadakis M, Chen R, et al. Neuroprotection by dimethyloxalylglycine following permanent and transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berger C, Stauder A, Xia F, Sommer C, Schwab S. Neuroprotection and glutamate attenuation by acetylsalicylic acid in temporary but not in permanent cerebral ischemia. Exp Neurol. 2008;210:543–548. doi: 10.1016/j.expneurol.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Britton P, Lu XC, Laskosky MS, Tortella FC. Dextromethorphan protects against cerebral injury following transient, but not permanent, focal ischemia in rats. Life Sci. 1997;60:1729–1740. doi: 10.1016/s0024-3205(97)00132-x. [DOI] [PubMed] [Google Scholar]
- 42.Weng YC, Kriz J. Differential neuroprotective effects of a minocycline-based drug cocktail in transient and permanent focal cerebral ischemia. Exp Neurol. 2007;204:433–442. doi: 10.1016/j.expneurol.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Hossmann KA. Pathophysiological basis of translational stroke research. Folia Neuropathol. 2009;47:213–227. [PubMed] [Google Scholar]
- 44.Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 45.Prado R, Watson BD, Zhao W, et al. L-arginine does not improve cortical perfusion or histopathological outcome in spontaneously hypertensive rats subjected to distal middle cerebral artery photothrombotic occlusion. J Cereb Blood Flow Metab. 1996;16:612–622. doi: 10.1097/00004647-199607000-00011. [DOI] [PubMed] [Google Scholar]
- 46.Yao H, Ginsberg MD, Watson BD, et al. Failure of MK-801 to reduce infarct volume in thrombotic middle cerebral artery occlusion in rats. Stroke. 1993;24:864–870. doi: 10.1161/01.str.24.6.864. [DOI] [PubMed] [Google Scholar]
- 47.Kitayama J, Kitazono T, Yao H, et al. Inhibition of Na+/H+ exchanger reduces infarct volume of focal cerebral ischemia in rats. Brain Res. 2001;922:223–228. doi: 10.1016/s0006-8993(01)03175-4. [DOI] [PubMed] [Google Scholar]
- 48.Takamatsu H, Tsukada H, Kakiuchi T, Tatsumi M, Umemura K. Changes in local cerebral blood flow in photochemically induced thrombotic occlusion model in rats. Eur J Pharmacol. 2000;398:375–379. doi: 10.1016/s0014-2999(00)00292-2. [DOI] [PubMed] [Google Scholar]
- 49.Henry TR, Votaw JR, Pennell PB, et al. Acute blood flow changes and efficacy of vagus nerve stimulation in partial epilepsy. Neurology. 1999;52:1166–1173. doi: 10.1212/wnl.52.6.1166. [DOI] [PubMed] [Google Scholar]
- 50.Conway CR, Chibnall JT, Tait RC. Vagus nerve stimulation for depression: a case of a broken lead, depression relapse, revision surgery, and restoration of patient response. Brain Stimul. 2008;1:227–228. doi: 10.1016/j.brs.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ben-Menachem E, Hamberger A, Hedner T, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20:221–227. doi: 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- 52.Neese SL, Sherill LK, Tan AA, et al. Vagus nerve stimulation may protect GABAergic neurons following traumatic brain injury in rats: An immunocytochemical study. Brain Res. 2007;1128:157–163. doi: 10.1016/j.brainres.2006.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 54.Zagon A, Kemeny AA. Slow hyperpolarization in cortical neurons: a possible mechanism behind vagus nerve simulation therapy for refractory epilepsy? Epilepsia. 2000;41:1382–1389. doi: 10.1111/j.1528-1157.2000.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 55.Schiene K, Bruehl C, Zilles K, et al. Neuronal hyperexcitability and reduction of GABAA-receptor expression in the surround of cerebral photothrombosis. J Cereb Blood Flow Metab. 1996;16:906–914. doi: 10.1097/00004647-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 56.Follesa P, Biggio F, Gorini G, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 57.Schabitz WR, Schwab S, Spranger M, Hacke W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1997;17:500–506. doi: 10.1097/00004647-199705000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Endres M, Fan G, Hirt L, et al. Ischemic brain damage in mice after selectively modifying BDNF or NT4 gene expression. J Cereb Blood Flow Metab. 2000;20:139–144. doi: 10.1097/00004647-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 59.Fiumelli H, Jabaudon D, Magistretti PJ, Martin JL. BDNF stimulates expression, activity and release of tissue-type plasminogen activator in mouse cortical neurons. Eur J Neurosci. 1999;11:1639–1646. doi: 10.1046/j.1460-9568.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- 60.Anonymous. Stroke--1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. doi: 10.1161/01.str.20.10.1407. [DOI] [PubMed] [Google Scholar]
- 61.Valtysson J, Hillered L, Andine P, Hagberg H, Persson L. Neuropathological endpoints in experimental stroke pharmacotherapy: the importance of both early and late evaluation. Acta Neurochir (Wien ) 1994;129:58–63. doi: 10.1007/BF01400874. [DOI] [PubMed] [Google Scholar]
- 62.Kraus T, Hosl K, Kiess O, et al. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm. 2007;114:1485–1493. doi: 10.1007/s00702-007-0755-z. [DOI] [PubMed] [Google Scholar]