Abstract
Influx of Ca2+ through L-type Ca2+ channels (LTCCs) contributes to numerous cellular processes in cardiomyocytes including excitation-contraction (EC) coupling, membrane excitability, and transcriptional regulation. Distinct subpopulations of LTCCs have been identified in cardiac myocytes, including those at dyadic junctions and within different plasma membrane microdomains such as lipid rafts and caveolae. These subpopulations of LTCCs exhibit regionally distinct functional properties and regulation, affording precise spatiotemporal modulation of L-type Ca2+ current (ICa,L). Different subcellular LTCC populations demonstrate variable rates of Ca2+-dependent inactivation and sometimes coupled gating of neighboring channels, which can lead to focal, persistent ICa,L. In addition, the assembly of spatially defined macromolecular signaling complexes permits compartmentalized regulation of ICa,L by a variety of neurohormonal pathways. For example, β-adrenergic receptor subtypes signal to different LTCC subpopulations, with β2-adrenergic activation leading to enhanced ICa,L through caveolar LTCCs and β1-adrenergic stimulation modulating LTCCs outside of caveolae. Disruptions in the normal subcellular targeting of LTCCs and associated signaling proteins may contribute to the pathophysiology of a variety of cardiac diseases including heart failure and certain arrhythmias. Further identifying the characteristic functional properties and array of regulatory molecules associated with specific LTCC subpopulations will provide a mechanistic framework to understand how LTCCs contribute to diverse cellular processes in normal and diseased myocardium.
Keywords: Cardiomyocyte, Calcium channel, Subcellular localization, Microdomain, Calcium signaling
1. Introduction
In the heart, voltage-dependent L-type Ca2+ channels (LTCCs) are essential to numerous cellular processes including excitability, excitation-contraction (EC) coupling, hormone secretion, and regulation of gene expression. Participation in such diverse functions demands that the influx of Ca2+ through L-type channels (L-type Ca2+ current, ICa,L) is tightly controlled and compartmentalized within the cardiac myocyte. It has long been recognized that discrete clusters of LTCCs exist along the sarcolemma, and studies in recent years have greatly extended our understanding of how specific subcellular localization impacts channel function and regulation by a variety of neurohormonal and second messenger pathways [1-6].
A number of important LTCC subpopulations have been identified in cardiomyocytes that associate with unique macromolecular signaling complexes and scaffolding proteins, which enables spatiotemporal modulation of ICa,L. These include channels that are localized to dyadic junctions as well as extradyadic channels that reside in biochemically distinct regions of surface membrane known as membrane microdomains. Plasma membrane microdomains, including lipid rafts and caveolae, exhibit unique lipid composition and protein components and coordinate numerous cellular functions including various signal transduction pathways and protein recycling [7-9]. Numerous signaling molecules have been localized to caveolae including components of the β2-adrenergic receptor/adenylyl cyclase/protein kinase A (PKA) cascade [5,6]. This review will highlight the evolving understanding of distinct subcellular populations of LTCCs in cardiomyocytes and their differing regulation and contributions to Ca2+ signaling in the heart.
2. LTCCs in the heart
2.1. Molecular composition of cardiac LTCCs
LTCCs are multimeric complexes consisting of a pore forming α1 subunit and auxiliary β, α2δ, and α subunits [10]. The α1 subunit serves as the main functional component of the channel complex and consists of four homologous domains (I-IV) each containing six transmembrane segments (S1-S6). Cav1.2 (α1C, encoded by the CACNA1C gene) is the predominant α1 subunit in ventricular myocardium, whereas both Cav1.2 and Cav1.3 (α1D, encoded by CACNA1D) are expressed in atrial tissue as well as nodal cells, where ICa,L contributes to automaticity [11-15]. Extensive alternative splicing of Cav1.2 has been reported, and these splice variants play unique roles in cardiovascular physiology, pharmacology, and disease [16,17]. One important example is alternative splicing of Cav1.2 within transmembrane segment IS6, which impacts sensitivity to the dihydropyridine class of LTCC blockers. Differential expression of these splice variants lead to higher or lower sensitivity in smooth and cardiac muscle, respectively [18,19].
Ca2+ channel auxiliary subunits further add to the functional diversity of LTCCs. The cytosolic β subunits promote trafficking of the channel complex to the plasma membrane and modulate gating properties of the channel [20-22]. The β subunits are encoded by four distinct genes (CACNB1-4), each of which undergoes alternative splicing to generate a total of 18 or more unique β subunit isoforms in human myocardium [23]. The α2δ subunits arise from a common precursor protein that is post-translationally cleaved and relinked via a disulfide bridge. The extracellular α2 peptide is heavily glycosylated and the δ peptide contains a single transmembrane domain [24]. Of the four α2δ subunits (encoded by CACNA2D1-4), α2δ-1-3 are expressed in atrial tissue whereas α2δ-1 and α2δ-2 are present in ventricular myocardium [25,26]. The α2δ subunits modify both channel gating properties and surface membrane expression of the L-type channel complex [20,27]. Ca2+ channel α subunits, of which eight exist (encoded by CACNG1-8), were originally demonstrated to associate with voltage-dependent Ca2+ channels in skeletal muscle and brain [28,29]. However, recent evidence suggests several α subunits including α4, α6, α7, and α8 are present in cardiac muscle and associate with the cardiac Cav1.2 channel complex, altering both activation and inactivation properties of the channel [30].
2.2. Distinct LTCC subpopulations in cardiac myocytes
2.2.1. Dyads
A critical subpopulation of LTCCs is that which participates in EC coupling. A number of studies applying immunoconfocal and electron microscopy techniques have demonstrated that a subset of LTCCs form dyadic complexes with Ca2+-release channels (ryanodine receptors) on apposing junctional sarcoplasmic reticulum (SR) [1,2,31,32]. Upon membrane depolarization, activation of these LTCCs leads to an influx of Ca2+ into the dyadic cleft space, which triggers the opening of ryanodine receptors and subsequent release of SR Ca2+ stores. This Ca2+-induced Ca2+ release (CICR) mechanism underlies the rise in free intracellular Ca2+ concentration ([Ca2+]i) that activates myofilament proteins leading to muscle contraction [33].
Studies have estimated that approximately 75% of LTCCs reside at dyad junctions in cardiac myocytes [34]. In mammalian ventricular cardiomyocytes, dyadic couplings occur predominantly within the transverse (T)-tubule network, which represents a complex system of interconnected membrane structures continuous with the extracellular space [35]. T-tubules extend deep into the myocyte at each myofibrillar Z-line, bringing the plasma membrane in close proximity with junctional SR through the width of the cell, thereby allowing synchronous Ca2+ release throughout the cell with each depolarization. However, as many as 25% of dyads are found at the surface sarcolemma in ventricular myocytes [35,36]. Studies in which the T-tubule system is isolated from the surface membrane using osmotic shock led to an 75-80% decrease in ICa,L without significantly altering SR Ca2+ load; therefore, it is hypothesized that although the majority of LTCCs reside in the T-tubular membrane, surface membrane LTCCs may play a particularly important role in SR Ca2+ loading [37].
Dyadic LTCCs are the target of numerous neurohormonal signaling pathways, most prominently the β-adrenergic/PKA signaling pathway, which increases contractility partially through enhancing ICa,L [38]. Whereas extradyadic LTCCs do not contribute directly to EC coupling, these channels likely play roles in several other cellular processes. These extradyadic channels are not found randomly throughout the sarcolemma, but associate with important signaling molecules in biochemically-distinct membrane microdomains [3,4,6,39].
2.2.2. Lipid rafts
The plasma membrane is a heterogeneous mixture of lipids, cholesterol, and proteins that can form localized regions that compartmentalize cellular processes. The tight packing of sphingolipids and cholesterol forms liquid-ordered microdomains popularly termed “lipid rafts”, which serve a number of important cellular functions including signal transduction and membrane trafficking [7,8]. Although the small (10-200 nm), heterogeneous, and dynamic nature of lipid rafts has made their visualization and characterization challenging, one common feature is that these microdomains resist solubilization with non-ionic detergents such as Triton X-100 at 4°C and can be found in low density fractions on density gradients [8,40,41]. A number of studies utilizing this biochemical methodology have demonstrated the presence of Cav1.2 in low density fractions from ventricular myocardium [5,42,43], strongly suggesting LTCCs localize to lipid raft domains.
2.2.3. Caveolae
Caveolae represent a subset of lipid rafts that are morphologically distinct structures appearing in electron micrographs as flask-shaped invaginations of membrane that are 50-100 nm in diameter. Caveolae are rich in cholesterol and are associated with the integral membrane protein caveolin [44]. Three genes (CAV1-3) encode six known caveolin subtypes (caveolin-1α and −1β; caveolin-2α, −2β, and −2α; and caveolin-3) that have varying tissue distribution [45]. Caveolin (Cav)-1 and Cav-2 are expressed in most cell types, whereas expression of Cav-3 is restricted to cardiac, skeletal, and smooth muscle types, as well as some types of neurons [45-47].
The lack of morphologically distinct caveolae in skeletal muscle from Cav-3 knockout mice highlights the importance of caveolin in caveolae formation [48]. Cholesterol is an additional component necessary for caveolar assembly and maintenance, because depletion of membrane cholesterol dramatically reduces caveolae number [49]. More recently, the cytosolic protein Polymerase I and Transcript Release Factor (PTRF), also known as Cavin, was also demonstrated to be required for caveolar biogenesis [50].
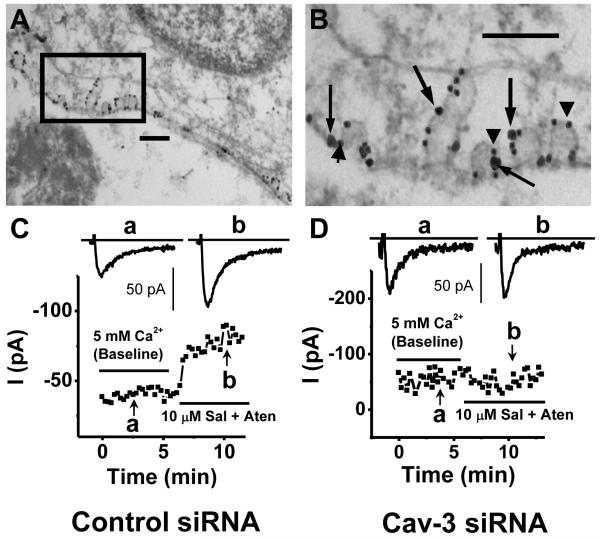
Several methods have been used to demonstrate the presence of LTCCs within caveolae in cardiomyocytes, exploiting the presence of Cav-3 as a marker to help differentiate the localization of proteins to caveolae versus non-caveolar lipid rafts. First, co-immunoprecipitation from ventricular myocytes using anti-Cav-3 or anti-Cav1.2 showed that the two proteins are associated [5,51]. Immunofluorescence studies of isolated ventricular myocytes using confocal microscopy also demonstrated extensive colocalization between Cav-3 and Cav1.2 [5,43]. Perhaps most strikingly, immunogold electron microscopy showed colabeling of Cav-3 and Cav1.2 in close association within morphologically distinct caveolae (Fig. 1A,B) [5,51].
Figure 1. Cardiomyocyte L-type Ca2+ channels localized to caveolae are regulated by β2-adrenergic receptor activation.
(A,B) Immunogold electron micrographs of mouse neonatal ventricular myocytes demonstrate co-localization of the Cav1.2 subunit of L-type Ca2+ channel (large particle, arrows) with Cav-3 (small particle, arrowheads) within caveolae. Scale bars represent 200 nm. (C) β2-adrenergic receptor activation with 10 μM salbutemol (Sal) plus 10 μM atenolol (Aten) increases peak ICa,L in neonatal mouse ventricular myocytes under control conditions. (D) β2-adrenergic stimulation of ICa,L is lost in cells in which caveolae are disrupted using Cav-3 siRNA. Copyright (2006) National Acadamy of Sciences, USA [5].
2.2.4. Caveolin-3 scaffolds
The presence of caveolins in cell types lacking morphologically distinct caveolae such as neurons has sparked interest in understanding the physiological role of caveolins independent of caveolae. It has been suggested that extra-caveolar caveolin forms scaffolds that regulate protein trafficking and signal transduction in some cell types [52]. Cav-3 scaffolds may be particularly important in cardiac myocytes, which have relatively few caveolae compared to other cell types such as endothelial cells and smooth muscle myocytes, yet express Cav-3 at high levels [52,53]. In adult rat cardiomyocytes, a significant amount of Cav-3 is found in heavy (non-buoyant) fractions, and immunoprecipition studies showed that Cav-3 associates with a number of signaling proteins including G-protein coupled receptors, adenylyl cyclase, and heterotrimeric G proteins even in heavy fractions [54]. These findings suggest Cav-3 may regulate signal transduction in sarcolemmal regions outside of caveolae in cardiac myocytes. However, no data yet directly demonstrate localization of LTCCs to noncaveolar Cav-3 scaffolds and additional approaches such as immunogold electron microscopy are needed to carefully investigate this possibility.
2.2.5. Nucleus
The carboxyl (C)-terminus of Cav1.2 undergoes proteolytic cleavage and serves as an autoinhibitory domain by reassociating with the truncated Cav1.2 subunit at the surface membrane [55-57]. Recently, the Cav1.2 C-terminus was also localized to the nucleus in cardiac myocytes, where it autoregulates transcription [58]. Western blotting of nuclear fractions showed expression of the cleaved Cav1.2 C-terminus in nuclear fractions, and GFP-tagged Cav1.2 C-terminus showed nuclear localization [58]. Interestingly, expression of various β subunits fused to GFP in ventricular myocytes also revealed significant localization of β4 in the nucleus; however, the significance of this is currently unclear [59].
2.2.6. Other subcellular compartments
Additional specialized compartments may exist that could prove important for LTCC modulation. For example, a recent study using electron tomography identified electron-dense bridges between dyadic clefts and the mitochondrial outer membrane in mouse ventricular myocardium, and ICa,L has been demonstrated to alter mitochondrial function either directly or through association with actin filaments [60-62]. This raises the possibility of a functional coupling between dyadic LTCCs and mitochondria. Similarly, a close spatial relationship exists between the T-tubule membrane, dyadic cleft, and the nuclear envelope [63]. However, the detailed identity and function of these different subcellular populations of LTCCs are largely unknown at this time.
Furthermore, the subcellular localization of LTCCs may be more complex than currently appreciated. There is a dynamic range in the size of dyadic junctional spaces, for instance, which could differentially impact LTCC function at individual dyads [60]. Likewise, subcompartmentalization of membrane microdomains into smaller nanodomains may be necessary to more precisely define their function and impact on LTCCs. For example, it is currently unknown whether subpopulations of LTCCs coupled to different signaling pathways coexist within the same caveola. Addressing these possibilities will require refinement of available biochemical and microscopy techniques.
3. Subcellular localization impacts LTCC function
With each heart beat, ICa,L activates ryanodine receptors to release SR Ca2+ stores, leading to a transient rise in global [Ca2+]i from ~100 nM during diastole to ~1 μM during systole, which activates myofilament proteins, producing contraction. How then, is specificity of Ca2+ signaling achieved within the cardiomyocyte? It is increasingly recognized that the localization of LTCCs to structurally or biochemically distinct subcellular regions affords functional and physical compartmentalization. Within these spatially defined areas, local [Ca2+] may differ significantly from bulk cytosolic [Ca2+]i, which modulates LTCC function and differentially regulates a number of Ca2+-dependent cellular processes [64,65].
3.1. Local effects on Ca2+-dependent inactivation of LTCCs
Upon opening, LTCCs undergo rapid voltage-dependent and Ca2+-dependent inactivation that limits the amount of Ca2+ entry during each action potential. The mechanism of Ca2+-dependent inactivation (CDI) involves tethering of the Ca2+ sensor protein calmodulin (CaM) to the carboxyl tail of Ca 1.2 [66,67]. Ca2+v ions near the mouth of the channel bind to CaM, which subsequently changes conformation to bind a nearby IQ motif, accelerating channel inactivation. The degree of CDI observed depends on local [Ca2+] and thus may differ among channels localized to various subcellular environments [68].
At the dyadic cleft, a spatially restricted area estimated between 4.39 × 105 nm3 and 1.5 × 106 nm3 in size, ICa,L and subsequent SR Ca2+ release combine to dramatically increase local [Ca2+] from approximately 100 nM during diastole to 100-600 μM during systole [60,69]. Elevated [Ca2+] in the dyadic space during CICR greatly enhances CDI of LTCCs, which impacts action potential duration in ventricular myocytes (Fig. 2) [70-75]. This was demonstrated in rat ventricular myocytes by depleting SR Ca2+ stores with thapsigargin to abolish CICR, which slowed CDI, resulting in a 40-70% increase in ICa,L during the action potential waveform and prolonging action potential duration [72].
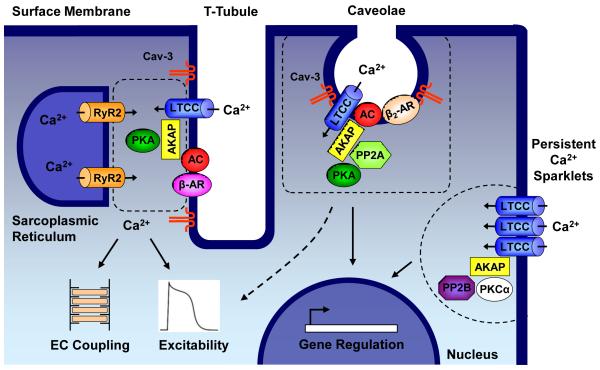
Figure 2. Schematic of L-type Ca2+ channel subpopulations and associated macromolecular signaling complexes that contribute to diverse processes within the cardiac myocyte.
Within T-tubules, some L-type Ca2+ channels (LTCCs) form dyadic junctions with ryanodine receptors (RyR2) on the apposing sarcoplasmic reticulum (SR). A small influx of Ca2+ through LTCCs triggers release of SR Ca2+ stores through RyR2, leading to the intracellular [Ca2+]i transient, which is essential for activation of myofilament proteins leading to contraction. β-adrenergic receptor (β-AR) stimulation of [Ca2+]i transients involves a signaling complex consisting of LTCCs, β-AR, adenylyl cyclase (AC), protein kinase A (PKA), caveolin-3 (Cav-3), and A-kinase anchoring proteins (AKAPs) [85]. Multiple AKAPs are important for regulating LTCC function in the heart including AKAP5 and AKAP15 [85,131]. Additional LTCC subpopulations in the surface sarcolemma are implicated in signaling to the nucleus to regulate the transcription of genes involved in cell survival or cardiac hypertrophy and may also contribute to membrane excitability. Within caveolae, LTCCs associate in a macromolecular protein complex with β2-AR, AC, PKA, protein phosphatase 2A (PP2A), and Cav-3. Caveolar LTCCs are locally stimulated by β2-AR activation [5]. Coupled gating of closely neighboring LTCCs results in persistent Ca2+ sparklets, which are enhanced by AKAPs and protein kinase C alpha (PKCα) and may serve as a local source of Ca2+ to stimulate calcineurin (PP2B) signaling to the nucleus [83,97].
Interestingly, LTCCs outside of T-tubules have been shown to exhibit slower inactivation kinetics, indicating surface membrane channels are less sensitive to SR Ca2+ release [74,76]. It is not clear whether subpopulations of surface membrane LTCCs localized to membrane microdomains such as lipid rafts and caveolae have a characteristic ‘signature’ of CDI. However, experiments using fluorescence-based Ca2+ sensors have indicated that subcaveolar [Ca2+] is higher than levels associated with the surrounding plasma membrane in endothelial cells [77]. Given the known clustering of LTCCs in lipid raft and caveolar microdomains in cardiomyocytes, it is possible that locally elevated subcaveolar [Ca2+] may contribute to unique CDI among caveolar LTCCs relative to channels residing outside of caveolae [5,43,51].
There is evidence that the conformational change of the CaM-bound C-terminus of Cav1.2 during CDI directly impacts Ca2+-dependent cellular processes such as excitation-transcription coupling and SR Ca2+ release [78]. For instance, activation of cAMP-responsive element-binding protein (CREB)-mediated gene transcription upon depolarization is dependent on a freely moving Cav1.2 C-terminus as well as the binding of CaM. This was demonstrated through experiments in which the Cav1.2 C-terminus was either immobilized to the plasma membrane by fusing it to a pleckstrin homology domain or made insensitive to CaM, both of which diminished CDI and abolished CREB activation despite an influx of Ca2+ and corresponding rise in [Ca2+]i [79]. These findings suggest at least one mechanism of excitation-transcription coupling is attributed more to the movement of CaM-bound Cav1.2 C-terminus than to ICa,L or changes in [Ca2+]i [78, 79]. Thus, overall differences in the degree and kinetics of CDI among subpopulations of LTCCs provide a mechanism to differentially regulate Ca2+ signaling processes within distinct subcellular domains of cardiac myocytes.
3.2. Coupled gating of LTCCs
Ca2+ influx through single LTCCs or small clusters of channels can be measured with high spatiotemporal resolution using total internal reflection fluorescence (TIRF) microscopy [80,81]. Whereas single L-type channels normally exhibit random, infrequent openings that permit small rises in submembrane [Ca2+] termed “Ca2+ sparklets”, some channels associate in highly active clusters that generate localized regions of significantly elevated [Ca2+] [82,83]. These “persistent Ca2+ sparklets” arise from the coupled opening of as many as six adjacent LTCCs [83]. Coupled gating of neighboring LTCCs associated with persistent Ca2+ sparklets is enhanced by the scaffold protein A-kinase anchoring protein (AKAP) 5 (also known as AKAP 79/150) as well as protein kinase C alpha (PKCα) [83]. Thus, subpopulations of LTCCs associating with AKAP5 and PKCα may serve as specialized Ca2+-signaling domains that are coupled to discrete cellular functions requiring locally high [Ca2+] [83,84]. Whether LTCCs giving rise to persistent Ca2+ sparklets localize to specific subcellular compartments remains unclear; however, AKAP5 has been shown to associate with Cav-3, indicating caveolae or Cav-3 scaffolds may serve an important role in modulating persistent Ca2+ sparklet activity [85]. Future work is needed to define the precise subcellular localization and physiological role of persistent Ca2+ sparklets in cardiac myocytes.
3.3. Membrane microdomains and excitation-transcription coupling
LTCCs provide a critical link between cellular excitability and gene regulation. Considering transcriptional regulation is on a time course of minutes to hours, it has remained puzzling how cardiac myocytes regulate Ca2+-dependent gene regulation given the rhythmic cycling of cytosolic [Ca2+]i with each heart beat. This has led some to propose that specific membrane microdomain compartments are responsible for Ca2+-dependent signal transduction to the nucleus [86,87].
CaM plays a significant role in coupling ICa,L to transcriptional regulation in cardiac myocytes, particularly in the activation of hypertrophic signaling [88,89]. Ca2+-CaM activates a number of transcriptional pathways, and the two most prominent in cardiac muscle involve either Ca2+-CaM-dependent protein kinases (CaMK) or the phosphatase calcineurin (protein phosphatase 2B, PP2B) [88]. The importance of ICa,L in stimulating hypertrophic remodeling was recently demonstrated in transgenic mice with cardiac-specific overexpression of the LTCC β2a subunit to increase ICa,L, which develop hypertrophy that is inhibited using blockers of LTCCs, CaMK, or calcineurin [90].
Relatively little is currently known regarding whether specific LTCC populations are responsible for signaling to the nucleus through the CaM-CaMK pathway. Interestingly, CaMKIIα has been localized to lipid rafts in neurons, and disruption of lipid rafts using methyl-β-cyclodextrin reduced phosphorylation of CaMKII substrates [91,92]. However, several studies have shown that overexpression of the nuclear localized CaMKIV and CaMKII-δB isoforms stimulate cardiomyocyte hypertrophy whereas the nonnuclear CaMKIIα does not seem to play a significant role in hypertrophic remodeling [93,94]. There is evidence that ICa,L triggers translocation of CaM from the cytosol to the nucleus, where it activates nuclear CaMKIV [95]. In cardiomyocytes, CaMKIV induces a hypertrophic response through activation of the transcription factor MEF2 [94].
Activation of the Ca2+-CaM-calcineurin pathway leads to dephosphorylation of members of the nuclear factor of activated T cells (NFAT) transcription factor family within the cytosol, which then translocate to the nucleus to regulate genes involved in cardiac hypertrophy [96]. In arterial smooth muscle cells and neurons, a macromolecular signaling complex between Cav1.2, AKAP5, and calcineurin couples ICa,L with NFAT-mediated gene transcription [97,98]. Recently, calcineurin has been demonstrated to associate with Cav1.2, AKAP5, and Cav-3 in ventricular myocytes [85]. This raises the possibility that LTCCs within caveolae or associated with Cav-3 scaffolds specifically couple to calcineurin-NFAT-mediated gene regulation (Fig. 2); however, little experimental evidence currently exists to directly support this hypothesis. It is worth noting that Cav-3 has a known role in cardiac hypertrophy, as knockout of Cav-3 leads to hypertrophic cardiomyopathy in mice [99].
3.4. Membrane microdomains and excitation-secretion coupling
LTCCs provide a critical influx of Ca2+ necessary for secretion of hormones in some cell types [100,101]. Cav1.2 associates with several soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins involved in exocytosis [102,103]. In pancreatic β-cells, it has been shown that the coupling of LTCCs to SNARE proteins permits locally elevated [Ca2+] near exocytotic machinery to trigger insulin secretion [102].
In atrial myocytes, LTCCs regulate secretion of atrial natriuretic peptide (ANP), a hormone with diuretic, natriuretic, and vasodilatory properties [104]. However, the precise role of ICa,L remains unclear. Some studies have shown that ICa,L stimulates ANP release, but other studies have suggested that ICa,L inhibits ANP secretion [105-112]. ANP release is also largely controlled by mechanical stretch, and ICa,L is coupled to the regulation of stretch-induced ANP secretion, suggesting there may be different roles for Ca2+ in basal versus stimulated release [112-114].
Little is known regarding the subcellular localization of ANP release machinery in atrial myocytes. However, several aspects of caveolae make this microdomain an attractive candidate for coordinating secretion of ANP. First, ANP has been localized to caveolae in atrial myocytes using electron microscopy [115]. Second, the localization of SNARE proteins to cholesterol-rich microdomains has been reported in various cell types and methyl-β-cyclodextrin treatment reduces exocytosis of secretory vesicles [116-118]. Finally, caveolae contain a subpopulation of LTCCs and are essential for translating mechanical stretch to activation of downstream signaling pathways, both of which impact the rate of ANP release [5,119]. Thus, it is possible that ANP release from atrial myocytes occurs in specialized membrane microdomains such as caveolae where LTCCs have preferred access to secretory machinery.
3.5. Modulation of ICa,L by membrane cholesterol and lipids
Relative to the bulk plasma membrane, lipid rafts and caveolae are enriched in sphingolipids, cholesterol, and phosphoinositides, which have been shown to modulate the functional properties of a number of ion channels [39,120]. Experimental approaches to investigate the role of cholesterol or lipids on ion channel function are often complicated because these interventions also target a large number of signaling molecules that regulate ion channel activity. However, there is some evidence that cholesterol directly modulates ICa,L. Exposure of smooth muscle cells to cholesterol-enriched liposomes led to a gradual increase in ICa,L and a positive shift in the voltage dependence of inactivation that was speculated to result from changes in membrane physical properties [121]. Importantly, lipid-altering strategies such as statin drugs or dietary supplementation with n-3 polyunsaturated fatty acids have shown promise as anti-arrhythmic therapy. These interventions are believed to exert effects via several mechanisms including modulation of ion channel expression and function [122-124]. The antiarrhythmic action of polyunsaturated fatty acids such as eicosapentaenoic acid has been partially attributed to a decrease in ICa,L [122,125]. Likewise, simvastatin has been shown to suppress ICa,L in mouse ventricular myocytes [126]. Studies have also shown that statins and diets rich in n-3 polyunsaturated fatty acids alter lipid raft and caveolar composition [127-130]. Future work is needed to investigate whether these lipid-altering therapies specifically result in changes in the localization, function, or regulation of LTCCs within membrane microdomains.
4. Unique regulation of LTCC subpopulations
Neurohormonal regulation of LTCCs is central to the ability of the heart to adapt to changing physiological needs by altering heart rate and contractility [131,132]. Membrane microdomains such as lipid rafts and caveolae are particularly well-suited for regulation of ICa,L because of the targeting of proteins involved in a variety of signaling cascades to these domains. Co-localization of signaling molecules with LTCCs enables highly localized and specific regulation of the channels.
4.1. Localization of LTCCs to a caveolar macromolecular signaling complex is required for β2-adrenergic regulation
The sympathetic nervous system drives the fight-or-flight response through activation of β-adrenergic receptors, and one of the essential downstream targets of β-adrenergic regulation is the LTCC [38]. Three subtypes of β-adrenergic receptors exist (β1-3) in myocardium and notable differences exist in the manner by which each receptor affects downstream pathways. For example, chronic activation of β1-adrenergic receptors results in cardiomyocyte apoptosis, whereas β2-adrenergic receptor stimulation is cardioprotective [133]. Furthermore, β1 receptor activation causes a global cellular response that includes PKA-mediated phosphorylation of multiple proteins involved in EC coupling including LTCCs and the SR protein phospholamban, yet β2 receptors signal locally to increase ICa,L without affecting other PKA-dependent processes such as phospholamban phosphorylation [134,135].
A number of recent studies have helped explain these divergent physiological responses observed with β1 versus β2-adrenergic receptor activation. A subpopulation of LTCCs was identified as part of a caveolar macromolecular complex with the β2-adrenergic receptor, adenylyl cyclase, Gαs, Gαi, and protein phosphatase 2A (PP2A) in ventricular myocytes [5]. Disruption of caveolae in neonatal mouse ventricular myocytes with either methyl-β-cyclodextrin or short interfering RNA (siRNA)-mediated knockdown of Cav-3 abolished β2-adrenergic stimulation of ICa,L (Fig. 1C,D) without effecting β1-adrenergic enhancement of ICa,L[5]. Similar studies utilizing methyl-β-cyclodextrin or intracellular application of Cav-3 antibody in adult rat ventricular myocytes showed disruption of normal β2 regulation of ICa,L [42]. These results demonstrate specific coupling of LTCCs with β2-adrenergic receptors within caveolae or in a complex scaffolded by Cav-3 and suggest that channels coupled to β1-adrenergic signaling molecules localize outside of cholesterol-rich membrane microdomains. Interestingly, ICa,L-mediated cardiomyocyte apoptosis resulting from β1-adrenergic activation involves CaMKII, which has not been reported in caveolae [136]. Thus, the specific coupling of caveolar LTCCs with β2-adrenergic receptors may stimulate pro-survival signaling (Fig. 2) [86,137].
Activation of Gαs-coupled receptors activates adenylyl cyclase, resulting in production of cAMP. Monitoring subcellular localization of cAMP pools using fluorescence resonance energy transfer (FRET)-based biosensors in ventricular myocytes has demonstrated that stimulation of β-adrenergic receptors using isoproterenol leads to cAMP accumulation within cytosolic and caveolar compartments, whereas activation of another Gαs-coupled receptor, the E-type prostaglandin receptor 4, results only in cytoplasmic cAMP production [138]. Thus, restricted production of cAMP within the caveolar compartment following β2-adrenergic receptor activation may explain the localized signaling between β2 receptors and LTCCs. This idea is consistent with studies in which disruption of caveolae with methyl-β-cyclodextrin led to more diffuse cAMP regulation after β2-adrenergic activation that included PKA phosphorylation of phospholamban [139]. Spatially restricted cAMP generation associated with β2 adrenergic activation may also involve coupling of β2 receptors with Gαi or localized expression of phosphodiesterase 4D isoforms [140,141].
4.2. AKAP5 essential for β-adrenergic stimulation of [Ca2+]i transient
Whereas selective activation of β2-adrenergic receptors within caveolae does not have far-reaching regulatory effects on phospholamban or contractile proteins, β1-adrenergic stimulation leads to global signaling events that increase contractility and speed relaxation. Recently, a macromolecular signaling complex involving the scaffolding protein AKAP5 and Cav-3 was demonstrated to be essential for β-adrenergic regulation of EC coupling proteins including LTCCs, ryanodine receptors and phospholamban [85]. Knockout of AKAP5 in mice abolished isoproterenol (a nonselective β-adrenergic receptor agonist) stimulation of [Ca2+]i transients, as well as phosphorylation of ryanodine receptor and phospholamban. Intriguingly, enhancement of whole cell ICa,L by isoproterenol remained intact in AKAP5 knockout mice. Further work demonstrated that in wild type mice, Cav1.2 channels associated with Cav-3 were preferentially phosphorylated at Serine 1928 (S1928) after treatment with isoproterenol whereas the fraction of Cav1.2 not associating with Cav-3 did not undergo significant phosphorylation. In AKAP5 knockout mice, however, the converse was observed as isoproterenol predominantly led to phosphorylation of non-Cav-3-associated Cav1.2 channels. Thus, loss of AKAP5 leads to β-adrenergic stimulation of ICa,L acting on a different subpopulation of LTCCs that does not communicate directly with ryanodine receptors. However, the physiological relevance of S1928 phosphorylation is debated as recent studies have demonstrated that S1928 is not necessary for the PKA-mediated increase in ICa,L [131,142,143]. Nevertheless, the authors provided important evidence to propose a model in which AKAP5 serves as a scaffold to assemble proteins involved in EC coupling with a β-adrenergic signaling complex within T-tubules, and interestingly this macromolecular signaling complex included Cav-3 (Fig. 2).
Questions remain regarding the precise subcellar localization of this AKAP5/Cav1.2/Cav-3 complex. In ventricular myocytes, caveolae are found in both surface and T-tubular sarcolemma but are absent from dyadic junctions [53,144]. A number of studies have demonstrated Cav-3 expression within the T-tubule system in ventricular myocytes, where Cav-3 has some degree of colocalization with Cav1.2 and ryanodine receptor [54,145]. Considering caveolar structures are excluded from dyads, the observation that Cav-3 colocalizes with both Cav1.2 and ryanodine receptor within the cell interior may suggest the presence of Cav-3 scaffolds at or very close to dyadic junctions. A recent study using a triple immunolabeling strategy revealed that a small but significant percentage (~3-5%) of Cav-3 colocalizes with both Cav1.2 and ryanodine receptor in atrial myocytes, suggesting Cav-3 is expressed at or adjacent to select dyads; however, atrial myocytes lack the highly developed T-tubule system seen in adult ventricular myocytes, which may lead to differences in dyad architecture between the two cell types [146,147]. Another study using confocal microscopy has reported little to no Cav-3 localization at dyad junctions in rat ventricular myocytes [148]. Because perturbation of cholesterol rich microdomains reduces [Ca2+]i transient amplitude and knockout of Cav-3 in mice leads to reduced myocardial contractility measured by fractional shortening, it seems possible that Cav-3 is expressed sufficiently close to dyadic junctions to play an important role in regulating EC coupling (Fig. 2) or alternatively influx of Ca2+ through distinct caveolar LTCCs contributes significantly to loading SR stores [42,99]. Additional strategies such as immunogold electron microscopy may yield insight into the localization of Cav-3 relative to dyadic Cav1.2 channels.
5. Targeting LTCCs to subcellular compartments
Considering spatially defined subpopulations of LTCCs regulate discrete cellular functions, understanding the mechanisms responsible for appropriate channel targeting to various subcellular compartments in cardiac myocytes is important, yet these pathways are largely undefined. Recent advances in our understanding of LTCC trafficking in cardiomyocytes will be briefly discussed.
5.1. Influence of Ca2+ channel auxiliary subunits on subcellular localization of LTCCs
The LTCC β subunits may play a significant role in defining subcellular L-type Ca2+ channel populations. Early evidence that β subunits can directly impact subcellular Ca2+ channel targeting came from studies in polarized epithelial cells, in which Cav2.1 subunits were trafficked to the basolateral membrane by β1a and β4 subunits but exhibited apical membrane localization when coexpressed with β2a [149]. Disparities in the localization of various β subunits have been also observed in cardiomyocytes. Western blots of canine ventricular membrane fractions and immunoconfocal microscopy of isolated ventricular myocytes demonstrated that β1b, β2, and β3 isoforms localize predominantly to T-tubule membranes while expression of β1a and β4 was detected more strongly at the surface sarcolemma [23]. Furthermore, adenovirus-mediated expression of various β subunits fused to GFP showed marked differences in subcellular localization among β subunit isoforms in cultured adult rat ventricular myocytes [59]. There is mounting evidence that caveolar localization of Cav1.2 channels requires an association between β2 subunits and Cav-3, suggesting β subunits play an active role in targeting L-type Ca2+ channel complexes in cardiac myocytes [150].
A role for α2δ subunits in subcellular targeting of LTCCs has also been proposed. The α2δ subunits undergo posttranslational modification to add glycosylphosphatidyl inositol (GPI) anchors, which are known to localize proteins to highly ordered microdomains such as lipid rafts and caveolae [151-152]. Another possibility is that trafficking of cardiac LTCCs to caveolae may involve Cav-3. Cav-1 has been demonstrated to direct trafficking of the voltage-dependent K+ channel Kv1.5 to cholesterol-rich rafts [153]. The skeletal muscle Cav1.1 LTCC subunit has been reported to interact directly with Cav-3; however, this has not been demonstrated with Cav1.2 [154]. Future work will be necessary to carefully address whether other auxiliary subunits are important for targeting LTCC complexes to specific membrane microdomains such as caveolae.
5.2. BIN1 targets channels to T-tubules in cardiac myocytes
Localization of LTCCs to dyadic junctions within T-tubule structures is essential for EC coupling, and mislocalization of channels caused by remodeling in heart failure is thought to contribute to impaired contractility. T-tubule biogenesis involves the membrane scaffolding protein BIN1, and recent studies have implicated BIN1 in targeting LTCCs to T-tubules [155,156]. BIN1 localized to T-tubules in human and mouse ventricular myocytes in that BIN1 tethers microtubule structures to coordinate anterograde trafficking of Cav1.2 channels to T-tubule structures [156]. Importantly, knockdown of BIN1 reduced plasmalemmal expression of Cav1.2 channels and diminished [Ca2+]i transients, suggesting fewer dyadic LTCCs [156].
5.3. Internalization and degradation of LTCCs
Although BIN1 plays an important role in anterograde trafficking of LTCCs to the plasma membrane, other mechanisms likely regulate channel retrieval and intracellular trafficking. There is some evidence for regulated internalization of LTCC complexes in cardiomyocytes; exposure of ventricular myocytes to isoproterenol for several minutes led to β-arrestin-1-mediated internalization of Cav1.2 into clathrin-coated vesicles [157]. However, it was unclear whether a specific subpopulation of LTCCs was targeted for internalization (i.e. bulk plasma membrane vs. caveolar); however, caveolar endocytosis is thought to be largely clathrin-independent, which suggests internalized channels are extracaveolar [158]. Furthermore, the destination of these internalized channels was not defined. Evidence exists for endocytic recycling of some cardiac ion channels to the surface membrane, and this process is normally regulated by the small GTPase Rab11 [159-161]. However, Rab11b has been demonstrated to target surface membrane LTCCs for degradation in cardiomyocytes [162]. Thus, recycling of cardiac LTCCs to the plasma membrane may occur via an alternative pathway, or internalized Cav1.2 channels may be preferentially degraded rather than recycled. In neurons, L-type Cav1.2 and Cav1.3 channels internalized in the presence of glutamate are targeted to the lysosome, which is thought to protect against excitotoxicity [163,164].
6. Altered microdomains disrupt LTCC function in cardiac disease
Dysregulation of LTCCs contributes to the pathophysiology of numerous heart diseases including heart failure, atrial fibrillation, and long and short QT syndromes [165-170]. Several reports have suggested that the geometry and protein composition of subcellular compartments associated with LTCC activity are altered in some cardiac diseases [171-174]. These changes could directly impact the function and regulation of LTCCs and contribute to defects in Ca2+ signaling, dysregulated EC coupling, and electrical instability [175].
6.1 Mutations in Cav1.2 and LTCC auxiliary subunits
Mutations in the genes encoding Cav1.2 and LTCC auxiliary subunits including β2b and α2δ-1 have been reported in patients with inherited arrhythmia disorders [165,169,176]. A number of mutations in Cav1.2, β2, and α2δ-1 lead to a loss-of-function phenotype characterized by dramatically reduced ICa,L. Although in most cases the reduction in ICa,L was attributed to changes in biophysical properties of the channel, one mutation in Cav1.2 (A39V) associated with short QT syndrome and sudden death disrupted LTCC trafficking [165,176]. It is unclear whether any of these reported loss-of-function mutations alter the specific subcellular localization of LTCCs. However given the potential role of auxiliary β and α2δ subunits in the subcellular targeting of LTCCs (see Section 5.1), it is possible these phenotypes could result from the mislocalization of critical LTCC subpopulations.
Timothy syndrome (TS) is a multisystem disorder characterized by ventricular arrhythmias and autism resulting from gain-of-function mutations (G406R, G402S) in Cav1.2 that interfere with voltage-dependent channel inactivation [169]. Interestingly, TS mutant G406R Cav1.2 channels have recently been demonstrated to exhibit increased propensity for coupled gating and persistent Ca2+ sparklets [83]. TS Cav1.2 channels in transgenic ventricular myocytes exhibited prominent localization at the surface sarcolemma and intercalated discs relative to T-tubules, suggesting these mutant channels represent a distinct subcellular population [167]. TS Cav1.2 channels appear to be abnormally coupled to AKAP5, undergoing frequent and prolonged openings that are eliminated by knockout of AKAP5 [167]. AKAP5 ablation also protects TS transgenic mice from arrhythmias, indicating persistent Ca2+ sparklet activity plays a significant role in the TS phenotype. Precisely where TS Cav1.2 channels target remains unclear, but caveolae represent an intriguing candidate due to the known association between AKAP5 and Cav-3 [85].
6.2. CAV3 mutations associated with arrhythmia and cardiomyopathy
Congenital long QT syndrome (LQTS) is a potentially lethal disorder associated with delayed cardiac repolarization, prolonged QT interval, and ventricular arrhythmias and arises due to mutations in a number of ion channels and scaffolding proteins [178]. Recently, mutations in the CAV3 gene were identified in a subset of LQTS patients (designated LQT9). Initial studies showed that the CAV3 mutations resulted in increased late Na+ current through Nav1.5 channels, which could prolong action potential duration [179]. Because LTCCs and important regulatory molecules such as β2-adrenergic receptors associate with Cav-3, alterations in ICa,L may also contribute to the pathophysiology of LQT9. However, additional information regarding the electrophysiological effects of LQTS CAV3 mutations is not available. Other mutations in CAV3 have been linked to familial hypertrophic cardiomyopathy in one family and dilated cardiomyopathy with AV conduction defects in another [180,181]. The molecular mechanisms leading to these phenotypes are not well understood.
In addition to CAV3, mutations in the gene encoding the caveolar protein PTRF/Cavin have also been linked to LQTS, sinus bradycardia, and supraventricular and ventricular tachycardias [182]. Further work will be required to understand the detailed mechanisms underlying cardiac arrhythmias and cardiomyopathies induced by mutations in caveolar proteins.
6.2. Remodeling in failing heart
Heart failure is characterized by weakened myocardial contractile force, partially due to abnormal EC coupling resulting in reduced SR Ca2+ release [171,183,184]. A number of studies have demonstrated extensive remodeling of the T-tubular system in failing heart, which likely contributes to inefficient EC coupling [167,185-187]. T-tubule structural remodeling in heart failure is associated with a significant (~50%) reduction in the density of LTCCs, and Cav1.2 channels within T-tubules may be specifically decreased compared to channels in the surface membrane [167,188]. It has also been suggested that T-tubule remodeling may alter the geometry of the dyadic cleft microdomain [171,174,189]. Alternatively, changes in T-tubular architecture and composition could potentially disrupt macromolecular signaling complexes and lead to dysregulation of LTCCs. Blunted β-adrenergic regulation of ICa,L has been observed in animal heart failure models and in human heart failure [167,190]. Changes in the relative density, localization, and coupling of β-adrenergic receptor subtypes have been observed [190-193]. For example, in a canine tachycardia-induced heart failure model, enhanced β2-adrenergic signaling through Gαi strongly blunted the increase in ICa,L by β1-adrenergic stimulation [190].
6.4. Atrial fibrillation
Chronic atrial fibrillation involves important structural and electrical changes including significant downregulation of ICa,L that results in shortened action potential duration and reduced atrial contractility [194,195]. Some studies have attributed the decrease in ICa,L to a reduction in transcription of Cav1.2 or various LTCC auxiliary subunits genes resulting in reduced mRNA and protein levels, whereas other reports have suggested impaired posttranslational modifications such as dephosphorylation of Cav1.2, altered channel trafficking, or enhanced degradation [196-200]. A decrease in T-tubule density was observed in atrial myocytes from a sheep model of atrial fibrillation, which may underlie dysynchronous CICR and reduced contractility [201]. However, it remains unclear whether specific populations of LTCCs are altered in atrial fibrillation.
7. Conclusions and future directions
In cardiac myocytes, LTCCs are localized to multiple distinct subcellular compartments that impact their function and regulation (Fig. 2). The significance of dyadic LTCCs in EC coupling has long been recognized, but other subpopulations, such as those localized to caveolae, are increasingly implicated in a variety of cellular functions and signaling pathways. Many cardiac diseases involve changes in subcellular architecture and organization, thus altered subcellular localization of LTCCs with associated changes in channel function properties can produce aberrant electrophysiology with resulting arrhythmias as well as dysregulation of various Ca2+-dependent cellular processes. Many gaps in our knowledge remain. For example, technical limitations still preclude detailed single channel studies of critical populations of LTCCs localized to T-tubules and thus out of reach from the patch pipette or TIRF microscope. Future studies will undoubtedly reveal additional details regarding how LTCCs and associated Ca2+-signaling molecules are assembled and targeted to defined subcellular compartments. Furthermore, a better understanding of the various subcellular populations of LTCCs may enable new therapeutic approaches for prevalent forms of heart disease such as heart failure and atrial fibrillation.
Highlights.
-
>
L-type Ca2+ channels regulate diverse cellular processes in the heart.
-
>
Different L-type Ca2+ channel subpopulations exist in cardiomyocytes.
-
>
Function and regulation of L-type Ca2+ channels depend on subcellular localization.
-
>
Altered localization of L-type Ca2+ channels plays a role in heart disease.
Acknowledgements
This work was supported by National Heart, Lung, and Blood Institute Grant R01 HL078878 (to T.J.K.) and American Heart Association Predoctoral Fellowship 10PRE2580002 (to J.M.B.).
Abbreviations
- LTCC
L-type Ca2+ channel
- EC
excitation-contraction
- ICa,L
L-type Ca2+ current
- SR
sarcoplasmic reticulum
- T-tubules
transverse tubules
- CICR
Ca2+-induced Ca2+ release
- [Ca2+]i
free intracellular Ca2+ concentration
- PKA
protein kinase A
- Cav
caveolin
- PTRF
Polymerase I and Transcript Release Factor
- C-terminus
carboxyl terminus
- CDI
Ca2+-dependent inactivation
- CaM
calmodulin
- CREB
cAMP-responsive element-binding protein
- TIRF
total internal reflection fluorescence
- AKAP
A-kinase anchoring protein
- PKCα
protein kinase C alpha
- CaMK
Ca2+-CaM-dependent kinase
- PP2B
protein phosphatase 2B
- NFAT
nuclear factor of activated T cells
- SNARE
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- ANP
atrial natriuretic peptide
- PP2A
protein phosphatase 2A
- FRET
fluorescence resonance energy transfer
- GPI
glycosylphosphatidyl inositol
- TS
Timothy syndrome
- LQTS
congenital long QT syndrome
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Carl SL, Felix K, Caswell AH, Brandt NR, Ball WJ, Jr, Vaghy PL, Meissner G, Ferguson DG. Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J Cell Biol. 1995;129:673–82. doi: 10.1083/jcb.129.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Sun XH, Protasi F, Takahashi M, Takeshima H, Ferguson DG, Franzini-Armstrong C. Molecular architecture of membranes involved in excitation-contraction coupling of cardiac muscle. J Cell Biol. 1995;129:659–71. doi: 10.1083/jcb.129.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Takagishi Y, Rothery S, Issberner J, Levi A, Severs NJ. Spatial distribution of dihydropyridine receptors in the plasma membrane of guinea pig cardiac myocytes investigated by correlative confocal microscopy and label-fracture electron microscopy. J Electron Microsc (Tokyo) 1997;46:165–70. doi: 10.1093/oxfordjournals.jmicro.a023504. [DOI] [PubMed] [Google Scholar]
- [4].Takagishi Y, Yasui K, Severs NJ, Murata Y. Species-specific difference in distribution of voltage-gated L-type Ca(2+) channels of cardiac myocytes. Am J Physiol Cell Physiol. 2000;279:C1963–9. doi: 10.1152/ajpcell.2000.279.6.C1963. [DOI] [PubMed] [Google Scholar]
- [5].Balijepalli RC, Foell JD, Hall DD, Hell JW, Kamp TJ. Localization of cardiac L-type Ca(2+) channels to a caveolar macromolecular signaling complex is required for beta(2)-adrenergic regulation. Proc Natl Acad Sci U S A. 2006;103:7500–5. doi: 10.1073/pnas.0503465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol. 2008;98:149–60. doi: 10.1016/j.pbiomolbio.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Maxfield FR. Plasma membrane microdomains. Curr Opin Cell Biol. 2002;14:483–7. doi: 10.1016/s0955-0674(02)00351-4. [DOI] [PubMed] [Google Scholar]
- [8].Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–72. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- [9].Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–94. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- [10].Catterall WA. Structure and function of voltage gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–55. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- [11].Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–3. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- [12].Takimoto K, Li D, Nerbonne JM, Levitan ES. Distribution, splicing and glucocorticoid-induced expression of cardiac alpha 1C and alpha 1D voltage-gated Ca2+ channel mRNAs. J Mol Cell Cardiol. 1997;29:3035–42. doi: 10.1006/jmcc.1997.0532. [DOI] [PubMed] [Google Scholar]
- [13].Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- [14].Mangoni ME, Couette B, Bourinet E, Platzer J, Reimer D, Striessnig J, Nargeot J. Functional role of L-type Cav1.3 Ca2+ channels in cardiac pacemaker activity. Proc Natl Acad Sci U S A. 2003;100:5543–8. doi: 10.1073/pnas.0935295100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang Q, Timofeyev V, Qiu H, Lu L, Li N, Singapuri A, Torado CL, Shin HS, Chiamvimonvat N. Expression and roles of Cav1.3 (α1D) L-type Ca2+ channel in atrioventricular node automaticity. J Mol Cell Cardiol. 2011;50:194–202. doi: 10.1016/j.yjmcc.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liao P, Yong TF, Liang MC, Yue DT, Soong TW. Splicing for alternative structures of Cav1.2 Ca2+ channels in cardiac and smooth muscles. Cardiovasc Res. 2005;68:197–203. doi: 10.1016/j.cardiores.2005.06.024. [DOI] [PubMed] [Google Scholar]
- [17].Yang Y, Chen X, Margulies K, Jeevanandam V, Pollack P, Bailey BA, Houser SR. L-type Ca2+ channel alpha 1c subunit isoform switching in failing human ventricular myocardium. J Mol Cell Cardiol. 2000;32:973–84. doi: 10.1006/jmcc.2000.1138. [DOI] [PubMed] [Google Scholar]
- [18].Welling A, Kwan YW, Bosse E, Flockerzi V, Hofmann F, Kass RS. Subunit-dependent modulation of recombinant L-type calcium channels. Molecular basis for dihydropyridine tissue selectivity. Circ Res. 1993;73:974–80. doi: 10.1161/01.res.73.5.974. [DOI] [PubMed] [Google Scholar]
- [19].Welling A, Ludwig A, Zimmer S, Klugbauer N, Flockerzi V, Hofmann F. Alternatively spliced IS6 segments of the alpha 1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res. 1997;81:526–32. doi: 10.1161/01.res.81.4.526. [DOI] [PubMed] [Google Scholar]
- [20].Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N. The roles of the subunits in the function of the calcium channel. Science. 1991;253:1553–7. doi: 10.1126/science.1716787. [DOI] [PubMed] [Google Scholar]
- [21].Kamp TJ, Pérez-García MT, Marban E. Enhancement of ionic current and charge movement by coexpression of calcium channel beta 1A subunit with alpha 1C subunit in a human embryonic kidney cell line. J Physiol. 1996;492:89–96. doi: 10.1113/jphysiol.1996.sp021291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chien AJ, Zhao X, Shirokov RE, Puri TS, Chang CF, Sun D, Rios E, Hosey MM. Roles of a membrane-localized beta subunit in the formation and targeting of functional L-type Ca2+ channels. J Biol Chem. 1995;270:30036–44. doi: 10.1074/jbc.270.50.30036. [DOI] [PubMed] [Google Scholar]
- [23].Foell JD, Balijepalli RC, Delisle BP, Yunker AM, Robia SL, Walker JW, McEnery MW, January CT, Kamp TJ. Molecular heterogeneity of calcium channel beta-subunits in canine and human heart: evidence for differential subcellular localization. Physiol Genomics. 2004;17:183–200. doi: 10.1152/physiolgenomics.00207.2003. [DOI] [PubMed] [Google Scholar]
- [24].Jay SD, Sharp AH, Kahl SD, Vedvick TS, Harpold MM, Campbell KP. Structural characterization of the dihydropyridine-sensitive calcium channel alpha 2-subunit and the associated delta peptides. J Biol Chem. 1991;266:3287–93. [PubMed] [Google Scholar]
- [25].Chu PJ, Best PM. Molecular cloning of calcium channel alpha(2)delta-subunits from rat atria and the differential regulation of their expression by IGF-1. J Mol Cell Cardiol. 2003;35:207–15. doi: 10.1016/s0022-2828(02)00313-9. [DOI] [PubMed] [Google Scholar]
- [26].Klugbauer N, Lacinová L, Marais E, Hobom M, Hofmann F. Molecular diversity of the calcium channel alpha2delta subunit. J Neurosci. 1999;19:684–91. doi: 10.1523/JNEUROSCI.19-02-00684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bangalore R, Mehrke G, Gingrich K, Hofmann F, Kass RS. Influence of L-type Ca channel alpha 2/delta-subunit on ionic and gating current in transiently transfected HEK 293 cells. Am J Physiol. 1996;270:H1521–8. doi: 10.1152/ajpheart.1996.270.5.H1521. [DOI] [PubMed] [Google Scholar]
- [28].Jay SD, Ellis SB, McCue AF, Williams ME, Vedvick TS, Harpold MM, Campbell KP. Primary structure of the gamma subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1990;248:490–2. doi: 10.1126/science.2158672. [DOI] [PubMed] [Google Scholar]
- [29].Klugbauer N, Dai S, Specht V, Lacinová L, Marais E, Bohn G, Hofmann F. A family of gamma-like calcium channel subunits. FEBS Lett. 2000;470:189–97. doi: 10.1016/s0014-5793(00)01306-5. [DOI] [PubMed] [Google Scholar]
- [30].Yang L, Katchman A, Morrow JP, Doshi D, Marx SO. Cardiac L-type calcium channel (Cav1.2) associates with {gamma} subunits. FASEB J. 2011;25:928–36. doi: 10.1096/fj.10-172353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Scriven DR, Dan P, Moore ED. Distribution of proteins implicated in excitation-contraction coupling in rat ventricular myocytes. Biophys J. 2000;79:2682–91. doi: 10.1016/S0006-3495(00)76506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gathercole DV, Colling DJ, Skepper JN, Takagishi Y, Levi AJ, Severs NJ. Immunogold-labeled L-type calcium channels are clustered in the surface plasma membrane overlying junctional sarcoplasmic reticulum in guinea-pig myocytes-implications for excitation-contraction coupling in cardiac muscle. J Mol Cell Cardiol. 2000;32:1981–94. doi: 10.1006/jmcc.2000.1230. [DOI] [PubMed] [Google Scholar]
- [33].Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- [34].Scriven DR, Asghari P, Schulson MN, Moore ED. Analysis of Cav1.2 and ryanodine receptor clusters in rat ventricular myocytes. Biophys J. 2010;99:3923–9. doi: 10.1016/j.bpj.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bers DM. Excitation-contraction coupling and cardiac contractile force. 2nd ed. Kluwer Academic Press; Dordrecht, Netherlands: 2001. [Google Scholar]
- [36].Orchard CH, Pásek M, Brette F. The role of mammalian cardiac t-tubules in excitation-contraction coupling: experimental and computational approaches. Exp Physiol. 2009;94:509–19. doi: 10.1113/expphysiol.2008.043984. [DOI] [PubMed] [Google Scholar]
- [37].Kawai M, Hussain M, Orchard CH. Excitation-contraction coupling in rat ventricular myocytes after formamide-induced detubulation. Am J Physiol. 1999;277:H603–9. doi: 10.1152/ajpheart.1999.277.2.H603. [DOI] [PubMed] [Google Scholar]
- [38].Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- [39].Dart C. Lipid microdomains and the regulation of ion channel function. J Physiol. 2010;588:3169–78. doi: 10.1113/jphysiol.2010.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–8. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- [41].Echarri A, Muriel O, Del Pozo MA. Intracellular trafficking of raft/caveolae domains: insights from integrin signaling. Semin Cell Dev Biol. 2007;18:627–37. doi: 10.1016/j.semcdb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- [42].Calaghan S, White E. Caveolae modulate excitation-contraction coupling and beta2-adrenergic signalling in adult rat ventricular myocytes. Cardiovasc Res. 2006;69:816–24. doi: 10.1016/j.cardiores.2005.10.006. [DOI] [PubMed] [Google Scholar]
- [43].Cavalli A, Eghbali M, Minosyan TY, Stefani E, Philipson KD. Localization of sarcolemmal proteins to lipid rafts in the myocardium. Cell Calcium. 2007;42:313–22. doi: 10.1016/j.ceca.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–82. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- [45].Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–67. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- [46].Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160–5. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- [47].Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–50. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Galbiati F, Engelman JA, Volonte D, Zhang XL, Minetti C, Li M, Hou H, Jr, Kneitz B, Edelmann W, Lisanti MP. Caveolin-3 null mice show a loss of caveolae, changes in the microdomain distribution of the dystrophin-glycoprotein complex, and t-tubule abnormalities. J Biol Chem. 2001;276:21425–33. doi: 10.1074/jbc.M100828200. [DOI] [PubMed] [Google Scholar]
- [49].Rothberg KG, Ying YS, Kamen BA, Anderson RG. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990;111:2931–8. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hill MM, Bastiani M, Luetterforst R, Kirkham M, Kirkham A, Nixon SJ, Walser P, Abankwa D, Oorschot VM, Martin S, Hancock JF, Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–24. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Shibata EF, Brown TL, Washburn ZW, Bai J, Revak TJ, Butters CA. Autonomic regulation of voltage-gated cardiac ion channels. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S34–S42. doi: 10.1111/j.1540-8167.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- [52].Head BP, Insel PA. Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 2007;17:51–7. doi: 10.1016/j.tcb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- [53].Gabella G. Inpocketings of the cell membrane (caveolae) in the rat myocardium. J Ultrastruct Res. 1978;65:135–47. doi: 10.1016/s0022-5320(78)90051-5. [DOI] [PubMed] [Google Scholar]
- [54].Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA. G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol Chem. 2005;280:31036–44. doi: 10.1074/jbc.M502540200. [DOI] [PubMed] [Google Scholar]
- [55].Gerhardstein BL, Gao T, Bünemann M, Puri TS, Adair A, Ma H, Hosey MM. Proteolytic processing of the C terminus of the alpha(1C) subunit of L-type calcium channels and the role of a proline-rich domain in membrane tethering of proteolytic fragments. J Biol Chem. 2000;275:8556–63. doi: 10.1074/jbc.275.12.8556. [DOI] [PubMed] [Google Scholar]
- [56].Gao T, Cuadra AE, Ma H, Bunemann M, Gerhardstein BL, Cheng T, Eick RT, Hosey MM. C-terminal fragments of the alpha 1C (CaV1.2) subunit associate with and regulate L-type calcium channels containing C-terminal-truncated alpha 1C subunits. J Biol Chem. 2001;276:21089–97. doi: 10.1074/jbc.M008000200. [DOI] [PubMed] [Google Scholar]
- [57].Hulme JT, Yarov-Yarovoy V, Lin TW, Scheuer T, Catterall WA. Autoinhibitory control of the CaV1.2 channel by its proteolytically processed distal C-terminal domain. J Physiol. 2006;576:87–102. doi: 10.1113/jphysiol.2006.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schroder E, Byse M, Satin J. L-type calcium channel C terminus autoregulates transcription. Circ Res. 2009;104:1373–81. doi: 10.1161/CIRCRESAHA.108.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marbán E, Yue DT. Novel functional properties of Ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–52. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hayashi T, Martone ME, Yu Z, Thor A, Doi M, Holst MJ, Ellisman MH, Hoshijima M. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. J Cell Sci. 2009;122:1005–13. doi: 10.1242/jcs.028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Viola HM, Hool LC. Cross-talk between L-type Ca2+ channels and mitochondria. Clin Exp Pharmacol Physiol. 2010;37:229–35. doi: 10.1111/j.1440-1681.2009.05277.x. [DOI] [PubMed] [Google Scholar]
- [62].Viola HM, Arthur PG, Hool LC. Evidence for regulation of mitochondrial function by the L-type Ca2+ channel in ventricular myocytes. J Mol Cell Cardiol. 2009;46:1016–26. doi: 10.1016/j.yjmcc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- [63].Escobar M, Cardenas C, Colavita K, Petrenko NB, Franzini-Armstrong C. Structural evidence for perinuclear calcium microdomains in cardiac myocytes. J Mol Cell Cardiol. 2011;50:451–9. doi: 10.1016/j.yjmcc.2010.11.021. [DOI] [PubMed] [Google Scholar]
- [64].Bauer PJ. The local Ca concentration profile in the vicinity of a Ca channel. Cell Biochem Biophys. 2001;35:49–61. doi: 10.1385/CBB:35:1:49. [DOI] [PubMed] [Google Scholar]
- [65].Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- [66].Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+ - dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–58. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- [67].Pitt GS, Zühlke RD, Hudmon A, Schulman H, Reuter H, Tsien RW. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J Biol Chem. 2001;276:30794–802. doi: 10.1074/jbc.M104959200. [DOI] [PubMed] [Google Scholar]
- [68].Haack JA, Rosenberg RL. Calcium-dependent inactivation of L-type calcium channels in planar lipid bilayers. Biophys J. 1994;66:1051–60. doi: 10.1016/S0006-3495(94)80886-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Langer GA, Peskoff A. Calcium concentration and movement in the dyadic cleft space of the cardiac ventricular cell. Biophys J. 1996;70:1169–82. doi: 10.1016/S0006-3495(96)79677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Sham JS. Ca2+ release-induced inactivation of Ca2+ current in rat ventricular myocytes: evidence for local Ca2+ signalling. J Physiol. 1997;500:285–95. doi: 10.1113/jphysiol.1997.sp022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Adachi-Akahane S, Cleemann L, Morad M. Cross-signaling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. J Gen Physiol. 1996;108:435–54. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Takamatsu H, Nagao T, Ichijo H, Adachi-Akahane S. L-type Ca2+ channels serve as a sensor of the SR Ca2+ for tuning the efficacy of Ca2+-induced Ca2+ release in rat ventricular myocytes. J Physiol. 2003;552:415–24. doi: 10.1113/jphysiol.2003.050823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Sipido KR, Callewaert G, Carmeliet E. Inhibition and rapid recovery of Ca2+ current during Ca2+ release from sarcoplasmic reticulum in guinea pig ventricular myocytes. Circ Res. 1995;76:102–9. doi: 10.1161/01.res.76.1.102. [DOI] [PubMed] [Google Scholar]
- [74].Brette F, Sallé L, Orchard CH. Differential modulation of L-type Ca2+ current by SR Ca2+ release at the T-tubules and surface membrane of rat ventricular myocytes. Circ Res. 2004;95:e1–7. doi: 10.1161/01.RES.0000135547.53927.F6. [DOI] [PubMed] [Google Scholar]
- [75].Alseikhan BA, DeMaria CD, Colecraft HM, Yue DT. Engineered calmodulins reveal the unexpected eminence of Ca2+ channel inactivation in controlling heart excitation. Proc Natl Acad Sci U S A. 2002;99:17185–90. doi: 10.1073/pnas.262372999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Brette F, Orchard C. Resurgence of cardiac t-tubule research. Physiology (Bethesda) 2007;22:167–73. doi: 10.1152/physiol.00005.2007. [DOI] [PubMed] [Google Scholar]
- [77].Isshiki M, Ying YS, Fujita T, Anderson RG. A molecular sensor detects signal transduction from caveolae in living cells. J Biol Chem. 2002;277:43389–98. doi: 10.1074/jbc.M205411200. [DOI] [PubMed] [Google Scholar]
- [78].Morad M, Soldatov N. Calcium channel inactivation: possible role in signal transduction and Ca2+ signaling. Cell Calcium. 2005;38:223–31. doi: 10.1016/j.ceca.2005.06.027. [DOI] [PubMed] [Google Scholar]
- [79].Kobrinsky E, Schwartz E, Abernethy DR, Soldatov NM. Voltage-gated mobility of the Ca2+ channel cytoplasmic tails and its regulatory role. J Biol Chem. 2003;278:5021–8. doi: 10.1074/jbc.M211254200. [DOI] [PubMed] [Google Scholar]
- [80].Navedo MF, Amberg GC, Votaw VS, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102:11112–7. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms underlying heterogeneous Ca2+ sparklet activity in arterial smooth muscle. J Gen Physiol. 2006;127:611–22. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–6. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- [83].Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, Scott JD, Santana LF. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ Res. 2010;106:748–56. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Santana LF, Navedo MF. Natural inequalities: why some L-type Ca2+ channels work harder than others. J Gen Physiol. 2010;136:143–7. doi: 10.1085/jgp.200910391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, Santana LF, McKnight GS. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ Res. 2010;107:747–56. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].George MS, Pitt GS. The real estate of cardiac signaling: location, location, location. Proc Natl Acad Sci U S A. 2006;103:7535–6. doi: 10.1073/pnas.0602389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Houser SR, Molkentin JD. Does contractile Ca2+ control calcineurin-NFAT signaling and pathological hypertrophy in cardiac myocytes? Sci Signal. 2008;1:pe31. doi: 10.1126/scisignal.125pe31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Maier LS, Bers DM. Calcium, calmodulin, and calcium-calmodulin kinase II: heartbeat to heartbeat and beyond. J Mol Cell Cardiol. 2002;34:919–39. doi: 10.1006/jmcc.2002.2038. [DOI] [PubMed] [Google Scholar]
- [89].Bers DM, Guo T. Calcium signaling in cardiac ventricular myocytes. Ann N Y Acad Sci. 2005;1047:86–98. doi: 10.1196/annals.1341.008. [DOI] [PubMed] [Google Scholar]
- [90].Chen X, Nakayama H, Zhang X, Ai X, Harris DM, Tang M, Zhang H, Szeto C, Stockbower K, Berretta RM, Eckhart AD, Koch WJ, Molkentin JD, Houser SR. Calcium influx through Cav1.2 is a proximal signal for pathological cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2011;50:460–70. doi: 10.1016/j.yjmcc.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tsui J, Inagaki M, Schulman H. Calcium/calmodulin-dependent protein kinase II (CaMKII) localization acts in concert with substrate targeting to create spatial restriction for phosphorylation. J Biol Chem. 2005;280:9210–6. doi: 10.1074/jbc.M407653200. [DOI] [PubMed] [Google Scholar]
- [92].Wang X, Tian QB, Okano A, Sakagami H, Moon IS, Kondo H, Endo S, Suzuki T. BAALC 1-6-8 protein is targeted to postsynaptic lipid rafts by its N-terminal myristoylation and palmitoylation, and interacts with alpha, but not beta, subunit of Ca/calmodulin-dependent protein kinase II. J Neurochem. 2005;92:647–59. doi: 10.1111/j.1471-4159.2004.02902.x. [DOI] [PubMed] [Google Scholar]
- [93].Ramirez MT, Zhao XL, Schulman H, Brown JH. The nuclear deltaB isoform of Ca2+/calmodulin-dependent protein kinase II regulates atrial natriuretic factor gene expression in ventricular myocytes. J Biol Chem. 1997;272:31203–8. doi: 10.1074/jbc.272.49.31203. [DOI] [PubMed] [Google Scholar]
- [94].Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TA, Overbeek P, Richardson JA, Grant SR, Olson EN. CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo. J Clin Invest. 2000;105:1395–406. doi: 10.1172/JCI8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- [96].Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, Molkentin JD. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol. 2002;22:7603–13. doi: 10.1128/MCB.22.21.7603-7613.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–75. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Nieves-Cintrón M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci U S A. 2008;105:15623–8. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, Tang B, Jelicks LA, Kitsis RN, Christ GJ, Factor SM, Tanowitz HB, Lisanti MP. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–97. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- [100].Marcantoni A, Baldelli P, Hernandez-Guijo JM, Comunanza V, Carabelli V, Carbone E. L-type calcium channels in adrenal chromaffin cells: role in pace-making and secretion. Cell Calcium. 2007;42:397–408. doi: 10.1016/j.ceca.2007.04.015. [DOI] [PubMed] [Google Scholar]
- [101].Drews G, Krippeit-Drews P, Düfer M. Electrophysiology of islet cells. Adv Exp Med Biol. 2010;654:115–63. doi: 10.1007/978-90-481-3271-3_7. [DOI] [PubMed] [Google Scholar]
- [102].Wiser O, Bennett MK, Atlas D. Functional interaction of syntaxin and SNAP-25 with voltage-sensitive L- and N-type Ca2+ channels. EMBO J. 1996;15:4100–10. [PMC free article] [PubMed] [Google Scholar]
- [103].Kobayashi T, Yamada Y, Fukao M, Tsutsuura M, Tohse N. Regulation of Cav1.2 current: interaction with intracellular molecules. J Pharmacol Sci. 2007;103:347–53. doi: 10.1254/jphs.cr0070012. [DOI] [PubMed] [Google Scholar]
- [104].Nishikimi T, Maeda N, Matsuoka H. The role of natriuretic peptides in cardioprotection. Cardiovasc Res. 2006;69:318–28. doi: 10.1016/j.cardiores.2005.10.001. [DOI] [PubMed] [Google Scholar]
- [105].Ruskoaho H, Toth M, Ganten D, Unger T, Lang RE. The phorbol ester induced atrial natriuretic peptide secretion is stimulated by forskolin and Bay K8644 and inhibited by 8-bromo-cyclic GMP. Biochem Biophys Res Commun. 1986;139:266–74. doi: 10.1016/s0006-291x(86)80108-5. [DOI] [PubMed] [Google Scholar]
- [106].Schiebinger RJ. Calcium, its role in isoproterenol-stimulated atrial natriuretic peptide secretion by superfused rat atria. Circ Res. 1989;65:600–6. doi: 10.1161/01.res.65.3.600. [DOI] [PubMed] [Google Scholar]
- [107].Saito Y, Nakao K, Morii N, Sugawara A, Shiono S, Yamada T, Itoh H, Sakamoto M, Kurahashi K, Fujiwara M, et al. Bay K 8644, a voltage-sensitive calcium channel agonist, facilitates secretion of atrial natriuretic polypeptide from isolated perfused rat hearts. Biochem Biophys Res Commun. 1986;138:1170–6. doi: 10.1016/s0006-291x(86)80405-3. [DOI] [PubMed] [Google Scholar]
- [108].McDonough PM, Stella SL, Glembotski CC. Involvement of cytoplasmic calcium and protein kinases in the regulation of atrial natriuretic factor secretion by contraction rate and endothelin. J Biol Chem. 1994;269:9466–72. [PubMed] [Google Scholar]
- [109].Ito T, Toki Y, Siegel N, Gierse JK, Needleman P. Manipulation of stretch-induced atriopeptin prohormone release and processing in the perfused rat heart. Proc Natl Acad Sci U S A. 1988;85:8365–9. doi: 10.1073/pnas.85.21.8365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].De Bold ML, De Bold AJ. Effect of manipulations of Ca2+ environment on atrial natriuretic factor release. Am J Physiol. 1989;256:H1588–94. doi: 10.1152/ajpheart.1989.256.6.H1588. [DOI] [PubMed] [Google Scholar]
- [111].Ruskoaho H, Vuolteenaho O, Leppäluoto J. Phorbol esters enhance stretch-induced atrial natriuretic peptide secretion. Endocrinology. 1990;127:2445–55. doi: 10.1210/endo-127-5-2445. [DOI] [PubMed] [Google Scholar]
- [112].Wen JF, Cui X, Ahn JS, Kim SH, Seul KH, Kim SZ, Park YK, Lee HS, Cho KW. Distinct roles for L- and T-type Ca(2+) channels in regulation of atrial ANP release. Am J Physiol Heart Circ Physiol. 2000;279:H2879–88. doi: 10.1152/ajpheart.2000.279.6.H2879. [DOI] [PubMed] [Google Scholar]
- [113].Doubell AF, Thibault G. Calcium is involved in both positive and negative modulation of the secretory system for ANP. Am J Physiol. 1994;266:H1854–63. doi: 10.1152/ajpheart.1994.266.5.H1854. [DOI] [PubMed] [Google Scholar]
- [114].Kim SH, Cho KW, Chang SH, Kim SZ, Chae SW. Glibenclamide suppresses stretch-activated ANP secretion: involvements of K+ATP channels and L-type Ca2+ channel modulation. Pflugers Arch. 1997;434:362–72. doi: 10.1007/s004240050409. [DOI] [PubMed] [Google Scholar]
- [115].Page E, Upshaw-Earley J, Goings GE. Localization of atrial natriuretic peptide in caveolae of in situ atrial myocytes. Circ Res. 1994;75:949–54. doi: 10.1161/01.res.75.5.949. [DOI] [PubMed] [Google Scholar]
- [116].Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–13. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Chamberlain LH, Burgoyne RD, Gould GW. SNARE proteins are highly enriched in lipid rafts in PC12 cells: implications for the spatial control of exocytosis. Proc Natl Acad Sci U S A. 2001;98:5619–24. doi: 10.1073/pnas.091502398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Salaün C, Gould GW, Chamberlain LH. Lipid raft association of SNARE proteins regulates exocytosis in PC12 cells. J Biol Chem. 2005;280:19449–53. doi: 10.1074/jbc.M501923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kawamura S, Miyamoto S, Brown JH. Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem. 2003;278:31111–7. doi: 10.1074/jbc.M300725200. [DOI] [PubMed] [Google Scholar]
- [120].Maguy A, Hebert TE, Nattel S. Involvement of lipid rafts and caveolae in cardiac ion channel function. Cardiovasc Res. 2006;69:798–807. doi: 10.1016/j.cardiores.2005.11.013. [DOI] [PubMed] [Google Scholar]
- [121].Sen L, Bialecki RA, Smith E, Smith TW, Colucci WS. Cholesterol increases the L-type voltage-sensitive calcium channel current in arterial smooth muscle cells. Circ Res. 1992;71:1008–14. doi: 10.1161/01.res.71.4.1008. [DOI] [PubMed] [Google Scholar]
- [122].Xiao YF, Sigg DC, Leaf A. The antiarrhythmic effect of n-3 polyunsaturated fatty acids: modulation of cardiac ion channels as a potential mechanism. J Membr Biol. 2005;206:141–54. doi: 10.1007/s00232-005-0786-z. [DOI] [PubMed] [Google Scholar]
- [123].Savelieva I, Camm J. Statins and polyunsaturated fatty acids for treatment of atrial fibrillation. Nat Clin Pract Cardiovasc Med. 2008;5:30–41. doi: 10.1038/ncpcardio1038. [DOI] [PubMed] [Google Scholar]
- [124].Sandesara CM, Roodneshin H, Sbaity S, Olshansky B. Antiarrhythmic effects of statins in heart failure. Heart Fail Clin. 2008;4:187–200. doi: 10.1016/j.hfc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- [125].Xiao YF, Gomez AM, Morgan JP, Lederer WJ, Leaf A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc Natl Acad Sci U S A. 1997;94:4182–7. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Vaquero M, Caballero R, Gómez R, Núñez L, Tamargo J, Delpón E. Effects of atorvastatin and simvastatin on atrial plateau currents. J Mol Cell Cardiol. 2007;42:931–45. doi: 10.1016/j.yjmcc.2007.03.807. [DOI] [PubMed] [Google Scholar]
- [127].Goebel J, Logan B, Forrest K, Mieczkowski A, Roszman TL, Wills-Karp M. Atorvastatin affects interleukin-2 signaling by altering the lipid raft enrichment of the interleukin-2 receptor beta chain. J Investig Med. 2005;53:322–8. doi: 10.2310/6650.2005.53610. [DOI] [PubMed] [Google Scholar]
- [128].Sánchez-Wandelmer J, Dávalos A, Herrera E, Giera M, Cano S, de la Peña G, Lasunción MA, Busto R. Inhibition of cholesterol biosynthesis disrupts lipid raft/caveolae and affects insulin receptor activation in 3T3-L1 preadipocytes. Biochim Biophys Acta. 2009;1788:1731–9. doi: 10.1016/j.bbamem.2009.05.002. [DOI] [PubMed] [Google Scholar]
- [129].Ma DW, Seo J, Davidson LA, Callaway ES, Fan YY, Lupton JR, Chapkin RS. n-3 PUFA alter caveolae lipid composition and resident protein localization in mouse colon. FASEB J. 2004;18:1040–2. doi: 10.1096/fj.03-1430fje. [DOI] [PubMed] [Google Scholar]
- [130].Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochimie. 2007;89:169–77. doi: 10.1016/j.biochi.2006.10.009. [DOI] [PubMed] [Google Scholar]
- [131].Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal. 2010;3:ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Xiang Y, Kobilka BK. Myocyte adrenoceptor signaling pathways. Science. 2003;300:1530–2. doi: 10.1126/science.1079206. [DOI] [PubMed] [Google Scholar]
- [133].Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. Adrenergic regulation of cardiac myocyte apoptosis. J Cell Physiol. 2001;189:257–65. doi: 10.1002/jcp.10024. [DOI] [PubMed] [Google Scholar]
- [134].Xiao RP, Cheng H, Zhou YY, Kuschel M, Lakatta EG. Recent advances in cardiac beta(2)-adrenergic signal transduction. Circ Res. 1999;85:1092–100. doi: 10.1161/01.res.85.11.1092. [DOI] [PubMed] [Google Scholar]
- [135].Kuschel M, Zhou YY, Spurgeon HA, Bartel S, Karczewski P, Zhang SJ, Krause EG, Lakatta EG, Xiao RP. beta2-adrenergic cAMP signaling is uncoupled from phosphorylation of cytoplasmic proteins in canine heart. Circulation. 1999;99:2458–65. doi: 10.1161/01.cir.99.18.2458. [DOI] [PubMed] [Google Scholar]
- [136].Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang T, Brown JH, Devic E, Kobilka BK, Cheng H, Xiao RP. Linkage of beta1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest. 2003;111:617–25. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–9. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- [138].Warrier S, Ramamurthy G, Eckert RL, Nikolaev VO, Lohse MJ, Harvey RD. cAMP microdomains and L-type Ca2+ channel regulation in guinea-pig ventricular myocytes. J Physiol. 2007;580:765–76. doi: 10.1113/jphysiol.2006.124891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Calaghan S, Kozera L, White E. Compartmentalisation of cAMP-dependent signalling by caveolae in the adult cardiac myocyte. J Mol Cell Cardiol. 2008;45:88–92. doi: 10.1016/j.yjmcc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- [140].Kuschel M, Zhou YY, Cheng H, Zhang SJ, Chen Y, Lakatta EG, Xiao RP. G(i) protein-mediated functional compartmentalization of cardiac beta(2)-adrenergic signaling. J Biol Chem. 1999;274:22048–52. doi: 10.1074/jbc.274.31.22048. [DOI] [PubMed] [Google Scholar]
- [141].De Arcangelis V, Liu R, Soto D, Xiang Y. Differential association of phosphodiesterase 4D isoforms with beta2-adrenoceptor in cardiac myocytes. J Biol Chem. 2009;284:33824–32. doi: 10.1074/jbc.M109.020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Ganesan AN, Maack C, Johns DC, Sidor A, O’Rourke B. Beta-adrenergic stimulation of L-type Ca2+ channels in cardiac myocytes requires the distal carboxyl terminus of alpha1C but not serine 1928. Circ Res. 2006;98:e11–8. doi: 10.1161/01.RES.0000202692.23001.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lemke T, Welling A, Christel CJ, Blaich A, Bernhard D, Lenhardt P, Hofmann F, Moosmang S. Unchanged beta-adrenergic stimulation of cardiac L-type calcium channels in Ca v 1.2 phosphorylation site S1928A mutant mice. J Biol Chem. 2008;283:34738–44. doi: 10.1074/jbc.M804981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Levin KR, Page E. Quantitative studies on plasmalemmal folds and caveolae of rabbit ventricular myocardial cells. Circ Res. 1980;46:244–55. doi: 10.1161/01.res.46.2.244. [DOI] [PubMed] [Google Scholar]
- [145].Scriven DR, Klimek A, Asghari P, Bellve K, Moore ED. Caveolin-3 is adjacent to a group of extradyadic ryanodine receptors. Biophys J. 2005;89:1893–901. doi: 10.1529/biophysj.105.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Walden AP, Dibb KM, Trafford AW. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J Mol Cell Cardiol. 2009;46:463–73. doi: 10.1016/j.yjmcc.2008.11.003. [DOI] [PubMed] [Google Scholar]
- [147].Schulson MN, Scriven DR, Fletcher P, Moore ED. Couplons in rat atria form distinct subgroups defined by their molecular partners. J Cell Sci. 2011;124:1167–74. doi: 10.1242/jcs.080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Jayasinghe ID, Cannell MB, Soeller C. Organization of ryanodine receptors, transverse tubules, and sodium-calcium exchanger in rat myocytes. Biophys J. 2009;97:2664–73. doi: 10.1016/j.bpj.2009.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Brice NL, Dolphin AC. Differential plasma membrane targeting of voltage dependent calcium channel subunits expressed in a polarized epithelial cell line. J Physiol. 1999;515:685–94. doi: 10.1111/j.1469-7793.1999.685ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Balijepalli RC, Foell JD, Wang J, Best JM, Kamp TJ. Targeting of Cav1.2 Channels to Caveolae Requires Cavβ Association with Caveolin-3 in the Cardiomyocytes. Circulation. 2008;118:S_339. Abstract. [Google Scholar]
- [151].Davies A, Kadurin I, Alvarez-Laviada A, Douglas L, Nieto-Rostro M, Bauer CS, Pratt WS, Dolphin AC. The alpha2delta subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci U S A. 2010;107:1654–9. doi: 10.1073/pnas.0908735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–44. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- [153].McEwen DP, Li Q, Jackson S, Jenkins PM, Martens JR. Caveolin regulates kv1.5 trafficking to cholesterol-rich membrane microdomains. Mol Pharmacol. 2008;73:678–85. doi: 10.1124/mol.107.042093. [DOI] [PubMed] [Google Scholar]
- [154].Couchoux H, Bichraoui H, Chouabe C, Altafaj X, Bonvallet R, Allard B, Ronjat M, Berthier C. Caveolin-3 is a direct molecular partner of the Ca(v)1.1 subunit of the skeletal muscle L-type calcium channel. Int J Biochem Cell Biol. 2011;43:713–20. doi: 10.1016/j.biocel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- [155].Lee E, Marcucci M, Daniell L, Pypaert M, Weisz OA, Ochoa GC, Farsad K, Wenk MR, De Camilli P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–6. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- [156].Hong TT, Smyth JW, Gao D, Chu KY, Vogan JM, Fong TS, Jensen BC, Colecraft HM, Shaw RM. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Lipsky R, Potts EM, Tarzami ST, Puckerin AA, Stocks J, Schecter AD, Sobie EA, Akar FG, Diversé-Pierluissi MA. beta-Adrenergic receptor activation induces internalization of cardiac Cav1.2 channel complexes through a beta-Arrestin 1-mediated pathway. J Biol Chem. 2008;283:17221–6. doi: 10.1074/jbc.C800061200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [158].Nichols BJ, Lippincott-Schwartz J. Endocytosis without clathrin coats. Trends Cell Biol. 2001;11:406–12. doi: 10.1016/s0962-8924(01)02107-9. [DOI] [PubMed] [Google Scholar]
- [159].Seebohm G, Strutz-Seebohm N, Birkin R, Dell G, Bucci C, Spinosa MR, Baltaev R, Mack AF, Korniychuk G, Choudhury A, Marks D, Pagano RE, Attali B, Pfeufer A, Kass RS, Sanguinetti MC, Tavare JM, Lang F. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ Res. 2007;100:686–92. doi: 10.1161/01.RES.0000260250.83824.8f. [DOI] [PubMed] [Google Scholar]
- [160].McEwen DP, Schumacher SM, Li Q, Benson MD, Iñiguez-Lluhí JA, Van Genderen KM, Martens JR. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. J Biol Chem. 2007;282:29612–20. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]
- [161].Hardel N, Harmel N, Zolles G, Fakler B, Klöcker N. Recycling endosomes supply cardiac pacemaker channels for regulated surface expression. Cardiovasc Res. 2008;79:52–60. doi: 10.1093/cvr/cvn062. [DOI] [PubMed] [Google Scholar]
- [162].Best JM, Foell JD, Buss CR, Delisle BP, Balijepalli RC, January CT, Kamp TJ. Small GTPase Rab11b regulates degradation of surface membrane L-type Cav1.2 channels. Am J Physiol Cell Physiol. 2011;300:C1023–33. doi: 10.1152/ajpcell.00288.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Mizuno F, Barabas P, Krizaj D, Akopian A. Glutamate-induced internalization of Ca(v)1.3 L-type Ca(2+) channels protects retinal neurons against excitotoxicity. J Physiol. 2010;588:953–66. doi: 10.1113/jphysiol.2009.181305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Tsuruta F, Green EM, Rousset M, Dolmetsch RE. PIKfyve regulates CaV1.2 degradation and prevents excitotoxic cell death. J Cell Biol. 2009;187:279–94. doi: 10.1083/jcb.200903028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Jr, Burashnikov E, Wu Y, Sargent JD, Schickel S, Oberheiden R, Bhatia A, Hsu LF, Haïssaguerre M, Schimpf R, Borggrefe M, Wolpert C. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–9. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Chen X, Piacentino V, 3rd, Furukawa S, Goldman B, Margulies KB, Houser SR. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ Res. 2002;91:517–24. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- [167].He J, Conklin MW, Foell JD, Wolff MR, Haworth RA, Coronado R, Kamp TJ. Reduction in density of transverse tubules and L-type Ca(2+) channels in canine tachycardia-induced heart failure. Cardiovasc Res. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- [168].Kamp TJ, He JQ. L-type Ca2+ channels gaining respect in heart failure. Circ Res. 2002;91:451–3. doi: 10.1161/01.res.0000035346.21625.4a. [DOI] [PubMed] [Google Scholar]
- [169].Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- [170].Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–36. doi: 10.1161/01.res.85.5.428. [DOI] [PubMed] [Google Scholar]
- [171].Gómez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–6. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- [172].Litwin SE, Zhang D, Bridge JH. Dyssynchronous Ca(2+) sparks in myocytes from infarcted hearts. Circ Res. 2000;87:1040–7. doi: 10.1161/01.res.87.11.1040. [DOI] [PubMed] [Google Scholar]
- [173].Banfi C, Brioschi M, Wait R, Begum S, Gianazza E, Fratto P, Polvani G, Vitali E, Parolari A, Mussoni L, Tremoli E. Proteomic analysis of membrane microdomains derived from both failing and non-failing human hearts. Proteomics. 2006;6:1976–88. doi: 10.1002/pmic.200500278. [DOI] [PubMed] [Google Scholar]
- [174].Heinzel FR, MacQuaide N, Biesmans L, Sipido K. Dyssynchrony of Ca2+ release from the sarcoplasmic reticulum as subcellular mechanism of cardiac contractile dysfunction. J Mol Cell Cardiol. 2011;50:390–400. doi: 10.1016/j.yjmcc.2010.11.008. [DOI] [PubMed] [Google Scholar]
- [175].Soeller C, Cannell MB. Numerical simulation of local calcium movements during L-type calcium channel gating in the cardiac diad. Biophys J. 1997;73:97–111. doi: 10.1016/S0006-3495(97)78051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Napolitano C, Antzelevitch C. Phenotypical manifestations of mutations in the genes encoding subunits of the cardiac voltage-dependent L-type calcium channel. Circ Res. 2011;108:607–18. doi: 10.1161/CIRCRESAHA.110.224279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Cheng EP, Yuan C, Navedo MF, Dixon RE, Nieves-Cintrón M, Scott JD, Santana LF. Restoration of Normal L-Type Ca2+ Channel Function During Timothy Syndrome by Ablation of an Anchoring Protein. Circ Res. 2011 Jun 23; doi: 10.1161/CIRCRESAHA.111.248252. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Ackerman MJ, Mohler PJ. Defining a new paradigm for human arrhythmia syndromes: phenotypic manifestations of gene mutations in ion channel- and transporter-associated proteins. Circ Res. 2010;107:457–65. doi: 10.1161/CIRCRESAHA.110.224592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Vatta M, Ackerman MJ, Ye B, Makielski JC, Ughanze EE, Taylor EW, Tester DJ, Balijepalli RC, Foell JD, Li Z, Kamp TJ, Towbin JA. Mutant caveolin-3 induces persistent late sodium current and is associated with long-QT syndrome. Circulation. 2006;114:2104–12. doi: 10.1161/CIRCULATIONAHA.106.635268. [DOI] [PubMed] [Google Scholar]
- [180].Hayashi T, Arimura T, Ueda K, Shibata H, Hohda S, Takahashi M, Hori H, Koga Y, Oka N, Imaizumi T, Yasunami M, Kimura A. Identification and functional analysis of a caveolin-3 mutation associated with familial hypertrophic cardiomyopathy. Biochem Biophys Res Commun. 2004;313:178–84. doi: 10.1016/j.bbrc.2003.11.101. [DOI] [PubMed] [Google Scholar]
- [181].Catteruccia M, Sanna T, Santorelli FM, Tessa A, Di Giacopo R, Sauchelli D, Verbo A, Lo Monaco M, Servidei S. Rippling muscle disease and cardiomyopathy associated with a mutation in the CAV3 gene. Neuromuscul Disord. 2009;19:779–83. doi: 10.1016/j.nmd.2009.08.015. [DOI] [PubMed] [Google Scholar]
- [182].Rajab A, Straub V, McCann LJ, Seelow D, Varon R, Barresi R, Schulze A, Lucke B, Lützkendorf S, Karbasiyan M, Bachmann S, Spuler S, Schuelke M. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 2010;6:e1000874. doi: 10.1371/journal.pgen.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Bito V, Heinzel FR, Biesmans L, Antoons G, Sipido KR. Crosstalk between L-type Ca2+ channels and the sarcoplasmic reticulum: alterations during cardiac remodelling. Cardiovasc Res. 2008;77:315–24. doi: 10.1093/cvr/cvm063. [DOI] [PubMed] [Google Scholar]
- [184].O’Rourke B, Kass DA, Tomaselli GF, Kääb S, Tunin R, Marbán E. Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res. 1999;84:562–70. doi: 10.1161/01.res.84.5.562. [DOI] [PubMed] [Google Scholar]
- [185].Balijepalli RC, Lokuta AJ, Maertz NA, Buck JM, Haworth RA, Valdivia HH, Kamp TJ. Depletion of T-tubules and specific subcellular changes in sarcolemmal proteins in tachycardia-induced heart failure. Cardiovasc Res. 2003;59:67–77. doi: 10.1016/s0008-6363(03)00325-0. [DOI] [PubMed] [Google Scholar]
- [186].Louch WE, Bito V, Heinzel FR, Macianskiene R, Vanhaecke J, Flameng W, Mubagwa K, Sipido KR. Reduced synchrony of Ca2+ release with loss of T-tubules-a comparison to Ca2+ release in human failing cardiomyocytes. Cardiovasc Res. 2004;62:63–73. doi: 10.1016/j.cardiores.2003.12.031. [DOI] [PubMed] [Google Scholar]
- [187].Lyon AR, MacLeod KT, Zhang Y, Garcia E, Kanda GK, Lab MJ, Korchev YE, Harding SE, Gorelik J. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci U S A. 2009;106:6854–9. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Horiuchi-Hirose M, Kashihara T, Nakada T, Kurebayashi N, Shimojo H, Shibazaki T, Sheng X, Yano S, Hirose M, Hongo M, Sakurai T, Moriizumi T, Ueda H, Yamada M. Decrease in the density of t-tubular L-type Ca2+ channel currents in failing ventricular myocytes. Am J Physiol Heart Circ Physiol. 2011;300:H978–88. doi: 10.1152/ajpheart.00508.2010. [DOI] [PubMed] [Google Scholar]
- [189].Heinzel FR, Bito V, Biesmans L, Wu M, Detre E, von Wegner F, Claus P, Dymarkowski S, Maes F, Bogaert J, Rademakers F, D’hooge J, Sipido K. Remodeling of T-tubules and reduced synchrony of Ca2+ release in myocytes from chronically ischemic myocardium. Circ Res. 2008;102:338–46. doi: 10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- [190].He JQ, Balijepalli RC, Haworth RA, Kamp TJ. Crosstalk of beta adrenergic receptor subtypes through Gi blunts beta-adrenergic stimulation of L-type Ca2+ channels in canine heart failure. Circ Res. 2005;97:566–73. doi: 10.1161/01.RES.0000181160.31851.05. [DOI] [PubMed] [Google Scholar]
- [191].Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–11. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- [192].Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S, et al. Beta 1- and beta 2 adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- [193].Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- [194].Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002;54:230–46. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- [195].Nattel S, Burstein B, Dobrev D. Atrial remodeling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 2008;1:62–73. doi: 10.1161/CIRCEP.107.754564. [DOI] [PubMed] [Google Scholar]
- [196].Yue L, Melnyk P, Gaspo R, Wang Z, Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ Res. 1999;84:776–84. doi: 10.1161/01.res.84.7.776. [DOI] [PubMed] [Google Scholar]
- [197].Qi XY, Yeh YH, Xiao L, Burstein B, Maguy A, Chartier D, Villeneuve LR, Brundel BJ, Dobrev D, Nattel S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res. 2008;103:845–54. doi: 10.1161/CIRCRESAHA.108.175463. [DOI] [PubMed] [Google Scholar]
- [198].Schotten U, Haase H, Frechen D, Greiser M, Stellbrink C, Vazquez Jimenez JF, Morano I, Allessie MA, Hanrath P. The L-type Ca2+-channel subunits alpha1C and beta2 are not downregulated in atrial myocardium of patients with chronic atrial fibrillation. J Mol Cell Cardiol. 2003;35:437–43. doi: 10.1016/s0022-2828(03)00012-9. [DOI] [PubMed] [Google Scholar]
- [199].Christ T, Boknik P, Wöhrl S, Wettwer E, Graf EM, Bosch RF, Knaut M, Schmitz W, Ravens U, Dobrev D. L-type Ca2+ current downregulation in chronic human atrial fibrillation is associated with increased activity of protein phosphatases. Circulation. 2004;110:2651–7. doi: 10.1161/01.CIR.0000145659.80212.6A. [DOI] [PubMed] [Google Scholar]
- [200].Brundel BJ, Ausma J, van Gelder IC, Van der Want JJ, van Gilst WH, Crijns HJ, Henning RH. Activation of proteolysis by calpains and structural changes in human paroxysmal and persistent atrial fibrillation. Cardiovasc Res. 2002;54:380–9. doi: 10.1016/s0008-6363(02)00289-4. [DOI] [PubMed] [Google Scholar]
- [201].Lenaerts I, Bito V, Heinzel FR, Driesen RB, Holemans P, D’hooge J, Heidbüchel H, Sipido KR, Willems R. Ultrastructural and functional remodeling of the coupling between Ca2+ influx and sarcoplasmic reticulum Ca2+ release in right atrial myocytes from experimental persistent atrial fibrillation. Circ Res. 2009;105:876–85. doi: 10.1161/CIRCRESAHA.109.206276. [DOI] [PubMed] [Google Scholar]