Abstract
The biophysical characterization of purified membrane proteins typically requires detergent mediated extraction from native lipid membrane environments. In the case of human G protein-coupled receptors (GPCRs), this process has been complicated by their conformational heterogeneity and the general lack of understanding the composition and interactions within the diverse human cellular membrane environment. Several successful GPCR structure determination efforts have shown that the addition of cholesterol analogs is often critical for maintaining protein stability. We have identified sterols that substantially increase the stability of the NOP receptor (ORL-1), a member of the opioid GPCR family, in a mixed micelle environment. Using dynamic light scattering and small-angle X-ray scattering, we have determined that the most thermal stabilizing sterol, cholesteryl hemmisuccinate, induces the formation of a bicelle-like micelle architecture when mixed with dodecyl maltoside detergent. Together with mutagenesis studies and recent GPCR structures, our results provide indications that stabilization is attained through a combination of specific sterol binding to GPCRs and modulation of micelle morphology.
Keywords: thermostability, cholesteryl hemisuccinate (CHS), cholesterol, opioid, NOP receptor (ORL-1), GPCR, SAXS
1. Introduction
Recent attempts at characterizing the Human Membrane Proteome identified approximately 6000 human membrane proteins constituting approximately 30% of the human genome [1, 2]. The characterization of membrane proteins typically requires their extraction from the lipid membrane bilayer, an environment that bestows considerable stabilization on these embedded proteins. Protein extraction from the membrane and its subsequent purification therefore represents a major obstacle that requires the use of detergents/surfactants, which assemble into globular micelles that adopt different geometrical shapes in solution [3, 4], and shield the hydrophobic core of membrane proteins from exposure to aqueous medium through formation of a protein-detergent complex (PDC) [5]. Finding suitable detergent(s) that will stabilize the protein’s structure and maintain its activity is therefore pivotal.
G protein-coupled receptors (GPCRs) represent the largest class of human membrane proteins involved in signal transduction (80%) [6], and remain a largely untapped reservoir of pharmaceutical targets as ~40% of all marketed drugs target only a small fraction of the 800+ members in this protein family [7, 8]. GPCRs share a common fold consisting of a seven transmembrane (TM) alpha helical bundle flanked by a N-terminus or a globular extracellular domain that plays a role in ligand recognition and/or binding in some receptors, and an amphipathic helix (helix VIII) followed by an intracellular domain at the C-terminus [9]. Common among the rhodopsin family of GPCRs is a palmitoylation site immediately following helix VIII, which anchors this region to the membrane bilayer and may target these proteins for partitioning into cholesterol rich rafts and caveolae [10, 11]. Analysis of the crystal structures of the β1 and β2 adrenergic receptors (β1AR and β2AR) has led to the identification and characterization of a specific cholesterol binding site at a cleft created by helices II, III, and IV [12–16]. Many residues within this specific binding site have been found to be conserved among the GPCR families, and thus this region has been termed the cholesterol consensus motif (CCM) [13].
The nonionic alkyl glucoside detergents, specifically n-Dodecyl-β-D-Maltoside (DDM), are the most widely used detergents for the isolation and purification of GPCRs. Above the critical micelle concentration (CMC), DDM micelles form oblate ellipsoids where the polar axis is shorter than the equatorial axes [3, 4, 17]. DDM mixtures with various additive detergents containing various acyl chain lengths or different head groups can be used to adjust the micelle’s physiochemical properties and dimensions for optimal membrane protein stability. Additionally, the use of detergent soluble cholesterol analogs is becoming increasingly popular, with numerous reports showing that these compounds may help protect GPCRs from thermal inactivation [13, 18–20]. Several successful GPCR structure determination efforts have shown that the addition of cholesterol analogs is often critical for maintaining receptor stability [13–15, 21–25]. However, it is unclear if the protective function of these sterol analogs is achieved through modulation of detergent micelle morphology and physiochemical properties, or through a specific sterol-protein interaction.
This study is focused on understanding the effect of cholesterol analogs on DDM/GPCR PDCs. In a case study, we show that the type of cholesterol analog supplemented to the PDC substantially influenced the thermostability of ORL-1 (Opioid Receptor Like -1; also known as NOP (nociceptin opioid peptide) receptor), a member of the opioid receptor (OR) GPCR family. All four opioid receptors have been shown to partition into lipid rafts, and methyl-β-cyclodextrin (MβCD) mediated depletion of cholesterol has been shown to modulate their downstream signaling [26–33]. Importantly, the ORs contain a tryptophan residue, Trp4.50 (superscript corresponds to the Ballesteros-Weinstein numbering [34]), located at the heart of a putative CCM, which is critical for π-stacking interactions with cholesterol’s sterol core [13]. The importance of ORL-1’s Trp4.50 residue has been underscored through mutagenesis studies. In an effort to understand the differences in effects attributed to the different cholesterol analogs, biophysical studies of various sterol/DDM micelle mixtures (free of protein) were conducted to measure differences in the mixed micelle morphologies. Using dynamic light scattering and small-angle X-ray scattering, we have determined that the most thermal stabilizing sterol, cholesteryl hemisuccinate, induces the formation of a bicelle-like architecture when mixed with DDM detergent.
2. Experimental Procedures (Materials and Methods)
2.1 ORL-1 Cloning, Expression and Purification
The wild type (WT) human ORL-1 gene (UniProt accession code P41146)was synthesized by DNA2.0 and then cloned into a modified pFastBac1 vector (Invitrogen) containing an expression cassette with an HA signal sequence followed by a FLAG tag at the N-terminus, and a PreScission protease site followed by a 10xHis tag at the C-terminus. Thirty amino acids were deleted from the C-terminus, which helped to increase receptor yield (data not shown). Recombinant baculoviruses were generated by using the Bac-to-Bac system (Invitrogen) and were used to infect Sf9 insect cells at a density of 2 × 106 cells/mL at a multiplicity of infection of 5. Infected cells were grown at 27 °C for 48 h prior to being harvested, and the cell pellets were stored at −80 °C.
Insect cell membranes were disrupted by dounce homogenization of cell pellets in a hypotonic buffer containing 25 mM HEPES [pH 7.5], 10 mM MgCl2, 20 mM KCl, and protease inhibitor cocktail (Roche). Extensive washing of the raw membranes was performed consecutively by repeated dounce homogenization and centrifugation in the same hypotonic buffer (~two times), followed by high osmotic buffer containing 1.0 M NaCl, 25 mM HEPES [pH 7.5], 10 mM MgCl2, and 20 mM KCl (~three-four times). Purified membranes were resuspended in 25 mM HEPES [pH 7.5], 20 mM KCl, and 30% (v/v) glycerol, flash-frozen with liquid nitrogen and stored at −80 °C until further use.
Purified membranes were thawed and incubated with 5 µM Compound-24 (C-24; 1-benzyl-N-{3-[spiroisobenzofuran-1(3H),40-piperidin-1-yl]propyl} pyrrolidine-2-carboxamide [35] was provided as a gift from Girolamo Calo, Remo Guerrini and Stephano Molinari at the University of Ferrara, Ferrara, Italy), 50 mM HEPES [pH 7.5], 500 mM NaCl, 20 mM KCl, and 5% glycerol (v/v), and incubated at 4 °C for 1 hour. Iodoacetamide (Sigma) was then added to the membranes at a final concentration of 1 mg/mL for another 15 minutes before solubilization with 10 mM DDM (~0.5% (w/v) n-dodecyl-β-D-maltopyranoside; Anatrace), and 2 mM CHS (~0.1% (w/v) cholesteryl hemisuccinate; Anatrace or Sigma) for three hours at 4 °C. The supernatant was isolated by centrifugation at 160,000 × g for 45 minutes, supplemented with 25 mM imidazole [pH 7.5], and incubated with TALON IMAC resin (Clontech) overnight at 4 °C. Typically, 0.75 mL of resin (slurry) per 1 L of original culture volume was used. After binding, the resin was divided and washed with 20 column volumes of wash buffer 1 (WB1: 50 mM HEPES [pH 7.5], 500 mM NaCl, 20 mM KCl, 10 mM MgCl2, 1 mM ATP, 10% glycerol (v/v), 25 mM imidazole, 5 µM C-24, 1 mM DDM supplemented with various sterols at the indicated concentration), and 25 column volumes of WB2 (same as WB1, but without ATP and MgCl2), before protein elution with 4 column volumes of elution buffer (EB: 50 mM HEPES [pH 7.5], 500 mM NaCl, 20 mM KCl, 10% glycerol (v/v), 250 mM imidazole, 5 µM C-24, 1 mM DDM supplemented with various sterols at the indicated concentrations). CHS (cholesteryl hemisuccinate) was purchased from Sigma or Anatrace; Façade (3α-hydroxy-7α,12α-di-((O-β-D-maltosyl)-2-hydroxyethoxy)-cholane) from Avanti; and CHAPS (3-[(3-Cholamidopropyl)-Dimethylammonio]-1-Propane Sulfonate/N,N-Dimethyl-3-Sulfo-N-[3-[[3α,5β,7α,12α)-3,7,12-Trihydroxy-24-Oxocholan-24-yl]Amino]propyl]-1-Propanaminium Hydroxide Inner Salt), CHAPSO (3-[(3-Cholamidopropyl)dimethylammonio]-2-Hydroxy-1-Propanesulfonat), Sodium Cholate (3α,7α,12α-Trihydroxy-5β-Cholan-24-oic Acid Monosodium Salt), Sodium deoxycholate (3α,12α-Dihydroxy-5β-Cholan-24-ic Acid, Monosodium Salt), BigCHAP (N,N'-bis-(3-D-Gluconamidopropyl)Cholamide), BigCHAP deoxy (N,N'-bis-(3-D-Gluconamidopropyl) Deoxycholamide, BigCHAP deoxy), and CHOBIMALT (β-cholesteryl-(maltosyl-β-(1,6)-maltoside)) were purchased from Anatrace. Exchange of the different DDM/sterol mixtures was verified through the correlation of retention time and the measured micelle sizes described below.
2.2 Thermostability Data Collection
Thermal stability data was collected using a modified procedure incorporating a thiol-reactive N-[4-(7-diethylamino-4-methyl-3-coumarinyl)phenyl]maleimide (CPM) fluorophore, which undergoes an increase in the fluorescence emission following interaction with Cysteine residues [36]. Based on amino acid threading onto the β2AR structure (PDB code 2RH1), ORL-1 contains seven buried Cys residues (out of thirteen found on this construct) in the TM core that become exposed and available for reaction with the CPM dye upon thermal unfolding/denaturation. Reactions contained 5 µM CPM and ~5 µg purified ORL-1 in a buffer (EB, but without imidazole) containing 50 mM HEPES [pH 7.5], 500 mM NaCl, 20 mM KCl, 10% glycerol (v/v), 5 µM C-24, 1 mM DDM and sterols at the indicated concentrations. Fluorescence of the CPM dye was measured on a Cary Eclipse Fluorescence Spectrophotometer using 387 nm excitation filter with a 2.5 nm bandpass and a 463 nm emission filter with a 10 nm bandpass. Measurements were made in 1 °C intervals from 20 – 90 °C with a ramp rate of 2 °C/min, and the background fluorescence of buffer in the absence of protein was subtracted. Midpoints of the thermal transitions (Tm) were obtained using a least squares non-linear regression analysis (GraphPad Prism) of fluorescence signal versus T plots to the following equation [37]:
where y is the observed fluorescence signal, yf and yu are the intercepts, mf and mu are the slopes of the pre- and post-transition baselines, Tm is the midpoint of the transition curve, T is the temperature in Kelvin, and R is the gas constant, and H is the enthalpy change for unfolding at Tm.
2.3 Light Scattering Measurements
Dynamic light scattering (DLS) of the detergent samples dissolved in 150 mM NaCl, 50mM TRIS [pH 8.0] with a DDM concentration of 5 mM and supplemented with various sterols was measured in a DynaPro (Wyatt, Santa Barbara, CA) instrument to obtain the apparent hydrodynamic radius of micelles, Rh. Experiments were performed in quartz cuvettes at 20 °C with 5 second acquisition times, and 50 acquisitions were collected to complete one measurement. The DLS data were analyzed using Dynamics 6.0 software using an assumed Rayleigh sphere model for the analysis.
2.4 SAXS Data Collection
Small angle X-ray scattering (SAXS) data were collected at the Bio-SAXS beamline BL4-2 of the Stanford Synchrotron Radiation Lightsource (SSRL, Stanford, CA). The beam size was slit-collimated to 0.2 × 0.2 mm, and the X-ray energy was calibrated to 11 KeV, which corresponds to a wavelength of λ=1.1271 Å. A Rayonix Mx225-He CCD detector was used at a sample to detector distance of 2 m, and the sample temperature was maintained at 20 °C. Testing of various CHS/DDM mixed detergent concentrations (5 mM to 200 mM DDM) revealed no change in micelle size (via Guinier analysis); therefore, to minimize inter-micelle scattering effects, the DDM concentration was a constant 5 mM (and supplemented with various sterols) for all reported data. All detergent samples were dissolved in 150 mM NaCl, 50 mM TRIS [pH 8.0], and were injected into a capillary for collection using automated liquid handling equipment. Twenty frames per sample with 1 second exposure times were collected and integrated radially, and the data from each sample were averaged, and subtracted from the background buffer in the absence of detergent. Guinier analysis was performed using Primus software (EMBL) [38], and MatLab software (MathWorks) was used for analysis of the two shell ellipsoid fitting by the methods described by Lipfert et al [4].
3. RESULTS
3.1 Cholesterol derivatives and ORL-1 thermostability
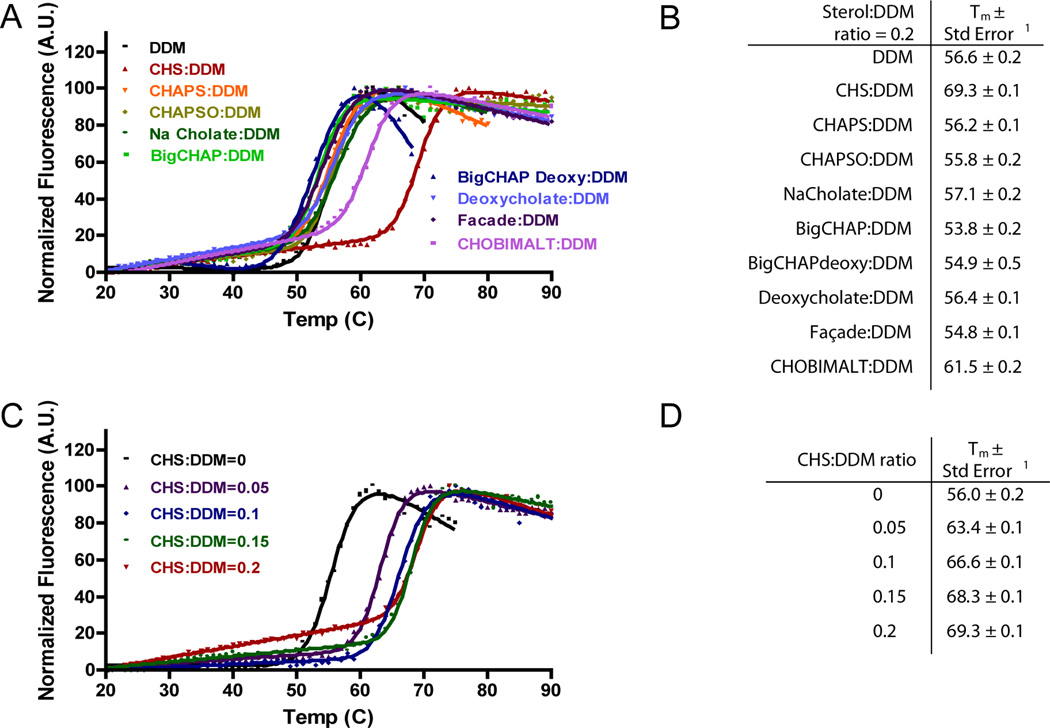
ORL-1 was expressed in Sf9 insect cells, and a thorough search for optimal detergents identified DDM as the best detergent for extraction and purification of this receptor (data not shown). To date, little is known about the thermostabilizing effect of sterol addition to OR PDCs. Because cholesterol is insoluble in the absence of lipids (unpublished observations), we tested the effect of cholesterol analogs mixed with DDM on their ability to promote the thermal stabilization of ORL-1 using a thiol-reactive fluorescent probe (CPM assay) [36]. The tested cholesterol analogs are soluble in detergent solutions by virtue of polar prosthetic groups attached to different portions of the tetracyclic sterol core (Figure 1). The sterol additives were dissolved in DDM at a molar ratio of 0.2:1 (sterol:DDM), and experimental determination of the CMC using a modified 1,6-diphenyl-1,3,5-hexatriene (DPH) fluorescence based assay [39] indicated that the sterol/DDM mixtures had a similar CMC to DDM alone (data not shown). Each sterol/DDM mixture was then exchanged at ~5 × the CMC on IMAC resin loaded with ORL-1, followed by measurement of the thermal stability of the different ORL-1/C-24/sterol/DDM complexes using the CPM assay. Thermostability of ORL-1 in the presence of most sterol/DDM mixtures was similar to DDM alone (Figure 2). However, in the presence of CHOBIMALT and CHS, the thermostability of ORL-1 was significantly improved by 5 and 15 °C, respectively (Figure 2).
Figure 1. Schematic diagram of cholesterol, cholesterol analogs and DDM.
Chemical structures of: cholesterol, with its four rings labeled A–D, DDM, the detergent used throughout these studies, and various cholesterol analogs. Notice that only CHS and CHOBIMALT have cholesterol like iso-octyl tails and planar steroid cores (by virtue of a double bond between C5 and C6 of ring B).
Figure 2. ORL-1 is thermally stabilized by the cholesterol analogs CHS and CHOBIMALT.
(A) (B) Thermal stability data for ORL-1/C-24 in the presence of various sterol:DDM mixtures at a ratio of 0.2:1. (C) (D) Thermal stability data for ORL-1/C-24 in the presence of different CHS:DDM ratios. 1Thermal transition temperatures (Tm) and standard errors are calculated from Equation 1 [47].
To better understand the influence of CHS on the thermostability of ORL-1, we measured its Tm at various CHS:DDM ratios (Figure 2). In the presence of CHS, all of the transitions were shifted to higher temperatures compared to ORL-1 PDCs containing only DDM. A maximal increase in thermal stability was observed at a CHS:DDM ratio of ~0.1; further increasing the CHS:DDM ratio resulted in a negligible increase in the transition temperature.
To determine if the CHS-dependent increase in thermostability is the result of a specific interaction with a cholesterol binding site on the receptor, we mutated Trp1754.50, located at the heart of the CCM, to an alanine residue. Fluorophore labeled antibody (α FLAG tag) based flow cytometry on Sf9 cells expressing either ORL-1 W1754.50A or WT-CCM ORL-1 suggested that expression of the receptor was not significantly affected by the mutation [40]. However, extracting the W175A protein from the membranes yielded low quality receptor as observed by analytical size exclusion chromatography, and the lack of a prominent thermal transition in the CPM assay suggested that many of the buried cysteines were exposed prior to heating the sample (data not shown).
3.2 Hydrodynamic radii of Sterol/DDM mixed micelles
To test whether the addition of cholesterol analogs to DDM would impact the physical characteristics (e.g. size) of the detergent micelle, we used Dynamic Light Scattering (DLS) to determine the hydrodynamic radius (Rh) of the different sterol/DDM mixtures. For DDM detergent micelles at 5 mM concentration the measured Rh = 35 Å, which is in agreement with the published radius of gyration value of 35 ± 1 Å [4]. A typical globular protein displaying a Rh = 35 Å would correspond to an apparent MW of 63 kDa (where MWapparent (kDa) = [0.168*Rh]2.3398; DynaPro manual), a value which is close to literature values for the mass of DDM micelles (70–72 kDa) [41, 42]. With the exception of CHS and CHOBIMALT, all sterol/DDM mixtures resulted in a decrease of the Rh values by 2–4 Å compared to DDM alone (Table 1). While the addition of CHOBIMALT to DDM had no effect on the Rh value, titration of CHS to DDM at increasing ratios resulted in an increase of the Rh; with CHS:DDM ratios of 0.1 and 0.2, the Rh increases by 3 and 6 Å, respectively, when compared to DDM micelles.
Table 1.
Detergent Micelle Measurements
| Detergent | DLS Rh (Å)1 | SAXS Rg (Å)2 | a1 (Å)3 | b1 (Å)3 | a2 (Å)3 | b2 (Å)3 |
|---|---|---|---|---|---|---|
| DDM | 35 | 33.2 ± 0.2 | 15.9 | 29.2 | 5.0 | 5.0 |
| CHS:DDM = 0.1 | 38 | 37.3 ± 0.1 | 16.5 | 36.0 | 8.4 | 5.1 |
| CHS:DDM = 0.2 | 41 | 44.8 ± 0.2 | 16.5 | 40.2 | 8.7 | 5.0 |
| CHAPS:DDM = 0.2 | 32 | 30.3 ± 0.1 | 15.5 | 26.5 | 5.1 | 5.1 |
| CHAPSO:DDM = 0.2 | 32 | 29.7 ± 0.2 | 15.4 | 26.5 | 5.1 | 5.1 |
| Na Cholate:DDM = 0.2 | 32 | 30.7 ± 0.2 | 15.3 | 26.6 | 4.9 | 4.9 |
| BigCHAP:DDM = 0.2 | 33 | 30.6 ± 0.2 | 15.4 | 26.5 | 5.3 | 5.3 |
| BigCHAPdeoxy:DDM = 0.2 | 32 | 31.9 ± 0.2 | 15.3 | 26.5 | 5.4 | 5.4 |
| Deoxycholate:DDM = 0.2 | 33 | 32.4 ± 0.3 | 15.0 | 28.1 | 5.0 | 5.0 |
| Façade:DDM = 0.2 | 31 | 29.6± 0.2 | 14.9 | 25.7 | 5.9 | 5.8 |
| CHOBIMALT:DDM=0.2 | 36 | N/A | N/A | N/A | N/A | N/A |
3.3 Micelle radius of gyration measured by SAXS
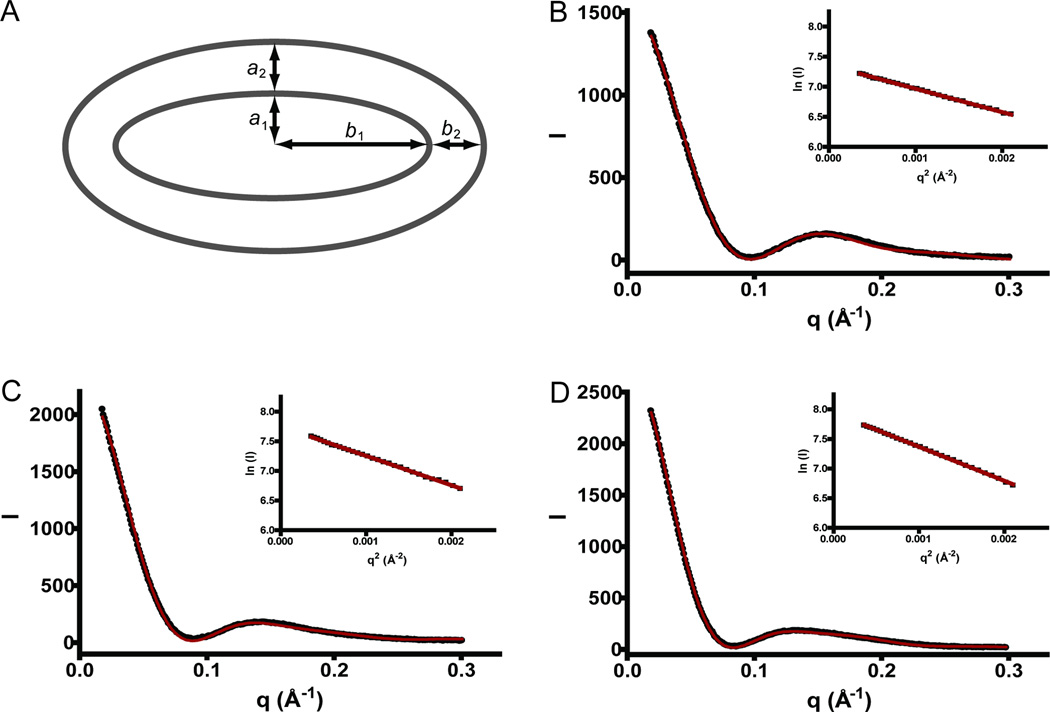
To further analyze the impact of sterols on DDM micelle size, we used SAXS to obtain the radii of gyration (Rg) of the different micelles using Guinier analysis (Figure 3). The calculated Rg for DDM in 150 mM NaCl was 33.2 ± 0.2 Å (Table 1), similar to previously published results [4]. The measured Rg for DDM determined by SAXS was about ~2 Å less than the Rh determined by DLS, and the same trend was also observed for most of the mixed micelles (Table 1). With the exception of CHS, the Rg measurements indicated that sterol mixed micelles are smaller than DDM alone. In contrast, the addition of CHS to DDM resulted in larger micelle dimensions confirming the results of the DLS experiments. To better understand the effect that CHS has on DDM micelles, we fit the full SAXS scattering profile to a two shell ellipsoid form factor model [4].
Figure 3. SAXS fitting reveals increasing micelle geometrical parameters in the presence of CHS.
(A) Cross-section of a two-shell oblate ellipsoid defining the geometrical parameters used for the two shell ellipsoid fits. Full SAXS scattering data (black spheres) fit to a two shell ellipsoid (red line) by the method described by Lipfert et al. [6] for micelles containing (B) DDM, (C) CHS:DDM, 0.1:1, (D) CHS:DDM, 0.2:1. Guinier representation (ln (I) versus q2 (Å−2) of the low angle data (black squares) and linear fit (red line) is plotted in the inset for the three datasets. Only the DDM and CHS/DDM data is included as a representative of all fits.
3.4 Ellipsoid Fit of SAXS data
The I versus q (Å−1) SAXS profiles of DDM and the mixed sterol/DDM micelles indicated the presence of a two-component ellipsoid model with the DDM maltoside head group likely constituting the outer shell and the hydrophobic alkyl detergent tail making up the inner shell (Figure 4) [4]. Previous studies showed that DDM micelles form oblate ellipsoids where the polar axis of rotation is smaller than the two equatorial axes [3, 4, 17]. We therefore fit the full SAXS profile to a two-shell ellipsoid model in order to determine the vector lengths of the two ellipsoid shells where a single minor a1 and two major b1 axes describe the inner shell, and a2 and b2 (parallel with the a1 and b1 vectors, respectively) represent the outer shell thickness (Figure 3). The fit of DDM micelle SAXS data revealed an a1 and b1 of 15.9 and 29.2 Å, respectively, and an a2 and b2 both equal to 5.0 Å. These results are in agreement with previously published parameters of DDM micelles [3, 4, 17]. Consistent with the Rg values, the hydrophobic core dimensions (a1 and b1) of micelles containing sterols with polar tails are smaller in magnitude, particularly along the b1 vector (Table 1). The outer shell dimensions (a2 and b2), likely composed of maltoside head groups and polar moieties from the cholesterol analogs, show differences that can be attributed to the sterol added. Addition of sterols with relatively large polar groups, such as BigCHAP, BigCHAPdeoxy, and Façade, to DDM revealed a modest increase in the a2 and b2 vector (note that CHOBIMALT was not analyzed by SAXS). The parameters obtained for the mixed CHS/DDM micelle were the most interesting. The addition of CHS to DDM induced a significant increase in the b1 parameter in a CHS concentration dependent manner (increase of 11 Å at a CHS:DDM ratio = 0.2), while a1 displayed only a modest increase in length by ~1 Å (at a ratio of 0.1 and 0.2, the CHS:DDM a1 = 16.5 Å). On the other hand, the outer shell b2 dimension did not change significantly in contrast with a2 which displayed a relatively large increase in thickness by ~3.5 Å. A model rationalizing these features and possible effects on the protein stability are discussed in the following section.
Figure 4. Models for DDM versus CHS:DDM micelles.
Addition of CHS to DDM micelles (A) results in altered mixed micelle morphologies (B). CHS inserts mostly in less curved regions of DDM micelles inducing a pseudo-bicelle architecture with the core forming a membrane–like bilayer disk consisting of DDM and CHS molecules flanked with a belt of DDM molecules. Rigid CHS molecules order DDM alkyl tails, resulting in a slight increase in the thickness of the hydrophobic slab, and decrease the area per DDM molecule, which results in reorientation of DDM maltoside head groups (yellow ovals), increasing the thickness of the hydrophilic layer.
4. DISCUSSION
Having evolved under pressure of natural selection, cholesterol is the primary sterol found in higher eukaryotes [43]. In the case of plants and fungi, cholesterol is replaced by phytosterols and ergosterol, respectively. The unique rigid planar structure of sterols imparts special properties on membranes by modulating its fluidity [44], thickness [45], curvature [46], and permeability [47]. Many of these “effects” are due to the increase in ordering of lipid acyl tails when packed against the rigid sterol tetracyclic backbone. Differential membrane physicochemical parameters have been implicated in the regulation of integral membrane protein function [48–50], and it is known that cholesterol modulates the function and activity of GPCRs in membrane bilayers [51]. Activation of rhodopsin induces a series of conformational intermediates that involve an expansion of the protein in the lipid bilayer, an event that is inhibited in membranes containing high cholesterol content due to an increase in lateral pressure [52, 53]. This finding is confirmed by studies on the oxytocin receptor that shows a more compact structure in the presence of cholesterol [54], suggesting that this stabilizing effect may be common. Lateral compression and hydrophobic matching between the lipid bilayer and a protein’s transmembrane core, parameters that are affected by cholesterol content, are critical for maintaining the structural integrity of embedded membrane proteins [55–57] (reviewed in [58]).
We examined the thermal stabilization of ORL-1 in DDM micelles supplemented with the different sterols shown in Figure 1. Our results indicated that the stability of ORL-1 is strongly dependent on the sterol with which it is reconstituted. Compared to DDM alone, CHOBIMALT increased the Tm by ~5 °C. However, in the presence of CHS we observed a much larger increase in the thermal transition temperature by almost ~15 °C compared to DDM alone (Figure 2). Although a previous study showed equal thermostabilization of κOR by CHOBIMALT and CHS [19], this could possibly be explained by the presence of CHOBIMALT’s large headgroup (discussed below) or from differences in the assay for thermal stability. We found the thermostabilizing effect of CHS to be dependent on its ratio with DDM; however, increasing amounts of CHS exceeding a CHS:DDM ratio of 0.1:1 did not result in any further stabilization of the receptor (Figure 2C & 2D). Previous studies have shown that CHS increases the thermostability and half-life of β2AR in detergent micelles [13, 59], and cholesterol has been shown to increase the melting temperature of β2AR in a membrane bilayer mimetic known as lipidic cubic phase (LCP) [60]. Similar results have been observed for other rhodopsin-like family members; cholesterol enhances the thermal stability of the oxytocin receptor [18], CHS addition to DDM increases the stability of the A2A adenosine receptor [20], and CHS and CHOBIMALT protect κOR from thermal inactivation [19]. Although ligand binding to proteins has a stabilizing effect that typically leads to an increase in thermostability [61, 62], the mechanism in which cholesterol and CHS are able to mediate GPCR thermostabilization is unclear.
Several GPCR crystal structures show cholesterol or CHS bound in a cleft formed by helices II, III, and IV [12–16]. Within this CCM cleft are several residues that favor cholesterol binding, the most notable of which is Trp4.50 [13]. Trp4.50 is 94% conserved among all GPCRs in the rhodopsin family and has been shown to interact with cholesterol rings through π-stacking interactions. All ORs contain the key Trp4.50 residue at the heart of the CCM, suggesting that cholesterol and appropriate analogs thereof potentially modulate the receptors through direct protein-sterol interactions. To explore if this effect stems from a specific interaction at the CCM, we mutated Trp1754.50 in ORL-1 to an alanine and attempted to measure the correlation between Tm at different CHS:DDM ratios. Despite flow cytometry data showing adequate expression of the W175A mutant ORL-1 in Sf9 cells [40], the protein extracted from the washed membranes displayed no thermal transitions and a poor SEC profile suggesting the protein was partially unfolded (data not shown). Albeit indirect, this finding suggested that the tryptophan within the CCM is critical for the integrity of the receptor. This could explain the observations that the four opioid receptors preferentially associate with cholesterol rich lipid rafts, and that MβCD-mediated cholesterol depletion has significant effects on signaling [26–33]. It has been shown that the δOR and µOR require cholesterol for proper agonist binding activity [26, 63, 64], and CHS is able to modulate agonist binding and efficacy to µOR, although purportedly through an increase in CHS:detergent microviscosity [48].
To understand the effects of sterols on the thermostability of ORL-1, we measured the size and shape of sterol-mixed micelles in the absence of protein using DLS and SAXS. Analysis of PDCs is not reported since the excess free micelle leads to sample heterogeneity, and the presence of protein invalidates the simplified two shell ellipsoid model used in the SAXS analysis. We found that the overall size of the micelles, as approximated by their hydrodynamic radius (Rh) or their radius of gyration (Rg), was dependent on the type of sterol mixed with DDM (Table 1). Whereas most studied sterols resulted in a smaller micelle size, CHOBIMALT had no apparent effect on the micelle size, which is consistent with the NMR based observations by Howell et al. [19]. In contrast, the properties of CHS were unique, since its addition to DDM resulted in an increase in the Rh and Rg in a concentration-dependent manner (Table 1).
More detailed information about the shape of the micelles was obtained from fitting the full SAXS profiles using a two-component scattering ellipsoid model with hydrophilic detergent head groups constituting an outer shell that surrounds an inner shell composed of the hydrophobic alkyl chains. The parameters obtained for the mixed CHS:DDM micelle indicated a swelling of the micelle; a substantial increase in the b1 radius of ~34% was observed, as was a modest but potentially significant increase in the length of a1 by ~4%. Comparison of the outer shell dimensions indicate that CHS promotes a significant increase in the a2 radius by ~70%, but does not change b2.
To explain these observations we propose the following model for mixed CHS/DDM micelles (Figure 4). CHS, like cholesterol and appropriate analogs [65], avoids highly curved regions and more readily inserts into the flatter parts of the micelle. The CHS sterol core intercalates in between the DDM hydrocarbon tails, thereby reducing the micelle curvature through the expansion of b1 due to favorable stacking interactions. The sterol mediated ordering effect on DDM hydrocarbon tails results in an increase in thickness of the hydrocarbon layer (parameter a1), allowing the central core of the micelle to resemble a membrane-like bilayer. The thickness of the outer micelle shell (parameter a2) is most likely increased as a consequence of two interacting forces. The hydrophobic-based condensing of DDM alkyl tails by CHS may cause DDM head groups to alter their orientation into a more extended configuration, and favorable electrostatic interactions between the DDM and CHS head groups likely contribute to the micelle packing and increase in a2 thickness. The bulkier head group of CHOBIMALT probably prevents condensing effects and electrostatic interactions with the maltoside head groups of DDM, providing an explanation for the smaller Rh value observed with this sterol/detergent mixture. NMR spectroscopy has demonstrated that the OH group of cholesterol participates in hydrogen bonding interactions with phospholipids [66], suggesting that the head groups of cholesterol analogs are important for similar interactions. In fact, studies with dipalmitoylphosphatidylcholine (DPPC) liposomes reconstituted with cholesterol or CHS indicate that the head groups of CHS and DPPC form both electrostatic and hydrogen bonding interactions causing greater membrane stabilization [67]. Thus, this model suggests that the arrangement of a CHS/DDM micelle resembles the architecture of a bicelle with DDM and CHS molecules forming a membrane-like bilayer disc flanked by a belt of DDM molecules.
Like cholesterol, only CHS and CHOBIMALT contain a double bond between C5 and C6 of the B- ring, which results in a more planar steroid core, enhanced packing against detergent acyl tails and putative specific binding sites on membrane proteins. In addition, all other studied sterols have hydrophilic groups attached either to the tetracyclic ring system or to the tail, thereby preventing their insertion into DDM micelles in the same orientation as CHS or CHOBIMALT, and thus reducing their potential for direct interactions with ORL-1 and other GPCRs. These factors likely combine to yield a more disordered DDM micelle that is smaller in size as evident from DLS and SAXS data, which is sufficient to destabilize GPCRs.
All DLS and SAXS measurements of the micelles were performed in the absence of embedded protein. However, superimposition of an oblate ellipsoid representing the inner hydrophobic shell of a CHS/DDM micelle (ratio = 0.2) with the high resolution β2AR crystal structure (PDB code 2RH1), clearly illustrates the spatial relationship of a GPCR’s PDC (Figure 5). Modeling of cholesterol against the outer bilayer half of the CCM illustrates a possible configuration that the sterol rings may adopt in the micelle (green cholesterol molecule in Figure 5).
Figure 5. Overlay of β2AR structure and the hydrophobic shell of a DDM and CHS:DDM micelle.
Superposition of β2AR structure (colored blue; PDB ID 2RH1) and the inner hydrophobic shell (colored gray) of a (A) (B) DDM micelle and a (C) (D) CHS:DDM micelle determined by SAXS (at a CHS:DDM ratio of 0.2:1). The palmitoyl acyl modification is colored cyan, three cholesterol molecules are colored magenta, Trp158 is colored yellow, and a modeled cholesterol molecule is colored green.
Consequently, our data and the model based on these data suggest that the stabilizing effect of CHS on ORL-1 embedded in mixed CHS/DDM micelles is likely two-fold. First, the bicelle-like structure of CHS/DDM micelles provides a more membrane-like environment, and second, CHS molecules insert in the hydrocarbon slab of the micelle in a similar orientation as cholesterol molecules insert in the membrane, which makes it possible for CHS to directly interact with cholesterol binding sites of ORL-1. We expect that these beneficial attributes should apply to most GPCRs and other membrane-embedded proteins. Taken together, these results suggest that unstable membrane proteins preferring DDM for extraction and purification, even those without CCMs, could benefit from the bicelle-like micelle properties provided by supplementation of CHS to this detergent.
5. Conclusion
A number of different sterol/DDM mixtures were tested for their ability to stabilize the opioid receptor ORL-1 for structural studies. Out of nine sterol-like detergents studied, only CHS and CHOBIMALT showed substantial thermostabilization of ORL-1. These two cholesterol analogs are chemically similar, each having a cholesterol-like tetracyclic ring system and non-polar tails, thereby permitting their penetration in the micelle core. The other seven sterols tested contained polar groups attached either to the ring system or to the hydrophobic tail and were likely incorporated within the micelle in configurations preventing direct sterol-ORL-1 interactions. CHS, the sterol that conferred the highest thermostability upon ORL-1, displayed the unique ability to increase the size of DDM micelles. Based on experimental data, we propose a model in which CHS molecules induce a bicelle-like architecture in the mixed CHS/DDM micelles. The membrane-like core of CHS/DDM micelles provides a more native stabilizing environment for membrane-embedded proteins and allows CHS molecules to directly interact with cholesterol binding sites on ORL-1. These attributes should extend to other GPCRs and proteins which are naturally embedded in a membrane bilayer environment. We therefore conclude that cholesterol analogs with planar steroid cores, hydrophobic tails, and small polar head groups attached to the hydroxyl group at the C3 position of ring-A should be the most effective in stabilizing membrane proteins in mixed micelles.
ACKNOWLEDGEMENT
The authors acknowledge the assistance of Tsutomu Matsui for support at SSRL BL 4.2; Jan Lipfert for access to the two-shell ellipsoid fitting script for MatLab; Girolamo Calo, Remo Guerrini and Stephano Molinari for the synthesis of Compound-24; and Angela Walker, Enrique Abola and Jeremiah S. Joseph for critical reading of the manuscript.
The abbreviations used are
- DPH
1,6-diphenyl-1,3,5-hexatriene
- Compound-24
1-benzyl-N-{3-[spiroisobenzofuran-1(3H),40-piperidin-1-yl]propyl} pyrrolidine-2-carboxamide
- β1AR
β1 adrenergic receptor
- β2AR
β2 adrenergic receptor
- CCM
cholesterol consensus motif
- CHS
cholesteryl hemisuccinate
- CPM
coumarin maleimide, N-[4-(7-diethylamino-4-methyl-3-coumarinyl)phenyl]maleimide
- CMC
Critical micelle concentration
- DPPC
dipalmitoylphosphatidylcholine
- DLS
dynamic light scattering
- GPCR
G protein-coupled receptor
- Rh
hydrodynamic radius
- IMAC
immobilized metal ion affinity chromatography
- LCP
lipidic cubic phase
- MβCD
methyl-β-cyclodextrin
- DDM
n-Dodecyl β-D-maltoside
- ORL-1
nociceptin opioid peptide receptor
- OR
opioid receptor
- PDC
protein-detergent complex
- Rg
radius of gyration
- SAXS
small-angle X-ray scattering
- Sf9
Spodoptera frugiperda cell line 9
- Tm
thermal transition temperature
- TM
transmembrane
- WT
wild type
Footnotes
This work was supported by the NIH Common Fund grant P50 GM073197 (RCS and VC) and NIH grant R01 GM089857 (VC). DW is supported by a Boehringer Ingelheim Fonds PhD Fellowship
REFERENCES
- 1.Fagerberg L, et al. Prediction of the human membrane proteome. Proteomics. 2010;10(6):1141–1149. doi: 10.1002/pmic.200900258. [DOI] [PubMed] [Google Scholar]
- 2.Almen MS, et al. Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 2009;7:50. doi: 10.1186/1741-7007-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dupuy CAX, Petipas C. Anomeric Effects on the Structure of Micelles of Alkyl Maltosides in Water. Langmuir. 1997;13:3965–3967. [Google Scholar]
- 4.Lipfert J, et al. Size and shape of detergent micelles determined by small-angle X-ray scattering. J Phys Chem B. 2007;111(43):12427–12438. doi: 10.1021/jp073016l. [DOI] [PubMed] [Google Scholar]
- 5.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta. 2004;1666(1–2):105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1(9):727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 8.Lin SH, Civelli O. Orphan G protein-coupled receptors: targets for new therapeutic interventions. Ann Med. 2004;36(3):204–214. doi: 10.1080/07853890310024668. [DOI] [PubMed] [Google Scholar]
- 9.Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7(4):339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 10.Moffett S, Brown DA, Linder ME. Lipid-dependent targeting of G proteins into rafts. J Biol Chem. 2000;275(3):2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- 11.Wang TY, Leventis R, Silvius JR. Partitioning of lipidated peptide sequences into liquid-ordered lipid domains in model and biological membranes. Biochemistry. 2001;40(43):13031–13040. doi: 10.1021/bi0112311. [DOI] [PubMed] [Google Scholar]
- 12.Cherezov V, et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science. 2007;318(5854):1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson MA, et al. A specific cholesterol binding site is established by the 2.8 A structure of the human beta2-adrenergic receptor. Structure. 2008;16(6):897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wacker D, et al. Conserved binding mode of human beta2 adrenergic receptor inverse agonists and antagonist revealed by X-ray crystallography. J Am Chem Soc. 2010;132(33):11443–11445. doi: 10.1021/ja105108q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warne T, et al. The structural basis for agonist and partial agonist action on a beta(1)-adrenergic receptor. Nature. 2011;469(7329):241–244. doi: 10.1038/nature09746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenbaum DM, et al. Structure and function of an irreversible agonist-beta(2) adrenoceptor complex. Nature. 2011;469(7329):236–240. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Malley MA, et al. The morphology and composition of cholesterol-rich micellar nanostructures determine transmembrane protein (GPCR) activity. Biophys J. 2011;100(2):L11–L13. doi: 10.1016/j.bpj.2010.12.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gimpl G, Fahrenholz F. Cholesterol as stabilizer of the oxytocin receptor. Biochim Biophys Acta. 2002;1564(2):384–392. doi: 10.1016/s0005-2736(02)00475-3. [DOI] [PubMed] [Google Scholar]
- 19.Howell SC, et al. CHOBIMALT: a cholesterol-based detergent. Biochemistry. 2010;49(44):9572–9583. doi: 10.1021/bi101334j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss HM, Grisshammer R. Purification and characterization of the human adenosine A(2a) receptor functionally expressed in Escherichia coli. Eur J Biochem. 2002;269(1):82–92. doi: 10.1046/j.0014-2956.2002.02618.x. [DOI] [PubMed] [Google Scholar]
- 21.Chien EY, et al. Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science. 2010;330(6007):1091–1095. doi: 10.1126/science.1197410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaakola VP, et al. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322(5905):1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimamura T, et al. Structure of the human histamine H1 receptor complex with doxepin. Nature. 2011;475(7354):65–70. doi: 10.1038/nature10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu B, et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330(6007):1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu F, et al. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332(6027):322–327. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andre A, et al. Membrane partitioning of various delta-opioid receptor forms before and after agonist activations: the effect of cholesterol. Biochim Biophys Acta. 2008;1778(6):1483–1492. doi: 10.1016/j.bbamem.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Butour JL, Corbani M, Meunier JC. Agonist-independent localization of the NOP receptor in detergent-resistant membrane rafts. Biochem Biophys Res Commun. 2004;325(3):915–921. doi: 10.1016/j.bbrc.2004.10.125. [DOI] [PubMed] [Google Scholar]
- 28.Ge X, Loh HH, Law PY. mu-Opioid receptor cell surface expression is regulated by its direct interaction with Ribophorin I. Mol Pharmacol. 2009;75(6):1307–1316. doi: 10.1124/mol.108.054064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang P, et al. Cholesterol reduction by methyl-beta-cyclodextrin attenuates the delta opioid receptor-mediated signaling in neuronal cells but enhances it in non-neuronal cells. Biochem Pharmacol. 2007;73(4):534–549. doi: 10.1016/j.bcp.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levitt ES, et al. Differential effect of membrane cholesterol removal on mu- and delta-opioid receptors: a parallel comparison of acute and chronic signaling to adenylyl cyclase. J Biol Chem. 2009;284(33):22108–22122. doi: 10.1074/jbc.M109.030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu Y, et al. Cholesterol Regulates {micro}-Opioid Receptor-Induced {beta}-Arrestin 2 Translocation to Membrane Lipid Rafts. Mol Pharmacol. 2011;80(1):210–218. doi: 10.1124/mol.110.070870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu W, et al. Localization of the kappa opioid receptor in lipid rafts. J Pharmacol Exp Ther. 2006;317(3):1295–1306. doi: 10.1124/jpet.105.099507. [DOI] [PubMed] [Google Scholar]
- 33.Zheng H, et al. Agonist-selective signaling is determined by the receptor location within the membrane domains. Proc Natl Acad Sci U S A. 2008;105(27):9421–9426. doi: 10.1073/pnas.0802253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballesteros JA, Weinstein H. Analysis and refinement of criteria for predicting the structure and relative orientations of transmembranal helical domains. Biophys J. 1992;62(1):107–109. doi: 10.1016/S0006-3495(92)81794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto Y, et al. Identification of a novel spiropiperidine opioid receptor-like 1 antagonist class by a focused library approach featuring 3D-pharmacophore similarity. J Med Chem. 2006;49(3):847–849. doi: 10.1021/jm0509851. [DOI] [PubMed] [Google Scholar]
- 36.Alexandrov AI, et al. Microscale fluorescent thermal stability assay for membrane proteins. Structure. 2008;16(3):351–359. doi: 10.1016/j.str.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Pace CN, et al. Conformational stability and thermodynamics of folding of ribonucleases Sa, Sa2 and Sa3. J Mol Biol. 1998;279(1):271–286. doi: 10.1006/jmbi.1998.1760. [DOI] [PubMed] [Google Scholar]
- 38.Konarev PV, VVV, Sokolova AV, Koch MHJ, Svergun DI. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Cryst. 2003;36:1277–1282. [Google Scholar]
- 39.Chattopadhyay A, London E. Fluorimetric determination of critical micelle concentration avoiding interference from detergent charge. Anal Biochem. 1984;139(2):408–412. doi: 10.1016/0003-2697(84)90026-5. [DOI] [PubMed] [Google Scholar]
- 40.Hanson MA, et al. Profiling of membrane protein variants in a baculovirus system by coupling cell-surface detection with small-scale parallel expression. Protein Expr Purif. 2007;56(1):85–92. doi: 10.1016/j.pep.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Strop P, Brunger AT. Refractive index-based determination of detergent concentration and its application to the study of membrane proteins. Protein Sci. 2005;14(8):2207–2211. doi: 10.1110/ps.051543805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.VanAken T, et al. Alkyl glycoside detergents: synthesis and applications to the study of membrane proteins. Methods Enzymol. 1986;125:27–35. doi: 10.1016/s0076-6879(86)25005-3. [DOI] [PubMed] [Google Scholar]
- 43.Mouritsen OG, Zuckermann MJ. What's so special about cholesterol? Lipids. 2004;39(11):1101–1113. doi: 10.1007/s11745-004-1336-x. [DOI] [PubMed] [Google Scholar]
- 44.Marsh D. Cholesterol-induced fluid membrane domains: a compendium of lipid-raft ternary phase diagrams. Biochim Biophys Acta. 2009;1788(10):2114–2123. doi: 10.1016/j.bbamem.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 45.Gandhavadi M, et al. Structure, composition, and peptide binding properties of detergent soluble bilayers and detergent resistant rafts. Biophys J. 2002;82(3):1469–1482. doi: 10.1016/S0006-3495(02)75501-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruner SM. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci U S A. 1985;82(11):3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haines TH. Do sterols reduce proton and sodium leaks through lipid bilayers? Prog Lipid Res. 2001;40(4):299–324. doi: 10.1016/s0163-7827(01)00009-1. [DOI] [PubMed] [Google Scholar]
- 48.Emmerson PJ, et al. Membrane microviscosity modulates mu-opioid receptor conformational transitions and agonist efficacy. J Neurochem. 1999;73(1):289–300. doi: 10.1046/j.1471-4159.1999.0730289.x. [DOI] [PubMed] [Google Scholar]
- 49.Stubbs CD, Smith AD. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- 50.Yeagle PL. Modulation of membrane function by cholesterol. Biochimie. 1991;73(10):1303–1310. doi: 10.1016/0300-9084(91)90093-g. [DOI] [PubMed] [Google Scholar]
- 51.Pucadyil TJ, Chattopadhyay A. Role of cholesterol in the function and organization of G-protein coupled receptors. Prog Lipid Res. 2006;45(4):295–333. doi: 10.1016/j.plipres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Niu SL, Mitchell DC, Litman BJ. Manipulation of cholesterol levels in rod disk membranes by methyl-beta-cyclodextrin: effects on receptor activation. J Biol Chem. 2002;277(23):20139–20145. doi: 10.1074/jbc.M200594200. [DOI] [PubMed] [Google Scholar]
- 53.Attwood PV, Gutfreund H. The application of pressure relaxation to the study of the equilibrium between metarhodopsin I and II from bovine retinas. FEBS Lett. 1980;119(2):323–326. doi: 10.1016/0014-5793(80)80281-x. [DOI] [PubMed] [Google Scholar]
- 54.Muth S, Fries A, Gimpl G. Cholesterol-induced conformational changes in the oxytocin receptor. Biochem J. 2011;437(3):541–553. doi: 10.1042/BJ20101795. [DOI] [PubMed] [Google Scholar]
- 55.Cantor R. Lateral pressures in cell membranes: a mechanism for modulation of protein function. J. Physiol. Chem. B. 1997;101:1723–1725. [Google Scholar]
- 56.Lundbaek JA, et al. Regulation of sodium channel function by bilayer elasticity: the importance of hydrophobic coupling. Effects of Micelle-forming amphiphiles and cholesterol. J Gen Physiol. 2004;123(5):599–621. doi: 10.1085/jgp.200308996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell DC, et al. Modulation of metarhodopsin formation by cholesterol-induced ordering of bilayer lipids. Biochemistry. 1990;29(39):9143–9149. doi: 10.1021/bi00491a007. [DOI] [PubMed] [Google Scholar]
- 58.Andersen OS, Koeppe RE., 2nd Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–130. doi: 10.1146/annurev.biophys.36.040306.132643. [DOI] [PubMed] [Google Scholar]
- 59.Yao Z, Kobilka B. Using synthetic lipids to stabilize purified beta2 adrenoceptor in detergent micelles. Anal Biochem. 2005;343(2):344–346. doi: 10.1016/j.ab.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Liu W, et al. LCP-Tm: an assay to measure and understand stability of membrane proteins in a membrane environment. Biophys J. 2010;98(8):1539–1548. doi: 10.1016/j.bpj.2009.12.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lo MC, et al. Evaluation of fluorescence-based thermal shift assays for hit identification in drug discovery. Anal Biochem. 2004;332(1):153–159. doi: 10.1016/j.ab.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 62.Pantoliano MW, et al. High-density miniaturized thermal shift assays as a general strategy for drug discovery. J Biomol Screen. 2001;6(6):429–440. doi: 10.1177/108705710100600609. [DOI] [PubMed] [Google Scholar]
- 63.Lagane B, et al. Role of sterols in modulating the human mu-opioid receptor function in Saccharomyces cerevisiae. J Biol Chem. 2000;275(43):33197–33200. doi: 10.1074/jbc.C000576200. [DOI] [PubMed] [Google Scholar]
- 64.Gaibelet G, et al. Cholesterol content drives distinct pharmacological behaviours of micro-opioid receptor in different microdomains of the CHO plasma membrane. Mol Membr Biol. 2008;25(5):423–435. doi: 10.1080/09687680802203380. [DOI] [PubMed] [Google Scholar]
- 65.Rukmini R, et al. Cholesterol organization in membranes at low concentrations: effects of curvature stress and membrane thickness. Biophys J. 2001;81(4):2122–2134. doi: 10.1016/S0006-3495(01)75860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soubias O, et al. Understanding sterol-membrane interactions, part II: complete 1H and 13C assignments by solid-state NMR spectroscopy and determination of the hydrogen-bonding partners of cholesterol in a lipid bilayer. Chemistry. 2004;10(23):6005–6014. doi: 10.1002/chem.200400246. [DOI] [PubMed] [Google Scholar]
- 67.Ding WX. Cholesteryl hemisuccinate as a membrane stabilizer in dipalmitoylphosphatidylcholine liposomes containing saikosaponin-d. Int J Pharm. 2005;300(1–2):38–47. doi: 10.1016/j.ijpharm.2005.05.005. [DOI] [PubMed] [Google Scholar]