Abstract
Background
Intraepidermal Langerhans cells (ILC) are difficult to differentiate from melanocytes under reflectance confocal microscopy (RCM) and their presence may simulate pagetoid spread of melanocytes on RCM images.
Objective
To correlate bright round and dendritic cells in a pagetoid pattern identified on RCM with findings of conventional histopathology and immunohistochemistry for lesions that were falsely diagnosed as melanoma by RCM.
Methods
This retrospective study included histopathologically proven nevi, imaged by RCM, which displayed bright cells in a pagetoid pattern (BCPP) under RCM, resulting in the incorrect RCM diagnosis of melanoma. Morphological comparisons were histopathologically proven melanomas displaying BCPP on RCM and biopsy-proven nevi without such cells on RCM.
Results
We identified 24 nevi that were falsely diagnosed as melanoma by RCM due to the presence of BCPP. These pagetoid cells on RCM corresponded on histopathology to ILC with a high density in 23 of the 24 nevi (95%) and to melanocytes in 7 of the 24 nevi (29%). Among 6 melanomas displaying BCPP on RCM, ILC with high density were observed histopathologically in 5 of the 6 cases (83%) and pagetoid melanocytes were seen in all 6 cases (100%).
Limitations
The results cannot be generalized to clinically banal-appearing nevi.
Conclusions
Although the finding of BCPP is a useful RCM feature for the diagnosis of melanoma it does not always imply the presence of pagetoid melanocytes but may at times, represent ILC.
Keywords: Langerhans cells, Pagetoid melanocytes, Pagetoid pattern, Reflectance confocal microscopy, CD1a, Melan-A
INTRODUCTION
The examination of the distribution and morphology of melanocytes under in vivo reflectance confocal microscopy (RCM) improves our ability to recognize early melanoma at the bedside 1–5. As with any imaging modality, however, RCM evaluation may give rise to false-positive or false-negative results, discordant with the final histopathologic diagnosis. Understanding the reasons for misdiagnoses is fundamental to furthering our knowledge about the advantages and limitations of RCM imaging.
The presence of melanocytes in the suprabasal layers of the epidermis, also known as melanocytes in a pagetoid pattern or spread, in many cases, constitutes a useful histopathologic criterion for the diagnosis of melanoma. However, there are situations in which nevi may also demonstrate pagetoid melanocytes on histopathology, therefore this feature should be assessed only in the context of a complete evaluation of the arvchitectural and cytologic features (e.g. size, symmetry and lateral circumscription) of a melanocytic lesion. A diagnosis of melanoma may be favored particularly when pagetoid spread of melanocytes is extensive, melanocytes are observed in the uppermost levels of the epidermis and at the lateral borders of the lesion, and when the melanocytes display cytological atypia 6, 7. Nevi, on the other hand, more often show focal and sparse pagetoid spread of melanocytes that lack cellular atypia6, 8, 9.
Using RCM, Pellacani et al. found the pagetoid spread of melanocytes to be a major criterion for the diagnosis of melanoma10. At times, however, the finding of bright cells in pagetoid pattern (BCPP) by RCM may be challenging to interpret since these cells may represent either melanocytes or intraepidermal langerhans cells (ILC)11, 12. ILC are dendritic cells that are typically found within the spinous layer of the epidermis, and which often exhibit a stellate morphology13. Langerhans cells vary in density within the human skin. On the head and neck, trunk and extremities LC can be found as high as 600–1000 per mm2. However, this number can be as low as 200 per mm2 in the palm, sole, and anogenital skin 13. ILC may simulate melanocytes on RCM. While ILC are difficult to visualize on H&E stained histopathologic tissue sections, immunohistochemical staining with CD1a reveals their presence as slender cells with processes that radiate from the cell body and extend between surrounding keratinocytes. On electron microscopy, the most reliable identifying feature of the LC is the presence of unique rod-shaped membrane bound granules in the cytoplasm known as Birbeck granules14, 15. The vast majority of Birbeck granules in LC are found within or in the vicinity of the Golgi region, but they can also be seen in other areas of the cytoplasm and in the dendritic processes15. LC are antigen-presenting cells for T lymphocytes and play an important role in the immune surveillance of the epidermis. LC have been observed in many benign and malignant tumors.16–20. Furthermore, alteration in density, distribution and morphology of LC have been reported with tumor progression19–23.
The aim of the present study was to correlate round and dendritic cells in a pagetoid pattern identified on RCM with findings on conventional histopathology and immunohistochemistry for lesions that were falsely diagnosed as melanoma by RCM.
METHODS
Sample
The patients for this retrospective analysis were selected from the pigmented lesion clinic at Memorial Sloan Kettering Cancer Center (MSKCC). Out of 463 nevi imaged between 2005 and 2009, we collected 24 samples from patients that were diagnosed as likely melanoma by RCM analysis based on the presence of bright roundish and/or dendritic cells in pagetoid pattern, but that proved to be nevi on histopathologic analysis. For morphological comparison, we included 6 biopsy-proven melanomas that showed BCPP on RCM as well as 9 samples from biopsy-proven nevi that were clinically atypical, but on RCM did not show BCPP.
RCM imaging and evaluation of images
RCM imaging was performed as part of an IRB-approved, ongoing multi-center prospective study titled “In-vivo Confocal Reflectance Microscopy for Pigmented Lesion Diagnosis.” All patients gave written consent for RCM examination of lesions. A commercially available, near infrared, RCM system (Vivascope 1500, Lucid Inc., Rochester, NY), which has been described previously 1, 24, was used for in vivo imaging of skin lesions. This system uses illumination with a near-infrared diode laser at 830 nm operating at a power of 5–10 mW. All RCM imaging was performed according to a standardized imaging protocol, which includes acquisition of the images with a digital camera, a dermoscopic camera, and the confocal microscope, biopsy of the lesions, and comparison of the captured images with the biopsy findings.
The retrospective evaluation of RCM images was jointly performed by two clinicians (P.H., A.S.). In the corresponding RCM images, BCPP were defined as bright roundish cells with dark nucleus or as bright dendritic cells within suprabasal layers. Cases displaying BCPP were evaluated for the following features (a) cell size, cells with a diameter less than 50 μm were considered “small” and cells that were 50 μm or greater were regarded as “large”; (b) Predominant cell shape was evaluated as “roundish”, “dendritic” or “pleomorphic” (when both roundish and dendritic cells were seen); (c) cell density was regarded as “slight” when less than 10 cells per mm2 were observed “ medium” for 11–20 cells per mm2 and “numerous” for more than 20 cells per mm2 10.
Histopathologic Study
In all samples included in this study, the lesion was biopsied following RCM imaging, formalin-fixed and paraffin embedded. Histopathological slides were routinely prepared and stained with hematoxylin-and-eosin (H&E); diagnoses were rendered during routine surgical pathology sign-out by a dermatopathologist. Subsequently, CD1a and Melan-A immunohistochemical stains were performed for the purpose of this study for all samples. In addition, specimens were also stained for cytokeratin-20, a specific marker for Merkel cells within the epidermis and related structures.
The presence of CD1a stained cells in the suprabasal layers of the lesional epidermis was considered positive for ILC. Positive cases were then stratified into “high” or “low” categories according to the density of LC where “low” corresponded to occasional, scattered LC, whereas “high” corresponded to numerous closely arranged LC. The lesions were also evaluated for the location of LC in the epidermis, and divided into those that were seen only as intercalated among basal keratinocytes (“basal”), versus those that were within the suprabasal layers.
Melanocytes in a pagetoid pattern were defined as the upward, discontinuous extension of melanocytes into the suprabasal layers of the epidermis. Histopathological criteria for melanocytes in a pagetoid pattern included the discontinuous extension of melanocytes from the junctional component, and the melanocyte location in the suprabasal epidermis at a level above that of the most superficial dermal papilla6; confirmed by positive staining for Melan-A. In addition, the density of dendrites of the pagetoid melanocytes was noted, and considered high when dendrites were intercalating between the majority of adjacent keratinocytes and intercellular bridges. The correlation of RCM-identified pagetoid cells to findings on histopathology and on immunohistochemistry was not blinded to the results of RCM or histopathologic analyses.
Analytical Methods
The data were analyzed for associations between the density of BCPP on RCM with the density of Langerhans Cells, and the density of pagetoid melanocytes. These analyses were performed both for the overall sample and each individual group controlling for LC density and groups as confounders in separate analyses. STATA version 10.1 was employed for the analysis of the results25. Due to small sample sizes available for this analysis, Pearson Chi-squared and Fisher-exact statistics are reported. p values less than α=0.05 were considered to be significant.
RESULTS
Overall, 39 melanocytic lesions from 39 patients (71.7% males, mean age: 51.2 ± 14.8) were included. Lesions were grouped into 3 categories (A, B, C) based on the histopathologic diagnosis and on the presence of BCPP on RCM analysis: A: nevi with BCPP on RCM (n = 24); B: nevi without BCPP on RCM (n = 9); C: melanomas with BCPP on RCM (n = 6). The trunk was the most commonly involved site for nevi and the extremities were most common for melanomas. The chief complaint was a history of change in lesions, in 24 nevi and in 4 melanomas (group A: 79%; group B: 56%; group C: 67%), versus the development of new lesions in the remainder. The 33 nevi were diagnosed on histopathology as junctional melanocytic nevi in 4, compound melanocytic nevi in 8, and dysplastic nevi in 21 samples. Inflammation was seen in higher proportions in group A, followed by group B, and C and this difference was significant (Fisher exact p = 0. 00). However, no significant association was found between LC density and inflammation (Fisher exact p = 0. 674).
LC were observed on histopathologic analysis in all 39 cases. LC in high density were seen in 23/24 lesions in group A nevi (96%) and 5/6 (83%) of the melanomas (group C); the difference was not significant (Fisher Exact p = 0.36). In all 24 lesions in group A, LC were located in the spinous as well as more superficial layers, while in 3/9 (33%) of group B nevi and in 3/6 (50%) of group C melanomas, LC were limited to basal layers and lower portion of spinous layers. Melan-A staining was positive in 29% of group A nevi, but in all group C melanomas (100%), (Fisher Exact p = 0.003). This difference was statistically significant. The density of dendrites of pagetoid melanocytes in melanomas was also significantly higher than nevi (Fisher Exact p = 0.005) (Table I). CK-20 staining was negative in all samples.
Table I.
Histopathological features and RCM characteristics of subgroups A, B, and C
| Subgroups | A. [n=24] | B. [n=9] | C. [n=6] | |
|---|---|---|---|---|
| Histopathology & RCM Characteristics | ||||
| Density of LC | High | 23/24 (95.8%) | 6/9 (66.6%) | 5/6 (83.3%) |
| Low | 1/24 (4.1%) | 3/9 (33.3%) | 1/6 (16.7%) | |
| Location of LC | Basal layer | 0/0 (00.0%) | 3/9 (33.3%) | 3/6 (50%) |
| Suprabasal layer | 24/24 (100%) | 6/9 (66.6%) | 3/6 (50%) | |
| Pagetoid melanocytes (PM) | Positive | 7/24 (29.1%) | 3/9 (33.3%) | 6/6 (100%) |
| Negative | 17/24 (70.8%) | 6/9 (66.6%) | 0/6 (0%) | |
| Density of dendrites in PM | High | 4/24 (16.6%) | 1/9 (11.1%) | 5/6 (83.3%) |
| Low | 20/24 (83.3%) | 8/9 (88.8%) | 1/6 (16.6%) | |
| BCPP size (RCM) | Small | 19/24 (79.1%) | 5/6 (83.3%) | |
| Large | 4/24 (16.6%) | 1/6 (16.6%) | ||
| BCPP density (RCM) | Slight | 10/24 (41.6%) | 1/6 (16.6%) | |
| Medium | 11/24 (45.8%) | 3/6 (50%) | ||
| Numerous | 3/24 (12.5%) | 2/6 (33.3%) | ||
| BCPP shape (RCM) | Roundish | 5/24 (20.8%) | 1/6 (16.6%) | |
| Dendritic | 4/24 (16.6%) | 0/6 (00.0%) | ||
| Pleomorphic | 15/24 (62.5%) | 5/6 (83.3%) | ||
The frequency and characteristics of BCPP, as observed on RCM, were reported in Table I. By definition, BCPP were observed on RCM in groups A nevi and group C melanomas. These cells were most frequently pleomorphic (both roundish and dendritic) (Fig 2B, 3B) in the two groups, followed by roundish cells (Fig 4B), and less commonly dendritic cells (Fig 1B). Small BCPP were observed in 19/24 (79%) of group A nevi and in 5/6 (83%) of group C melanomas (Fisher Exact p = 0.66). Numerous BCPP were seen in 2/6 (33%) of group C melanomas (Fig 5B), whereas the majority (87%) of group A nevi displayed slight to medium density of BCPP.
Case 2.
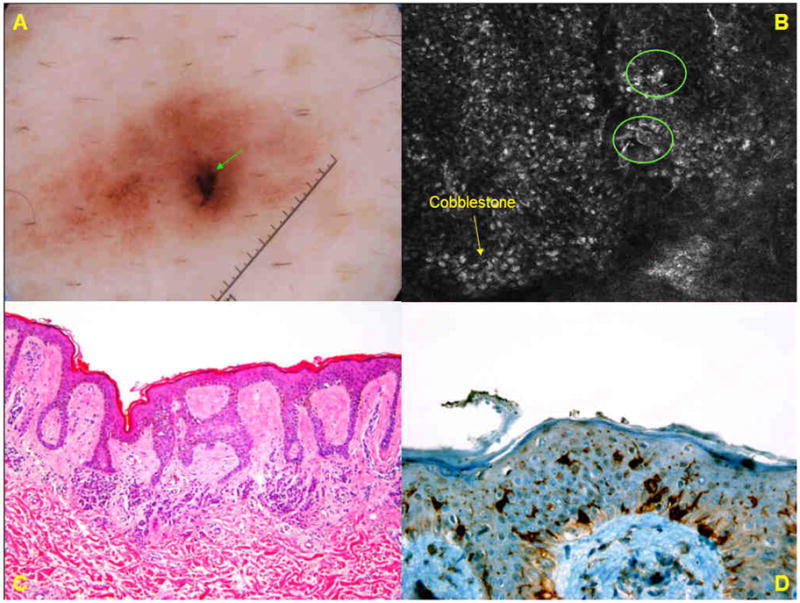
A. Dermoscopic image of a brown lesion (group A) on the abdomen of 61-year-old male shows an ill-demarcated lesion with a patchy network and an eccentric, peripheral black blotch. B. Basic RCM image (0.5mm × 0.5 mm) at the level of the spinous layer, from the dermoscopically-identified eccentric blotch, demonstrates a cobblestone pattern (→) in which few small, bright pleomorphic cells (❍) are seen in pagetoid pattern. C. Histopathologic analysis (H&E; original magnification ×50) shows a compound melanocytic nevus. There is mostly-lentiginous proliferation of melanocytes along the dermo-epidermal junction, but melanocytes in pagetoid pattern are not seen. D. Immunohistochemistry for CD1a reveals scattered intraepidermal Langerhans cells.
Case 4.
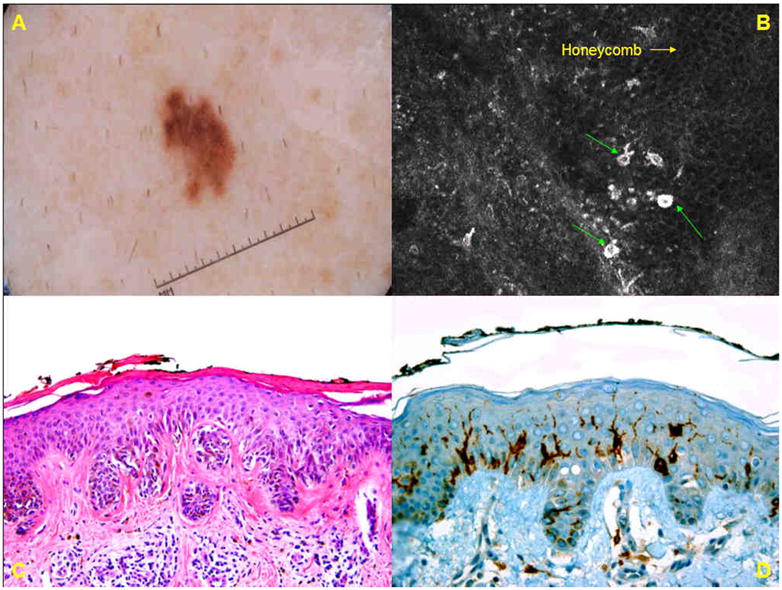
A: Dermoscopic image of a 5-mm brown lesion (group A) on the shoulder of a 45-year-old male reveals a patchy reticulated network with focal loss of network at one pole. B. Basic RCM image (0.5mm × 0.5 mm) at the level of the spinous layer displays an irregular cobblestone pattern with few small roundish and gamma-shaped bright cells (→) in pagetoid pattern. C. Histopathologic analysis (H&E; original magnification × 100) reveals a compound nevus with proliferation of monomorphous melanocytes in regular nests at the dermo-epidermal junction, and in the papillary dermis, and reticular dermis. D. CD1a staining reveals scattered Langerhans cells in the spinous layer.
Case 1.
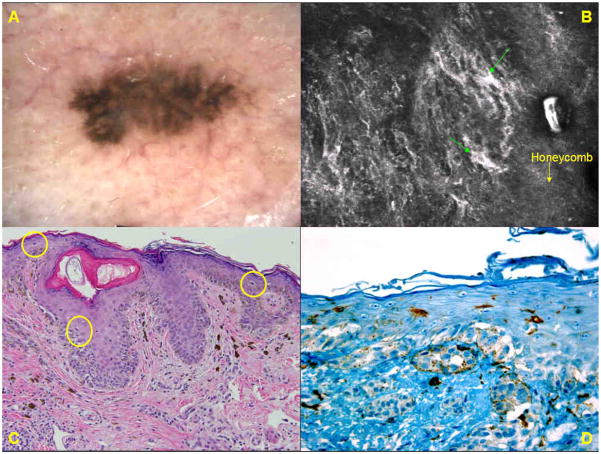
A. Dermoscopic image of an asymmetric pigmented lesion (group A) on the lower back of a 57-year man reveals biaxial asymmetry of colors and structures and a multi-component global pattern with foci showing negative network, blue–white structures, and peripheral structureless areas. Clinically and dermoscopically, the lesion was suspicious for melanoma. B. RCM basic image (0.5mm × 0.5mm) at the level of spinous layer shows small bright dendritic cells and dendritic processes (→) in medium density. C. Histopathologic analysis (H&E; original magnification ×200) shows a compound dysplastic nevus; the proliferation of melanocytes at the dermo-epidermal junction is mostly nested, cells in pagetoid pattern are not seen, and all of the melanocytes have small, oval nuclei. D. Immunohistochemistry for Melan-A displayes a few melanocytes in the basal layer.
Case 5.
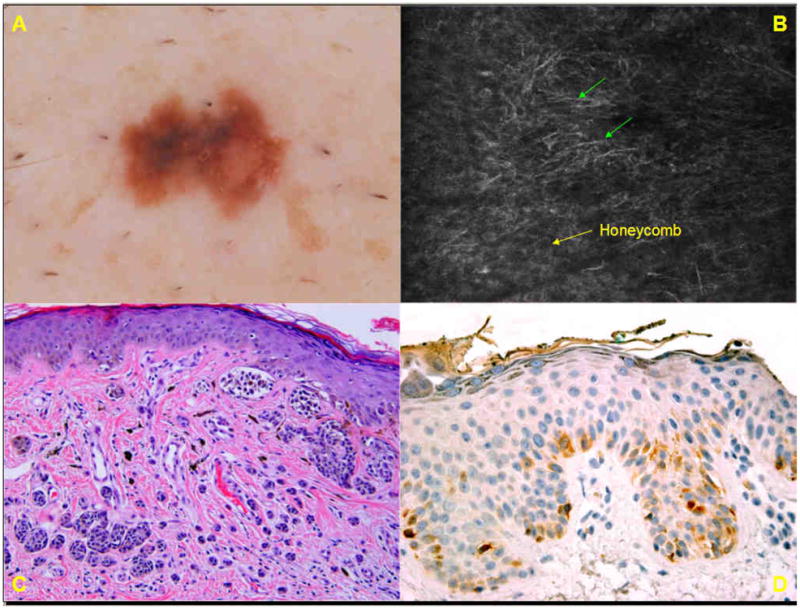
A. Dermoscopic image of a 3×6 mm irregular, brown macule (group C) on the cheek of a 72-year-old male displays a multi-component pattern with an atypical network and blue-white structures. B. Basic RCM image (0.5mm × 0.5mm) at the spinous layer shows a disarranged pattern with numerous large, bright dendritic cells (→) in a pagetoid pattern. C. Histopathologic analysis (H&E; original magnification ×50) reveals a dense proliferation of melanocytes as solitary units and irregular nests at the dermo-epidermal junction and extending down adnexal structures. Solitary melanocytes in pagetoid pattern (❍) are seen. The final diagnosis was melanoma, superficial Clark level IV. D. Immunohistochemistry for CD1a reveals a few intraepidermal Langerhans cells.
The association of density between BCPP on RCM and the density of Langerhans Cells was neither significant in groups A or C, nor the over all sample (Group A Pearson Chi2 = 1.46, Fisher Exact p = 0.542; Group C Pearson Chi2 = 6.0, Fisher Exact p = 0.167; Overall Pearson Chi2 = 3.7, Fisher Exact p = 0.276). The association between BCPP density on RCM and the density of pagetoid melanocytes was not significant in the over all sample (Pearson Chi2 = 4.48, Fisher Exact p = 0.137) (Table 2). This association was also analyzed by controlling LC density as a confounder. For the high density LC group, however, this association did approach significance (Pearson Chi2 = 5.47, Fisher Exact p = 0.07).
Table II.
Association of the BCPP density with the density of Langerhans cells and pagetoid melanocytes in the samples that show pagetoid pattern on RCM (group A and C)
| BCPP density on RCM | Pearson Chi2 | Fisher Exact p value | ||||
|---|---|---|---|---|---|---|
| Slight | Medium | Numerous | ||||
| Density of LC | High | 9 | 14 | 5 | 3.7013 | 0.276 |
| Low | 2 | 0 | 0 | |||
| Pagetoid melanocytes | Positive | 2 | 8 | 3 | 4.4867 | 0.137 |
| Negative | 9 | 6 | 2 | |||
DISCUSSION
Several investigators have demonstrated the utility of RCM in differentiating melanoma from benign pigmented lesions 3, 26–32. One of the most robust RCM features associated with melanoma was shown to be the presence of pagetoid cells in the spinous layer10, 26. In previous studies, the finding of bright roundish nucleated cells or bright dendritic cells on RCM were thought to correlate with melanocytes3, 26–32; however, these studies did not confirm that pagetoid cells identified on RCM were indeed melanocytes. Applying these RCM criteria in the clinic, we have encountered lesions that proved to be nevi on histopathology (Fig 1C, 2C, 3C, 4C) that demonstrate BCPP on RCM (Fig 1B, 2B, 3B, 4B).
Case 3.

A. Dermoscopic image of a 7-mm brown-black lesion (group A) on the abdomen of a 60 year-old male shows a multi-component pattern with peripheral network and central blue-white structures. The lesion was clinically and dermoscopically suspicious for melanoma. B. Basic RCM image (0.5mm × 0.5mm) at the dermo-epidermal junction and basal layer displays a disarranged pattern with bright dendritic and roundish nucleated cells (→) in medium density. C.: Histopathologic analysis (H&E; original magnification ×100) reveals a compound nevus, characterized by a proliferation of melanocytes as solitary units or small and large nests situated at the dermoepidermal junction and in the papillary dermis. The melanocytes have small oval monomorphous nuclei and scant cytoplasm. D. Staining for Melan-A reveals numerous melanocytes in the basal and spinous layers.
The lack of diagnostic specificity of pagetoid cells is a well-recognized issue in histopathologic analysis, particularly since this pattern of spread can be found in the melanocytes of acral nevi, congenital nevi, Spitz nevi, inflamed or traumatized nevi, and recurrent nevi6, 8, 9. However, in our experience, in most of the nevi that display cells in pagetoid pattern on RCM, pagetoid melanocytes were not the culprit. In the present study, we found that the RCM finding of BCPP (group A) most often correlated with the presence of ILC on histopathology though not to a significant degree (Table II). While CD1a staining for ILC was positive in all lesions, Melan A staining for melanocytes was positive in a minority (29%) of Group A nevi. We also determined that epidermal Merkel cells did not contribute to the RCM-identified cells in pagetoid pattern.
We compared nevi that showed BCPP on RCM (group A) to nevi without such cells on RCM (group B). Interestingly, both groups showed LC by immunohistochemistry. However, in group A nevi, the density of LC was higher than in group B, and the LC were located notably higher in the spinous layers in all of group A nevi, but only rarely in group B nevi. We speculate that, in group A nevi, the higher density and anatomic location of LC makes them more conspicuous on RCM examination. An atrophic or attenuated epidermis may further add to this phenomenon. The alternative explanation may be that LC in group A are functionally different from LC in group B, making them more refractive under RCM. For example, changes in distribution and density of Birbeck granules in LC may influence refractive index in vivo33, 34. We specifically found inflammation to be significantly higher in group A compared to group B. Additionally, it is possible that LC uptake melanosomes in certain melanocytic lesions, 35–38 and that these melanosomes within LC influence reflectivity.
In addition, we compared attributes of RCM-identified cells in pagetoid pattern between nevi and melanomas, with the hope of finding subtle differences in cellular morphology that distinguish LC from melanocytes on RCM. We did not find reproducible differences in cellular morphology between group A nevi and group C melanomas. Surprisingly, LC in our study were frequently pleomorphic among group A nevi; while we expected LC to present as dendritic cells with fine, long dendritic processes, we saw LC in some cases presenting as round nucleated cells on RCM. Indeed, LC have previously been shown to differ in their dendrite morphology when found in association with tumors18, 19, 21. For instance, Gibson et al. described that LC in tumors were rounded and had shorter, thicker and plump dendrites in comparison to normal skin, where dendrites were longer, delicate, thin and showed extensive arborization19. Similarly, Gatter described loss of dendrites in LC overlying BCC18. The size of BCPP also failed to discriminate between group A nevi and group C melanomas on RCM: large BCPP were present in 4/24 (16%) of nevi and in 1/6 of melanomas (16%). The most helpful RCM parameter in distinction of melanoma from nevi was the density of BCPP, which were slight to medium in nevi, but medium to numerous in melanomas (Fig 5B). Another striking difference was the histopathologic correlation; while BCPP seen with group A nevi correlated to melanocytes in a minority of cases, all group C melanomas showed suprabasal melanocytes that stained with Melan A. High density LC were also present in 83% of melanomas, and therefore, RCM-identified cells in the pagetoid pattern among melanomas could either represent LC or melanocytes.
Few studies have addressed the issue of the histopathologic correlates of round nucleated and dendritic cells seen on RCM. In line with our findings, Segura et al found that RCM-identified dendritic cells within pigmented basal cell tumor nests and in the overlying epidermis were indistinguishable from each other11. By immunohistochemical analysis, the dendritic cells in the overlying epidermis corresponded to LC, whereas those within tumor aggregates proved to be melanocytes11. Gerger et al. characterized dendrite-like structures in the epidermis of melanocytic skin tumors and suggested that they may have different significance according to their number, size and pattern3. Their results show that dendrite-like structures with a complex branching pattern are frequently seen in melanomas, and less frequently in benign nevi, where they tend to be smaller and more delicate3. Simple branching dendrites were described as having minor importance for diagnostic decision due to the low sensitivity (34.8%) and specificity (1.6%), while complex branching dendrites had 64.8% sensitivity and 100% specificity for melanoma3. However, in this study, dendrite-like structures were presumed to represent melanocytes, while the results of our study show that some of these dendritic cells may have been, in fact, LC.
Variations in LC density and distribution have been described in various skin tumors, including melanomas18–23. For example, Stene et al. found decreased number and density of LC overlying “deeply invasive” melanomas in comparison to in situ or “early invasive” melanomas23. Similar findings of variations of LC density and distribution in the epidermis overlying in-situ and thin melanomas vs. “deeply invasive” melanomas were confirmed by Toriyama and colleagues22. Facchetti et al. examined two melanoma cases with a peripheral radial growth and a central vertical growth20. They observed increased number of epidermal LC in periphery of the biopsy, while almost complete disappearance of epidermal LC in the vertical growth part of the lesion20. All our melanomas were melanoma in situ, superficial spreading type, having high number of pagetoid melanocytes in their pathology; this may explain the presence of LC in high frequency (83%) on histopathologic analysis. It is of interest, also, that these LC tended to be distributed within the lower levels of the epidermis as opposed to those involving their benign melanocytic counterparts.
Other factors that influence the density of LC in skin include aging, UV exposure, environmental antigens, and medications39–42. Aging and UV exposure reduces the number of LC in the skin13. In animal models, low dose UV-B treatment mediated by TNFα has been shown to reduce LC and alter the morphology of these cells by shortening or eliminating their dendrites to result in a more rounded cells43. We assume that the selection of samples from actinically damaged skin is more likely to demonstrate fewer Langerhans cells. Additionally, it has been demonstrated that UV radiation and the application of corticosteroids induce the loss of LC surface markers, which reduces the detection of LC in the biopsy samples44, 45.
This study has several limitations. Since this is an observational study, the data provided on comparison groups (group B and C) are to highlight morphological contrasts. The comparison groups have not been selected apriori to qualify as controls. In addition the small sample size of the comparison group was a major impediment to our statistical analyses, since it caused large standard errors making it difficult to obtain statistical significance. Large-scale studies with appropriately selected controls can shed further light on the histopathological correlation of BCPP on RCM. The study also does not provide data on the estimated prevalence of “pagetoid” cells that are not originally melanocytic, amongst lesions that are examined by RCM. Detection of various pagetoid cells requires specific immunohistochemistry or marker-specific types of staining46. However, we only employed Immunohistochemistry staining methods for LC, pagetoid melanocytes and Merkle cells. Interestingly, the certainty of presence of LC in the samples can be increased by application of other Langerhans staining techniques including both cytoplasmic markers and membrane markers such as ATPase, B-glucuronidase and Iab surface antigens since each of these staining identify different number of cells39. Finally, it is clear that the results of the study cannot be generalized to banal-appearing nevi in primary care settings, as the excised cases were clinically predetermined by referring specialists to be suspicious for melanoma.
In conclusion, this study highlights a pitfall in RCM imaging of melanocytic lesions. In RCM, bright cells in the spinous layer do not necessarily signify the presence of pagetoid melanocytes but may represent epidermal LC. While LC can sometimes mimic pagetoid melanocytes on RCM and lead to a false positive diagnosis of melanoma, this is an infrequent occurrence, and such lesions should be removed based on RCM suspicion. RCM evaluation of melanocytic lesions requires the evaluation of all cellular and architectural characteristics of the lesions.
Capsule Summary.
Bright cells in a pagetoid pattern (BCPP) are a major RCM criterion for melanoma diagnosis.
We evaluated the histology of nevi falsely classified as melanoma based on the presence of BCPP. We also analyzed BCPP in melanomas. BCPP corresponded most frequently to Langerhans cells in nevi and to melanocytes in melanomas. There were no reproducible differences in morphology of BCPP between the nevi and melanomas.
Equivocal melanocytic lesions with BCPP on RCM should be excised.
Acknowledgments
Funding source: Supported in part by a grant from National Institute of Health, Bethesda, MD. Grant ID: 2R44CA058054-04
We thank Babak Mohit, MPH from the Johns Hopkins Bloomberg School of Health for his assistance with the data analysis.
Abbreviations and acronyms
- RCM
Reflectance Confocal Microscopy
- ILC
Intraepidermal Langerhans cells
- LC
Langerhans Cells
- H&E
Hematoxylin-eosin
- BCPP
Bright cells in a pagetoid pattern
- PM
Pagetoid Melanocytes
Footnotes
Conflict of interest: The original grant was obtained by Lucid Inc., Rochester, New York for the study of “In-vivo Confocal Reflectance Microscopy for Pigmented Lesion Diagnosis”. Technical equipment from Lucid Inc. was employed for this study. No known financial conflicts of interests exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Anderson RR. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995 Jun;104(6):946–952. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- 2.Yamashita T, Kuwahara T, Gonzalez S, Takahashi M. Non-invasive visualization of melanin and melanocytes by reflectance-mode confocal microscopy. J Invest Dermatol. 2005 Jan;124(1):235–240. doi: 10.1111/j.0022-202X.2004.23562.x. [DOI] [PubMed] [Google Scholar]
- 3.Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005 Mar;124(3):493–498. doi: 10.1111/j.0022-202X.2004.23569.x. [DOI] [PubMed] [Google Scholar]
- 4.Pellacani G, Cesinaro AM, Longo C, Grana C, Seidenari S. Microscopic in vivo description of cellular architecture of dermoscopic pigment network in nevi and melanomas. Arch Dermatol. 2005 Feb;141(2):147–154. doi: 10.1001/archderm.141.2.147. [DOI] [PubMed] [Google Scholar]
- 5.Curiel-Lewandrowski C, Williams CM, Swindells KJ, et al. Use of in vivo confocal microscopy in malignant melanoma: an aid in diagnosis and assessment of surgical and nonsurgical therapeutic approaches. Arch Dermatol. 2004 Sep;140(9):1127–1132. doi: 10.1001/archderm.140.9.1127. [DOI] [PubMed] [Google Scholar]
- 6.Haupt HM, Stern JB. Pagetoid melanocytosis. Histologic features in benign and malignant lesions. Am J Surg Pathol. 1995 Jul;19(7):792–797. doi: 10.1097/00000478-199507000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Massi G. Melanocytic nevi simulant of melanoma with medicolegal relevance. Virchows Arch. 2007 Sep;451(3):623–647. doi: 10.1007/s00428-007-0459-7. [DOI] [PubMed] [Google Scholar]
- 8.Stern JB, Haupt HM. Pagetoid melanocytosis: tease or tocsin? Semin Diagn Pathol. 1998 Aug;15(3):225–229. [PubMed] [Google Scholar]
- 9.Petronic-Rosic V, Shea CR, Krausz T. Pagetoid melanocytosis: when is it significant? Pathology. 2004 Oct;36(5):435–444. doi: 10.1080/00313020412331285345. [DOI] [PubMed] [Google Scholar]
- 10.Pellacani G, Cesinaro AM, Seidenari S. Reflectance-mode confocal microscopy for the in vivo characterization of pagetoid melanocytosis in melanomas and nevi. J Invest Dermatol. 2005 Sep;125(3):532–537. doi: 10.1111/j.0022-202X.2005.23813.x. [DOI] [PubMed] [Google Scholar]
- 11.Segura S, Puig S, Carrera C, Palou J, Malvehy J. Dendritic cells in pigmented basal cell carcinoma: a relevant finding by reflectance-mode confocal microscopy. Arch Dermatol. 2007 Jul;143(7):883–886. doi: 10.1001/archderm.143.7.883. [DOI] [PubMed] [Google Scholar]
- 12.Busam KJ, Marghoob AA, Halpern AC. Melanoma diagnosis by confocal microscopy: promises and pitfalls. J Invest Dermatol. 2005;125:vii–ix. doi: 10.1111/j.0022-202X.2005.23865.x. [DOI] [PubMed] [Google Scholar]
- 13.Modlin RL, Kim J, Maurer D, Bangert C, Sting G. Innate and adaptive immunity in the skin. In: Wolff K, Goldsmith LA, Katz SI, Gilgres B, Paller AS, Leffell D, editors. Fitzpatrick’s Dermatology in General Medicine. Vol. 1. New York, NY: McGraw-Hill Companies, Inc; 2008. p. 107. [Google Scholar]
- 14.Chu AC. Histiocytoses. In: Champion R, Burton J, Burns D, Breathnach S, editors. Rook/Wilkinson/Ebling Textbook of Dermatology. 6. III. Malden, MA: Blackwell Science, Inc; 1998. p. 2313. [Google Scholar]
- 15.Breathnach SM. The Langerhans cell. Br J Dermatol. 1988 Oct;119(4):463–469. doi: 10.1111/j.1365-2133.1988.tb03249.x. [DOI] [PubMed] [Google Scholar]
- 16.Yamaji K, Matsui M, Saida T. Increased densities of Langerhans cells in the epidermis of skin tumors. J Dermatol. 1987;14:20–24. doi: 10.1111/j.1346-8138.1987.tb02989.x. [DOI] [PubMed] [Google Scholar]
- 17.Sterry W, Henseler T, Pehlemann FW, Schlegel D. Relative increase of Langerhans cells in ‘banal’ and dysplastic melanocytic naevi. Br J Dermatol. 1987 Apr;116(4):511–515. doi: 10.1111/j.1365-2133.1987.tb05870.x. [DOI] [PubMed] [Google Scholar]
- 18.Gatter KC, Morris HB, Roach B, Mortimer P, Fleming KA, Mason DY. Langerhans’ cells and T cells in human skin tumours: an immunohistological study. Histopathology. 1984 Mar;8(2):229–244. doi: 10.1111/j.1365-2559.1984.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 19.Gibson GE, O’Grady A, Kay EW, Leader M, Murphy GM. Langerhans cells in benign, premalignant and malignant skin lesions of renal transplant recipients and the effect of retinoid therapy. J Eur Acad Dermatol Venereol. 1998 Mar;10(2):130–136. [PubMed] [Google Scholar]
- 20.Facchetti F, de Wolf-Peeters C, de Greef H, Desmet VJ. Langerhans cells in various benign and malignant pigment-cell lesions of the skin. Arch Dermatol Res. 1984;276(5):283–287. doi: 10.1007/BF00404618. [DOI] [PubMed] [Google Scholar]
- 21.Smolle J, Soyer HP, Ehall R, Bartenstein S, Kerl H. Langerhans cell density in epithelial skin tumors correlates with epithelial differentiation but not with the peritumoral infiltrate. J Invest Dermatol. 1986 Oct;87(4):477–479. doi: 10.1111/1523-1747.ep12455529. [DOI] [PubMed] [Google Scholar]
- 22.Toriyama K, Wen DR, Paul E, Cochran AJ. Variations in the distribution, frequency, and phenotype of Langerhans cells during the evolution of malignant melanoma of the skin. J Invest Dermatol. 1993 Mar;100(3):269S–273S. doi: 10.1111/1523-1747.ep12470135. [DOI] [PubMed] [Google Scholar]
- 23.Stene MA, Babajanians M, Bhuta S, Cochran AJ. Quantitative alterations in cutaneous Langerhans cells during the evolution of malignant melanoma of the skin. J Invest Dermatol. 1988 Aug;91(2):125–128. doi: 10.1111/1523-1747.ep12464142. [DOI] [PubMed] [Google Scholar]
- 24.Rajadhyaksha M, Gonzalez S, Zavislan JM, Anderson RR, Webb RH. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999 Sep;113(3):293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 25.Stata Statistical Software [computer program]. Version 10.1. College Station, TX; StataCorp LP: 2007. [Google Scholar]
- 26.Pellacani G, Guitera P, Longo C, Avramidis M, Seidenari S, Menzies S. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007 Dec;127(12):2759–2765. doi: 10.1038/sj.jid.5700993. [DOI] [PubMed] [Google Scholar]
- 27.Guitera P, Pellacani G, Longo C, Seidenari S, Avramidis M, Menzies SW. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009 Jan;129(1):131–138. doi: 10.1038/jid.2008.193. [DOI] [PubMed] [Google Scholar]
- 28.Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008 Feb;158(2):329–333. doi: 10.1111/j.1365-2133.2007.08389.x. [DOI] [PubMed] [Google Scholar]
- 29.Gerger A, Hofmann-Wellenhof R, Samonigg H, Smolle J. In vivo confocal laser scanning microscopy in the diagnosis of melanocytic skin tumours. Br J Dermatol. 2009 Mar;160(3):475–481. doi: 10.1111/j.1365-2133.2008.08995.x. [DOI] [PubMed] [Google Scholar]
- 30.Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006 Jul 1;107(1):193–200. doi: 10.1002/cncr.21910. [DOI] [PubMed] [Google Scholar]
- 31.Gerger A, Wiltgen M, Langsenlehner U, et al. Diagnostic image analysis of malignant melanoma in in vivo confocal laser-scanning microscopy: a preliminary study. Skin Res Technol. 2008 Aug;14(3):359–363. doi: 10.1111/j.1600-0846.2008.00303.x. [DOI] [PubMed] [Google Scholar]
- 32.Langley RG, Walsh N, Sutherland AE, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology. 2007;215(4):365–372. doi: 10.1159/000109087. [DOI] [PubMed] [Google Scholar]
- 33.Wolff K. The fine structure of the Langerhans cell granule. J Cell Biol. 1967 Nov;35(2):468–473. doi: 10.1083/jcb.35.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishima Y. Melanosomes in phagocytic vacuoles in Langerhans cells. Electron microscopy of keratin-stripped human epidermis. J Cell Biol. 1966 Aug;30(2):417–423. doi: 10.1083/jcb.30.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tobin DJ. A possible role for Langerhans cells in the removal of melanin from early catagen hair follicles. Br J Dermatol. 1998 May;138(5):795–798. doi: 10.1046/j.1365-2133.1998.02215.x. [DOI] [PubMed] [Google Scholar]
- 36.Hemmi H, Yoshino M, Yamazaki H, et al. Skin antigens in the steady state are trafficked to regional lymph nodes by transforming growth factor-beta1-dependent cells. Int Immunol. 2001 May;13(5):695–704. doi: 10.1093/intimm/13.5.695. [DOI] [PubMed] [Google Scholar]
- 37.Fryer PR, Pope FM. Accumulation of membrane-bound melanosomes occurs in Langerhans cells of patients with the Leopard syndrome. Clin Exp Dermatol. 1992 Jan;17(1):13–15. doi: 10.1111/j.1365-2230.1992.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 38.Bartosik J. Melanosome complexes and melanin macroglobules in normal human skin. Acta Derm Venereol. 1991;71(4):283–286. [PubMed] [Google Scholar]
- 39.Rittman BR, Hill MW, Rittman GA, Mackenzie IC. Age-associated changes in Langerhans cells of murine oral epithelium and epidermis. Arch Oral Biol. 1987;32(12):885–889. doi: 10.1016/0003-9969(87)90102-6. [DOI] [PubMed] [Google Scholar]
- 40.Thiers BH, Maize JC, Spicer SS, Cantor AB. The effect of aging and chronic sun exposure on human Langerhans cell populations. J Invest Dermatol. 1984 Mar;82(3):223–226. doi: 10.1111/1523-1747.ep12260055. [DOI] [PubMed] [Google Scholar]
- 41.Gilchrest BA, Murphy GF, Soter NA. Effect of chronologic aging and ultraviolet irradiation on Langerhans cells in human epidermis. J Invest Dermatol. 1982 Aug;79(2):85–88. doi: 10.1111/1523-1747.ep12500031. [DOI] [PubMed] [Google Scholar]
- 42.Lynch DH, Gurish MF, Daynes RA. Relationship between epidermal Langerhans cell density ATPase activity and the induction of contact hypersensitivity. J Immunol. 1981 May;126(5):1892–1897. [PubMed] [Google Scholar]
- 43.Vermeer M, Streilein JW. Ultraviolet B light-induced alterations in epidermal Langerhans cells are mediated in part by tumor necrosis factor-alpha. Photodermatol Photoimmunol Photomed. 1990 Dec;7(6):258–265. [PubMed] [Google Scholar]
- 44.Aberer W, Schuler G, Stingl G, Honigsmann H, Wolff K. Ultraviolet light depletes surface markers of Langerhans cells. J Invest Dermatol. 1981 Mar;76(3):202–210. doi: 10.1111/1523-1747.ep12525745. [DOI] [PubMed] [Google Scholar]
- 45.Berman B, France DS, Martinelli GP, Hass A. Modulation of expression of epidermal Langerhans cell properties following in situ exposure to glucocorticosteroids. J Invest Dermatol. 1983 Mar;80(3):168–171. doi: 10.1111/1523-1747.ep12533397. [DOI] [PubMed] [Google Scholar]
- 46.Kohler S, Rouse RV, Smoller BR. The differential diagnosis of pagetoid cells in the epidermis. Mod Pathol. 1998 Jan;11(1):79–92. [PubMed] [Google Scholar]