Abstract
Objective
Endothelial cell (EC) migration is essential for arterial healing after angioplasty. Oxidized low-density lipoproteins and oxidative stress decrease EC migration in vitro. The objective of this study was to determine the effect of hypercholesterolemia and oxidative stress on EC healing after an arterial injury.
Methods and Results
C57Bl/6 wild-type mice were placed in one of eight groups: chow diet (n = 11), high cholesterol (HC) diet (n = 11), chow diet plus paraquat (n = 11), HC diet plus paraquat (n = 11), chow diet plus N-acetylcysteine (NAC) (n = 11), HC diet plus NAC (n = 11), chow diet plus paraquat and NAC (n = 11), and HC diet plus paraquat and NAC (n = 11)After two weeks on the assigned diet with or without NAC, the carotid artery was injured using electrocautery. Animals in the paraquat groups were given 1 mg/kg intraperitoneally to increase oxidative stress. After 120 hours, Evans Blue dye was infused intravenously to stain the area of the artery that remained deendothelialized. This was used to calculate the percentage of reendothelialization. Plasma and tissue samples were analyzed for measures of oxidative stress.
The HC diet increased oxidative stress and reduced EC healing compared with a chow diet, with EC covering 26.8 ± 2.8% and 48.1 ± 5.2% (P < .001) of the injured area, respectively. Administration of paraquat decreased healing in both chow and HC animals to 18.1 ± 3.5% (P < .001) and 9.8% ± 4.6% (P < .001), respectively. Pretreatment with NAC (120 mmol/L in drinking water) for 2 weeks prior to injury, to decrease oxidative stress, improved EC healing to 39.9 ± 5.7% (P < .001) in hypercholesterolemic mice and to 30.7 ± 3.6% (P < .001) in the paraquat group. NAC treatment improved healing to 24.6% ± 3.4% (P < .001) in hypercholesterolemic mice treated with paraquat.
Conclusion
Reendothelialization of arterial injuries is reduced in hypercholesterolemic mice, and is inversely correlated with oxidative stress. An oral antioxidant decreases oxidative stress and improves EC healing.
Clinical Relevance
Vascular injury following cardiovascular intervention including cardiac and peripheral arterial angioplasty and stenting is associated with inflammation and oxidative stress. Hypercholesterolemia is also associated with increased oxidative stress. Oxidative stress, regardless of the source, induces cellular dysfunction in endothelial and smooth muscle cells that reduces healing after arterial injury. Decreasing oxidative stress with an exogenously administered antioxidant can improve endothelial cell healing, and this is important to control intimal hyperplasia and reduce the thrombogenicity of the vessel.
Keywords: Endothelium, hypercholesterolemia, antioxidant, angioplasty, reactive oxygen species, vascular injury, endothelial migration
INTRODUCTION
Angioplasty is commonly performed for the treatment of coronary and peripheral arterial disease, and endothelial cell (EC) migration is critical for luminal healing after arterial injury. The long-term patency following angioplasty is limited by thrombosis and restenosis, with the latter due to vessel recoil, constrictive remodeling, and neointimal hyperplasia.1 Decreased EC migration into denuded areas contributes to prolonged surface thrombogenicity and development of intimal hyperplasia, and this may result in failure of arterial interventions. Factors affecting EC migration have been studied extensively in vitro, but the relevance in vivo is not clear. The failure rate of angioplasties increases in the setting of hypercholesterolemia, but the mechanisms involved are not completely understood.
Studies of arterial injuries in animal models have added to the understanding of the pathophysiologic events accompanying angioplasties and have identified factors contributing to increased intimal hyperplasia but have largely ignored EC migration. Aortic intimal thickening following balloon angioplasty is significantly greater in hypercholesterolemic rabbits than in chow-fed rabbits.2 Hypercholesterolemia is associated with elevated circulating levels of oxidized low-density lipoprotein (oxLDL), increased oxLDL at the site of injury compared with chow-fed animals,3 and elevated plasma lysophosphatidylcholine (lysoPC, a product of lipid oxidation and the major lysophospholipid of oxLDL).
OxLDL stimulates smooth muscle cell (SMC) migration and proliferation,4, 5 and inhibition of EC migration in vitro.6 The antimigratory effects of oxLDL and lysoPC, that for much of oxLDL’s antimigratory activity,7 can be reversed with superoxide dismutase and NAD(P)H inhibitors in vitro.8 These in vitro studies suggest a possible role for hypercholesterolemia and ROS in limiting endothelial healing after an injury in vivo. The present study was undertaken to assess the applicability of these in vitro findings to arterial healing in vivo. The effect of hypercholesterolemia and oxidative stress on EC migration was evaluated after a denuding arterial injury, and the ability of an orally administered antioxidant to preserve EC migration in vivo was determined.
MATERIALS AND METHODS
Study groups
Six-week-old male C57Bl/6 wild-type mice were randomized to one of eight study groups: 1) chow diet, 2) high cholesterol (HC) Western diet (Harlan Teklad, Madison, WI) containing 21% milk fat and 0.2% cholesterol by weight, 3) chow diet plus paraquat (methyl viologen, Sigma-Aldrich, St. Louis, MO), 4) HC diet plus paraquat, 5) chow diet plus N-acetylcysteine (NAC), 6) HC diet plus NAC, 7) chow diet plus paraquat and NAC, and 8) HC diet plus paraquat and NAC. Paraquat, which causes oxidative stress,9 was administered at dose of 1 mg/kg intraperitoneally at the time of carotid injury. NAC (120 mmol/L, pH 7.0), a cell-permeable antioxidant with direct and indirect antioxidant activity,10, 11 was administered in drinking water starting two weeks prior to injury and continued to the completion of the study. All animals were maintained on the assigned diet and treatment throughout the study. The animal study protocol was approved by the Institutional Animal Care and Use Committee. All procedures and care complied with the American Association of Laboratory Animal Care guidelines and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1996).
Carotid injury
Carotid injury was performed when mice were eight weeks of age by modified perivascular electrical injury12 or air-drying injury.13 The mice were anesthetized with an intraperitoneal injection of ketamine 80 mg/kg (Fort Dodge, Fort Dodge, IA) and xylazine 5 mg/kg (Vedco, St, Joseph, MO). The right common carotid artery was isolated and injured by applying electrical current or air drying. The electrical injury was produced by applying bipolar electrocautery using a Surgistat II electrosurgical generator (Valleylab, Boulder, CO) and custom bipolar forceps (Elmed Inc., Addison, IL) with 4 mm wide tips. Two watts of power were applied to the artery for 3 seconds. For the air-drying injury, the proximal common and internal carotid arteries were encircled with silk sutures. The external carotid artery was ligated, a 30-gauge needle was inserted into the proximal external carotid artery, and an exit hole was made at the proximal end of the common carotid. The common carotid was flushed with saline to remove blood. Then, air was injected at 20 mL/min for five minutes to denude the endothelium. The silk sutures were removed from the common and internal carotid arteries to restore blood flow through the area of injury.
Carotid artery harvest and analysis
At 120 hours after electrical injury or 108 hours after air injury, mice were anesthetized, and blood was collected from the inferior vena cava. Evans Blue (5% in PBS, 100 μL) 14 was injected into the inferior vena cava and allowed to circulate for 10 minutes to delineate the deendothelialized area of the right carotid artery. The vessels were perfusion-fixed for 10 minutes by intracardiac infusion of paraformaldehyde (4% in distilled water, pH 7.0, 30 mL). The right common carotid artery was excised, opened longitudinally, and pinned flat on a wax-coated plate. The specimen was imaged using Scion Image (Scion Corp., Frederick, MD) for analysis with NIH Image software and the area of unhealed artery, that stained with Evans Blue, was measured. Scanning electron microscopy was used on a subset of carotid arteries to verify that Evans Blue accurately reflected the deendothelialized artery. The length of the injury was 4 mm in length in the electrocautery injury and defined as extending 4 mm distally from the air exit hole in the common carotid artery in the air-drying model. The results of the endothelial cell migration for both methods of injury were verified by a reviewer blinded to the treatment group.
Plasma assays
Blood was obtained at the time of carotid artery removal to measure total cholesterol, lysoPC, and thiobarbituric acid reactive substances (TBARS). A cholesterol oxidase method (Infinity Cholesterol Reagent, Thermo Fisher Scientific, Inc., Waltham, MA) was used to determine the total cholesterol concentration. Plasma lysoPC concentration was determined by an enzymatic method using the Azwell LPC Assay Kit (Cosmo Bio USA Inc., Carlsbad, CA). Lipid peroxides were measured as TBARS using a spectrofluorometric method (λex = 515 nm, λem = 553 nm).15
Immunostaining for macrophages and tissue oxidized LDL
The presence of macrophages and oxLDL was assessed by immunohistochemistry on paraffin-embedded sections of the right and left carotid arteries using antibody to MAC-3 (1:50, BD Pharmingen, Franklin Lakes, NJ) or hypochlorite-oxLDL (1:2000, Calbiochem, 428035, Gibbstown, NJ), respectively. The total numbers of cells, as well as the number of positively stained macrophages, were counted. The data were reported as the percentage of macrophages in a 0.05 mm2 area. OxLDL was used as a marker of tissue level oxidative stress. Three observers blinded to the treatment group qualitatively scored the oxLDL staining on a scale of 0 to 4.
Statistical analysis
Results were represented as the mean ± standard deviation (SD). Statistical analysis was performed by student t-test or analysis of variance (ANOVA) followed by Tukey’s posthoc multiple comparison using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA), and P ≤ .05 was considered statistically significant.
RESULTS
A total of 108 8-week old C57Bl/6 mice were included in this study, including 88 mice that underwent an electrical injury and 20 that underwent an air-drying injury. The demographics for mice that underwent the electrical injury are detailed in Table I. There was no significant difference in age among the treatment groups, but the weight of mice receiving a HC diet tended to be higher than mice on a chow diet. No mice that underwent an electrical injury died during the procedure or thrombosed the injured artery.
Table I.
Mouse demographics and plasma data for mice that underwent carotid electrical injury (presented as mean ± standard deviation)
| Group | N | Age (days) | Weight (g) | Cholesterol (mmol/L) | LysoPC (μmol/L) | TBARS (μmol MDA/L) |
|---|---|---|---|---|---|---|
| Chow | 11 | 57 ± 1.9 | 23.0 ± 1.5a | 2.54 ± 0.19c | 593 ± 49a,e,f | 0.36 ± 0.02a,k |
| Chow + Paraquat | 11 | 57 ± 1.9 | 24.4 ± 1.8 | 2.34 ± 0.14c | 627 ± 54 | 0.81 ± 0.05l |
| Chow + NAC | 11 | 56 ± 1.9 | 23.3 ± 1.7b | 2.46 ± 0.16c | 480 ± 42 | 0.36 ± 0.02 |
| Chow + Paraquat + NAC | 11 | 57 ± 1.6 | 24.0 ± 1.8 | 2.51 ± 0.14c | 642 ± 44 | 0.66 ± 0.03 |
| High Cholesterol | 11 | 58 ± 0.3 | 25.6 ± 1.7 | 3.75 ± 0.32 | 851 ± 60g,h,i | 0.62 ± 0.04g,h,i |
| HC + Paraquat | 11 | 58 ± 1.5 | 24.9 ± 1.5 | 3.69 ± 0.24d | 933 ± 75j | 0.95 ± 0.04j |
| HC + NAC | 11 | 57 ± 1.7 | 24.2 ± 1.9 | 4.01 ± 0.31 | 748 ± 72 | 0.56 ± 0.03 |
| HC + Paraquat + NAC | 11 | 56 ± 1.8 | 24.9 ± 2.0 | 3.80 ± 0.16 | 788 ± 62 | 0.72 ± 0.04 |
HC = High cholesterol
NAC = N-acetylcysteine
LysoPC = Lysophosphatidylcholine
TBARS = Thiobarbituric acid reactive substances
Significant differences (P ≤ .05) are designated as:
Between chow and high cholesterol
Between chow + NAC and high cholesterol
Between all chow groups and all high cholesterol groups
Between HC + paraquat and HC + NAC
Between chow and chow + NAC
Between chow and chow + paraquat + NAC
Between high cholesterol and HC + paraquat
Between high cholesterol and HC + NAC
Between high cholesterol and HC + paraquat + NAC
Between HC + paraquat and HC + paraquat + NAC
Between chow and chow + paraquat
Between chow + paraquat and chow + paraquat + NAC
Effect of hypercholesterolemia on reendothelialization after injury
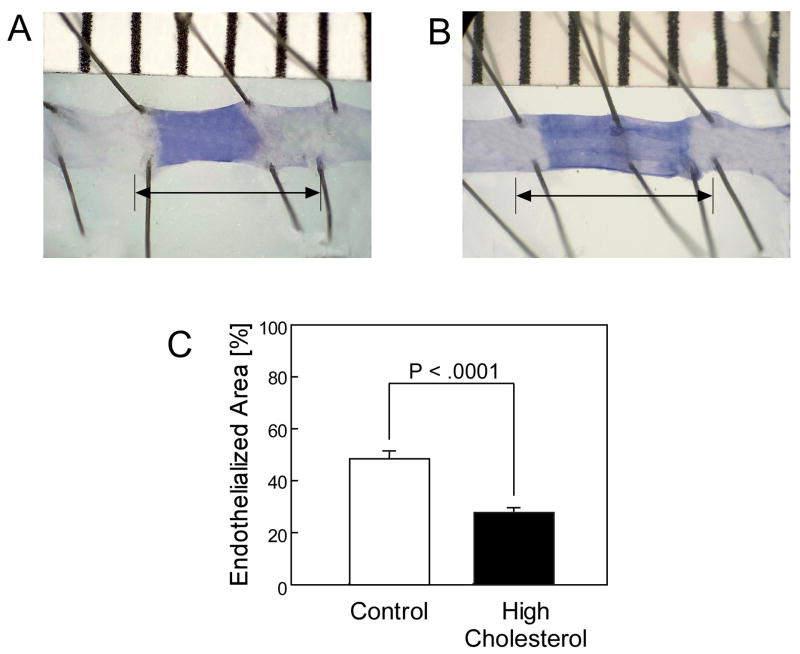
Diet had a dramatic impact on plasma cholesterol level and reendothelialization rate. Plasma cholesterol levels were significantly higher in the HC mice than the chow group, being 3.68 ± 0.32 mmol/L and 2.53 ± 0.19 mmol/L, respectively (P <.001) (Table I). After the electrical injury, mice on the chow diet healed 48.1% ± 5.2% of the injured area by 120 hours (Fig. 1, A & C), but reendothelialization in the HC group was significantly decreased with coverage of 26.8% ± 2.8% (P <.0001) (Fig. 1, B & C) (Table II). EC healing was observed to extend farther from the proximal edge of uninjured artery than the distal, suggesting that EC migration proceeded more efficiently in line with blood flow.
Fig. 1.
Representative carotid arteries 120 hours after carotid electrical injury for mice in the chow group (A) and the high cholesterol group (B). The stained area represents the denuded section of artery. The arrow represents the length of the original injury used to calculate the reendothelialized area. Reendothelialization following carotid electrical injury is reported as the area reendothelialized relative to the total area of injury and expressed as the mean ± standard error for mice in the chow group (n = 5) and in the high cholesterol group (n = 5).
Table II.
Endothelial cell migration and tissue oxidized LDL scores for mice that underwent carotid electrical injury (presented as mean ± standard deviation)
| Group | N EC Migration | EC Migration (%) | N OxLDL Score Injured | OxLDL score Injured Artery | N OxLDL Score Uninjured | OxLDL score Uninjured Artery |
|---|---|---|---|---|---|---|
| Chow | 5 | 48.1 ± 5.2a,b,c | 5 | 1.8 ± 0.9a,b | 5 | 1.4 ± 0.2a,b |
| Chow + Paraquat | 5 | 18.0 ± 3.5d | 5 | 3.1 ± 0.4h | 5 | 2.0 ± 0.8 |
| Chow + NAC | 5 | 70.4 ± 7.6 | 5 | 3.0 ± 1.9 | 5 | 1.9 ± 0.4 |
| Chow +Paraquat + NAC | 5 | 30.7 ± 3.6 | 4 | 2.8 ± 0.4h | 5 | 2.0 ± 0.4 |
| High Cholesterol | 5 | 26.8 ± 2.8e,f | 5 | 3.0 ± 0.5f,h | 5 | 2.2 ± 0.2 |
| HC + Paraquat | 5 | 9.8 ± 4.6g | 5 | 3.0 ± 0.5h | 5 | 2.1 ± 0.8 |
| HC + NAC | 5 | 39.9 ± 5.7 | 6 | 2.8 ± 0.5 | 4 | 2.6 ± 0.6 |
| HC + Paraquat + NAC | 5 | 24.6 ± 3.4 | 6 | 2.4 ± 0.7 | 5 | 2.1 ± 0.3 |
HC = High cholesterol
NAC = N-acetylcysteine
EC = Endothelial cell
oxLDL = Oxidized low-density lipoprotein
Significant differences (P ≤ .05) are designated as:
Between chow and high cholesterol
Between chow and chow + paraquat
Between chow and chow + NAC
Between chow + paraquat and chow + paraquat + NAC
Between high cholesterol and HC + paraquat
Between high cholesterol and HC + NAC
Between HC + paraquat and HC + paraquat + NAC
Between injured artery and uninjured artery within same diet and treatment group
Because of the concern that a transmural injury might alter the pattern of EC migration, an additional 20 mice underwent a carotid air-drying injury the focus of the study was endothelial healing and the effect of a transmural injury on EC migration was unknown, a carotid air-drying injury model was also used. Three mice were excluded from the carotid air-drying injury portion of the study due to arterial thrombosis between the time of injury and arterial harvest. The rate of thrombosis with the air injury is within the expected range for this model. Chow-fed mice reendothelialized 69.2% ± 25.8% (Fig. 2, A & C) of the injured area at 108 hours compared with 44.6% ± 22.2% reendothelialization in HC diet mice (P =.03) (Fig. 2, B & C) (Table II). Since both forms of injury showed a similar pattern of results, the decision was made to use the electrical injury for future studies because it created a more consistent injury.
Fig. 2.
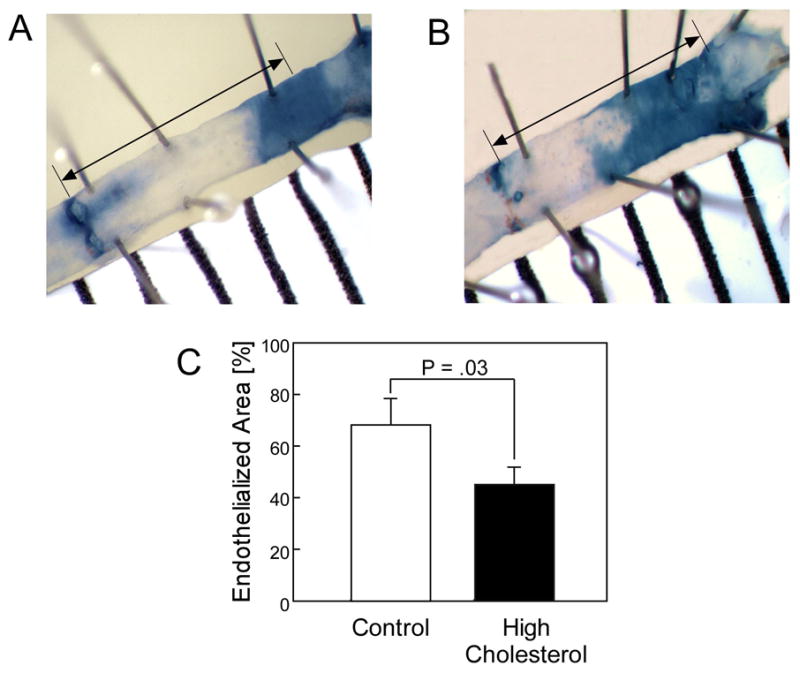
Representative carotid arteries 108 hours after carotid air injury for mice in the chow group (A) and high cholesterol group (B). The stained area represents the denuded section of artery. The arrow represents the length of the original injury used to calculate the reendothelialized area. Reendothelialization following carotid air injury is reported as the area reendothelialized relative to a 4 mm length of the original injury and expressed as the mean ± standard error for mice in the chow group (n = 7) and in the high cholesterol group (n = 10).
As markers of oxidative stress, plasma TBARS and lysoPC levels, and tissue oxLDL levels were determined. Both TBARS and lysoPC levels were significantly higher in mice on the HC diet compared with chow-fed mice (Table I). The tissue oxLDL scores were not significantly different between the injured and uninjured arteries in chow-fed mice, with the scores being 1.8 ± 0.9 (n = 5) and 1.4 ± 0.2 (n = 5), respectively (Table II). However, in hypercholesterolemic mice the tissue oxLDL scores were significantly higher in the injured artery compared to the uninjured artery with scores of 3.0 ± 0.5 (n = 5) and 2.0 ± 0.2 (n = 5) (P =.01), respectively (Table II). For both the injured and uninjured carotid arteries, the oxLDL score was significantly higher in the hypercholesterolemic mice compared to the normocholesterolemic mice (P =.02 and P <.001, respectively) (Table II).
Cellularity and macrophage infiltration were assessed in the injured and uninjured carotid arteries. The number of cells and the percentage of macrophages in corresponding areas were not significantly different between the chow-fed and hypercholesterolemic animals, but within each dietary group, the percentage of macrophages in the injured right carotid artery was significantly higher than in the uninjured left carotid artery (P ≤.001 for both the chow and HC groups) (Table III).
Table III.
Cellularity and macrophage accumulation in injured and uninjured carotid arteries in mice that underwent a carotid electrical injury (presented as mean ± standard deviation)
| Group | Injured Artery | Uninjured Artery | ||||
|---|---|---|---|---|---|---|
| N | Total Cellsa | % Macrophageb | N | Total Cellsa | % Macrophageb | |
| Chow | 5 | 342 ± 49c | 65 ± 15f | 5 | 347 ± 64 | 11 ± 8 |
| Chow + Paraquat | 5 | 249 ± 55d | 69 ± 8f | 5 | 358 ± 50 | 16 ± 12 |
| Chow + NAC | 6 | 286 ± 60 | 44 ± 26f | 6 | 369 ± 76 | 14 ± 2 |
| Chow + Paraquat + NAC | 6 | 268 ± 65 | 63 ± 8f | 6 | 325 ± 50 | 9 ± 5 |
| High Cholesterol | 4 | 314 ± 53 | 57 ± 15f | 4 | 357 ± 82 | 10 ± 4 |
| HC + Paraquat | 6 | 282 ± 51 | 69 ± 10f | 5 | 328 ± 65 | 19 ± 7 |
| HC + NAC | 5 | 202 ± 53e | 62 ± 11f | 5 | 301 ± 71 | 9 ± 6 |
| HC + Paraquat + NAC | 5 | 274 ± 97 | 61 ± 15f | 5 | 351 ± 63 | 8 ± 6 |
HC = High cholesterol
NAC = N-acetylcysteine
Significant differences (P ≤ .05) are designated as:
Total cell count/0.05 mm2
Percent of total number of cells identifies as macrophages/0.05 mm2 Significant differences (P ≤ .05) are designated as:
Total cells between chow and chow + paraquat injured arteries
Total cells between chow + paraquat injured and uninjured arteries
Total cells between HC + NAC injured and uninjured arteries
% Macrophages between injured and uninjured arteries with in same diet treatment group
Effect of antioxidant therapy on reendothelialization after arterial injury
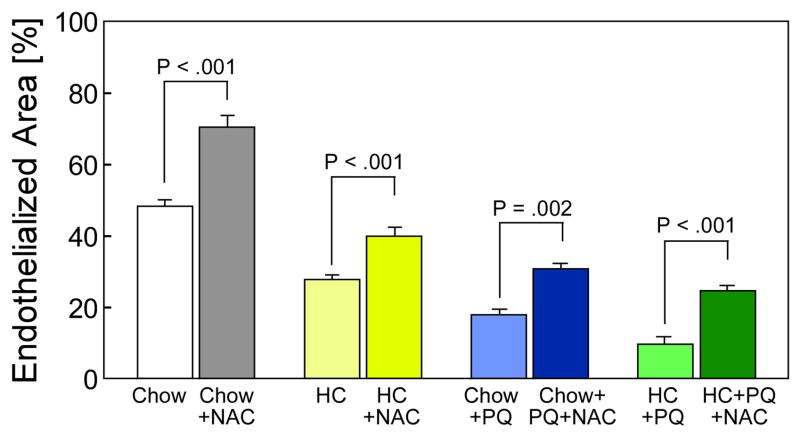
Overall, hypercholesterolemic mice had higher levels of global and local oxidative stress, as measured by plasma TBARS, plasma lysoPC, and tissue oxLDL, and had significantly lower reendothelialization after arterial injury than mice on a chow diet. To explore the role of increased oxidative stress in decreased arterial healing in hypercholesterolemic mice, the effect of an exogenous antioxidant, NAC, on arterial healing was studied. NAC treatment significantly increased endothelial healing in normocholesterolemic mice from 48.1% ± 5.2% to 70.4 ± 7.6% (P <.001) (Fig. 3). In hypercholesterolemic mice, treatment with NAC also significantly increased reendothelialization from 26.8% ± 2.8% to 39.9% ± 5.7% (P <.001) (Fig. 3). NAC did not alter the cellularity or macrophage infiltration of normocholesterolemic or hypercholesterolemic mice and did not decrease the TBARS levels in normocholesterolemic mice, but NAC treatment significantly decreased the TBARS level in HC mice (Table I). NAC administration also significantly decreased plasma lysoPC levels in both chow-fed animals and hypercholesterolemic mice (Table I). Treatment with NAC did not affect the tissue oxLDL score in normocholesterolemic mice, but decreased the oxLDL score in hypercholesterolemic mice from 3.0 ± 0.5 to 2.1 ± 0.5 (P =.01) (Table II). Thus, NAC treatment reduced the global and local indicators of oxidative stress and significantly improved endothelial healing in hypercholesterolemic mice.
Fig. 3.
Reendothelialization of mouse carotid arteries 120 hours after electrical injury reported as the area reendothelialized relative to the total area of injury and expressed as the mean ± standard error for mice in each group: chow (n = 5), chow plus N-acetylcysteine (NAC, n = 5), high cholesterol (HC, n = 5), HC plus NAC (n = 5), chow plus paraquat (PQ, n = 5), chow plus PQ plus NAC (n = 5), HC plus PQ (n = 5), and HC plus PQ plus NAC (n = 5).
Effect of a known oxidative stress on arterial healing
To further investigate the role of oxidative stress in endothelial healing, paraquat, a known generator of oxidative stress,9 was administered to mice at the time of arterial injury. Administration of paraquat (1 mg/kg) did not produce any signs of systemic toxicity, but significantly decreased reendothelialization of the injured artery from 48.1% ± 5.2% to 18.0% ± 3.5% (P <.001) (Table II) in chow-fed mice. Pretreatment with NAC resulted in significant improvement in EC healing with 30.7% ± 3.6% coverage by 120 hours (P =.002 compared to paraquat-treated mice)(Fig. 3) (Table II). Paraquat administration caused a significant increase in global oxidative stress as assessed by TBARS levels, but NAC significantly reduced this level (Table I). As with hypercholesterolemia, administration of paraquat led to a significant increase in tissue oxLDL score from 1.8 ± 0.9 in the chow group to 3.0 ± 0.4 in the chow + paraquat group (P = 0.01) (Table II). Administration of a known source of oxidative stress significantly increased measures of systemic and local oxidative stress and significantly decreased reendothelialization after arterial injury. Pretreatment with an antioxidant decreased indicators of global, but not local, oxidative stress and increased reendothelialization in mice treated with paraquat.
Effect of multiple sources of oxidative stress on reendothelialization after arterial injury
The effect of multiple sources of oxidative stress, HC diet and paraquat, on reendothelialization after arterial injury, and NAC’s ability to reverse the deleterious effects, was also assessed. The combination of a HC diet and paraquat resulted in a significant decrease in reendothelialization compared to mice that received paraquat or a HC diet alone, with reendothelialization covering only 9.8% ± 4.6% in the HC plus paraquat group, 18.0% ± 3.5% (P <.001) in the chow plus paraquat group, and 26.8% ± 2.8% (P <.001) in the HC group (Fig. 3). For mice in the HC diet plus paraquat group, NAC significantly improved reendothelialization from 9.8% ± 4.6% to 24.6% ± 3.4% (P <.001) (Fig. 3) (Table II). The combination of a HC diet and paraquat resulted in a significant increase in TBARS and plasma lysoPC levels, which was reduced by pretreatment with NAC (Table I). Exposure to multiple sources of oxidative stress resulted in increased markers of systemic oxidative stress and decreased reendothelialization after arterial injury. Pretreatment with NAC lowered measures of oxidative stress and improved reendothelialization. Across all treatment groups in the current study, a significant inverse correlation exists between EC migration and levels of both TBARS (r = −.901, P <.001) and plasma lysoPC (r = −.644, P <.001).
DISCUSSION
Our study shows that hypercholesterolemia and oxidative stress adversely impact EC healing in vivo, just as oxidative stress and oxidatively-modified lipids and lipoproteins impair EC migration in vitro.8,6 In vitro studies suggest that lipids and lipoproteins do not inhibit EC migration unless they are oxidatively modified.6 OxLDL increases superoxide production by EC in vitro and elevated superoxide levels inhibit EC migration.8 Previous studies show increased superoxide production in the aorta of hypercholesterolemic rabbits compared with chow-fed rabbits,16 and increased plasma lysoPC in cholesterol-fed pigs.17 The current study shows higher systemic oxidative stress as reflected by plasma TBARS and lysoPC levels and higher levels of local oxidative stress as reflected by the tissue oxLDL scores in cholesterol-fed mice compared with controls. Thus, the impaired EC healing in hypercholesterolemic animals may be due to the associated increased oxidative stress.
In this study, a HC diet inhibits reendothelialization in wild-type mice regardless of the type of injury, endothelial denudation by air drying or transmural electrical injury. The study evaluates early healing and results suggest that hypercholesterolemia causes an oxidative stress and inhibits early EC migration following arterial injury. The effect on late healing was not addressed. Lau et al. 18 found no difference in reendothelialization of balloon-injured aortas or aortic content of oxidized lipids between control and hypercholesterolemic rabbits at 6 weeks after injury, raising the possibility that EC healing in hypercholesterolemic animals can catch up to controls.
An elevation in plasma cholesterol alone is not sufficient to impair EC migration. A recent study by Ii et al.19 showed greater reendothelialization following wire-induced carotid denudation in apolipoprotein E knockout (ApoE−/−) mice than in wild type mice. Similarly, previous studies performed in our lab showed no difference in reendothelialization following a carotid air-drying injury in LDL receptor knockout (LDLr−/−) and wild-type mice on a HC diet despite dramatic differences in plasma cholesterol (data not shown). The lack of effect of increased plasma cholesterol on EC healing could be due to differences in the lipoprotein profile or altered lipid handling by the EC themselves, with impaired uptake of cholesterol. LDLr−/− and ApoE−/− mice have higher levels of plasma cholesterol but no significant difference in tissue cholesterol levels compared with wild-type mice.20 Our study addresses the impact of diet-induced hypercholesterolemia on arterial healing in wild-type mice with normal lipid handling. These findings may be more relevant to the usual patient consuming a high fat diet than would models utilizing ApoE−/− or LDLr−/− mice.
Oxidative stress and inflammation in response to vascular injury is well established.21, 22 A low level of ROS may be required to initiate EC migration,23 but a pathologic level of superoxide production overwhelms the endogenous antioxidant defense mechanisms and inhibits EC migration in vitro.8 In vivo, elevated superoxide production has been documented from 24 hours to 14 days after balloon injury.21, 22 The source of the ROS has not been identified with certainty, but may be from macrophages that infiltrate the damaged vessel where they are activated and produce ROS, providing the conditions for the formation of oxidized lipids. In the current study, there is a significant increase in the macrophage infiltration in the injured right carotid artery compared with the uninjured left carotid artery for all study groups, as would be expected after injury. We saw no difference in macrophage infiltration at 5 days among the various experimental groups, and NAC administration improved healing in all groups, suggesting that increased ROS production delays EC healing.
Antioxidants improve EC migration in vitro and in vivo. Our previous in vitro studies show that specific antioxidants restore the migration of EC incubated with oxLDL.8 Several studies have shown that exogenous antioxidants can reduce intimal hyperplasia after arterial injury, but their effect on EC migration has received little attention. NAC reduces the inflammatory response and intimal thickening after arterial balloon injury in rabbits.24, 25 Probucol promotes reendothelialization of balloon-injured rabbit aortas and reduces intimal thickening at the injury site.18 The mechanism of increased EC regeneration is not clear because probucol decreases serum and tissue cholesterol, as well as the tissue levels of oxidized lipids in the injured aorta, and also increases nitric oxide bioactivity, probably by inhibiting the increased superoxide production in the vascular wall following injury.18 Increasing SOD expression by gene transfer to the balloon-injured aortic wall in rabbits results in more rapid reendothelialization,26 suggesting that endogenous antioxidant activity improves EC migration. Our studies suggest that administration of an exogenous antioxidant also improves EC migration.
In the current study, reendothelialization after arterial injury is inversely related to oxidative stress. Pretreatment with NAC significantly reduces oxidative stress and improves reendothelialization of an arterial injury in hypercholesterolemic mice and mice administered paraquat. NAC does not alter plasma total cholesterol levels as has been reported in studies of mice on a 2% cholesterol, 0.5% cholic acid diet.27 NAC has both direct and indirect antioxidant activity,10, 11 inactivating ROS and hypochlorite directly by reduction to form NAC radicals10 and increasing the quantity of glutathione, the most abundant intracellular antioxidant. Glutathione reacts with peroxynitrite to prevent its accumulation, and the resultant membrane lipid peroxidation, protein denaturation, and DNA damage.28 NAC also decreases the expression of several proinflammatory cytokines by preventing activation of NF-κB.29 In chow-fed mice, NAC improves EC healing even though measured oxidative stress is not altered, perhaps a reflection of the lack of sensitivity of the assays or timing of the measurements, potentially missing an early reduction in oxidative stress. Improved EC healing is not likely the result of a direct stimulatory effect of NAC because NAC does not increase EC migration in vitro (data not shown). The inverse correlation between EC healing and measures of oxidative stress suggests that the beneficial effect of NAC in vivo is related to its antioxidant activity.
Multiple sources of ROS may be present at the time of arterial injury in vivo, and this study suggests that their effect on EC healing might be additive. This has implications in patients with cardiovascular disease who have multiple risk factors for atherosclerosis. In addition to hypercholesterolemia, other risk factors for atherogenesis, such as cigarette smoking and hyperglycemia, elevate systemic oxidative stress.30, 31 Increased oxidative stress has been demonstrated in humans following an after arterial injury, specifically a coronary angioplasty.32 The inflammatory response and oxidative stress after stent placement is greater than after balloon angioplasty alone, perhaps due to the presence of a foreign body, and is accompanied by a greater accumulation of oxidized lipids.33 These multiple sources of oxidative stress may adversely impact EC healing in the clinical setting. Our studies suggest that an appropriate antioxidant is capable of improving EC healing.
The current study supports the relevance of our in vitro studies on EC migration to in vivo arterial healing. An increase in lipid oxidation products or oxidative stress impairs EC migration in vitro and in vivo. Oxidative stress and inflammation accompany the vascular injury associated with all cardiovascular interventions including angioplasty, stent placement, and graft implantation. Hypercholesterolemia results in increased levels of ROS and oxidative stress. Oxidative stress, regardless of the source, results in the generation of potent biologic agents that induce cellular dysfunction and adversely impact healing of arterial injuries. Decreasing oxidative stress improves EC healing, which is important to control SMC proliferation and to reduce thrombogenicity of the vessel lumen by providing an actively anti-thrombogenic surface. Our studies suggest that EC healing can be improved by avoiding hypercholesterolemia and decreasing oxidative stress by antioxidant administration. The relevance of these studies to the clinical arena will need to be determined by clinical trials.
Acknowledgments
This project was supported by Grant Numbers HL41187, HL64357, and F32HL090205 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
Competition of Interest: Nil
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sdringola S, Assali A, Anderson HV, Smalling RW. Restenosis: Relationship with Thrombosis. Curr Interv Cardiol Rep. 2000 Nov;2(4):285–92. [PubMed] [Google Scholar]
- 2.Kisanuki A, Asada Y, Hatakeyama K, Hayashi T, Sumiyoshi A. Contribution of the endothelium to intimal thickening in normocholesterolemic and hypercholesterolemic rabbits. Arterioscler Thromb. 1992;12:1198–205. doi: 10.1161/01.atv.12.10.1198. [DOI] [PubMed] [Google Scholar]
- 3.Theilmeier G, Quarck R, Verhamme P, Bochaton-Piallat M-L, Lox M, Bernar H, et al. Hypercholesterolemia impairs vascular remodelling after porcine coronary angiplasty. Cardiovasc Res. 2002;55:385–95. doi: 10.1016/s0008-6363(02)00409-1. [DOI] [PubMed] [Google Scholar]
- 4.Autio I, Jaakkola O, Solakivi T, Nikkari T. Oxidized low-density lipoprotein is chemotactic for arterial smooth muscle cells in culture. FEBS Lett. 1990;277:247–9. doi: 10.1016/0014-5793(90)80857-f. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee S. Role of oxidized human plasma low density lipoproteins in atherosclerosis: effects on smooth muscle cell proliferation. Mol Cell Biochem. 1992;111:143–7. doi: 10.1007/BF00229586. [DOI] [PubMed] [Google Scholar]
- 6.Murugesan G, Chisolm GM, Fox PL. Oxidized low density lipoprotein inhibits the migration of aortic endothelial cells in vitro. J Cell Biol. 1993;120:1011–9. doi: 10.1083/jcb.120.4.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murugesan G, Fox PL. Role of lysophosphatidylcholine in the inhibition of endothelial cell motility by oxidized low density lipoprotein. J Clin Invest. 1996;97:2736–44. doi: 10.1172/JCI118728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Aalst JA, Zhang D-M, Miyazaki K, Colles SM, Fox PL, Graham LM. Role of reactive oxygen species in inhibition of endothelial cell migration by oxidized low-density lipoprotein. J Vasc Surg. 2004;40:1208–15. doi: 10.1016/j.jvs.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Suntres Z. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 10.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6:593–7. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- 11.Witko-Sarsat V, Gausson V, Nguyen A-T, Touam M, Drüeke T, Santangelo F, et al. AOPP-induced activation of human nuetrophil and monocyte oxidative metabolism: a potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003;64:82–91. doi: 10.1046/j.1523-1755.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 12.Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-a but not estrogen receptor-b. Circulation. 2001;103:423–8. doi: 10.1161/01.cir.103.3.423. [DOI] [PubMed] [Google Scholar]
- 13.Simon DI, Chen ZP, Seifert P, Edelman ER, Ballantyne CM, Rogers C. Decreased neointimal formation in Mac-1−/− mice reveals a role for inflammation in vascular repair after angioplasty. J Clin Invest. 2000;105:293–300. doi: 10.1172/JCI7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindner V, Fingerle J, Reidy MA. Mouse model of arterial injury. CircRes. 1993;73:792–6. doi: 10.1161/01.res.73.5.792. [DOI] [PubMed] [Google Scholar]
- 15.Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976;15:212–6. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- 16.Keaney JF, Jr, Xu A, Cunningham D, Jackson T, Frei B, Vita JA. Dietary probucol preserves endothelial function in cholesterol-fed rabbits by limiting vascular oxidative stress and superoxide generation. J Clin Invest. 1995;95:2520–9. doi: 10.1172/JCI117953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Keyzer D, Karabina SA, Wei W, Geeraert B, Stengel D, Marsillach J, et al. Increased PAFAH and oxidized lipids are associated with inflammation and atherosclerosis in hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol. 2009 Dec;29(12):2041–6. doi: 10.1161/ATVBAHA.109.196592. [DOI] [PubMed] [Google Scholar]
- 18.Lau AK, Leichtweis SB, Hume P, Mashima R, Hou JY, Chaufour X, et al. Probucol promotes functional reendothelialization in balloon-injured rabbit aortas. Circulation. 2003;107:2031–6. doi: 10.1161/01.CIR.0000062682.40051.43. [DOI] [PubMed] [Google Scholar]
- 19.Ii M, Takeshita K, Ibusuki K, Luedemann C, Wecker A, Eaton E, et al. Notch signaling regulates endothelial progenitor cell activity during recovery from arterial injury in hypercholesterolemic mice. Circulation. 2010 Mar 9;121(9):1104–12. doi: 10.1161/CIRCULATIONAHA.105.553917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dietschy JM, Turley SD. Control of cholesterol turnover in the mouse. J Biol Chem. 2002 Feb 8;277(6):3801–4. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Niculescu R, Wang D, Patel S, Davenpeck KL, Zalewski A. Increased NAD(P)H oxidase and reactive oxygen species in coronary arteries after balloon injury. Arterioscler Thromb Vasc Biol. 2001;21:739–45. doi: 10.1161/01.atv.21.5.739. [DOI] [PubMed] [Google Scholar]
- 22.Nunes GL, Robinson K, Kalynych A, King SB, III, Sgoutas DS, Berk BC. Vitamins C and E inhibit O2− production in the pig coronary artery. Circulation. 1997;96:3593–601. doi: 10.1161/01.cir.96.10.3593. [DOI] [PubMed] [Google Scholar]
- 23.Moldovan L, Moldovan NI, Sohn RH, Parikh SA, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86:549–57. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- 24.Mass H, Pirazzi B, Gonzalez P, Collazo V, Fitzovich D, Avakian E. N-acetylcysteine diminishes injury induced by balloon angioplasty of the carotid artery in rabbits. Biochem Biophys Res Commun. 1995;215:613–8. doi: 10.1006/bbrc.1995.2508. [DOI] [PubMed] [Google Scholar]
- 25.Ghigliotti G, Mereto E, Eisenberg PR, Martelli A, Orsi P, Sini D, et al. N-acetyl-cysteine reduces neointimal thickening and procoagulant activity after balloon-induced injury in abdominal aortae of New Zealand White rabbits. Thromb Haemost. 2001;85:724–9. [PubMed] [Google Scholar]
- 26.Laukkanen MO, Kivelä A, Rissanen T, Rutanen J, Karkkainen MK, Leppanen O, et al. Adenovirus-mediated extracellular superoxide dismutase gene therapy reduces neointimal formation in balloon-denuded rabbit aorta. Circulation. 2002;106:1999–2003. doi: 10.1161/01.cir.0000031331.05368.9d. [DOI] [PubMed] [Google Scholar]
- 27.Korou LM, Agrogiannis G, Pantopoulou A, Vlachos IS, Iliopoulos D, Karatzas T, et al. Comparative antilipidemic effect of N-acetylcysteine and sesame oil administration in diet-induced hypercholesterolemic mice. Lipids Health Dis. 2010;9:23. doi: 10.1186/1476-511X-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Ciccolo A, Centorrino T, et al. Protective effects of n-acetylcysteine on lung injury and red blood cell modification induced by carrageenan in the rat. FASEB J. 2001;15:1187–200. doi: 10.1096/fj.00-0526hyp. [DOI] [PubMed] [Google Scholar]
- 29.Geudens N, Van De Wauwer C, Neyrinck AP, Timmermans L, Vanhooren HM, Vanaudenaerde BM, et al. N-acetyl cysteine pre-treatment attenuates inflammatory changes in the warm ischemic murine lung. Journal of Heart and Lung Transplantation. 2007;26:1326–32. doi: 10.1016/j.healun.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995 May 4;332(18):1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 31.Cosentino F, Hishikawa K, Katusic ZS, Luscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation. 1997;96:25–8. doi: 10.1161/01.cir.96.1.25. [DOI] [PubMed] [Google Scholar]
- 32.Coghlan JG, Flitter WD, Holley AE, Norell M, Mitchell AG, Ilsley CD, et al. Detection of free radicals and cholesterol hydroperoxides in blood taken from the coronary sinus of man during percutaneous transluminal coronary angioplasty. Free Radic Res Commun. 1991;14(5–6):409–17. doi: 10.3109/10715769109093429. [DOI] [PubMed] [Google Scholar]
- 33.Kawamoto R, Yamashita A, Nishihira K, Furukoji E, Hatakeyama K, Ishikawa T, et al. Different inflammatory response and oxidative stress in neointimal hyperplasia after balloon angioplasty and stent implantation in cholesterol-fed rabbits. Pathology-Research & Practice. 2006;202:447–56. doi: 10.1016/j.prp.2005.12.011. [DOI] [PubMed] [Google Scholar]