Abstract
Event-related oscillations (EROs) are heritable electrophysiological measures associated with cognitive activity and have been shown to be particularly informative for the genetic analysis of substance dependence and other psychiatric disorders. In the present study associations between the cortical event-related oscillations (EROs) elicited by affective stimuli, and the diagnosis of ASPD or CD (ASPD/CD) were investigated, and heritability and linkage analyses conducted in 662 individuals residing in an American Indian community. Results from this study found that participants with ASPD/CD showed increased alpha ERO energy in centro-parietal leads in the 0 to 250 ms time window in response to all three emotional expressions (sad, neutral and happy faces). Participants with ASPD/CD also showed increased alpha ERO energy in centro-parietal leads in the 400 to 700 ms time window in response to happy and neutral faces. Variance components analysis suggested a significant familial component to each of the described ERO phenotypes. Although a follow-up genome-wide linkage analysis failed to detect significant evidence of genetic linkage for any of these phenotypes, centro-parietal alpha energy in response to happy faces showed suggestive evidence of linkage to chromosome 1p36.31 (LOD = 2.40), in an area found in previous studies to be associated with externalizing phenotypes. Findings from this study suggest greater activation of neural circuits required to perform a facial recognition task in participants with ASPD/CD. The observed increase in alpha ERO energy may represent a heritable endophenotype associated with select externalizing disorders in this population.
Keywords: American Indians, Conduct disorder, Antisocial personality disorder, Event-related oscillations, Emotion recognition, Heritability
1. Introduction
Electrophysiological measures [e.g., electroencephalogram (EEG), event-related potentials (ERPs) and event-related oscillations (EROs)] have been particularly informative endophenotypes for the genetic analysis of substance dependence and other psychiatric disorders (Begleiter & Porjesz, 2006). For example, there is evidence to suggest that individuals with antisocial personality disorder (ASPD) or conduct disorder (CD) exhibit reductions in the amplitude of the P3 component of the event-related potential (ERP) (Bauer & Hesselbrock, 2003; Bauer et al., 1994; Gao & Raine, 2009; Gilmore et al., 2010; Patrick et al., 2006). Additionally, a study conducted in an American Indian community suggested reductions in the amplitudes of two P300-like components (P350 and P450) were due, in part, to the presence of externalizing disorders (Ehlers et al., 2007).
Findings from the Collaborative Study on Genetics of Alcoholism (COGA) project have achieved significant progress identifying EROs associated with the human P3 component and several genes potentially involved in their regulation (Begleiter & Porjesz, 2006; Porjesz et al., 2005; Rangaswamy & Porjesz, 2008). For instance, studies have demonstrated that delta and theta EROs are important contributors to the generation of the human P3 component (Rangaswamy & Porjesz, 2008). In one such study, time-frequency analysis was used to decompose the averaged P3 response in individuals diagnosed with externalizing disorders, and a significant association between delta activity and externalizing psychopathology was found (Gilmore et al., 2010). Data from that study suggest that delta activity in the time interval of the P2-N2-P3 ERP complex may serve as an endophenotype for externalizing disorders. Association studies have supported this conclusion demonstrating that delta EROs show associations with genes previously associated with alcohol dependence and other externalizing disorders such as the muscarinic acetylcholine receptor (M2) gene (CHRM2) (Dick et al., 2008; Jones et al., 2004, 2006; Wang et al., 2004).Thus, an improved understanding of the relations between EROs and externalizing disorders could provide insight into the genetic factors involved in the etiology of such psychopathology.
The study of neural responses to emotional facial expressions has also been shown to provide an index of changes in brain functioning in individuals diagnosed with externalizing disorders such as ASPD and CD (Deely et al., 2006; Dolan & Fullam, 2006; Marsh & Blair, 2008; Marsh et al., 2008; Passamonti et al., 2010). Studies have shown that facial emotion processing is an essential component of effective socialization and social interaction (Corden et al., 2006; Fridlund, 1991) and failure to be properly guided by social cues of others plays an important role in the development of antisocial behaviors (Blair, 2003; Marsh & Blair, 2008). A meta-analysis of 20 studies of children and adults found that antisocial individuals were less accurate than non-antisocial individuals at recognizing facial expressions of six different emotions (fear, sadness, surprise, disgust, anger, happiness) and were particularly impaired in recognizing facial expressions of fear (Marsh & Blair, 2008). It has been also suggested that these face processing deficits might reflect involvement of the amygdala or cortical regions with connections to the amygdala, such as the dorsal anterior cingulate cortex or the ventromedial prefrontal cortex in antisociality (Marsh et al., 2008).
Given that identifying neurophysiological endophenotypes associated with ASPD and CD may further our understanding of the genetic substrates associated with externalizing disorders, we sought to examine the relations between antisocial behavior and EROs and ERPs generated in cortical sites in response to three different emotional expressions (sad, neutral, happy faces) using a face emotion recognition task. We subsequently performed linkage analysis of select neurophysiological phenotypes to demonstrate their utility in molecular genetic research.
2. Methods
2.1. Participants
Participants were 662 American Indians recruited from eight geographically contiguous reservations in southern California with a total population of about 3,000 individuals as part of an ongoing family-based investigation of the genetic influences on substance use phenotypes. Of the 662 participants, 83 were the only individual from their family included in the present study. The remaining 582 participants belonged to 98 families ranging in size from 2 to 16 members (average 5.11 ± 3.75). Participants responded to fliers posted at the Indian Health Clinic, tribal halls, local schools, and local businesses and were also recruited by word-of-mouth. Eligible participants were between the ages of 18 and 70 years of age and of at least one-sixteenth American Indian heritage as determined by their federal Indian blood quantum. The protocol for the study was approved by the Institutional Review Board of The Scripps Research Institute (TSRI), and the Indian Health Council, a tribal review group overseeing health issues for the reservations where the recruitments were undertaken. Written informed consent was obtained from each participant after the procedures had been fully explained. Participants were compensated for their time spent in the study.
2.2. Clinical Measures and Diagnoses
The Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) was used to gather demographic information and make a lifetime diagnosis of the combined phenotype of ASPD or CD (ASPD/CD) according to DSM-III-R criteria (Bucholz et al., 1994; Hesselbrock et al., 1999). The SSAGA has been successfully used in Native American populations previously (Hesselbrock et al., 2000; Wall et al., 2003). Personnel from the COGA project trained all interviewers participating in the study. A research psychiatrist/addiction specialist made all best final diagnoses.
2.3. Face Emotion Recognition Task
The present study used a face emotion recognition task (Erwin et al., 1992) that was adapted for use in an ERP paradigm and previously described (Orozco & Ehlers, 1998). The stimuli were digital photographs of happy, neutral and sad faces presented on a computer screen for 1000 ms with an inter-trial interval of 1000–1500 ms. Thirty-six different faces (12 each of happy, neutral, and sad) were presented in random order for a total of 216 trials. Participants were instructed to depress separate counters whenever a happy or sad face was displayed (36 trials each) and not to respond to neutral faces (144 trials). The number of male and female faces presented was also equally distributed among neutral, sad and happy stimuli.
2.4. General Electrophysiological Procedures
Acquisition and analyses of ERPs were done as previously described (Ehlers et al., 2006, 2007). Briefly, seven channels of ERP data (FZ, CZ, PZ, F3, F4, F7, and F8, referenced to linked ear lobes with a forehead ground, international 10–20 system) were obtained; signals were amplified and transferred on-line to a PC. ERP trials were digitized at a rate of 256 Hz. The N1 (75–150 ms), P350 (250–500 ms) and P450 (475–600 ms) components of the ERP were quantified as previously described (Ehlers et al., 2006, 2007). For target stimuli, only trials with correct identification were included in the averaging. The baseline was determined by averaging the 100 ms of prestimulus activity obtained for each trial. All peaks were quantified by one investigator and verified by a second investigator, both of whom were blind to participants’ characteristics.
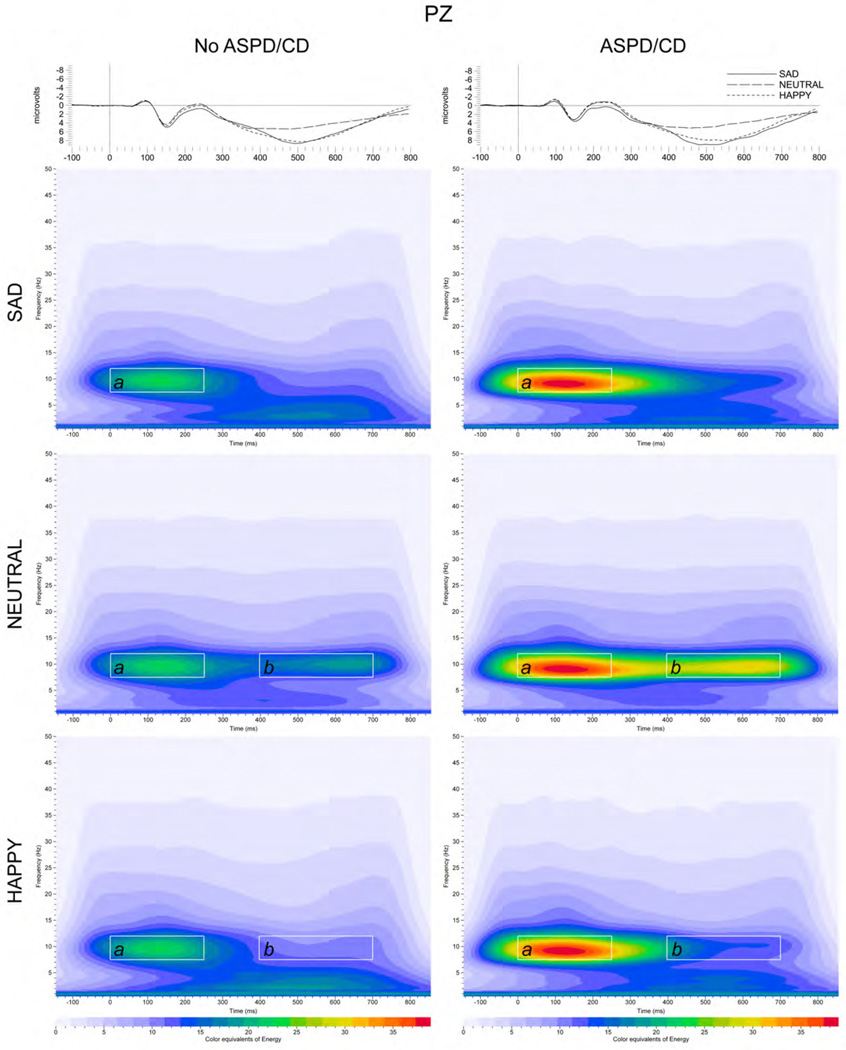
Data from each trial generated by the stimuli were processed by a time-frequency analysis algorithm, which utilizes the S-transform (Stockwell et al., 1996), a generalization of the Gabor transform (Gabor, 1946). To quantify S transform magnitudes, a region of interest (ROI) was identified by specifying the band of frequencies and the time interval contained in the rectangular ROI. ERO energy was determined as the measure of the energy values in the ROI (energy summed over time and frequency). Baseline corrected post-stimulus activity (900 ms) was calculated by subtracting pre-stimulus ERO energy values (100 ms) from the post-stimulus ROI values, as previously described (Padmanabhapillai et al., 2006). The ROI frequencies were: delta (0.75 – 4.25 Hz), theta (4.25 – 7.25 Hz), alpha (7.25 – 11.75 Hz) and beta (11.75 – 19.75 Hz). The ROI time intervals were based on early (0 – 250 ms) and late (400 – 700 ms) ERO peaks identified in the grand averages (see Figures 1 and 2).
Figure 1.
Time-frequency responses of evoked alpha band energy distribution to sad, neutral and happy faces in participants without ASPD/CD (A, B, C) and with ASPD/CD (D, E, F) participants at the Pz electrode position. Time-frequency ROI windows (in white squares) shown were 0 – 250 ms (a) and 400 – 700 ms (b) The inset shows representative ERP grand averages from participants without ASPD/CD (left) and with ASPD/CD (right) groups in response to sad, neutral and happy faces.
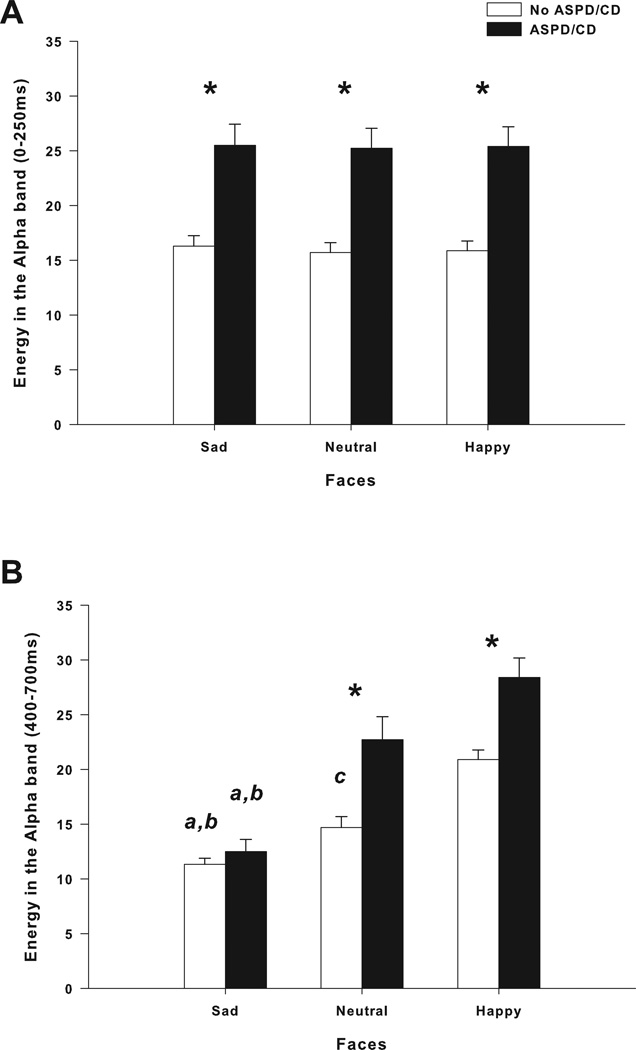
Figure 2.
Mean amplitude values of ERO energy for alpha bands in response to sad, neutral and happy faces in centro-parietal leads. (A). ASPD/CD participants showed an increase in alpha ERO energy in the 0 – 250 ms time window in response to sad, neutral and happy faces. (B). ASPD/CD participants showed an increase in alpha ERO energy in the 400 – 700 ms time window in response to neutral and happy, but not sad faces. Greater alpha ERO energy was found in response to happy faces than to sad and neutral faces in participants without ASPD/CD participants. Alpha ERO energy was greater in response to neutral faces than to sad faces in participants without ASPD/CD. Greater alpha ERO energy was found in response to happy faces than to sad faces in ASPD/CD participants.* Significant differences between participants with and without ASPD/CD; a Significant differences between sad and neutral faces; b Significant differences between sad and happy faces; c Significant differences between neutral and happy faces (p < 0.004).
2.5 Genotyping
Of the 662 participants, 381 participants with both genotype and phenotype data were used in the genetic analyses. Genotypes were available for 381 subjects based on protocols described in previous reports (Ehlers et al., 2010a, 2010b). Briefly, DNA isolated from whole blood was genotyped for a panel of 791 autosomal microsatellite polymorphisms (Weber & May, 1989) according to procedures recommended by the manufacturer (HD5 version 2.0; Applied Biosystems, Foster City, CA). This marker set has an average distance of 4.6 cM between adjacent markers. Allele frequencies for linkage analysis were determined from unrelated founders in the present population. Prior to analysis, genotypes were subjected to extensive quality control. This included using genotype data to confirm pedigree structures using PREST (McPeek & Sun, 2000), detection of Mendelian errors using Pedcheck (O'Connell & Weeks, 1998), and the error-checking algorithm implemented in Merlin (Abecasis et al., 2002) to identify inconsistent genotypes across family relationships beyond simple Mendelian errors. These checks resulted in the removal of six individuals due to pedigree structure errors, 772 genotypes due to Mendelian errors, and 508 genotypes due to other inconsistencies across family members. In total 273,598 genotypes (99.5%) were accepted for analysis.
2.6. Data Analysis
To analyze the ERP components (N1, P350 and P450) and ERO energy, a principal component analysis (PCA) was performed (SPSS, Inc., Chicago IL) over the seven electrode locations in response to the three faces (neutral, sad, happy) in the face emotion recognition task, as previously described (Ehlers et al., 2001). The electrode sites loading on the first factor were the frontal leads (FZ, F3, F4, F7, F8) electrode sites loading on the second factor were the two more centro-posterior leads (CZ, PZ). Participants’ age and gender were included as potential covariates and retained if they accounted for a significant portion of the total variance.
ERP amplitudes and latencies and ERO energy for the two components identified in the PCA (frontal leads, centro-parietal leads) were evaluated using a two-way analysis of variance (ANOVA). A two-way ANOVA was also used to determine the effects of a diagnosis of ASPD/CD on response accuracy (percent correct response) among the three emotional facial expressions within each group. Group (ASPD/CD vs. No ASPD/CD) was assessed as a between subject factor. Face (neutral, sad, happy) was assessed as within subject repeated measures factor. When appropriate, post hoc analysis of the two-way ANOVA utilized independent one-way ANOVAs to assess group differences. Repeated measures one-way ANOVA was performed to determine within group differences in ERO energy among sad, neutral and happy faces. For all repeated measures analyses, Greenhouse-Geisser corrected p-values were reported to account for violations of sphericity. To minimize the probability of a Type I error occurring due to multiple comparisons, Bonferroni corrections were used (p < 0.004). When appropriate, post hoc analysis of repeated measures one-way ANOVA utilized the Tukey HSD test (p < 0.05). Fisher's exact test for dichotomous variables and ANOVA for continuous variables were used to evaluate potential differences in demographic variables between the groups evaluated (p < 0.01).
Linkage analyses were conducted using the variance components approach implemented in SOLAR v4.2.0 (Almasy & Blangero, 1998; Southwest Foundation for Biomedical Research (S.F.B.R.), 2011) for those phenotypes that showed a relation with antisocial behavior. Participants' age at the time of evaluation and gender were evaluated as covariates and included in all analyses when appropriate. As a preliminary step in the linkage analysis, SOLAR partitions the variation in the trait to be analyzed into a familial component (h2) that provides an estimate of the additive genetic influences contributing to the trait and a non-shared environmental component (e2). Each of the EEG phenotypes yielded an h2 estimate that was significantly greater than 0 as determined using a Student's t-test (p < 0.05), and thus were considered appropriate for linkage analysis. Gene-dropping simulations were conducted for 100,000 replicates and used to derive empirical p-values for the observed linkage peaks. A critical p-value of 0.000022 was used to identify genome-wide significant linkage (Lander & Kruglyak, 1995), and a more liberal p-value of 0.001 was used to identify suggestive evidence for linkage.
3. Results
3.1. Descriptive Data
Demographic data on the 662 participants are presented in Table 1. The sample contained more female participants than males. An overall significant difference between participants with ASPD/CD and those without ASPD/CD was found for age and education (Table 1). Demographic comparisons between participants with and without ASPD/CD revealed no significant differences between the groups of American Indian heritage.
Table 1.
Demographic characteristics of participants in the facial discrimination task as function of a personal history of ASPD/CD
| Demographic Variable |
No ASPD/CD | ASPD/CD |
|---|---|---|
| Age (years) | 32.3 ± 0.6 | 28.9 ± 1* |
| Gender (n) a | ||
| Male | 191 | 85 |
| Female | 340 | 46 |
| Years of education | 11.7 ± 0.1 | 11.2 ± 0.1* |
p < 0.01
The sample contained more female participants than males (females: n = 386, 58%; males: n=276, 42%); p < 0.01.
Values are x̄ ± SEM
3.2. Performance in the Face Emotion Recognition Task
There was no significant difference in response accuracy (percent correct response) between groups. Two-way ANOVA showed no significant differences in response accuracy to sad (ASPD/CD: 85 ± 1.3%; No ASPD/CD: 85 ± 0.7%), neutral (ASPD/CD: 92 ± 1.0%; No ASPD/CD: 93 ± 0.5%) and happy (ASPD/CD: 89 ± 1.0%; No ASPD/CD: 90 ± 0.4%) faces.
3.3. ERP Responses in Adults with ASPD/CD
No significant associations were found between a personal history of ASPD/CD and N1, P350 or P450 component amplitudes and latencies in either the frontal or centro-parietal leads to any of the emotional facial expressions (data not shown). Grand averages of grand-mean ERPs of sad, neutral and happy faces for centro-parietal regions are illustrated in Figure 1. Given the negative results, linkage analyses of the ERP phenotypes were not conducted.
3.4. ERO Responses in Adults with ASPD/CD
3.4.1. ERO mean energy in the 0–250 ms window
Two-way repeated measures ANOVA revealed a significant Group × Face interaction in the centro-parietal alpha band in response to sad, neutral and happy faces (F(1.7,950) = 19.8, p < 0.005) (Figure 2A). Post hoc comparisons showed that participants with ASPD/CD exhibited a significant increase in ERO energy in response to sad, neutral and happy faces compared to participants without ASPD/CD (F’s(1,653) < 13.6, p < 0.001). No differences in centro-parietal alpha ERO energy levels among sad, neutral and happy faces were found in participants with either ASPD/CD or without ASPD/CD (Figure 2A). The h2 estimates for the power of the centro-parietal alpha band in response to sad, neutral, and happy faces were significantly greater than 0 suggesting genetic contributions to the measured ERO energies. Although there were several loci that approached the threshold for suggestive evidence of linkage, no loci yielded a p-value < 0.001 (see Table 2). No significant associations were found between ASPD/CD and frontal ERO energy in the delta, theta and beta bands (data not shown). Linkage analyses were not conducted for these bands.
Table 2.
Results of frontal and centro-parietal alpha ERO analyses
| Time Window |
Brain Region |
Emotional Valence |
ERO energy differences between participant groups by emotional stimuli1 |
ERO energy differences across emotional stimuli by participant group2 |
Heritability | Linkage Findings | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASPD/CD | Controls | h2 | p | Chr | Position (cM) |
LOD score |
p | ||||
| 0–250 ms | F | H | No difference | 0.32 | <0.0001 | No LOD scores >2.00 | |||||
| N | No difference | H=N=S | H=N=S | 0.43 | <0.0001 | No LOD scores >2.00 | |||||
| S | No difference | 0.33 | <0.0001 | No LOD scores >2.00 | |||||||
| C-P | H | ASPD/CD>Controls | 0.46 | <0.0001 | 6 | 54 | 2.22 | 0.0025 | |||
| N | ASPD/CD>Controls | 0.32 | <0.0001 | No LOD scores >2.00 | |||||||
| S | ASPD/CD>Controls | H=N=S | H=N=S | 0.35 | <0.0001 | 2 | 128 | 2.24 | 0.0025 | ||
| 3 | 99 | 2.36 | 0.0020 | ||||||||
| 12 | 107 | 2.06 | 0.0036 | ||||||||
| 17 | 101 | 2.35 | 0.0021 | ||||||||
| 400–700 ms | F | H | No difference | 0.43 | <0.0001 | No LOD scores >2.00 | |||||
| N | No difference | 0.41 | <0.0001 | 13 | 95 | 2.01 | 0.0101 | ||||
| S | No difference | 0.44 | <0.0001 | 2 | 175 | 2.16 | 0.0031 | ||||
| H=N=S | H=N=S | 4 | 61 | 2.33 | 0.0020 | ||||||
| 8 | 81 | 2.17 | 0.0030 | ||||||||
| 14 | 44 | 2.16 | 0.0031 | ||||||||
| 20 | 102 | 2.62 | 0.0012 | ||||||||
| 22 | 58 | 2.30 | 0.0022 | ||||||||
| C-P | H | ASPD/CD>Controls | 0.34 | <0.0001 | 1 | 9 | 2.40 | 0.0009 | |||
| N | ASPD/CD>Controls | H=N>S | H>N>S | 0.22 | <0.0001 | No LOD scores >2.00 | |||||
| S | No difference | 0.29 | <0.0001 | 1 | 9 | 2.79 | 0.0021 | ||||
| 12 | 125 | 2.06 | 0.0036 | ||||||||
The age and gender of the participant were assessed as potential covariates in the models. ASPD/CD = Antisocial Personality Disorder/Conduct Disorder. h2 = genetic heritability. F = Frontal and C-P = Centro-parietal. H = Happy faces, N = Neutral faces, and S = Sad faces. Controls = participants without ASPD or CD diagnosis. Chr = chromosome. cM = centimorgan. LOD = logarithm of the odds. Significant age differences in heritability were found in all models (p < 0.05). Significant gender differences in heritability were found in most models (p < 0.05), except in the frontal regions of the 0 – 250 ms time interval in response to all three emotional expressions. Bold text indicates evidence of linkage, p-value < 0.001, 1 - > symbol significant difference between participant groups (p<0.004), 2 - > symbol indicates significant difference between these categories of emotional stimuli (p<0.004 and post hoc (p<0.05)).
In contrast, two-way repeated measures ANOVA did not show a significant Group × Face interaction in the frontal alpha band in response to sad, neutral and happy faces. Additional statistical analyses performed using independent One-way ANOVAs with a Bonferroni correction (p < 0.008) found that participants with ASPD/CD showed a significant increase in ERO energy in the frontal alpha band in response to sad, neutral and happy faces relative to participants without ASPD/CD (data not shown). While the h2 estimates for the power of the frontal alpha band in response to sad, neutral, and happy faces were significantly greater than 0 suggesting genetic contributions to the measured ERO energies, linkage analysis failed to identify any significant or suggestive evidence of genetic linkage (see Table 2). No significant associations were found between ASPD/CD and frontal ERO energy in the delta, theta and beta bands (data not shown). Thus, linkage analyses were not conducted for these bands.
3.4.2. ERO mean energy in the 400 – 700 ms window
Two-way repeated measures ANOVA showed a significant Group × Face interaction in the centro-parietal alpha band in response to sad, neutral and happy faces (F(1.3,719) = 6.7, p < 0.01) (Figure 2B). Post hoc comparisons showed that participants with ASPD/CD exhibited a significant increase in ERO energy in response to sad, neutral and happy faces compared to participants without ASPD/CD (F’s(1,653) < 14.0, p < 0.005)(Figure 2B). Differences among centro-parietal alpha ERO energy levels in response to sad, neutral and happy faces were found in participants without ASPD/CD (F(2,1577) = 53.6, p < 0.004). Post hoc analyses indicated that centro-parietal alpha ERO energy in response to happy faces was significantly greater than alpha energy obtained in response to sad and neutral faces (p < 0.05; Figure 2B). Post hoc tests also showed greater centro-parietal alpha ERO energy in response to neutral than sad faces in participants without ASPD/CD (p < 0.05; Figure 2B). Differences among centro-parietal alpha ERO energy in response to the three emotional facial expressions were also found in participants with ASPD/CD (F(2,389) = 8.1, p < 0.004). Post hoc analyses showed lower centro-parietal alpha ERO energy in response to sad faces than to neutral and happy faces (p < 0.05; Figure 2B). No differences in alpha ERO energy were found between neutral and happy faces (Figure 2B).
Consistent with the ERO findings presented thus far, the h2 estimates for the power of the centro-parietal alpha band in response to sad, neutral, and happy faces were significantly greater than 0 suggesting genetic contributions to the measured ERO energies. Although there were several loci that approached the threshold for suggestive evidence of linkage, only a single locus yielded a p-value < 0.001 (see Table 2). Centro-parietal alpha energy in response to happy faces showed suggestive evidence of linkage to chromosome 1 at 9 cM (LOD = 2.40, pointwise empirical p = 0.0009).
Two-way repeated measures ANOVA showed a significant Group × Face interaction in the centro-parietal beta band in response to sad, neutral and happy faces (F(1.8,1044) = 3.4, p < 0.05). In contrast to findings in the centro-parietal alpha band, post hoc comparisons showed that participants with ASPD/CD only exhibited a significant increase in ERO energy in response to neutral faces compared to participants without ASPD/CD (F(1,650) = 5.9, p < 0.05; ASPD/CD: 16.6 ± 1.1; No ASPD/CD: 13.6 ± 0.6). No significant associations were found between ASPD/CD and frontal ERO energy in the delta and theta bands (data not shown). Linkage analyses were not conducted for delta, theta and beta bands. Time-frequency representations of grand-mean ERO energy of sad, neutral and happy faces for the alpha frequency band in the 0 – 250 ms and 400 – 700 ms time windows are illustrated for centro-parietal regions in Figures 1.
In contrast, two-way repeated measures ANOVA did not show a significant Group × Face interaction in the frontal alpha band in response to sad, neutral and happy faces. Additional statistical analyses performed using independent One-way ANOVAs with a Bonferroni correction (p < 0.008) found that participants with ASPD/CD showed a significant increase in ERO energy in the frontal alpha band in response to neutral and happy faces relative to participants without ASPD/CD, but not to sad faces (data not shown). The h2 estimates for the power of the frontal alpha band in response to sad, neutral, and happy faces were significantly greater than 0 suggesting genetic contributions to the measured ERO energies. Although there were several loci that approached the threshold for suggestive evidence of linkage, no loci yielded a p-value < 0.001 (see Table 2). Notably, the sad and neutral faces, which did not show a difference between the ASPD/CD and control groups, showed the strongest linkage evidence, whereas happy faces failed to yield any evidence for linkage. No significant associations were found between ASPD/CD and frontal ERO energy in the delta, theta and beta bands (data not shown). Linkage analyses were not conducted for these bands.
4. Discussion
The present investigation extended our initial studies of neurophysiological endophenotypes in American Indian adults by characterizing the association between ASPD/CD with cortical EROs and ERPs during a face emotion recognition task. The results indicate that ASPD/CD participants showed increased alpha ERO energy in centro-parietal leads in the 0 to 250 ms time window in response to sad, neutral and happy faces when compared to participants without ASPD/CD. These findings also suggest that ASPD/CD participants exhibited increased centro-parietal alpha ERO energy in the 400 to 700 ms time window in response to neutral and happy faces compared to participants without ASPD/CD, but not in response to sad faces. Together, these findings suggest that cortical alpha ERO energy is increased among ASPD/CD participants under a variety of conditions during an emotional facial expression identification task as compared to participants without ASPD/CD. Additionally, the present study demonstrated that these alpha ERO energy phenotypes show significant evidence of familial transmission, which coupled with the described group differences, suggests that these alpha ERO energy phenotypes may serve as useful endophenotypes for ASPD/CD.
As described, ASPD/CD participants exhibited an increase in centro-parietal alpha ERO energy in the 400 to 700 ms time window in response to neutral and happy faces, but not sad faces when compared to controls. It has been proposed that alpha activity may reflect neural processing that is necessary for attention and memory operations (Kolev et al., 2001; Maltseva et al., 2000). This notion is supported by evidence that increases in alpha activity are associated with task demands and attention effort (Klimesch et al., 1999, 2000; Spencer & Polich, 1999). These findings suggest that participants with ASPD/CD in the present study may have required greater effort to make the detections of emotional facial expressions. Previous studies have demonstrated that participants with antisocial behaviors may have reduced accuracy for recognizing emotional facial expressions (Marsh & Blair, 2008), thus differences in response accuracy between studies may be due to task difficulty or the population under study.
Results from the present study population also showed that participants with ASPD/CD exhibit changes in alpha ERO energy that are different from recent findings showing an association between externalizing disorders and delta activity in the time interval of the P2-N2-P3 ERP (Gilmore et al., 2010). One possible explanation for these contrasting results is the use of different paradigms to generate the EROs. For instance, Gilmore et al. (2010) used a rotated-heads visual oddball task that presented two types of visual stimuli (standard [oval view of head] and target [superior view of head] stimuli) with different probabilities of occurrence (33.5% and 66.5%, respectively). In contrast, the present study used a face emotion recognition task that presented three types of visual stimuli (target [happy faces]; non-target [neutral faces]; and, target [sad faces]) with different probabilities of occurrence (16.7%, 66.6% and 16.7%). While there are some similarities between the P450 component generated by the face emotion recognition task and the P3b component generated by the visual oddball task used by Gilmore and colleagues (Gilmore et al., 2010), participants in the present study had to discriminate among three different emotional facial expressions by identifying whether a face is sad, neutral or happy. Whether our contrasting findings are due to the neural responses to the emotional valence of the facial expressions is not known.
Finally, results from the present study suggest that ERO energy in the alpha frequency band is heritable. Although a follow-up genome-wide linkage analysis failed to detect significant evidence of genetic linkage for any of these phenotypes, centro-parietal alpha energy in response to happy faces in the 400–700 ms time window showed suggestive evidence of linkage to chromosome 1p36.31 (LOD = 2.40). This locus also showed a relation with centro-parietal alpha energy in response to sad faces in the same time window. This region was previously implicated in a genome-wide linkage scan of conduct disorder conducted in the COGA sample (Dick et al., 2004), and together with the findings of the present study, suggests there may be a genetic locus in this region of chromosome 1 that influences both ASPD/CD behavior and alpha ERO energy. These results would also seem to support the conclusion that centro-parietal alpha ERO energy may index the genetic risk underlying ASPD/CD, and thus serve as a useful endophenotype for ASPD/CD.
The results of this study should be interpreted in the context of several limitations. First, the findings may not generalize to other ethnic populations. Second, only retrospective and cross-sectional data on ASPD/CD were assessed. Third, it is notable that participants were nested within families, but this could not be addressed in the study analyses due to the extended and varied structure of the family pedigrees. Nonetheless, this represents a limitation of the present report as correlated data among participants can lead to reduced standard errors and an increase in Type I error. Despite these limitations, this report represents an important step in an ongoing investigation to determine genetic and environmental factors associated with substance use disorders and related psychiatric disorders in this high risk and understudied ethnic group. Further studies are needed to determine whether the increase in frontal and centro-parietal alpha ERO energy associated with the diagnosis of ASPD/CD reflects compensatory activation of neural circuits associated with the processing of attention and memory operations required to perform the face emotion recognition task used in the present study.
Acknowledgements
We thank Dr. David Gilder, Derek Wills, Phil Lau, Susan Lopez, Linda Corey, Agnes Whitton and Greta Berg for assistance in data collection and analyses.
Role of funding source
Funding for this study was provided by National Institutes of Health, NIAAA Grant AA010201 and to National Center on Minority Health and Health Disparities (NCMHD) (to C.L.E.), NIDA 030976 (to C.L.E., I.G., W.S), the NIAAA and NIDA had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
No conflict declared.
Contributors
Cindy L. Ehlers was responsible for the study design. Evie Phillips was responsible for electrophysiological data collection. Cindy L. Ehlers and Ian R. Gizer were responsible for coding the clinical data. Ian R. Gizer and Wendy S. Slutske were responsible for the genetic analyses. Jose R. Criado and Ian R. Gizer conducted all statistical analyses. Jose R. Criado, Cindy L. Ehlers, Wendy S. Slutske and Ian R. Gizer were responsible for the preparation of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nature Genetics. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. American Journal of Human Genetics. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LO, O'Connor S, Hesselbrock VM. Frontal P300 decrements in antisocial personality disorder. Alcoholism: Clinical and Experimental Research. 1994;18:1300–1305. doi: 10.1111/j.1530-0277.1994.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Brain maturation and subtypes of conduct disorder: interactive effects on P300 amplitude and topography in male adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:106–115. doi: 10.1097/00004583-200301000-00017. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Genetics of human brain oscillations. International Journal of Psychophysiology. 2006;60:162–171. doi: 10.1016/j.ijpsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Neurobiological basis of psychopathy. British Journal of Psychiatry. 2003;182:5–7. doi: 10.1192/bjp.182.1.5. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. Journal of Studies on Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Corden B, Critchley HD, Skuse D, Dolan RJ. Fear recognition ability predicts differences in social cognitive and neural functioning in men. Journal of Cognitive Neuroscience. 2006;18:889–897. doi: 10.1162/jocn.2006.18.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado JR, Ehlers CL. Electrophysiological responses to affective stimuli in Southwest California Indians: relationship to alcohol dependence. Journal of Studies on Alcohol and Drugs. 2007;68:813–823. doi: 10.15288/jsad.2007.68.813. [DOI] [PubMed] [Google Scholar]
- Deeley Q, Daly E, Surguladze S, Tunstall N, Mezey G, Beer D, Ambikapathy A, Robertson D, Giampietro V, Brammer MJ, Clarke A, Dowsett J, Fahy T, Phillips ML, Murphy DG. Facial emotion processing in criminal psychopathy. Preliminary functional magnetic resonance imaging study. British Journal of Psychiatry. 2006;189:533–539. doi: 10.1192/bjp.bp.106.021410. [DOI] [PubMed] [Google Scholar]
- Dick DM, Li TK, Edenberg HJ, Hesselbrock V, Kramer J, Kuperman S, Porjesz B, Bucholz K, Goate A, Nurnberger J, Foroud T. A genome-wide screen for genes influencing conduct disorder. Molecular Psychiatry. 2004;9:81–86. doi: 10.1038/sj.mp.4001368. [DOI] [PubMed] [Google Scholar]
- Dick DM, Aliev F, Wang JC, Grucza RA, Schuckit M, Kuperman S, Kramer J, Hinrichs A, Bertelsen S, Budde JP, Hesselbrock V, Porjesz B, Edenberg HJ, Bierut LJ, Goate A. Using Dimensional Models of Externalizing Psychopathology to Aid in Gene Identification. 2008;65:310–318. doi: 10.1001/archpsyc.65.3.310. [DOI] [PubMed] [Google Scholar]
- Dolan M, Fullam R. Face affect recognition deficits in personality-disordered offenders: association with psychopathy. Psychological Medicine. 2006;36:1563–1569. doi: 10.1017/S0033291706008634. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Visual P3 findings in Mission Indian youth: relationship to family history of alcohol dependence and behavioral problems. Psychiatry Research. 2001;105:67–78. doi: 10.1016/s0165-1781(01)00313-4. [DOI] [PubMed] [Google Scholar]
- Ehlers C, Hurst S, Phillips E, Gilder D, Dixon M, Gross A, Lau P, Yehuda R. Electrophysiological responses to affective stimuli in american indians experiencing trauma with and without PTSD. Annals of the New York Academy of Sciences. 2006;1071:125–136. doi: 10.1196/annals.1364.011. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Finnerman G, Gilder D, Lau P, Criado J. P3 components and adolescent binge drinking in Southwest California Indians. Neurotoxicology and Teratology. 2007;29:153–163. doi: 10.1016/j.ntt.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Phillips E, Wilhelmsen KC. EEG alpha phenotypes: linkage analyses and relation to alcohol dependence in an American Indian community study. BMC Medical Genetics. 2010a;11:43. doi: 10.1186/1471-2350-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Gizer IR, Schuckit MA, Wilhelmsen KC. Genome-wide scan for self-rating of the effects of alcohol in American Indians. Psychiatric Genetics. 2010b;20:221–228. doi: 10.1097/YPG.0b013e32833add87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin RJ, Gur RC, Gur RE, Skolnick B, Mawhinney-Hee M, Smailis J. Facial emotion discrimination: I Task construction and behavioral findings in normal subjects. Psychiatry Research. 1992;42:231–240. doi: 10.1016/0165-1781(92)90115-j. [DOI] [PubMed] [Google Scholar]
- Fridlund AJ. Evolution and facial action in reflex, social motive, and paralanguage. Biological Psychology. 1991;32:3–100. doi: 10.1016/0301-0511(91)90003-y. [DOI] [PubMed] [Google Scholar]
- Gabor D. Theory of Communication. Institute of Electrical Engineers Journal. 1946;93:429–457. [Google Scholar]
- Gao Y, Raine A. P3 event-related potential impairments in antisocial and psychopathic individuals: a meta-analysis. Biological Psychology. 2009;82:199–210. doi: 10.1016/j.biopsycho.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology. 2010;47:123–132. doi: 10.1111/j.1469-8986.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock M, Easton C, Bucholz KK, Schuckit M, Hesselbrock V. A validity study of the SSAGA--a comparison with the SCAN. Addiction. 1999;94:1361–1370. doi: 10.1046/j.1360-0443.1999.94913618.x. [DOI] [PubMed] [Google Scholar]
- Hesselbrock VM, Segal B, Hesselbrock MN. Alcohol dependence among Alaska Natives entering alcoholism treatment: a gender comparison. Journal of Studies on Alcohol. 2000;61:150–156. doi: 10.15288/jsa.2000.61.150. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC, Dick DM, Hinrichs A, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O'Connor SJ, Schuckit MA, Stimus AT, Tischfield JA, Reich T, Begleiter H. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. International Journal of Psychophysiology. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Dick D, Goate A, Hinrichs A, Rice JP, Wang JC, Bauer LO, Crowe R, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Rohrbaugh J, Schuckit MA, Tischfield J, Edenberg HJ, Begleiter H. A cholinergic receptor gene (CHRM2) affects event-related oscillations. Behavioral Genetics. 2006;36:627–639. doi: 10.1007/s10519-006-9075-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Schwaiger J, Auinger P, Winkler T. 'Paradoxical' alpha synchronization in a memory task. Brain Research: Cognitive Brain Research. 1999;7:493–501. doi: 10.1016/s0926-6410(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Doppelmayr M, Rohm D, Pollhuber D, Stadler W. Simultaneous desynchronization and synchronization of different alpha responses in the human electroencephalograph: a neglected paradox? Neuroscience Letters. 2000;284:97–100. doi: 10.1016/s0304-3940(00)00985-x. [DOI] [PubMed] [Google Scholar]
- Kolev V, Yordanova J, Schurmann M, Basar E. Increased frontal phase-locking of event-related alpha oscillations during task processing. International Journal of Psychophysiology. 2001;39:159–165. doi: 10.1016/s0167-8760(00)00139-2. [DOI] [PubMed] [Google Scholar]
- Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nature Genetics. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Maltseva I, Geissler HG, Basar E. Alpha oscillations as an indicator of dynamic memory operations - anticipation of omitted stimuli. International Journal of Psychophysiology. 2000;36:185–197. doi: 10.1016/s0167-8760(99)00093-8. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Blair RJ. Deficits in facial affect recognition among antisocial populations: a meta-analysis. Neuroscience and Biobehavioral Reviews. 2008;32:454–465. doi: 10.1016/j.neubiorev.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair RJ. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry. 2008;165:712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- McPeek MS, Sun L. Statistical tests for detection of misspecified relationships by use of genome-screen data. American Journal of Human Genetics. 2000;66:1076–1094. doi: 10.1086/302800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. American Journal of Human Genetics. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco S, Ehlers CL. Gender differences in electrophysiological responses to facial stimuli. Biological Psychiatry. 1998;44:281–289. doi: 10.1016/s0006-3223(97)00487-3. [DOI] [PubMed] [Google Scholar]
- Padmanabhapillai A, Tang Y, Ranganathan M, Rangaswamy M, Jones KA, Chorlian DB, Kamarajan C, Stimus A, Kuperman S, Rohrbaugh J, O'Connor SJ, Bauer LO, Schuckit MA, Begleiter H, Porjesz B. Evoked gamma band response in male adolescent subjects at high risk for alcoholism during a visual oddball task. International Journal of Psychophysiology. 2006;62:262–271. doi: 10.1016/j.ijpsycho.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Goodyer IM, Hurford G, Hagan CC, Rowe JB, Calder AJ. Neural abnormalities in early-onset and adolescence-onset conduct disorder. Archives of General Psychiatry. 2010;67:729–738. doi: 10.1001/archgenpsychiatry.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clinical Neuropathology. 2005;116:993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. Uncovering genes for cognitive (dys)function and predisposition for alcoholism spectrum disorders: a review of human brain oscillations as effective endophenotypes. Brain Research. 2008;1235:153–171. doi: 10.1016/j.brainres.2008.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwest Foundation for Biomedical Research (S.F.B.R) Sequential Oligogenic Linkage Analysis Routines. San Antonio, TX: SOLAR; [Last updated 2011. Last accessed 07.19.11]. http://solar.txbiomedgenetics.org/download.html. [Google Scholar]
- Spencer KM, Polich J. Poststimulus EEG spectral analysis and P300: attention, task, and probability. Psychophysiology. 1999;36:220–232. [PubMed] [Google Scholar]
- Stockwell RG, Mansinha L, Lowe RP. Localization of the complex spectrum: The S Transform. IEEE Trans on Signal Processing. 1996;44:998–1001. [Google Scholar]