Abstract
Zebrafish larvae are ideally suited for high-throughput analyses of vertebrate behavior. The larvae can be examined in multiwell plates and display a range of behaviors during early development. Previous studies have shown that zebrafish larvae display a preference for the edge of the well and several lines of evidence suggest this edge preference (thigmotaxis) may be a measure of anxiety. In the present study, we further examined the relation between edge preference and anxiety by imaging zebrafish larvae exposed to three psychoactive drugs diazepam (Valium), fluoxetine (Prozac), and caffeine. The edge preference was first examined in a five-fish assay, with and without visual stimuli. Diazepam, a benzodiazepine that binds to GABA receptors, reduced the larval edge preference, with or without visual stimuli. In contrast, fluoxetine, a selective serotonin reuptake inhibitor, did not affect the edge preference. Caffeine increased the preference for the edge in response to visual stimuli. Similar effects were observed in a two-fish assay; diazepam-exposed larvae showed a reduced edge preference and caffeine exposed larvae showed an increased edge preference. These results suggest that the edge preference in zebrafish larvae is a measure of anxiety and further illustrate that the pharmaceuticals used in the study have different mechanisms of action. Although there are substantial differences between zebrafish and human brains, our results indicate that the signals that regulate anxiety are similar on a molecular level. We propose that high-throughput assays in zebrafish may be used to uncover genetic or environmental factors that cause anxiety disorders and may contribute to the development of novel strategies to prevent or treat such disorders.
Keywords: Zebrafish, Anxiety, Diazepam, Fluoxetine, High-throughput, Thigmotaxis
1. Introduction
Zebrafish are an emerging model system in behavioral neuroscience [1]. Sophisticated genetic and optical tools are available to examine neural patterns in the brain and automated imaging systems have been developed for high-throughput analyses of behaviors such as fear and anxiety, social behavior, and learning and memory [2]. Large-scale screens can be carried out using adult zebrafish [3, 4]. However, there is a particular growing interest in zebrafish embryos, which can be collected and tested in much larger quantities and due to their rapid development into free-swimming larvae.
Zebrafish larvae hatch from their chorion between 2 and 3 days post fertilization (dpf). By 4-5 dpf, the larvae inflate their swim bladder and start to exhibit a broad range of behaviors, including hunting, avoidance, escape, phototaxis, and thigmotaxis, which are readily quantified in automated assays [5-7]. The larvae are small enough that they can be imaged in multiwell plates. For example, zebrafish embryos and larvae have recently been utilized for high-throughput studies to determine the quantitative effects of small molecules on rest, wake, and motor behavior [8, 9]. In these studies, thousands of small molecules and compounds were tested in a short period of time. Other behaviors have been examined using automated imaging systems that analyze larval interactions or analyze the response to local visual stimuli [6]. It was shown that larvae exposed to visual stimuli (a red ‘bouncing ball’) avoid the stimulus by moving to the opposite side of the well (avoidance) and by moving towards the edge of the well (thigmotaxis). Several lines of evidence suggest that this latter behavior, thigmotaxis, is a measure of anxiety [10, 11].
Recently, an increasing number of behavioral methods to detect fear and anxiety in rodents and adult zebrafish have been developed. The studies in zebrafish often utilize high-throughput detection of behaviors such as shoaling, erratic movements, freezing, and jumping in response to pharmaceuticals, visual stimuli representing a predator, or alarm substances from wounded fish [12-14]. One specific anxiety-related behavior is thigmotaxis, which has been studied in detail in both rodents and zebrafish [12, 13]. In rodents, a standard assay for testing novel anxiolytics is the elevated plus maze [15]. The maze has two enclosed arms and two open arms and the time spent in the enclosed arms is a measure for anxiety. Similarly, zebrafish may swim along the walls or the bottom of the tank. These behaviors have been examined in adult zebrafish, using pharmaceuticals that have known anxiolytic (decreased anxiety) or anxiogenic (increased anxiety) properties in humans. When adult zebrafish are transferred to a novel tank, they initially dive to the bottom of the tank and start to explore the upper layers of the tank after a period of adjustment. It is thought that the novel environment induces anxiety and this idea has been validated using pharmaceuticals, such as the anxiolytic diazepam (Valium), which reduces the time that the fish spend on the bottom of the tank [16]. Scototaxis, a preference for darkness, is also indicative of anxiety in adult zebrafish. If adult zebrafish are given chronic fluoxetine (Prozac), an anti-depressant with anxiolytic properties, the zebrafish spend significantly more time in the white area compared to controls, whereas caffeine had the opposite effect, producing anxiogenic behaviors with zebrafish spending less time in the white arena [17]. Overall, these studies suggest that the signaling pathways that regulate anxiety are conserved in vertebrate species.
In the present study, we use a similar strategy to the one that was successful in adult fish, i.e. zebrafish larvae were exposed to known psychoactive drugs in order to examine the relation between thigmotaxis and anxiety.
2. Materials and Methods
2.1 Zebrafish
Adult wild type zebrafish were originally obtained from Carolina Biological and are maintained at Brown University as a genetically diverse outbred strain. This wild type line is similar to the AB line in the larval edge preference (unpublished results). However, to avoid measuring specific behaviors that are introduced by many years of inbreeding in a laboratory setting, we used the outbred line for all experiments in this study. The fish were kept in a mixed male and female population on a 14 hour light / 10 hour dark cycle and were fed a combination of flake food and frozen brine shrimp. Embryos were collected from the tanks at ‘dawn’ and were raised at 28.5°C in egg water, containing 60 mg/L sea salt (Instant Ocean) in deionized water and 0.25 mg/L methylene blue to inhibit fungal growth in the culture medium. Embryos were grown at a density of 250 embryos per 2L of egg water in Aquatic Habitats breeder tanks. Dead embryos were removed and egg water was replaced every other day up until the day of behavioral analysis at 7 days post fertilization (dpf).
2.2 Exposure to pharmaceuticals
At 7 dpf, zebrafish larvae were exposed to diazepam (Sigma Aldrich D0899) at ranges of 0.05 mg/L - 5 mg/L, fluoxetine (Sigma Aldrich F132) at ranges of 0.2 - 2 mg/L, and caffeine (Sigma Aldrich C0750) at ranges of 10 - 100 mg/L. For the five-fish assay, the highest drug dosages used in the current study correspond to the lowest doses that elicited behavioral changes when used in previous studies [16, 18, 19]. Caffeine was dissolved directly in egg water. Diazepam and fluoxetine were dissolved in DMSO and stored at −20°C. At the onset of the experiment, the stock solutions in DMSO were diluted in egg water to their final concentrations. The corresponding DMSO concentrations were used as a solvent control. Embryos were exposed to the pharmaceuticals for two hours prior to behavioral analysis and kept in the same solutions during the behavioral assays, which lasted one hour. The drug exposure time was chosen as an acute exposure to affect neural function, since chronic exposures during the embryonic and larval stages might interfere with neural patterning and brain development. Possibly the effect of chronic exposures on neural function could be examined in older fish, when neural patterning and brain development have been completed. Chronic exposures have not been examined in this study, but could be very interesting with pharmaceuticals such as fluoxetine, which are most effective in humans during longer treatments.
2.3 Image collection
Our laboratory recently developed a high-throughput imaging system for automated analyses of behavior in zebrafish larvae [6]. Briefly, this system includes a 15 megapixel digital camera mounted on the top shelf of a tall cabinet. The 7 dpf zebrafish larvae were imaged in 6-well or 12-well plates (Corning Costar 3506 and 3512). The multiwell plates were placed on the bottom of the cabinet, on top of a laptop screen. The laptop is used both to provide a white background and to present visual stimuli to the zebrafish larvae (Fig. 1 A-B). A single imaging unit can image four multiwell plates simultaneously. The optics of the multiwell plates was improved by utilizing an agarose ring on the outer edges of each of the wells. In the 6-well plates, each well was filled with 5 ml agarose (0.5% w/v in deionized water); after the agarose hardened, the center portion of the agarose was stamped out to create a 27 mm diameter × 5 mm deep swimming area surrounded by an agarose ring. In the 12-well plates, each well was filled with 1.5 ml agarose and the center was stamped out to create a 14 mm diameter × 3 mm deep swimming area.
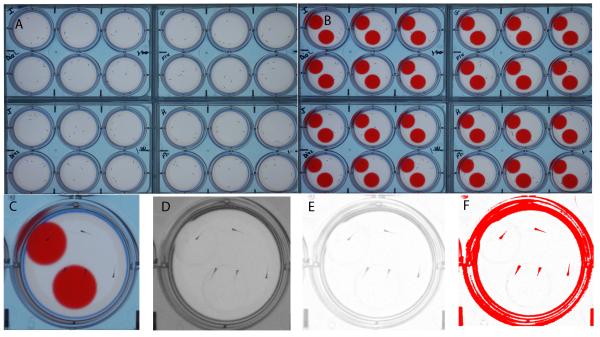
Figure 1. The five-fish assay for measuring avoidance behavior and edge preference.
A) 7-day-old zebrafish larvae are imaged in four 6-well plates containing five larvae per well. The larvae are first imaged for 30 minutes without visual stimuli. B) The same larvae are then imaged for 30 minutes in the presence of visual stimuli, a PowerPoint presentation with a ‘bouncing’ red ball in the top half of the well and a stationary red ball in the bottom half of the well. C) The high-resolution color images show the location and orientation of the larvae. D) Larval behavior is examined by automated image analysis in ImageJ. First, the visual stimuli are removed from the image by splitting the color channels. E) Dark areas in the background are removed by background subtraction. F) Larvae are separated from the background using a threshold for dark objects. Small air bubbles and specks of dust are removed with a filter for object size and the X,Y coordinates of the larvae are measured by particle analysis. The X, Y coordinates are imported in MS excel to calculate how often the larvae are located in the bottom half of the well, away from the bouncing ball (avoidance) and how often the larvae are located along the edge of the well. Scale bar = 1 cm.
2.4 Five-fish behavioral assay
The five-fish behavioral assay was carried out as described previously [6]. This assay is useful because it has a low well-to-well variability, which increases the statistical power of the experiment. The assay provides quantitative information on the number of larvae down in the well and on the edges of the well. Briefly, five zebrafish larvae, kept in their respective treatments, were placed in each well of a 6-well plate. We used an interval sampling method to record the location of the larvae in the wells, i.e. images were collected every 30 seconds for one hour. During the first half hour larvae were imaged on a white background without visual stimuli and during the second half hour larvae were imaged while exposed to visual stimuli. The visual stimuli were created in Microsoft PowerPoint, using an animated presentation with a red moving disc (“bouncing ball”) in the upper half of the well and a red stationary ball in the bottom half of the well (Figure 1B). A red bouncing ball moved continuously from left to right and back and is an aversive stimulus that the larvae avoid by moving away from the ball and demonstrating a preference for the edge (thigmotaxis) [6]. A stationary ball was used to counter-balance for brightness and color of the red bouncing ball. The RGB values for the red balls were 255, 0, 0. This color value was chosen because it is easily removed during the image analysis (Fig. 1C-F).
2.5 Two-fish behavioral assay
Two zebrafish larvae, kept in their respective treatments, were placed in each well of a 12-well plate. This assay is useful because it gives a quick and reliable measure of the distance between the two larvae in the well and allows us to determine the number of larvae that are up or down in the well or on the edge of the well. Previous studies have shown that it is possible to calculate distances between adult zebrafish in small groups of fish [14, 20]. Our system is currently not set up for measuring distances between five zebrafish larvae; although, it would be interesting to develop such capabilities in future research. The 12-well plates were imaged on a white background without visual stimuli. We used an interval sampling method to determine how often larvae were located on the edge of the well and how often the two larvae were located in the same quadrant of the well. Images were collected every 60 seconds for one hour.
2.6 Swim speed
In order to acquire data on swim speed, one zebrafish larva was placed in each well of a 12-well plate (one-fish assay). With only one larva per well, our system can automatically calculate the swim speed from the X,Y coordinates of the larva in each image. The 12-well plates were imaged on a white background without visual stimuli, using a 6 second interval between images. We tested the swim speed of larvae exposed to diazepam, fluoxetine, and caffeine at various concentrations and examined activity of larvae exposed to the anesthetic Tricaine (MS222) at a 0.016% w/v concentration. In all cases, the exposures started 2 hours before the imaging experiments and continued during the imaging experiments.
2.7 Automated image analysis using ImageJ
The acquired images were analyzed in ImageJ, an image analysis package that can be downloaded free of charge from the National Institutes of Health (http://rsb.info.nih.gov/ij/). We have developed an automated ImageJ macro that measures the location and orientation of 7 dpf zebrafish larvae in a large number of images [6]. Briefly, the macro removes the visual stimuli by splitting the color channels, applies a threshold for dark objects to separate the larvae from the background, and identifies the larvae by particle analysis (Fig 1C-F). The macro generates a ‘Results’ file which consists of a long list of coordinates, showing the midpoint of the well, well number, and the X,Y coordinates of the larval centroids. The Results file is then opened in Microsoft Excel for further analysis.
2.8 Data analysis in MS Excel
The X,Y coordinates of the larval centroids were compared to the X,Y coordinates of the well’s midpoint as described previously [6, 21, 22]. If the Y coordinate of a larva is smaller than the Y coordinate of the midpoint of a well, the larva is in the upper half of the well (‘up’). If not, the larva is located in the lower half of the well (‘down’). When a visual stimulus is presented, ‘up’ is close to the stimulus and ‘down’ is away from the stimulus. ‘Up’ or ‘down’ in the dish is not a vertical measure, rather a horizontal one. A larva is considered to be in the ‘center’ if the distance between the larva and the midpoint of the well is smaller than the square root of the radius of the swimming area. If not, the larva is considered to be located on the ‘edge’. In both cases, up:down and edge:center are matched for area. Thus, there is a 50% chance that a larva is located down and a 50% chance that a larva is located on the edge if the larvae are distributed randomly in the swimming area. Previous studies have shown that 7 dpf larvae display a preference for the edge of the well and avoid the bouncing ball in the upper half of the well [5, 6].
2.9 Statistical Analyses
The statistical analyses were carried out in MS Excel. Excel’s ‘COUNTIFS’ function was used to count how often larvae were located down in the well and how often the larvae are located on the edge of the well. Since larvae in the five-fish and two-fish assays may interact, the data was analyzed on a per well basis (n = number of wells). This assures that the measured values are independent. Average values (± standard error of the mean) were calculated for all wells within an experimental group. Differences in behavior before and during the visual stimuli were tested for significance using a two-tailed t-test with unequal variance. Similarly, differences between pharmaceutical exposures (diazepam, fluoxetine, and caffeine) and the corresponding controls (DMSO and egg water) were tested for significance using a two-tailed t-test with unequal variance. The asterisks in the graphs indicate a significant difference between the pharmaceutical exposures and the corresponding egg water or DMSO controls.
3. Results
3.1 Effects of diazepam in the five-fish bouncing ball assay
The effect of diazepam on avoidance behavior was examined in the five-fish bouncing ball assay, by measuring how often the larvae are down in the well away from the moving stimulus (Fig. 2A). The five-fish bouncing ball assay was chosen as it is more robust than the one- or two-fish bouncing ball assays [6]. Control larvae, exposed to the solvent DMSO, displayed a significant avoidance response to the bouncing ball stimulus (t (94) = 4.47, p=2.10−5, n=48 wells). Larvae were 52.3% (±1.5) down in the well prior to the visual stimuli and 61.8% (±1.5) down in the well when shown the visual stimuli. A slightly lower avoidance response was observed in diazepam exposed larvae (n=36 wells), i.e. larvae were 51.8% (±2.2) down in the well prior to the visual stimuli and 56.9% (±2.7) down in the well during the visual stimuli. The differences between the DMSO and diazepam-exposed groups were not significant (t (66) = .162 and t (55) = 1.57, p>0.05), suggesting that diazepam has a minimal effect on the larvae’s ability to respond to aversive stimuli.
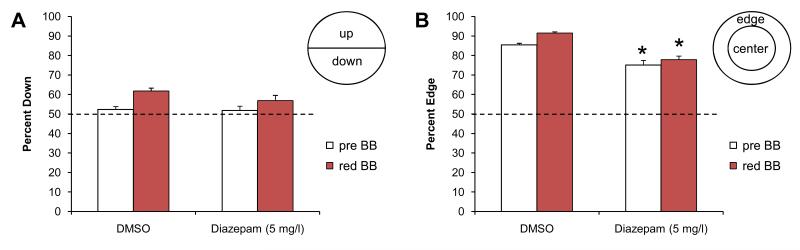
Figure 2. Diazepam-exposed larvae in the five fish bouncing ball assay.
A) Control larvae exposed to the solvent DMSO show an avoidance response to the red bouncing ball. Before the bouncing ball stimulus, larvae are located down in the well 52.3% of the time. This percentage increased to 61.8% in the presence of a bouncing ball stimulus. A similar avoidance response was observed in larvae exposed to diazepam (Valium). B) Control larvae exposed to the solvent DMSO show a strong preference for the edge of the well, i.e. larvae are located on the edge 85.4% of the time. This edge preference increased to 91.5% in the presence of a bouncing ball stimulus. Larvae exposed to diazepam show a reduced edge preference, both before and during the bouncing ball stimulus. Up:down and edge:center were matched for area. The dotted line indicates the expected value based on a random 50:50 distribution. Pre BB = before the bouncing ball stimulus, red BB = during the bouncing ball stimulus. Asterisks indicate a significant change compared to the DMSO control (p<0.001).
The same five-fish assay was used to measure how often the larvae were located on the edge (Fig. 2B). Control larvae, exposed to the solvent DMSO, displayed a strong preference for the edge of the well. Larvae were 85.4% (±0.9) on the edge prior to the visual stimuli and 91.5% (±0.7) on the edge when shown the visual stimuli. This increase in edge preference in response to the bouncing ball is significant (t (89) = 5.39, p=6.10−7, n=48 wells). The diazepam-exposed larvae were 75.1% (±2.4) on the edge prior to the visual stimuli and 77.9% (±1.8) on the edge during the visual stimuli. Thus, diazepam significantly suppressed the larvae’s preference for the edge, both prior to (t (45) = 4.08, p=2.10−4) and during the visual stimuli (t (46) = 7.18, p=5.10−9, n=36 wells). Based on these results, we conclude that diazepam affects the larvae’s preference for the edge of the well.
3.2 Effects of fluoxetine in the five-fish bouncing ball assay
The effect of fluoxetine on avoidance behavior was measured in the five-fish bouncing ball assay (Fig 3A). Control larvae, exposed to the solvent DMSO, displayed a significant avoidance response to the bouncing ball stimulus (t (69) = 4.88, p=7.10−6, n=36 wells). Larvae were 52.7% (±1.4) down in the well prior to the visual stimuli and 63.1% (±1.6) down in the well when shown the visual stimuli. Fluoxetine-exposed larvae were 48.6% (±3.0) down in the well prior to the visual stimuli and 56.0% (±2.7) down in the well during the visual stimuli (n=36 wells). The 56% down is a significant reduction compared to the DMSO control (t (57) = 2.29, p=0.03), indicating that fluoxetine affects avoidance behaviors in zebrafish larvae.
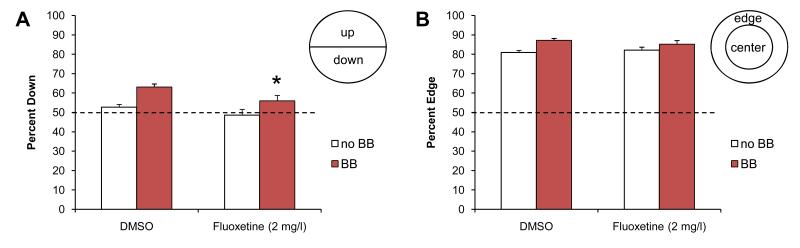
Figure 3. Fluoxetine-exposed larvae in the five-fish bouncing ball assay.
A) Avoidance behaviors of larvae exposed to DMSO and fluoxetine (Prozac). The fluoxetine exposed larvae show a reduced avoidance response (p<0.001). B) The edge preference in the fluoxetine exposed larvae is similar to the edge preference in the DMSO controls. The dotted line indicates the expected value based on a random 50:50 distribution. Pre BB = before the bouncing ball stimulus, red BB = during the bouncing ball stimulus. Asterisk indicates a significant change compared to the DMSO control (p<0.05).
Fluoxetine did not have a significant effect on the larvae’s edge preference (Fig. 3B). Control larvae, exposed to the solvent DMSO, were 80.9% (±1.1) on the edge prior to exposure to the visual stimuli and 87.2% (±1.0) on the edge during the visual stimuli (n=36 wells). Fluoxetine-exposed larvae were 82.1% (±1.6) on the edge prior to the visual stimuli and 85.2% (±1.9) on the edge during the visual stimuli (n=36 wells). These results suggest that fluoxetine has a minimal effect on the larvae’s edge preference.
3.3 Effects of caffeine in the five fish bouncing ball assay
The effect of caffeine on avoidance behavior was measured in the five-fish bouncing ball assay (Fig. 4A). Control larvae in egg water displayed a significant avoidance response to the bouncing ball stimulus (t (117) = 6.85, p=4.10−10, n=60 wells). Larvae were 53.6% (±1.0) down in the well prior to the visual stimuli and 64.0% (±1.1) down in the well during the visual stimuli. Caffeine-exposed larvae were 48.7% (±3.7) down in the well prior to the visual stimuli and 54.3% (±3.4) down in the well during the visual stimuli (n=35 wells). The 54.3% down is a significant reduction compared to the egg water control (t (43) = 2.7, p=0.001), indicating that caffeine affects avoidance behaviors in zebrafish larvae.
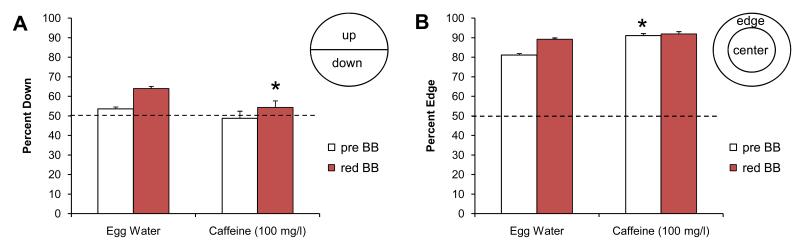
Figure 4. Caffeine-exposed larvae in the five fish assay.
A) Larvae exposed to caffeine show a significantly reduced avoidance response. B) The caffeine-exposed larvae show a significant increase in their edge preference. The dotted line indicates the expected value based on a random 50:50 distribution. Pre BB = before the bouncing ball stimulus, red BB = during the bouncing ball stimulus. Asterisks indicate significant changes compared to the egg water controls (p<0.01).
The effect of caffeine on edge preference was examined in the same five-fish assay (Fig. 4B). Control larvae in egg water were 81.1% (±0.8) on the edge prior to the visual stimuli and 89.2% (±0.7) on the edge during the visual stimuli. The increase in edge preference in response to the bouncing ball is significant (t (113) = 7.61, p=9.10−12, n=60 wells). Caffeine-exposed larvae were 91.1% (±1.0) on the edge prior to the visual stimuli and 91.9% (±1.2) on the edge during the visual stimuli (n=35 wells). These edge preferences prior to and during the visual stimuli are significantly higher than the corresponding egg water controls (t (74) = 7.50, p=1.10−10 and t (58) = 2.03, p=0.047, respectively). These results indicate that caffeine exaggerates the larvae’s edge preference.
Overall, the data from the five-fish assay show that diazepam, fluoxetine, and caffeine each affect larval behavior differently. Diazepam reduces the % edge, fluoxetine reduces the % down, and caffeine reduces the % down and increases the % edge. The data also show that the % down may not be a straightforward measure of anxiety, since the % down was reduced both after exposure with the anxiolytic fluoxetine and the anxiogenic caffeine. In contrast, the % edge may provide information on both reduced and increased anxiety. Larvae are less on the edge after exposure to the anxiolytic diazepam and more on the edge after exposure to the anxiogenic caffeine. An additional advantage of measuring the % edge is that it can also be acquired using larvae placed in 12-well plates without visual stimuli.
3.4 Effects of diazepam, fluoxetine, and caffeine in the two-fish assay
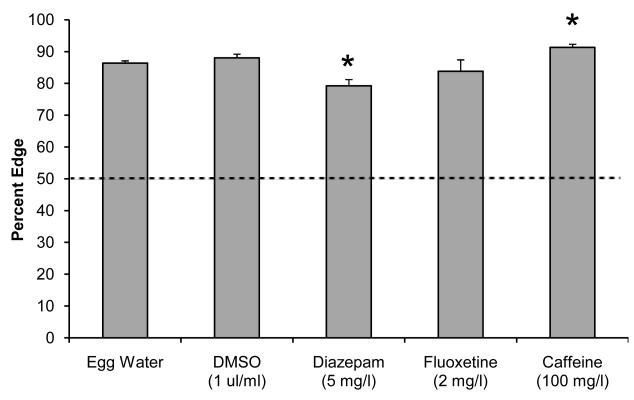
The two-fish assay was carried out in 12-well plates without visual stimuli (Fig. 5). This assay provides information on the % edge and can be used to examine larval interactions [22]. In both the egg water and DMSO controls, larvae show a clear preference for the edge (Fig. 6). Larvae in egg water were located 86.4% (±0.7, n=143 wells) on the edge and control larvae exposed to the solvent DMSO were located 88.0% (±1.2, n=48) on the edge. Diazepam-exposed larvae were located 79.2% (±2.0, n=48 wells) on the edge, a significant reduction compared to the DMSO controls (t (77) = 3.80, p=3.10−4). Fluoxetine did not have a significant effect on the edge preference (t (27) = 1.14, p>0.05). Caffeine-exposed larvae were located 91.3% (±1.0, n= 48) on the edge, a significant increase compared to the egg water controls (t (94) = 3.91, p=2.10−4). We also examined how often the larvae were together in the same quadrant. The expected value in a random distribution is 25% vs. an observed 14.9% (±0.5, n=143 wells) in the egg water controls and 13.3% (±0.9, n=48 wells) in the DMSO controls. However, none of the pharmaceuticals tested showed a significant difference compared to controls (p>0.05). Overall, the results of the two-fish assay are consistent with the five-fish assay in regards to edge preference; diazepam exposure leads to a reduced edge preference and caffeine exposure leads to an increased edge preference.
Figure 5. The two-fish assay for measuring edge preference and social avoidance.
A) 7-day-old zebrafish larvae are imaged in four 12-well plates containing two larvae per well. The larvae are imaged for one hour on a white background. B) Larval locations are measured by automated image analysis. C) Overlay of the well and measured larvae. The X,Y coordinates of the larvae are imported in MS Excel to calculate how often the larvae are located on the edge of the well and how often the larvae are located together in the same quadrant of the well. Scale bar = 1 cm.
Figure 6. Edge preference in the two-fish assay.
Zebrafish larvae display a strong edge preference in the two fish assay. The edge preference is reduced in larvae exposed to diazepam and is increased in larvae exposed to caffeine. Asterisks indicate a significant change compared to the corresponding controls (p<0.001).
3.5 Swim speed
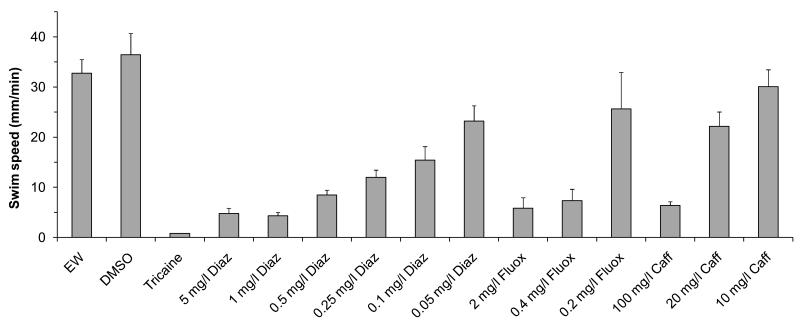
Since the psychoactive drugs used in our study can tend to have sedative effects, we examined whether the observed edge effects might be explained by a reduction in swim speed. The swim speed was measured in a one-fish assay using a 12-well plate without visual stimuli (Fig. 7). The egg water and DMSO-control larvae displayed swim speeds of 32.8 mm/min (±2.7, n=34 wells) and 36.4 mm/min (±4.2, n=36 wells), respectively. Larvae exposed to 5 mg/l diazepam, 2 mg/l fluoxetine, or 100 mg/l caffeine, the same concentrations used in the previous experiments, displayed a significant reduction in swim speed (t (39) = 7.28, p=1.10−8, t (45) = 6.48, 6.10−8, t (37) = 9.39, 2.10−11, respectively). Diazepam reduced the speed to 4.8 mm/min (±1.0, n=12 wells), fluoxetine reduced the swim speed to 5.8 mm/min (±2.1, n=11 wells), and caffeine reduced the swim speed to 6.4 mm/min (±0.7, n=12 wells). However, in all exposure groups the larvae were able to swim and the swim speed was significantly higher (t (11) = 3.86), p=3.10−3, t (10) = 2.40, 0.04, t (11) = 7.52, 1.10−5) than the slow 0.8 mm/min drift (±0.02, n=12 wells) that can be observed in tricaine-anesthetized larvae. We conclude that the swim speed is affected by all pharmaceutical treatments, but that the observed edge effects cannot be explained by a reduction in swim speed. Namely, diazepam and caffeine both reduce the swim speed, but have opposite effects of each other with respect to the % edge.
Figure 7. Swim speed.
7-day-old larvae were imaged in 12-well plates containing one larva per well. Larvae exposed to 5 mg/l diazepam (diaz), 2 mg/l fluoxetine (fluox), or 100 mg/l caffeine (caff) display swim speeds that are much lower than the egg water or DMSO controls, but not as low as the subtle drift that can be observed in tricaine-anesthetized larvae. When larvae are exposed to 0.05 mg/l diazepam, 0.2 mg/l fluoxetine, or 20 mg/l caffeine, the larvae display swim speeds that are at least half of the swim speed in the egg water and DMSO controls.
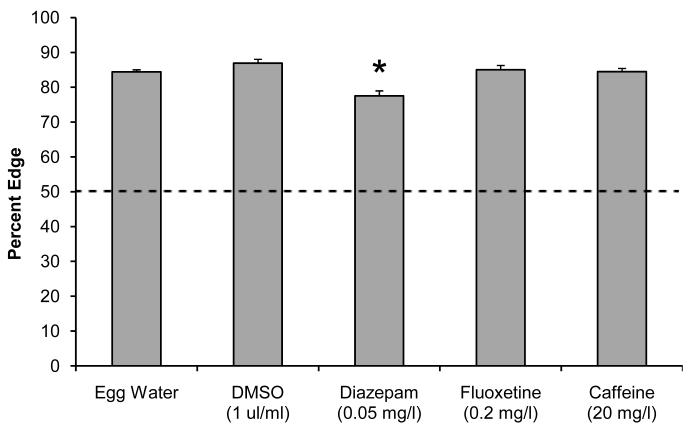
The swim speed experiments raise the question if edge effects can be observed at near-normal swim speeds. To address this question, we first reduced the pharmaceutical concentrations to a level where the larvae display a near-normal swim speed, i.e. a swim speed that is at least half of the swim speed observed in the egg water controls. This was accomplished with 0.05 mg/l diazepam, 0.2 mg/l fluoxetine, and 20 mg/l caffeine (Fig. 7). We then used the two-fish assay to examine the % edge and % together at these low pharmaceutical concentrations. Larvae in egg water were located 84.4% (±0.6, n=247 wells) on the edge and control larvae exposed to the solvent DMSO were located 86.9% (±1.1, n=71 wells) on the edge (Fig. 8). Fluoxetine- and caffeine-exposed larvae displayed an edge preference that was similar to the corresponding controls. In contrast, diazepam-exposed larvae were located 77.5% (±1.5, n=45 wells) on the edge, a significant reduction compared to the DMSO controls (t (90) = 5.05, p=2.10−6). The % together was significantly reduced after exposure to 20 mg/l caffeine (t (199) = 5.69, p=4.10−8). Larvae in egg water were together in the same quadrant 13.3% (±0.4, n=247 wells) of the time, and larvae exposed to caffeine were located together 8.5% (±0.7, n=114) of the time. These results indicate that specific behavioral defects can be observed at a near-normal swim speed using low concentrations of diazepam and caffeine.
Figure 8. Exposure to low concentrations of diazepam, fluoxetine, and caffeine.
Zebrafish larvae were exposed to 0.05 mg/l diazepam, 0.2 mg/l fluoxetine, or 20 mg/l caffeine and imaged in 12-well plates containing two larvae per well. Larvae exposed to 0.05 mg/l diazepam display a reduced preference for the edge (p<0.001).
4. Discussion
Previous studies have suggested that avoidance behaviors and thigmotaxis in zebrafish may be a measure of anxiety [5, 10, 23, 24]. In the present study, we further examined the relation between these behaviors and anxiety by imaging 7-day-old zebrafish larvae exposed to the psychoactive drugs diazepam (Valium), fluoxetine (Prozac), and caffeine. We show that two-hour exposures to diazepam, fluoxetine, and caffeine induce significant changes in both avoidance behaviors and thigmotaxis. Thigmotaxis may be the most reliable measure of anxiety, since the larvae displayed either reduced or increased edge preferences, depending on the pharmaceutical treatment administered.
Avoidance behaviors were examined by quantifying the percentage of larvae ‘down’ in the dish when using a bouncing ball stimulus, which the 7-day-old larvae may perceive as a large predator or the shadow of a large predator. Diazepam-treated larvae showed an avoidance response similar to the controls. In contrast, fluoxetine and caffeine-exposed larvae displayed a reduced avoidance response. The differences between the diazepam and fluoxetine are likely due to the fact that these drugs target different neuronal signaling pathways [23]. Diazepam (Valium) is a benzodiazepine that binds to neuronal GABA receptors. It has a sedative and anxiolytic effect and is most widely prescribed for anxiety and sleep disorders, and panic attacks [25]. In addition, because this drug enhances the actions of GABA, it produces muscle relaxant effects and sedation depending on the dose administered illustrated by the reduction in swim speed of the larvae after exposure. Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) and most commonly used as an anti-depressant even though is it sometimes used to treat anxiety and obsessive-compulsive disorders [26]. Because caffeine can affect a number of behaviors, its effect is more difficult to interpret. Caffeine is an antagonist of adenosine receptors and has been shown to act as a stimulant at lower doses, but higher doses have been shown to decrease locomotor activity [27]. In addition, caffeine produces anxiogenic effects in humans, adult zebrafish, and mice [19, 28, 29]. We anticipated that caffeine-treated larvae would show an increased avoidance response, but instead the caffeine-treated larvae showed a reduced avoidance response. Possibly, caffeine triggers another predator avoidance strategy, such as freezing or hiding on the edge, similar to the response seen with adult zebrafish in the novel tank diving test [19].
The edge preference (thigmotaxis behavior) was examined in a five-fish bouncing ball assay and in a two-fish assay without visual stimuli. In both assays, the edge preference was reduced by different dosages of diazepam, was not affected by fluoxetine, and was increased by caffeine at a high dose. The caffeine-induced preference for the edge was similar to the bouncing ball induced preference for the edge, suggesting that the two stimuli may be comparable in their anxiogenic effect. Our results may also be similar to studies in adult zebrafish, where caffeine evokes anxiety when measured by an increase in white avoidance behavior [30]. Because larvae show significantly less thigmotaxis behavior after exposure to the anxiolytic diazepam but not to the anti-depressant fluoxetine, this suggests that our high-throughput assay is extremely useful for the detection of anxiety-related behaviors in zebrafish larvae and that the behavior is easily quantified by our high-throughput system. As a caveat, diazepam, fluoxetine, and caffeine all reduced the larval swim speed in varying levels depending upon the dosage administered. However, this reduction of swim speed cannot explain the observed edge effects, i.e. diazepam and caffeine cause a similar reduction of swim speed and have an opposite edge effect. Despite the slower swim speed in these groups, the activity levels were all well above those of larvae treated with MS-222.
Overall, the pharmaceuticals had very different effects on avoidance behaviors and edge preference. Avoidance behaviors were suppressed by fluoxetine and caffeine, and the edge preference was suppressed by diazepam and increased by caffeine. These differences are likely due to the fact that these pharmaceuticals target different neuronal signaling pathways. In addition, these differences may be explained on a cognitive level, i.e. it is possible that the different pharmaceutical profiles and behavioral measures reflect differences between fear and anxiety. An imminent threat such as a mock predator is likely to induce fear, and the anticipation of an unspecified threat is likely to induce anxiety [23]. Thus, the diazepam-treated larvae may retain their normal fear for a nearby stimulus, but have less general anxiety. On the other hand, the fluoxetine-treated larvae may be less fearful of the mock predator, but have a normal general level of anxiety. If correct, the two behavioral assays could potentially be used to screen for novel anxiolytics.
In conclusion, our studies show that the signals that drive avoidance and thigmotaxis in zebrafish larvae are similar to the signals that control anxiety in humans. The high-throughput assays developed in our laboratory may be used to screen for genes, environmental toxicants, or pharmaceuticals that cause anxiety disorders. In addition, the same assays may be used to develop novel strategies to prevent or treat these disorders.
Highlights.
Our high-throughput assay is able to detect anxiety-related behavior in zebrafish
Zebrafish larvae naturally avoid a visual moving stimulus and show thigmotaxis
Zebrafish larvae treated with diazepam show decreased thigmotaxis
Zebrafish larvae treated with fluoxetine show decreased avoidance
Zebrafish larvae treated with caffeine show increased thigmotaxis
Acknowledgements
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, R01HD060647) and the National Institute of Environmental Health Sciences (NIEHS, R03ES017755).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References
- [1].Mathur P, Guo S. Use of zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiol Dis. 2010;40:66–72. doi: 10.1016/j.nbd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gerlai R. High-throughput behavioral screens: the first step towards finding genes involved in vertebrate brain function using zebrafish. Molecules. 2010;15:2609–22. doi: 10.3390/molecules15042609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Blackburn JS, Liu S, Raimondi AR, Ignatius MS, Salthouse CD, Langenau DM. High-throughput imaging of adult fluorescent zebrafish with an LED fluorescence macroscope. Nat Protoc. 2011;6:229–41. doi: 10.1038/nprot.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Norton W, Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neuroscience. 2010;11:90. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Colwill RM, Creton R. Locomotor behaviors in zebrafish (Danio rerio) larvae. Behav Processes. 2011;86:222–9. doi: 10.1016/j.beproc.2010.12.003. (NIHMS264983) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pelkowski S, Kapoor M, Richendrfer H, Wang X, Colwill RM, Creton R. A novel high-throughput imaging system for automated analyses of avoidance behavior in zebrafish larvae. Behav Brain Res. 2011;223:135–44. doi: 10.1016/j.bbr.2011.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tierney KB. Behavioural assessments of neurotoxic effects and neurodegeneration in zebrafish. Biochim Biophys Acta. 2011;1812:381–9. doi: 10.1016/j.bbadis.2010.10.011. [DOI] [PubMed] [Google Scholar]
- [8].Kokel D, Bryan J, Laggner C, White R, Cheung CYJ, Mateus R, et al. Rapid behavior-based identification of neuroactive small molecules in the zebrafish. Nat Chem Biol. 2010;6:231–7. doi: 10.1038/nchembio.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rihel J, Prober DA, Arvanites A, Lam K, Zimmerman S, Jang S, et al. Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation. Science. 2010;327:348–51. doi: 10.1126/science.1183090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Champagne DL, Hoefnagels CCM, de Kloet RE, Richardson MK. Translating rodent behavioral repertoire to zebrafish (Danio rerio): Relevance for stress research. Behav Brain Res. 2010;214:332–42. doi: 10.1016/j.bbr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- [11].Miller BH, Schultz LE, Gulati A, Su AI, Pletcher MT. Phenotypic Characterization of a Genetically Diverse Panel of Mice for Behavioral Despair and Anxiety. PLoS ONE. 2010;5:e14458. doi: 10.1371/journal.pone.0014458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gerlai R. Zebrafish antipredatory responses: A future for translational research? Behav Brain Res. 2010;207:223–31. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maximino C, de Brito TM, da Silva Batista AW, Herculano AM, Morato S, Gouveia A., Jr Measuring anxiety in zebrafish: A critical review. Behav Brain Res. 2010;214:157–71. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- [14].Miller NY, Gerlai R. Shoaling in zebrafish: what we don’t know. Rev Neurosci. 2011;22:17–25. doi: 10.1515/RNS.2011.004. [DOI] [PubMed] [Google Scholar]
- [15].Pellow S, Chopin P, File SE, Briley M. Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- [16].Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Maximino C, da Silva AWB, Gouveia A, Jr, Herculano AM. Pharmacological analysis of zebrafish (Danio rerio) scototaxis. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:624–31. doi: 10.1016/j.pnpbp.2011.01.006. [DOI] [PubMed] [Google Scholar]
- [18].Airhart MJ, Lee DH, Wilson TD, Miller BE, Miller MN, Skalko RG. Movement disorders and neurochemical changes in zebrafish larvae after bath exposure to fluoxetine (PROZAC) Neurotoxicol Teratol. 2007;29:652–64. doi: 10.1016/j.ntt.2007.07.005. [DOI] [PubMed] [Google Scholar]
- [19].Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Buske C, Gerlai R. Shoaling develops with age in Zebrafish (Danio rerio) Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1409–15. doi: 10.1016/j.pnpbp.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Colwill RM, Creton R. Automated Imaging of Avoidance Behavior in Larval Zebrafish. In: Kalueff AV, Cachat JM, editors. Neuromethods. Springer Science+Business Media, LLC; New York: 2011. p. 2010. [Google Scholar]
- [22].Creton R. Automated analysis of behavior in zebrafish larvae. Behav Brain Res. 2009;203:127–36. doi: 10.1016/j.bbr.2009.04.030. [DOI] [PubMed] [Google Scholar]
- [23].Colwill RM, Creton R. Imaging escape and avoidance behavior in zebrafish larvae. Rev Neurosci. 2011;22:63–73. doi: 10.1515/RNS.2011.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dadda M, Domenichini A, Piffer L, Argenton F, Bisazza A. Early differences in epithalamic left-right asymmetry influence lateralization and personality of adult zebrafish. Behav Brain Res. 2010;206:208–15. doi: 10.1016/j.bbr.2009.09.019. [DOI] [PubMed] [Google Scholar]
- [25].PubChem., NIH; National Library of Medicine; Diazepam: 2006. [Google Scholar]
- [26].Wong DT, Bymaster FP, Engleman EA. Prozac (fluoxetine, lilly 110140), the first selective serotonin uptake inhibitor and an antidepressant drug: twenty years since its first publication. Life Sci. 1995;57:411–41. doi: 10.1016/0024-3205(95)00209-o. [DOI] [PubMed] [Google Scholar]
- [27].Finn IB, Holtzman SG. Tolerance to caffeine-induced stimulation of locomotor activity in rats. J Pharmacol Exp Ther. 1986;238:542–6. [PubMed] [Google Scholar]
- [28].El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. The anxiogenic-like effect of caffeine in two experimental procedures measuring anxiety in the mouse is not shared by selective A2A adenosine receptor antagonists. Psychopharmacology. 2000;148:153–63. doi: 10.1007/s002130050037. [DOI] [PubMed] [Google Scholar]
- [29].Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40:1243–55. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- [30].Maximino C, Lima MG, Olivera KRM, Picanço-Diniz DLW, Herculano AM. Adenosine A1, but not A2, Receptor Blockade Increases Anxiety and Arousal in Zebrafish. Basic Clin Pharmacol Toxicol. 2011 doi: 10.1111/j.1742-7843.2011.00710.x. [DOI] [PubMed] [Google Scholar]