Abstract
Uridine adenosine tetraphosphate (Up4A) has been recently identified as a novel and potent endothelium-derived contracting factor and contains both purine and pyrimidine moieties, which activate purinergic P2X and P2Y receptors. The present study was designed to compare contractile responses to Up4A and other nucleotides such as ATP (P2X/P2Y agonist), UTP (P2Y2/P2Y4 agonist), UDP (P2Y6 agonist), and α,β-methylene ATP (P2X1 agonist) in different vascular regions [thoracic aorta, basilar, small mesenteric, and femoral arteries] from deoxycorticosterone acetate-salt (DOCA-salt) and control rats. In DOCA-salt rats [vs. control uninephrectomized (Uni) rats]: (1) in thoracic aorta, Up4A-, ATP-, and UTP-induced contractions were unchanged; (2) in basilar artery, Up4A-, ATP-, UTP- and UDP-induced contractions were increased, and expression for P2X1, but not P2Y2 or P2Y6 was decreased; (3) in small mesenteric artery, Up4A-induced contraction was decreased and UDP-induced contraction was increased; expression of P2Y2 and P2X1 was decreased whereas P2Y6 expression was increased; (4) in femoral artery, Up4A-, UTP-, and UDP-induced contractions were increased, but expression of P2Y2, P2Y6 and P2X1 was unchanged. The α,β-methylene ATP-induced contraction was bell-shaped and the maximal contraction was reached at a lower concentration in basilar and mesenteric arteries from Uni rats, compared to arteries from DOCA-salt rats. These results suggest that Up4A-induced contraction is heterogenously affected among various vascular beds in arterial hypertension. P2Y receptor activation may contribute to enhancement of Up4A-induced contraction in basilar and femoral arteries. These changes in vascular reactivity to Up4A may be adaptive to the vascular alterations produced by hypertension.
Keywords: contraction, dinucleotide, DOCA-salt, EDCF, extracellular nucleotides, P2 receptor
Introduction
Arterial hypertension is associated with vasomotor dysfunction, which manifests in part as decreased endothelium-dependent relaxation and also as altered vascular smooth muscle function [1–5]. Although endothelium-derived relaxing factors (EDRFs) play a crucial role in the regulation of circulatory vasomotion, experimental evidence supports the concept that endothelium-derived contracting factors (EDCFs) also regulate vascular function, which becomes particularly important in the hypertensive process [2,3]. Impaired endothelium-dependent vasomotor function in hypertensive animal models has been attributed to both reduced nitric oxide (NO) bioavailability and enhanced endothelium-dependent contraction [2,3,6]. Alterations in the bioavailability and actions of endothelium-derived factors may contribute to changes in resting blood pressure. Although a strategy for suppressing endothelial dysfunction (i.e., increasing EDRFs signaling and/or reducing EDCFs signaling) may become a therapeutic target for hypertensive disease, the detailed mechanisms by which endothelial factors regulate the cardiovascular system are not fully understood.
The dinucleotide uridine adenosine tetraphosphate (Up4A) is a novel potent non-peptidic EDCF that is assumed to play an important role in the regulation of vascular tone [7], and may be a potent inducer of pro-inflammatory responses in the vascular wall [8]. Up4A is released from the endothelium in response to acetylcholine (ACh), the calcium ionophore (A23187), endothelin-1, adenosine triphosphate (ATP), and uridine triphosphate (UTP) [7]. Since the identification of Up4A in 2005 [7], Up4A has been shown to modulate vascular tone in rat aorta [9], pulmonary arteries [10], and perfused kidney [7,11], and aorta [12] and renal afferent arterioles [13] isolated from mouse. Up4A possesses both purine and pyrimidine moieties, which are known to potentially activate purinoceptors.
Purinoceptors are present in most organ systems in the body, and there is accumulating evidence that they play an important role in contractile tone of various arteries including cerebral arteries [14,15], thoracic aorta [16], mesenteric [17,18] and femoral arteries [19]. Purinoceptors have been classified into two subtypes: P1 and P2 receptors, based on their pharmacological properties and molecular cloning [20]. Adenosine and its phosphate derivatives, ATP and ADP, have been identified as the endogenous ligands for P1 and P2 receptors, respectively [20]. The P2 receptors are divided into two families: such as P2X (ligand-gated ion channels) and P2Y receptors (G protein-coupled receptors) [20–24]. So far, seven P2X receptor subtypes (P2X1-P2X7) and eight P2Y receptor subtypes (P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11–P2Y14) have been reported [20–24]. P2X receptors are exclusively activated by ATP, whereas P2Y receptors respond to both purine (ATP and ADP) and pyrimidine (UTP and UDP) nucleotides [20–24]. Specifically, ATP is a ligand for P2X1–P2X7, P2Y2, P2Y11, and P2Y13 receptors, whereas UTP is a ligand for P2Y2 and P2Y4 receptors [20–24]. UDP preferentially activates P2Y6 receptors [25,26].
So far, the studies of Up4A-mediated responses have been carried out only in normal animals, and there is no study to indicate the vascular effects of Up4A under pathophysiological conditions, such as arterial hypertension. We recently found that Up4A-induced contraction is increased in renal artery but not in pulmonary artery isolated from deoxycorticosterone acetate (DOCA)-salt rats, a salt-dependent experimental model of arterial hypertension [27]. However, regional specific differences of Up4A and purinoceptor signaling in arterial hypertension are largely unknown/unexplored. Since circulating levels of Up4A are strongly associated with juvenile hypertension [28], Up4A may contribute to the early development of primary hypertension and to the development of hypertension-associated vascular abnormalities.
Hypertension leads to vascular complications in various regions including cerebrovascular [29,30] and peripheral vasculatures [31,32]. For instance, chronic hypertension leads to alteration of vascular responses both in resistance (e.g. small mesenteric artery, which plays an important role in blood pressure regulation [33,34]) and conductance arteries (e.g. basilar artery, which may make important contributions to total cerebral vascular resistance and is a major determinant of local microvascular pressure [35,36], thoracic aorta [3,4,33,37], femoral artery, which supplies blood to skeletal muscles of the lower extremity). Purinoceptor signaling may play an important role in regulation of vascular tone [14–19, 38]. However, the effects of arterial hypertension on Up4A arterial responsiveness are yet to be determined.
For the present study, we hypothesized that regional differences in Up4A-induced vasoconstriction would be identified in hypertension animals. To test this hypothesis, we designed experiments to characterize regional differences to Up4A in DOCA-salt rats, a salt-dependent experimental model of arterial hypertension [33,37,39]. Basilar, thoracic aorta, small mesenteric and femoral arteries were used based on previous reports showing that hypertension leads to altered reactivity in these vessels and their heterogenous contribution to total peripheral resistance as well as their importance for local control of blood flow. Responses to Up4A and other purinoceptor agonists were determined in these four different arteries from DOCA-salt rat and its control uninephrectomized (Uni) rats. Mechanisms involved in differential vascular regional responses to Up4A were also investigated.
2. Materials and methods
2.1. Drugs and solutions
Acetylcholine (ACh), DOCA, phenylephrine (PE), ATP, UTP, UDP, and α,β-methylene ATP were all purchased from Sigma Chemical Co. (St. Louis, MO, USA). Up4A was obtained from Biolog Life Science Institute (Bremen, Germany). Drugs were dissolved in sterile HPLC grade water. The antibody against P2Y2 was obtained from Abcam (Cambridge, MA, USA) and its respective blocking peptide was from Neuromics (Edina, MN, USA). The antibody against P2Y6 and its respective blocking peptide were obtained from Enzo Life Sciences (Plymouth Meeting, PA, USA). The antibody against P2X1 and its respective blocking peptide were obtained from Alomone Labs (Jerusalem, Israel).
2.2. Animals and experimental design
Male Wistar rats (8 weeks-old, 250–340 g; Harlan Laboratories, Indianapolis, IN, USA) were used in this study. All procedures were performed in accordance with the Guiding Principles in the Care and Use of Animals and approved by the Georgia Health Sciences University Committee on the Use of Animals in Research and Education. The animals were housed two or three per cage on a 12-h light/dark cycle and fed a standard chow diet with water ad libitum.
DOCA-salt hypertension was induced in rats as previously described [35,36]. Briefly, all animals were uninephrectomized under anesthesia and were given no further treatment (Uni rats) or received 1% NaCl plus 0.2% KCl in the drinking water and DOCA silastic pellets (0.2 g/kg), which were implanted subcutaneously in the scapular region (DOCA-salt rats). The duration of treatment was 4 to 5 weeks.
One week before euthanasia, systolic blood pressure (SBP) was measured by the tail-cuff method using a CODA system (Kent Scientific Corporation). At the end of 4 to 5 weeks of treatment, basilar artery, thoracic aorta, second-order mesenteric artery, and femoral artery were removed and submitted to experimental procedures.
2.3. Measurement of isometric force
Vascular isometric force was recorded as previously described [33,37]. After euthanasia, basilar artery, thoracic aorta, femoral artery, and the mesentery were isolated and placed in an ice-cold physiologic salt solution (PSS) containing (mM): 130.0 NaCl, 4.7 KCl, 14.9 NaHCO3, 5.5 glucose, 1.18 KH2PO4, 1.17 MgSO47H2O, 1.6 CaCl22H2O, and 0.026 EDTA. Each artery (thoracic aorta, basilar and femoral arteries, and second-order branches of mesenteric artery) was carefully cleaned of fat and connective tissue under a stereomicroscope. The arteries were then cut into 2 mm rings (several rings were obtained from each animal) and mounted on an isometric Mulvany-Halpen myograph (Danish MyoTech; Aarhus, Denmark) filled with 5 mL PSS and continuously gassed with 95% O2- 5% CO2 while maintaining temperature at 37°C and recorded by a PowerLab 8/SP data acquisition system (ADInstruments). The rings were stretched until an optimal resting tension, which was established from preliminary length-active tension curves, of 2.0 mN (basilar artery), 30 mN (thoracic aorta), 2.3 mN (femoral artery), or 2.0 mN (mesenteric artery), and then allowed to equilibrate for at least 45 min. Arterial integrity was assessed by contracting the segments with a depolarizing concentration of potassium chloride (KCl, 120 mM) and subsequently, with PE (10−6 or 10−5 M) followed by relaxation with ACh (10−6or 10−5 M).
After washing and a new stabilization, Up4A (10−9 – 3×10−5 M), ATP (10−8 - 10−3 M), UTP (10−8 - 10−3 M), UDP (10−8 - 10−3 M) or α,β-methylene-ATP (10−8 – 3×10−5 M) was added cumulatively to the bath until a maximal response was achieved. After the addition of a chosen concentration of the agonist, a plateau response was allowed to develop before the addition of the next concentration.
In order to avoid the possibility of purinoceptor desensitization, rings were exposed to only one purinoceptor agonist. To investigate vasoconstriction to a receptor-independent stimulation, concentration-response curves to KCl (10 – 80 mM) were performed. Accordingly, after the concentration-response curve for a purinoceptor agonist, rings were washed several times with PSS until the tension returned to baseline. Subsequently, the concentration-response curve to KCl was constructed.
2.4. Western blotting for P2 receptor
The protein levels of P2Y2, P2Y6, and P2X1 receptors were quantified using immunoblotting procedures, essentially as described before [27,33,37]. Basilar, mesenteric, and femoral arteries from DOCA-salt and Uni rats were isolated, cleaned from fat, dissected, and frozen in liquid nitrogen. Proteins [basilar artery: 5 µg (for P2X1) or 15 µg (for P2Y2 or P2Y6), mesenteric artery: 20 µg, and femoral artery: 15 µg] extracted from each artery were separated by electrophoresis on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Nonspecific binding sites were blocked with 5% skim milk in Tris-buffered saline solution with Tween (0.1%) for 1 h at 24°C. Membranes were then incubated with antibodies overnight at 4°C. Antibodies were as follows: anti-P2Y2 (1:500; Abcam), anti-P2Y6 (1:500; Enzo Life Sciences), anti- P2X1 (1:500; alomone labs), and β-actin (1:15,000; Sigma). Specificity of the antibody was assessed using blocking peptides according to the manufacturer’s instructions. After incubation with secondary antibodies, signals were revealed with chemiluminesence, visualized by autoradiography, and quantified densitometrically. Results were normalized by β-actin expression and expressed as a percentage of control. The β-actin expression was similar between DOCA-salt and Uni groups in each artery (data not shown).
2.5. Data analysis and statistics
Arterial contractions recorded from the myograph were expressed as changes in the displacement from baseline in mN and were represented as percent maximum contraction to 120 mM KCl (% contraction). The contractions induced by 120 mM KCl were similar between DOCA-salt and Uni groups in each group of arterial rings (Table 1). Agonist concentration-response curves were fitted using a nonlinear interactive fitting program (Graph Pad Prism 5.0; GraphPad Software Inc., San Diego, CA, USA). Data are expressed as means ± SEM. Statistical analysis of the concentration-response curves was performed by using 2-way analysis of variance (ANOVA) for comparison between the groups. When compared to control Uni group, data were analyzed using ANOVA with post-hoc Bonferroni testing or Student’s t-test. Western blot data were analyzed by 1-sample t test. The values of P < 0.05 were considered statistically significant.
Table 1.
Force induced by 120 mM KCl in various arterial rings from Uni and DOCA-salt rats.
| Artery | Uni (mN) | DOCA-salt (mN) |
|---|---|---|
| Basilar | 6.5 ± 0.4 (32) | 5.5 ± 0.5 (34) |
| Thoracic aorta | 25.4 ± 1.4 (25) | 26.1 ± 1.3 (22) |
| Second-order Mesenteric | 18.8 ± 0.8 (40) | 17.6 ± 0.8 (37) |
| Femoral | 19.0 ± 1.9 (28) | 14.7 ± 1.4 (29) |
Values are means ± SEM and represent contractile responses (in mN) to 120 mM KCl. Number of determinations is shown within parentheses.
3. Results
3.1. General parameters
At 3 or 4 weeks, DOCA-salt rats displayed higher systolic blood pressure in comparison with Uni rats (192 ± 3 mmHg, n=34 vs. 134 ± 2 mmHg, n=37, P < 0.001; respectively). At the time of the experiment, the body weight of the DOCA-salt rats was lower than that of Uni rats (333.2 ± 7.4 g, n=34 vs. 412.3 ± 4.9 g, n=37, P < 0.001; respectively).
3.2. Contractile response induced by isotonic K+
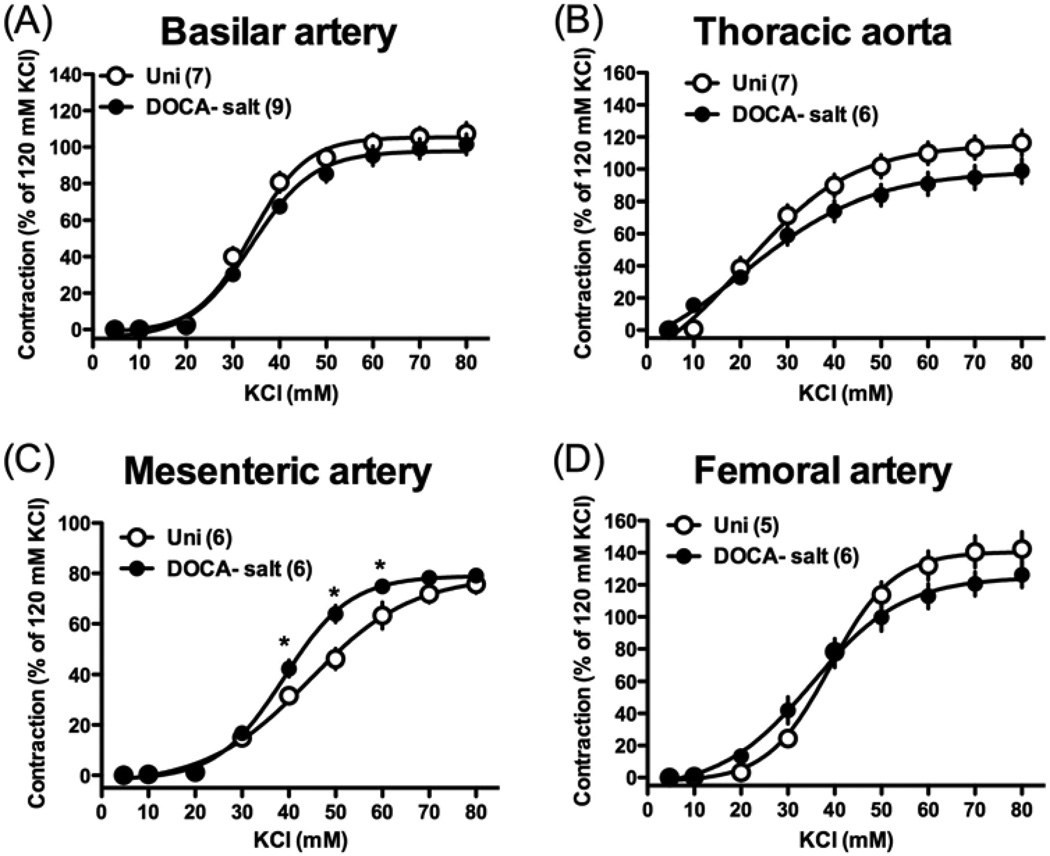
To investigate a contractile response induced by receptor-independent stimulation, we performed concentration-response curves to KCl, which is known to cause membrane depolarization and stimulate Ca2+ entry through voltage-gated Ca2+ channels [40]. As shown in Fig. 1, exposure of all arterial rings to isotonic high K+ solution (10–80 mM) led to a concentration-dependent increase in tension in the normotensive Uni and hypertensive DOCA-salt groups. The maximal contractile responses induced by high K+ (Emax; % of 120 mM KCl-induced contraction) were similar in basilar artery [101.5 ± 5.6 %, (n=9) and 107.5 ± 5.9 %, (n=7), P > 0.05], thoracic aorta [98.8 ± 7.7 %, (n=6) and 116.4 ± 7.9 %, (n=7), P > 0.05], mesenteric artery [79.1 ± 2.2 %, (n=6) and 75.7 ± 3.5 %, (n=6), P > 0.05], and femoral artery [126.2 ± 7.8 %, (n=6) and 142.3 ± 10.7 %, (n=5), P > 0.05] from DOCA-salt and Uni rats, respectively. The EC50 values (mM) for the high K+-induced contractions were similar in basilar artery [35.5 ± 1.7, (n=9) and 31.5 ± 2.5, (n=7), P > 0.05], thoracic aorta [28.2 ± 1.6, (n=6) and 25.6 ± 2.0, (n=7), P > 0.05], mesenteric artery [38.4 ± 1.6, (n=6) and 41.7 ± 1.7, (n=6), P > 0.05], and femoral artery [36.6 ± 2.0, (n=6) and 39.9 ± 0.02, (n=5), P > 0.05] from DOCA-salt and Uni rats, respectively.
Fig. 1.
Concentration-response curves for isotonic high-K+ solution in basilar artery (A), thoracic aorta (B), mesenteric artery (C), and femoral artery (D) from DOCA-salt hypertensive and control Uni rats. Data are means ± SEM. Number of determinations is shown within parentheses. *P < 0.05, DOCA-salt vs. Uni group.
3.3. Contractile response induced by Up4A
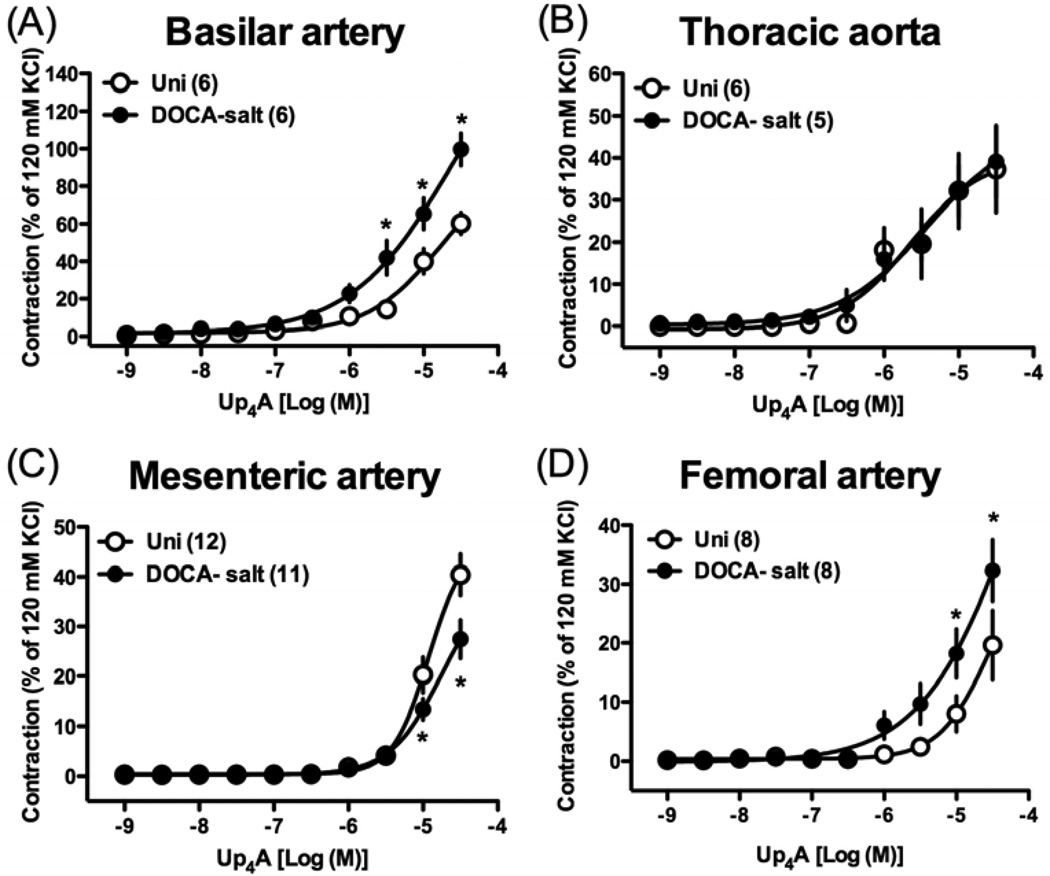
Cumulative administration of Up4A (10−9 – 3×10−5 M) induced concentration-dependent contractions in all arterial rings from both DOCA-salt and Uni rats (Fig. 2). In DOCA-salt rats (vs. Uni rats), Up4A-induced contractile response was significantly increased in basilar artery (Fig. 2A), unchanged in thoracic aorta (Fig. 2B), significantly decreased in mesenteric artery (Fig. 2C), and significantly increased in femoral artery (Fig. 2D).
Fig. 2.
Concentration-response curves for Up4A in basilar artery (A), thoracic aorta (B), mesenteric artery (C), and femoral artery (D) from DOCA-salt hypertensive and control Uni rats. Data are means ± SEM. Number of determinations is shown within parentheses. *P < 0.05, DOCA-salt vs. Uni group.
3.4. Contractile response induced by other nucleotides
Since Up4A contains both purine and pyrimidine moieties, we next investigated whether contractile responses induced by other nucleotides were altered in these four different arteries from DOCA-salt rats.
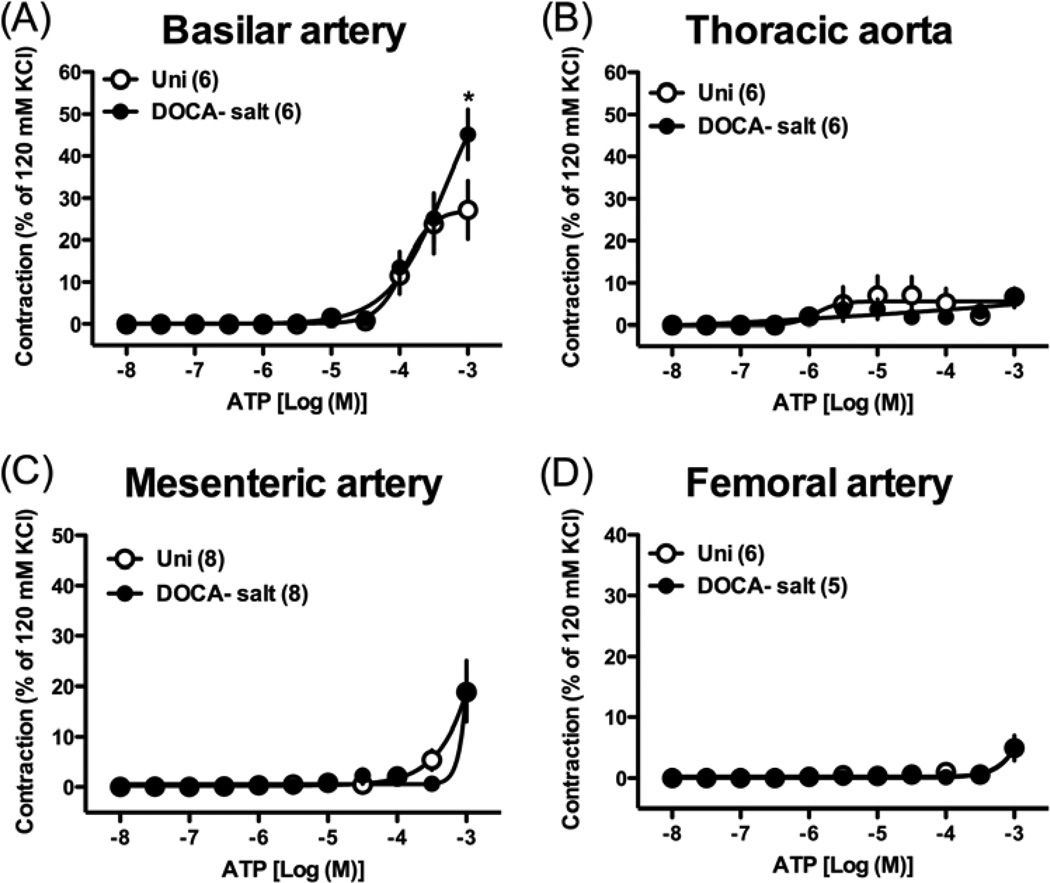
The contractile response induced by the maximal concentration of ATP (i.e., 10−3 M) was significantly augmented in basilar arteries from DOCA-salt rats compared to that in than in arteries from Uni rats (Fig. 3A). Exposure of thoracic aorta (Fig. 3B), mesenteric (Fig. 3C), and femoral arteries (Fig. 3D) to ATP produced very weak contractions, which were similar between the two groups.
Fig. 3.
Concentration-response curves for ATP in basilar artery (A), thoracic aorta (B), mesenteric artery (C), and femoral artery (D) from DOCA-salt hypertensive and control Uni rats. Data are means ± SEM. Number of determinations is shown within parentheses. *P < 0.05, DOCA-salt vs. Uni group.
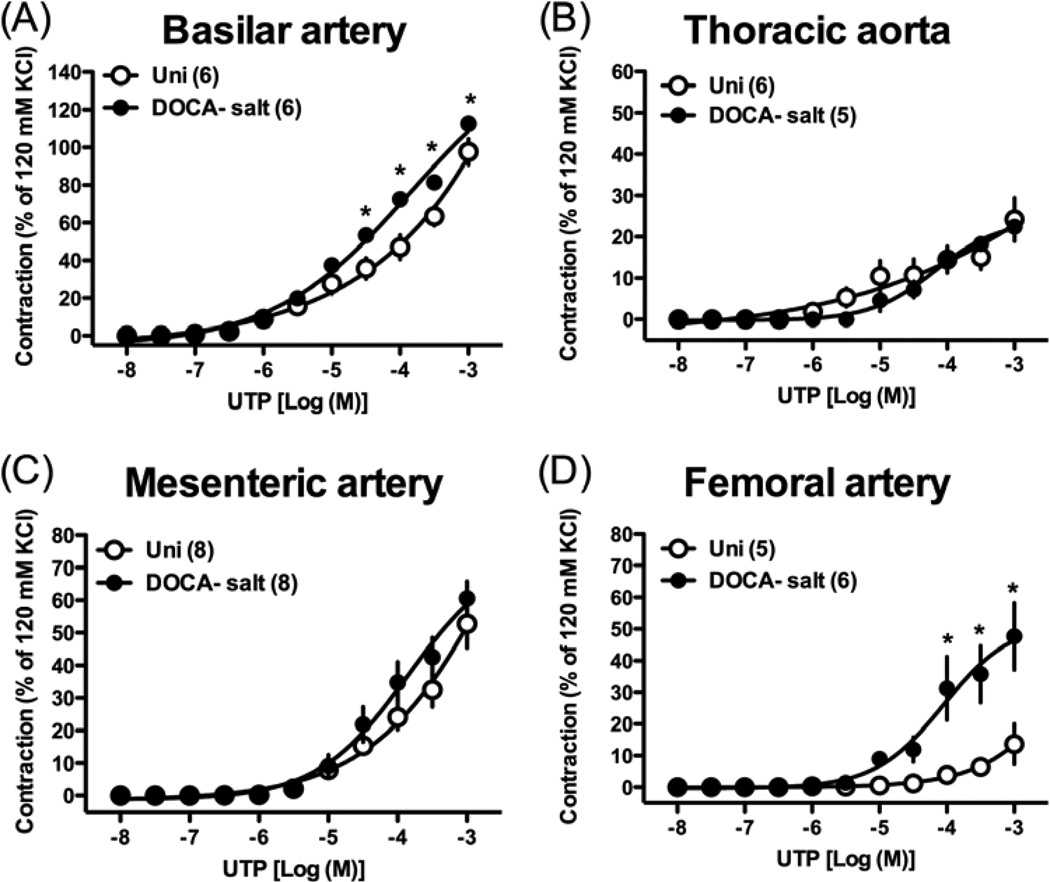
The contractile response induced by UTP was significantly increased in basilar (Fig. 4A) and femoral (Fig. 4D) arterial rings from DOCA-salt rats compared to those in rings from Uni rats. On the other hand, UTP-induced contraction was similar in thoracic aorta (Fig. 4B) and mesenteric artery (Fig. 4C) between the two groups.
Fig. 4.
Concentration-response curves for UTP in basilar artery (A), thoracic aorta (B), mesenteric artery (C), and femoral artery (D) from DOCA-salt hypertensive and control Uni rats. Data are means ± SEM. Number of determinations is shown within parentheses. *P < 0.05, DOCA-salt vs. Uni group.
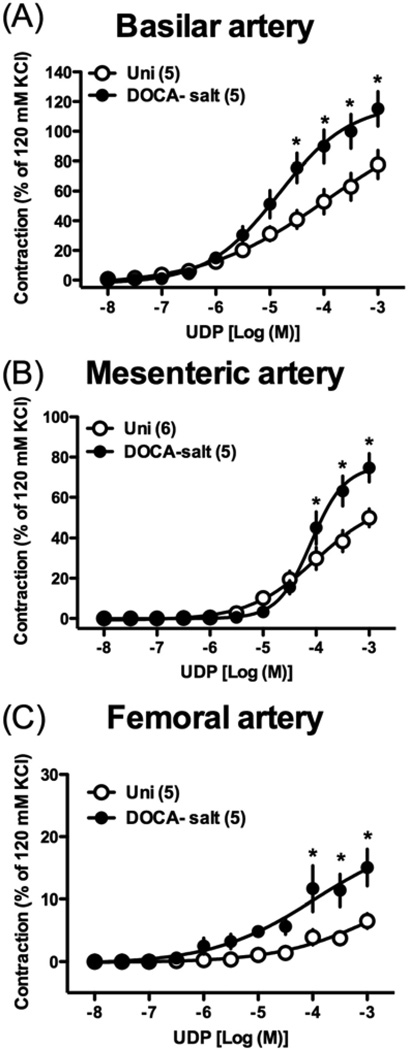
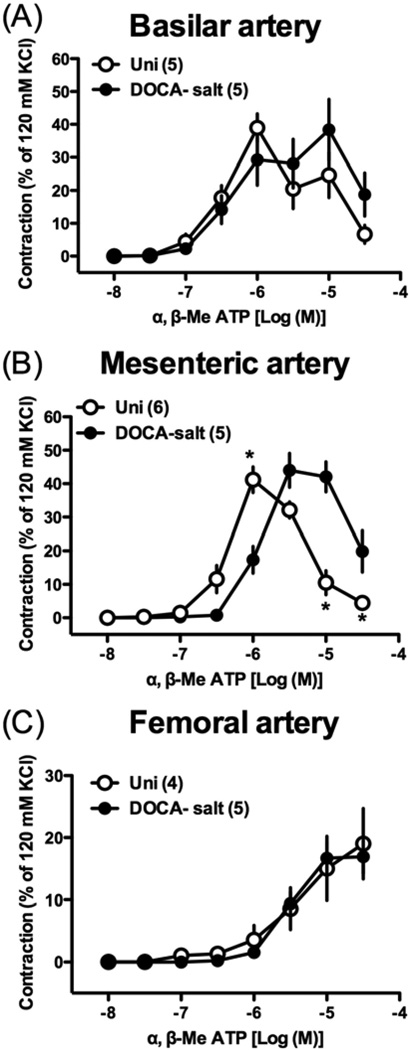
We further investigated the effects of UDP [endogenous P2Y receptor agonist that preferentially activates P2Y6 [25,26] (Fig. 5)] and α,β-methylene ATP [selective P2X1 agonist [41,42] (Fig. 6)] in basilar, mesenteric, and femoral arteries from DOCA-salt rats, which displayed altered responses to Up4A. UDP-induced contraction was significantly greater in basilar (Fig. 5A), mesenteric (Fig. 5B), and femoral (Fig. 5C) arteries from DOCA-salt rats (vs. Uni rats). On the other hand, α,β-methylene ATP-induced contraction was similar in basilar (Fig. 6A) and femoral (Fig. 6C) arteries between the two groups. However, the maximal contractile response of α,β-methylene ATP-induced contraction was reached at a lower concentration in basilar and mesenteric arteries from Uni rats, compared to arteries from DOCA-salt rats (Figs. 6A,B). The concentration-response curve to α,β-methylene ATP was bell-shaped in basilar (Fig. 6A) and mesenteric (Fig. 6B) arteries.
Fig. 5.
Concentration-response curves for UDP in basilar artery (A), mesenteric artery (B), and femoral artery (C) from DOCA-salt hypertensive and control Uni rats. Data are means ± SEM. Number of determinations is shown within parentheses. *P < 0.05, DOCA-salt vs. Uni group.
Fig. 6.
Concentration-response curves for α,β-methylene ATP in basilar artery (A), mesenteric artery (B), and femoral artery (C) from DOCA-salt hypertensive and control Uni rats. Data are means ± SEM. Number of determinations is shown within parentheses. *P < 0.05, DOCA-salt vs. Uni group.
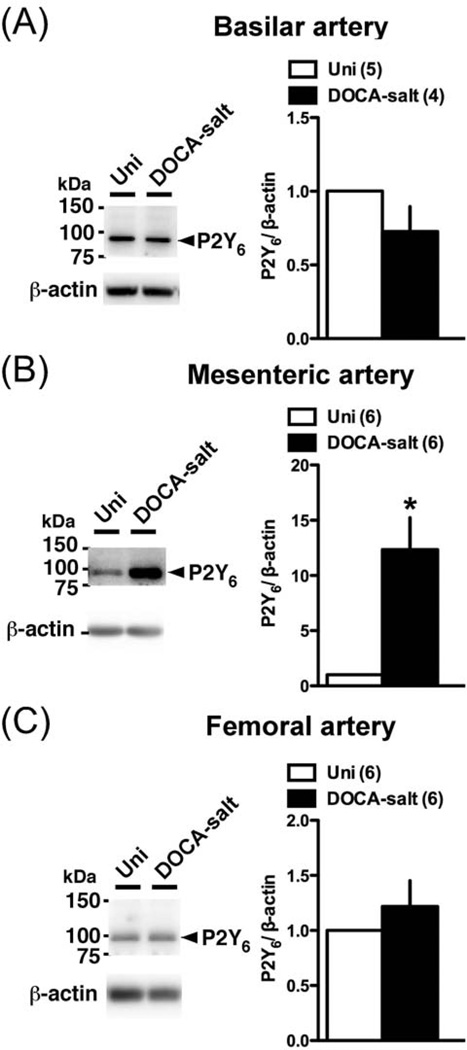
3.5. Protein expression of P2 receptors
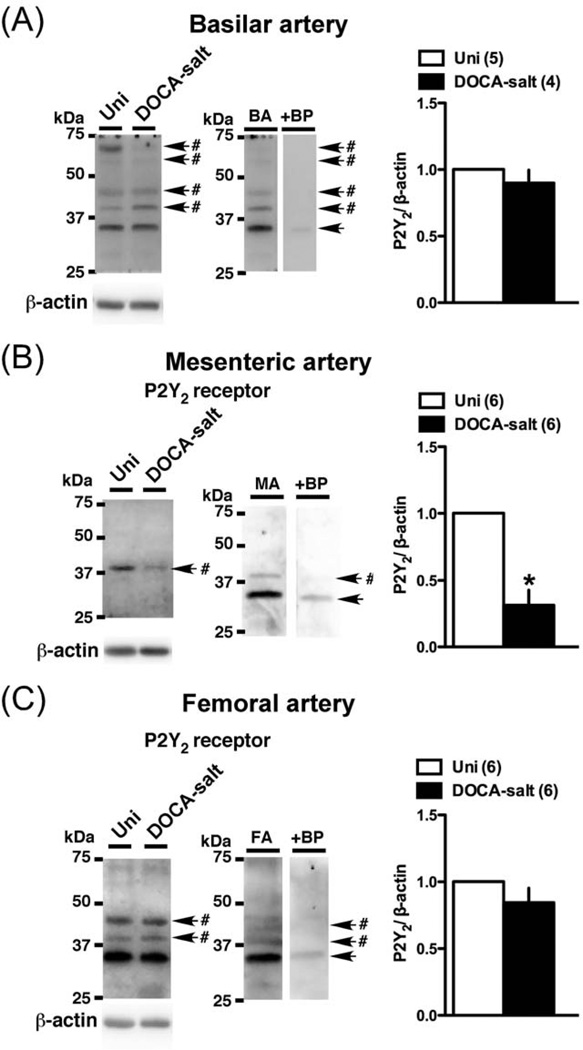
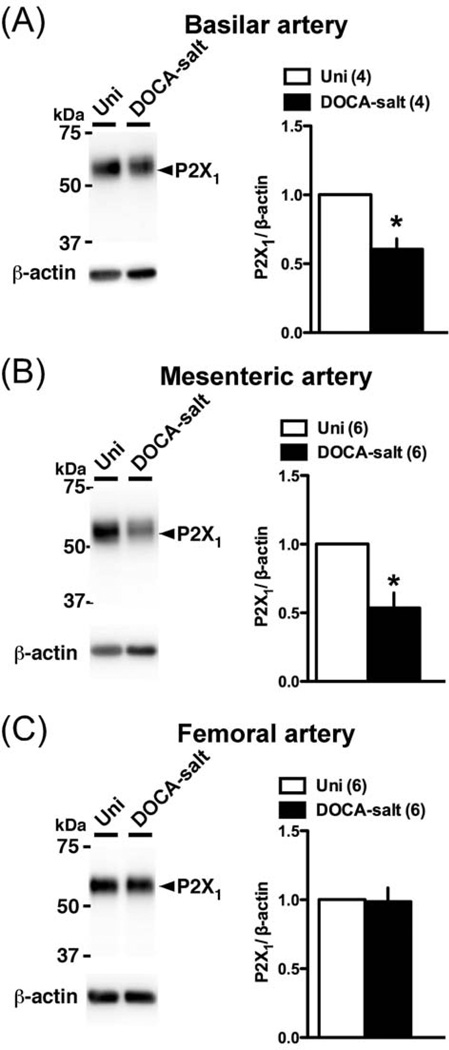
Conceivably, an alteration in P2 receptor expression could underlie the altered Up4A-mediated constriction in basilar, mesenteric, and femoral arteries. Accordingly, we investigated P2 receptors protein expression in these arteries (Figs. 7–9). The P2Y2 polyclonal antibody detected multiple bands, with two bands between 50 and 75 kDa in the basilar artery, two other bands between 37 and 50 kDa (one in mesenteric artery) or (two in basilar and femoral arteries), and one band corresponding to approximately 35 kDa. Multiple bands for P2Y2 receptor have been reported in previous studies [43–45], and could result from posttranscriptional modification of protein, oligomerization of the receptor, or formation of heteromers with other proteins [44,45]. Importantly, the band between the 37 and 75 kDa disappeared, but the band at ~35 kDa remained when the primary antibody was pre-incubated with the appropriate antigenic peptide (Fig. 7). The bands of P2Y6 (Fig. 8) and P2X1 (Fig. 9) were completely lost using each blocking peptide (data not shown). Protein expressions of P2Y2 (Fig. 7A; analyzed sum of four multiple bands, except ~35kDa) and P2Y6 (Fig. 8A) were similar between basilar arteries from DOCA-salt and Uni rats. On the other hand, P2X1 (Fig. 9A) receptor expression was significantly reduced in DOCA-salt basilar artery. Interestingly, an altered expression profile was observed in mesenteric artery from DOCA-salt rats (vs. Uni rats) as follows; reduced P2Y2 (Fig. 7B) and P2X1 (Fig. 9B), and increased P2Y6 (Fig. 8B). In femoral artery, protein expression of P2Y2 (Fig. 7C; analyzed sum of two multiple bands, except ~35kDa), P2Y6 (Fig. 8C), and P2X1 (Fig. 9C) receptors was similar between arteries from DOCA-salt and Uni rats.
Fig. 7.
Western blots for P2Y2 receptor in basilar artery (A), mesenteric artery (B), and femoral artery (C) from DOCA-salt and Uni rats. Left: representative Western blots for P2Y2 and β-actin. #Band disappeared after the incubation of the primary antibody with the respective blocking peptide. BP, blocking peptide. Right: bands were quantified as described in Materials and methods. Ratios were calculated for the optical density of each receptor over that of β-actin. Number of determinations is shown within parentheses. *P < 0.05, DOCA-salt vs. Uni group.
Fig. 9.
Western blots for P2X1 receptor in basilar artery (A), mesenteric artery (B), and femoral artery (C) from DOCA-salt and Uni rats. Left: representative Western blots for P2X1 and β-actin. Right: bands were quantified as described in Materials and methods. Ratios were calculated for the optical density of each receptor over that of β-actin. Number of determinations is shown within parentheses. *P < 0.05, DOCA-salt vs. Uni group.
Fig. 8.
Western blots for P2Y6 receptor in basilar artery (A), mesenteric artery (B), and femoral artery (C) from DOCA-salt and Uni rats. Left: representative Western blots for P2Y6 and β-actin. Right: bands were quantified as described in Materials and methods. Ratios were calculated for the optical density of each receptor over that of β-actin. Number of determinations is shown within parentheses. *P < 0.05, DOCA-salt vs. Uni group.
4. Discussion
Earlier studies have demonstrated that the dinucleotide Up4A produces constriction in various arteries from animals under normal conditions (viz. non-pathophysiological state) [7, 9–13]. In the present study, we have demonstrated that regional differences in Up4A-mediated contraction are observed in DOCA-salt rats, a salt-dependent experimental model of arterial hypertension.
Purinergic receptors are present in most organ systems in the body, and there is accumulating evidence that they play an important role in contractile tone of arteries, as observed in the present study (viz. cerebral arteries [14,15], thoracic aorta [16], mesenteric arteries [17,18], and femoral arteries [19]). Several reports suggest that the activation of both P2X and P2Y receptors on smooth muscle cells leads to constriction in various arteries [21–23]. However, the relative importance of different P2 receptor subtypes in vascular smooth muscle for contraction is complex and delineation of the contributions of specific subtypes is complicated by the lack of highly selective agonists/antagonists. Available data suggest that ionotropic P2X1 receptors and G protein-coupled P2Y2, P2Y4, and P2Y6 receptors mainly contribute to nucleotide-mediated contraction [21–23]. Up4A has both purine and pyrimidine moieties, which can potentially activate purinergic receptors. In rat pulmonary artery, Up4A leads to contraction via P2Y receptors [10]. In perfused rat kidney, Up4A-induced vasoconstriction depends not only on the activation of the P2X1 receptor [7] but also on the activation of the P2Y2 receptor [11]. Therefore, in the present study, we investigated whether DOCA-salt hypertension is associated with abnormal Up4A-induced vasoconstriction. Vasoconstriction activated by other purinoceptors ligands such as ATP (P2X/P2Y agonist), UTP (P2Y2/P2Y4 agonist), UDP (P2Y6 agonist), and α,β-Me ATP (P2X1 agonist), and vascular purinoceptor expression (viz. P2Y2, P2Y6, and P2X1) were also examined.
In the thoracic aorta, the Up4A-induced contraction was similar between DOCA-salt hypertensive and normotensive Uni rats. Moreover, ATP- and UTP-induced contractions were also similar between the two groups. These results suggest that the contractile responses induced by P2 receptor activation are not influenced by chronic systemic hypertension in this representative large conduit artery.
We found that Up4A-induced contraction was increased in basilar and femoral arteries, whereas contraction to Up4A was reduced in mesenteric arteries from DOCA-salt hypertensive rats, compared to Uni rats. To further investigate vascular purinoceptor signaling, we examined contractile responses induced by ATP, UTP, UDP, and α,β-Me ATP. Pharmacological studies coupled with expression of cloned receptors in rats indicate that P2Y2 and P2Y4 are selective for ATP and UTP, whereas P2Y6 favors UDP [17,46]. α,β-Me ATP is a full agonist at arterial smooth muscle P2X receptors and responses are rapidly inactivated in the continued presence of the agonist [47]. This native phenotype closely resembles that of recombinant P2X1 receptors, which are expressed at high levels by arterial smooth muscle cells [48]. In the present study, we found that in basilar, mesenteric, and femoral arteries from DOCA-salt hypertensive rats (vs. Uni group): that 1) ATP-induced contraction, which induces a relatively weak in accordance to previous reports [10,49], is increased in basilar artery, but not in mesenteric and femoral arteries, 2) UTP-induced contraction is increased in basilar and femoral arteries, but not in mesenteric artery, 3) UDP-induced contraction is increased in basilar, mesenteric, and femoral arteries, and 4) higher concentrations of α,β-Me ATP are needed to contraction in mesenteric arteries, whereas contraction induced by α,β-Me ATP is not changed in femoral artery. These results suggest that enhanced Up4A-induced contraction in basilar and femoral arteries from hypertensive rats may be attributable to P2Y signaling rather than P2X1 signaling.
In the present study, we found in DOCA-salt group (vs. Uni group) that 1) receptor expression of P2Y2 and P2Y6 did not change, although P2X1 receptor expression was decreased in basilar artery, 2) downregulation of P2Y2 and P2X1 and upregulation of P2Y6 were seen in mesenteric artery, and 3) the receptor expression of P2Y2, P2Y6, and P2X1 did not change in femoral artery. These results suggest that the enhancement of Up4A-induced contraction in basilar and femoral arteries may alter down-stream pathways upon P2Y receptor stimulation rather than receptor expression. Various down-stream pathways (which are related to vasoconstriction) are activated by P2Y receptor stimulation, such as MAPK and Rho kinase [8,50]. Moreover, several reports have demonstrated that these signaling pathways are altered in arteries from DOCA-salt hypertensive animals [5,35,51]. Indeed, our recent study showed that enhanced Up4A-induced contraction in DOCA-salt renal artery was reduced by an ERK pathway inhibitor, and Up4A-stimulated ERK activation was increased in renal artery from DOCA-salt rats [27].
In contrast to enhanced Up4A-induced contraction in basilar and femoral arteries, a reduced response to Up4A was observed in mesenteric artery from DOCA-salt rats. Although downregulation of P2Y2 and P2X1 may partially contribute to the reduced Up4A-induced contraction in mesenteric artery from DOCA-salt rats, other receptor(s), including an unknown receptor for Up4A may also contribute to this response, since ATP- and UTP-induced contractions were similar in mesenteric arteries from DOCA-salt and Uni rats. Since augmented P2Y6 signaling (UDP-induced contraction and P2Y6 upregulation) was seen in DOCA-salt mesenteric artery, decreased Up4A-induced response is not due to decreased P2Y6-mediated signaling. The existence of specific dinucleotide phosphate receptors has been proposed [52,53]. However, the specificity of dinucleotide receptors and their implication into multiple extracellular signals are still beginning to be understood.
In the present study, we demonstrated that arterial hypertension is associated with heterogenous Up4A-induced arterial contraction. The total peripheral resistance (TPR) is increased in the DOCA-salt rat model [54]. In the present study, Up4A-induced contraction was similar in thoracic aorta, which has a small contribution to TPR, from control and DOCA-salt rats. On the other hand, in small mesenteric artery (which greatly contributes to TPR), Up4A-induced contraction was lower in the DOCA-salt group. Considering our data, we speculate that the vasoconstriction induced by this dinucleotide does not contribute to increased peripheral resistance in this hypertensive model. Arterial hypertension is one of the main risk factors for cerebrovascular diseases [55]. In the cerebral circulation, large arteries such as the basilar artery make important contributions to total cerebral vascular resistance and are major determinants of local microvascular pressure [35]. On the other hand, the femoral arteries supply blood to the muscles of the lower extremities, which may play an important role in regulation of the peripheral arterial resistance. Enhanced Up4A-induced contraction in these arteries may contribute to the increased resistance of cerebral and peripheral circulation during chronic arterial hypertension. Up4A, therefore, potentially plays an important role in the regulation of vascular tone. Further study using other experimental models of arterial hypertension (e.g. angiotensin II-induced hypertension), and at various stages of hypertension (initial and established phases), as well as additional in vitro and in vivo techniques, will lead to a better understanding of the pathophysiological roles of Up4A.
There is a limitation of the present study that should be mentioned. The concentrations of extracellular nucleotides are regulated by ectonucleotidases (e.g., nucleoside triphosphate diphosphohydrolase-1 and -2) and these enzymes are expressed in the vasculature [56, 57]. The enzymatic breakdown of dinucleoside polyphosphates leads to generation of mononucleotides and nucleotides that, in turn, are biologically active. A possible interpretation of the present results is that metabolic inter-conversion of nucleotides may be altered in the vasculature of hypertensive animals. Indeed, there is evidence that ecto-ATPase activity is increased in platelets from hypertensive patients [58]. However, the relationship between purinergic ligand-receptor interaction (activation) and vascular tone is complex since not only dinucleotide but also its metabolites can affect vascular tone. Further studies will be required to investigate these points.
5. Conclusions
In summary, the present results suggest that the existence of vascular regional differences in Up4A-induced contraction in DOCA-salt hypertensive rats. Understanding the regulation of vascular tone by Up4A and its signal-transduction may be of extreme value to understanding the pathophysiology of hypertension. We believe that our findings should stimulate further interest in the Up4A system as a potential therapeutic target in hypertension-associated vascular dysfunctions.
Acknowledgements
This study was supported in part by the NIH (Grants: R01 HL071138 and R01 DK083685), and by the Naito Foundation Japan.
Footnotes
Conflict of interest disclosure.
No conflict of interest to disclose.
References
- 1.Bohr DF, Webb RC. Vascular smooth muscle membrane in hypertension. Annu Rev Pharmacol Toxicol. 1988;28:389–409. doi: 10.1146/annurev.pa.28.040188.002133. [DOI] [PubMed] [Google Scholar]
- 2.Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder. Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 3.Vanhoutte PM, Feletou M, Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ajay M, Achike FI, Mustafa MR. Modulation of vascular reactivity in normal, hypertensive and diabetic rat aortae by a non-antioxidant flavonoid. Pharmacol Res. 2007;55:385–391. doi: 10.1016/j.phrs.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Lee DL, Webb RC, Jin L. Hypertension and RhoA/Rho-kinase signaling in the vasculature: highlights from the recent literature. Hypertension. 2004;44:796–799. doi: 10.1161/01.HYP.0000148303.98066.ab. [DOI] [PubMed] [Google Scholar]
- 6.Denniss SG, Jeffery AJ, Rush JW. RhoA-Rho kinase signaling mediates endothelium- and endoperoxide-dependent contractile activities characteristic of hypertensive vascular dysfunction. Am J Physiol Heart Circ Physiol. 2010;298:H1391–H1405. doi: 10.1152/ajpheart.01233.2009. [DOI] [PubMed] [Google Scholar]
- 7.Jankowski V, Tolle M, Vanholder R, Schonfelder G, van der Giet M, Henning L, et al. Uridine adenosine tetraphosphate: a novel endothelium-derived vasoconstrictive factor. Nat Med. 2005;11:223–227. doi: 10.1038/nm1188. [DOI] [PubMed] [Google Scholar]
- 8.Schuchardt M, Prufer J, Prufer N, Wiedon A, Huang T, Chebli M, et al. The endothelium-derived contracting factor uridine adenosine tetraphosphate induces P2Y(2)-mediated pro-inflammatory signaling by monocyte chemoattractant protein-1 formation. J Mol Med. 2011;89:799–810. doi: 10.1007/s00109-011-0750-6. [DOI] [PubMed] [Google Scholar]
- 9.Linder AE, Tumbri M, Linder FF, Webb RC, Leite R. Uridine adenosine tetraphosphate induces contraction and relaxation in rat aorta. Vascul Pharmacol. 2008;48:202–207. doi: 10.1016/j.vph.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gui Y, Walsh MP, Jankowski V, Jankowski J, Zheng XL. Up4A stimulates endothelium-independent contraction of isolated rat pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2008;294:L733–L738. doi: 10.1152/ajplung.00403.2007. [DOI] [PubMed] [Google Scholar]
- 11.Tolle M, Schuchardt M, Wiedon A, Huang T, Klockel L, Jankowski J, et al. Differential effects of uridine adenosine tetraphosphate on purinoceptors in the rat isolated perfused kidney. Br J Pharmacol. 2010;161:530–540. doi: 10.1111/j.1476-5381.2010.00914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen PB, Hristovska A, Wolff H, Vanhoutte P, Jensen BL, Bie P. Uridine adenosine tetraphosphate affects contractility of mouse aorta and decrease blood pressure in conscious rats and mice. Acta Physiol. 2010;200:171–179. doi: 10.1111/j.1748-1716.2010.02135.x. [DOI] [PubMed] [Google Scholar]
- 13.Jankowski V, Patzak A, Herget-Rosenthal S, Tran TN, Lai EY, Gunthner T, et al. Uridine adenosine tetraphosphate acts as an autocrine hormone affecting glomerular filtration rate. J Mol Med. 2008;86:333–340. doi: 10.1007/s00109-008-0306-6. [DOI] [PubMed] [Google Scholar]
- 14.Malmsjo M, Hou M, Pendergast W, Erlinge D, Edvinsson L. The stable pyrimidines UDPbetaS and UTPgammaS discriminate between contractile cerebrovascular P2 receptors. Eur J Pharmacol. 2003;458:305–311. doi: 10.1016/s0014-2999(02)02787-5. [DOI] [PubMed] [Google Scholar]
- 15.Miyagi Y, Kobayashi S, Nishimura J, Fukui M, Kanaide H. Dual regulation of cerebrovascular tone by UTP:P2U receptor-mediated contraction and endothelium-dependent relaxation. Br J Pharmacol. 1996;118:847–856. doi: 10.1111/j.1476-5381.1996.tb15477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guns PJ, Korda A, Crauwels HM, Van Assche T, Robaye B, Boeynaems JM, et al. Pharmacological characterization of nucleotide P2Y receptors on endothelial cells of the mouse aorta. Br J Pharmacol. 2005;146:288–295. doi: 10.1038/sj.bjp.0706326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ralevic V, Burnstock G. Effects of purines and pyrimidines on the rat mesenteric arterial bed. Circ Res. 1991;69:1583–1590. doi: 10.1161/01.res.69.6.1583. [DOI] [PubMed] [Google Scholar]
- 18.Demel SL, Galligan JJ. Impaired purinergic neurotransmission to mesenteric arteries in deoxycorticosterone acetate-salt hypertensive rats. Hypertension. 2008;52:322–329. doi: 10.1161/HYPERTENSIONAHA.108.110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kluess HA, Buckwalter JB, Hamann JJ, DeLorey DS, Clifford PS. Frequency and pattern dependence of adrenergic and purinergic vasoconstriction in rat skeletal muscle arteries. Exp Physiol. 2006;91:1051–1058. doi: 10.1113/expphysiol.2006.034694. [DOI] [PubMed] [Google Scholar]
- 20.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 21.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burnstock G. Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol Rep. 2008;60:12–20. [PubMed] [Google Scholar]
- 23.Burnstock G. Purinergic regulation of vascular tone and remodeling. Auton Autacoid Pharmacol. 2009;29:63–72. doi: 10.1111/j.1474-8673.2009.00435.x. [DOI] [PubMed] [Google Scholar]
- 24.Burnstock G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 25.Guns PJ, Hendrickx J, Van Assche T, Fransen P, Bult H. P2Y receptors and atherosclerosis in apolipoprotein E-deficient mice. Br J Pharmacol. 2010;159:326–336. doi: 10.1111/j.1476-5381.2009.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, Boeynams JM, et al. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol. 2008;74:777–784. doi: 10.1124/mol.108.046904. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto T, Tostes RC, Webb RC. Uridine adenosine tetraphosphate-induced contraction is increased in renal but not pulmonary arteries from deoxycorticosterone acetate-salt hypertensive rats. Am J Physiol Heart Circ Physiol. 2011;301:H409–H417. doi: 10.1152/ajpheart.00084.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jankowski V, Meyer AA, Schlattmann P, Gui Y, Zheng XL, Stamcou I, et al. Increased uridine adenosine tetraphosphate contractions in plasma of juvenile hypertensives. Arterioscler Thromb Vasc Biol. 2007;27:1776–1781. doi: 10.1161/ATVBAHA.107.143958. [DOI] [PubMed] [Google Scholar]
- 29.Miller AA, Budzyn K, Sobey CG. Vascular dysfunction in cerebrovascular disease: mechanisms and therapeutic intervention. Clin Sci. 2010;119:1–17. doi: 10.1042/CS20090649. [DOI] [PubMed] [Google Scholar]
- 30.Daneshtalab N, Smeda JS. Alterations in the modulation of cerebrovascular tone and blood flow by nitric oxide synthase in SHRsp with stroke. Cardiovasc Res. 2010;86:160–168. doi: 10.1093/cvr/cvp395. [DOI] [PubMed] [Google Scholar]
- 31.Mori Y, Ohyanagi M, Koida S, Ueda A, Ishiko K, Iwasaki T. Effects of endothelium-derived hyperpolarizing factor and nitric oxide on endothelial function in femoral resistance arteries of spontaneous hypertensive rats. Hypertens Res. 2006;29:187–195. doi: 10.1291/hypres.29.187. [DOI] [PubMed] [Google Scholar]
- 32.Kanagy NL, Webb RC. Increased responsiveness and decreased expression of G proteins in deoxycorticosterone hypertension. Hypertension. 1996;27:740–745. doi: 10.1161/01.hyp.27.3.740. [DOI] [PubMed] [Google Scholar]
- 33.Giachini FR, Sullivan JC, Lima VV, Carneiro FS, Fortes ZB, Pollock DM, et al. Extracellular signal-regulated kinase 1/2 activation, via downregulation of mitogen-activated protein kinase phosphatase 1, mediates sex differences in deoxycorticosterone-salt hypertension vascular reactivity. Hypertension. 2010;55:172–179. doi: 10.1161/HYPERTENSIONAHA.109.140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Giachini FR, Lima VV, Carneiro FS, Tostes RC, Webb RC. Decreased cGMP level contributes to increased contraction in arteries from hypertensive rats: role of phosphodiesterase 1. Hypertension. 2011;57:655–663. doi: 10.1161/HYPERTENSIONAHA.110.164327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 36.Kamouchi M, Kitazono T, Nagao T, Fujishima M, Ibayashi S. Role of CA(2+)-activated K+ channels in the regulation of basilar arterial tone in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2002;29:575–581. doi: 10.1046/j.1440-1681.2002.03688.x. [DOI] [PubMed] [Google Scholar]
- 37.Lima VV, Giachini FR, Choi H, Carneiro FS, Carneiro ZN, Fortes ZB, et al. Impaired vasodilator activity in deoxycorticosterone acetate-salt hypertension is associated with increased protein O-GlucNAcylation. Hypertension. 2009;53:166–174. doi: 10.1161/HYPERTENSIONAHA.108.116798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kluess HA, Buckwalter JB, Hamann JJ, Clifford PS. Elevated temperature decreases sensitivity of P2X purinergic receptors in skeletal muscle arteries. J Appl Physiol. 2005;99:995–998. doi: 10.1152/japplphysiol.00319.2005. [DOI] [PubMed] [Google Scholar]
- 39.Ebrahim Z, Yellon DM, Baxter GF. Attenuated cardioprotective response to bradykinin, but not classical ischaemic preconditioning, in DOCA-salt hypertensive left ventricular hypertrophy. Pharmacol Res. 2007;55:42–48. doi: 10.1016/j.phrs.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Khalil RA, Breemen C. Mechanisms of calcium mobilization and homeostatis in vascular smooth muscle and their relevance to hypertension. In: Laragh JH, Brenner BM, editors. Hypertesnion: pathophysiology, diagnosis and management. New York: Raven Press; 1995. pp. 523–540. [Google Scholar]
- 41.Harhun MI, Povstyan OV, Gordienko DV. Purinoceptor-mediated current in myocytes from renal resistance arteries. Br J Pharmacol. 2010;160:987–997. doi: 10.1111/j.1476-5381.2010.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inscho EW, Cook AK, Webb RC, Jin LM. Rho-kinase inhibition reduces pressure-mediated autoregulatory adjustments in afferent arteriolar diameter. Am J Physiol Renal Physiol. 2009;296:F590–F597. doi: 10.1152/ajprenal.90703.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim CH, Kim SS, Choi JY, Shin JH, Kim JY, Namkung W, et al. Membrane-specific expression of functional purinergic receptors in normal human nasal epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L835–L842. doi: 10.1152/ajplung.00285.2003. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Karlsson L, Moses S, Hultgardh-Nisson A, Andersson M, Boma C, et al. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Flores RV, Hernandez-Perez MG, Aquino E, Garrad RC, Weisman GA, Gonzalez FA. Agonist-induced phosphorylation and desensitization of the P2Y2 nucleotide receptor. Mol Cell Biochem. 2005;280:35–45. doi: 10.1007/s11010-005-8050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boarder MR, Hourani SM. The regulation of vascular function by P2 receptors: multiple sites and multiple receptors. Trends Pharmacol Sci. 1998;19:99–107. doi: 10.1016/s0165-6147(98)01170-5. [DOI] [PubMed] [Google Scholar]
- 47.Lewis CJ, Evans RJ. Lack of run-down of smooth muscle P2X receptor currents recorded with the amphotericin permeabilized patch technique, physiological and pharmacological characterization of the properties of mesenteric artery P2X receptor ion channels. Br J Pharmacol. 2000;131:1659–1666. doi: 10.1038/sj.bjp.0703744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gever JR, Cockayne DA, Dillon MP, Burnstock G, Ford AP. Pharmacology of P2X channels. Pfluger Arch. 2006;452:513–587. doi: 10.1007/s00424-006-0070-9. [DOI] [PubMed] [Google Scholar]
- 49.Malmsjo M, Adner M, Harden TK, Pendergast W, Edvinsson L, Erlinge D. The stable pyrimidines UDPbetaS and UTPgammaS discriminate between the P2 receptors that mediate vascular contraction and relaxation of the rat mesenteric artery. Br J Pharmacol. 2000;131:51–56. doi: 10.1038/sj.bjp.0703536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Vaillant N, Gadeau AP, Desgranges C, et al. P2Y(1), P2Y(2), P2Y(4), and P2Y(6) receptors are coupled to Rho and Rho kinase activation in vascular myocytes. Am J Physiol Heart Circ Physiol. 2000;278:H1751–H1761. doi: 10.1152/ajpheart.2000.278.6.H1751. [DOI] [PubMed] [Google Scholar]
- 51.Wehrwein EA, Northcott CA, Loberg RD, Watts SW. Rho/Rho kinase and phosphoinositide 3-kinase are parallel pathways in the development of spontaneous arterial tone in deoxycorticosterone acetate-salt hypertension. J Pharmacol Exp Ther. 2004;309:1011–1019. doi: 10.1124/jpet.103.062265. [DOI] [PubMed] [Google Scholar]
- 52.Delicado EG, Miras-Portugal MT, Carrasquero LM, Leon D, Perez-Sen R, Gualix J. Dinucleotide polyphosphates and their interaction with other nucleotide signaling pathways. Plfugers Arch. 2006;452:563–572. doi: 10.1007/s00424-006-0066-5. [DOI] [PubMed] [Google Scholar]
- 53.Flores NA, Stavrou BM, Sheridan DJ. The effects of diadenosine polyphosphate on the cardiovascular system. Cardiovasc Res. 1999;42:15–26. doi: 10.1016/s0008-6363(99)00004-8. [DOI] [PubMed] [Google Scholar]
- 54.Yu M, Gopalakrishnan V, McNeill JR. Role of endothelin and vasopressin in DOCA-salt hypertension. Br J Pharmacol. 2001;132:1447–1454. doi: 10.1038/sj.bjp.0703958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Collins R, Peto R, MacMahon S, Hebert P, Fiebach NH, Eberlein KA, et al. Blood pressure, stroke, and coronary heart disease. Part 2, Short-term reductions in blood pressure: overview of randomised drug trials in their epidemiological context. Lancet. 1990;335:827–838. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- 57.Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D’Orleans-Juste P, et al. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res. 2010;85:204–213. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lunkes GI, Lunkes D, Stefanello F, Morsch A, Morsch VM, Mazzanti CM, et al. Enzymes that hydrolyze adenine nucleotides in diabetes and associated pathologies. Thromb Res. 2003;109:189–194. doi: 10.1016/s0049-3848(03)00178-6. [DOI] [PubMed] [Google Scholar]