Abstract
Lim domain only protein 2 (Lmo2) is a transcription factor that plays a critical role in the development of T-acute lymphoblastic leukemia (T-ALL). A previous report established a link between Lmo2 expression and the nuclear presence of oncogenic Janus kinase 2 (JAK2), a non-receptor protein tyrosine kinase. The oncogenic JAK2 kinase phosphorylates histone H3 on Tyr 41 that leads to the relief of Lmo2 promoter repression and subsequent gene expression. Similar to JAK2, constitutive activation of lymphocyte-specific protein tyrosine kinase (Lck) has been implicated in lymphoid malignancies. However, it is not known whether oncogenic Lck regulates Lmo2 expression through a similar mechanism. We show here that Lmo2 expression is significantly elevated in T cell leukemia LSTRA overexpressing active Lck kinase and in HEK 293 cells expressing oncogenic Y505FLck kinase. Nuclear localization of active Lck kinase was confirmed in both Lck-transformed cells by subcellular fractionation and immunofluorescence microscopy. More importantly, in contrast to oncogenic JAK2, oncogenic Lck kinase does not result in significant increase in histone H3 phosphorylation on Tyr 41. Instead, chromatin immunoprecipitation experiment shows that oncogenic Y505FLck kinase binds to the Lmo2 promoter in vivo. This result raises the possibility that oncogenic Lck may activate Lmo2 promoter through direct interaction.
Keywords: Lck, Lmo2, protein tyrosine kinase, nuclear localization, gene regulation
Introduction
Lim domain only protein 2 (Lmo2) is a transcription factor that plays a critical role in both hematopoietic development and vascular remodeling [1,2]. Lmo2 was first identified because of its association with chromosomal translocations that characterize T-acute lymphoblastic leukemia (T-ALL) and is believed to be one of the major oncogenes involved in the development of T-ALL [2]. Lmo2 is aberrantly expressed in high percentage of T-ALL patients [3,4]. The regulation of Lmo2, therefore, plays a critical role in leukemogenesis [5]. While Lmo2 regulation has been partially characterized in normal hematopoiesis, molecular mechanisms responsible for its abnormal expression in leukemic cells remains largely uncharacterized. A previous report established a link between Lmo2 expression and the nuclear presence of oncogenic Janus kinase 2 (JAK2), a non-receptor protein tyrosine kinase [6]. However, it is unclear whether other oncogenic protein tyrosine kinases regulate Lmo2 expression through a similar mechanism.
Lymphocyte-specific protein tyrosine kinase (Lck) is a Src family kinase expressed predominantly in T cells and is essential for T cell development and activation [7,8]. In humans, the Lck gene is located at a site of frequent chromosomal aberrations [9]. Similar to JAK2, overexpression and constitutive activation of Lck has been implicated in lymphoid and nonlymphoid malignancies [10,11]. Lck activity is modulated by tyrosine phosphorylation at the positive regulatory Tyr 394 and the negative regulatory Tyr 505 [12]. Mutation of Tyr 505 to Phe (Y505FLck) results in a constitutively active kinase that is oncogenic and transforms fibroblasts in culture [13,14]. Our previous studies have characterized the molecular mechanisms adopted by oncogenic Lck to induce transformation. We have shown that Lck-transformed cells exhibit persistent activation of the JAK kinases and downstream signal transducer and activator of transcription (STAT) proteins [15]. Constitutive STAT5 activation contributes to Lck-induced cell proliferation and resistance to apoptosis [16]. However, blocking STAT5 activity cannot fully reverse Lck-mediated cellular transformation. It points to the existence of STAT5-independent mechanisms involved in malignant transformation by the oncogenic Lck kinase.
Src family kinases are primarily membrane-associated or cytosolic and transmit extracellular signals to the cell nucleus via secondary messengers [17]. Recent reports have described the nuclear localization of Lyn and Fyn, where they function as mediators of euchromatin hypocondensation induced by growth factors [18,19]. Tyrosine kinase-induced reorganization of chromatin structure can potentially result in regulation of gene expression as seen in the case of nuclear oncogenic JAK2 [6]. Supporting this theory, nuclear Src was found to elevate c-Myc expression through direct binding to the promoter region of c-Myc gene in consort with epidermal growth factor receptor (EGFR) and STAT3 in pancreatic cancer cells [20]. It becomes increasingly evident that signaling molecules exert additional functions in distinct intracellular organelles. Here, we examine the nuclear localization of oncogenic Lck and, specifically, address its role in regulating Lmo2 gene expression that may potentially promote Lck-induced oncogenesis.
Materials and methods
Cell lines and culture conditions
Two mouse T cell lines, BYDP and LSTRA, were maintained in RPMI supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in a 5% CO2 incubator [21]. T-REx-293/Y505FLck cell line was established by stable transfection of T-REx-293 with pcDNA5/TO/Y505FLck/myc-His and maintained in DMEM as described previously [16]. Unless specified otherwise, Y505FLck expression was induced by treating T-REx-293/Y505FLck cells with 5 ng/ml of doxycycline (Sigma-Aldrich, St. Louis, MO) for 1 day.
Subcellular fractionation
T-REx-293/Y505FLck cells were washed twice in phosphate-buffered saline, resuspended in hypotonic lysis buffer and then homogenized by passing through a 27-gauge needle. LSTRA cells were resuspended in hypotonic lysis buffer containing 0.5% NP-40. Cytosolic and nuclear fractions from both cell types were obtained through differential centrifugation as described previously [22]. Cytosol was concentrated using vacuum centrifugation to approximately 1/10th the original volume. Nuclear proteins were extracted using RIPA lysis buffer. Purity of fractions was verified by immunoblotting of specific markers. RIPA lysis buffer was also used to prepare whole cell lysates.
Immunoprecipitation and western blotting
For immunoprecipitation experiments, equal amounts of total proteins from the fractions were adjusted to isotonic condition. Samples were then incubated with anti-Lck antibody and western blotting was performed as described previously [23]. Anti-Lamin B1 and anti-pY41 histone H3 antibodies were purchased from Abcam (Cambridge, MA). Antibodies specific for glyceraldehydes 3-phosphate dehydrogenase (GAPDH) and Tyr 394-phosphorylated Lck were from Cell Signaling (Danvers, MA). Anti-Lck and anti-Myc tag antibodies were from Millipore (Billerica, MA) while anti-histone H3 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody dilutions were prepared as recommended by the manufacturers. Signals were detected using either the Luminata Forte Western chemiluminescence reagent (Millipore, Billerica, MA) or by the LI-COR Odyssey infrared imaging system (Lincoln, NE).
Immunofluorescence microscopy
Both LSTRA and doxycycline-treated T-REx-293/Y505FLck cells were processed for immunostaining as described elsewhere [21,22]. Immunostaining was performed with anti-Lck polyclonal antibody (Millipore) and Alexa Fluor 594-conjugated donkey anti-rabbit antibody (Invitrogen Inc., Carlsbad, CA). Nuclei were visualized by DAPI staining (Invitrogen Inc.). Stained cells were viewed with appropriate filters using a fluorescence microscope with a Nikon Metamorph digital imaging system.
Real-time RT-PCR analysis
Total RNAs were extracted by TRIzol (Invitrogen Inc.), treated with RQ1 RNase-free DNase (Promega, Madison, WI), and then reverse transcribed using High Capacity cDNA Reverse transcription Kit (Applied Biosystems Inc., Foster City, CA) into cDNAs. Quantitative real-time PCR using SYBR Green chemistry (Applied Biosystems Inc.) was performed according to standard protocol using an annealing temperature of 60°C for all primer sets. Relative fold values were obtained using ΔΔCT method by normalization to β-actin. Primers for human lmo2 are 5′-CGGCGCCTCTACTACAAACT-3′ (forward) and 5′-GAATCCGCTTGTCACAGGAT-3′ (reverse). Primers for mouse lmo2 are 5’-GACTATCTCAGGCTTTTTGGTC-3’ (forward) and 5’-CACTCCAGGTGATACACTTTGT-3’ (reverse). Primers for human β-actin are 5’-CGCAGAAAACAAGATGAGATTG- 3’ (forward) and 5’-ACCTTCACCGTTCCAGTTTTTA-3’ (reverse). Primers for mouse β-actin are 5’-TTCGTTGCCGGTCCACA-3’ (forward) and 5’-ACCAGCGCAGCGATATCG-3’ (reverse).
Chromatin immunoprecipitation (ChIP)
T-REx-293/Y505FLck cells were formaldehyde crosslinked and subjected to chromatin immunoprecipitation using the EZ-ChIP Magna G kit (Millipore) as per manufacturer's instructions. Immunoprecipitation was performed using anti-Myc 4A6 monoclonal antibody (Millipore) with mouse IgG (Millipore) as a background control. Quantitative real-time PCR was performed on the eluted DNA using the following primers that span the promoter region of lmo2: 5’-CCAGACAAACTCAAATAACGTACACA-3’ (forward) and 5’- AGTGGGTACCATTGTCCCTGTT-3’ (reverse).
Statistical analysis
For quantitative analysis, data are presented as mean ± SD from three independent experiments. The significance of differences was analyzed by student t-test. Differences were considered significant when p<0.05.
Results and discussion
Elevated Lmo2 gene expression in Lck-transformed cells
The oncogenic protein tyrosine kinase Lck and the transcription factor Lmo2 are both implicated in malignant transformation. To determine if oncogenic Lck can modulate Lmo2 expression, we examined two well-characterized Lck-transformed cell lines: LSTRA and T-REx-293/Y505FLck. LSTRA is a mouse T cell leukemia that overexpresses endogenous Lck kinase and exhibits high levels of Lck kinase activity. To analyze the effects of exogenous Lck in nonlymphoid cells, we established the T-REx-293 system to express the constitutively active Y505FLck kinase in a regulated manner. Treatment of T-REx-293/Y505FLck with doxycycline results in maximal expression of Y505FLck within 24 hours [16]. Induced expression of Y505FLck also leads to morphological transformation and elevated cell cycle progression in HEK 293 cells (not shown).
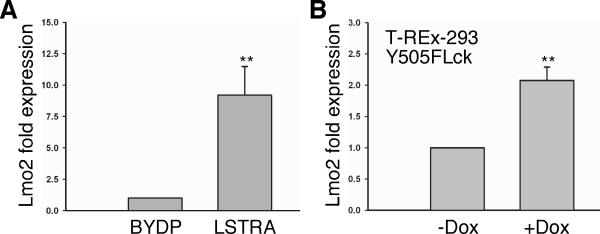
We used quantitative real-time PCR to measure the expression levels of Lmo2. As shown in Fig. 1A, LSTRA cells have a significant increase of Lmo2 transcripts when compared to mouse BYDP T cells that express regular levels of Lck. Similarly, doxycycline-induced expression of Y505FLck also led to enhanced Lmo2 gene expression in HEK 293 cells (Fig. 1B). These results demonstrate that both endogenous and exogenous oncogenic Lck kinases contribute to elevated Lmo2 expression in lymphoid and non-lymphoid cells, respectively. It raises the possibility that Lmo2 is a potential target gene associated with tumorigenesis downstream of the Lck oncogene.
Fig. 1.
Elevated Lmo2 expression in Lck-transformed cells. (A) Total RNA was isolated from mouse BYDP and LSTRA T cells, reverse transcribed, and then subjected to real-time PCR analysis using primers specific for mouse Lmo2 and actin mRNAs. Data from triplicates were normalized to actin and expressed as fold change to BYDP cells. (B) T-REx-293/Y505FLck cells were either left untreated (-) or treated (+) with doxycycline (Dox) to induce the expression of Y505FLck. RNA was isolated and real-time PCR was performed using primers specific for human Lmo2 and actin mRNAs. Data from triplicates were normalized to actin and expressed as fold change to cells that were not treated with doxycycline. Statistical analyses show the results from three independent studies, ** p<0.01.
We showed previously that STAT5 is constitutively active in various Lck-transformed cells [15,16]. However, there is no functional STAT5-binding site identified in the promoter region of Lmo2 gene. It suggests that Lck induces Lmo2 expression through a STAT5-independent mechanism. The observation that oncogenic Lck induces Lmo2 expression in HEK 293 lacking endogenous STAT5 (Fig. 1B) further supports this hypothesis. The data is also consistent with our earlier report that mechanisms other than STAT5-dependent gene activation contribute to Lck-mediated oncogenesis [16].
Nuclear localization of oncogenic Lck kinase
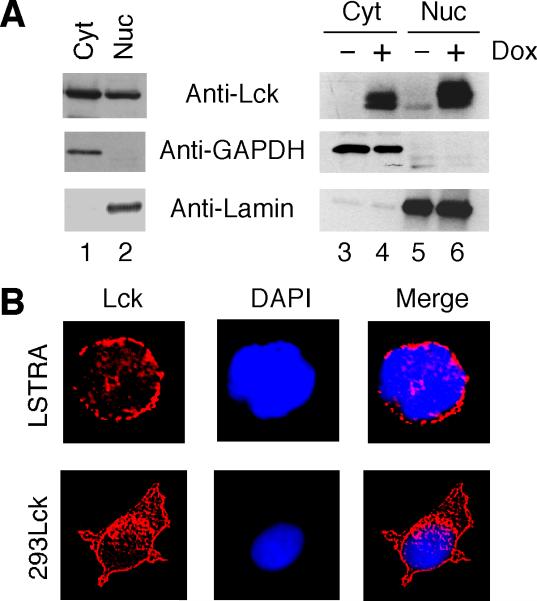
The finding that oncogenic JAK2V617F in the nucleus upregulates Lmo2 expression in human leukemic cells [6] prompted us to hypothesize a role of nuclear Lck in activating the Lmo2 gene. Like other Src family kinases, Lck is usually localized near the plasma membrane of T cells and is activated upon T cell receptor stimulation [17]. To determine if oncogenic Lck is present in the nucleus, both LSTRA leukemic cells and HEK 293 expressing the constitutively active Y505FLck kinase were subjected to subcellular fractionation. As shown in Fig. 2A, a significant amount of Lck is present in the nuclear fractions of both LSTRA (lane 2) and doxycycline-treated T-REx-293/Y505FLck (lane 6) cells. Immunoblotting for GAPDH (cytosolic marker) and Lamin B1 (nuclear marker) confirmed the absence of cytosolic contamination in the nuclear fraction. We also conducted immunofluorescence microscopy to confirm the nuclear localization of Lck. As shown in Fig. 2B, nuclear localization of Lck is clearly visible in the two-color merge image.
Fig. 2.
Nuclear localization of oncogenic Lck kinase. (A) Cytosolic (Cyt) and nuclear (Nuc) fractions were isolated from LSTRA cells (lanes 1-2) as well as T-REx-293/Y505FLck cells either untreated or treated with doxycycline (lanes 3-6). Normalized total proteins were analyzed by immunoblotting with antibodies specific for Lck, GAPDH and Lamin B1. (B) LSTRA and doxycycline-treated T-REx-293/Y505FLck (293Lck) cells were stained for Lck (red) and nuclei were visualized by DAPI staining (blue). Magnification is 200X for LSTRA and 60X for HEK 293 cells.
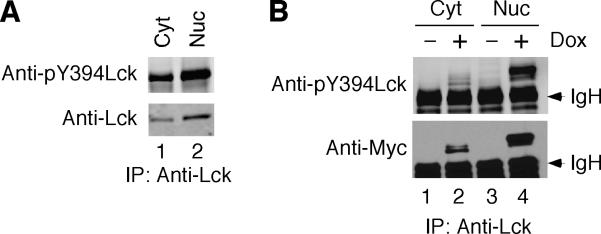
A constitutively active Y505FLck mutant has been reported to possess reduced kinase activity upon enforced nuclear translocation in rat fibroblast cells [24]. To evaluate the activity of nuclear Lck, we examined the phosphorylation status of the positive regulatory Y394. High levels of Y394 phosphorylation were detected in nuclear Lck isolated from both LSTRA cells (Fig. 3A) and HEK 293 cells after doxycycline induction (Fig. 3B). These data support the notion that both endogenous and exogenous Lck kinases are active in the nuclear compartment.
Fig. 3.
Phosphorylation of positive regulatory tyrosine of nuclear Lck. Cytosolic and nuclear fractions were isolated from LSTRA (A) and T-REx-293/Y505FLck (B) cells as described for Fig. 2. Normalized total proteins were immunoprecipitated with anti-Lck antibody, followed by immunoblotting with antibody specific for Tyr 394-phosphorylated Lck. The membrane was stripped and then reblotted for total Lck using anti-Lck (A) or anti-Myc tag (B) antibody. The arrowheads mark the positions of immunoglobulin heavy chain (IgH).
Interferon α-induced nuclear localization of Lck in Jurkat T cells is hypothesized to play a role in differentiating downstream responses of specific extracellular stimuli [25]. It highlights the importance of protein subcellular localization for initiating specific cellular signaling cascades during different physiological events. Additionally, nuclear presence of Lck has been reported in breast cancer cells where Lck is thought to play a role in activating transcription factor Sp1 [26]. Our finding of oncogenic Lck in the nuclei of LSTRA and HEK 293 cells further demonstrate the role of nuclear Lck in lymphoid and non-lymphoid malignancies.
Effect of Lck on histone H3 phosphorylation at Tyr 41
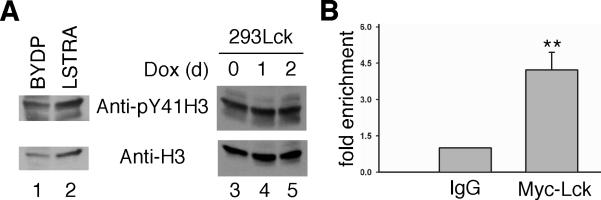
Nuclear JAK2-mediated tyrosine phosphorylation of histone H3 at Tyr 41 causes structural reorganization of the chromatin that results in increased transcriptional activity at the Lmo2 promoter site [6]. To determine if nuclear Lck uses a similar mechanism to directly regulate Lmo2 transcription, total proteins from BYDP and LSTRA cells were examined by immunoblotting. As shown in Fig. 4A, the levels of both Tyr 41-phosphorylated and total histone H3 were elevated in LSTRA leukemia as compared to the BYDP control (compare lanes 1 and 2). Quantitative analysis shows that the stoichiometry of histone H3 phosphorylation is not significantly different between the two cells. Consistent with these results, there was no change of histone H3 phosphorylation at Tyr 41 in T-REx-293/Y505FLck cells treated with doxycycline (Fig. 4A, compare lanes 3-5). These findings suggest that oncogenic Lck and JAK2 may utilize different mechanisms to upregulate Lmo2 levels during oncogenic transformation.
Fig. 4.
The effect of Lck on histone H3 phosphorylation and Lmo2 gene promoter. (A) Whole cell lysates were prepared from BYDP, LSTRA and T-REx-293/Y505FLck without or with doxycycline treatment. Normalized total proteins were subjected to sequential immunoblotting using antibodies specific for Tyr 41-phosphorylated histone H3 and total histone H3. (B) Doxycycline-treated T-REx-293/Y505FLck cells were subjected to chromatin immunoprecipitation with control IgG or anti-Myc tag antibody, followed by real-time PCR using primers specific for the promoter region of human Lmo2. Data from triplicates are presented as fold enrichment in Lck binding to the Lmo2 promoter region. ** p<0.01.
Phosphorylation of histone H3 at Tyr 41 has been shown to prevent binding of heterochromatin protein 1α and result in the relief of transcriptional repression of the Lmo2 gene promoter [6]. While expression of oncogenic Lck kinase does not lead to enhanced histone H3 phosphorylation at Tyr 41 (Fig. 4A), it does not exclude its potential contribution in Lmo2 expression. The constitutive histone H3 phosphorylation at Tyr 41 may be important in maintaining the open chromatin configuration to facilitate binding of other nuclear factors in the assembly of a functional transcriptional complex on the Lmo2 gene promoter.
In vivo binding of Lck to the promoter region of Lmo2
Other protein tyrosine kinases, such as Src and EGFR, have been reported to translocate to the nucleus and regulate gene expression by binding directly to gene promoters in human cancer cells [20]. To assess if nuclear Lck can directly bind the promoter region of Lmo2, we performed chromatin immunoprecipitation in T-REx-293/Y505FLck cells treated with doxycycline to induce expression of Myc-tagged oncogenic Lck. Quantitative real-time PCR was performed using primers specific for human Lmo2 promoter region on control IgG and Myc tag-immunoprecipitated DNA. As seen in Fig. 4B, there is a significant enrichment in Myc-tagged Y505FLck binding to Lmo2 promoter region over the IgG control. This observation raises the possibility that oncogenic Lck may activate Lmo2 promoter through direct interaction.
While the Lmo2 promoter lacks STAT5-binding sites, functional Ets factor binding sites have been identified in the proximal promoter region [5]. It remains to be determined whether Lck binds to the Lmo2 promoter independently of Ets factors. Ets family transcription factors are downstream of the mitogen-activated protein kinase pathway and can be activated by extracellular signal-regulated kinase (ERK) [27]. Expression of oncogenic Lck kinase induces ERK phosphorylation and activation (data not shown). Therefore, we cannot exclude the possibility that Lck and Ets factors may act in concert on the Lmo2 gene promoter to activate its transcription.
In summary, we have identified a novel function of oncogenic Lck in the nucleus that is likely to support its transforming potential. We report here that Lmo2 expression is elevated in Lck-transformed lymphoid and non-lymphoid cells. Mechanistically, oncogenic Lck kinase translocates to the nucleus and binds to the Lmo2 gene promoter. In our previous studies, we have shown the involvement of both STAT5-dependent and STAT5-independent pathways in Lck-mediated cellular transformation. Nuclear presence of Lck and its induction of Lmo2 expression may contribute to malignant transformation independent of STAT5 activation. Therefore, oncogenic Lck can initiate multiple signaling cascades that are likely to be controlled by the spatial localization of the oncoprotein.
Research Highlights.
Lmo2 expression is elevated in Lck-transformed cells.
Both endogenous and exogenous Lck localize in the nucleus.
Nuclear Lck is active in Lck-transformed cells.
Lck binds to the promoter region of Lmo2 gene in vivo.
In contrast to JAK2, Lck does not increase histone H3 phosphorylation on Tyr 41.
Acknowledgments
We thank Dr. Mohanan Veettil in Dr. Bala Chandran's lab for assistance in immunofluorescence microscopy. This work was supported in part by National Institutes of Health Grant CA107210 and RFUMS H. M. Bligh Cancer Research Fund (to C.L.Yu).
Abbreviations used
- Lmo2
Lim domain only protein 2
- T-ALL
T-acute lymphoblastic leukemia
- JAK
Janus kinase
- Lck
lymphocyte-specific protein tyrosine kinase
- STAT
signal transducer and activator of transcription
- EGFR
epidermal growth factor receptor
- GAPDH
glyceraldehydes 3-phosphate dehydrogenase
- ChIP
chromatin immunoprecipitation
- ERK
extracellular signal-regulated kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCormack MP, Rabbitts TH. Activation of the T-cell oncogene LMO2 after gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2004;350:913–922. doi: 10.1056/NEJMra032207. [DOI] [PubMed] [Google Scholar]
- 2.Nam CH, Rabbitts TH. The role of LMO2 in development and in T cell leukemia after chromosomal translocation or retroviral insertion. Mol. Ther. 2006;13:15–25. doi: 10.1016/j.ymthe.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Oram SH, Thoms JA, Pridans C, Janes ME, Kinston SJ, Anand S, Landry JR, Lock RB, Jayaraman PS, Huntly BJ, Pimanda JE, Gottgens B. A previously unrecognized promoter of LMO2 forms part of a transcriptional regulatory circuit mediating LMO2 expression in a subset of T-acute lymphoblastic leukaemia patients. Oncogene. 2010;29:5796–5808. doi: 10.1038/onc.2010.320. [DOI] [PubMed] [Google Scholar]
- 4.Rabbitts TH, Axelson H, Forster A, Grutz G, Lavenir I, Larson R, Osada H, Valge-Archer V, Wadman I, Warren A. Chromosomal translocations and leukaemia: a role for LMO2 in T cell acute leukaemia, in transcription and in erythropoiesis. Leukemia. 1997;11(Suppl 3):271–272. [PubMed] [Google Scholar]
- 5.Landry JR, Kinston S, Knezevic K, Donaldson IJ, Green AR, Gottgens B. Fli1, Elf1, and Ets1 regulate the proximal promoter of the LMO2 gene in endothelial cells. Blood. 2005;106:2680–2687. doi: 10.1182/blood-2004-12-4755. [DOI] [PubMed] [Google Scholar]
- 6.Dawson MA, Bannister AJ, Gottgens B, Foster SD, Bartke T, Green AR, Kouzarides T. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature. 2009;461:819–822. doi: 10.1038/nature08448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sefton BM. The lck tyrosine protein kinase. Oncogene. 1991;6:683–686. [PubMed] [Google Scholar]
- 8.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 9.Marth JD, Disteche C, Pravtcheva D, Ruddle F, Krebs EG, Perlmutter RM. Localization of a lymphocyte-specific protein tyrosine kinase gene (lck) at a site of frequent chromosomal abnormalities in human lymphomas. Proc .Natl. Acad. Sci. USA. 1986;83:7400–7404. doi: 10.1073/pnas.83.19.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majolini MB, Boncristiano M, Baldari CT. Dysregulation of the protein tyrosine kinase LCK in lymphoproliferative disorders and in other neoplasias. Leuk. Lymphoma. 1999;35:245–254. doi: 10.3109/10428199909145727. [DOI] [PubMed] [Google Scholar]
- 11.Wright DD, Sefton BM, Kamps MP. Oncogenic activation of the Lck protein accompanies translocation of the LCK gene in the human HSB2 T-cell leukemia. Mol. Cell. Biol. 1994;14:2429–2437. doi: 10.1128/mcb.14.4.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin GS. The hunting of the Src. Nat. Rev. Mol. Cell Biol. 2001;2:467–475. doi: 10.1038/35073094. [DOI] [PubMed] [Google Scholar]
- 13.Amrein KE, Sefton BM. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc. Natl. Acad. Sci. USA. 1988;85:4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marth JD, Cooper JA, King CS, Ziegler SF, Tinker DA, Overell RW, Krebs EG, Perlmutter RM. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck) Mol. Cell. Biol. 1988;8:540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu C-L, Jove R, Burakoff SJ. Constitutive activation of the Janus kinase-STAT pathway in T lymphoma overexpressing the Lck protein tyrosine kinase. J. Immunol. 1997;159:5206–5210. [PubMed] [Google Scholar]
- 16.Shi M, Cooper JC, Yu C-L. A constitutively active Lck kinase promotes cell proliferation and resistance to apoptosis through signal transducer and activator of transcription 5b activation. Mol. Cancer Res. 2006;4:39–45. doi: 10.1158/1541-7786.MCR-05-0202. [DOI] [PubMed] [Google Scholar]
- 17.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol. Rev. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi A, Obata Y, Fukumoto Y, Nakayama Y, Kasahara K, Kuga T, Higashiyama Y, Saito T, Yokoyama KK, Yamaguchi N. Nuclear localization of Src-family tyrosine kinases is required for growth factor-induced euchromatinization. Exp. Cell Res. 2009;315:1117–1141. doi: 10.1016/j.yexcr.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda K, Nakayama Y, Togashi Y, Obata Y, Kuga T, Kasahara K, Fukumoto Y, Yamaguchi N. Nuclear localization of Lyn tyrosine kinase mediated by inhibition of its kinase activity. Exp. Cell Res. 2008;314:3392–3404. doi: 10.1016/j.yexcr.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Jaganathan S, Yue P, Paladino DC, Bogdanovic J, Huo Q, Turkson J. A functional nuclear epidermal growth factor receptor, SRC and Stat3 heteromeric complex in pancreatic cancer cells. PLoS One. 2011;6:e19605. doi: 10.1371/journal.pone.0019605. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Chueh F-Y, Leong K-F, Yu C-L. Mitochondrial translocation of signal transducer and activator of transcription 5 (STAT5) in leukemic T cells and cytokine-stimulated cells. Biochem. Biophys. Res. Commun. 2010;402:778–783. doi: 10.1016/j.bbrc.2010.10.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chueh F-Y, Leong K-F, Cronk RJ, Venkitachalam S, Pabich S, Yu C-L. Nuclear localization of pyruvate dehydrogenase complex-E2 (PDC-E2), a mitochondrial enzyme, and its role in signal transducer and activator of transcription 5 (STAT5)-dependent gene transcription. Cell. Signal. 2011;23:1170–1178. doi: 10.1016/j.cellsig.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venkitachalam S, Chueh F-Y, Leong K-F, Pabich S, Yu C-L. Suppressor of cytokine signaling 1 interacts with oncogenic lymphocyte-specific protein tyrosine kinase. Oncol. Rep. 2011;25:677–683. doi: 10.3892/or.2011.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yurchak LK, Hardwick JS, Amrein K, Pierno K, Sefton BM. Stimulation of phosphorylation of Tyr394 by hydrogen peroxide reactivates biologically inactive, non-membrane-bound forms of Lck. J. Biol. Chem. 1996;271:12549–12554. doi: 10.1074/jbc.271.21.12549. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed Z, Beeton CA, Williams MA, Clements D, Baldari CT, Ladbury JE. Distinct spatial and temporal distribution of ZAP70 and Lck following stimulation of interferon and T-cell receptors. J. Mol. Biol. 2005;353:1001–1010. doi: 10.1016/j.jmb.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Chakraborty G, Rangaswami H, Jain S, Kundu GC. Hypoxia regulates cross-talk between Syk and Lck leading to breast cancer progression and angiogenesis. J. Biol. Chem. 2006;281:11322–11331. doi: 10.1074/jbc.M512546200. [DOI] [PubMed] [Google Scholar]
- 27.Svensson S, Jirström K, Rydén L, Roos G, Emdin S, Ostrowski MC, Landberg G. ERK phosphorylation is linked to VEGFR2 expression and Ets-2 phosphorylation in breast cancer and is associated with tamoxifen treatment resistance and small tumours with good prognosis. Oncogene. 2005;24:4370–4379. doi: 10.1038/sj.onc.1208626. [DOI] [PubMed] [Google Scholar]