Abstract
MT1-MMP (membrane type 1-matrix metalloproteinase) plays important roles in cell growth and tumor invasion via mediating cleavage of MMP2/gelatinase A and a variety of substrates including type I collagen. BST-2 (bone marrow stromal cell antigen 2) is a membrane tetherin whose expression dramatically reduces the release of a broad range of enveloped viruses including HIV from infected cells. In this study, we provided evidence that both transient and IFN-α induced BST-2 could decrease the activity of MMP2 via binding to cellular MT1-MMP on its C- terminus and inhibiting its proteolytic activity; and finally block cell growth and migration. Zymography gel and Western-blot experiments demonstrated that BST-2 decreased MMP2 activity, but no effect on the expression of MMP2 and MT1-MMP genes. Confocal and immunoprecipitation data showed that BST-2 co-localized and interacted with MT1-MMP. This interaction inhibited the proteolytic enzyme activity of MT1-MMP, and blocked the activation of proMMP2. Experimental results of C-terminus deletion mutant of MT1-MMP showed that activity of MMP2 was no change and also no interaction existed between the mutant and BST-2 after co-transfection with the mutant and BST-2. It meant that C-terminus of MT1-MMP played a key role in the interaction with BST-2. In addition, cell growth in 3-D type I collagen gel lattice and cell migration were all inhibited by BST-2. Taken together, BST-2, as a membrane protein and a tetherin of enveloped viruses, was a novel inhibitor of MT1-MMP and could be considerable as an inhibitor of cancer cell growth and migration on clinic.
Keywords: MT1-MMP, BST-2, MMP2, Type I collagen
INTRODUCTION
MT1-MMP (Membrane-type matrix metalloproteinase 1), a member of the MMP super-family, is a key protein in physiological and pathological processes from normal cell development to cancer cell growth (Seiki, 2002 and 2003; Hiraoka et al., 1998; Zhou et al., 2000). Main function of MT1-MMP is as a cell surface proteinase and it displays a broad spectrum of activity against ECM components such as type I and II collagen, fibronectin, vitronectin, laminin, fibrin and proteoglycan (Ohuchi et al., 1997; d’Ortho et al., 1997). Various cell types employ MT1-MMP to alter their surrounding environment during angiogenesis, tissue remodeling, tumor invasion, and metastasis (Hotary et al., 2000; Lehti et al., 2002; Koshikawa et al., 2000). There are three methods to regulate the activity of MT1-MMP: the first is via binding to its endogenous inhibitors, such as TIMP-2 and TIMP3 (Will et al., 1996; Bigg et al., 2001); the second is via protein trafficking between the cytoplasm and plasma membrane (including regulated by other proteins, such as Clathrin, Caveolin, Src, Rab8, etc.) (Gálvez et al., 2004; Jiang et al., 2001; Uekita et al., 2001; Remacle et al., 2003; Nyalendo et al., 2007; Bravo-Cordero et al., 2007); the third is via transcriptional and translational control by the related factors. Transcription of MT1-MMP can be regulated by signal pathways, such as the Wnt-β-catenin-Tcf4 signal pathway (Hlubek et al., 2004; Nawrocki-Raby et al., 2003; Liu et al., 2010).
BST-2 (bone marrow stromal cell antigen 2; also known as PDCA-1, CD137 and HM1.24) is a type II transmembrane protein (the N-terminus is in the cytoplasm) whose lumenal C-terminus is modified with a glycosylphosphatidylinositol (GPI) membrane anchor (Kupzig et al., 2003). The cytoplasmic domain of BST-2 could interact/bind directly or indirectly with other proteins and regulate their activities and functions (Rollason et al., 2007 and 2009). It is newly found that BST-2 was identified as a host cell ‘restriction factor’ counteracted by the HIV-1 accessory protein Vpu to limit the spread of enveloped viruses (Neil et al., 2008; Van Damme et al., 2008).
In this study, we found that BST-2 co-localized and interacted with MT1-MMP in cells, and formed granular complexes in cellular cytoplasm. After interaction, the proteolytic enzyme activity of bound MT1-MMP with BST-2 was inhibited and could not activate pro-MMP2 protein into active MMP2; and finally resulted in inhibition of cell growth in 3-D type I collagen lattice and cell migration. Furthermore, our experimental data showed that C-terminus of MT1-MMP played an important role in the interaction between MT1-MMP and BST-2. It meant that BST-2 might use its cytoplasmic N-terminus to interact with the cytoplasmic C-terminal domain of MT1-MMP.
METHODS AND MATERIALS
Cell culture and Transfection
All tissue culture reagents were purchased from BRL-GIBCO. Both of cell lines, MDCK and HT1080, were obtained from American type culture collection (ATCC) and subcloned subsequently. Subline MDCK-umn (Pei, 1999) was epithelial-like in cell shape and grew well in DMEM and was used throughout the experiments. The cells were maintained in DMEM supplemented with 10% fetal bovine sera, L-glutamine (2mM) and streptomycin/penicillin (50 units/ml). HT1080 cells were maintained as described (Pei, 1999; Pei and Weiss, 1996). All cells were cultured in a growth chamber with 5% CO2/95% air at 37°C.
Before transfection, cells were seeded and cultured in 5% FBS medium for 24h. The wild-type and mutant DNA constructs were transfected into various cells by Lipofectamine 2000 using protocols as described by the manufacture (Invitrogen Inc. CA, USA).
Plasmids, Antibodies and chemicals
pcDNA3.1(+)uni-MT1-MMP, MT1-MMP/ΔC (C-terminus deletion mutant) and MT1-MMP/ΔTM (transmembrane domain deletion mutant) were constructed as described previously (d’Ortho et al., 1997; Hotary et al., 2000) and kept by our lab. pcDNA3.1(+)uni-HA-BST-2 was constructed by using general PCR methods. The PCR primers for BST-2 are: forward 5’ accggatccatggcatctacttcgtatgac 3’, and reverse 5’ aacctcgagctgcagcagagcgctgag 3’; the restriction enzymes used were BamH I and Xho I. The PCR products were connected into pcDNA3.1(+)uni-HA vector which was constructed and kept by our lab (HA tag located just behind Xho I site).
A rabbit polyclonal antibody against purified MT1-MMP catalytic domain was raised by immunizing rabbits and affinity-purified as described previously (Lehti et al., 2002; Jiang et al., 2001), and a mouse polyclonal antibody against HA and a goat antibody against BST-2 were purchased from Santa Cruz Biotechnology, Carlsbad, CA, USA. The antibody anti-β-Actin was purchased from Cell Signaling Technology (Danvers, MA, USA). Lipofectamine 2000 was purchased from Invitrogen Corporation. Type I collagen was purchased from Collaborative Research, Bedford, MA. Immnoprecipitation kit was purchased from Promega Inc. (Madison, WI, USA). Alexa Fluor® 488 goat anti-rabbit IgG, Alexa Fluor® 594 goat anti-mouse IgG and Alexa Fluor® 594 rabbit anti-goat IgG were purchased from Invitrogen Inc. IFN-α (type I interferon alpha) was purchased from Sigma-Aldrich Inc. and used with a final concentration 2,000U/mL in all experiments. Other general chemicals were purchased from Sigma-Aldrich or Fisher (Pittsburgh, PA, USA).
Generation of Stable cell line
pcDNA3.1(+)uni-MT1-MMP plasmids were transfected into MDCK cells by using Lipofectamine 2000, and stable clones were selected in the presence of 1mg/mL G418 as described previously (Jiang and Pei, 2003). The stable clones were screened for proMMP2 activation by a zymography assay and western blot analysis by using the anti-MT1-MMP catalytic domain antibody. At least two representative clones were selected for the experiments.
Zymography, Western Blotting and immunoprecipitation
Zymography gel assay was performed as described before (Jiang and Pei, 2003). In brief, cells were cultured in 12-well plate and transfected as indicated in figures. After 24h, media was changed into DMEM containing 5% FBS (the source of the proMMP2). After another 24 hours, the media were harvested and cleared by centrifugation at 12,000 rpm for 10 min, and then subjected to analysis by SDS-PAGE impregnated with 1 mg/ml gelatin as described previously (Pei, 1999). The gels were incubated at 37°C overnight, stained with Coomassie Blue, destained, and then scanned. For Western blotting and immunoprecipitation experiments, transfected cells were cultured in media with 5µM of the MMP inhibitor GM6001 to prevent auto-degradation as described in figures. At 48h after transfection, cells were harvested and lysed in lysis buffer (50mM Tris-HCl, pH7.5, 150mM NaCl, 0.25% sodium deoxycholate, 0.1% Nonidet P-40 and a mixture of protease inhibitors), cleared by centrifugation; and the supernants were used for western blotting assay with relative antibodies or for immunoprecipitation by using kit as described on its introductions. Western blotting analysis of immunoprecipitation was done with specific antibodies as described in the figures.
Reverse Transcription PCR (RT–PCR)
Total cellular RNA was extracted from snap-frozen cell pellets using the Trizol reagent (Life Technologies, Inc., Burlington, Ontario, Canada) according to the manufacturer’s instructions. Two µg of total RNA were reverse transcribed by using a reaction mixture containing 50 mM Tris-HCl (pH 8.3), 30 mM KCl, 8 mM MgCl2, 10 mM DTT, 100 ng oligo(dT)12–18, 40 units of RNase inhibitor, 1 mM deoxynucleotide triphosphates, and eight units of avian myeloblastosis virus reverse transcriptase (all from Pharmacia Biotech, Baie D’Urfe, Quebec, Canada). The mixture was incubated for 10 min at 25°C, then for 45 min at 42°C, and finally for 5 min at 95°C. The PCR primers used were as follows: for MT1-MMP: sense 5’-CGC TAC GCC ATC CAG GGT CTC AAA-3’, antisense 5’-CGG TCA TCA TCG GGC AGC ACA AAA-3’ (expected product 497 bp); for BST-2: sense 5′-CCT GCT CGG CTT TTC GCT TGA ACA T-3′, antisense 5′-CGG AGG GAG GCT CTG GAG GGA GAC-3′ (expected product 199bp); for beta-Actin: sense 5’-GGA CTT CGA GCA AGA GAT GG-3’, antisense 5’-AGC ACT GTG TTG GCG TAC AG-3’ (expected product 234 bp). The cDNA was amplified using 35 cycles, each of 30 s at 94°C, 30 s at 55°C and 30 s at 72°C, followed by a 10min incubation at 72°C. In the preliminary experiments, it was confirmed that under these conditions, the RT-PCR products were obtained during the exponential phase of the amplification curve. The amplified DNA fragments were analyzed by electrophoresis in 1.2% agarose gels.
Growth of MDCK and HT1080 Cells in Three-dimensional Type I collagen Lattice
Cells (including MDCK, HT1080 and stable MDCK) were seeded in 6-well plates and transfected with plasmids as indicated in figures by using Lipofectamine 2000. At 24h after transfection, the same amount of cells (1×103) were mixed with 500 µl of type I collagen (2.5 mg/ml) and allowed to gel at 37°C for half an hour in 24-well plates to give rise to three-dimensional collagen lattice. Fresh medium containing 10% FBS was added to wells and changed every 2 days. One week later, cells were photographed with a video camera at the University of Minnesota Biomedical Image Processing Laboratories as described previously (Kang et al., 2000).
Migration Assay of MDCK and HT1080 Cells
Cell motility was tested using a scratch wound assay as described before (Mercure et al., 2008). In brief, equal numbers of MDCK or HT1080 or stable MDCK cells were cultured in 6-well plates in 5%FBS DMEM and transfected with plasmids as indicated in individual figures. Media was changed into a full media (DMEM containing 10% FBS) at 24h after transfection. When the plates were grown to almost confluency in the full media (containing 10% FBS), a scratch was made along the axis of each well using a pipette tip. Cells were washed three times with PBS buffer, and media were changed after scratching. Cells migrated into the scratch were photographed at 60 h.
Immunostaining and Confocal Microscopy
Cells were grown on glass coverslips and transfected with the indicated plasmids in figures. After being cultured with GM6001 (5µM) for 48h, cells were fixed with 4% polyformaldehyde for 20 min and incubated with PBS containing 0.1% Triton X-100 for 5min. After blocking with 3% goat serum in PBS, 0.2 µg/mL anti-MT1-MMP antibody (rabbit), anti-HA tag antibody (mouse), and anti-BST2 antibody (goat) were added to the cells, respectively; and then were incubated at 4 °C overnight. Secondary antibodies, including Alexa Fluor® 594 labeled goat anti-mouse IgG, Alexa Fluor® 594 labeled rabbit anti-goat IgG and Alexa Fluor® 488 labeled goat anti-rabbit IgG were used to detect the primary antibody. Confocal microscopy was carried out in the Biomedical Image Processing laboratories at the University of Minnesota using a Bio-Rad MRC 1024 system attached to an Olympus microscope (Melville, NY) with a 60X oil objective. The images were processed in Photoshop 7.0 (Adobe, San Jose, CA).
Statistical analysis
Experiments were carried out with three or four replicates. Data were presented as mean ± SD or SEM as shown in the figures. All data were assessed by SPSS 11.5 Software.
RESULTS
Inhibition of activity of MMP2 by BST-2
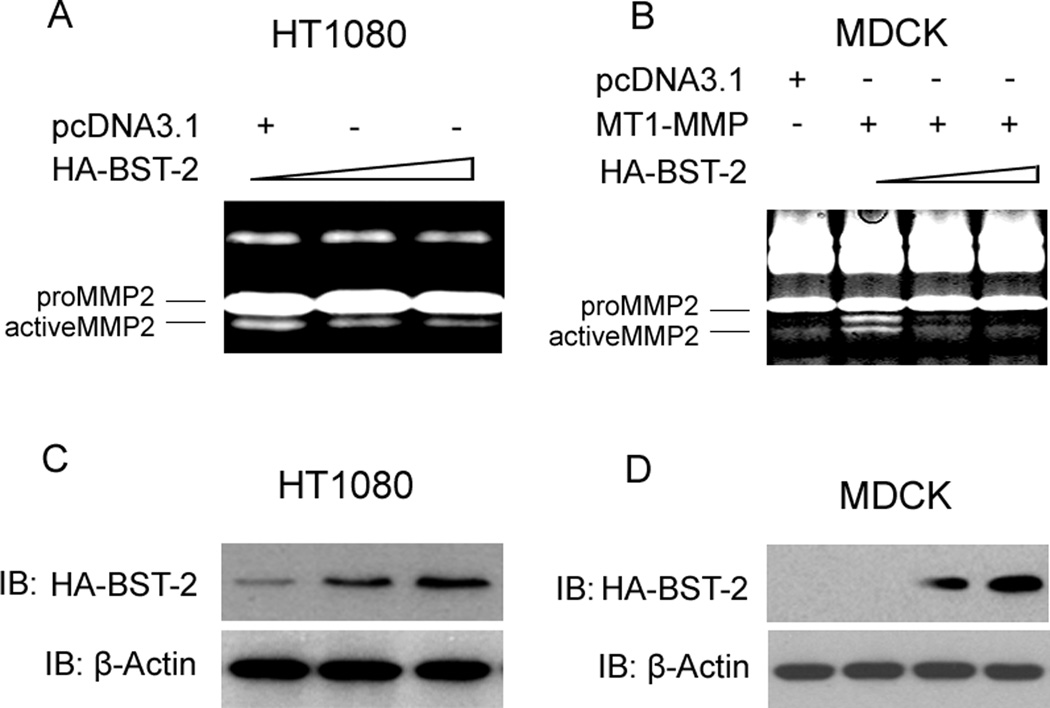
To study the effects of BST-2 on MMP2 activity, we employed HT1080 cell line which expresses endogenous MT1-MMP and MDCK cell line which doesn’t express endogenous MT1-MMP as the cellular modes. Our experimental data showed that the activity of MMP2 was significant decreased after transfecting plasmid pcDNA3.1-HA-BST-2 into HT1080 cells (fig. 1A), and the same way was happened in MDCK cells when co-transfected with MT1-MMP and HA-BST-2 (fig. 1B) by compared with control cells (transfected with pcDNA3.1 vector in HT1080 cells and with MT1-MMP in MDCK cells). Furthermore, with the increase of pcDNA3.1-HA-BST-2 plasmids transfected into cells, the decrease of MMP2 activity displayed a dependent trend with the increased protein level of HA-BST-2 (fig. 1A, 1B, 1C and 1D). These results suggested that BST-2 could down-regulate the amount of active MMP2 and MMP2 activity in both expressed endogenous MT1-MMP HT1080 cells and transient MT1-MMP MDCK cells. In addition, it has been well charactered that proMMP2 is a specific substrate of MT1-MMP and only MT1-MMP can cleave and activate proMMP2 into active MMP2 (d’Ortho et al., 1997). So, all of these results indicated that the regulation of MMP2 activity by BST-2 might be related to MT1-MMP.
Figure 1. Inhibition of MMP2 activity by transfected BST-2 in HT1080 and MDCK cells.
(A) Zymography gel assay of the effect of BST-2 on the activity of MMP2 in HT1080 cells. HA-BST-2 was transfected in a gradient; and MMP2 activity was decreased in a dose dependent manner. (B) Zymography gel assay of the effect of BST-2 on the activity of MMP2 in MDCK cells. MT1-MMP co-transfected with HA-BST-2 into cells, and BST-2 was transfected in a gradient; and MMP2 activity was decreased in a dose dependent manner. (C) Western-blot assay of the extracts of HT1080 cells after transfection of HA-BST-2. (D) Western-blot assay of the extracts of MDCK cells after transfection of HA-BST-2.
Effect of BST-2 on the expression of MT1-MMP
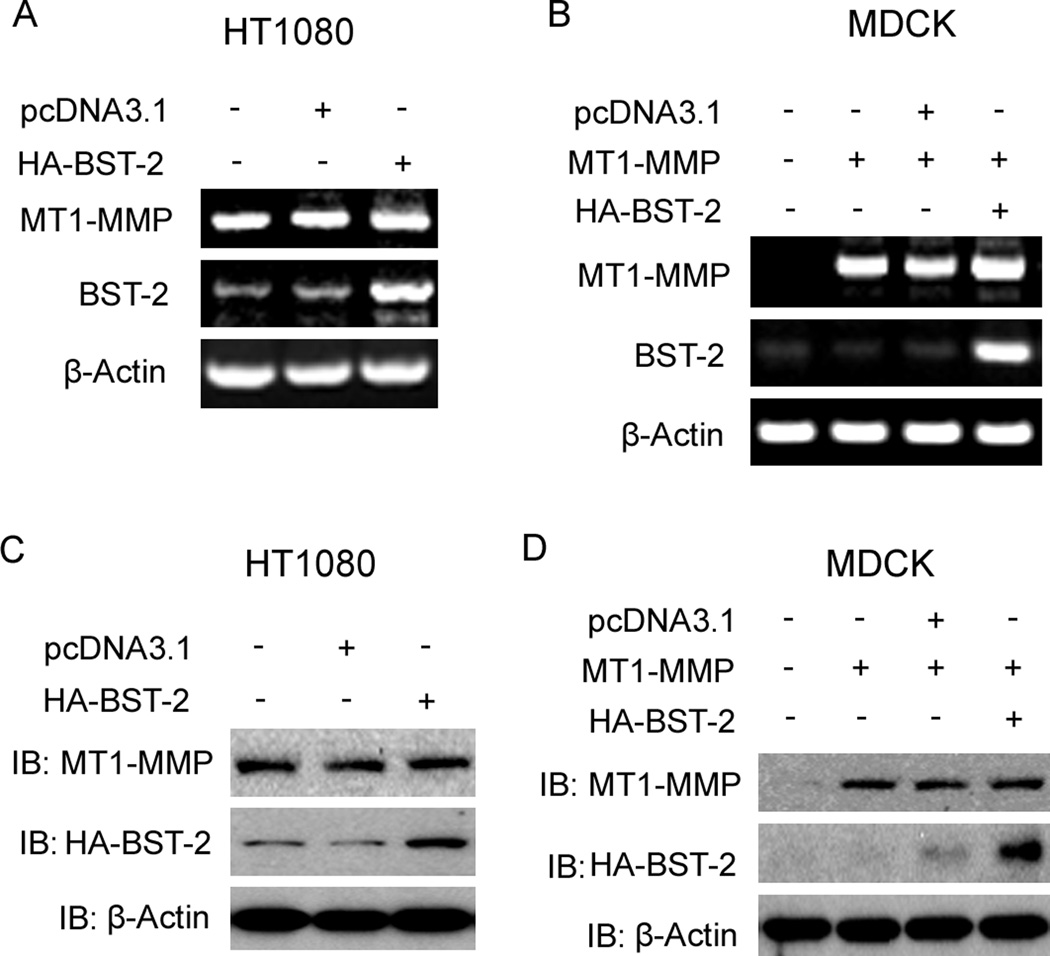
MT1-MMP is produced in cytoplasm, and then activated and transferred to cell surface by trafficking process. MT1-MMP is a proteolytic enzyme and has many substrates; and proMMP2 is a typical substrate of MT1-MMP. Cellular surface MT1-MMP can activate proMMP2 by cleaving proMMP2 into active form MMP2 (Seiki, 2002 and 2003). Based on these, we employed several experiments to investigate if BST-2 decreased the activity of MMP2 via blocking the expression of MT1-MMP. From our results, RT-PCR data showed that mRNA levels of MT1-MMP were not changed both in HT1080 cells and MT1-MMP transient MDCK cells after transfection of BST-2 (fig. 2A, 2B); and the western-blot data also showed that protein levels of MT1-MMP were not changed by transfected BST-2 (fig. 2C, 2D). These results demonstrated that BST-2 couldn’t affect the expression of MT1-MMP not only on transcription level, but also on protein level. It meant that BST-2 might down-regulate MT1-MMP activity by interacting with it.
Figure 2. Effect of BST-2 on the expression of MT1-MMP.
(A) RT-PCR assay results of MT1-MMP, BST-2, and β-Actin (as control) after transfection of HA-BST-2 in HT1080 cells. (B) RT-PCR assay results of MT1-MMP, BST-2, and β-Actin (as control) after co-transfection of MT1-MMP with HA-BST-2 in MDCK cells. (C) Protein levels of MT1-MMP, HA-BST-2 and β-Actin (as control) using Western-blot assay after transfection of HA-BST-2 in HT1080 cells. (D) Protein levels of MT1-MMP, HA-BST-2 and β-Actin (as control) using Western-blot assay after co-transfection of MT1-MMP with HA-BST-2 in MDCK cells.
Co-localization and interaction of BST-2 and MT1-MMP
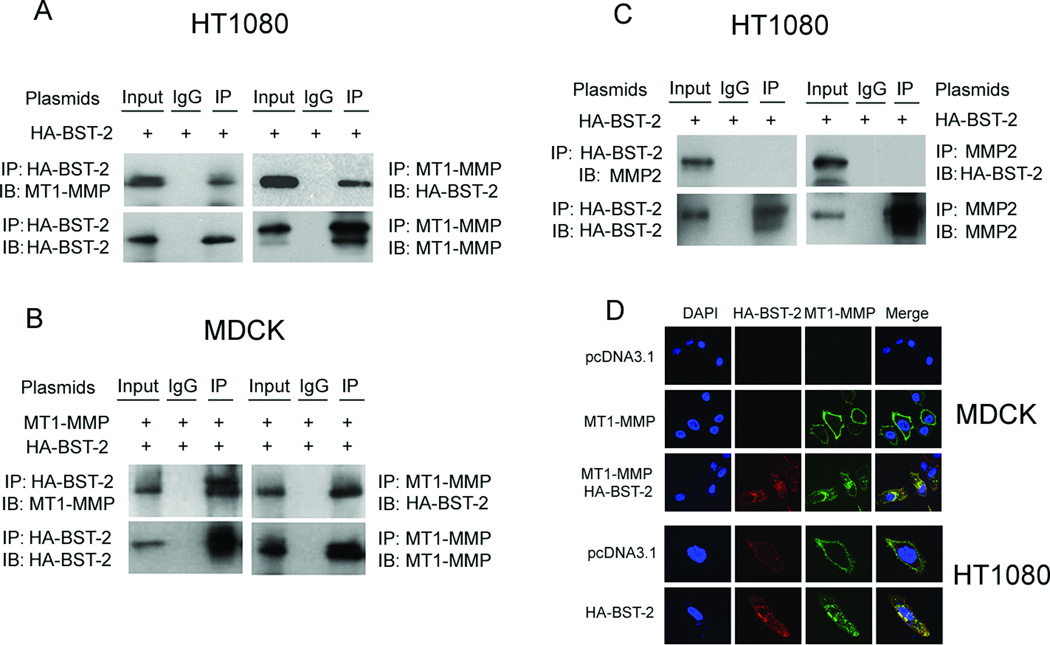
To determine if BST-2 inhibited the activity of MT1-MMP via an interaction between them, immunoprecipitation and confocal microscopy experiments were carried out. MDCK cells were co-transfected with MT1-MMP and HA-BST-2, and HT1080 cells were transfected with HA-BST-2 alone. At 48 h after transfection, cells were collected and cell lysates were subjected to immunoprecipitation with either an anti-MT1-MMP antibody or an anti-HA tag antibody. As demonstrated in Figure 3A, in HT1080 cells, endogenous MT1-MMP was detected in the protein complex immunoprecipitated by the anti-HA tag antibody; reciprocally, transient HA-BST-2 was detectable in the protein complex immunoprecipitated by the anti-MT1-MMP antibody (Fig. 3A). In MDCK cells, transient MT1-MMP and HA-BST-2 were also detectable in both protein complexes immunoprecipitated by anti-HA tag antibody and anti-MT1-MMP antibody, respectively (fig. 3B). Furthermore, in MT1-MMP stable MDCK cells, the immunoprecipitated results were the same as transient MDCK cells with co-transfection of MT1-MMP and HA-BST-2 (data not shown).
Figure 3. Co-localization and interaction of BST-2 with MT1-MMP in cells.
HT-1080 cells were only transfected with HA-BST-2, whereas MDCK cells were co-transfected with MT1-MMP and HA-BST-2; and then cells cultured in the full medium containing 5µM GM6001. At 48 h after transfection, cells were collected and lysed in immunoprecipitation (IP) buffer. Cell lysates were subjected to co-IP with an anti-MT1-MMP antibody/anti-MMP2 antibody (A, B and C; right panels) or an anti-HA-BST-2 antibody (A, B and C; left panels) or nonspecific IgG. Immunoprecipitated proteins were analyzed by western blot using antibodies as indicated. (D) MDCK cells were transfected with pcDNA3.1, MT1-MMP or co-transfected with MT1-MMP and HA-BST-2, whereas HT1080 cells were transfected with pcDNA3.1 or HA-BST-2, respectively. Transfected cells were cultured in the full medium containing 5µM GM6001 in 24-well plate with glass slide. At 48h after transfection, immunofluorescence chemistry experiments were performed using the anti-MT1-MMP antibody (green) and/or anti-HA-BST-2 antibody (red) to detect cells expressing MT1-MMP and/or HA-BST-2, and to detect the co-localization of MT1-MMP and BST-2 or not.
Besides expressing endogenous MT1-MMP, HT1080 cells also express endogenous proMMP2 (Liu et al., 2010). So we here employed HT1080 cells to investigate if the interaction existed between BST-2 and proMMP2. The experimental data showed that HA-BST-2 and proMMP2 were not detectable in both protein complexes immunoprecipitated by anti-HA tag antibody and anti-MMP2 antibody, respectively (fig. 3C).
Protein interactions can also be tested by immunostaining using Confocal Microscopy. Our confocal results of HT1080 cells transfected with HA-BST-2 alone and MDCK cells co-transfected with MT1-MMP and HA-BST-2 showed that MT1-MMP was predominately co-localized with BST-2 in granular arrays in the cytoplasm and no co-localization in the nucleus (fig.3D).
All of these results demonstrated that BST-2 co-localize and interacted with MT1-MMP and blocked the proteolytic enzyme activity of MT1-MMP, and further resulted in decreased activation of proMMP2.
Cytoplasmic tail (C-terminal domain) of MT1-MMP was responsible for the effect of BST-2 on MT1-MMP
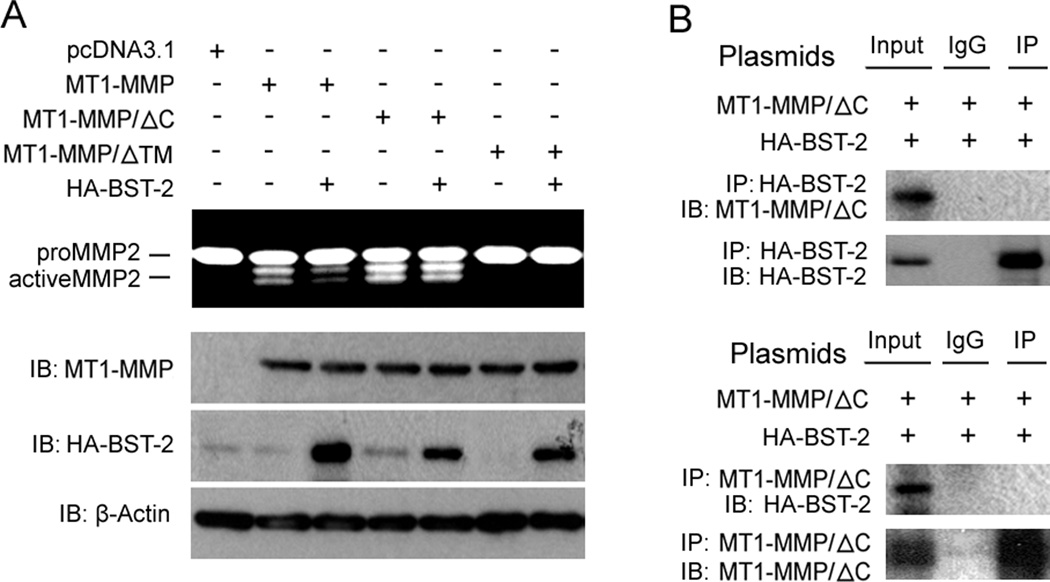
Previous reports have shown that the cytoplasmic tail domain of MT1-MMP (C-terminal domain) plays an important role in the regulation and trafficking of MT1-MMP; and transmembrane domain of MT1-MMP (TM) was also important in the regulation of MT1-MMP activity (Jiang et al., 2001; Wang et al., 2004; Nyalendo et al., 2007). Here we used the cytoplasmic tail domain deletion mutant of MT1-MMP (MT1-MMP/ΔC) and transmembrane domain deletion mutant of MT1-MMP (MT1-MMP/ΔTM) to test if the C-terminal tail and TM domain were the key domains of MT1-MMP in the interaction effect of BST-2 on the activity of MT1-MMP. Our data show that the activity of MT1-MMP/ΔC was not affected by BST-2 when co-transfected HA-BST-2 with MT1-MMP/ΔC in MDCK cells (fig. 4A), and imunoprecipitation data also showed that HA-BST-2 and MT1-MMP were not detectable in both protein complexes immunoprecipitated by anti-HA tag antibody and anti-MT1-MMP antibody, respectively (fig. 4B). It meant that the interaction between MT1-MMP/ΔC and HA-BST-2 was not existed; and indicated that the interaction between MT1-MMP and BST-2 related to C-terminal domain of MT1-MMP. MT1-MMP had no activity when deleting TM domain (fig. 4A); but immunoprecipitation data demonstrated that HA-BST-2 and MT1-MMP/ΔTM could be detected in both protein complexes immunoprecipitated by anti-HA tag antibody and anti-MT1-MMP antibody, respectively (data not shown). So it’s impossible that effect of BST-2 on MT1-MMP was via binding to TM domain of MT1-MMP. Taken together, these results here indicated that C-terminal domain of MT1-MMP played a key role in the interaction between BST-2 and MT1-MMP and TM domain of MT1-MMP was not involved in the interaction.
Figure 4. Effects of C-terminal cytoplasmic domain of MT1-MMP on the regulation of the activity of MT1-MMP by BST-2.
(A) Two sets of MDCK cells were co-transfected with HA-BST-2 and MT1-MMP/ΔC or MT1-MMP/ΔTM as indicated. After transfection, cells were cultured in 24-well plate with 5% FBS media (one set of cells was added 5µM GM6001 in media). 48h later, media of cultured cells with no GM6001 were harvested for zymography gel assay, and cells with GM6001 in media were lysed for Western blotting assay. (B) MDCK cells were co-transfected with MT1-MMP/ΔC and HA-BST-2, and then cells cultured in the full medium containing 5µM GM6001. At 48h after transfection, cells were collected and lysed in IP buffer. Cell lysates were subjected to IP and Western blotting as in figure 3.
Effects of BST-2 on cell growth and cell migration
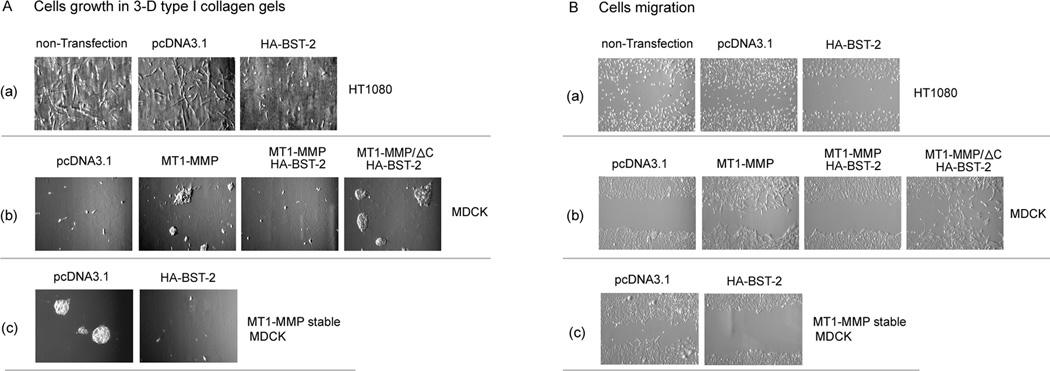
It is already characterized that MT1-MMP plays a positively important role in cell growth in 3-D type I collagen gels and in cell migration via activating proMMP2 (Seiki, 2002 and 2003; Hiraoka et al., 1998; Zhou et al., 2000). From our data above, BST-2 could inhibit the activities of MT1-MMP and MMP2 in HT1080 and MDCK cells, so it should subsequently block the enhancement of MT1-MMP on cell growth and cell migration. To identify this point, we did cell growth and cell migration experiments. In HT1080 cells, cell growth was significant inhibited by transfected HA-BST-2 in 3-D type I collagen gels (fig. 5A (a)). In MDCK cells, we found that transfected MT1-MMP greatly enhanced cell growth in 3-D type I collagen gels (Fig 5A (b)); however, the enhancement of cell growth was blocked by co-transfecting HA-BST-2 (fig 5A (b)). In MDCK/MT1-MMP stable cell line, the effect of transfected BST-2 on MT1-MMP enhanced cell growth was the same as in MT1-MMP transiently transfected MDCK cells (fig.5A(c)).
Figure 5. Effects of BST-2 on cell migration and cell growth in 3-D collagen gel.
(A) HT1080 cells were transfected with pcDNA3.1 or HA-BST-2; MDCK cells were transfected with pcDNA3.1, MT1-MMP, co-transfected with MT1-MMP and HA-BST-2, or co-transfected with MT1-MMP/ΔC and HA-BST-2; and MT1-MMP stable MDCK cells were transfected with pcDNA3.1 or HA-BST-2, respectively. 24h after transfection, the cells were trypsined and equal amount cells of each transfection (including non-transfection cells) were mixed with type I collagen (2.5mg/ml), and then cultured in the incubator in 24-well plates, and after 7 days, the mysts grown from cells in three-dimensional type I collagen gel lattice were photographed. (B) HT1080, MDCK, and MT1-MMP stable MDCK cells were transfected as the same as above in 6-well plate with full media. After cells grew to 90% conference, cell migration assays were performed by using a scratch wound assay method as described in materials and methods. The scratch wounds and the migration cells in the scratch were photographed at 60h after scratching (the border areas of the scratch wound are marked by two white lines as noted in the figures).
Effect of BST-2 on the role of MT1-MMP in cell migration was also examined. In confluent monolayer cell wound assays, the migration of HT1080 cells was greatly inhibited by transfected HA-BST-2 alone by comparing with cells of non-transfection and transfection with pcDNA3.1 vector (fig. 5B(a)). MDCK cells transfected with MT1-MMP migrated at a greater rate than control cells transfected with pcDNA3.1 vector and cells co-transfected with MT1-MMP and HA-BST-2; but cells co-transfected with MT1-MMP/ΔC and HA-BST-2 still migrated at a high rate. Thus, cell migration enhanced by transient MT1-MMP in MDCK cells was also inhibited by BST-2 (fig. 5B(b)); and MDCK/MT1-MMP stable cells were the same as transient MDCK cells (fig. 5B(c)).
These data indicated that BST-2 greatly decreased the enhancement of MT1-MMP on cell migration and cell growth in 3-D type I collagen gels via interacting with MT1-MMP in both HT1080 and MDCK cells; and further identified that this interaction depended on C-terminal tail domain of MT1-MMP.
Effect of IFN-α induced BST-2 on expression of MT1-MMP and activity of MMP2 in HT1080 cells
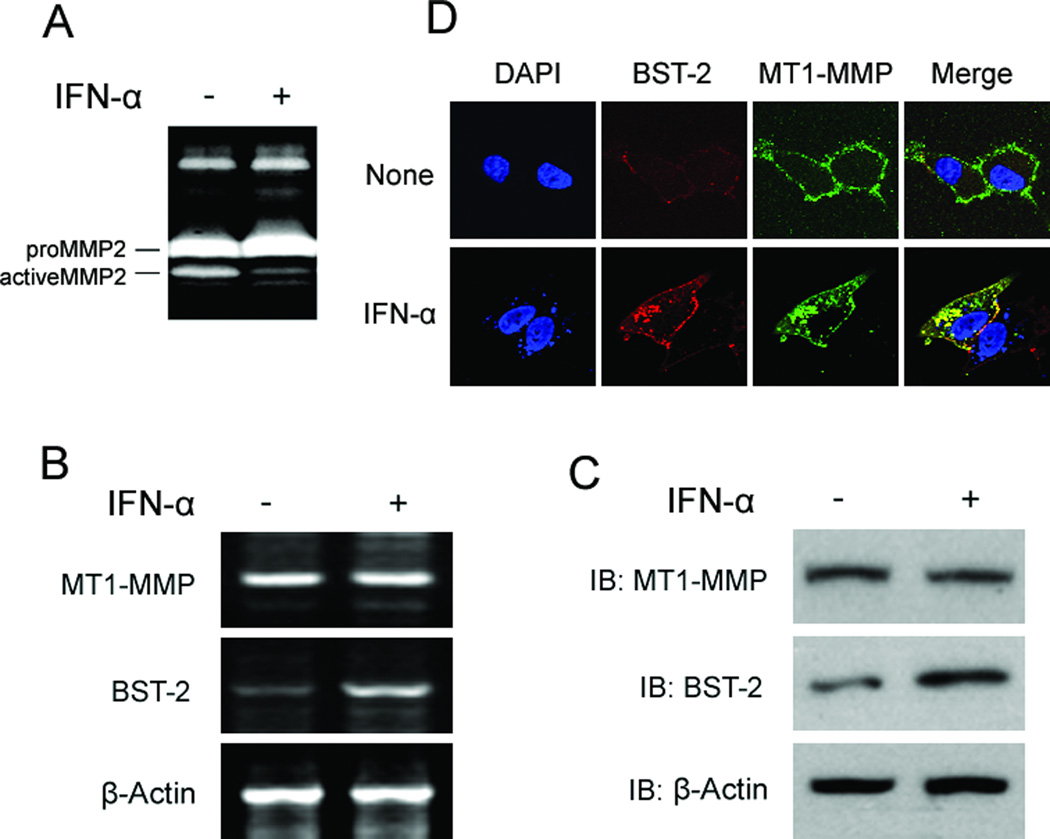
It was reported that IFN-α (type I interferon alpha) could induce the expression of BST-2 in HT1080 cells (Neil et al., 2008). So we employed HT1080 cells to test if the induced endogenous BST-2 also blocked MMP2 activity via binding MT1-MMP and inhibiting its proteolytic enzyme activity. Cells were seeded and cultured in 12-well plates; and 12h later, media was changed with fresh 5% FBS DMEM, and treated with IFN-α (finally concentration: 2,000 U/mL) for another 24 hours (for western blot and RT-PCR experiments, cells were also treated with GM6001). Media were harvested for zymography assay; cells (treated with GM6001) were harvested for western-blot or RT-PCR assays. For Confocal experiments, cells were cultured in 12-well plate with slides and transfected as indicated in figures; and treated with IFN-α and GM6001; and then did Confocal experiment as described in Methods. Our data showed that MMP2 activity treated with IFN-α was significantly decreased by compared with non-treated control (fig.6A). Western-blots and RT-PCR results showed that BST-2 protein and mRNA levels were all increased after treated with IFN-α; but no effect on MT1-MMP (fig. 6B and 6C). Confocal data showed that INF-α induced endogenous BST-2 was co-localized with endogenous MT1-MMP and mainly existed in cytoplasm in granular array forms (fig. 6D). These results demonstrated that IFN-α induced BST-2 in HT1080 cells could also interact with MT1-MMP and inhibit its proteolytic enzyme activity; and finally resulted in decreasing the amount and activity of active MMP2.
Figure 6. INF-α induced BST-2 also co-localized and interacted with MT1-MMP, and decreased MMP2 activity in HT1080 cells.
HT1080 cells were seeded in 12-well plates with 5% FBS DMEM for 12 hours, and then treated with IFN-α (2,000 U/mL) for another 24 hours. Media were harvested for zymography assay (A); cells were harvested for RT-PCR experiments (B) and western-blot assay (C). (D) For Confocal experiment, cells seeded in 12-well plate with slides and treated as above; 24h later, cells were fixed with 4% polyformaldehyde and done Confocal experiment as described in Materials and Methods.
DISCUSSION
In this study, we found that BST-2 could inhibit growth and migration of specific cells which expressed MT1-MMP. Our experimental data showed that membrane protein BST-2 co-localized and interacted with MT1-MMP, and inhibited the proteolytic enzyme activity of MT1-MMP and the activation of proMMP2; finally blocked cell growth and migration. It demonstrated that we found a novel down-regulator and a novel down-regulation mode of MT1-MMP activity, which was via MT1-MMP binding to BST-2 and resulted in decreasing the amount and activity of active MMP2. This regulation mode, same as most other regulation modes, was also related to and depended on the important C-terminal tail domain of MT1-MMP.
It was well identified that MT1-MMP/proMMP2/activeMMP2 activation pathway plays a key role in cancer cell migration in vitro and tumor metastasis in vivo (Seiki, 2003; Zhou et al., 2000). In clinic, some regulators and regulation modes of MT1-MMP were selected as targets of new found chemical drugs which were tested to therapy some tumors. Up to now, there are several regulators and regulation models of MT1-MMP found and reported, including binding to its inhibitors, trafficking between cell surface and cytoplasm, and transcriptional regulation by transcription factors. In this study, we showed that BST-2, as a new regulator of MT1-MMP, could co-localize and interact with MT1-MMP and inhibit its activity; and further block cell growth and migration by decreasing the activation of proMMP2 into active MMP2. From the confocal data, we found that BST-2 bound to MT1-MMP and formed granular arrays and transferred into cytoplasm; thus decreased the amount of MT1-MMP on the cell surface (fig. 3D and 6D). In addition, IFN-α induced endogenous BST-2 was also co-localized and associated with endogenous MT1-MMP in HT1080 cells, and further inhibited the activity of MMP2 (fig.6). Furthermore, we also examined the expression of TIMP-2, which plays a key role in activating proMMP2, by using qRT-PCR; and found that mRNA and protein levels of TIMP-2 were all not changed by transfection of BST-2. It meant that TIMP-2 was not involved in the inhibition of the proteolytic enzyme activity of MT1-MMP by BST-2 (supplemental data fig.s1A). These results indicated that we found a new regulation pathway of MT1-MMP and MMP2 activities; and meant that BST-2 was a negative regulator of MT1-MMP and might be a valuable considering factor in clinical cancer therapy via inhibiting tumor metastasis in vivo.
BST-2 has two mainly bio-functions in cells: the first is that BST-2 is identified as a host cell ‘restriction factor’ counteracted the accessory protein Vpu of enveloped viruses on cell surface and then limits the spread of enveloped viruses (Evans et al., 2010); the second is that BST-2 is preferentially overexpressed on multiple myeloma cells and several cancer cells, and played a key role in tumor progression (Wang et al., 2009; Wainwright et al., 2011). It means that BST-2 is helpful for cell progression in several myeloma and cancer cells. In this study, we found that BST-2 could inhibit cell growth and cell migration in MT1-MMP expressed cell lines. From our strong data, transient BST-2 or IFN-α induced endogenous BST-2 all co-localized and interacted with MT1-MMP when they co-expressed in cells; and blocking the expression of BST-2 by using BST-2 siRNA pool could abrogate the inhibition of MMP-2 activity by BST-2 induced by IFN (supplemental data fig.s1B). Based on this interaction, activity and biological functions of MT1-MMP were negatively regulated and the activation of proMMP2 was thus blocked. This negative regulation of activation pathway, BST-2┨MT1-MMP→MMP2, finally inhibited cell growth and cell migration promoted by MT1-MMP and MMP2. In addition, it was same as most of other membrane proteins that BST-2 could be endocytosis mediated by the trafficking proteins, like clathrin (Rollason et al., 2007; Masuyama et al., 2009). Here our results strongly supported that the interaction complex included BST-2 and MT1-MMP could form granular particles and transfer into cytoplasm (fig.3D and fig.6D). This transfer or trafficking might be also related to clathrin and/or caveolin (need to be further identified). Furthermore, we could imagine that the bio-function of BST-2, as a host cell “restriction factor”, might be also regulated by the binding of MT1-MMP. As to what was the effect of MT1-MMP on the function of BST-2, it should be investigated in the next steps.
C-terminal tail domain and transmembrane domain (TM domain) of MT1-MMP are important domains in regulating its activity and bio-functions (Jiang et al., 2001; Wang et al., 2004; Nyalendo et al., 2007). Our experimental data showed that the MMP2 activity of cells transfected with C-terminal tail domain deletion mutant of MT1-MMP (MT1-MMP/ΔC) was not affected by BST-2, while wild-type MT1-MMP was affected by BST-2 (fig. 4); and immunoprecipitation data also showed that MT1-MMP/ΔC could not interact with BST-2 in co-transfected MDCK cells (fig. 4). These results demonstrated that C-terminal tail domain was still the key part of MT1-MMP in the interaction between MT1-MMP and BST-2. It indicated that BST-2 bound to MT1-MMP through the C-terminal tail domain of MT1-MMP and maybe cytoplasmic N-terminal domain of BST-2. The TM domain (transmembrane domain) of MT1-MMP was not related to the interaction between MT1-MMP and BST-2, but still affected the activity of MT1-MMP.
In summary, the most novel finding here is that BST-2, as a new regulator, can directly interact with MT1-MMP to form granular complex entering into cytoplasm and thus inhibit the cell surface proteolytic enzyme activity of MT1-MMP; and finally inhibit cell growth and cell migration via decreasing activity of MMP2. This implies that BST-2 may have a regulatory role in the subcellular spatial segregation of MT1-MMP, which may lead its transport to cytoplasm from plasma membrane and thus regulate its proteolytic activity on many substrates, including proMMP2.
Supplementary Material
ACKNOWLEDGEMENTS
This project was supported by the grants: the National Nature Science Foundation of China (Nos. 81072433, 31071000), and NIH Grant (CA114418-01A2). We would like to give our thanks to Cynthia L Croy (Department of Pharmacology, University of Minnesota) and Shuangquan Zhang (Life Science College, Nanjing Normal University) for technical assistance and research discussion.
REFERENCES
- Bigg HF, Morrison CJ, Butler GS, Bogoyevitch MA, Wang Z, Soloway PD, Overall CM. Tissue inhibitor of metalloproteinases-4 inhibits but does not support the activation of gelatinase A via efficient inhibition of membrane type 1-matrix metalloproteinase. Cancer Res. 2001;61:3610–3618. [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Marrero-Diaz R, Megías D, Genís L, García-Grande A, García MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Ortho MP, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997;250:751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. BST-2/tetherin: a new component of the innate immune response to enveloped viruses. Trends Microbiol. 2010;18:388–396. doi: 10.1016/j.tim.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gálvez BG, Matías-Román S, Yáñez-Mó M, Vicente-Manzanares M, Sánchez-Madrid F, Arroyo AG. Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol Biol Cell. 2004;15:678–687. doi: 10.1091/mbc.E03-07-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka N, Allen E, Apel IJ, Gyetko MR, Weiss SJ. Matrix metalloproteinases regulate neovascularization by acting as pericellular fibrinolysins. Cell. 1998;95:365–377. doi: 10.1016/s0092-8674(00)81768-7. [DOI] [PubMed] [Google Scholar]
- Hlubek F, Spaderna S, Jung A, Kirchner T, Brabletz T. Beta-catenin activates a coordinated expression of the proinvasive factors laminin-5 gamma2 chain and MT1-MMP in colorectal carcinomas. Int J Cancer. 2004;108:321–326. doi: 10.1002/ijc.11522. [DOI] [PubMed] [Google Scholar]
- Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Lehti K, Wang X, Weiss SJ, Keski-Oja J, Pei D. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc Natl Acad Sci USA. 2001;98:13693–13698. doi: 10.1073/pnas.241293698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Pei D. Distinct roles of catalytic and pexin-like domains in membrane-type matrix metalloproteinase (MMP)-mediated pro-MMP-2 activation and collagenolysis. J Biol Chem. 2003;278:38765–38771. doi: 10.1074/jbc.M306618200. [DOI] [PubMed] [Google Scholar]
- Kang T, Yi J, Yang W, Wang X, Jiang A, Pei D. Functional characterization of MT3-MMP in transfected MDCK cells: progelatinase A activation and tubulogenesis in 3-D collagen lattice. FASEB J. 2000;14:2559–2568. doi: 10.1096/fj.00-0269com. [DOI] [PubMed] [Google Scholar]
- Koshikawa N, Giannelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupzig S, Korolchuk V, Rollason R, Sugden A, Wilde A, Banting G. Bst-2/HM1.24 is a raft-associated apical membrane protein with an unusual topology. Traffic. 2003;4:694–709. doi: 10.1034/j.1600-0854.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- Lehti K, Lohi J, Juntunen MM, Pei D, Keski-Oja J. Oligomerization through hemopexin and cytoplasmic domains regulates the activity and turnover of membrane-type 1 matrix metalloproteinase. J Biol Chem. 2002;277:8440–8448. doi: 10.1074/jbc.M109128200. [DOI] [PubMed] [Google Scholar]
- Liu P, Yang J, Pei J, Pei D, Wilson MJ. Regulation of MT1-MMP activity by β-catenin in MDCK non-cancer and HT1080 cancer cells. J Cell Physiol. 2010;225:810–821. doi: 10.1002/jcp.22292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama N, Kuronita T, Tanaka R, Muto T, Hirota Y, Takigawa A, Fujita H, Aso Y, Amano J, Tanaka Y. HM1.24 is internalized from lipid rafts by clathrin-mediated endocytosis through interaction with alpha-adaptin. J Biol Chem. 2009;284:15927–15941. doi: 10.1074/jbc.M109.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercure MZ, Ginnan R, Singer HA. CaM kinase II delta2-dependent regulation of vascular smooth muscle cell polarization and migration. Am J Physiol Cell Physiol. 2008;294:C1465–C1475. doi: 10.1152/ajpcell.90638.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrocki-Raby B, Gilles C, Polette M, Martinella-Catusse C, Bonnet N, Puchelle E, Foidart JM, Van Roy F, Birembaut P. E-Cadherin mediates MMP down-regulation in highly invasive bronchial tumor cells. Am J Pathol. 2003;163:653–661. doi: 10.1016/S0002-9440(10)63692-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Nyalendo C, Michaud M, Beaulieu E, Roghi C, Murphy G, Gingras D, Béliveau R. Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: role in endothelial and tumor cell migration. J Biol Chem. 2007;282:15690–15699. doi: 10.1074/jbc.M608045200. [DOI] [PubMed] [Google Scholar]
- Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- Pei D, Weiss SJ. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J Biol Chem. 1996;271:9135–9140. doi: 10.1074/jbc.271.15.9135. [DOI] [PubMed] [Google Scholar]
- Pei D. Identification and characterization of the fifth membrane-type matrix metalloproteinase MT5-MMP. J Biol Chem. 1999;274:8925–8932. doi: 10.1074/jbc.274.13.8925. [DOI] [PubMed] [Google Scholar]
- Remacle A, Murphy G, Roghi C. Membrane type I-matrix metalloproteinase (MT1-MMP) is internalised by two different pathways and is recycled to the cell surface. J Cell Sci. 2003;116:3905–3916. doi: 10.1242/jcs.00710. [DOI] [PubMed] [Google Scholar]
- Rollason R, Korolchuk V, Hamilton C, Schu P, Banting G. Clathrin-mediated endocytosis of a lipid-raft-associated protein is mediated through a dual tyrosine motif. J Cell Sci. 2007;120:3850–3858. doi: 10.1242/jcs.003343. [DOI] [PubMed] [Google Scholar]
- Rollason R, Korolchuk V, Hamilton C, Jepson M, Banting G. A CD317/tetherin-RICH2 complex plays a critical role in the organization of the subapical actin cytoskeleton in polarized epithelial cells. J Cell Biol. 2009;184:721–736. doi: 10.1083/jcb.200804154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M. The cell surface: the stage for matrix metalloproteinase regulation of migration. Curr Opin Cell Biol. 2002;14:624–632. doi: 10.1016/s0955-0674(02)00363-0. [DOI] [PubMed] [Google Scholar]
- Seiki M. Membrane-type 1 matrix metalloproteinase: a key enzyme for tumor invasion. Cancer Lett. 2003;194:1–11. doi: 10.1016/s0304-3835(02)00699-7. [DOI] [PubMed] [Google Scholar]
- Uekita T, Itoh Y, Yana I, Ohno H, Seiki M. Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J Cell Biol. 2001;155:1345–1356. doi: 10.1083/jcb.200108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Nie J, Pei D. Mint-3 regulates the retrieval of the internalized membrane-type matrix metalloproteinase, MT5-MMP, to the plasma membrane by binding to its carboxyl end motif EWV. J Biol Chem. 2004;279:51148–51155. doi: 10.1074/jbc.M400264200. [DOI] [PubMed] [Google Scholar]
- Wainwright DA, Balyasnikova IV, Han Y, Lesniak MS. The expression of BST2 in human and experimental mouse brain tumors. Exp Mol Pathol. 2011;91:440–446. doi: 10.1016/j.yexmp.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Nishioka Y, Ozaki S, Jalili A, Abe S, Kakiuchi S, Kishuku M, Minakuchi K, Matsumoto T, Sone S. HM1.24 (CD317) is a novel target against lung cancer for immunotherapy using anti-HM1.24 antibody. Cancer Immunol Immunother. 2009;58:967–976. doi: 10.1007/s00262-008-0612-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will H, Atkinson SJ, Butler GS, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996;271:17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Apte SS, Soininen R, Cao R, Baaklini GY, Rauser RW, Wang J, Cao Y, Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci USA. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.