Abstract
In the clinical setting, repeated exposures (10–30) to low-doses of ionizing radiation (≤ 200 cGy), as seen in radiotherapy for cancer, causes fatigue. Almost nothing is known, however, about the fatigue inducing effects of a single exposure to environmental low-dose ionizing radiation that might occur during high-altitude commercial air flight, a nuclear reactor accident or a solar particle event (SPE). To investigate the short-term impact of low-dose ionizing radiation on mouse biobehaviors and neuroimmunity, male CD-1 mice were whole body irradiated with 50 cGy or 200 cGy of gamma or proton radiation. Gamma radiation was found to reduce spontaneous locomotor activity by 35% and 36%, respectively, 6 h post irradiation. In contrast, the motivated behavior of social exploration was un-impacted by gamma radiation. Examination of pro-inflammatory cytokine gene transcripts in the brain demonstrated that gamma radiation increased hippocampal TNF-α expression as early as 4 h post-irradiation. This was coupled to subsequent increases in IL-1RA (8 h and 12 h post irradiation) in the cortex and hippocampus and reductions in activity-regulated cytoskeleton-associated protein (Arc) (24 h post irradiation) in the cortex. Finally, restraint stress was a significant modulator of the neuroimmune response to radiation blocking the ability of 200 cGy gamma radiation from impairing locomotor activity and altering the brain-based inflammatory response to irradiation. Taken together, these findings indicate that low-dose ionizing radiation rapidly activates the neuroimmune system potentially causing early onset fatigue-like symptoms in mice.
Keywords: Innate immunity, Co, Cs, Interleukin-1 (IL-1), prodromal stage, acute radiation syndrome (ARS)
Introduction
The impact of ionizing radiation on behavior and neuroimmunity is an emerging field. Currently, the primary focus is on clinically delivered radiation therapy to oncology patients and the consequent adverse biobehavioral impact these critical treatments engender (Bower et al. 2009, Hofman et al. 2007, James 2006). Therapeutic radiation involves delivery of relatively high doses (30–80 Gy/14–60 days) administered focally and strategically to limit treatment of uninvolved normal tissues (Lawrence et al. 2008). The majority of patients receiving radiotherapy over the past century have been treated with electrons, x-rays (high energy photons) or gamma-rays (high energy photons). The distinction between x- and gamma-rays from a radiotherapy perspective relates to the source of the photons: x-rays originate from outer electrons and gamma rays originate from atomic nuclei. In radiotherapy applications, however, both x- and gamma-rays are photons in the 1–20 MeV energy range. The physical properties of these forms of radiation cause maximal energy deposition (dose) to occur early in the tissue particle track at depths of 0.5–4 cm. Recently, there has been renewed interest in treating patients with heavier charged particles such as protons, which deposit the majority of their dose toward the end of the tissue particle track at depths up to 20–30 cm. This affords more focused delivery of dose to deeply seated neoplasms with less radiation being administered to tissues more distal to the target (Evaluation Subcommittee of ASTRO’s Emerging Technologies Committee). In addition to the differences in macroscopic dose distribution, photons and protons create disparate microscopic dose distributions due to dissimilar linear energy transfer (LET) coefficients. Importantly, differences in microdosimetric track structure may cause photons and protons to have qualitatively and quantitatively unequal dose-toxicity profiles (Cengel et al. 2010).
In contrast, environmental radiation exposure is ubiquitous, of very low-dose (approximately 6.2 mGy/year) (Schauer and Linton 2009), whole body and comprised of a mix of particle types that include photons, electrons, protons and heavy ions. This environmental dosage, however, can jump significantly at altitudes that commercial aircraft fly (approximately 6.30–6.79 μGy/h (Mohler 2003)), in manned space exploration (approximately 50–100 μGy/day during interplanetary travel and 25–50 μGy/day on planetary surfaces (Cucinotta and Durante 2006)) or during a severe nuclear reactor accident such as occurred at Fukishima Daiichi Nuclear Power Plant in Japan 2011 (ground air as high as 1 Gy) (Makihijani 2011, Weissman 2011). Environmental radiation can be markedly compounded during a solar particle event (SPE) with doses reaching 1.4 Gy/h for skin, 0.8 Gy/h for eyes and 8 cGy for bone marrow in data modeling studies from the August 1972 SPE (Parsons and Townsend 2000). In addition, SPE irradiation is primarily comprised of relatively superficially penetrating protons with energies less than 50 MeV. The energy spectra a specific SPE, however, is highly variable and some SPES have had a greater proportion of deeply penetrating, higher energy protons. With the anticipation of expanded near space/space tourism/travel, nuclear power plant construction and threat of nuclear terrorism, the population at risk for total body radiation exposures in the range of 0.5–2 Gy are likely to increase appreciably.
Total body exposure to ionizing radiation can lead to acute radiation syndrome (ARS) that includes the initial prodromal stage defined by nausea, vomiting and diarrhea (N-V-D stage) (Donnelly et al. 2010). An underappreciated component of the prodromal stage is neuroimmune system-mediated sickness symptoms often described as feelings of unease and weakness with an associated lack of motivation and energy (Hofman et al. 2007, Marquette et al. 2003, Young 1987). Like other maladies associated with weariness and malaise, radiation-induced fatigue is a complex interplay of mental, emotional and physical biobehaviors that are often ignored due to concerns over the manifest illness stage and, ultimately, survival. The first radiation-induced behavioral effects involving the dose and type(s) of radiation present in SPEs were delineated in animals (predominantly primates) during the 1970s and 80s. Memory and cognition testing in monkeys irradiated at dose rates of 0.3, 0.8 and 1.8 Gy/min, (total dose of 10.0 Gy) showed that hampered performance occurred in 81% of animals at 1.8 Gy/min but only in 7% of animals at 0.3 Gy/min. Thus, the effective dose for radiation-induced performance deficits was estimated to occur at doses of 3 Gy or less (Bogo 1988). In addition, behavioral test complexity appeared impacted by ionizing radiation with tasks requiring greater physical exertion being affected more (Bogo 1988). As for rodents, conditioned taste aversion could be induced at doses as low as 0.25 Gy (Bogo 1988). Interestingly, 3 Gy of proton radiation caused a decrease in latency to fall in rotarod testing and loss of acoustic startle habituation (Pecaut et al. 2002). In sum, almost all studies reporting on the behavioral impact of low-dose radiation (≤10 Gy) examined endpoints of days to weeks post radiation. Therefore, almost nothing is known about the immunobehavioral impact of low-dose ionizing radiation within hours after exposure.
Methods
Materials
All reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except as noted. RNAlater (AM7021) and RiboPure Blood Kits (AM1928) were purchased from Ambion (Austin, TX). QIAGEN RNeasy Lipid Tissue Mini Kits (Cat No. 74804) were purchased from QIAGEN (Valencia, CA). Reverse transcription kits and primers for qPCR were purchased from Applied Biosystems (Foster City, CA). Plastic containment cubes (AMAC530C) were purchased from AMAC Plastic Products (Petaluma, CA).
Animals
Animal use was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council). Male 6-wk old CD-1 mice (n=632) were obtained from Taconic (Hudson, NY). Mice were group housed (4 × cage) in standard shoebox cages (length 28 cm; width 17 cm; height 12.5 cm) and allowed water and standard rodent chow ad libitum. Mice where maintained in an environmentally controlled room on a 12 h dark/light cycle (1900 h-0700 h) at a temperature of 72° F and a humidity of 45–55%. Mice tested were 7–8 wks of age. Video recording of animal behavior was performed under red light using a Sony HDR-XR500V Night Shot capable video camera (San Diego CA, USA).
Radiation exposure
Mice were singly housed for 12 h prior to irradiation or sham irradiation. 137Cs irradiated mice were exposed to gamma radiation for no more than 10 min at a dose rate of 44.50 ± 0.1 cGy/min (high dose rate) to doses of 50 cGy or 200 cGy. 137Cs radiation was delivered using a Nordion GammaCell 40-Series 1 Irradiator (Ottawa, Canada). 60Co irradiated mice were exposed to gamma radiation at a dose rate of 0.5 ± 0.01 cGy/min (low-dose rate). 60Co radiation was delivered using an Atomic Energy of Canada Eldorado Model ‘G’ irradiator (Ottawa, Canada). Proton irradiated mice were exposed to proton radiation at a dose rate of either 50.0 ± 0.1 cGy/min (high-dose rate) or 0.5 ± 0.01 cGy/min (low dose rate). Proton radiation was delivered using the horizontal clinical beam line at the Loma Linda University Medical Center (LLUMC, Loma Linda CA). Protons were tailored to have a similar macroscopic dose distribution as 60Co produced photons, which are considered to be the standard by which other forms of therapeutic radiation are compared (Cengel et al. 2010, Maks et al. 2011). Sham irradiated mice were treated identically to irradiated mice except the radiation source remained shielded. Irradiation occurred 3 h prior to the start of the dark cycle (1600 h).
Restraint
Immediately before radiation exposure or sham irradiation exposure, all subject mice were individually placed inside 7.25 cm × 4.10 cm × 4.10 cm well-ventilated containment cubes. These cubes did not provide absolute restraint and mice were able to move minimally. In minimally restrained mice, time of confinement did not exceed 10 min (restraint-10 mice). For mice subjected to more sustained restraint, confinement in the containment cube was 240 min (restraint-240 mice). Therefore, restraint-10 mice were irradiated while simultaneously confined (total confinement/irradiation duration ≤ 10 min). Restraint-240 mice were either: 1) irradiated while simultaneously confined (confined/irradiation duration, 10 min) then allowed to remain in the containment cube sans irradiation for an additional 230 min (total confinement, 240 min) or 2) irradiated while simultaneously confined (total confinement/irradiation duration, 240 min). Following any form of restraint, mice were returned to their home cage.
Social exploration
Social exploration was performed as we have previously described (Johnson et al. 2007). Social exploration testing was initiated 10 min after irradiation/restraint exposure. At the time points indicated a 3–4 wk-old novel conspecific juvenile mouse of the same sex (challenge mouse) was introduced into the home cage of the subject mouse. The challenge mouse was confined in a 7.62 × 7.62 × 7.62 cm square metal mesh enclosure. Testing duration was 5 min and a new challenge mouse was used to test each subject mouse at every time point examined. Investigation/exploration was evaluated from the video record and was considered as nose-to-enclosure contact.
Locomotion
Spontaneous locomotor activity was measured as we have previously described (Lavin et al. 2011). Immediately after irradiation/restraint exposure and at the time points indicated mice were video recorded in their home cage for 5 min. Movement was quantified using EthoVision XT 7 (Noldus Information Technology, Leesburg VA). Parameters examined included distance moved (cm) and velocity of movement (cm/s).
qPCR
Following behavioral testing, mice were sacrificed via CO2 asphyxiation. For blood collection, cardiac puncture was executed using a Becton Dickinsin (BD) 26G × 3/8 inch needle (Franklin Lakes, NJ). Drawn blood was anti-coagulated in EDTA containing Microtainers (BD, Cat No. 365974). After anticoagulation, 0.4 mL of blood was mixed in 1.3 mL of RNAlater. Total RNA was extracted using RiboPure Blood Kits per manufacturer’s instructions. For brain collection, brains were harvested and either perfused or not perfused (un-perfused) (as indicated) with ice cold PBS to remove blood as we have done previously (Lavin 2011). Perfusion studies were performed to determine the contribution, if any, of blood based gene transcripts to brain biomarker detection. Where indicated cortex, hippocampus, hypothalamus and cerebellum were separately dissected from perfused brains as we have done previously (Lavin 2011). In all brain isolations, olfactory bulb was not included for study. Total RNA was extracted using a QIAGEN RNeasy Lipid Tissue Mini Kit per the manufacturer’s instructions. After all RNA extractions reverse transcription was performed with an Applied Biosystem high-capacity cDNA reverse transcription kit (Cat No. 4368813). As indicated, qPCR utilized the following TaqMan (Applied Biosystems) gene expression primers: IL-1β (Mm99999061_mH), TNF-α (Mm00443258_m1), IL-1RA (Mm01337566_m1), activity-regulated cytoskeleton-associated protein (Arc) (Mm00479619_m1), IL-6 (Mm01210733_m1), IL-1α (Mm_99999060_m1) and IFN-γ (Mm99999071_m1). qPCR was performed on a 7900 HT Fast real-time PCR system (Applied Biosystems) using TaqMan universal PCR master mix (Cat No. 4318157). To normalize gene expression, a parallel amplification of endogenous glyceraldehydes-3-phosphate dehydrogenase (Mm03302249_g1) was performed. Relative quantitative evaluation of target gene levels was performed by comparing ΔCt’s, where Ct was the threshold concentration.
Statistics
All data are presented as mean ± SEM. Data were analyzed using SAS 9.2 (SAS Institute, Inc., Cary NC). To test for statistical differences, a one-way or two-way ANOVA was used with or without repeated measurements where needed. Tukey’s test was used for post-hoc pair-wise multiple comparison procedures. Where needed and indicated, raw data was transformed to attain normal distribution. Also, where indicated, a Freidman’s two-way ANOVA for non-parametric analysis was used when nonparametric data was unable to be transformed to a normal distribution. All statistical analysis included testing for time point × dose, restraint × dose or perfusion × dose interactions. Statistical significance was denoted at p<0.05.
Results
Gamma radiation but not proton radiation reduces mouse locomotor activity
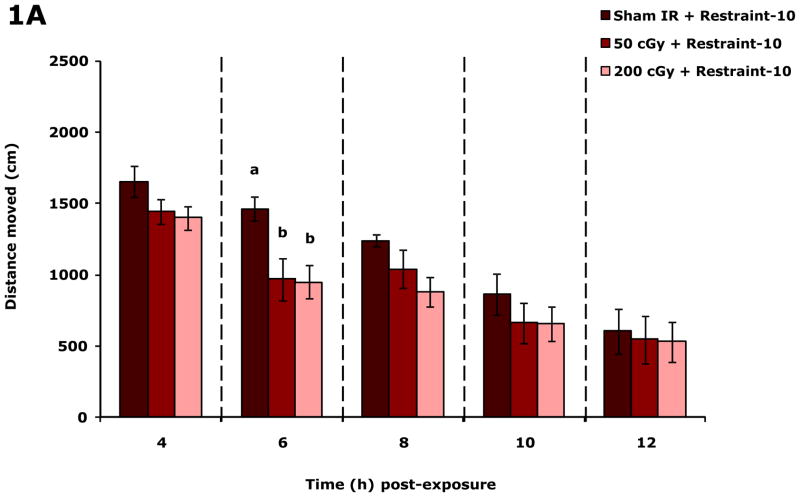
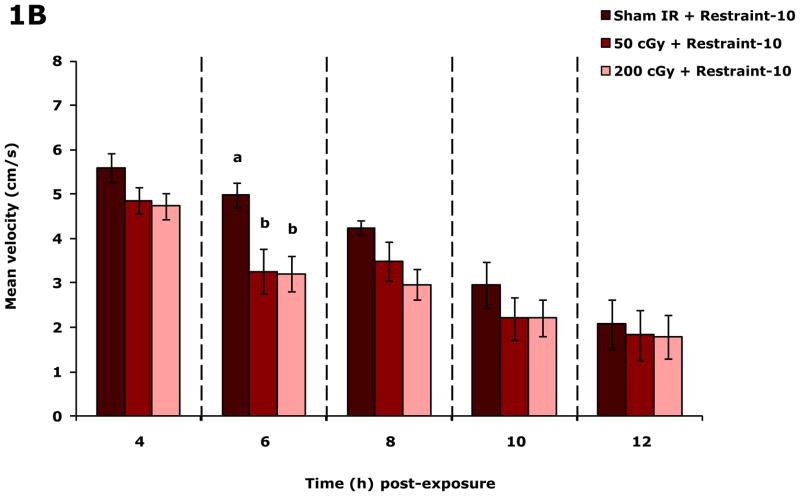
Figure.1A shows that restraint-10 mice exposed to 50 or 200 cGy of gamma radiation (44.5 ± 0.1 cGy/min) had, respectively, a 33.8% and 35.1% reduction in spontaneous distance moved (locomotion) 6 h post irradiation compared to sham irradiated mice. Figure.1B shows that mean velocity of movement (velocity) was reduced 6 h post irradiation at both 50 and 200 cGy of gamma radiation by 34.7% and 35.7%, respectively. When 50 or 200 cGy of gamma radiation was delivered at approximately 1/100 the dose rate (0.5 ± 0.01 cGy/min), mouse locomotion/velocity was not impacted at 0, 2, 4, 6, 8 or 24 h after irradiation (data not shown). Similarly, when 50 or 200 cGy of proton radiation was used (dose rate of either 0.5 ± 0.01 cGy/min or 50.0 ± 0.1 cGy/min), mouse locomotion/velocity was not impacted at 0, 2, 4, 6, 8 or 24 h after irradiation (data not shown). Social exploration was not affected by either 50 or 200 cGy of gamma or proton radiation (regardless of dose rate) at 0, 2, 4, 6, 8 and 24 post irradiation (Table S1).
Figure 1. Gamma radiation but not proton radiation reduces mouse locomotor activity.
Restraint-10 mice were exposed to 50 or 200 cGy of gamma radiation (44.5 ± 0.1 cGy/min) as indicated. Spontaneous locomotor activity and velocity of movement were measured at the time points indicated post irradiation. Results are expressed as means ± s.e.m.; n = 8. (A) Distance moved (cm): main effects of dose (P < 0.001) and time point (P < 0.001); 6 h time point: P < 0.05, sham IR v. 50 cGy (1463.8 ± 83.7 v. 968.4 ± 147.6) and sham IR v. 200 cGy (1463.8 ± 83.7 v. 950.5 ± 117.2). (B) Velocity of movement (cm/s): main effects of dose (P < 0.001) and time point (P < 0.001); 6 h time point: P < 0.05, sham IR v. 50 cGy (5.0 ± 0.3 v. 3.3 ± 0.5) and sham IR v. 200 cGy (5.0 ± 0.3 v. 3.2 ± 0.4). Bars without a common superscript are different (P < 0.05).
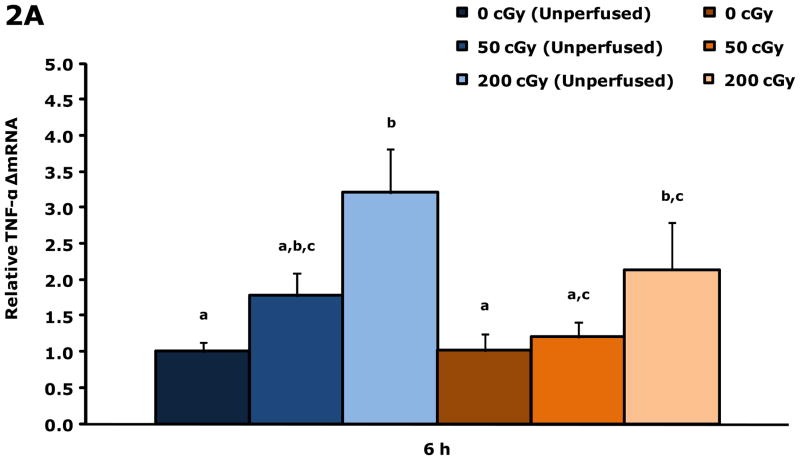
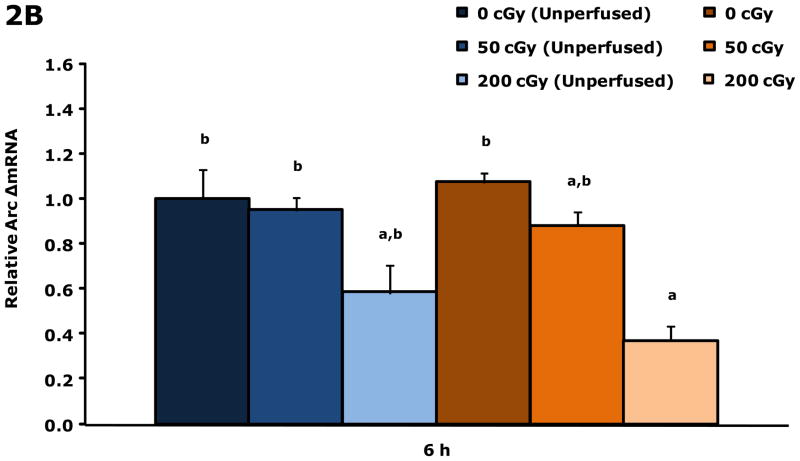
Gamma radiation up-regulates gene transcripts for TNF-α and Arc in whole brains 6 h post irradiation
Figure.2A shows that unperfused and perfused brains from restraint-10 mice exposed to 200 cGy of gamma radiation (44.5 ± 0.1 cGy/min) had a 3.2-fold and 2.1-fold increase in TNF-α gene transcripts, respectively, 6 h post irradiation. Whole brain gene transcript expressions for IL-1α, IL-1β, IL-1RA, IL-6 and IFN-γ were not impacted by gamma radiation at this time point. Figure 2B demonstrates that in perfused brains restraint-10 mice exposed to 200 cGy of gamma radiation (44.5 ± 0.1 cGy/min) had a 0.37-fold decrease in Arc 6 h post irradiation.
Figure 2. Gamma radiation up-regulates gene transcripts for TNF-α and Arc in whole brain 6 h post irradiation.
Restraint-10 mice were exposed to 50 or 200 cGy of gamma radiation (44.5 ± 0.1 cGy/min) as indicated. qPCR was used to quantify mRNAs from un-perfused and perfused whole brains as indicated 6 h post irradiation. Results are expressed as relative fold change in mRNA expression (ΔmRNA), means ± s.e.m.; n = 4–6. (A) TNF-α: main effect of dose (P < 0.001) and perfusion (P = 0.042); P < 0.05, 200 cGy (unperfused) v. sham IR (unperfused), sham IR, 50 cGy (3.205 ± 0.252 v. 1.000 ± 0.166, 1.017 ± 0.286, 1.201 ± 0.241, respectively); P < 0.05, 200 cGy v. sham IR (unperfused), sham IR (2.139 ± 0.387 v. 1.000 ± 0.166, 1.017 ± 0.286, respectively). (B) Arc: main effect of dose (P = 0.003); P < 0.05, 200 cGy v. sham IR (unperfused), 50 cGy (unperfused), sham IR (0.370 ± 0.076 v. 1.000 ± 0.239, 0.951 ± 0.211, 1.074 ± 0.107, respectively).
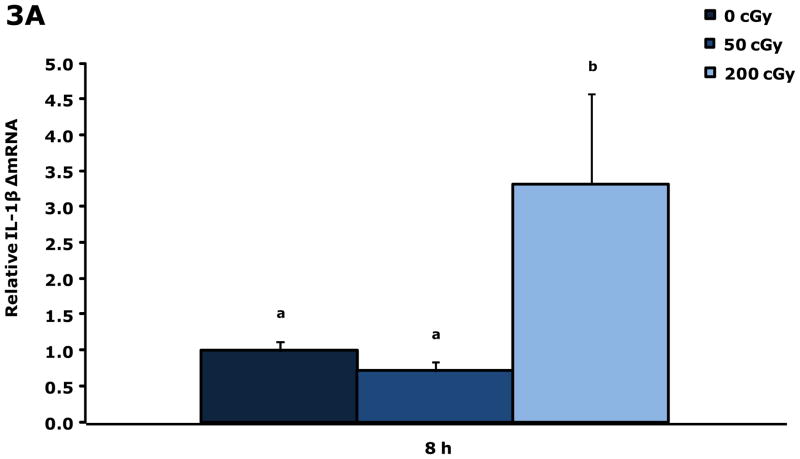
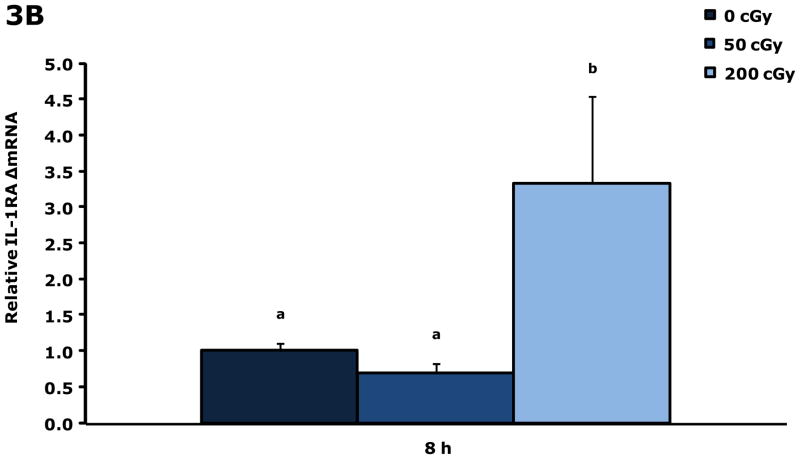
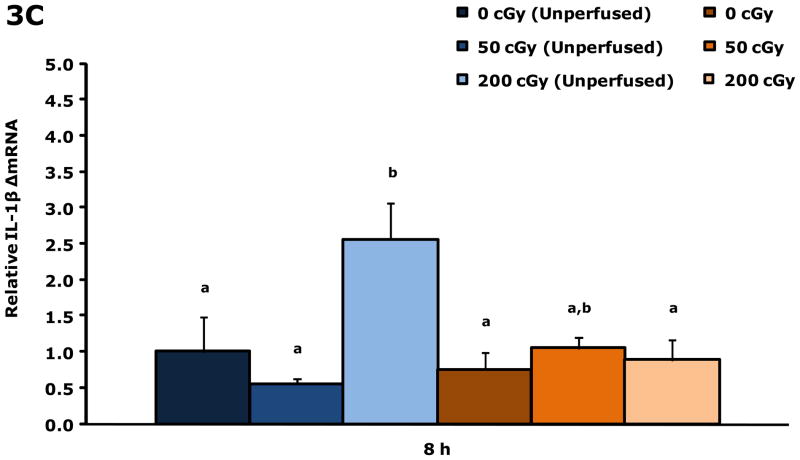
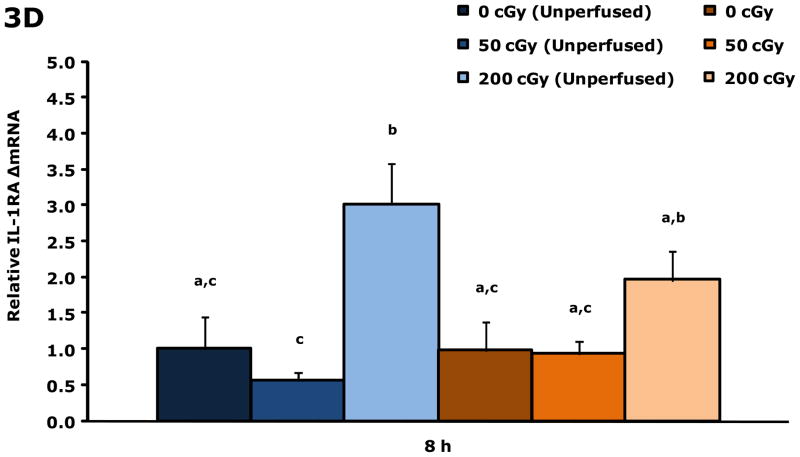
Gamma radiation up-regulates gene transcripts for IL-1β and IL-1RA in blood 8 h post irradiation
Figure. 3A&B show that blood from restraint-10 mice exposed to 200 cGy of gamma radiation (44.5 ± 0.1 cGy/min) had a 3.3-fold and 3.3-fold increase in IL-1β and IL-1RA gene transcripts, respectively, 8 h post irradiation. Blood gene transcript expressions for IL-1α, TNF-α, IL-6, and IFN-γ were not impacted by gamma radiation at this time point or at 4 h post gamma irradiation. IL-1α was increased 2.1-fold at 6 h by 200 cGy of gamma radiation (data not shown), however, IL-1β, IL-1RA, TNF-α, IL-6, and IFN-γ were not. Figure. 3C&D demonstrate that up-regulation of IL-1β and IL-1RA gene transcripts in whole brain at 8 h post gamma irradiation are due to blood in the brain.
Figure 3. Gamma radiation up-regulates gene transcripts for IL-1β and IL-1RA in blood 8 h post irradiation.
Restraint-10 mice were exposed to 50 or 200 cGy of gamma radiation (44.5 ± 0.1 cGy/min) as indicated. qPCR was used to quantify mRNAs from blood and un-perfused and perfused whole brains as indicated 8 h post irradiation. Results are expressed as relative fold change in mRNA expression (ΔmRNA), means ± s.e.m.; n = 4–6. (A) IL-1β: main effect of dose (P < 0.001); P < 0.05, 200 cGy v. sham IR, 50 cGy (3.312 ± 0.468 v. 1.000 ± 0.182, 0.711 ± 0.254, respectively). (B) 8 h IL-1RA: main effect of dose (P < 0.001); P < 0.05, 200 cGy v. sham IR, 50 cGy (3.335 ± 0.446 v. 1.000 ± 0.146, 0.690 ± 0.273, respectively). (C) IL-1β: main effect of dose (P = 0.006), dose-perfusion interaction (P = 0.002); P < 0.05, 200 cGy (unperfused) v. sham IR (unperfused), 50 cGy (unperfused), sham IR, 50 cGy (2.555 ± 0.267 v. 1.000 ± 0.570, 0.557 ± 0.182, 0.755 ± 0.407, 1.052 ± 0.189, respectively). (D) IL-1RA: main effect of dose (P < 0.001); P < 0.05, 200 cGy (unperfused) v. sham IR (unperfused), 50 cGy (unperfused), sham IR, 50 cGy (3.013 ± 0.255 v. 1.000 ± 0.531, 0.570 ± 0.244, 0.982 ± 0.488, 0.935 ± 0.258, respectively); P = 0.009, 200 cGy v. 50 cGy (unperfused) (1.961 ± 0.267 v. 0.570 ± 0.244). Bars without a common superscript are different (P < 0.05).
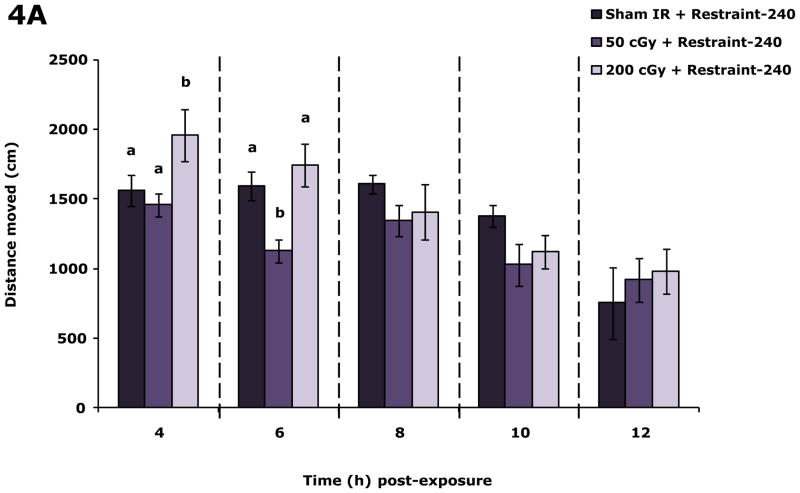
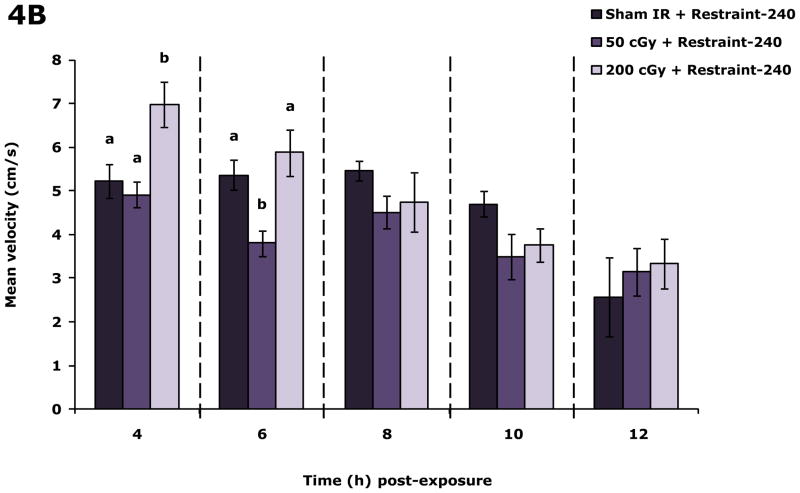
Restraint inhibits the impact of 200 cGy gamma radiation on locomotor activity
Fig. 4A demonstrates that in restraint-240 mice 200 cGy of radiation (44.5 ± 0.1 cGy/min) increased locomotion 4 h post irradiation by 20.3% and 25.4%, respectively, compared to sham irradiated restraint-240 mice and restraint-240 mice exposed to 50 cGy of gamma radiation. Likewise, 4 h post irradiation velocity was increased in restraint-240 mice exposed to 200 cGy of gamma radiation by 25.7% and 45.7%, respectively, compared to sham irradiated restraint-240 mice and restraint-240 mice exposed to 50 cGy of gamma radiation. At 6 h post irradiation mice exposed to 50 cGy had a 29.2% and 35.3% decrease in locomotion compared to sham and 200 cGy gamma irradiated mice and a 29.6% and 35.6% reduction in velocity, respectively. When restraint-240 mice were compared to restraint-10 mice, restraint-240 mice exposed to 200 cGy of gamma radiation (44.5 ± 0.1 cGy/min) moved 39.5%, 83.5% and 59.1% farther at 4, 6, and 8 h post irradiation, respectively (Table S2). Non-irradiated restraint-240 mice and restraint-10 mice moved similarly except at the 10 h time point (Table S2).
Figure 4. Restraint-240 inhibits the impact of 200 cGy gamma radiation on locomotor activity.
Restraint-240 mice were exposed to 50 or 200 cGy of gamma radiation (44.5 ± 0.1 cGy/min) as indicated. Spontaneous locomotor activity and velocity of movement were measured at the time points indicated post irradiation. Results are expressed as means ± s.e.m.; n = 8–16. (A) Distance moved (cm): main effects of dose (P = 0.004) and time point (P < 0.001), dose-time point interaction (P = 0.036); 4 h time point: P ≤ 0.05, sham IR + restraint-240 v. 200 cGy + restraint-240 (1559.1 ± 111.5 v. 1955.8 ± 188.1), 50 cGy + restraint-240 v. 200 cGy + restraint-240 (1457.3 ± 85.6 v. 1955.8 ± 188.1); 6 h time point: P < 0.05, sham IR + restraint-240 v. 50 cGy + restraint-240 (1592.9 ± 101.8 v. 1127.7 ± 82.0), 50 cGy + restraint-240 v. 200 cGy + restraint-240 (1127.7 ± 82.0 v. 1743.9 ± 151.2). (B) Velocity of movement (cm/s): main effects of dose (P =0.003) and time point (P < 0.001), dose-time point interaction (P = 0.013); 4h time point: P < 0.05, sham IR + restraint-240 v. 200 cGy + restraint-240 (5.2 ± 0.4 v. 7.0 ± 0.5), 50 cGy + restraint-240 v. 200 cGy + restraint-240 (4.9 ± 0.3 v. 7.0 ± 0.5); 6 h time point: P < 0.05, sham IR + restraint-240 v. 50 cGy + restraint-240 (5.4 ± 0.4 v. 3.8 ± 0.3), 50 cGy + restraint-240 v. 200 cGy + restraint-240 (3.8 ± 0.3 v. 5.9 ±0.5). Bars without a common superscript are different (P < 0.05).
Restraint of gamma irradiated mice impacts TNF-α, IL-1RA and Arc gene expression differentially in cerebral hippocampus, hypothalamus, cortex, and cerebellum at 4, 8, 12 and 24 h post irradiation
Table 1 shows that when restraint-10 mice and restraint-240 mice were exposed to either 50 or 200 cGy of gamma radiation (44.5 ± 0.1 cGy/min) differential gene transcript expression of TNF-α, IL-1RA and Arc occurred that was dependent on brain region, restraint duration and dose of gamma radiation.
Table 1.
Impact of high-dose rate gamma irradiation on TNF-α, IL-1RA and Arc gene transcripts in cerebral hippocampus, hypothalamus, cortex, and cerebellum at 4, 8, 12 and 24 h post irradiation.
| Brain region | Time post-irradiation | Restraint-10
|
Restraint-240
|
|||||
|---|---|---|---|---|---|---|---|---|
| Gene | Sham IR | 50 cGy | 200 cGy | Sham IR | 50 cGy | 200 cGy | ||
| Hippocampus | 4 h | TNF-α*# | 1.000 (0.253)a,c | 1.144 (0.133)a,c | 1.535 (0.211)a | 0.394 (0.261)b,c | 0.599 (0.075)c | 1.326 (0.450)a |
| Hippocampus | 4 h | Arc# | 1.000 (0.132)a | 0.886 (0.098)a | 0.974 (0.147)a | 1.730 (0.172)b | 1.303 (0.184)a,b | 1.326 (0.177)a,b |
| Hypothalamus | 4 h | TNF-α*# | 1.000 (0.320)a | 0.985 (0.260)a,b | 1.019 (0.180)a | 1.235 (0.355)b,c | 1.248 (0.127)c | 1.260 (0.339)a |
| Hypothalamus | 4 h | IL-1RA# | 1.000 (0.437)a,b | 0.668 (0.199)a,b | 1.313 (0.137)a | 0.630 (0.381)a,b | 0.560 (0.194)b | 0.683 (0.275)a,b |
| Cortex | 4 h | TNF-α*#† | 1.000 (0.237)a | 1.076 (0.137)a | 1.226 (0.184)a | 0.420 (0.220)b | 0.516 (0.110)b | 1.096 (0.356)a |
| Cortex | 4 h | Arc*# | 1.000 (0.154)a,b,c | 0.573 (0.157)a | 0.667 (0.127)a,c | 1.732 (0.375)b | 1.426 (0.227)b | 1.199 (0.272)b,c |
| Cerebellum | 4 h | TNF-α*# | 1.000 (0.121)a | 0.963 (0.131)a | 1.164 (0.198)a | 0.522 (0.146)b | 0.465 (0.233)b | 0.822 (0.352)a,b |
| Cerebellum | 4 h | Arc#§ | 1.000 (0.062)a | 0.923 (0.042)a | 0.895 (0.092)a | 0.532 (0.083)c | 0.573 (0.130)b,c | 0.786 (0.147)a,b |
| Hippocampus | 8 h | TNF-α* | 1.000 (0.127)a | 1.338 (0.094)a | 2.093 (0.136)b | 1.002 (0.146)a | 1.200 (0.131)a | 1.965 (0.138)b |
| Hippocampus | 8 h | IL-1RA* | 1.000 (0.491)a,c | 0.909 (0.204)a | 1.880 (0.203)b,c | 1.537 (0.249)a,b,c | 1.008 (0.203)a,c | 2.280 (0.103)b |
| Hippocampus | 8 h | Arc# | 1.000 (0.127)a,b | 0.972 (0.108)a,b | 1.124 (0.159)a | 0.904 (0.141)a,b | 0.782 (0.094)b | 0.869 (0.106)a,b |
| Hypothalamus | 8 h | Arc#† | 1.000 (0.088)a | 1.066 (0.087)a | 0.770 (0.194)a,b | 0.610 (0.111)b,c | 0.473 (0.205)c | 0.674 (0.118)b,c |
| Cortex | 8 h | TNF-α* | 1.000 (0.224)a,b | 1.092 (0.094)a,b | 1.645 (0.264)a | 0.732 (0.140)b | 1.395 (0.336)a | 1.454 (0.212)a |
| Cortex | 8 h | IL-1RA*§ | 1.000 (0.159)a | 1.109 (0.067)a | 3.043 (0.196)b | 1.199 (0.183)a | 1.188 (0.157)a,b | 2.630 (0.085)b |
| Hippocampus | 12 h | TNF-α*† | 1.000 (0.176)a | 1.497 (0.146)b,c | 1.890 (0.136)c | 1.123 (0.123)a,b | 1.173 (0.111)a,b | 2.409 (0.152)c,d |
| Hippocampus | 12 h | IL-1RA* | 1.000 (0.261)a | 1.873 (0.241)b | 2.391 (0.210)b | 1.731 (0.167)a,b | 1.692 (0.275)a,b | 2.991 (0.123)b |
| Cortex | 12 h | TNF-α*# | 1.000 (0.191)a | 1.131 (0.172)a,b | 1.732 (0.149)b | 1.131 (0.202)a,b | 1.154 (0.165)a,b | 2.899 (0.205)c |
| Cortex | 12 h | IL-1RA*†§ | 1.000 (0.076)a | 1.157 (0.055)a | 2.454 (0.067)b | 1.374 (0.113)a | 1.228 (0.117)a | 3.499 (0.113)b |
| Hippocampus | 24 h | TNF-α* | 1.000 (0.110)a | 1.109 (0.071)a,b | 1.529 (0.158)b,c | 1.050 (0.070)a,b | 1.166 (0.263)a,b | 1.807 (0.147)c |
| Hippocampus | 24 h | IL-1RA* | 1.000 (0.218)a,b | 0.804 (0.306)a | 1.133 (0.159)a,b | 0.894 (0.175)a,b | 1.049 (0.337)a,b | 1.524 (0.097)b |
| Cortex | 24 h | TNF-α* | 1.000 (0.227)a | 1.108 (0.100)a,c | 1.803 (0.154)b,c | 1.073 (0.146)a | 1.139 (0.254)a,c | 2.332 (0.184)b |
| Cortex | 24 h | IL-1RA*† | 1.000 (0.215)a,b | 0.662 (0.169)a | 1.054 (0.141)a,b | 0.697 (0.164)a | 0.855 (0.304)a | 1.580 (0.123)b |
| Cortex | 24 h | Arc*† | 1.000 (0.191)a | 0.648 (0.123)a,b,c | 0.431 (0.060)c | 0.564 (0.126)b,c | 0.678 (0.170)a,b | 0.747 (0.258)a,b |
Results are expressed as relative fold change in mRNA expression (ΔmRNA), means (s.e.m.); n = 6.
P < 0.05, significant main effect of dose.
P < 0.05, significant main effect of restraint-240.
P < 0.05, significant dose-restraint-240 interaction. Results within individual rows without a common superscript letter are different (P < 0.05).
indicates data was transformed using ((Original value)2)1/4).
Discussion
Near continuous exposure to environmental ionizing radiation of a very low-dose rate is omnipresent. With certain occupations and in certain circumstances, dose rate can increase such that a modest dose of radiation (200 cGy) is received in a relatively short period of time (>8 hr). In humans, these exposures are increasing in frequency (nuclear accidents) and becoming better recognized (SPEs). When ionizing radiation doses are significant in duration or energy to cause ARS, prodromal stage symptoms of nausea, vomiting and diarrhea (NVD) (Donnelly at al. 2010, James 2006) occur manifesting within hours (Donnelly et al. 2010). Fatigue is either underappreciated or unreported because prodromal stage research has focused on NVD as this symptom triad is perceived as predicative of severe organ damage and demise (Donnelly et al. 2010). In humans, radiation-induced NVD typically requires a minimum dose of 70 cGy, although mild symptoms may be observed at doses of 30 cGy (Centers for Disease Control 2010). Radiation-induced fatigue has been best studied in relationship to radiation therapy where loss of energy and malaise is a common side effect (Bower 2007). Serious cancer treatment-associated fatigue, however, usually manifests gradually (Portenoy and Itri 1999) compounding with delivery of repeated fractionated doses of 200 cGy that over the course of therapy (up to 8 wks), can deliver up to 80 Gy. In general, CNS function is not felt to be impacted at single radiation doses of ≤ 200 cGy (Porter and Kaplan 2006) and if fatigue is documented in a single 100–200 cGy exposure it is usually tallied during the illness phase which for a dose ≤ 200 cGy would occur nearly a month post exposure (Porter and Kaplan 2006). Overall, a single isolated exposure of less than ≤ 200 cGy is deemed recoverable without supportive care (Porter and Kaplan 2006) which is underscored by the establishment of 5 cSv (equivalent to 5 cGy of gamma radiation) as the annual occupational radiation exposure limit by the Nuclear Regulatory Commission (NRC) (Code of Federal Regulations 1998).
As delineated above, little work has been performed examining the early impact (<24 h) of low-dose (≤ 2 Gy) ionizing radiation on the neuroimmune system. Fig. 1 demonstrates that a single dose of gamma radiation as low as 50 cGy reduces mouse locomotor activity 6 h after exposure. When social exploration was examined at radiation doses of 50 cGy and 200 cGy neither dose impaired mouse exploratory behavior (Table S1). These findings indicate that low-dose ionizing gamma radiation appears to perturb unmotivated behaviors to a greater extent than motivated behaviors. Such results are similar to findings we have observed in high-fat diet (HFD) fed mice where the HFD state causes a decrease in spontaneous locomotion (Lavin et al. 2011) that is not reflected by a loss of social exploration (Sherry et al. 2009). Customarily, strong activators of the neuroimmune system like lipopolysaccharide (LPS) induce both locomotor retardation and social withdrawal (Dantzer 2004). As we have shown, HFD-feeding appears to be a very weak stimulator of CNS inflammation (Lavin 2011). Consequently, from a biobehavioral standpoint low-dose radiation is at best a very weak immediate activator of neuroimmunity.
To demonstrate that the irradiation given could activate the neuroimmune system, pro-inflammatory cytokine gene transcripts were measured in whole brains from mice administered both 50 cGy and 200 cGy of gamma radiation. Fig. 2 shows that TNF-α is increased 6 h post irradiation in mice receiving 200 cGy. When un-perfused brains were compared to perfused brains, 50 cGy increased TNF-α transcripts in un-perfused brains. To determine if this boost in transcripts was due to an increase in blood TNF-α transcripts, whole blood TNF-α transcripts were examined and found to be unaffected by gamma radiation. Blood, however, did show significant transcript up-regulation of the inflammatory bioactives IL-1β and IL-1RA 8 h post 200 cGy gamma irradiation (Fig. 3) and these blood transcripts were present in the brain (Fig. 3). Taken together these findings indicate that low-dose gamma radiation activates the neuroimmune system relatively rapidly. How radiation triggers this response is not clear.
Previous work with high-dose radiation in mice (15 Gy) has demonstrated that irradiation of the body without irradiating the head induces up-regulation of proinflammatory cytokine transcripts in the brain (Marquette et al. 2003). This work was designed to support the concept that a stimulated peripheral innate immune system could communicate with the brain as is seen with peripheral LPS administration (Dantzer 2004). Although not designed to test this question, our work is supportive in that blood present in the brain contains cells with increased IL-1β gene transcripts. Further support for this concept was seen in the proton radiation experiments because proton irradiation at both 50 cGy and 200 cGy did not disturb either locomotion or social exploration (data not shown, Table S1, respectively). Proton radiation is of higher energy than gamma radiation (Ni et al. 2011) and contributes more significantly to SPEs (Cengel et al. 2010, Ni et al. 2011). Given this higher energy, we expected proton radiation to impact behavior more than gamma radiation. Proton radiation, however, has a different linear energy transfer profile than gamma radiation. The Bragg peak for gamma radiation is likely more toward the skin surface as opposed to proton radiation where the Bragg peak is likely skewed to the interior of the animal. This difference in ionization location further supports the potential importance of peripheral immune activation to radiation-induced neuroimmune activation. Interestingly, blood also showed an up-regulation of IL-1RA transcripts indicating (in conjunction with the IL-1β transcript data noted above) that the IL-1 arm of the innate immune system is an early pathway activated by radiation. Organ systems with high radiation sensitivity include the hematopoietic system where radiation increases mitochondrial-dependent reactive oxygen species (ROS) generation (Xiao and Whitnall 2009). Importantly, ROSs have been shown recently to stimulate the NALP3 inflammasome (one of three inflammasomes responsible for activation of caspase-1) which is required for the conversion of pro-IL-1β into mature secretable IL-1β (Schroder et al. 2010). Curiously, little work has been performed examining radiation and inflammasome activation. Given the reduction of locomotion observed after 50 cGy of radiation and the lack of pro-inflammatory transcript changes, radiation-dependent triggering of the inflammasome with production of mature IL-1β, as seen with the NALP3 inflammasome and uric acid (Martinon et al. 2006), seems likely. TNF-α gene transcript up-regulation may be secondary to the IL-1β signal if that signal is significantly robust.
How pro-inflammatory cytokines induce fatigue is still imprecise. Although CNS IL-1 (Patarca 2001) and TNF-α are implicated (Cavadini et al. 2007, Foley 2007), the mechanistic connection remains elusive. Some believe the indoleamine-2,3-dioxygenase (IDO) pathway may be important (Capuron et al. 2011) because altered serotonergic (5-hydroxytryptamine, (5-HT)) neurotransmission is identified in patients with chronic fatigue syndrome (Smith et al. 2008). In addition, pro-inflammatory cytokines, especially TNF-α, provoke the brain-based IDO pathway to convert the serotonin precursor tryptophan to kynurenine which affords production of potential neurotoxic kynurenine metabolites (Dantzer 2008, Dantzer et al. 2011). With radiation-induced fatigue, however, the IDO pathway seems an unlikely player because blockade of 5-HT is a key first line defense for the inhibition of radiation-induced nausea and vomiting in radiotherapy patients (Monroe et al. 2008) and there is no clear evidence that inhibition of these prodromal ARS symptoms with 5-HT antagonists ameliorates subsequent radiation-induced fatigue.
Low dose-rate gamma radiation was also examined and found not to perturb locomotor or social exploratory behavior (data not shown, Table S1, respectively). The most likely explanations for these observations were that low-dose rate radiation (50 cGy and 200 cGy of radiation delivered over 240 min as opposed to 10 min as in Fig. 1 (high-dose rate)) did not impart significant energy to activate the neuroimmune system or that the mice in the low-dose rate experiments were restrained for 240 min as opposed to 10 min. To investigate these possibilities, more prolonged restraint experiments were performed in which mice were delivered 50 cGy and 200 cGy (both at high-dose rate) during the first 10 min of restraint then left restrained for an additional 230 min. Fig. 4 reveals that restraint-240 ameliorated the effect of 200 cGy on locomotion but not that of 50 cGy. Unexpectedly, restraint-240 produced hyper-mobility in mice irradiated with 200 cGy suggesting that restraint-240 may sensitize mice to biobehavioral stimuli like it does for cutaneous hypersensitivity (Flint and Tinkle 2001). Importantly, restraint-240 had no impact on locomotor activity of sham irradiated mice (data not shown) which is consistent with the majority of work examining restraint (Buybitsky and Mostofsky 2009). Taken together these findings indicate that early radiation-induced alterations in biobehaviors requires a threshold dose rate that can be potentially modulated by the stress response. Furthermore, since the bulk of the time restrained was spent after irradiation these results point to a radiation priming-like interaction which occurs at 200 cGy but not at 50 cGy because the 50 cGy irradiated restraint-240 mice behaved like restraint-10 50 cGy irradiated mice (Fig. 1).
In general, the overall impact of restraint tends towards immune suppression (Buybitsky and Mostofsky 2009). Table 1 shows that immediately after restraint-240 (4 hr post-irradiation) sham irradiated restraint-240 mice had a decrease in hippocampal, hypothalamic, cortical and cerebellar TNF-α transcripts compared to sham irradiated restraint-10 mice. 200 cGy irradiation prevented this down-regulation while 50 cGy had little effect. As time post-irradiation progressed, TNF-α transcripts increased showing a movement toward resolution by 24 hr post irradiation. Unlike whole brain at 8 h post irradiation (Fig 3D), brain regions showed region-specific increases in IL-1RA gene transcripts. At 8 h post-irradiation, hippocampal IL-1RA gene expression was increased especially in the cortex (Table 1). Peak IL-1RA expression occurred near 12 hrs post-irradiation with resolution before 24 hrs. IL-1RA expression patterns somewhat mimicked that of TNF-α but were expressed in the brain later and resolved quicker. This pattern fits with a NALP3 inflammasome driven biobehavioral process (Johnson et al. 2007).
Finally, activity-regulated cytoskeletal-associated protein (Arc) was up-regulated by restraint-240 in the hippocampus as previously reported (Mikkelsen and Larsen 2006). Arc is an immediate early gene induced in hippocampal and parietal neurons following behavioral experiences best tied to maintenance of long-term potentiation and spatial memory consolidation (Rosi et al. 2008). Inflammation and proinflammatory cytokines, including TNF-α, are shown to reduce Arc gene transcript expression (Centonze et al. 2010). Unexpectedly, restraint-240 led to decreased Arc in the cerebellum. What function Arc has in the cerebellum is not clear but it may play a role in cerebellar associative learning as evoked in such responses as eyeblink conditioning (Kim and Thompson 2011). 200 cGy gamma irradiation led to a decrease in whole brain Arc gene transcripts at 6 hr. Brain region analysis (Table 1) showed that this effect was transient in that it was not evident at 4 hr or 8 hr post irradiation. At 24 hr post irradiation (restraint-10 200 cGy), Arc transcripts were reduced in the cortex which may fit with post-radiation cognitive deficits that can manifest after whole brain irradiation, although acute radiation encephalopathy is rare at doses under 300 cGy (Soussain et al. 2009). Taken together these findings indicate that low-dose gamma radiation affects the cortex and hippocampus of mice with changes that can last at least 24 hr post-irradiation. Up-regulated TNF-α appears linked to down-regulated Arc. Since manned space and commercial air travel carry a significant risk of SPE exposure in conjunction with physical and psychological stress (extensively studied in astronauts (Morphew 2001) and more recently recognized in airline passengers (economy class syndrome (Dalen 2003, Grajewski et al. 2011))), restraint stress may modulate early radiation-induced fatigue due to brain-based immunosuppression (DeLano and Mallery 1998). However, this restraint-240-dependent reduction in TNF-α gene expression is short-lived and cortical TNF-α is higher in irradiated restraint-240 animals at 12 hr and 24 hr post irradiation when compared to restraint-10 animals. More work is required to understand if and/or how early neuroimmune activation contributes to radiation-induced fatigue-like responses, especially when conjoined to the stress response.
Supplementary Material
Acknowledgments
Support: This research was supported the National Institutes of Health (DK064862, NS058525 and AA019357 to G.G.F.) and by the NSBRI CARR grant. The NSBRI is funded through NASA NCC 9-58
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bogo V. Radiation: behavioral implications in space. Toxicology. 1988;49:299–307. doi: 10.1016/0300-483x(88)90012-1. [DOI] [PubMed] [Google Scholar]
- Bower JE. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain, Behav, Immun. 2007;21:863–871. doi: 10.1016/j.bbi.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: recent developments. Neurosci Biobehav Rev. 2009;33:1089–1098. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Capuron L, Schroecksnadel S, Féart C, Aubert A, Hiqueret D, Barberger-Gateau P, Layé S, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.12.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Cavadini G, Petrzilka S, Kohler P, Jud C, Tobler I, Birchler T, Fontana A. TNF-α suppresses the expression of clock genes by interfering with E-box-mediated transcription. Proc Natl Acad Sci U S A. 2007;104:12843–12848. doi: 10.1073/pnas.0701466104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengel KA, Diffenderfer ES, Avery S, Kennedy AR, McDonough J. Using electron beam radiation to simulate the dose distribution for whole body solar particle event proton exposure. Radiat Environ Biophys. 2010;49:715–721. doi: 10.1007/s00411-010-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Acute radiation syndrome: A fact sheet for physicians. 2010 Retrieved September 23, 2010, from http://www.bt.cdc.gov/radiation/arsphysicianfactsheet.asp#2.
- Centonze D, Muzio L, Rossi S, Furlan R, Bernardi G, Martino G. The link between inflammation, synaptic transmission and neurodegeneration in multiple sclerosis. Cell Death Differ. 2010;17:1083–1091. doi: 10.1038/cdd.2009.179. [DOI] [PubMed] [Google Scholar]
- Code of Federal Regulations, 1998 ed. Standards for Protection Against Radiation – Subpart C: Occupational Dose Limits. Title 10, Pt. 203.
- Cucinotta FA, Durante M. Cancer risk from exposure to galactic cosmic rays: implications for space exploration by human beings. Lancet Oncol. 2006;7:431–435. doi: 10.1016/S1470-2045(06)70695-7. [DOI] [PubMed] [Google Scholar]
- Dalen JE. Economy class syndrome. Arch Intern Med. 2003;163:2674–2676. doi: 10.1001/archinte.163.22.2674. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: a neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36:426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLano RM, Mallery SR. Stress-related modulation of central nervous system immunity in a murine model of herpes simplex encephalitis. J Neuroimmunol. 1998;89:51–58. doi: 10.1016/s0165-5728(98)00087-3. [DOI] [PubMed] [Google Scholar]
- Donnelly EH, Nemhauser JB, Smith JM, Kazzi ZN, Farfán EB, Chang AS. Acute radiation syndrome: assessment and management. South Med J. 2010;103:541–544. doi: 10.1097/SMJ.0b013e3181ddd571. [DOI] [PubMed] [Google Scholar]
- Evaluation Subcommittee of ASTRO’s Emerging Technologies Committee. An evaluation of proton beam therapy. n.d Retrieved from http://www.astro.org/HealthPolicy/EmergingTechnology/EvaluationProjects/documents/ProtonBeamReport.pdf.
- Flint MS, Tinkle SS. C57BL/6 mice are resistant to acute restraint modulation of cutaneous hypersensitivity. Toxicol Sci. 2001;62:250–256. doi: 10.1093/toxsci/62.2.250. [DOI] [PubMed] [Google Scholar]
- Foley JF. Sleepy time with TNF-α. Sci STKE. 2007;398:tw280. [Google Scholar]
- Grajewski B, Waters MA, Yong LC, Tseng C, Zivkovich Z, Cassinelli RT., II Airline pilot cosmic radiation and circadian disruption exposure assessment from logbook and company records. Ann Occup Hyg. 2011;55:465–475. doi: 10.1093/annhyg/mer024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- James DC. Radiation emergencies: a nurse can lead. J Radiol Nurs. 2006;25:101–105. [Google Scholar]
- Johnson DR, O’Connor JC, Hartman ME, Tapping RI, Freund GG. Acute hypoxia activates the neuroimmune system, which diabetes exacerbates. J Neurosci. 2007;27:1161–1166. doi: 10.1523/JNEUROSCI.4560-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Thompson RF. c-Fos, Arc and Stargazin expression in rat eyeblink conditioning. Behav Neurosci. 2011;125:117–123. doi: 10.1037/a0022328. [DOI] [PubMed] [Google Scholar]
- Lavin DN, Joesting JJ, Chiu GS, Moon ML, Meng J, Dilger RN, Freund GG. Fasting induces an anti-inflammatory effect on the neuroimmune system, which a high-fat diet prevents. Obesity. 2011 doi: 10.1038/oby.2011.73. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence TS, Ten Haken RK, Giaccia A. Principles of Radiation Oncology Chapter 21. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. Cancer Principles & Practice of Oncology. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 307–336. [Google Scholar]
- Makhijani A. Post-tsunami situation at the Fukushima Daiichi nuclear power plant in Japan: facts, analysis and some potential outcomes. Institute for Energy and Environmental Research release. 2011 March 14–16 [Google Scholar]
- Maks CJ, Wan XS, Ware JH, Romero-Weaver AL, Sanzari JK, Wilson JM, Rightnar S, Wroe AJ, Koss P, Gridley DS, Slater JM, Kennedy AR. Analysis of white blood cell counts in mice after gamma- or proton-radiation exposure. Radiat Res. 2011;176(2):170–176. doi: 10.1667/RR2413.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquette C, Linard C, Galonnier M, Van Uye A, Mathieu J, Gourmelon P, Clarençon D. IL-1beta, TNF-alpha and IL-6 induction in the rat brain after partial-body irradiation: role of vagal afferents. Int J Radiat Biol. 2003;79:777–785. doi: 10.1080/09553000310001610998. [DOI] [PubMed] [Google Scholar]
- Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Larsen MH. Effects of stress and adrenalectomy on activity-regulated cytoskeleton protein (Arc) gene expression. Neurosci Lett. 2006;403:239–243. doi: 10.1016/j.neulet.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Mohler SR. Galactic radiation exposure during commercial flights: is there a risk? Can. Med Assoc J. 2003;168:1157–1158. [PMC free article] [PubMed] [Google Scholar]
- Monroe AT, Reddy SC, Gibbs GL, White GA, Peddada AV. Factors associated with radiation-induced nausea and vomiting in head and neck cancer patients treated with intensity modulated radiation therapy. Radiother Oncol. 2008;87:188–194. doi: 10.1016/j.radonc.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Morphew ME. Psychological and human factors in long duration space flight. McGill J Med. 2001;6:74–80. [Google Scholar]
- Ni H, Balint K, Zhou Y, Gridley DS, Maks C, Kennedy AR, Weissman D. Effect of solar particle event radiation on gastrointestinal tract bacterial translocation and immune activation. Radiat Res. 2011;175:485–492. doi: 10.1667/RR2373.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JL, Townsend LW. Interplanetary crew dose rates for the August 1972 solar particle event. Radiat Res. 2000;153:729–733. doi: 10.1667/0033-7587(2000)153[0729:icdrft]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Patarca R. Cytokines and chronic fatigue syndrome. Ann N Y Acad Sci. 2001;933:185–200. doi: 10.1111/j.1749-6632.2001.tb05824.x. [DOI] [PubMed] [Google Scholar]
- Pecaut MJ, Haerich P, Zuccarelli CN, Smith AL, Zendejas ED, Nelson GA. Behavioral consequences of radiation exposure to simulated space radiation in the C57BL/6 mouse: open field, rotorod and acoustic startle. Cogn Affect Behav Neurosci. 2002;2:329–340. doi: 10.3758/cabn.2.4.329. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Itri LM. Cancer-related fatigue: guidelines for evaluation and management. Oncologist. 1999;4:1–10. [PubMed] [Google Scholar]
- Porter RS, Kaplan JL. The Merck Manual of Diagnosis and Therapy. (18) 2006;chap 317 sec. 21. Retrieved June 20, 2011, from http://www.merckmanuals.com/professional/sec21/ch317/ch317a.html.
- Rosi S, Andres-Mach M, Fishman KM, Levy W, Ferguson RA, Fike JR. Cranial irradiation alters the behaviorally induced immediate-early gene Arc (activity-regulated cytoskeleton-associated protein) Cancer Res. 2008;68:9763–9770. doi: 10.1158/0008-5472.CAN-08-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer DA, Linton OW. National Council on Radiation Protection and Measurements report shows substantial medical exposure increase. Radiology (Oak Brook, IL, U S) 2009;253:293–296. doi: 10.1148/radiol.2532090494. [DOI] [PubMed] [Google Scholar]
- Schroder K, Zhou R, Tschopp J. The NLRP3 inflammasome: a sensor for metabolic danger? Science. 2010;327:296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- Sherry CL, Kim SS, Freund GG. Accelerated recovery from acute hypoxia is due to obesity-associated up-regulation of Interleukin-1 receptor antagonist. Endocrinology. 2009;150:2660–2667. doi: 10.1210/en.2008-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Dimulescu I, Falkenberg VR, Narasimhan S, Heim C, Vernon SD, Rajeevan MS. Genetic evaluation of the serotonergic system in chronic fatigue syndrome. Psychoneuroendocrinology. 2008;33:188–197. doi: 10.1016/j.psyneuen.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Soussain C, Ricard D, Fike JR, Mazeron J, Psimanas D, Delattre J. CNS complications of radiotherapy and chemotherapy. Lancet. 2009;374:1639–1651. doi: 10.1016/S0140-6736(09)61299-X. [DOI] [PubMed] [Google Scholar]
- Weissmann G. Fukushima Daiichi and Icarus: the human factor in a meltdown (Sv = 1J/kg.w) FASEB J. 2011;25:1777–1780. doi: 10.1096/fj.11-0601ufm. [DOI] [PubMed] [Google Scholar]
- Xiao M, Whitnall MH. Pharmacological countermeasures for the acute radiation syndrome. Curr Mol Pharmacol. 2009;2:122–133. doi: 10.2174/1874467210902010122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.