Abstract
In addition to the classic genomic mechanism of steroid action mediated by activation of intracellular nuclear receptors, there is now extensive evidence that steroids also activate receptors on the cell surface to initiate rapid intracellular signaling and biological responses that are often nongenomic. Recent progress in our understanding of rapid, cell surface-initiated actions of estrogens, progestins, androgens and corticosteroids and the identities of the membrane receptors that act as their intermediaries is briefly reviewed with a special emphasis on studies in teleost fish. Two recently discovered novel proteins with seven-transmembrane domains, G protein-coupled receptor 30 (GPR30), and membrane progestin receptors (mPRs) have the ligand binding and signaling characteristics of estrogen and progestin membrane receptors, respectively, but their functional significance is disputed by some researchers. GPR30 is expressed on the cell surface of fish oocytes and mediates estrogen inhibition of oocyte maturation. mPRα is also expressed on the oocyte cell surface and is the intermediary in progestin induction of oocyte maturation in fish. Recent results suggest there is cross-talk between these two hormonal pathways and that there is reciprocal down-regulation of GPR30 and mPRα expression by estrogens and progestins at different phases of oocyte development to regulate the onset of oocyte maturation. There is also evidence in fish that mPRs are involved in progestin induction of sperm hypermotility and anti-apoptotic actions in ovarian follicle cells. Nonclassical androgen and corticosteroid actions have also been described in fish models but the membrane receptors mediating these actions have not been identified.
Keywords: nongenomic steroid actions, nonclassical steroid actions, cell-surface initiated steroid actions, GPR30, membrane progestin receptors, membrane steroid receptors, fish
1. Introduction
The majority of extracellular signaling molecules, including protein and peptide hormones, and growth factors do not readily diffuse through the plasma membrane into the cell and therefore exert their effects by activating specific receptors on the cell surface. Many of these receptors have a similar seven transmembrane (7-TM) domain structure, are coupled to G proteins, and are members of the G protein-coupled receptor (GPCR) superfamily [19, 88,192]. Hormonal responses mediated by GPCRs in target cells involve activation of G proteins and alterations in intracellular second messenger pathways [19,192]. Small lipophilic molecules such as steroid hormones, thyroid hormones and vitamin D are the exception as they can readily diffuse through the cell membrane [153] and exert their actions through binding to intracellular receptors belonging to the nuclear steroid receptor superfamily which act as ligand-activated transcription factors [236]. Hormonal activation of nuclear receptors results in translocation of the hormone-receptor complexes to the nucleus where they bind to hormone response elements on the promoter regions of genes and recruit coregulators, resulting in alterations in the rates of gene transcription and translation [126,133,236]. This classic genomic mechanism of steroid action involving new mRNA and protein synthesis is relatively slow, typically occurring over a time scale of hours. This mechanism is therefore fundamentally different from that of hormones acting through 7-TM receptors, where the response is often nongenomic and involves rapid activation of intracellular signal transduction pathways within a few minutes. Although most research has focused on the classic (i.e. genomic) mechanism of steroid action, a growing body of evidence has been obtained over the past 15–20 years that steroid hormones, thyroid hormones and vitamin D, like the larger extracellular signaling molecules, also exert cell surface-initiated, rapid (i.e. nonclassical) hormone actions through binding to membrane receptors and activation of intracellular second messenger pathways [56,150,183,246]. Moreover, many of these nonclassical steroid actions are nongenomic. Some recent advances in our understanding of steroid hormone actions initiated at the cell surface and the identities of the membrane receptors that mediate them are briefly reviewed in this paper, with examples from studies with fish models. Although nonclassical steroid actions involving activation of second messengers have been also been described for receptors residing in other cellular compartments such as the cytoplasm [22], they will not be discussed in detail here.
2. Nonclassical steroid actions
Initial evidence that steroids, in addition t o their classic genomic actions, can also elicit rapid, cell surface-mediated responses that are often nongenomic was obtained over 40 years ago. Szego and Davis showed in 1967 that 17β-estradiol rapidly elevates cAMP levels in the rat uterus [203]. On the basis of their results the authors proposed the existence of membrane-associated steroid receptors, but another decade of research was required to obtain direct evidence that specific estrogen binding sites are present on the cell surface [175]. During the same period it was shown by Masui and Markert that progesterone induction of oocyte maturation in amphibians does not involve transcription and can be induced in enucleated oocytes, from which the authors concluded that the progesterone receptor must reside outside the nucleus [129]. In the same year another group of investigators reported that whereas progesterone in the external medium induced oocyte maturation, microinjection of progesterone into oocytes was ineffective [195]. It was subsequently demonstrated that progesterone linked to a polymer which cannot pass through the cell membrane was an effective inducer of oocyte maturation [71]. The demonstration by Sadler and Maller that progesterone inhibits adenylyl cyclase activity in isolated oocyte plasma membranes provided further evidence that progesterone can act on the surface of oocytes to induce an intracellular response [186]. In the following year these authors identified progesterone binding sites on oocyte membranes by photoaffinity labeling [187]. Evidence for nongenomic actions of steroids and the existence of membrane receptors was reviewed in the early 1980s [47,204], but the existence of nonclassical steroid actions was not widely accepted and over the next 15 years only modest progress was made.
Research interest in nonclassical steroid actions has increased dramatically since the mid-1990s and has resulted in major advances in our understanding of this steroid mechanism. Rapid activation of a broad range of second messengers and alterations in intracellular ion concentrations have been described in a wide variety of cells for all classes of steroid hormones [56,150,183,246], as well as for vitamin D and thyroid hormones [37,148,189]. Some of these nonclassical steroid actions, such as progesterone-induced increases in intracellular free calcium concentrations in mammalian sperm, are extremely rapid, occurring within a few seconds of steroid addition [18]. Intracellular sites of steroid action via nuclear steroid receptors to alter second messenger pathways have also been described [22,138]. Thus, nuclear steroid receptors in an extranuclear location (e.g. cytosol) as well as membrane receptors are involved in mediating these responses. Putative membrane receptors implicated in mediating these cell-surface steroid actions include nuclear receptor or nuclear receptor-like proteins [81,163,181,247], novel seven-transmembrane (7-TM) and single-membrane receptors unrelated to members of the nuclear receptor superfamily [55,182,222,260,262] and receptors with different characteristics from those of any known receptors [109,178]. In addition, it has been demonstrated that steroids bind to other classes of membrane receptors such as GABA, oxytocin and opioid-like receptors to alter neuronal functions [65, 74, 152]. Direct, receptor-independent actions of steroids on plasma membranes to alter their fluidity have been demonstrated, although this is often observed only at high steroid concentrations [56]. Finally, recently identified alternative mechanisms, such as those involving mitochondrial translocation of receptors, may be important for mediating some nonclassical steroid actions [46]. Therefore, while the importance of nonclassical steroid actions has recently become broadly recognized, the diversity of mechanisms and putative receptors mediating these actions complicates their investigation. The presence of nuclear receptors mediating classical steroid actions in most of the cells where nonclassical steroid actions have been identified presents an additional challenge in designing experiments that will yield unequivocal results.
Biochemical binding studies have identified steroid binding sites for all classes of steroids, thyroid hormones and vitamin D on the plasma membranes of a broad range of cells, tissues and animal models which have the characteristics of specific steroid membranes receptors. For example, specific membrane receptors for estrogens have been identified in mammalian brain, breast cancer tissues and fish testes [39,113,127,258], for glucocorticoids in amphibian and bird brains and mammalian livers [152,191,235], for androgens in synaptosomes and fish ovaries [27,234], for progestogens on fish and amphibian oocytes [112,165], in bovine ovaries [179], and on mammalian and fish sperm membranes [11,213], for progesterone metabolites in breast cancer cells [250], for vitamin D on intestinal epithelial cells [148], and for thyroid hormones on erythrocytes [26]. However, the lack of information until recently on the identity, functional characteristics and molecular structures of membrane steroid receptors has slowed progress in understanding critical molecular aspects of steroid actions via this alternative mechanism and a more widespread appreciation of its significance [245]. In the past decade two putative novel 7-TM steroid receptors, membrane progesterone receptor alpha (mPRα) and G-protein coupled receptor 30 (GPR30), were discovered. In addition, several functional motifs have been identified on nuclear receptors which are of potential importance in their membrane expression and intracellular signaling [81,182,222,260,262]. Although considerable progress has been made in the past few years, the functional characterization of mPRs, GPR30 and membrane–bound nuclear steroid receptors is incomplete, and there is considerable controversy over their importance in mediating nonclassical steroid actions [104,107,155,210,212,261].
3. Nonclassical estrogen actions and estrogen receptors
In addition to their classic genomic actions, estrogens also rapidly activate intracellular signaling pathways in a wide variety of cell types and target tissues through binding to receptors on the cell membrane or attached to it. Cell surface-initiated, rapid estrogen actions identified in mammalian models include increases in cAMP and MAPkinase activity in estrogen-responsive breast cancers [3], and calcium flux in the developing human oocyte [209]. Some rapid estrogen actions, such as those identified in vascular endothelial cells [102], rat pituitary cells [247], as well as some of those in breast cancer cells [168,181], appear to be mediated by nuclear estrogen receptors (ERs) or truncated forms of the ER expressed near the cell surface. In addition, there is a growing body of evidence that some rapid nongenomic estrogen actions in several other cell and tissue models, including hypothalamic and hippocampal neurons [77,178], rat pancreatic islets [144], adipocytes [96], and human breast cancer cells [59], do not involve ERs and instead seem to be mediated by novel membrane estrogen receptors.
3.1 GPR30
The potential involvement of the orphan GPCR-like protein, GPR30 (G protein-coupled receptor 30) in mediating estrogen actions was first proposed by Filardo and coworkers based on their observations that estrogens cause rapid activation of second messengers (cAMP, ERK) in a human breast cancer cell line, SKBR3 cells, that lack ERα and ERβ, but express GPR30 [59]. Subsequently, our research group and Prossnitz’s independently demonstrated in 2004 that human GPR30 (or GPER) displays high affinity estrogen binding and has the binding characteristics of a membrane estrogen receptor [182,222]. Estrogen was shown to act through human GPR30 on SKBR3 and GPR30-transfected cells to activate a stimulatory G protein (Gs), resulting in increased adenylyl cyclase activity and cAMP production by their plasma membranes [60, 222]. In addition, estrogens have been shown to act through GPR30 to release epidermal growth factor EGF-related ligands and transactivate the EGF receptor (EGFR) [57,59,60,61]. The development of a purported selective GPER agonist, G-1, that does not activate ERs, has greatly facilitated research on GPR30 functions in many estrogen target tissues that co-express ERs [21]. Extensive research on GPR30 over the past five years has implicated the putative receptor in the development or progression of breast, endometrial, and ovarian cancers [1,58,197], and in a broad range of physiological functions in mammals, including neurotransmitter and neuroendocrine regulation [149,254], protection against autoimmunity [243], trauma-hemorrhage of the liver [91], ischemic stress in the heart [164], lipid metabolism and cardiovascular tone [78], insulin secretion [128], and primordial follicle formation [244].
3.2 Fish Models
Nonclassical estrogen actions have also been described in teleost gonads and gametes. Estrogen was found to decrease hCG-stimulated androgen synthesis in Atlantic croaker testis via a cell surface-initiated, nongenomic mechanism, because it was induced by 17β-estradiol conjugated to BSA, which cannot pass through the cell membrane, and was not blocked by transcription and translation inhibitors [85][113]. A high affinity (Kd 1.6 nM), low capacity, displaceable, and specific 17β-estradiol binding site was identified on croaker testicular membranes with the characteristics of an estrogen membrane receptor [113]. The testicular estrogen membrane receptor also binds diethylstilbestrol (DES) and the antiestrogens, tamoxifen and ICI 182,780, and has a ligand binding specificity similar to the ER in the cytoplasm in this species [113,114]. However, the observation that ICI 182,780 does not act as an antiestrogen, but instead decreases testicular androgen production, suggests that the membrane estrogen receptor mediating these effects may be unrelated to the ER. The identity of this receptor remains unknown since its binding characteristics differ from those of GPR30 which is abundantly expressed in croaker and zebrafish testes [111,161].
3.2.1 Estrogen inhibition of oocyte maturation
Recently we discovered a nonclassical action of estrogens to inhibit spontaneous as well as gonadotropin and maturation inducing steroid-induced oocyte maturation in croaker and zebrafish oocytes [161,158]. Although there had been a few earlier reports of inhibitory effects of estrogens on oocyte maturation in teleost fishes [93], the potential role of estrogens in regulating the onset of oocyte maturation in teleosts had not been explored further. The finding that treatment of follicle-enclosed croaker and zebrafish oocytes with aromatase inhibitors caused marked increases in spontaneous oocyte maturation in vitro, which was reversed by estrogen treatment, provided further evidence that oocyte maturation was under inhibitory control by estrogens [158,159,161]. Subsequent experiments with denuded zebrafish oocytes demonstrated that estrogens of follicular cell origin exert their effects directly on the oocytes to inhibit meiotic maturation [159,174]. In addition, experiments with BSA-conjugated 17β-estradiol and a transcription inhibitor showed that the estrogen action is via a cell surface-mediated, nongenomic mechanism [174].
3.2.2 Role of GPR30 in inhibition of oocyte maturation
Clear evidence has been obtained that this inhibitory action of estrogens on oocyte maturation is mediated by GPR30. The receptor protein is expressed on the surface of Atlantic croaker and zebrafish oocytes but is absent in the surrounding follicle cells [158,159] (Figure 1A, B). GPR30 has been cloned from a croaker cDNA library and the recombinant protein expressed on HEK-293 cells has the characteristics of human GPR30, including high affinity (Kd 2.7 nM), low capacity, displaceable binding specific for 17β-estradiol and the purported GPR30-specific agonist, G-1, but not for the ER ligand, DES [161, 222]. These steroid binding characteristics are almost identical to those of the estrogen receptor characterized on croaker oocyte plasma membranes [161]. The finding that G-1 and the ER antagonist ICI 182,780 mimics the inhibitory effects of 17β-estradiol on oocyte maturation, whereas the ERα- and ERβ-selective agonists PPT (propylpyrazole) and DPN (diarylpropionitrile) are ineffective [161, 212], further supports a role for GPR30 in mediating these effects. Finally, the demonstration that knockdown of GPR30 expression by microinjection of GPR30 antisense oligonucleotides into zebrafish oocytes blocked the inhibitory effects of estrogens on oocyte maturation [161] (Figure 2A), whereas microinjection with ER antisense was ineffective, confirms that GPR30 and not ERα is the intermediary in this nongenomic inhibitory estrogen mechanism [159].
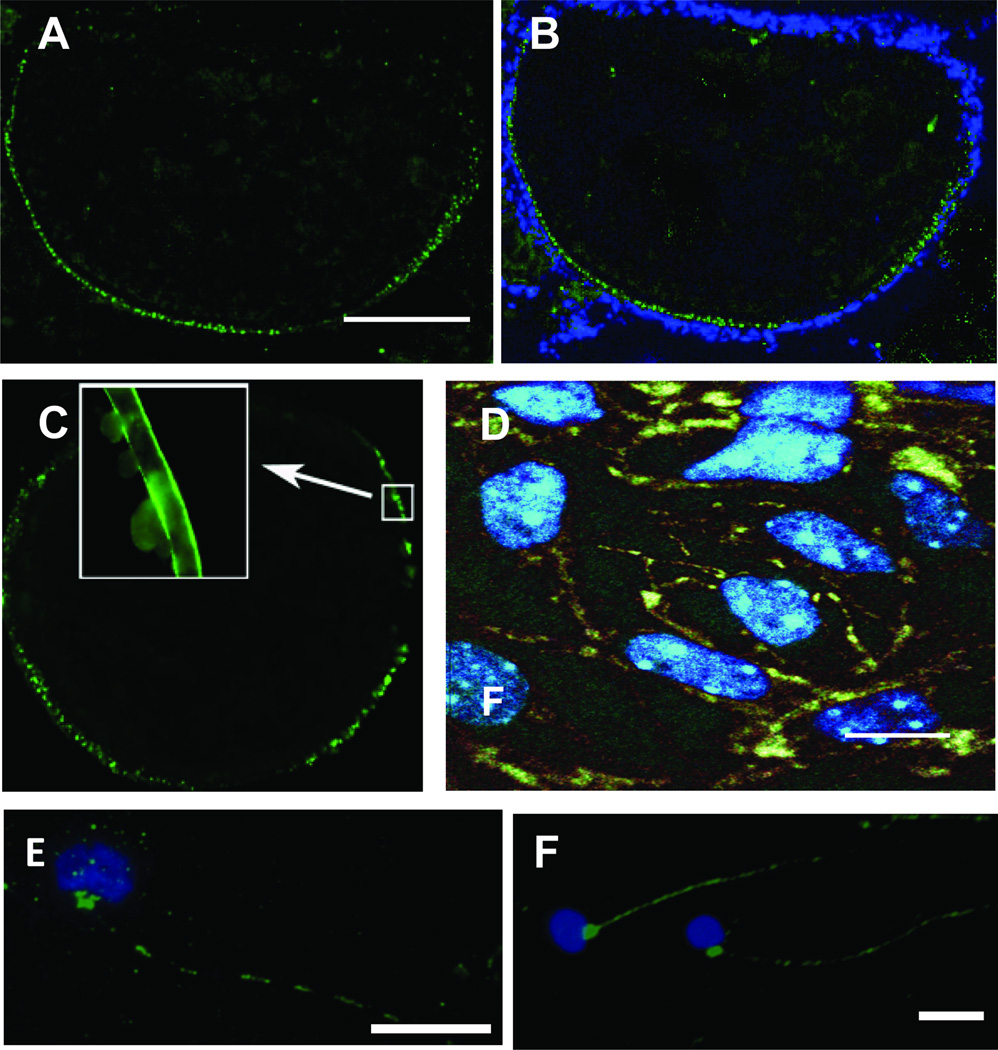
Figure 1.
Immunofluorescent localization of GPR30 (A,B), mPRα (C–E) and olfactory G protein (Golf, F) in teleost oocytes, ovarian follicles and sperm using specific GPR30, mPRα and Golf antibodies. A. GPR30 localization on late-stage vitellogenic zebrafish oocytes in ovarian tissue cryosections. Scale bar=100 µm. B. Co-staining with DAPI of nuclear DNA of zebrafish follicular cells. Reproduced from Pang and Thomas, Dev. Biol. 342 (2010) 194–206 [159], Figure 2, with permission. C. mPRα localization on zebrafish oocytes using a mPRα antibody. Insert, high magnification showing mPRα on oocyte membrane. Reproduced from Hanna et al. J. Endocrinol. 190 (2006) 247–260 [82], Figure 2, with permission. D. mPRα localization in Atlantic croaker granulosa and theca cell cultures using the mPRα antibody and DAPI nuclear staining. Scale bar= 10 µm. From Dressing et al., Endocrinology 151 (2010) 5916–5926 [44], Figure 1, with permission. E, F. Immunocytochemistry of Atlantic croaker sperm using mPRα (E) and Golf (F) antibodies and DAPI nuclear staining. Scale bar=5µm. From Tubbs and Thomas, Endocrinology 150 (2009) 473–484 [239], Figure 7, with permission.
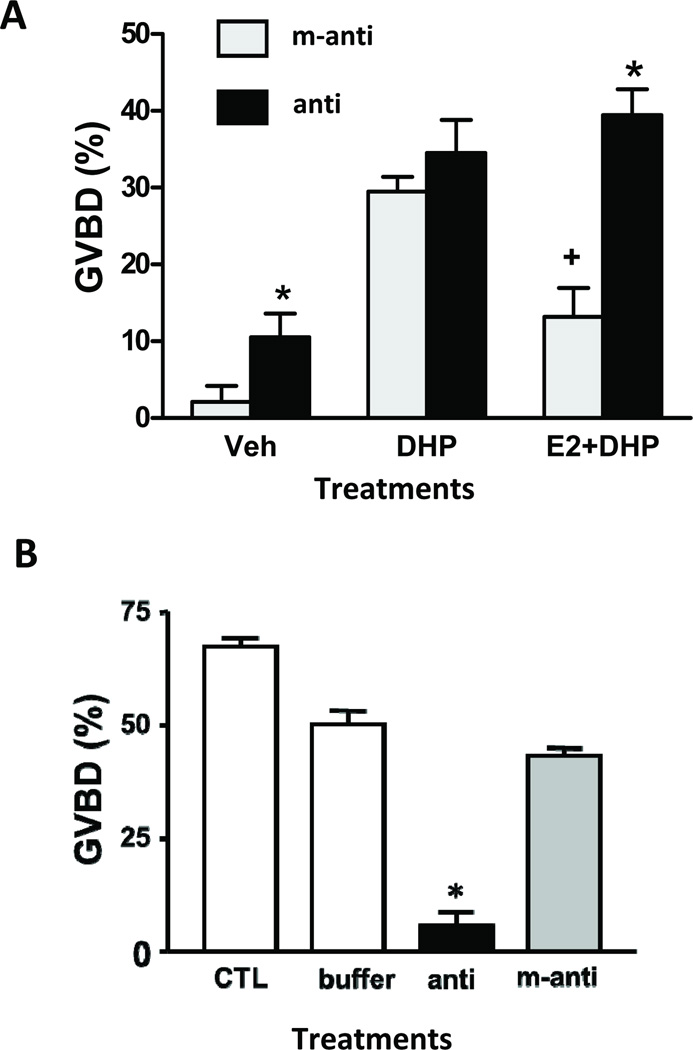
Figure 2.
Effects of microinjection of antisense morpholino oligonucleotides to GPR30 (A) and mPRα (B) on maturation (% GVBD) of fully grown zebrafish oocytes in response to hormonal treatments. A. Oocyte maturation in response to DHP and inhibition by 17β-estradiol. Veh: no hormonal treatment, DHP: 10 nM DHP alone, E2+DHP: 10 nM DHP + 110 nM 17β-estradiol, anti: zebrafish GPR30 antisense, m-anti: GPR30 mis-antisense. *, P<0.05 compared to respective mis antisense control group. + P< 0.05 compared with DHP treated groups. From Pang et al., Endocrinology 149 (2008) 3410–4326 [161], Figure 9, with permission. B. Oocyte maturation in response to 50 nM DHP. CTL, control uninjected oocytes, buffer, injected with buffer alone, anti, zebrafish mPRα-antisense, m-anti, zebrafish mPRα-mis-antisense. From Zhu et al, Proc. Natl. Acad. Sci. 100 (2003) 2231–2236 [262], Figure 7, with permission.*, P<0.05 compared to other treatments groups..
The signal transduction pathways activated by estrogens through GPR30 in croaker and zebrafish are the same as those described earlier in human breast cancer cells [159,161,222]. Binding of 17β-estradiol or G-1 to croaker and zebrafish GPR30 results in activation of a stimulatory G-protein (Gs) and increased cAMP production, presumably through G-protein alpha subunit activation of adenylyl cyclase (AC) [159,161]. An action of endogenous estrogens to maintain high levels of cAMP in teleost oocytes through a GPR30/Gs alpha subunit/AC/cAMP pathway is a plausible mechanism through which they could maintain meiotic arrest. Induction of oocyte maturation in fish and amphibians by progestins involves a marked decrease in cAMP levels [95,186], whereas pharmacologically-induced increases in cAMP levels prevent progestin-induced oocyte maturation [40,124,125]. Similarly, estrogens attenuate the progestin-induced decrease in cAMP levels and the induction of oocyte maturation in croaker and zebrafish [159,161]. Moreover, cAMP levels in zebrafish oocytes are decreased by removal of the follicle layer and inhibition of aromatase activity, both of which are associated with increases in spontaneous maturation [159,161]. Whereas all these results support a role for Gs alpha subunit signaling and increased cAMP levels in maintaining meiotic arrest, a recent study also implicates G protein βγ subunit-dependent signaling involving transactivation of epidermal growth factor receptor (Egfr) in this estrogen action in zebrafish oocytes, similar to that identified earlier in human breast cancer cells [59,174]. Both egfr mRNA and Egfr protein were detected in denuded zebrafish oocytes. The effects of specific inhibitors and stimulators of different components of the EGFR pathway on the inhibitory actions of estrogens on oocyte maturation were examined [174]. Both the intracellular tyrosine kinase (Src) inhibitor,PP2, and a matrix metalloproteinase (MMP) inhibitor, ilomastat, which prevents the release of heparin-bound epidermal growth factor, increased spontaneous oocyte maturation, whereas the MMP activator, interleukin-1α, decreased spontaneous OM. Several inhibitors of EGFR (ErbB1) and extracellular-related kinase 1 and 2 MAP2K1/2 (MEK1/2) also increased spontaneous OM. In addition, 17β-estradiol and the GPR30 specific ligand, G-1, increased phosphorylation of Mapk3/1, and this was abrogated by simultaneous treatment with the EGFR inhibitor. On the basis of these results it is proposed that estrogen binding to GPR30 results in activation of a stimulatory G protein (Gs). The Gs βγ subunit transactivates Egfr through Src and MMP which results in the phosphorylation of Mapk3/1 to inhibit oocyte maturation [174] (for pathway see Figure 5A). This is the first evidence that epidermal growth factor receptor signaling in vertebrate oocytes can prevent meiotic progression.
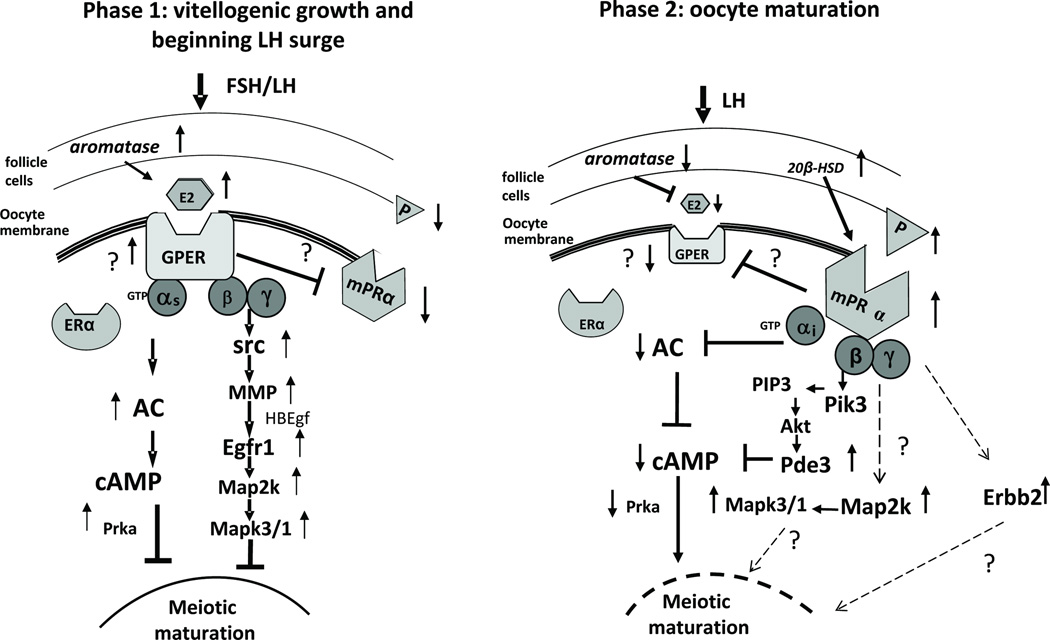
Figure 5.
Proposed model of the dual control of the onset of oocyte maturation in teleosts by estrogens and progestins acting through GPR30 and mPRα, respectively, at different stages of oocyte development. Phase 1: vitellogenesis and beginning of preovulatory LH surge. Phase 2: oocyte maturation. ? : evidence for pathway preliminary or equivocal. Details of model are described in the text. Redrawn from Pang and Thomas, Dev Biol. 342 (2010) 194–206 [159], Figure 8, and Peyton and Thomas, Biol. Reprod. 85 (2011) 42–50 [174], with permission.
4. Nonclassical progestin actions and progestin receptors
Nonclassical progestin actions have been identified in gametes, neural tissues, and reproductive tissues as well as in nonreproductive tissues such as vascular smooth muscles and lymphocytes. The nongenomic actions of progestins to induce meiotic maturation of amphibian and fish oocytes are well known and have been studied extensively over the past 30 years [94,125, 145,147,229]. Two progestins, 17,20β-dihydroxy-4-pregnen-3-one (DHP) and 17, 20β, 21-trihydroxy-4-pregnen-3-one (20β-S) have been identified as the major maturation inducing steroids (MIS) in teleosts [145,146,226]. In contrast, the physiological importance of progesterone as the MIS in amphibians has recently been questioned by Lutz et al. [120]. These investigators showed that testosterone can also induce meiotic maturation of Xenopus oocytes and is the major steroid produced by late maturation stage ovarian follicles [120]. Progestins also exert nonclassical actions to induce maturation of vertebrate sperm [228]. Progesterone and 20β-S induce hypermotility of mammalian and fish sperm, respectively, within a minute of hormone addition [9, 90,119,211,223,241], which is associated with equally rapid changes in intrasperm cAMP and Ca2+ levels [211,239]. In addition progesterone induces the acrosome reaction in mammalian sperm [241], but it remains unclear whether the acrosome reaction and sperm hypermotility share a common progesterone-mediated pathway [10,154]. All of these progestin actions are nongenomic because sperm are considered to be transcriptionally inactive. The brain is a major site of rapid actions by progesterone and progestin neurosteroids [8]. For example, the secretion of luteinizing hormone is down-regulated within a few minutes of progesterone administration in progesterone receptor knockout (PRKO) mice [194]. Similarly, the teleost progestin, 20β-S, rapidly decreases gonadotropin releasing hormone secretion from Atlantic croaker hypothalamic tissue slices in vitro [223]. Progesterone also induces rapid changes in sexual behavior and protein kinase C activation in the hypothalamus in rodents [8,38]. In addition to the well-known genomic actions of progestins on apoptosis and cell survival, they have also been shown to influence these processes in cancer cells and ovarian follicle cells through nongenomic mechanisms [17,44,49]. Smooth muscle cells in nonreproductive tissues such as the colon and cardiovascular system are also sites of nonclassical progesterone actions [142,253]. The results of several studies also suggest that progesterone causes immunosuppression via a nonclassical mechanism [35,48].
The identities of the receptors mediating many of these nonclassical progestin actions remain unclear. Binding moieties with the characteristics of progestin membrane receptors have been described in a variety of vertebrate tissues and cells including fish and amphibian oocytes [16,103,112,116,165,256], bovine ovaries and luteal cells [134,179], rat Leydig cells [185], mammalian and fish sperm membranes[18,54,118,176,213,227], pig liver microsomes [136], and for progesterone and progesterone metabolites in breast cancer cells [45,250]. However, progesterone has also been shown to act on receptors intracellularly to induce rapid signaling cascades inside the cell [12,22,66,231]. Despite extensive research on oocyte maturation in amphibians, considerable controversy surrounds the identity of the steroid receptor mediating this nongenomic steroid action, and nuclear progestin receptor (PR) or PR-like receptors [12,231], the androgen receptor (AR) [120], as well as a novel membrane progestin receptor, mPRβ [97], have been proposed as intermediaries. The PR has been clearly implicated in the activation of intracellular second messengers in a variety of cells, including breast cancer cells [22,193]. Some rapid responses to progesterone such as lordosis persist in PRKO mice and progesterone signaling in human T cells which lack the PR, suggesting they are mediated through novel progesterone membrane receptors [64, 43]. In addition, antiapoptotic nonclassical actions of progestins on granulosa cells have been described that do not involve the PR [44,49,171,208]. A variety of receptor proteins have been implicated in these cell-surface initiated progestin actions including GABAA and oxytocin receptors that can also bind progesterone in neural tissues [65,74], and two novel receptor families which are unrelated to any previously described receptor proteins, membrane progestin receptors (mPRs) and progesterone membrane receptor component 1 (PGRMC1) in granulosa and breast and ovarian cancer cells [44,45,170,171].
4.1 Membrane progestin receptors (mPRs)
In 2003 we reported the discovery of a novel cDNA which encoded a 352 amino acid 40kDa protein in spotted seatrout ovaries with the characteristics of the MIS membrane receptor regulating oocyte maturation, named membrane progestin receptor alpha (mPRα) [262]. In addition we identified homologous genes to seatrout mPRα in other vertebrates [260]. Several important criteria were met in these initial studies for preliminary designation of mPRs as functional membrane progestin receptors. The protein has a plausible structure because hydrophilicity and transmembrane analysis of the deduced amino acid sequence showed the protein has seven transmembrane domains, which is characteristic of GPCRs and other cell-surface receptors [19]. Immunocytochemistry of seatrout ovarian follicles using a seatrout mPRα antibody showed the protein is localized primarily on the oocyte plasma membrane, as expected for a membrane receptor. Importantly, specific progestin binding characteristic of a hormone receptor (progestin specificity, high affinity, limited capacity, displaceable) to the recombinant protein produced in a bacterial (E.coli) expression system [262], and subsequently confirmed in membranes prepared from human breast cancer cells (MDA-MB-231 cells) transfected with the seatrout cDNA [221] was clearly demonstrated. Moreover, progestins, but not estrogen and androgens, caused rapid induction of signal transduction pathways in mPR-transfected cells which involved activation of MAPkinase (Map2k) and down-regulation of adenylyl cyclase activity [262]. Subsequent studies have confirmed that both wild-type and recombinant seatrout mPRα is coupled to and activates an inhibitory G protein, consistent with its identity as the MIS receptor [210, 221]. Evidence was obtained for gonadotropin-induced upregulation of the seatrout mPRα during oocyte maturation, which supports its proposed biological function as the receptor mediating this progestin action [262]. The requirement for mPRα for oocyte maturation was confirmed in zebrafish in which oocyte maturation in response to the MIS, DHP, was blocked by microinjection of morpholino antisense oligonucleotides to zebrafish mPRα [262] (Figure 2B).
In a second paper published in the same year we reported the identification of 13 closely-related genes in other vertebrates that could be separated into three clades on the basis of sequence identity and phylogenetic analysis, called α, β and γ subtypes [260]. The three mPRs showed differential expression in reproductive tissues such as the ovary, testis, placenta, uterus, brain, and nonreproductive tissues such as the kidney and intestinal tissues in mammals [260]. The mPRs belong to the progestin and adipoQ receptor (PAQR) vertebrate family of 7-TM proteins, with no structural or sequence homology to nuclear steroid receptors, or GPCRs [206,221]. Specific progestin receptor binding has been demonstrated with recombinant vertebrate mPRα (PAQR7), mPRβ (PAQR8), mPRγ (PAQR5) and two additional members of the PAQR family, mPRδ (PAQR6) and mPRε (PAQR9) produced in prokaryotic (E.coli), mammalian, and yeast expression systems by several research groups [6,7,82,97,196,221,233,260,262]. The recombinant mPRs expressed in mammalian expression systems display high affinity binding (Kd 3–7 nM). Most of the research conducted to date on the characteristics and functions of mPRs have focused on the alpha subtype which is the most abundant mPR expressed in a wide range of human tissues [106,206,210]. Experiments with pertussis toxin as well co-immunoprecipitation studies have shown that in nearly all the tissues and cells examined, mPRα activates and is coupled to an inhibitory G protein (Gi), and progestin activation of the Gi α subunit causes down-regulation of adenylyl cyclase activity [44,100,194,210,221]. In contrast, recent studies indicate mPRα is coupled to a stimulatory olfactory G protein (Golf) in croaker sperm and cAMP production by sperm membranes is rapidly increased after progestin addition [239]. The βγ subunit of the inhibitory G protein also participates in signal transduction through mPRα, since upregulation of PI3K/Akt, MAPkinase and p38MAPkinase has been reported after progestin treatment in cells expressing wild-type and recombinant mPRα [44,82,100,157]. Limited studies with mPRβ, which is often co-expressed in the same cells as mPRα [43,194] suggest it is also coupled to an inhibitory G protein (Gi) [100]. Currently, information is lacking on the signal transduction pathways activated through mPRγ, mPRδ and mPRε.
The presence of functional mPRs and mPR-dependent signaling in mammalian cells, such as myometrial cells, lymphocytes, hypothalamic GnRH neurons, sperm and breast cancer cells, suggests they mediate progestin functions during labor, in immune responses, in the neuroendocrine control of the reproductive cycle, in sperm motility, and the development of breast cancer, respectively [45,100,160,194,228]. Evidence has been obtained that progesterone inhibits the transition of human breast cancer cells from epithelial to the invasive mesenchyme type through mPRs [263], and signals through mPRs in ovarian cancer cells [33]. However, in many cases definitive proof that mPRs regulate these processes is lacking. For example, human sperm motility and male fertility is related to the abundance of the mPRα protein on the sperm midpiece [228], like it is in fish species [239], but selective knockdown of mPRα expression with siRNA to confirm its role in progestin stimulation of hypermotility cannot be used with sperm because they are transcriptionally inactive. The recent identification of a selective mPR agonist that does not activate the nuclear PR, Org OD-02-0, has provided a valuable new tool for investigating mPR-specific functions in vertebrate cells and tissues [101].
4.2 Progesterone receptor membrane component 1 (PGRMC1)
Progesterone receptor membrane component 1 (PGRMC1) is a 194 amino acid 26–28kDa protein with a single transmembrane domain which was cloned from porcine smooth muscle cells and has an identical terminal amino acid sequence to a steroid binding protein purified earlier from porcine livers [55,136]. PGRMC1 and the closely related PGRMC2 belong to the membrane-associated progesterone receptor (MAPR) family [31]. The PGRMC1 gene has been cloned by other investigators and given many other names relating to its wide variety of proposed functions including axonal guidance during embryogenesis, steroid synthesis and metabolism, cholesterol regulation, alteration of reproductive behaviors, endocytosis, a component of a progesterone receptor with plasminogen activator inhibitor RNA binding protein-1 (PAIRBP-1), and an adapter protein [31,115]. Extensive studies in granulosa cells indicate an involvement of PGRMC1 in progesterone inhibition of apoptosis [171]. Recently, the protein has also been implicated in apoptosis in ovarian cancer cells [170]. PGRMC1 has been shown to interact with a variety of proteins in addition to PAIRBP-1 including SCAP and Insig-1, which are involved in sterol synthesis [31,210], and steroidogenesis activator polypeptide [115]. A surprising variety of ligands have been shown or suggested to bind to PGRMC1, including heme [141,200], cholesterol and steroids with 21 carbons, glucocorticoids and progestins [31]. However, the ability of PGRMC1 alone to bind progesterone and act as a progestin membrane receptor has not been clearly demonstrated [115,210,231]. Although transfection of PGRMC1 into CHO cells [54] and overexpression in spontaneously immortalized granulosa cells (SIGCs) caused an increase in specific [3H]-progesterone binding to the microsomal fraction [54], no specific [3H]-progesterone binding was detected to recombinant PGRMC1 produced in a prokaryotic (E.coli) expression system, whereas the recombinant protein was able to bind heme, suggesting a functional protein was produced by this recombinant expression system [140].
4.3 Fish Models
Nonclassical progestin actions have been identified in fish oocytes, sperm, ovarian follicle cells, and hypothalamus [210]. One of the most extensively investigated and well characterized models of nongenomic steroid actions initiated at the cell surface is the induction of oocyte meiotic maturation in fish and amphibians by progestin hormones [125,147]. Investigations on the effects of steroids on oocyte maturation in fish began in the 1960s and over the following decade progestogens and 11-deoxycorticosteroids were identified as the most effective steroids [72]. Evidence that this steroid action is nongenomic and does not require new mRNA synthesis was first reported in 1969 by Dettlaff and Skoblina who showed that a transcription inhibitor does not block progesterone induction of oocyte maturation in sturgeon [42]. In contrast, nonclassical progestin actions on sperm, ovarian follicle cells and the hypothalamus have only been identified within the past 15 years [44,211,216,223].
4.3.1 Oocyte maturation
As discussed in an earlier section (3.22), progestin induction of the final phase of oocyte maturation in fish, called the germinal vesicle breakdown phase [167], is associated with a decrease in cAMP levels [95,147]. Several lines of evidence suggest this decrease in cAMP is mediated through activation of a pertussis toxin-sensitive inhibitory G protein (Gi), and subsequent down-regulation of adenylyl cyclase activity [156,229,255]. First, it was shown that treatment with pertussis toxin blocks both the progestin (20β-S) down-regulation of adenylyl cyclase activity and induction of GVBD [156]. Then it was demonstrated that 20β-S treatment increases [35S]GTPγS binding to ovarian membranes which was immunoprecipitated with antibodies directed against the alpha subunits of inhibitory G (Gi 1–3) proteins, indicating that 20β-S activates an inhibitory G-protein [156]. The ligand binding affinity of receptors coupled to G proteins is reduced by agents such as pertussis toxin and GTPγS that uncouple the G proteins from the receptor [151]. Therefore, the finding that progestin binding to ovarian membranes was reduced by pretreatment with pertussis toxin and GTPγS, suggests that the MIS receptor is directly coupled to inhibitory G-proteins [156,255]. These results suggest that the resulting down-regulation of adenylyl cyclase activity leads to a reduction in protein kinase A (Prka) activation. Inhibition of Prka is reported to be sufficient to induce oocyte maturation in the Indian catfish [80], whereas the cell permeable protein kinase inhibitors were ineffective in inducing or enhancing 20β-S-induced oocyte maturation in Atlantic croaker [157]. However, in croaker and striped bass inhibitors of the phosphatidylinositol 3-kinase (Pi3k)/Akt signal transduction pathway block 20β-S-induced oocyte maturation [157,248]. Based on these results it was proposed that the βγ subunits of the heterotrimeric G protein also recruit Pi3k to the plasma membrane to catalyze the formation of phosphatidylinositol-3,4,5-trisphosphate (PIP3). PIP3 in turn binds to serine/threonine kinase Akt resulting in its activation and subsequent alteration of down-stream effectors such as phosphodiesterases (Pde), which breakdown cAMP [157]. Thus, MIS binding to its receptor and activation of an inhibitory G protein could potentially lead to decreased intracellular concentrations of cAMP via multiple signaling pathways to release the oocytes from meiotic arrest and complete maturation. Finally, preliminary evidence has been obtained that Erbb2 signaling is also involved in DHP-induced oocyte maturation in zebrafish, since the ERBB2 inhibitors, AG879 and RG13022, attenuated the DHP stimulation of GVBD of zebrafish oocytes [174].
A high affinity (Kd 3.5nM), limited capacity (Bmax 0.1–1.0 pmole/g ovary), displaceable, specific binding site for progestins in fish ovarian plasma membranes with rapid rates of association and dissociation, characteristics typical of steroid membrane receptors, was first described in spotted seatrout [165]. The receptor showed greatest binding affinity for 20β-S, the MIS in seatrout [165,226]. Moreover, a close correlation was seen between the relative binding affinities of various progestins for the seatrout progestin membrane receptor and their agonist or antagonist activities in a GVBD bioassay [215], further evidence of its identity as the MIS receptor in this species. Receptors with high binding affinities for progestins have since been identified in ovarian and oocyte membranes prepared from rainbow trout, arctic char and yellow tail [16,180,256]. As expected, the receptors in rainbow trout and arctic char show higher binding affinities for the salmonid MIS, DHP, than for any other progestins [16,256]. A several-fold increase in receptor concentrations was observed in spotted seatrout ovaries during GVBD in vivo [224]. Interestingly, this increase in seatrout receptor concentrations is associated with the development of oocyte maturational competence (i.e. ability to respond to 20β-S and complete GVBD) and is induced by gonadotropin treatment [167,224]. Moreover, similar increases in ovarian or oocyte MIS membrane receptor concentrations have been observed in rainbow trout, arctic char and yellowtail after gonadotropin treatment [16,180,256]. These studies showing that the increase in progestin receptor concentration coincides with the oocytes becoming responsive to the MIS suggests that the control of MIS receptor abundance is an important physiological process regulating the induction of oocyte maturation in teleost fishes.
4.3.2 Role of mPRs in the control of oocyte maturation
The evidence presented in the original paper on mPRs that MIS induction of oocyte maturation in teleosts is mediated through these receptors [262] has been supported by the results of subsequent studies in several teleost models in which both mPRα and mPRβ have been shown to be involved [82,83,84,223,232,233]. The mPRα protein is expressed on the plasma membranes of seatrout, croaker, goldfish and zebrafish oocytes (Figure 1C) and its expression is upregulated in these species during oocyte maturation [83,232,237,260]. This upregulation of mPRα by gonadotropin during the initial phase of oocyte maturation is associated with the development of maturational competence [166,167]. Strong evidence that mPRα is involved in the development of oocyte maturational competence has been obtained from studies which showed that microinjection with antisense oligonucleotides to mPRα into zebrafish and goldfish oocytes during the priming phase blocked the subsequent GVBD response to the MIS in these species, DHP [232,260] (Figure 2B). Parallel studies in zebrafish with antisense oligonucleotides to mPRβ, which is also present in zebrafish oocytes [83], suggest it is also involved in priming of zebrafish oocytes [84,223]. However, overexpression of mPRα accelerated maturation of zebrafish oocytes, whereas overexpression of mPRβ did not (Figure 3A) [84]. Interestingly, the acceleration of oocyte maturation occurred in the absence of MIS treatment and was accompanied with Erk phosphorylation (Figure 3B) [84]. Other studies using pharmacological agents that selectively activate mPRα or inhibit G-protein and second messenger pathways are consistent with the hypothesis that MIS induction of oocyte maturation is mediated by mPRα and involves activation of a pertussis toxin-sensitive inhibitory G protein (Gi) and its associated second messengers [156]. Both recombinant seatrout mPRα and the wildtype receptor in seatrout oocyte membranes co-immunoprecipitate with Gi proteins [210,221], suggesting they are coupled to Gi. This is supported by experiments showing that treatment of seatrout oocytes and cells expressing the recombinant receptor with 20β-S caused a decrease in the production of cAMP, presumably due to down-regulation of adenylyl cyclase activity, a progestin action blocked by pretreatment with pertussis toxin, a specific inhibitor of Gi activation [157,262]. Importantly, microinjection of seatrout oocytes with pertussis toxin also blocked progestin induction of oocyte maturation [157]. It is concluded from these studies that the MIS induces oocyte maturation in teleosts by activating mPRα, and in some species also by activating mPRβ, which is coupled to an inhibitory G protein, resulting in a decline in cAMP levels in the oocyte, thereby releasing it from meiotic arrest and permitting maturation to proceed.
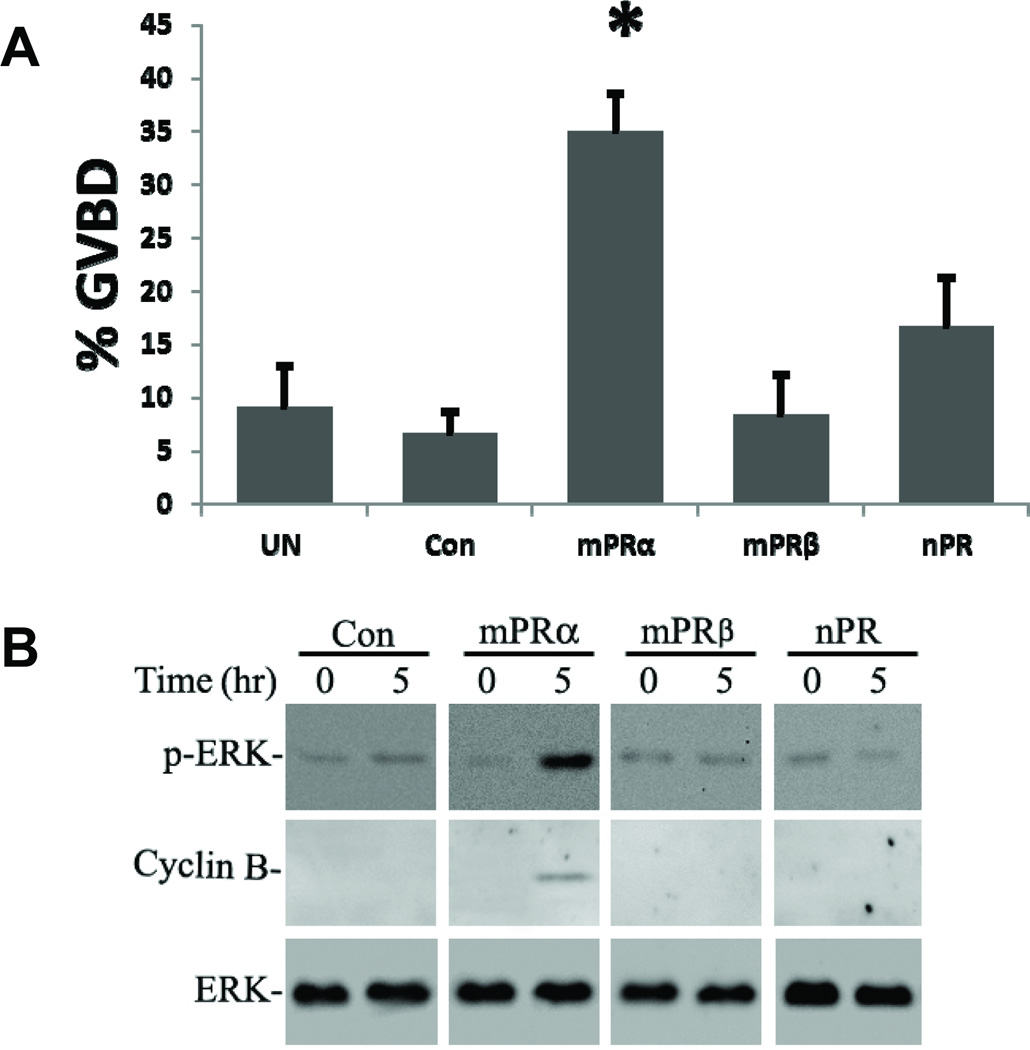
Figure 3.
Effects of overexpression of mPRα, mPRβ and PR in mature zebrafish follicle-enclosed oocytes on spontaneous oocyte maturation (A) and the activities of meiotic signaling molecules (B) in the absence of exogenous DHP stimulation. A. Oocyte maturation (GVBD) was assessed 5h following microinjection with the receptor transcripts. Controls (Con) were injected with EGFP transcripts. UN: uninjected controls. * significantly different (P<0.05) from Con and UN control groups. B. Western blot analysis of MAPK activity (p-ERK: phosphorylated MAPK) and production of cyclin B protein. Total ERK was used as a loading control. Reproduced from Hannna and Zhu, Mol. Cell. Endocrinol. 37 (2011) 80–90 [84], Figure 4, with permission.
4.3.3 Sperm motility
Recently a rapid, cell surface-mediated, nongenomic action of MISs to induce sperm hypermotility upon release into the surrounding water was discovered on Atlantic croaker sperm [211,216,223]. Fish sperm have to be hypermotile, which involves a high rate of turning, in order to enter the oocytes through the micropyle, a specialized pore in the fish oocyte plasma membrane, and fertilize them. Treatment of croaker and flounder sperm with physiologically relevant concentrations (10–200 nM) of 20β-S for 1–5min caused concentration-dependent increases in the percentage of motile sperm, sperm velocity and sperm turning rate (hypermotility) [223,238,239]. This stimulatory action on sperm motility is specific for 20β-S and several less potent progestins and is associated with rapid increases in cAMP and intrasperm calcium levels [211,238,239]. Similar stimulatory effects of 20β-S on sperm motility have been reported for two closely related species, red drum and spotted seatrout, and also a flatfish species, southern flounder [238,239,240], suggesting that this mechanism of progestin induction of sperm hypermotility is widespread amongst teleosts. Recent studies have shown that membrane adenylyl cyclase is rapidly activated in croaker sperm by 20β-S, resulting in increased cAMP levels within 1 minute, and that treatment with blockers of adenylyl cyclase prevent both the increase in cAMP levels and the increase in sperm motility in response to 20β-S treatment [239]. Interestingly, the 20β-S-induced increase in adenylyl cyclase activity in croaker sperm is associated with activation of a member of the stimulatory G protein family, the olfactory G protein (Golf) on the sperm midpiece [239]. Hormonal activation of olfactory G proteins had not been reported previously in any vertebrate tissues.
4.3.4 Role of mPRs in sperm motility
Clear evidence has been obtained that this direct effect of 20β-S on fish sperm to induce hypermotility is mediated by mPRα. Progestin membrane receptors with high affinity, limited capacity progestin binding with steroid specificities consistent with their identities as mPRα have been biochemically characterized on the plasma membranes of Atlantic croaker, spotted seatrout and southern flounder [213,216,227,238,239]. In addition, mPRα mRNA is expressed in croaker, seatrout and flounder sperm [238,239,240], and the mPRα protein has been detected on the sperm membranes of these three teleost species [219,227,238,240,262]. The mPRα proteins are localized primarily to the midpiece of croaker (Figure 1E), seatrout and flounder sperm [238,239,240] consistent with their proposed role in mediating progestin stimulation of hypermotility. The finding that there is a loss in the binding affinity of [3H]-20β-S to croaker and flounder sperm membranes after uncoupling of G proteins by pretreatment with excess GTPγ-S [238,239], and that 20β-S treatment causes G protein activation [239,240], suggests the progestin receptors on sperm are coupled to G proteins, as has been shown for mPRs in other tissues [210]. However, mPRα is coupled to an olfactory stimulatory G-protein (Golf) in croaker sperm and is expressed on the sperm midpiece (Figure 1E) [239], whereas it has been shown to couple to a pertussis toxin-sensitive inhibitory G protein, Gi, in all the other tissues investigated [210]. Importantly, the abundance of the mPRα protein on croaker, seatrout and flounder sperm is related to their motilities, with sperm displaying low motility having lower concentrations of the mPRα protein than sperm exhibiting high motility [238,239,240]. In addition, hormonal upregulation of mPRα protein expression on croaker sperm membranes by in vivo treatment with a gonadotropin releasing hormone analog is associated with increased sperm motility [239]. Taken together, these results suggest a major role for mPRα in mediating progestin stimulation of fish sperm motility through activation of a stimulatory G protein.
4.3.5 Anti-apoptotic actions and the role of mPRα
The MIS, 20β-S, has also been shown to activate signal transduction pathways in Atlantic croaker ovarian follicle cells to inhibit apoptosis [44]. A high affinity, low capacity single binding site has been identified on the plasma membranes of croaker granulosa/theca cell co-cultures that displays high binding affinity for 20β-S, progesterone and the specific mPR agonist Org OD 02-0, but low binding affinity for the potent PR agonist, R5020, suggesting that the progestin binding is mediated solely through mPRs. Studies showing that specific [3H]20β-S membrane receptor binding was significantly reduced by treatment with pertussis toxin, which causes uncoupling of inhibitory G proteins from receptors resulting in decreases in their ligand binding affinities [44], is consistent with its identity as mPRα [210,251]. Immunocytochemistry revealed that the mPRα protein is primarily localized on the plasma membranes of both granulosa and theca cells (Figure 1D). Treatment with 20β-S caused a rapid pertussis toxin-sensitive decrease in cAMP levels, suggesting that mPRα is coupled to an inhibitory G protein (Gi), which was confirmed in a co-immunoprecipitation experiment. Thus the signaling mediated through mPRα in croaker granulosa and theca cells involving activation of an inhibitory G protein is similar to that observed in most other vertebrate cell models [210]. The inhibition of serum starvation-induced cell death and apoptosis of granulosa and theca cells induced by 20β-S was mimicked by Org OD 02-0, but not by R5020, indicating that the apoptotic action is mediated through mPRα and not through PR. Knockdown of mPRα and PR by transfection with siRNAs for the receptors confirmed that the antiapoptotic action of 20β-S is mediated through mPRα. Finally, 20β-S caused rapid phosphorylation of Erk and Akt in the follicle cells which is a potential mechanism by which mPR inhibits apoptosis, since both ERK and Akt are known to be involved in apoptotic pathways in mammals [199].
5. Nonclassical androgen actions and membrane receptors
Rapid, cell surface-initiated, nongenomic androgen actions and the membrane receptors thought to mediate them have been described in a variety of vertebrate tissues [62,137], although these nonclassical androgen actions have received less attention than those for estrogens and progestins. Rapid increases in intracellular calcium levels have been reported in mouse T cells which lack a nuclear androgen receptor (AR) in response to BSA-conjugated testosterone which cannot pass through the cell membrane, suggesting this response is mediated through a novel membrane receptor [252]. The calcium response is inhibited by a pertussis toxin-sensitive pathway, suggesting the involvement of an inhibitory G protein [108,252]. Rapid alterations in intracellular calcium levels have also been observed in a variety of other cells including vascular tissue, cardiac myocytes and skeletal muscle [50,172,242], and human prostate and neuroblastoma cell lines [51,121]. Androgens also influence other second messengers such as regulation of intracellular levels of diacylglycerol (DAG), inositol-3-phosphate (IP3), cAMP and phosphorylation of ERK through a cell-surface mediated mechanism [108,137]. These rapid, nongenomic androgen responses have been implicated in a wide range of responses including apoptosis and actin polymerization in prostate cancer cells [86,98,162] neurite outgrowth [51], and protection against myocardial ischemia [32]. However, some of these effects were only observed at high androgen concentrations so their physiological significance remains unclear [137].
Androgen binding to plasma membranes has been partially characterized in T cells and also in mammalian endothelial cells, osteoblasts, prostate cells, glial cells and macrophages [5,14,98,108]. Rapid, nongenomic actions have been observed in cells that express the AR and can be modulated by AR ligands such as flutamide, suggesting they may be mediated through the AR [73,259]. However, nonclassical androgen actions initiated a the cell surface have also been observed in cells that do not express the AR, indicating the likely presence of membrane androgen receptors unrelated to the AR [13,14,86].
5.1 Fish Models
Nonclassical androgen actions have also been identified in teleost ovaries and follicle cells. Androgens inhibited gonadotropin-stimulated 17β-estradiol production from Atlantic croaker ovarian fragments in vitro, while treatment with the nuclear AR antagonist, cyproterone acetate, did not reverse the androgen inhibition [28]. The finding that BSA-conjugated dihydrotestosterone exerted similar inhibitory effects on estrogen production suggests this androgen action is initiated at the cell surface. Furthermore, co-treatment with actinomycin D did not block the inhibitory response, indicating that the androgens are acting through a nongenomic mechanism [28]. Recently, testosterone has also been shown to promote apoptosis of croaker granulosa/theca cells through a cell surface-mediated mechanism that probably does not involve the AR because AR agonists such as mibolerone were ineffective [15]. Taken together, these findings suggest the presence of a nontraditional androgen receptor in croaker ovaries.
The first comprehensive biochemical characterization of the binding characteristics of an androgen membrane receptor was conducted with Atlantic croaker ovarian tissue [27]. The ovarian membrane protein has a high affinity (Kd−15.3 nM), limited capacity (Bmax2.8 pmol/mg protein) and a displaceable single binding site specific for androgens, all characteristics of steroid receptors. These binding characteristics differ somewhat from those of an androgen-binding moiety detected on rainbow trout olfactory membranes (Kd 0.5 nM, Bmax 30–60fmol/mg protein [177]. The steroid specificity of the croaker ovarian membrane receptor differs from that of the ARs, showing no affinity for R1881 and mibolerone, whereas they bind to the AR previously characterized in the ovary of this species with relative binding affinities of 62% and 42%, respectively [27,201,202]. Surprisingly, the membrane androgen receptor displays high binding affinity for progesterone, although R5020 and RU 486 and the fish MISs 20β-S and 17,20β-DHP do not compete for androgen binding to the receptor [27,219]. Moreover, the rates of ligand association/dissociation to the membrane receptor are much more rapid than they are to the AR, further suggesting that the membrane bound androgen receptor is unrelated to ARs. Testosterone treatment of ovarian cell membranes causes G protein activation [27], in agreement with the earlier findings in T cells [108], indicating that the membrane androgen receptor might be a G protein-coupled receptor (GPCR). Separation of solubilized ovarian membrane proteins on a DEAE column, and subsequent determination of the approximate molecular weight of the major protein present in the eluted fraction containing the androgen binding activity indicates it has a molecular weight of approximately 40kDa, within the molecular weight range of GPCRs [219]. Currently the identity of the ~40kDa protein in croaker ovaries with androgen binding activity is being investigated.
6. Nonclassical corticosteroid actions and membrane receptors
The rapid response of the hypothalamus-pituitary-adrenal axis to stressors, particularly those that are life-threatening, is often necessary for survival and adaptation to changing environmental conditions. It is not surprising therefore that a wide range of rapid, nongenomic responses to the corticosteroid stress hormones have been reported, particularly in neural tissues [41,123,207]. For example, corticosteroids suppress courtship behavior in roughskinned newts within a few minutes [143]. They also cause rapid changes in neuronal activity, monoamine levels [117], second messengers, vasopressin secretion and CRF-induced ACTH secretion [89]. Rapid corticosteroid actions have also been reported in lymphocytes and neutrophils [20,110]. Corticosteroids act via nonclassical mechanisms to induce a variety of intracellular responses including activation of PI3K [79] and phosphorylation of protein kinase B, Akt, c-Src and MAPKs [130,198]. High concentrations of corticosteroids have been shown to directly affect membrane fluidity in lymphocytes, breast cancer cells and kidney epithelial cells [20]. However, the most cell surface-initiated corticosteroid actions identified to date in the brain are likely mediated through membrane receptors, since specific corticosteroid binding sites have been identified on the plasma membranes of neuronal cells in salamanders, birds and mammals [30,152,207,234]. The identity of the membrane corticosteroid receptor(s) remains unclear. A nuclear glucocorticoid receptor (GR)-like protein has been detected using GR antibodies on the membrane of lymphoma cells [68,69], whereas the specificity of the corticosteroid receptor on salamander neuronal membranes differs from that of the GR in that corticosterone binding is inhibited by nalaxolone, an opioid antagonist [52]. Binding of corticosterone to the salamander neuronal membrane was modulated by guanyl nucleotides and is associated with a 63kDa protein, suggesting that the receptor is unrelated to the GR and is coupled to a G protein [53,151]. Similarly, the finding that glucocorticoid modulation of voltage-gated Ca2+ currents in mammalian neurons is blocked by pertussis toxin and protein kinase C inhibitors, suggest this action involves an inhibitory G protein and PKC [87].
Nonclassical actions of aldosterone have also been described in a variety of aldosterone targets including renal cells, vascular smooth muscle cells, cardiomyocytes and the gastrointestinal tract [67,105,116,132,190,230]. For example vasoconstriction of glomerular arterioles occurs within 5 minutes of aldosterone treatment [2]. Signaling pathways such as epsilon protein kinase c, protein kinase D and Src kinase are rapidly activated in renal cells [29,132,139]. Aldosterone-induced initial trafficking of the epithelial sodium channel which involves c-Src-dependent transactivation of EGFR [230]. Membrane binding sites for aldosterone have also been identified on leucocytes and livers [4,116,135]. The majority of studies suggest these effects are mediated by the classical mineralocorticoid receptor (MR) [29,132,139], and are supported by studies of Grossmann et al. who showed MR-transfected cells were responsive to nongenomic aldosterone actions [76]. On the other hand, the finding that the effects of aldosterone are not blocked by the MR antagonist spironolactone and persist in MR-knockout mice suggests at least some of these nonclassical aldosterone receptor actions are mediated through a novel membrane receptor [85,249]. A recent study has implicated the putative membrane estrogen receptor, GPR30, in aldosterone mediated phosphorylation of ERK and JNK [75]. However, aldosterone binding sites have not been demonstrated on GPR30 so the nature of its purported involvement in nonclassical aldosterone signaling remains unclear.
6.1 Fish Models
Euryhaline teleosts often experience marked changes in environmental salinity, particularly in estuaries which have large tidal ranges, which are associated with transient disturbances of the osmolality and ionic composition of the blood. These fishes rapidly restore homeostasis by hormonal regulation of the drinking rate and the loss or gain of salts across epithelial tissues. Adaptation to freshwater (hypo-osmotic environment) is chiefly controlled by prolactin [188], whereas adaptation to sea water (hyper-osmotic environment) is regulated primarily by cortisol and the growth hormone signaling pathway [131]. Recent studies with a euryhaline species, Mozambique tilapia, indicate that cortisol can rapidly down-regulate the secretion of prolactin from the pituitary gland through a nonclassical mechanism of corticosteroid action, thereby facilitating adaptation to hyper-osmotic environments. Borski and co-workers demonstrated that prolactin secretion from tilapia pituitaries was significantly reduced within 20 minutes of incubation with cortisol [23] (Figure 4). A cortisol-induced reduction in prolactin secretion was also observed in the presence of the protein synthesis inhibitor cyclohexamide [24], and was mimicked with a plasma membrane impenetrable ligand, cortisol-21 hemisuccinate conjugated to BSA [25], indicating that cortisol is acting through a nongenomic action initiated at the cell surface. In addition, significant reductions in Ca2+ and cAMP levels in prolactin cells (lactotrophs) are observed within 20 minutes of incubation with cortisol, suggesting that cortisol decreases the activity of these osmotically sensitive cells [23,24]. Interestingly, this reduction in intracellular Ca2+ activity is associated with a reduction of voltage-gated calcium channel activity [92]. These findings suggest that cortisol can decrease lactotroph functions within 20 minutes in addition to its well-known actions on epithelial ion transport, causing the freshwater adaptation mechanisms mediated by prolactin to be rapidly down-regulated, thereby accelerating the restoration of osmotic and ionic homeostasis.
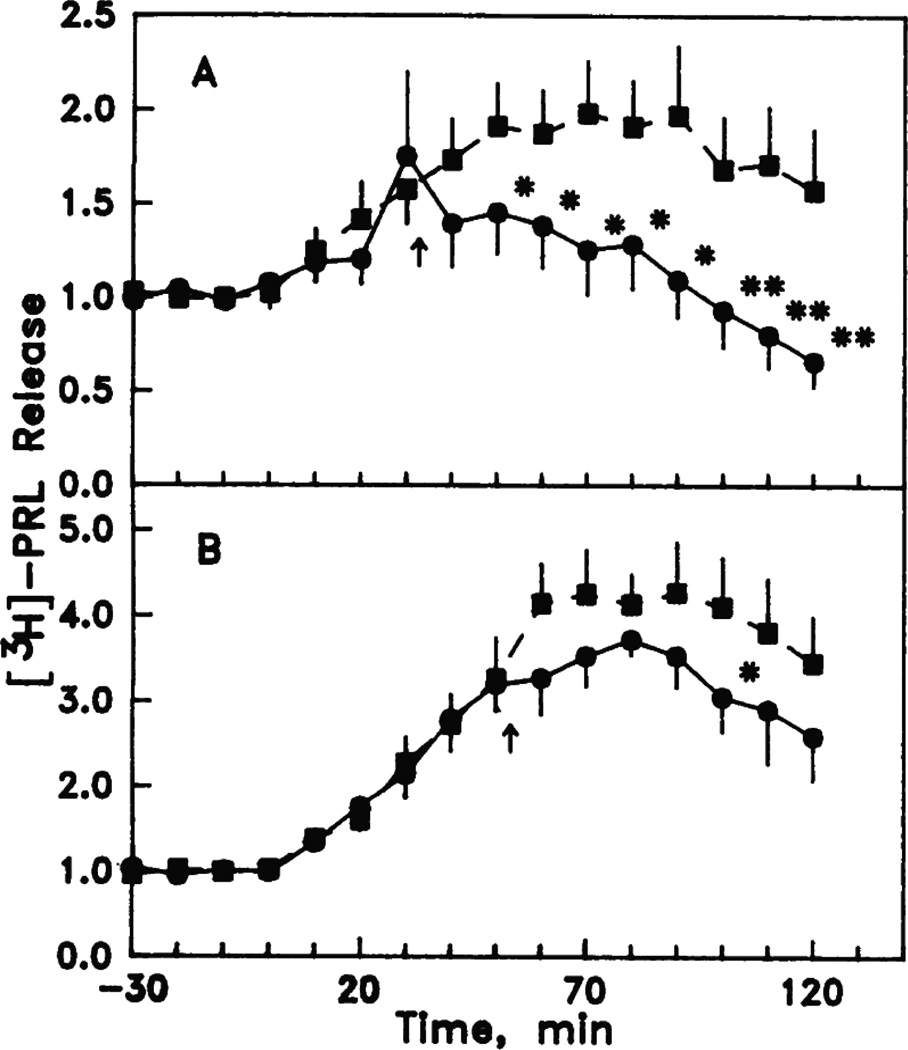
Figure 4.
Time-course effects of 200 nM cortisol (A) and 50 nM cortisol (B) on the release of [3H]prolactin from tilapia rostral pars distalis in a perifusion system. Prolactin release was induced by perifusing hypo-osmotic medium at time 0 and maintaining it until the end of the experiment. The response to hypo-osmotic treatment alone is shown as solid squares. The introduction of cortisol in shown by the arrows and the response is shown as solid circles. *, P <0.05, **P<0.01 compared to hypo-osmotic treatment alone. Reproduced from Borski et al., Proc. Natl. Acad.Sci. 88 (1991) 2758–2762 [23], Figure 2 with permission.
7. Cross-talk between steroid signaling pathways involving membrane receptors
7.1 mPRs and PR
Evidence has been obtained by Karteris and collaborators that activation of mPRs alters transactivation activity of the PR in human myometrial cells [100]. PR transactivation was demonstrated by assessing binding of the PR to a glucocorticoid-responsive element (GRE) linked to a luciferase reporter vector. Dexamethasone, progesterone and R5020 caused transactivation of the PR, as expected, in myocytes with high levels of PR expression. Pretreatment with pertussis toxin, which prevents Gi signaling through mPRs, partially blocked the response to progesterone, but not to the other two steroids which do not bind to the mPRs. Furthermore, the transactivation responses to progesterone were greatly attenuated in myocytes treated with siRNAs for mPRα and mPRβ [100]. These studies demonstrate that progesterone acts through an mPR-dependent pathway as well as through PR to transactivate the PR in human myocytes. The authors proposed that activation of mPRs during early pregnancy amplifies progesterone actions through PR-B to maintain the myometrium in a quiescent state. These results provided the first evidence that novel steroid membrane receptors can influence the transactivation activities of nuclear receptors. Potential alteration of PR transactivation through mPRs was also detected in myometrial cells at term through altering the expression of co-activators. Treatment of these myocytes which have elevated mPR expression with progesterone conjugated to BSA caused transient down-regulation of SRC2 mRNA and protein expression, which was attenuated in cells that had been transfected with siRNA for mPRα but not mPRβ. These results suggest that the progesterone-induced down-regulation of the SRC2 gene is at least partially mediated through mPRα and could contribute to the functional progesterone withdrawal in the human myometrium during labor [100].
7.2 mPRs and GPR30
Recently, evidence has been obtained for reciprocal regulation of mPRs and GPR30 by estrogens and DHP in zebrafish oocytes to regulate the onset of oocyte maturation [159]. Treatment with 17β-estradiol and the GPR30 agonist, G-1, caused a rapid down-regulation of mPRα protein expression and an increase in GPR30 protein in zebrafish oocyte membranes, whereas treatment with DHP and the mPR-selective agonist Org OD 02-0 caused an opposite effect, increasing mPRα expression and decreasing that of GPR30. These changes in protein levels were accompanied by parallel changes in mPRα and GPR30 mRNA expression. On the basis of these results and earlier studies, a model has been proposed for the dual control of the onset of oocyte maturation in teleosts by progestins and estrogens acting through GPR30 and mPRα, respectively, at different phases of oocyte development [159] (Figure 5). During the first phase at the end of vitellogenic oocyte growth, and at the beginning of the LH surge in some species, the ovarian follicles produce large amounts of estrogens and minor quantities of progestins, resulting in activation of the inhibitory pathway regulating oocyte maturation, but not the stimulatory pathway. The estrogens act through GPR30 to activate a stimulatory G protein (Gs) resulting in stimulation of adenylyl cyclase activity and increases in cAMP production. The high levels of cAMP maintain meiotic arrest of fish oocytes, possibly through downstream signaling molecules such as protein kinase A. A recent study shows that the βγ subunits of Gs also contribute to this inhibition through transactivation of Egfr through Src, MMP and HBEgf [174]. In addition, estrogens upregulate GPR30 expression to potentiate the inhibitory pathway and down-regulate mPRα expression to block the stimulatory one through activation of GPR30 via unknown pathways. As a result, the oocytes remain in meiotic arrest (Figure 5A). In the second phase during oocyte maturation several hours later, the steroidogenic pathway has switched to the production of progestins and estrogen production has declined. The increase in progestin concentrations causes upregulation of mPRα expression resulting in activation of the stimulatory pathway controlling oocyte maturation. At the same time progestins through mPRα cause down-regulation of the GPR30 expression, which together with the reduction in estrogen levels, causes suppression of the inhibitory pathway. As discussed previously, progestin binding to mPRα results in activation of an inhibitory G protein (Gi) and decreases in adenylyl cyclase activity and cAMP levels leading to inhibition of protein kinase A, thereby releasing the oocyte from meiotic arrest and allowing oocyte maturation to proceed [156,229,255]. In croaker, the βγ subunits of Gi are also involved in mediating the stimulation of oocyte maturation through activation of a Pi3k/Akt-dependent pathway, resulting in upregulation of phosphodiesterase activity and increased degradation of cAMP [157]. There is also preliminary evidence for an involvement of EGFR2 signaling [174]. MAPkinase (Map2k) is also activated resulting in Mapk3/1 phosphorylation during oocyte maturation [84,157]. However, both the receptor mediating this signaling pathway and its role in progestin induction of oocyte maturation are unclear at present [84,157,220] (Figure 5B).
8. Current controversies
It is perhaps not surprising that considerable controversy exists in this emerging field, particularly the physiological roles of novel putative steroid membrane receptors such as mPRs and GPR30 whose functions as steroid receptors were first proposed only 6–8 years ago [182, 222,262]. It has often proven to be difficult to induce sufficient cell-surface expression of recombinant hormone receptors in mammalian expression systems in order to demonstrate specific receptor binding and signal transduction [210,261]. Typically, cell surface expression of hormone receptors is highly regulated, with only a small proportion of the total present on the cell membrane, and knowledge of the adaptor proteins and culture conditions that promote cell-surface expression is often lacking [173]. Expression of putative steroid receptors represents a particular challenge because high amounts of specific binding to the receptor are required to distinguish it from the relatively high levels of nonspecific binding of these lipophilic molecules to the lipid-rich cell membranes. Continued selection over 6–8 weeks of stably transfected cells showing high protein expression mPRα on the cell membrane (e.g. by limiting dilution or by cell sorting) was found to be necessary to demonstrate progestin binding and signaling with seatrout mPRα [210]. Consequently several research groups have not been able to demonstrate cell surface expression of mPRs and GPR30 in recombinant homologous expression systems, as well as the accompanying specific steroid receptor binding in the plasma membrane fractions [104,155]. Even if these researchers had obtained significant surface expression of the putative receptor proteins, it is unlikely that specific receptor binding could have been demonstrated because nonstandard receptor protocols for both mPR and GPR30 were employed, which have been shown to be ineffective for measurement of these receptors [210,212]. On the basis of these negative results, Brosens and coworkers and Otto et al. have forcibly argued that mPR and GPR30 do not function as steroid membrane receptors, respectively, but they did not provide any data suggesting alternative functions for these proteins. Of course, negative data alone do not provide a strong basis for rejecting a hypothesis, particularly when they challenge the independent findings showing specific steroid binding of the recombinant proteins produced in prokaryotic, yeast and mammalian expression systems from several laboratories, such as those obtained for mPRs [6,7,82,196,221,233,260,262]. The possibility that mPRs bind progestins is now acknowledged by the Brosens group, although the ability of mPRs to activate through G proteins is still questioned in their recent review [70]. Similarly, the ability of GPR30 to bind estrogens and act as an intermediary in estrogen activation of signal transduction pathways has been independently confirmed by several research groups [59,91,111,161,182,222,243]. However, despite the existence of a large body of experimental data supporting a role for GPR30 as an intermediary in the rapid, nongenomic actions of estrogens, this proposed function of GPR30 as a membrane estrogen receptor has been challenged by several research groups on the basis of their negative results [99,107,155,168]. Based on the results of a recent study it was proposed that estrogen signaling in SKBR3 breast cancer c ells expressing wild-type GPR30 and in HEK293 cells transfected with human GPR30 is mediated through a N-terminal truncated variant of ERα, named ERα-36, not through GPR30 [99]. However, this conclusion is not supported by unpublished findings in our laboratory.
9. Role of comparative endocrinology
Comparative endocrinology studies in which the characteristics of novel putative steroid membrane receptors are compared between representatives of distantly-related vertebrate groups can be valuable for resolving many of these current controversies and for determining the fundamental, evolutionarily conserved functions of these proteins. For example, a comparison of the functional characteristics of recombinant spotted seatrout and human membrane progestin receptor alphas (mPRα) expressed in human breast cancer cells showed that both are expressed on the plasma membrane and display similar high affinity, specific progestin binding typical of steroid receptors [221]. Also, it was demonstrated that the seatrout and human mPRαs activate the same signal transduction pathway in these cells through an inhibitory G protein (Gi), resulting in down-regulation of adenylyl cyclase activity, and are directly associated with Gi proteins [221], consistent with the results of studies with wildtype mPRαs in seatrout and human cells [100,156,210]. Finally, a comparison of the relative binding affinities of mammalian and teleostean progestin hormones for recombinant human, seatrout, zebrafish and goldfish mPRαs expressed in the same human breast cancer cell line revealed that in each case the recombinant receptor showed highest binding affinity for the physiologically important hormone in that species and showed low binding affinity for the other vertebrate progestin hormones [82,210,221,233]. Thus, human mPRα shows highest binding affinity for progesterone, seatrout highest affinity for 20βS, and zebrafish and goldfish greatest binding affinity for DHP. The finding that these four recombinant proteins expressed in the same expression system have different progestin specificities suggests that specific progestin binding is an intrinsic property of these proteins, consistent with their proposed functions as progestin receptors [210].
A similar comparative approach has been used to investigate whether recombinant Atlantic croaker GPR30 has similar receptor and signaling characteristics as recombinant human GPR30 when it is expressed in the same cell line [161,222]. The receptor binding characteristics of plasma membranes from human- and croaker-transfected HEK-293 cells are very similar in that both display high affinity (human: Kd 3.3 nM, croaker: Kd 2.7 nM) specific 17β-estradiol binding characteristic of estrogen receptors [212]. Moreover, both human and croaker GPR30 are coupled to stimulatory G proteins (Gs) and activate adenylyl cyclase [161,222]. Finally, the estrogen binding, signaling and physiological functions of GPR30 occur in zebrafish oocytes which lack any truncated forms of any ERα, arguing against a role for ERα-36 in estrogen nonclassical actions in other vertebrates. Our findings suggest that the estrogen binding and estrogen signaling functions of GPR30 arose early in vertebrate evolution, more than 200 million years ago, and are fundamental, conserved physiological functions of GPR30 in vertebrates.
10. Concluding remarks
Over the last decade there have been significant advances in our understanding of nonclassical steroid actions and the membrane receptors that mediate them. Two novel putative 7-TM receptors, mPRα and GPR30, have been identified and their ligand binding, G protein interactions, and signaling pathways investigated in detail. However, the characterization of these novel 7-TM receptors is incomplete and questions remain concerning their functional characteristics and their physiological roles in steroid hormone regulation of reproductive and nonreproductive processes in health and disease. For example, the structural requirements for steroid binding and G protein coupling to these putative 7-TM receptors are unknown. Site-directed mutagenesis of amino acids potentially involved in ligand binding to mPRs and GPR30 is currently being conducted in order to develop and evaluate plausible models of their ligand binding pockets. A mechanistic explanation of the sites of ligand and G protein interactions with mPRs and GPR30 at the molecular level is one of the most critical remaining criteria that need to be met in order to fully establish them as steroid membrane receptors.
Knowledge of the mechanisms by which mPRs and GPR30 are transported to the cell membrane, how receptor abundance on the cell membrane is regulated, and how they are internalized and cycled upon ligand binding is also required in order to understand how the receptors function in target cells. Recent studies suggest that internalization of mPRs and GPR30 involves clathrin-dependent and trans-Golgi-proteasome pathways, respectively [34, 63]. Only limited information is currently available on the hormonal regulation of these 7-TM receptors and its functional significance. In addition to regulation of mPR and GPR30 abundance by estrogens and progestins [100,159,222,262], there is recent evidence that members of the growth factor beta (TGF-β) superfamily also regulate mPR expression in zebrafish oocytes [205]. It is necessary to demonstrate distinct and critical functions for mPRs and GPR30 in regulating reproductive and other processes in vertebrates in order to establish their physiological importance. However, studies on the functions of these receptors are frequently complicated by co-expression of ERs and PRs, and by PGRMC1 or ERα36. Specific agonists are potentially valuable pharmacological tools for investigating novel 7-TM receptor-dependent functions in cells expressing multiple receptors, but the specificity of the GPR30 agonist, G-1, has been questioned [99]. Although selective down-regulation of these receptors using siRNA and antisense oligonucleotides have proven valuable for demonstrating their critical roles in oocyte maturation and other processes [161](Figure 2), gene knockout in mice models has not been successfully employed with these receptors to date. Conflicting and equivocal results have been obtained after knockout of GPR30 [122]. This knockout approach is complicated for the mPRs, because there are five subtypes (α,β,γ,δ,ε; PAQR5–9) that could potentially assume the functions of the deleted gene, and has not been attempted to date. It is anticipated that significant progress will be made within the next few years in addressing many of these questions concerning the roles of mPRs and GPR30 as steroid membrane receptors of physiological importance.
Nonclassical steroid actions mediated by nuclear steroid receptors have not been identified in fish models to date. A comparative approach could resolve some of the questions concerning the functional motifs proposed for nuclear receptor localization to the cell membrane and activation of Src [22,169]. The presence of the palmytilation motif in the ligand binding region of human nuclear steroid receptors proposed to be involved in their association with the cell membrane [169], has not been investigated in nonmammalian vertebrate species [261]. The proline rich motif in the amino terminal of human PR-B involved in activation of Src [22] is not conserved in other species [261]. Investigations of whether nuclear steroid receptors mediate nonclassical steroid actions in fish models and the functional motifs involved would provide an indication of the importance and conservation of this proposed mechanism of nonclassical steroid action in vertebrates.
The relationship between initial nonclassical steroid actions in target cells and the longer term classical steroid responses needs to be better understood. Some nonclassical steroid actions such as rapid changes in behavior or gamete function seem to be largely independent of genomic steroid actions. In other cell models such as signaling in myometrial cells and apoptosis in granulosa cells the limited evidence available suggests both steroid mechanisms act together in a coordinated fashion. However, for the majority of nonclassical steroid actions described to date, the integration of this initial cellular response with the slower classical one involving changes in gene transcription and translation remains unclear.
Finally, there is a growing body of evidence that xenobiotic chemicals which cause endocrine disruption through binding to nuclear receptors can also interfere with steroid actions mediated through membrane receptors including mPRs and GPR30 [36,184,214,217,218,225,233,257]. Therefore, it is important that environmental toxicologists and regulatory agencies also consider endocrine disruption through interference with nonclassical steroid actions when investigating environmental contaminants and making regulatory decisions.
Acknowledgments
Research on mPRα conducted by Thomas and coworkers was funded by Grant ESO ESO12961 from the National Institutes of Health Grant and on croaker sperm by National Research Initiative Competitive Grant No.2006-35203-17130 and Agriculture and Food Initiative Competitive Grant no 2009-65203-05757 from the USDA National Institute of Food and Agriculture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17β-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–1866. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- 2.Arima S, Kohagura K, Xu HL, Sugawara A, Abe T, Satoh F, Takeuchi K, Ito S. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J.Am.Soc.Nephrol. 2003;14:2255–2263. doi: 10.1097/01.asn.0000083982.74108.54. [DOI] [PubMed] [Google Scholar]
- 3.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene expression. Proc.Natl. Acad. Sci. USA. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armanini D, Strasser T, Weber PC. Characterization for aldosterone binding sites in circulating human mononuclear leucocytes. Am. J. Physiol. 1985;248:388–390. doi: 10.1152/ajpendo.1985.248.3.E388. [DOI] [PubMed] [Google Scholar]
- 5.Armen TA, Gay CV. Simultaneous detection and functional response of testosterone and estradiol receptors in osteoblast plasma membranes. J. Cell. Biochem. 2000;79:620–627. [PubMed] [Google Scholar]
- 6.Ashley RL, Arreguin-Arevalo JA, Nett TM. Binding characteristics of the ovine membrane progesterone receptor alpha and expression of the receptor during the estrous cycle. Rep. Biol.Endocrinol. 2009;7:42. doi: 10.1186/1477-7827-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashley RL, Clay CM, Farmerie TA, Niswender GD, Nett TM. Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology. 2006;147:4151–4159. doi: 10.1210/en.2006-0002. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK. Nonclassical mechanisms of progesterone action in the brain: I. Protein kinase C activation in the hypothalamus of female rats. Endocrinology. 2008;14:5509–5517. doi: 10.1210/en.2008-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldi E, Krausz C, Luconi M, Bonaccorsi L, Maggi M, Forti G. Actions of progesterone on human sperm: A model of non-genomic effects of steroids. J. Steroid Biochem. Mol. Biol. 1995;53:199–203. doi: 10.1016/0960-0760(95)00046-3. [DOI] [PubMed] [Google Scholar]
- 10.Baldi E, Luconi M, Bonaccorsi L, Forti G. Signal transduction pathways in human spermatozoa. J. Reprod. Immunol. 2002;53:121–131. doi: 10.1016/s0165-0378(01)00089-4. [DOI] [PubMed] [Google Scholar]
- 11.Baldi E, Luconi M, Bonaccorsi L, Maggi M, Francavilla S, Gabriel A, Properzi G, Forti G. Nongenomic progesterone receptor on human spermatozoa: biochemical aspects and clinical implications. Steroids. 1999;64:143–148. doi: 10.1016/s0039-128x(98)00100-7. [DOI] [PubMed] [Google Scholar]
- 12.Bayaa M, Booth RA, Sheng Y, Liu XJ. The classical progesterone receptor induces Xenopus oocyte maturation through a nongenomic mechanism. Proc. Natl. Acad. Sci. USA. 2000;97:12607–12612. doi: 10.1073/pnas.220302597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benten WP, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, Mossmann H, Wunderlich F. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999;13:123–133. doi: 10.1096/fasebj.13.1.123. [DOI] [PubMed] [Google Scholar]
- 14.Benten WP, Lieberherr M, Stamm O, Wrehlke C, Guo Z, Wunderlich F. Testosterone signaling through internalizable surface receptors in androgen receptor-free macrophages. Mol. Biol. Cell. 1999;10:3113–3123. doi: 10.1091/mbc.10.10.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berg AH, Thomas P. Isolation and characterization of a cDNA from Atlantic croaker (Micropogonias undulatus) ovaries encoding a novel protein with the characteristics of a membrane androgen receptor. Biol. Reprod. SI. 2007:136. [Google Scholar]
- 16.Berg AH, Thomas P, Olsson P-E. Characterization of a membrane progestin receptor in artic charr ovaries. Reprod. Biol. Endocrinol. 2005;3:64. doi: 10.1186/1477-7827-3-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertelsen EL, Endresen PC, Orbo A, Sager G. Non-genomic cell growth inhibition by progesterone: cell cycle retardation and induction of cell death. Anticancer Res. 2004;24:3749–3755. [PubMed] [Google Scholar]
- 18.Blackmore PF, Neulen J, Lattanzio F, Beebe SJ. Cell surface binding sites for progesterone mediated calcium-uptake in human sperm. J. Biol. Chem. 1991;266:18655–18659. [PubMed] [Google Scholar]
- 19.Bockaert J, Pin JP. Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J. 1999;16:1723–1729. doi: 10.1093/emboj/18.7.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boldizsar F, Talaber G, Szabo M, Bartis D, Palinkas L, Nemeth P, Berki T. Emerging pathways of non-genomic glucocorticoid (GC) signaling in T cells. Immunobiology. 2010;215:521–526. doi: 10.1016/j.imbio.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Bologa CG, Revankar CM, Young M, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat. Chem. Biol. 2006;2:207–212. doi: 10.1038/nchembio775. [DOI] [PubMed] [Google Scholar]
- 22.Boonyaratnakonkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinase. Mol. Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 23.Borski RJ, Helms LM, Richman NH, 3rd, Grau EG. Cortisol rapidly reduces prolactin release and cAMP and 45Ca2+ accumulation in the cichlid fish pituitary in vitro. Proc. Natl. Acad. Sci. USA. 1999;88:2758–2762. doi: 10.1073/pnas.88.7.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borski RJ, Hyde GN, Fruchtman S. Signal transduction mechanisms mediating rapid, nongenomic effects of cortisol on prolactin release. Steroids. 2002;67:539–548. doi: 10.1016/s0039-128x(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 25.Borski RJ, Hyde GN, Fruchtman S, Tai WS. Cortisol suppresses prolactin release through a non-genomic mechanism involving interactions with the plasma membrane. Comp. Biochem. Physiol. B, Biochem. Mol. Biol. 2001;129:533–541. doi: 10.1016/s1096-4959(01)00358-x. [DOI] [PubMed] [Google Scholar]
- 26.Botta JA, De Mendoza D, Morero RD, Farias RN. High affinity L-triiodothyronine binding sites on washed erythrocyte membranes. J. Biol. Chem. 1983;258:6690–6692. [PubMed] [Google Scholar]
- 27.Braun A, Thomas P. Biochemical characterization of a membrane androgen receptor in the ovary of the Atlantic croaker. Biol. Reprod. 2004;71:146–155. doi: 10.1095/biolreprod.103.025825. [DOI] [PubMed] [Google Scholar]
- 28.Braun AM, Thomas P. Androgens inhibit estradiol-17β synthesis in Atlantic croaker (Micropogonias undulatus) ovaries by a nongenomic mechanism initiated at the cell surface. Biol. Reprod. 2003;69:1642–1650. doi: 10.1095/biolreprod.103.015479. [DOI] [PubMed] [Google Scholar]
- 29.Braun S, Losel R, Wehling M, Boldyreff B. Aldosterone rapidly activates Src kinase in M-1 cells involving the mineralocorticoid receptor and HSP84. FEBS Lett. 2004;570:69–72. doi: 10.1016/j.febslet.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 30.Breuner CW, Orchinik M. Seasonal regulation of membrane and intracellular corticosteroid receptors in the house sparrow brain. J. Neuroendocrinol. 2001;13:412–420. doi: 10.1046/j.1365-2826.2001.00646.x. [DOI] [PubMed] [Google Scholar]
- 31.Cahill MA. Progesterone receptor membrane component 1: an integrative review. J. Steroid Biochem. Mol. Biol. 2007;105:16–36. doi: 10.1016/j.jsbmb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Calles F, Stromer H, Schwinger RH, Bolk B, Hu K, Frantz S, Leopold A, Beer S, Allolia B, Bonz AW. Administration of testosterone is associated with a reduced susceptibility to myocardial ischemia. Endocrinology. 2003;144:4478–4483. doi: 10.1210/en.2003-0058. [DOI] [PubMed] [Google Scholar]
- 33.Charles NA, Thomas P, Lange CA. Signaling events mediated by membrane progesterone receptors (mPRs) in ovarian cancer cells. Hormones and Cancer. 2010;1:167–176. doi: 10.1007/s12672-010-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng S-HB, Quinn J, Graeber CT, Filardo E. Downmodulation of GPR30/GPER from the cell surface occurs via a transGolgi-proteasome pathway. J. Biol. Chem. 2011;286:22441–22455. doi: 10.1074/jbc.M111.224071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chien EJ, Chang C-P, Lee W-F, Su T-H, Wu C-H. Nongenomic immunosuppressive actions of progesterone inhibits PHA-induced alkalinization and activation in T cells. J. Cell. Biochem. 2006;99:292–304. doi: 10.1002/jcb.20858. [DOI] [PubMed] [Google Scholar]
- 36.Das S, Thomas P. Pesticides interfere with the nongenomic action of a progestogen on meiotic maturation by binding to its plasma membrane receptor on fish oocytes. Endocrinology. 140:1953–1956. doi: 10.1210/endo.140.4.6781. 199. [DOI] [PubMed] [Google Scholar]
- 37.Davis PJ, Davis FB. Nongenomic actions of thyroid hormones. Thyroid. 1996;6:497–504. doi: 10.1089/thy.1996.6.497. [DOI] [PubMed] [Google Scholar]
- 38.DeBold JF, Frye CA. Genomic and nongenomic actions of progesterone on the control of female hamster sexual behavior. Horm. Behav. 1994;28:445–453. doi: 10.1006/hbeh.1994.1042. [DOI] [PubMed] [Google Scholar]
- 39.Deecher DC, Siggard P, Frail DE, O’Connor LT. Characterization of a membrane-associated estrogen receptor in a rat hypothalamic cell line (D12) Endocrine. 2003;22:211–223. doi: 10.1385/ENDO:22:3:211. [DOI] [PubMed] [Google Scholar]
- 40.DeManno DA, Goetz FW. Steroid-induced final maturation in brook trout (Salvelinus fontinalis) oocytes in vitro: the effects of forskolin and phosphodiesterase inhibitors. Biol. Reprod. 1987;36:1321–1332. doi: 10.1095/biolreprod36.5.1321. [DOI] [PubMed] [Google Scholar]
- 41.De Kloet ER, Karst H, Joels M. Corticosteroid hormones in the central stress response: Quick-and-slow. Front. Neuroendocrinol. 2008;29:268–272. doi: 10.1016/j.yfrne.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 42.Dettlaff TA, Skoblina MN. The role of germinal vesicle in the process of oocyte maturation in Anura and Acipenseridae. An. Embryol. Morphog. 1969 Suppl1:133–151. [Google Scholar]
- 43.Dosiou C, Hamilton AE, Pang Y, Overgaard MT, Tulac S, Dong J, Thomas P, Guidice LC. Expression of membrane progesterone receptors (mPRs) on human T lymphocytes and Jurkat cells and activation of G proteins by progesterone. J. Endocrinol. 2008;196:67–77. doi: 10.1677/JOE-07-0317. [DOI] [PubMed] [Google Scholar]
- 44.Dressing G, Pang Y, Dong J, Thomas P. Progestin signaling through mPRα in Atlantic croaker granulosa/theca cell co-cultures and its involvement in progestin inhibition of apoptosis. Endocrinology. 2010;151:5914–5928. doi: 10.1210/en.2010-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dressing GE, Thomas P. Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids. 2007;72:111–115. doi: 10.1016/j.steroids.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 46.Du J, McEwen B, Manji HK. Glu cocorticoid receptors modulate mitochondrial function - A novel mechanism of neuroprotection. Commun. Integrat. Biol. 2009;2:350–352. doi: 10.4161/cib.2.4.8554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duval D, Durant S, Homo-Delarche F. Non-genomic effects of steroids. Interactions of steroid molecules with membrane structures and functions. Biochimica Biophysica Acta. 1983;737:409–442. doi: 10.1016/0304-4157(83)90008-4. [DOI] [PubMed] [Google Scholar]
- 48.Ehring GR, Kerschbaum HH, Eder C, Neben AL, Fanger CM, Khoury RM, Negulescu PA, Cahalan MD. A nongenomic mechanism for progesterone-mediated immunosuppression: inhibition of K+ channels, Ca2+ signaling, and gene expression in T lymphocytes. J. Exp. Med. 1998;188:1593–1602. doi: 10.1084/jem.188.9.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engmann L, Losel R, Wehling M, Peluso JJ. Progesterone regulation of human granulosa/luteal cell viability by an RU486-independent mechanism. J. Clin. Endocrinol. Metab. 2006;91:4962–4968. doi: 10.1210/jc.2006-1128. [DOI] [PubMed] [Google Scholar]
- 50.Estrada M, Liberona JL, Miranda M, Jaimovich E. Aldosterone- and testosterone-mediated intracellular calcium response in skeletal muscle cell cultures. Am. J. Physiol. Endocrinol. Metab. 2000;279:E132–E139. doi: 10.1152/ajpendo.2000.279.1.E132. [DOI] [PubMed] [Google Scholar]
- 51.Estrada M, Uhlen P, Ehrlich BE. Ca2+ oscillations induced by testosterone enhance neurite growth. J. Cell Science. 2006;119:733–743. doi: 10.1242/jcs.02775. [DOI] [PubMed] [Google Scholar]
- 52.Evans SJ, Murray TF, Moore FL. A subset of kappa opioid ligands bind to the membrane glucocorticoid receptor in an amphibian brain. Endocrinology. 2000;141:2294–2300. doi: 10.1210/endo.141.7.7587. [DOI] [PubMed] [Google Scholar]
- 53.Evans SJ, Murray TF, Moore FL. Partial purification and biochemical characterization of a membrane glucocorticoid receptor from an amphibian brain. J. Steroid Biochem. Mol. Biol. 2000;72:209–221. doi: 10.1016/s0960-0760(00)00031-5. [DOI] [PubMed] [Google Scholar]
- 54.Falkenstein E, Heck M, Gerdes D, Grube D, Christ M, Weigel M, Buddhikot M, Meizel S, Wehling M. Specific progesterone binding to a membrane protein and related nongenomic effects on Ca2+-fluxes in sperm. Endocrinology. 1999;140:5999–6002. doi: 10.1210/endo.140.12.7304. [DOI] [PubMed] [Google Scholar]
- 55.Falkenstein E, Meyer C, Eisen C, Sciba PC, Wehling M. Full length cDNA sequence of progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 1996;229:86–89. doi: 10.1006/bbrc.1996.1761. [DOI] [PubMed] [Google Scholar]
- 56.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones--a focus on rapid, nongenomic effects. Pharmacol. Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- 57.Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J. Steroid Biochem. Mol. Biol. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 58.Filardo EJ, Graeber CT, Quinn JA, Resnick MB, Giri D, DeLellis RA, Steinhoff MM, Sabo E. Distribution of GPR30, a seven-transmembrane spanning estrogen receptor, in primary breast cancer and its association with clinopathologic determinants of tumor progression. Clin. Cancer Res. 2006;12:6359–6366. doi: 10.1158/1078-0432.CCR-06-0860. [DOI] [PubMed] [Google Scholar]
- 59.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via transactivation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 60.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol. Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 61.Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. TRENDS Endocrinol. Metab. 2005;16:362–367. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 62.Foradori CD, Weiser MJ, Handa RJ. Non-genomic actions of androgens. Front. Neuroendocrinol. 2008;29:169–181. doi: 10.1016/j.yfrne.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Foster H, Reynolds A, Stenbeck G, Dong J, Thomas P, Karteris E. Internalization of membrane progesterone receptor alpha (mPRα) after treatment with progesterone: potential involvement of a clathrin-dependent pathway. Mol. Med. Report. 2010;3:27–35. doi: 10.3892/mmr_00000214. [DOI] [PubMed] [Google Scholar]
- 64.Frye CA, Lydon JP, O’Malley BW, Pfaff DW. Mid-aged and aged wild-type and progestin receptor knockout (PRKO) mice demonstrate rapid progesterone and 3alpha,5alpha-THP-facilitiated lordosis. Psychopharmacology. 2006;185:423–432. doi: 10.1007/s00213-005-0300-4. [DOI] [PubMed] [Google Scholar]
- 65.Frye CA, Petralia SM. Progestins have actions through GABA A receptors. In: Watson CS, editor. The Identities of Steroid Membrane Receptors. Boston: Kluwer Academic Publishers; 2003. pp. 65–168. [Google Scholar]
- 66.Fu X-D, Giretti MS, Baldacci C, Garibaldi S, Flamini M, Sanchez AM, Gadducci A, Genazzani AR, Simoncini T. Extra-nuclear signaling of progesterone receptor to breast cancer cell movement and invasion through the actin cytoskeleton. Plos One. 2008;3:e2790. doi: 10.1371/journal.pone.0002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Funder JW. The nongenomic actions of aldosterone. Endocrine Rev. 2005;26:313–321. doi: 10.1210/er.2005-0004. [DOI] [PubMed] [Google Scholar]
- 68.Gametchu B. Glucocorticoid receptor-like antigen in lymphoma cell membranes: correlation with cell lysis. Science. 1987;236:456–461. doi: 10.1126/science.3563523. [DOI] [PubMed] [Google Scholar]
- 69.Gametchu B, Watson CS, Wu S. Use of receptor antibodies to demonstrate membrane glucocorticoid receptor in cell from human leukemic patients. FASEB J. 1993;7:1283–1293. doi: 10.1096/fasebj.7.13.8405814. [DOI] [PubMed] [Google Scholar]
- 70.Gellersen B, Fernandes MS, Brosens JJ. Non-genomic progesterone actions in female reproduction. Human Reproduction Update. 2009;15:119–138. doi: 10.1093/humupd/dmn044. [DOI] [PubMed] [Google Scholar]
- 71.Godeau JF, Schorderet-Slatkine S, Hubert P, Baulieu E-E. Induction of maturation in Xenopus laevis oocytes by a steroid linked to a polymer. Proc. Natl. Acad. Sci. USA. 1978;75:2353–2357. doi: 10.1073/pnas.75.5.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goetz FW. Hormonal control of oocyte final maturation and ovulation in fishes. In: Hoar WS, Randal DJ, Donaldson EM, editors. Fish Physiology. vol. 9B. New York: Academic Press; 1993. pp. 116–170. [Google Scholar]
- 73.Gorczynska E, Handelsman DJ. Androgens rapidly increase the cytosolic calcium in Sertoli cells. Endocrinology. 1995;136:2052–2059. doi: 10.1210/endo.136.5.7720654. [DOI] [PubMed] [Google Scholar]
- 74.Grazzini E, Guillon G, Mouilliac B, Zing HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392:509–512. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- 75.Gros R, Ding Q, Sklar LA, Prossnitz ER, Arterburn JB, Chorazyczewski J, Feldman RD. GPR30 expression is required for the mineralocorticoid receptor-independent rapid vascular effects of aldosterone. Hypertension. 2011;57:442–451. doi: 10.1161/HYPERTENSIONAHA.110.161653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grossmann C, Benesic A, Krug AW, Freudinger R, Mildenberger S, Gassner B, Gekle M. Human mineralocorticoid receptor expression renders cells responsive for nongenotropic aldosterone actions. Mol. Endocrinol. 2005;19:1697–1710. doi: 10.1210/me.2004-0469. [DOI] [PubMed] [Google Scholar]
- 77.Gu Q, Korach KS, Moss RL. Rapid action of 17 beta-estradiol on kainate-induced currents in hippocampal neurons lacking intracellular estrogen receptors. Endocrinology. 1999;140:660–666. doi: 10.1210/endo.140.2.6500. [DOI] [PubMed] [Google Scholar]
- 78.Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perz-Dominiguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role for G protein-coupled estrogen receptor for vascular function and obesity. Circ. Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activity of endothelial nitric oxide synthase. Nat. Med. 2002;8:473–479. doi: 10.1038/nm0502-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haider S, Baqri SS. Role of cyclic AMP-dependent protein kinase in oocyte maturation of the catfish, Clarias batrachus. J. Exp. Zool. 2002;292:587–593. doi: 10.1002/jez.10102. [DOI] [PubMed] [Google Scholar]
- 81.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Extranuclear steroid receptors: nature and actions, Endocr. Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
- 82.Hanna R, Pang Y, Thomas P, Zhu Y. Cell surface expression, progestin binding and rapid nongenomic signaling of zebrafish membrane progestin receptors α and β in transfected cells. J. Endocrinol. 2006;190:247–260. doi: 10.1677/joe.1.06694. [DOI] [PubMed] [Google Scholar]
- 83.Hanna R, Zhu Y. Expression of membrane progestin receptors in zebrafish (Danio rerio) oocytes, testis and pituitary. Gen. Comp. Endocrinol. 2009;161:153–157. doi: 10.1016/j.ygcen.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 84.Hanna RN, Zhu Y. Controls of meiotic signaling by membrane or nuclear progestin receptor in zebrafish follicle-enclosed oocytes. Mol. Cell. Endocrinol. 2011;337:80–90. doi: 10.1016/j.mce.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 85.Haseroth K, Gerdes D, Berger S, Furing M, Gunther A, Herbst C, Christ M, Wehling M. Rapid nongenomic effects of aldosterone in mineralocorticoid-receptor knockout mice. Biochem. Biophys. Res. Commun. 1999;266:257–261. doi: 10.1006/bbrc.1999.1771. [DOI] [PubMed] [Google Scholar]
- 86.Hatzoglou A, Kampa M, Kogia C, Charalampogoulos I, Theodoropoulos PA, Anezinis P, Dambaki C, Papakonstanti EA, Stathopoulos EN, Gravanis A, Castanas E. Membrane androgen receptor activation induces apoptotic regression of human prostate cancer cells in vitro and in vivo. J. Clin. Endocrinol. Metab. 2005;90:893–903. doi: 10.1210/jc.2004-0801. [DOI] [PubMed] [Google Scholar]
- 87.He LM, Zhang CG, Zhou Z, Xu T. Rapid inhibitory effects of corticosterone on calcium influx in rat dorsal root ganglion neurons. Neuroscience. 2003;116:325–333. doi: 10.1016/s0306-4522(02)00568-7. [DOI] [PubMed] [Google Scholar]
- 88.Hien P, Bunëmann M. Coupling mode of receptors and G-proteins. Naunyn-Schmied. Arch. Pharmacol. 2009;379:435–443. doi: 10.1007/s00210-008-0383-7. [DOI] [PubMed] [Google Scholar]
- 89.Hinz B, Hirschelmann R. Rapid non-genomic feedback effects of glucocorticoids on CRF-induced ACTH secretion in rats. Pharm. Res. 2000;17:1273–1277. doi: 10.1023/a:1026499604848. [DOI] [PubMed] [Google Scholar]
- 90.Ho H, Suarez S. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction. 2001;122:519–526. doi: 10.1530/rep.0.1220519. [DOI] [PubMed] [Google Scholar]
- 91.Hsieh YC, Yu HP, Frink M, Suzuki T, Choudry MA, Schwacha MG, Chaudry IH. G protein-coupled receptor 30-dependent protein kinase A pathway is critical in nongenomic effects of estrogen in attenuating liver injury after trauma-hemorrhage. Am. J. Physiol. 2007;170:1210–1218. doi: 10.2353/ajpath.2007.060883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hyde GN, Seale AP, Grau EG, Borski RJ. Cortisol rapidly suppresses intracellular calcium and voltage-gated calcium channel activity in prolactin cells of the tilapia (Oreochromis mossambicus) Am. J. Physiol. Endocrinol. Metab. 2003;256:E626–E633. doi: 10.1152/ajpendo.00088.2003. [DOI] [PubMed] [Google Scholar]
- 93.Jalabert B. Modulation of 17-alpha-hydroxy-20-beta-dihydroprogesterone or gonadotropic extract activity on the in vitro intrafollicular maturation of rainbow trout (Salmo gairdnerii) oocytes by various steroids lacking maturing activity. C. R. Acad. Sci. Hebd. Seances Acad. Sci. D. 1975;281:811–814. 1975. [PubMed] [Google Scholar]
- 94.Jalabert B, Bry C, Breton B, Campbell C. Effect of 17 alpha hydroxy-20 beta dihydroprogesterone and progesterone on maturation and ovulation in vivo and on the plasma level of gonadotropic hormone t-GtH in rainbow trout Salmo gairdneri. C. R. Acad. Sci. Hebd Seances Acad. Sci. D. 1976;283:1205–1208. [PubMed] [Google Scholar]
- 95.Jalabert B, Finet B. Regulation of oocyte maturation in the rainbow trout, Salmo gairdneri: role of cyclic AMP in the mechanism of action of the maturation inducing steroid (MIS), 17α hydroxy, 20β-dihydroprogesterone. Fish. Physiol. Biochem. 1986;2:65–74. doi: 10.1007/BF02264074. [DOI] [PubMed] [Google Scholar]
- 96.Jaubert AM, Mehebik-Mojaat N, Lacasa D, Sabourault D, Guidicelli Y, Ribiere C. Nongenomic estrogen effects on nitric oxide synthase activity in rat adipocytes. Endocrinology. 2007;148:2444–2445. doi: 10.1210/en.2006-1329. [DOI] [PubMed] [Google Scholar]
- 97.Josefsberg B-YL, Lewellyn AL, Thomas P, Maller JL. The role of Xenopus membrane progesterone receptor β in mediating the effect of progesterone on oocyte maturation. Mol. Endocrinol. 2007;21:664–673. doi: 10.1210/me.2006-0256. [DOI] [PubMed] [Google Scholar]
- 98.Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos S, Stournaras C, Castanas E. The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors, which increase PSA secretion and modify actin cytoskeleton. FASEB J. 2002;16:1429–1431. doi: 10.1096/fj.02-0131fje. [DOI] [PubMed] [Google Scholar]
- 99.Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. Involvement of estrogen receptor variant ER-alpha36, not GPR30, in nongenomic estrogen signaling. Mol. Endocrinol. 2010;24:709–721. doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol. Endocrinol. 2006;20:1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- 101.Kelder J, Azevedo R, Pang Y, de Vlieg J, Dong J, Thomas P. Comparison between steroid binding to the human progestin membrane receptor α subtype and to the human progestin nuclear receptor: correlations with physicochemical properties assessed by comparative molecular field analyses. Steroids. 2010;75:314–322. doi: 10.1016/j.steroids.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim KH, Moriarty K, Bender JR. Vascular cell signaling by membrane estrogen receptors. Steroids. 2009;73:864–869. doi: 10.1016/j.steroids.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.King W, Ghosh S, Thomas P, Sullivan CV. A receptor for the oocyte maturation inducing hormone 17α,20β,21-trihydroxy-4-pregnen-3-one on ovarian membranes of striped bass. Biol. Reprod. 1997;56:266–271. doi: 10.1095/biolreprod56.1.266. [DOI] [PubMed] [Google Scholar]
- 104.Krietsch T, Fernandes MS, Kero J, Losel R, Heyens M, Lam E-WF, Huhtaniemi I, Brosens JJ, Gellersen B. Human homologs of the putative G protein-coupled membrane progestin receptors mPR [alpha}, [beta}, and [gamma} localize to the endoplasmic reticulum and are not activated by progesterone. Mol. Endocrinol. 2006;20:3146–3164. doi: 10.1210/me.2006-0129. [DOI] [PubMed] [Google Scholar]
- 105.Krug AW, Pojoga LH, Williams GH, Adler GK. Cell membrane-associated aldosterone receptor? : new evidence. Hypertension. 2011;57:1019–1025. doi: 10.1161/HYPERTENSIONAHA.110.159459. [DOI] [PubMed] [Google Scholar]
- 106.Labombarda F, Meffre D, Delespierre B, Krivokapic-Blondiaux S, Chastre A, Thomas P, Pang Y, Lydon JP, Gonzalez S1J, De Nicola AF, Schumacher M, Guennoun R. Expression mapping of membrane progesterone receptors (mPRs) in the mouse spinal cord. Neuroscience. 2010;166:94–106. doi: 10.1016/j.neuroscience.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 107.Levin ER. G protein-coupled receptor 30: estrogen receptor or collaborator? Endocrinology. 2009;154:1563–1565. doi: 10.1210/en.2008-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lieberherr M, Grosse B. Androgens increase intracellular calcium concentration and inositol 1,4,5-triphosphate and diacylglycerol formation via a pertussis toxin-sensitive G-protein. J. Biol. Chem. 1994;269:7217–7223. [PubMed] [Google Scholar]
- 109.Liu D, Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric oxide synthetase by a specific plasma membrane receptor coupled to Galpha(i2,3) J. Biol. Chem. 2002;277:21379–21388. doi: 10.1074/jbc.M200491200. [DOI] [PubMed] [Google Scholar]
- 110.Liu L, Wang YX, Zhou J, Long F, Sun HW, Liu Y, Chen YZ, Jiang CL. Rapid non-genomic inhibitory effects of glucocorticoids on human neutrophil degranulation. Inflamm. Res. 2005;54:37–41. doi: 10.1007/s00011-004-1320-y. [DOI] [PubMed] [Google Scholar]
- 111.Liu X, Zhu P, Sham KW, Yuen JM, Xie C, Zhang Y, Liu Y, Li S, Huang X, Cheng CH, Lin H. Identification of a membrane estrogen receptor in zebrafish with homology to mammalian GPER and its high expression in early germ cells of the testis. Biol. Reprod. 2009;80:1253–1261. doi: 10.1095/biolreprod.108.070250. [DOI] [PubMed] [Google Scholar]
- 112.Liu Z, Patiño R. High-affinity binding of progesterone to the plasma membrane of Xenopus oocytes: characteristics of binding and hormonal and developmental control. Biol. Reprod. 1993;49:980–988. doi: 10.1095/biolreprod49.5.980. [DOI] [PubMed] [Google Scholar]
- 113.Loomis AK, Thomas P. Effects of estrogens and xenoestrogens on androgen production by Atlantic croaker testes in vitro: evidence for a nongenomic action mediated by an estrogen membrane receptor. Biol. Reprod. 2000;62:995–1004. doi: 10.1095/biolreprod62.4.995. [DOI] [PubMed] [Google Scholar]
- 114.Loomis AK, Thomas P. Binding characteristics of estrogen receptor (ER) in Atlantic croaker (Micropogonias undulatus) testis: different affinity for estrogens and xenobiotics from that of hepatic ER. Biol. Reprod. 1999;61:51–60. doi: 10.1095/biolreprod61.1.51. [DOI] [PubMed] [Google Scholar]
- 115.Losel RM, Besong D, Peluso JJ, Wehling M. Progesterone receptor membrane component 1-many tasks for a versatile protein. Steroids. 2008;73:929–934. doi: 10.1016/j.steroids.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 116.Losel RM, Wehling M. Classic versus non-classic receptors for nongenomic mineralocorticoid responses: emerging evidence. Front. Neuroendocrinol. 2008;29:258–267. doi: 10.1016/j.yfrne.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 117.Lowry CA, Burke KA, Renner KJ, Moore FL, Orchink M. Rapid changes in monoamine levels following administration of corticotrophin-releasing factor or corticosterone are localized in the dorsomedial hypothalamus. Horm. Behav. 2001;39:195–205. doi: 10.1006/hbeh.2001.1646. [DOI] [PubMed] [Google Scholar]
- 118.Luconi M, Bonaccorsi L, Maggi M, Pecchioli P, Krausz C, Forti G, Baldi E. Identification and characterization of functional nongenomic progesterone receptors on human sperm membrane. J. Clin. Endocrinol. Metab. 1998;83:877–885. doi: 10.1210/jcem.83.3.4672. [DOI] [PubMed] [Google Scholar]
- 119.Luconi M, Francavilla F, Porazzi I, Macerola B, Forti G, Baldi E. Human spermatozoa as a model for studying membrane receptors mediating rapid nongenomic effects of progesterone and estrogens. Steroids. 2004;69:553–559. doi: 10.1016/j.steroids.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 120.Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR. Evidence that androgens are the primary steroids produced by Xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc. Natl. Acad. Sci. USA. 2001;98:13728–13733. doi: 10.1073/pnas.241471598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lyng FM, Jones GR, Rommerts FFG. Rapid androgen actions on calcium signaling in rat Sertoli cells and two human prostatic cell lines: similar biphasic responses between 1 picomolar and 100 nanomolar concentrations. Biol. Reprod. 63:736–747. doi: 10.1095/biolreprod63.3.736. 200. [DOI] [PubMed] [Google Scholar]
- 122.Maggiolini M, Picard D. The unfolding stories of GPR30, a new membrane bound estrogen receptor. J Endocrinol. 2010;204:105–114. doi: 10.1677/JOE-09-0242. [DOI] [PubMed] [Google Scholar]
- 123.Makara GB, Haller J. Non-genomic effects of glucocorticoids in the neural system-Evidence, mechanisms and implications. Progr. Neurobiol. 2001;65:367–390. doi: 10.1016/s0301-0082(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 124.Maller JL. Regulation of amphibian oocyte maturation. Cell. Diff. 1985;16:211–221. doi: 10.1016/0045-6039(85)90570-6. [DOI] [PubMed] [Google Scholar]
- 125.Maller JL. The elusive progesterone receptor in Xenopus oocytes. Proc. Natl. Acad. Sci. USA. 2001;98:8–10. doi: 10.1073/pnas.98.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mangelsdorf DF, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Márquez DC, Pietras RJ. Membrane-associated binding sites for estrogen contribute to growth regulation of human breast cancer cells. Oncogene. 2001;20:5420–5430. doi: 10.1038/sj.onc.1204729. [DOI] [PubMed] [Google Scholar]
- 128.Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daskiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150:687–698. doi: 10.1210/en.2008-0623. [DOI] [PubMed] [Google Scholar]
- 129.Masui Y, Markert CL. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J. Exp. Zool. 1971;177:129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- 130.Mathews L, Berry A, Ohanina J, Garside H, Ray D. Caveolin mediates rapid glucocorticoid effects and couples glucocorticoid action to the antiproliferative program. Mol. Endocrinol. 2008;22:1320–1330. doi: 10.1210/me.2007-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McCormick SD. Endocrine control of osmoregulation in teleost fish. Amer. Zool. 2001;41:781–794. [Google Scholar]
- 132.McEneaney V, Harvey BJ, Thomas W. Aldosterone rapidly activates protein kinase D via a mineralocorticoid receptor/EGFR trans-activation pathway in the M1 kidney CCD cell line. J. Steroid Biochem. Mol Biol. 2007;107:180–190. doi: 10.1016/j.jsbmb.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 133.McKenna NJ, O'Malley BW. Minireview: nuclear receptor coactivators--an update. Endocrinology. 2002;143:2461–2465. doi: 10.1210/endo.143.7.8892. [DOI] [PubMed] [Google Scholar]
- 134.Menzies GS, Howland K, Rae MT, Bramley TA. Stimulation of specific binding of [3H]-progesterone to bovine luteal cell-surface membranes: specificity of digitonin. Mol. Cell. Endocrinol. 1999;153:57–69. doi: 10.1016/s0303-7207(99)00091-x. [DOI] [PubMed] [Google Scholar]
- 135.Meyer C, Christ M, Wehling M. Char acterization and solubilization of novel aldosterone binding proteins in porcine liver microsomes. Eur. J. Biochem. 1995;229:736–740. doi: 10.1111/j.1432-1033.1995.tb20521.x. [DOI] [PubMed] [Google Scholar]
- 136.Meyer C, Schmid R, Scriba PC, Wehling M. Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Euro. J. Biochem. 1996;239:726–731. doi: 10.1111/j.1432-1033.1996.0726u.x. [DOI] [PubMed] [Google Scholar]
- 137.Michels G, Hoppe UC. Rapid actions of androgens. Front. Neuroendocrinol. 2008;29:182–198. doi: 10.1016/j.yfrne.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 138.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Balancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17:101–111. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mihailidou AS, Mardini M, Funder JW. Rapid, nongenomic effects of aldosterone in the heart mediated by episolon protein kinase C. Endocrinology. 2004;145:773–780. doi: 10.1210/en.2003-1137. [DOI] [PubMed] [Google Scholar]
- 140.Min L, Stushkevich V, Harnastai IN, Iwamoto H, Gilep AA, Takemori H, Usanov SA, Nanaka Y, Hori H, Vinson GP, Okamoto M. Molecular identification of adrenal inner zone antigen as a heme-binding protein. FEBS J. 2005;272:5832–5843. doi: 10.1111/j.1742-4658.2005.04977.x. [DOI] [PubMed] [Google Scholar]
- 141.Min L, Takemori H, Nonaka Y, Katoh Y, Doi J, Horike N, Osamu H, Raza FS, Vinson GP, Okamato M. Characterization of the adrenal-specific antigen IZA (inner zone antigen) and its role in the steroidogenesis. Mol. Cell. Endocrinol. 2004;215:143–146. doi: 10.1016/j.mce.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 142.Minshall RD, Puvcnik D, Browne DL, Hermemeyer K. Nongenomic vasodilator action of progesterone on primary coronary arteries. J. Appl. Physiol. 2008;92:701–706. doi: 10.1152/japplphysiol.00689.2001. [DOI] [PubMed] [Google Scholar]
- 143.Moore FL, Miller IJ. Stress-induced inhibition of sexual behavior: corticosterone inhibits courtship behaviors of a male amphibian (Taricha granulosa) Horm. Behav. 1984;18:400–410. doi: 10.1016/0018-506x(84)90026-6. [DOI] [PubMed] [Google Scholar]
- 144.Nadal A, Ropero AB, Laribi O, Maillet M, Fuentes E, Soria B. Nongenomic actions of estrogens and xenoestrogens by binding at a plasma membrane receptor unrelated to estrogen receptor alpha and estrogen receptor beta. Proc. Natl. Acad. Sci. USA. 2000;97:11603–11608. doi: 10.1073/pnas.97.21.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nagahama Y. 17α,20β-Dihydroxy-4-pregnen-3-one, a maturation-inducing hormone in fish oocytes: Mechanisms of synthesis and action. Steroids. 1997;62:190–196. doi: 10.1016/s0039-128x(96)00180-8. [DOI] [PubMed] [Google Scholar]
- 146.Nagahama Y, Adachi S. Identification of maturation-inducing steroid in the teleost, the Amago salmon (Oncorhynchus rhodurus) Dev. Biol. 1985;109:428–435. doi: 10.1016/0012-1606(85)90469-5. [DOI] [PubMed] [Google Scholar]
- 147.Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008;50:S195–S219. doi: 10.1111/j.1440-169X.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- 148.Nemere I, Dormanen MC, Hammond MW, Okamura WH, Norman AW. Identification of a specific binding protein for 1α, 25-dihydroxyvitamin D3 in basal-lateral membranes of chick intestinal epithelium and relationship to transcaltachia. J. Biol. Chem. 1994;269:23750–23756. [PubMed] [Google Scholar]
- 149.Noel SD, Keen KI, Baumann Dl, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in the rapid effects of estrogens on LHRH neurons. Mol. Endocrinol. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Norman AW, Mizwicki MT, Norman DP. Steroid-hormone rapid actions, membrane receptors and a conformational ensemble model. Nat. Rev. Drug. Discov. 2004;3:27–41. doi: 10.1038/nrd1283. [DOI] [PubMed] [Google Scholar]
- 151.Orchinik M, Murray T, Franklin P, Moore F. Guanyl nucleotides modulate binding to steroid receptors in neuronal membranes. Proc. Nat. Acad. Sci. USA. 1992;89:3830–3834. doi: 10.1073/pnas.89.9.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Orchinik M, Murray MF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848–1853. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- 153.Oren I, Fleishma SJ, Kessel A, Ben-Tai N. Free diffusion of steroid hormones across biomembranes: a simplex search with implicit solvent calculations. Biophysical J. 2004;87:768–779. doi: 10.1529/biophysj.103.035527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Osman RA, Andria ML, Jones AD, Meizel S. Steroid induced exocytosis: the human sperm acrosome reaction. Biochem. Biophys. Res. Commun. 1989;160:828–833. doi: 10.1016/0006-291x(89)92508-4. [DOI] [PubMed] [Google Scholar]
- 155.Otto C, Rhode-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzmeier K-H. G protein coupled receptor localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 156.Pace MC, Thomas P. Activation of a pertussis toxin-sensitive, inhibitory G-protein is necessary for steroid-mediated oocyte maturation in spotted seatrout. Dev. Biol. 2005;285:70–79. doi: 10.1016/j.ydbio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 157.Pace MC, Thomas P. Steroid-induced oocyte maturation in Atlantic croaker (Micropogonias undulatus) is dependent on activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Biol. Reprod. 2005;73:988–996. doi: 10.1095/biolreprod.105.041400. [DOI] [PubMed] [Google Scholar]
- 158.Pang YF, Thomas P. Involvement of estradiol-17β and its membrane receptor, G protein coupled receptor 30 (GPR30) in regulation of oocyte maturation in zebrafish, Danio rerio. Gen. Comp. Endocrinol. 2009;161:58–61. doi: 10.1016/j.ygcen.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pang YF, Thomas P. Role of G protein-coupled estrogen receptor 1, GPR30, in inhibition of oocyte maturation in zebrafish by endogenous estrogens. Dev. Biol. 2010;342:194–206. doi: 10.1016/j.ydbio.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pang YF, Thomas P. Progesterone signals through membrane progesterone receptors (mPRs) in MDA-MB-468 and mPR-transfected MDA-MB-231 breast cancer cells which lack full-length and C-terminal truncated isoforms of the nuclear progesterone receptor. Steroids. 2011;76:921–928. doi: 10.1016/j.steroids.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pang YF, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker GPR30 and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149:3410–3426. doi: 10.1210/en.2007-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Papadopoulou N, Papakonstanti EA, Kallergi G, Alevizopoulos K, Stournaras C. Membrane androgen receptor activation in prostate and breast tumor cells: molecular signaling and clinical impact. IUBMB Life. 2009;61:56–61. doi: 10.1002/iub.150. [DOI] [PubMed] [Google Scholar]
- 163.Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 164.Patel VH, Chen J, Adya J, Ramanjaneya M, Karteris E, Thomas P, Randeva HS. G-protein coupled estrogen receptor 1 expression in rat and human heart: Protective role during ischaemic stress. Int. J. Mol. Med. 26:193–199. doi: 10.3892/ijmm_00000452. 20100. [DOI] [PubMed] [Google Scholar]
- 165.Patiño R, Thomas P. Characterization of membrance receptor activity for 17α20β,21-tryhydroxy-4-pregnen-3-one in ovaries of spotted seatrout (Cynoscion nebulosus) Gen. Comp. Endocrinol. 1990;78:204–217. doi: 10.1016/0016-6480(90)90007-9. [DOI] [PubMed] [Google Scholar]
- 166.Patiño R, Thomas P. Effects of gonadotropin on ovarian intrafollicular processes during the development of oocyte maturational competence in a teleost, the Atlantic croaker: evidence for two distinct stages of gonadotropin control of final oocyte maturation. Biol. Reprod. 1990;43:818–827. doi: 10.1095/biolreprod43.5.818. [DOI] [PubMed] [Google Scholar]
- 167.Patiño R, Yoshizaki G, Thomas P, Kagawa H. Gonadotropic control of ovarian follicle maturation: the two-stage concept and its mechanisms. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2001;129:427–439. doi: 10.1016/s1096-4959(01)00344-x. [DOI] [PubMed] [Google Scholar]
- 168.Pedram A, Razandi A, Levin ER. Nature of functional receptors at the plasma membrane. Mol. Endocrinol. 2006;20:196–209. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 169.Pedram A, Razandi M, Sainson R, Kim J, Hughes C, Levin E. A conserved mechanism for steroid receptor translocation to the plasma membrane. J. Biol. Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 170.Peluso JJ. Progesterone signaling mediated through progesterone receptor membrane component-1 in ovarian cells with special emphasis on ovarian cancer. Steroids. 2011;76:903–909. doi: 10.1016/j.steroids.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone's antiapoptotic action. Endocrinology. 2006;147:3133–3140. doi: 10.1210/en.2006-0114. [DOI] [PubMed] [Google Scholar]
- 172.Perusquia M, Stalone JN. Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am. J. Physiol. Heart Circ.Physiol. 2010;298:1522–1539. doi: 10.1152/ajpheart.00753.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J. Biol. Chem. 2000;275:13727–13736. doi: 10.1074/jbc.275.18.13727. [DOI] [PubMed] [Google Scholar]
- 174.Peyton C, Thomas P. Involvement of epidermal growth factor receptor signaling in estrogen inhibition of oocyte maturation mediated through the G protein-coupled estrogen receptor (Gper) in zebrafish (Danio rerio) Biol. Reprod. 2011;85:42–50. doi: 10.1095/biolreprod.110.088765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pietras RJ, Szego CM. Specific oestrogen binding sites at the outer surfaces of endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 176.Pietrobon EO, Monclus MDLA, Alberdi AJ, Fornes MW. Progesterone receptor availability in mouse spermatozoa during epididymal transit and capacitation: ligand blot detection of progesterone-binding protein. J. Androl. 2003;24:612–620. doi: 10.1002/j.1939-4640.2003.tb02713.x. [DOI] [PubMed] [Google Scholar]
- 177.Pottinger TG, Moore A. Characterization of putative steroid receptors in the membrane, cytosol, and nuclear fractions from the olfactory tissue of brown trout. Fish. Physiol. Biochem. 1997;16:45–63. [Google Scholar]
- 178.Qiu J, Bosch MA, Tobias SC, Grandy TK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rae MT, Menzies GS, McNeilly AS, Woad K, Webb R, Bramley TA. Specific non-genomic, membrane-localized binding sites for progesterone in the bovine corpus luteum. Biol. Reprod. 1988;58:1394–1406. doi: 10.1095/biolreprod58.6.1394. [DOI] [PubMed] [Google Scholar]
- 180.Rahman MA, Ohta K, Yoshikuni M, Nagahama Y, Chuda H, Matsuyama M. Characterization of ovarian membrane receptor for 17,20β-dihydroxy-4-pregnen-3-one, a maturation-inducing hormone in yellowtail, Seriola quinqueradiata. Gen. Comp. Endocrinol. 2001;127:71–79. doi: 10.1016/s0016-6480(02)00026-6. [DOI] [PubMed] [Google Scholar]
- 181.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol. Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 182.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 183.Revelli A, Massobrio M, Tesarik J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr. Rev. 1998;19:3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- 184.Ropero AB, Alonsa-Magdalena P, Ripoll C, Fuentes E, Nadal A. Rapid endocrine disruption, Environmental estrogen actions triggered outside the nucleus. J. Steroid Biochem. Mol. Biol. 2006;103:163–169. doi: 10.1016/j.jsbmb.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 185.Rossato M, Nogara A, Merico M, Ferlin ACF. Identification of functional binding sites for progesterone in rat Leydig cell plasma membrane - the role of second messengers. Steroids. 1999;64:168–175. doi: 10.1016/s0039-128x(98)00104-4. [DOI] [PubMed] [Google Scholar]
- 186.Sadler SE, Maller JL. Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J. Biol. Chem. 1981;256:6368–6373. [PubMed] [Google Scholar]
- 187.Sadler SE, Maller JL. Identification of a steroid receptor on the surface of Xenopus oocytes by photoaffinity labeling. J. Biol. Chem. 1982;257:355–361. [PubMed] [Google Scholar]
- 188.Sakamoto T, McCormick SD. Prolactin and growth hormone in fish osmoregulation. Gen. Comp. Endocrinol. 2006;147:24–30. doi: 10.1016/j.ygcen.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 189.Scapin S, Leoni S, Pagnuolo S, Gnocchi D, De vito P, Luly P, Pedersen JZ, Incerpi S. Short-term effects of thyroid hormones during development: focus on signal transduction. Steroids. 2010;75:576–584. doi: 10.1016/j.steroids.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 190.Schmidt BMW. Rapid non-genomic effects of aldosterone on the renal vasculature. Steroids. 2008;73:961–965. doi: 10.1016/j.steroids.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 191.Schmidt KL, Malish JL, Breuner CW, Soma KK. Corticosterone and cortisol binding sites in plasma, immune organs and brain of developing zebrafinches: I intracellular and membrane-associated receptors. Brain Behav. Immun. 2010;24:908–918. doi: 10.1016/j.bbi.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 192.Schoneberg T, Schulz A, Gudermann T. The structural basis of G-protein-coupled receptor function and dysfunction in human diseases. Rev. Physiol. Biochem. Pharmacol. 2002;144:143–327. [PubMed] [Google Scholar]
- 193.Skildum A, Favre E, Lange CA. Progesterone receptors induce cell cycle progression via mitogen-activated protein kinases. Mol. Endocrinol. 2005;19:327–339. doi: 10.1210/me.2004-0306. [DOI] [PubMed] [Google Scholar]
- 194.Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE. Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology. 2009;150:3833–3844. doi: 10.1210/en.2008-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Smith LD, Ecker RE. The interaction of steroids with Rana pipens oocytes in the induction of maturation. Dev. Biol. 1971;25:232–247. doi: 10.1016/0012-1606(71)90029-7. [DOI] [PubMed] [Google Scholar]
- 196.Smith JL, Kupchak BR, Garitaonandia I, Haong LK, Maina AS, Regall LM, Lyons LK. Heterologous expression of human mPRα, mPRβ and mPRγ in yeast confirms their ability to function as membrane progesterone receptors. Steroids. 2008;73:1160–1173. doi: 10.1016/j.steroids.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, Prossnitz ER. GPR30: a novel indicator of poor survival in endometrial cancer. Am. Soc. Obst. Gynecol. 2007;196:386e1–386e11. doi: 10.1016/j.ajog.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 198.Solito E, Mulla A, Morris JF, Christian HC, Flower RJ, Buckingham JC. Dexamethasone induces rapid serine phosphorylation and membrane translocation of annexin 1 in a human folliculostellate cell line via a novel nongenomic mechanism involving the glucocorticoid receptor, protein kinase c, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase. Endocrinology. 2003;144:1164–1174. doi: 10.1210/en.2002-220592. [DOI] [PubMed] [Google Scholar]
- 199.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J. Cell. Molec. Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Song J, Vinarov D, Tyler EM, Shahan MN, Tyler RC, Marley JL. Hypothetical protein At2g24940.1 from Arabidopsis thaliana has a cytochrome b5 like fold. J. Biomol. NMR. 2004;30:215–218. doi: 10.1023/b:jnmr.0000048943.34504.29. [DOI] [PubMed] [Google Scholar]
- 201.Sperry TS, Thomas P. Characterization of two nuclear androgen receptors in Atlantic croaker: comparison of their biochemical properties and binding specificities. Endocrinology. 1999;140:1602–1611. doi: 10.1210/endo.140.4.6631. [DOI] [PubMed] [Google Scholar]
- 202.Sperry T, Thomas P. Androgen binding profiles of two distinct nuclear androgen receptors in Atlantic croaker (Micropogonias undulatus) J. Steroid Biochem. Mol. Biol. 2000;73:93–103. doi: 10.1016/s0960-0760(00)00069-8. [DOI] [PubMed] [Google Scholar]
- 203.Szego CM, Davis JS. Adenosine 3,’5’-monophosphate in rat uterus: acute elevation by estrogen. Proc. Natl. Acad. Sci. USA. 1967;58:1711–1718. doi: 10.1073/pnas.58.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Szego CM, Pietras RJ. Membrane recognition and effector sites in steroid hormone action. In: Litwack G, editor. Biochemical Actions Hormones. vol 8. New York: Academic Press; 1981. pp. 307–463. [Google Scholar]
- 205.Tan Q, Zagrodny A, Bernaudo S, Peng C. Regulation of membrane progestin receptors in the zebrafish ovary by gonadotropin, activin, TGF-β, and BMP-15. Mol. Cell. Endocrinol. 2009;312:72–79. doi: 10.1016/j.mce.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 206.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J. Mol. Evol. 2005;61:372–380. doi: 10.1007/s00239-004-0375-2. [DOI] [PubMed] [Google Scholar]
- 207.Tasker JG, Di S, Mlacher-Lopes R. Rapid central corticosteroid effects: evidence for membrane glucocorticoid receptors in the brain. Integr. Comp. Biol. 2005;45:665–671. doi: 10.1093/icb/45.4.665. [DOI] [PubMed] [Google Scholar]
- 208.Telleria CM, Stocco CO, Stati AO, Deis RP. Progesterone receptor is not required for progesterone action in the rat corpus luteum of pregnancy. Steroids. 1989;64:760–766. doi: 10.1016/s0039-128x(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 209.Tesarik J, Mendoza C. Nongenomic effects of 17beta-estradiol on maturing oocytes: relationship to oocyte developmental potential. J. Clin. Endocrinol. Metab. 1995;80:1438–1443. doi: 10.1210/jcem.80.4.7714121. [DOI] [PubMed] [Google Scholar]
- 210.Thomas P. characteristics of membrane progesterone alpha (mPRα) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front. Neuroendocrinol. 2008;29:292–312. doi: 10.1016/j.yfrne.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Thomas P. Rapid, nongenomic steroid actions initiated at the cell surface: lessons from studies with fish. Fish Physiol. Biochem. 2003;28:3–12. [Google Scholar]
- 212.Thomas P, Alyea R, Pang Y, Peyton C, Dong J, Berg AH. Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER 1) in mammals and fish. Steroids. 2010;75:595–602. doi: 10.1016/j.steroids.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Thomas P, Breckenridge-Miller D, Detweiler C. Binding characteristics and regulation of the 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S) receptor on testicular and sperm plasma membranes of spotted seatrout (Cynoscion nebulosus) Fish Physiol. Biochem. 1997;17:109–116. [Google Scholar]
- 214.Thomas P, Breckenridge-Miller D, Detweiler C. The teleost sperm membrane progestogen receptor: interactions with xenoestrogens. Mar. Environ. Res. 1998;46:163–167. [Google Scholar]
- 215.Thomas P, Das S. Correlation between binding affinities of C21 steroids for the maturation-inducing steroid membrane receptor in spotted seatrout ovaries and their agonist and antagonist activities in an oocyte maturation bioassay. Biol. Reprod. 1997;57:999–1007. doi: 10.1095/biolreprod57.5.999. [DOI] [PubMed] [Google Scholar]
- 216.Thomas P, Das S, Breckenridge–Miller D, Detweiler C. Characterization and regulation of a progestin receptor on Atlantic croaker sperm membranes. In: Kawashima S, Kikoyama S, editors. Advances in Comparative Endocrinology. Bologna: Monduzzi Editore; 1997. pp. 1381–1385. [Google Scholar]
- 217.Thomas P, Doughty K. Disruption of rapid, nongenomic steroid actions by environmental chemicals: Interference with progestin stimulation of sperm motility in Atlantic croaker. Environ. Sci. Technol. 2004;38:6328–6332. doi: 10.1021/es0403662. [DOI] [PubMed] [Google Scholar]
- 218.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006;102:107–109. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 219.Thomas P, Dressing G, Pang Y, Berg H, Tubbs C, Benninghoff A, Doughty K. Progestin, estrogen and androgen G-protein coupled receptors in fish gonads. Steroids. 2006;71:310–316. doi: 10.1016/j.steroids.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 220.Thomas P, Harris C, Pang Y. The roles of different types of progestin receptors in oocyte maturation of zebrafish. Biol. Reprod. Sp Iss SI Abs. 2008;705:220. [Google Scholar]
- 221.Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C. Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor alpha subtypes and their evolutionary origins. Endocrinology. 2007;148:705–718. doi: 10.1210/en.2006-0974. [DOI] [PubMed] [Google Scholar]
- 222.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 223.Thomas P, Pang Y, Zhu Y, Detweiler C, Doughty K. Multiple rapid progestin actions and progestin membrane receptor subtypes in fish. Steroids. 2004;69:567–573. doi: 10.1016/j.steroids.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 224.Thomas P, Pinter J, Das S. Upregulation of the maturation-inducing steroid membrane receptor in spotted seatrout ovaries by gonadotropin during oocyte maturation and its physiological significance. Biol. Reprod. 2001;64:21–29. doi: 10.1095/biolreprod64.1.21. [DOI] [PubMed] [Google Scholar]
- 225.Thomas P, Sweatman J. Interference by atrazine and bisphenol A with progestin binding to the ovarian progestin membrane receptor and induction of oocyte maturation in Atlantic croaker. Mar. Environ. Res. 2008;66:1–2. doi: 10.1016/j.marenvres.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 226.Thomas P, Trant JM. Evidence that 17α,20β,21-trihydroxy-4-pregnen-3-one is a maturation-inducing steroid in spotted seatrout. Fish Physiol. Biochem. 1989;7:185–191. doi: 10.1007/BF00004706. [DOI] [PubMed] [Google Scholar]
- 227.Thomas P, Tubbs C, Detweiler C, Das S, Ford L, Breckenridge-Miller D. Binding characteristics, hormonal regulation and identity of the sperm membrane progestin receptor in Atlantic croaker. Steroids. 2005;70:427–433. doi: 10.1016/j.steroids.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 228.Thomas P, Tubbs C, Garry VF VF. Progestin functions in vertebrate gametes mediated by membrane progestin receptors (mPRs): Identification of mPRα on human sperm and its association with sperm motility. Steroids. 2009;74:614–621. doi: 10.1016/j.steroids.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 229.Thomas P, Zhu Y, Pace M. Progestin membrane receptors involved in the meiotic maturation of teleost oocytes: a review with some new findings. Steroids. 2002;67:511–517. doi: 10.1016/s0039-128x(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 230.Thomas W, McEneaney V, Harvey BJ. Aldosterone-induced signaling and cation transport in the distal nephron. Steroids. 2008;73:979–984. doi: 10.1016/j.steroids.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 231.Tian T, Kim S, Heilig E, Ruderman JV. Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation. Proc. Nat. Acad. Sci. USA. 2000;97:14358–14363. doi: 10.1073/pnas.250492197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Tokumoto M, Nagahama Y, Thomas P, Tokumoto T. Cloning and identification of a membrane progestin receptor in goldfish ovaries and evidence it is an intermediary in oocyte meiotic maturation. Gen. Comp. Endocrinol. 2006;145:101–108. doi: 10.1016/j.ygcen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 233.Tokumoto T, Tokumoto M, Thomas P. Interactions of diethylstilbestrol (DES) and DES analogues with membrane progestin receptor α (mPRα) and the correlation with their nongenomic progestin activities. Endocrinology. 2007;148:3459–3467. doi: 10.1210/en.2006-1694. [DOI] [PubMed] [Google Scholar]
- 234.Towle AC, Sze PY. Steroid binding to synaptaptic plasma membrane: differential binding of glucocorticoids and gonadal steroids. J. Steroid Biochem. 1983;18:135–143. doi: 10.1016/0022-4731(83)90079-1. [DOI] [PubMed] [Google Scholar]
- 235.Trueba M, Vallejo AI, Rodriquez I, Ibarrola I, Sancho MJ, Marino A, Macarulla JM. Evidence for the presence of specific binding sites for corticoids in mouse plasma membranes. J. Membr. Biol. 1989;108:115–124. doi: 10.1007/BF01871023. [DOI] [PubMed] [Google Scholar]
- 236.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Ann. Rev. Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 237.Tubbs C, Pace M, Thomas P. Expression and gonadotropin regulation of membrane progestin receptor alpha in Atlantic croaker (Micropogonias undulatus) gonads: role in gamete maturation. Gen. Comp. Endocrinol. 2010;165:144–154. doi: 10.1016/j.ygcen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 238.Tubbs C, Tan W, Shi B, Thomas P. 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S) stimulation of sperm hypermotility and identification of 20β-S binding and membrane progestin receptor alpha on southern flounder sperm (Paralichthys lethostigma) Gen, Comp. Endocrinol. 2011;170:629–639. doi: 10.1016/j.ygcen.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 239.Tubbs C, Thomas P. Progestin signaling through an olfactory G protein and membrane progestin receptor-α in Atlantic croaker sperm: potential role in induction of sperm hypermotility. Endocrinology. 2009;150:473–484. doi: 10.1210/en.2008-0512. [DOI] [PubMed] [Google Scholar]
- 240.Tubbs C, Thomas P. Functional characteristics of membrane progestin receptor alpha (mPRα) subtypes: A review with new data showing mPRα expression in seatrout sperm and its association with sperm motility. Steroids. 2008;73:935–941. doi: 10.1016/j.steroids.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 241.Uhler ML, Leung A, Chan SYN, Wang C. Direct effects of progesterone and antiprogesterone on human sperm hyperactivated motility and acrosome reaction. Fert. Steril. 1992;58:1191–1198. [PubMed] [Google Scholar]
- 242.Vicencio JM, Ibarra C, Estrada M, Chiong M, Soto D, Parra V, Diaz-Araya G, Jaimovich E, Lavandero S. Testosterone induces an intracellular calcium increase by a non-genomic mechanism in cultured rat cardiac myocytes. Endocrinology. 2006;147:1386–1395. doi: 10.1210/en.2005-1139. [DOI] [PubMed] [Google Scholar]
- 243.Wang C, Dehghani B, Li Y, Kaler LJ, Proctor T, Vandenbank AA, Offner H. Membrane estrogen receptor regulates experimental autoimmune encephalomyelitis through upregulation of programmed cell death. J. Immunol. 2009;182:3294–3303. doi: 10.4049/jimmunol.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang C, Prossnitz ER, Roy SK. G Protein coupled estrogen receptor expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology. 2008;149:4452–4461. doi: 10.1210/en.2008-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Watson CS. Preface. In: Watson CS, editor. The Identities of Membrane Steroid Receptors. Boston: Kluwer Academic Publishers; 2003. pp. xi–xiv. [Google Scholar]
- 246.Watson CS, Gametchu B. Membrane-initiated steroid actions and the proteins that mediate them. Proc. Soc. Exp Biol. Med. 1999;220:9–19. doi: 10.1046/j.1525-1373.1999.d01-2.x. [DOI] [PubMed] [Google Scholar]
- 247.Watson CS, Norfleet AM, Pappas TC, Gametchu B. Rapid actions of estrogens in GH3/B6 pituitary tumor cells via a plasma membrane version of estrogen receptor-alpha. Steroids. 1999;64:5–13. doi: 10.1016/s0039-128x(98)00107-x. [DOI] [PubMed] [Google Scholar]
- 248.Weber G, Sullivan C. In vitro hormone induction of final oocyte maturation in striped bass (Morone saxatilis) follicles is inhibited by blockers of phosphatidylinositol 3-kinase activity. Comp. Biochem. Physiol. B. 2001;129:467–473. doi: 10.1016/s1096-4959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 249.Wehling M, Kasmayr J, Theisen K. Rapid effects of mineralocorticoids on sodium-proton exchanger: genomic or nongenomic pathway? Am. J. Physiol. 1991;260:E719–E726. doi: 10.1152/ajpendo.1991.260.5.E719. [DOI] [PubMed] [Google Scholar]
- 250.Weiler PJ, Wiebe JP. Plasma membrane receptors for the cancer regulating progesterone metabolites, 5a-pregnane-3,20-dione and 3a-hydroxy-4-pregnen-20-one in MCF7 breast cancer cells. Biochem. Biophys. Res. Commun. 2000;272:731–737. doi: 10.1006/bbrc.2000.2847. [DOI] [PubMed] [Google Scholar]
- 251.West RE, Moss J, Vaughn M, Lui T, Lui TY. Pertussis toxin catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J. Biol. Chem. 1985;260:14428–14430. [PubMed] [Google Scholar]
- 252.Wunderlich F, Benten WP, Lieberherr M, Guo Z, Stamm O, Wrehlke C, Sekeris CE, Mossmann H. Testosterone signaling in T cells and macrophages. Steroids. 2002;67:535–538. doi: 10.1016/s0039-128x(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 253.Xiao Z-L, Cao W, Biancani P, Behar J. Nongenomic effects of progesterone on the contraction of smooth muscle cells from the guinea pig colon. Am J. Physiol. Gastrointest. Liv. Physiol. 2006;290:G1008–G1015. doi: 10.1152/ajpgi.00382.2005. [DOI] [PubMed] [Google Scholar]
- 254.Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, Prossnitz ER, Battaglia G, Doncarlos LL, Muma NA. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158:1599–1607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Yoshikuni M, Nagahama Y. Involvement of an inhibitory G protein in the signal transduction pathway of maturation-inducing hormone (17α,20β-dihydroxy-4-pregnen-3-one) action in rainbow trout (Oncorhynchus mykiss) oocytes. Dev. Biol. 1994;166:615–622. doi: 10.1006/dbio.1994.1341. [DOI] [PubMed] [Google Scholar]
- 256.Yoshikuni M, Shibata N, Nagahama Y. Specific binding of[3H]17α,20β-dihydroxy-4-pregnen-3-one to oocyte cortices of rainbow trout (Oncorhynchus mykiss) Fish Physiol. Biochem. 1993;11:15–24. doi: 10.1007/BF00004546. [DOI] [PubMed] [Google Scholar]
- 257.Yu X, Filardo EJ, Shaikh ZA. The membrane estrogen receptor GPR30 mediates cadmium-induced proliferation of breast cancer cells. Tox. Appl. Pharmacol. 2010;245:83–90. doi: 10.1016/j.taap.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 258.Zheng J, Ali A, Ramirez VD. Steroids conjugated to bovine serum albumin as tools to demonstrate specific steroid neuronal membrane binding sites. Psychiatry Neurosci. 1996;21:187–197. [PMC free article] [PubMed] [Google Scholar]
- 259.Zhu X, Li H, Liu J-P, Funder JW. Androgen stimulates mitogen-activated protein kinase in human breast cancer cells. Mol. Cell. Endocrinol. 1999;152:199–206. doi: 10.1016/s0303-7207(99)00031-3. [DOI] [PubMed] [Google Scholar]
- 260.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc. Natl. Acad. Sci. 2003;100:2237–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Zhu Y, Hanna RN, Schaaf MJM, Spaink HP, Thomas P. Candidates for membrane progestin receptors in vertebrates- past approaches and future challenges. Comp. Biochem. Physiol. C, Toxicol. Pharmacol. 2008;148:381–389. doi: 10.1016/j.cbpc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 262.Zhu Y, Rice CD, Pang YF, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc. Nat. Acad. Sci. U.S.A. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zuo L, Li W, You S. Progesterone reverses the mesenchymal phenotypes of basal phenotype breast cancer cells by a membrane progesterone receptor alpha mediated pathway. Breast Cancer Res. 2010;12:R34. doi: 10.1186/bcr2588. [DOI] [PMC free article] [PubMed] [Google Scholar]