Abstract
The mechanisms of nonclassical export of signal peptide-less proteins remain insufficiently understood. Here we demonstrate that stress-induced unconventional export of FGF1, a potent and ubiquitously expressed mitogenic and proangiogenic protein, is associated with and dependent on the formation of membrane blebs and localized cell surface exposure of phosphatidylserine. In addition, we found that the differentiation of promonocytic cells results in massive FGF1 release, which also correlates with membrane blebbing and exposure of phosphatidylserine. These findings indicate that the externalization of acidic phospholipids could be used as a pharmacological target to regulate the availability of FGF1 in the organism.
Keywords: FGF1, nonclassical secretion, phosphatidylserine exposure, blebbing, PLSCR1
Introduction
Many extracellular proteins do not have a signal peptide in their primary structure, and thus cannot be secreted through the classical export pathway involving the endoplasmic reticulum (ER) and Golgi apparatus. Instead, they use unconventional export mechanisms that are not fully understood (Grieve and Rabouille, 2011; Nickel and Seedorf, 2008; Prudovsky et al., 2008). Among the nonclassically released proteins are the most ubiquitously expressed members of the FGF family, FGF1 and FGF2 that play important roles in tissue repair, angiogenesis, inflammation, and tumorigenesis (Dorey and Amaya; Korc and Friesel, 2009; Ornitz and Itoh, 2001). FGF1 is released from fibroblastic cells under various conditions of cellular stress (Ananyeva et al., 1997; Jackson et al., 1992; Mouta Carreira et al., 2001; Shin et al., 1996)as a part of a copper-dependent (Landriscina et al., 2001a)multiprotein complex that also includes sphingosine kinase 1 (Soldi et al., 2007), the 40 kDa form of synaptotagmin 1 (LaVallee et al., 1998) and the small calcium-binding protein S100A13 (Landriscina et al., 2001b). Previously, we found that under conditions of cellular stress, FGF1 migrates from the internal areas of cytoplasm to the vicinity of the cell membrane (Prudovsky et al., 2002). However, it remained unclear whether FGF1 export proceeds through the entire cell surface or occurs in limited membrane domains.
In the present study, we demonstrate that under stress conditions in fibroblastic cells, FGF1 is exported through cell membrane domains characterized by the formation of protrusions and externalization of phosphatidylserine (PS), an acidic phospholipid that under normal conditions is confined to the inner leaflet of the plasma membrane. Moreover, small molecular compounds that inhibit externalization of PS also repressed FGF1 export. An artificial increase in interactions between the cell membrane and the underlying peripheral actin cytoskeleton significantly suppressed FGF1 secretion, thereby confirming a role for membrane blebbing in FGF1 export. In addition, we found that FGF1 export from differentiated U937 promonocytic leukemia cells also correlates with membrane blebbing and PS externalization. Chelation of intracellular Ca2+ resulted in the inhibition of both PS exposure and FGF1 export in differentiated U937 cells. Although U937 differentiation is accompanied by a dramatic increase in the expression of phospholipid scramblase 1 (PLSCR1), a protein originally proposed to mediate Ca2+ -activated transbilayer movement of phospholipids resulting in cell surface exposure of PS(Sims and Wiedmer, 2001), we show that the expression of PLSCR1 is unrelated to FGF1 export under these conditions.
Materials and Methods
Cell Culture
Murine NIH 3T3 cells (ATCC, Manassas, VA) were maintained in DMEM (HyClone, Logan, UT) supplemented with 10% bovine calf serum (HyClone). U937 cells (ATCC) were grown in RPMI medium (HyClone) supplemented with 10% fetal calf serum. U937 cells stably transfected with FGF1 (Mandinova et al., 2003)or FGF1:GFP were grown in medium supplemented with 400 μg/ml geneticin (Invitrogen, Carlsbad, CA).
Genetic constructs
FGF1 cloned in the pMEXneo expression vector (Jackson et al., 1992)was recloned into the SalI and EcoRI restriction sites of the pcDNA3/HA vector (Clontech, Mountain View, CA) thus forming FGF1:HA. Then, FGF1:HA was cloned into the multiple cloning site (MCS) of the shuttle vector pAdlox (generous gift of Lisa Phipps, Somatix Therapy Corporation, CA).
Mouse PLSCR1:GFP was expressed in the pMiG retroviral vector (Nanjundan et al., 2003).
FGF1:GFP cloned in the pcDNA 3.1 vector was a kind gift of Andrew Baird (Human BioMolecular Research Institute, San Diego, CA).
Constitutively active (CA) mutant T567D and wild type (WT) ezrin, both with the 3′ attachment of the VSVG tag (Algrain et al., 1993) were excised using Hind III and XbaI enzymes from the pC6 vector. They were then cloned into the MCS of the TRE2Hygro expression vector (Clontech), in which Hind III and XbaI restriction sites were introduced by PCR mutagenesis.
CMVt-rtTA construct was a kind gift of John Hiscott (McGill University, Montreal).
Human PLSCR1 shRNA construct and control shRNA cloned in the pGFP-V-RS retroviral vector were obtained from Origene (Rockville, MD). The following target sequence inPLSCR1 mRNA was used: 5′-TGAAAGTCTCCTCAGGAAATCTGAAGTCT-3′ (Zhao et al., 2004).
Production of viruses and viral transfection
Recombinant FGF1:HA adenovirus was produced, purified, and titered as described (Duarte et al., 2008). Briefly, CRE8 cells were transfected with SfiI-digested pAdlox-derived constructs, and infected with the ψ5 virus. The lysates were prepared 2 days after infection. The virus was passed twice through CRE8 cells, and purified from the second passage using a cesium density gradient. The virus was quantified by optical density at 260 nm, and the bioactivity was determined by a plaque-forming unit assay. Adenoviral transduction was performed in serum-free DMEM with approximately 103 viral particles/cell in the presence of poly-D-Lysine hydrobromide (Sigma) (5×103 molecules/viral particle) for 2 h at 37°C. Then the adenovirus-containing medium was removed and replaced with serum-containing medium. The cells were plated for experiments 24–48 hours after transduction.
PLSCR1:GFP and control GFP retroviruses were produced in the Recombinant Viral Vector Core of MMCRI. The packaging cell line Bosc was transfected with the PLSCR1:GFP or GFP coding retroviral constructs using polybrene. Conditioned medium from the 2 day culture of the producer cell line was collected and filtered to remove cell debris. Actively growing recipient cells were incubated for 2 h with retrovirus-containing conditioned medium in the presence of 5μg/ml hexadimethrine bromide. The medium was replaced with fresh growth medium after 2 h.
Generation of stable cell transfectants
To achieve inducible expression of ezrin, the NIH 3T3 cells were cotransfected with CMVt-rtTAand WT ezrin/pTRE2Hygro, CA ezrin/PTRE2Hygro or empty pTRE2Hygro vector using the Fugene reagent (Roche, Nutley, NJ) according to the manufacturer’s protocol. Transfected cell clones were selected in the medium containing 2 μg/ml puromycin (Sigma, St. Louis, MO) and 50 mg/ml hygromycin (Roche). To assess the inducibility of ezrin expression, cells of individual clones were plated on glass coverslips and incubated for 48 h in medium containing 0 or 10 μg/ml doxycycline (Sigma). The cells were formalin fixed and the doxycycline-inducible expression of WT or CA ezrin was verified by immunofluorescence using anti-VSVG antibodies (Sigma) followed by secondary FITC-conjugated antibodies (Vector Laboratories).
U937 cells expressing FGF1 (Mandinova et al., 2003) were retrovirally transfected with PLSCR1 shRNA and control shRNA, and selected in medium containing 2 μg/ml puromycin (Sigma, St Louis, MO). After three weeks of selection, GFP fluorescence was observed in 10–20 % of the surviving cells. GFP-positive cells were then selected by flow cytometry (FACSVantage, BD) in the Flow Cytometry Core of MMCRI.
U937 cells expressing FGF1 (Mandinova et al., 2003) were retrovirally transduced with PLSCR1:GFP or GFP. Three days after transduction, GFP fluorescence was observed in 5% of the cells. The GFP-positive cells were selected using flow cytometry and further propagated. Cell populations with at least 90% of GFP-positive cells were used for experiments.
U937 cells were transfected with FGF1:GFP using the Nucleofector II system (Koeln, Germany) and special Amaxa transfection buffer C. After three weeks of selection in the medium with 800 μg/ml G418, GFP fluorescence was observed in 5–10% of the surviving cells. GFP-positive cells were then selected using flow cytometry.
Fluorescence microscopy
To simultaneously assess the externalization of FGF1 and PS, NIH 3T3, cells transduced with FGF1:HA were incubated for 20 min at 4°C with anti-HA monoclonal antibodies (Covance, Princeton, NJ) and FITC-conjugated Annexin V (Invitrogen) in buffer composed of 10 mM HEPES (pH 7.5), 140 mM NaCl and 2.5 mM CaCl2. Next, the cells were fixed in 10% neutral formalin, and externalized FGF1 was detected by using the CY3-conjugated anti-mouse IgG antibody (Sigma).
Alternatively, the cells were incubated for 20 min at 4°C with anti-HA monoclonal antibodies (Covance) together with the PS-targeting chimeric antibody, bavituximab (Peregrine Pharmaceuticals Inc., Tustin, CA), in PBS supplemented with 1% bovine serum albumin. Bavituximab binds to PS in a β2-glycoprotein I-dependent manner (Luster et al., 2006). Next, the cells were fixed in 10% neutral formalin, externalized FGF1 was detected using the CY3-conjugated anti-mouse IgG antibody (Sigma), and externalized PS was detected by using FITC-conjugated anti- human IgG antibody (Invitrogen).
To visualize external and internal cell membranes, the cells were fixed for 10 min in 10% neutral formalin, incubated 30 min in blocking buffer (PBS with 5% bovine serum albumin and 0.1% Triton X100), and then stained with 5,5′-Phe2-DilC18(3) or Vybrant (both from Invitrogen) in the blocking buffer. Nuclei were stained with TO-PRO3 (Invitrogen). To visualize the actin cytoskeleton, fixed and permeabilized cells were stained with Alexa488-conjugated phalloidin (Invitrogen).
To simultaneously visualize externalized and intracellular FGF1:HA, the transduced NIH 3T3 cells were incubated for 20 min at 4°C with FITC-conjugated anti-HA antibodies (Sigma) in buffer composed of 10mM Hepes (pH 7.5), 140 mM NaCl, and 2.5 mM CaCl2, then fixed with 10% neutral formalin, permeabilized with 0.1% Triton X100, incubated with anti-HA rabbit monoclonal antibodies (Covance), and then with the CY3-conjugated anti-rabbit IgG antibodies (Sigma).
To visualize the externalized PS in U937 cells, we incubated live cells with phycoerythrin- conjugated Annexin V (Invitrogen). The visualization of externalized FGF1:GFP in stably transfected U937 cells was achieved by incubation of live cells with E6 monoclonal anti-GFP antibodies (Invitrogen) followed by formalin fixation and staining with CY3-conjugated anti-mouse IgG antibodies.
Fluorescent preparations were embedded in Vectashield (Vector Laboratories, Burlingame, CA), and the images were taken using the Leica SP1 confocal microscope at the MMCRI confocal microscopy facility.
Analysis of PLSCR1 expression
RT-PCR analysis of PLSCR1 expression was performed with total RNA isolated from U937 cells using the RNeasy kit (Qiagen, Valencia, CA). Total RNA (1 μg) was used as a template for the PCR reaction performed with the Platinum Tap One Step RT-PCR Kit (Invitrogen). The following PCR primers were utilized: forward 5′-GGTGCCTGTTTCCTCATTGAC-3′ and reverse 5′-GTCCTTTTTCTCAAATTGAC-3′. β-actin expression served as a control for RNA loading. The amplification products were visualized by 1.5% agarose gel electrophoresis and ethidium bromide staining.
Analysis of FGF1 release
The NIH 3T3 cells were used to study FGF1 release 48 h after adenoviral transduction with FGF1. The heat shock-induced FGF1 release assay was performed by incubation of the cells at 42°C for 110 minutes in serum-free DMEM containing 5 U/ml of heparin (Sigma), as previously described (Jackson et al., 1992). Control cultures were incubated at 37°C for the same period. Conditioned media were collected, briefly centrifuged at 1000 g to remove detached cells and FGF1 was isolated for immunoblot analysis using heparin-Sepharose chromatography as described. A similar method was used to study the export of stably transfected FGF1 from adherent differentiated U937 cells. To assess the FGF1 release from nonadherent U937 cells, the cells were collected by centrifugation and then resuspended in serum-free DMEM containing 5 U/ml of heparin.
Cell viability was assessed by measuring lactate dehydrogenase (LDH) activity in the medium using the Promega CytoTox kit (Promega, Madison, WI).
To analyze the association of released FGF1 with microparticles and exosomes, the conditioned media were pre-centrifuged for 10 min at 1000 g to remove detached cells and then supernatants were centrifuged for 1 h at 100,000 g. The supernatants were removed and pellets were resuspended in 1 ml PBS containing 0.1% Triton X 100 to lyse the precipitated vesicles. FGF1 was isolated from the supernatants and pellet lysates using heparin chromatography.
Results
1. Stress-induced export of FGF1 occurs in the limited domains of the cell surface characterized by membrane protrusions
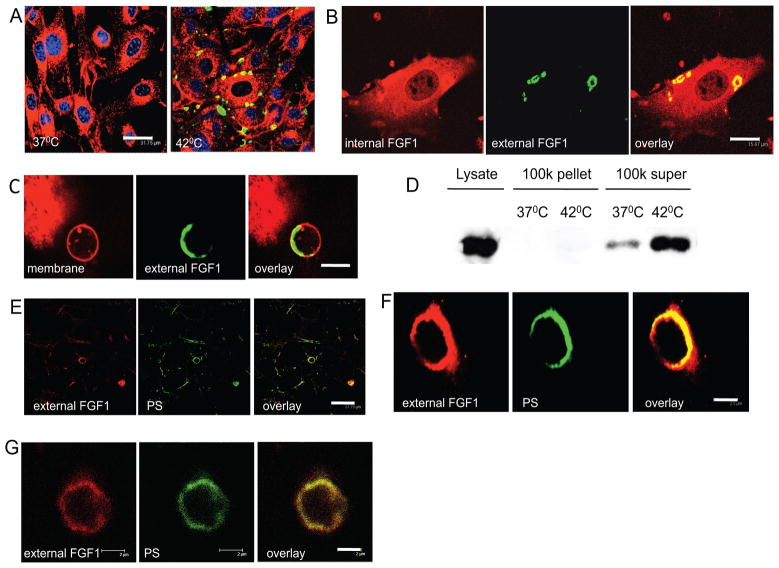
To investigate the topologic distribution of FGF1 that is exported to the cell surface, NIH 3T3 cells were adenovirally transduced with FGF1:HA and incubated for 110 min in DMEM at 42°C (heat shock) or 37°C (control). The cells were incubated at 4°C for an additional 20 min in isotonic HEPES buffer with Ca2+ and FITC-conjugated mouse monoclonal anti-HA antibodies, and then fixed. Following the fixation, the cells were permeabilized and stained for intracellular FGF1 (using rabbit anti-HA antibodies followed by CY3-conjugated secondary antibodies) or membranes (using 5,5′-Phe2-DilC18(3) or Vybrant lipid stains). As expected, non-stressed cells were largely negative for externalized FGF1. The stressed cells exhibited distinct 0.5–8 μm round or oval domains that were positive for externalized FGF1 (Figure 1A). Typically, these domains were also characterized by an increased concentration of intracellular FGF1 (Figure 1B). Membrane staining and confocal studies at high magnification demonstrated that portals of FGF1 release were usually associated with membrane blebs (Figure 1C). The expression of FGF1 at the cell surface was stress-dependent and transient. Indeed, 2 h after the cells were returned to37°C, the FGF1-HA positivity of cell surface was mostly lost (data not shown). The presence of externalized FGF1 on cell membrane protrusions could indicate that FGF1 is released into the medium as a component of the detached blebs. Indeed, bleb-derived microparticles are involved in the export of multiple biologically active proteins (Al-Nedawi et al., 2009; Hendrix et al.; Sabatier et al., 2009). To examine this possibility, membrane particles were collected by ultracentrifugation from media conditioned by heat shocked FGF1-expressing NIH 3T3 cells as described for FGF2 by Nickel et al. (Seelenmeyer et al., 2008). The resulting pellet and supernatant were examined for the presence of FGF1. Almost all of the released FGF1 was found in the supernatant (Figure 1D). Based on these results, we conclude that cell surface protrusions represent the portals but not the vehicles for stress-induced FGF1 export.
Figure 1. Stress-induced export of FGF1 is associated with the formation of membrane protrusions and externalization of PS.
Each figure is representative of four independent experiments. (A) Visualization of released FGF1 at the cell surface. NIH 3T3 cells adenovirally transduced with FGF1:HA were incubated for 110 min at 37 or 42°C. The cells were then treated for 20 min at 4°C with FITC-conjugated anti-HA antibodies (green), fixed with neutral formalin and permeabilized. The cell membranes were stained with the 5,5′-Phe2-DilC18(3) dye (red) and nuclei with the TOPRO-3 dye (blue). Bar - 31.75 μm. (B) Simultaneous visualization of internal and externalized FGF1 in a heat-shocked cell. After heat shock, the cells were stained with FITC- conjugated anti-HA antibodies (green), fixed, permeabilized, and stained for internal FGF1 using rabbit anti-HA antibodies followed by CY3-conjugated secondary antibodies (red). Yellow -overlay. Bar - 15.87μm. (C) FGF1 exported during heat shock is localized on a large membrane bleb (high magnification). Stained as in “A.” Red -membrane. Green -FGF1:HA. Bar -7μm. (D). Released FGF1 is not associated with detached vesicular structures. After heat shock, cell culture medium was collected, pre-centrifuged at 1000 g and then centrifuged at 100,000 g. FGF1 from the supernatant and pellet were collected as described in the “Materials and Methods” and detected by immunoblotting. 100% of media supernatant, and precipitate, and 10% of cell lysate were loaded onto the gel. (E) FGF1-positive cell surface domains contain externalized PS. NIH 3T3 cells adenovirally transduced with FGF1:HA were incubated for 110 min at 42°C, followed by an additional incubation for 20 min at 4°C with anti-HA antibodies and FITC-Anx V (green). The cells were fixed, and externalized FGF1 was revealed by using CY3-conjugated secondary antibodies (red). Yellow -overlay. Bar -31.75 μm. (F) An individual bleb with co-localization of externalized PS and FGF1, high magnification. The cells were processed as in “E.” Bar – 2.5 μm. (G) An individual bleb showing co-localization of externalized FGF1 and PS. The cells were processed similar to “E” except that an alternative PS detecting agent, bavituximab, was used to detect externalized PS. Bound bavituximab was detected with FITC-conjugated antibodies to human IgG. Bar – 2.0 μm.
2. FGF1 export domains contain externalized PS
FGF1 and other members of FGF1 release complex bind PS with high affinity (Prudovsky et al., 2008; Tarantini et al., 1995). We have shown that FGF1 specifically destabilizes bilayers composed of acidic phospholipids, such as PS, phoshatidylglycerol and phosphatidylinositol (Graziani et al., 2006). Thus, we hypothesized that stress conditions, resulting in the movement of PS from inner to outer plasma membrane leaflet might provide a mechanism for transport of FGF1 from the cytosol into the external medium (Prudovsky et al., 2008). To assess this hypothesis for nonclassical export of FGF1, we incubated stressed and control cells with a mixture of anti-HA antibodies and FITC-labeled Annexin V, which binds to externalized PS. After fixation, the cells were incubated with CY3-conjugated secondary antibodies. Confocal microscopy revealed that the FGF1 export domains were PS positive (Figure 1E,F). These results were confirmed with bavituximab (Marconescu and Thorpe, 2008) that binds and stabilizes complexes of PS and β2-glycoprotein I (Luster et al., 2006). Bleb-like structures on the surface of heat shocked cells were coincidently stained with both anti-HA antibodies and bavituximab (Figure 1G). These results suggest that FGF1 export occurred in conjunction with the externalization of PS. The stress conditions we used were strictly controlled and did not induce cell death as assessed by the absence of LDH release induction in all supernatants and by DAPI staining used to detect the formation of apoptotic nuclear fragments (data not shown).
3. Chemical compounds inhibiting PS externalization repress stress-induced FGF1 export
Our next aim was to determine if there is a causal relationship between cell surface PS exposure and stress-induced FGF1 export. The asymmetric distribution of PS between inner and outer plasma membrane leaflets is believed to be regulated by multiple membrane-associated enzymes (Lenoir et al., 2007). The most extensively studied are: (1) aminophospholipid translocase (Seigneuret and Devaux, 1984), a Type IV ATPase that serves to sequester PS to the inner surface of the plasma membrane by catalyzing its movement from outer to inner leaflet; and (2) phosholipid scramblase 1 (PLSCR1) (Sims and Wiedmer, 2001), a membrane-associated protein that was isolated and characterized based upon its ability to mediate collapse of transbilayer phospholipid asymmetry through Ca2+-activated bidirectional transbilayer movement of PS and other lipids in reconstituted proteoliposomes, and was proposed to serve this same “phospholipid scramblase” function in the plasma membrane. However, no chemical compound has been Identified that targets a specific PS translocating enzyme and thus induce or inhibit PS exposure.
At the same time, several chemicals were reported to suppress PS externalization. To explore the functional relationship between movement of PS to the cell surface and export of FGF1 into the cell medium, we undertook to identify inhibitors of both processes. Taurine is a non-essential amino acid that is a membrane-stabilizing and cytoprotective (Anderheggen et al., 2006) agent, which is present in high concentrations in animal organisms and used as an anti- inflammatory nutrient supplement (Yamori et al., 2010). Using in vivo staining with phycoerythrin- conjugated annexin V, we found that taurine caused an inhibition of heat shock-induced PS externalization in NIH 3T3 cells (Figure 2A). Using heparin chromatography followed by immunoblotting, we found that taurine also induced a concentration-dependent inhibition of FGF1 export from NIH 3T3 cells (Figure 2B).
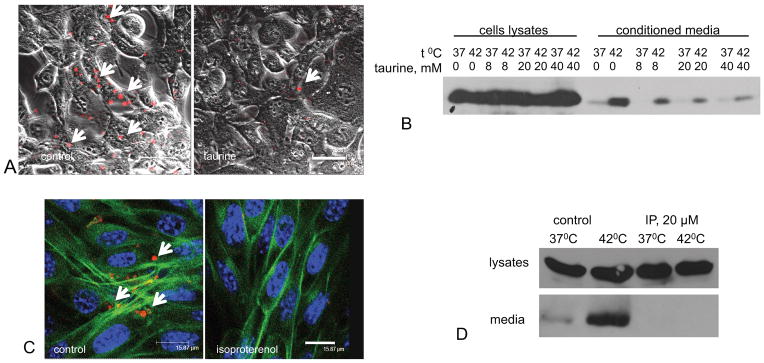
Figure 2. Chemical agents repressing PS exposure inhibit stress-induced FGF1 export.
Each figure is representative of three independent experiments. (A). Taurine inhibits PS externalization. NIH 3T3 cells were incubated with or without 40 mM taurine (Sigma) for 2h at 37°C in DMEM plus 10 % serum, followed by an additional 110 min 42°C in DMEM without serum. Next, the cells were incubated for 20 min at 4°C with annexin V conjugated to phycoerythrin. The cells were examined under a confocal microscope; the combined phase contrast and phycoerythrin fluorescence (red) images are presented. PS externalization is marked with arrows. Bar -20 μm. (B). Taurine inhibits heat shock-induced FGF1 export in a concentration-dependent manner. NIH 3T3 cells adenovirally transduced with FGF1 were incubated with or without 8, 20, or 40 μM taurine (Sigma) for 2 h at 37°C in DMEM plus 10 % fetal calf serum, followed by an additional incubation for 110 min at 37°C or 42°C in DMEM without serum. FGF1 was isolated from conditioned media and detected by immunoblotting. (C) Isoproterenol inhibits PS externalization. NIH 3T3 cells were incubated with or without 20 μM isoproterenol (Sigma) for 2 h at 37°C in DMEM plus 10 % serum and then for additional 110 min at 42°C in DMEM without serum. The cells were then incubated for 20 min at 4°C with annexin V:phycoerythrin (red), formalin fixed, permeabilized, stained with Alexa 488- conjugated phalloidin (green), and examined using a confocal microscope. PS externalization is marked with arrows. Bar - 15.87 μm. (D). Isoproterenol inhibits heat shock-induced FGF1 export. NIH 3T3 cells adenovirally transduced with FGF1 were incubated with or without 20 μM isoproterenol for 2 h at 37°C in DMEM plus 10 % fetal calf serum, followed by an additional incubation for 110 min at 37°C or 42°C in DMEM without serum. FGF1 was isolated from conditioned media and detected using immunoblotting.
In addition, the β-adrenergic receptor agonist, isoproterenol, has been reported to inhibit cell surface PS exposure (Lang et al., 2005). We found that similar to taurine, this compound inhibited PS exposure and FGF1 export (Figure 2C,D).
Thus, two unrelated molecules were demonstrated to suppress both PS externalization and FGF1 export.
4. Strengthening the binding between plasma membrane and the submembrane cytoskeleton inhibitsFGF1 export
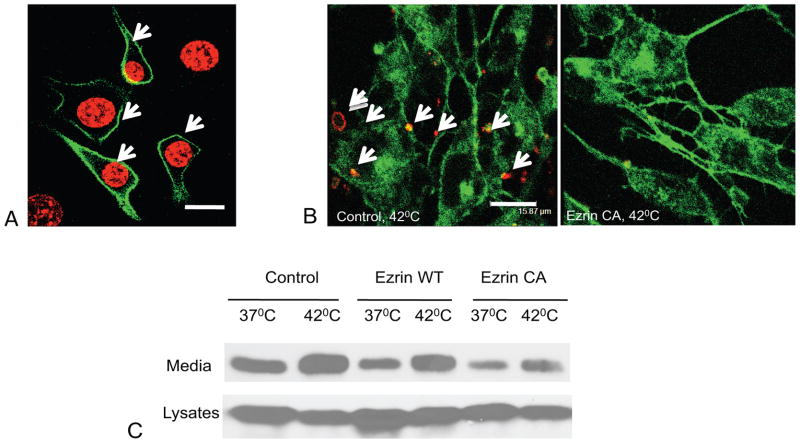
Bleb formation relies on the local weakening of the connection between the plasma membrane and submembrane actin (Charras et al., 2009; Fackler and Grosse, 2008). The cell membrane is bound to actin by the proteins of ERM family, one of which is ezrin phosphorylated at threonine 567(Arpin et al., 2011). The expression of the constitutively active (CA) T567D mutant of ezrin results in the stable binding of actin cytoskeleton to the cell membrane (Fievet et al., 2007). We generated NIH 3T3 cells that inducibly express WT or CA ezrin after doxycycline stimulation (Figure 3A). We compared FGF1 release from the doxycycline-stimulated NIH 3T3 cells cotransfected with rtTA and either WT ezrin, CA ezrin or empty vector (Figure 3C). Doxycycline caused considerable FGF1 export from all these cells at normal temperature, which may be due to its stressful effect on mitochondria (van den Bogert et al., 1986). However, heat shock significantly increased FGF1 secretion in doxycycline-treated cells. We found that in the cells expressing CA ezrin the export of FGF1 was suppressed both at 37 and 42°C (Figure 3C). Cells expressing CA ezrin also exhibited a decreased externalization of PS after heat shock (Figure 3B). These data support the importance of bleb formation (which is dependent on the decrease of cytoskeleton-membrane interaction) for the export of FGF1.
Figure 3. Expression of constitutively active ezrin decreases stress-induced PS externalization and attenuates FGF1 export.
Each figure is representative of three independent experiments. (A). Peripheral distribution of CA ezrin in stably transfected cells. NIH 3T3 cells stably cotransfected with rtTA and CA ezrin under control of the Tet promoter were stimulated for 48 h with 10 μg/ml doxycycline, formalin fixed, and stained for CA ezrin (anti-VSVG antibodies followed by FITC-conjugated secondary antibodies, green) and DNA (TO-PRO3, red). Confocal image. Peripheral ezrin is marked with arrows. Bar -15.87μm. (B). Decrease of PS externalization in cells expressing CA ezrin. NIH 3T3 cells stably transfected with rtTA, and either the empty pTRE2Hygro vector or the CA ezrin vector were stimulated for 48 h with doxycycline, and incubated for 110 min at 42°C. The live cells were then stained with annexin V:phycoerythrin (red), formalin fixed, stained with Alexa-488 conjugated phalloidin (green), and examined by confocal microscopy. PS externalization is marked with arrows. Bar -15.87μm (C). CA ezrin decreases FGF1 export. NIH 3T3 cells cotransfected with rtTA and either the empty pTRE2Hygro vector, WT ezrin, or CA ezrin were adenovirally transduced with FGF1, stimulated for 48 h with doxycycline, and then incubated for 110 min at 37°C or 42°C in DMEM without serum. FGF1 was isolated from conditioned media and detected by immunoblotting.
5. Bleb formation and externalization of PS correlate with FGF1 export in differentiated promonocytic leukemia cells
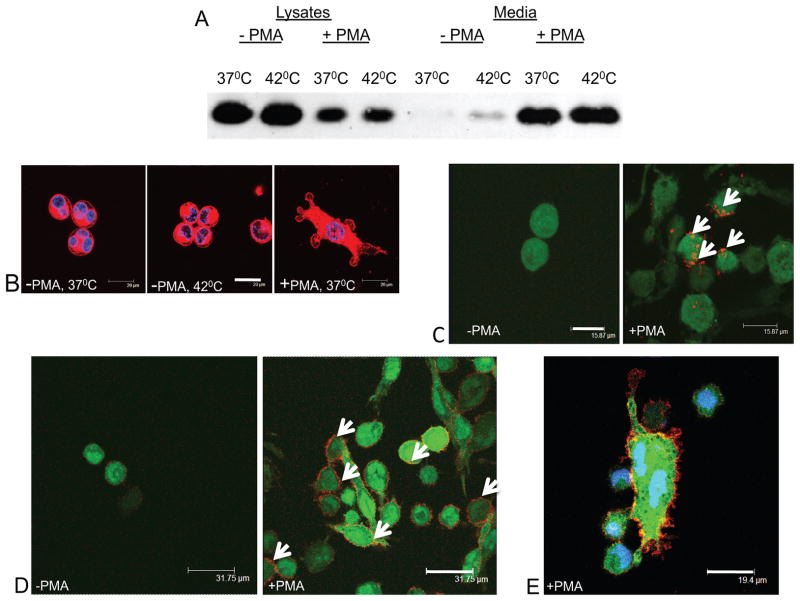
The correlation between FGF1 export and blebbing in fibroblastic cells prompted us to explore whether a similar connection can be observed in a promonocyte culture, U937. Therefore, we used U937 cells stably transfected with FGF1. We assessed FGF1 export at 37 and 42°C in undifferentiated U937 cells and in cells induced to undergo a macrophage-like differentiation by treatment with 150 nM PMA for 48 h (Figure 4A). Although undifferentiated cells growing in suspension exhibited a barely detectable FGF1 export, adherent differentiated cells were characterized by a massive FGF1 release (Figure 4A). Interestingly, heat shock only marginally induced FGF1 secretion from differentiated cells (Figure 4A), and lactate dehydrogenase release assays did not reveal cell damage under either PMA treatment or heat shock (results not shown). Undifferentiated U937 cells had a smooth surface both at normal temperature and at the heat shock (Figure 4B). In contrast, adherent differentiated U937 cells formed numerous membrane blebs (Figure 4B). In addition, PMA-treated U937 cells exhibited localized externalization of PS (Figure 4C). To visualize the release of FGF1 to the cell surface, we used U937 cells stably transfected with FGF1:GFP. In this experiment, externalized FGF1 was detected using monoclonal anti-GFP antibodies followed by CY3-conjugated secondary antibodies, while internal FGF1 was detected by GFP fluorescence (Figure 4D,E). As expected, undifferentiated U937 cells did not show significant externalization of FGF1, while pronounced FGF1 externalization was observed in differentiated cells. Unlike heat shocked NIH 3T3, we did not observe distinct domains enriched in externalized FGF1 in differentiated U937 cells. This is apparently due to the diffusion of externalized FGF1 across the cell surface during the prolonged differentiation period, which is characterized by high mobility of cell membrane. In contrast, the localized distribution of externalized PS could be explained by its relatively short lifetime on cell surface.
Figure 4. Phorbol ester-induced differentiation of promonocytic leukemia cells is accompanied by FGF1 export, membrane blebbing, and PS externalization.
Each figure is representative of four independent experiments. (A). PMA treatment induces FGF1 export from U937 cells. U937 cells stably transfected with FGF1 were treated or not treated with 150 nM PMA for 48 h, and then incubated for 110 min at 37°C or 42°C. FGF1 was isolated from conditioned media and detected using immunoblotting. 100% medium and 10% cell lysate were loaded per lane. (B). PMA treatment induces bleb formation in U937 cells. Control and PMA-treated U937 cells were formalin fixed and stained with the Vybrant membrane stain (red). The nuclei were stained with TOPRO3 (blue). Confocal images. Bar – 20 μm. (C). PMA-induced differentiation of U937 cells is accompanied by the externalization of PS. PMA-treated and untreated U937 cells transfected with FGF1:GFP (green) were incubated for 20 min with phycoerythrin-conjugated Annexin V (red), formalin fixed, and examined by confocal microscopy. PS externalization is marked with arrows. Bar - 15.87μm (D,E). Externalization of FGF1 in PMA-treated U937 cells. PMA-treated and untreated U937 cells transfected with FGF1:GFP (green) were incubated for 20min with monoclonal anti-GFP antibodies (red), formalin fixed, incubated with CY3-conjugated secondary antibodies, and examined by confocal microscopy. FGF1 externalization is marked with arrows. Bar – 31.75μm. E: PMA-treated U937 transfected with FGF1:GFP, higher magnification. Processed as in “D.” Additional staining of nuclei with TOPRO-3 (blue). Bar -19μm.
In conclusion, these experiments demonstrate that macrophage-like differentiation results in strong FGF1 export, which correlates with membrane blebbing and PS externalization.
6. Intracellular calcium, but not PLSCR1 is required for FGF1 export from differentiated U937 cells
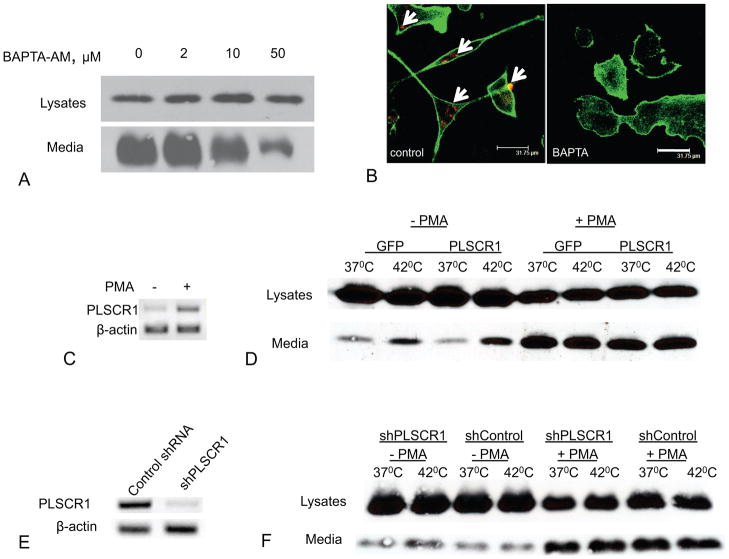
Because differentiation of U937 cells is accompanied by increased cytosolic calcium concentration (Klein et al., 1990), which can stimulate PS externalization (Vance and Steenbergen, 2005), we assessed the effect of the cell-permeable calcium chelator, BAPTA-AM, on FGF1 export from PMA-treated U937 cells. We found that BAPTA-AM inhibited both FGF1 export (Figure 5A) and PS exposure (Figure 5B) in these cells. Interestingly, copper chelator tetrathiomolybdate (TM), which inhibits FGF1 export from stressed fibroblasts (Landriscina et al., 2001a), failed to suppress FGF1 secretion from differentiated U937 macrophages (Supplementary Figure 1).
Figure 5. Intracellular calcium but not PLSCR1 is required for FGF1 export from U937 cells.
Each figure is representative of three independent experiments. (A). BAPTA-AM inhibits FGF1 export from U937 cells in a concentration-dependent manner. U937 cells stably transfected with FGF1 were treated with 150 nM PMA for 48 h, and then incubated for 110 min at 37°C in presence or absence of BAPTA-AM. FGF1 was isolated from conditioned media and detected using immunoblotting. 100% medium and 2% cell lysate were loaded per lane. (B). BAPTA-AM inhibits PS externalization in differentiated U937 cells. PMA-treated U937 cells were preincubated for 2 h with or without 50 μM BAPTA-AM and then incubated for 20 min with phycoerythrin-conjugated Annexin V (red), formalin fixed, permeabilized, stained with Alexa 488-phalloidin (green) and studied using confocal microscopy. PS externalization is marked with arrows. Bar – 31.75μm. (C). Undifferentiated U937 cells have low PLSCR1 expression, which is enhanced with PMA treatment. RNA was isolated from undifferentiated U937 cells and U937 cells treated for 48 h with 150 nM PMA. RT-PCR was performed using PLSCR1 and β-actin (control) primers. (D). Over-expression of PLSCR1 does not enhance FGF1 export from U937 cells. U937 cells stably transfected with PLSCR1:GFP or with GFP (control) were treated or not with 150 nM PMA for 48 h, and incubated for 110 min at 37°C or 42°C. FGF1 was isolated from conditioned media and detected by immunoblotting. 100% medium and 10% cell lysate were loaded per lane. (E). Specific shRNA decreases PLSCR1 expression in U937 cells. RNA was isolated from PMA-treated U937 cells stably transfected with PLSCR1 shRNA or control shRNA. RT PCR was performed using PLSCR1 and β-actin (control) primers. (F). shRNA knockdown of PLSCR1 does not affect FGF1 export from U937 cells. U937 cells stably transfected with FGF1 and cotransfected with PLSCR1 shRNA or control shRNA were treated or not with 150 nM PMA for 48 h, and then incubated for 110 min at 37°C or 42°C. FGF1 was isolated from conditioned media and detected using immunoblotting.
PLSCR1 is an endofacial plasma membrane protein proposed to mediate intracellular Ca2+- activated transbilayer movement of plasma membrane phospholipids, resulting in PS exposure at the cell surface (Sims and Wiedmer, 2001). Chen et al. showed that undifferentiated U937had low expression of PLSCR1, which was dramatically enhanced upon the induction of cell differentiation (Huang et al., 2006). We confirmed these results using RT-PCR with specific PLSCR1 primers (Figure 5C). The marked contrast of undifferentiated U937 that do export very low amounts of FGF1 versus PMA-differentiated U937 that extensively release FGF1 suggested that this up-regulation of FGF1 export upon cell differentiation might be directly related to the concomitant up-regulation of PLSCR1 expression. To examine this possibility, U937 cells stably overexpressing PLSCR1:GFP were generated. We found that the over-expression of PLSCR1 failed to stimulate FGF1 release from heat shocked undifferentiated U937 cells, and did not enhance FGF1 secretion stimulated by PMA (Figure 5D). Furthermore, we produced U937 cells in which PLSCR1 had been stably knocked down (Figure 5E). We found that PLSCR1 knockdown had no effect on FGF1 secretion in PMA-treated cells (Figure 5F).
Discussion
Unlike spontaneous FGF2 export that occurs through submicron-sized non-raft membrane patches evenly distributed through the cell surface (Engling et al., 2002), stress-induced FGF1 secretion proceeds through large membrane domains. The co-localization of externalized FGF1 and PS in these domains corroborates our earlier hypothesis that stress-induced translocation of acidic phospholipids could be a driving force of stress-dependent FGF1 export (Prudovsky et al., 2008). We demonstrated that FGF1 not only selectively binds PS (Tarantini et al., 1995)but also efficiently destabilizes artificial membranes composed of acidic phospholipids, such as PS, phosphatidylglycerol and phosphatidylinositol, but not zwitterionic phosphatidylcholine (Graziani et al., 2006). The 40 kDa form of synaptotagmin 1, a critical member of the FGF1 export complex, which efficiently binds PIP2 (Sutton et al., 1995), permeabilizes artificial bilayers composed of phosphatidylinositol but not of phosphatidylcholine. Moreover, mutations of FGF1 and 40 kDa synaptotagmin 1, which abolish the destabilization of acidic phospholipid liposomes, strongly inhibit the stress-induced release of these proteins. In addition, another member of FGF1 export pathway, sphingosine kinase 1 (Stahelin et al., 2005) binds PS with high affinity. The transmembrane translocation of PS occurs both in the plasma membrane under cell stress and in the membranes of the trans-Golgi network under normal conditions (Fairn et al., 2011). The mechanisms underlying these processes are not well understood. Schroit et al. (Mirnikjoo et al., 2009) demonstrated that PS externalization in apoptotic cells is a result of lysosome fusion with the plasma membrane. However, we found that the lysosomotrophic amine, chloroquine, as well as a caspase I inhibitor failed to suppress PS exposure and FGF1 export in heat shocked NIH 3T3 cells (data not shown). Interestingly, the co-localization with exposed PS has been observed for another non-classically exported protein, epimorphin (Hirai et al., 2007).
We found that two chemical compounds, the nonessential amino acid taurine and the agonist of β-adrenergic receptors isoproterenol, efficiently suppressed both the stress-induced externalization of PS and FGF1 export. Interestingly, taurine exhibits anti-inflammatory activity (Yamori et al., 2010)and inhibits the growth of certain tumor types (Neary et al., 2010). Although the molecular mechanisms underlying the biological effects of taurine are unknown, it has been used as a beneficial nutritional supplement(Yamori et al., 2010). Based on our results, we suggest that the inhibition of nonclassical export of mitogenic and pro-inflammatory proteins at least partially underlies the biological effects of taurine.
The localization of externalized FGF1 in the protrusions of the cell membrane could indicate that FGF1 export proceeds as a result of the detachment of cytoplasmic blebs. However, our experiments in which microparticles were removed from conditioned medium by ultracentrifugation showed that FGF1 is not associated with detached blebs or released exosomes, and is present in the supernatant. Interestingly, Nickel et al. demonstrated similar results using fractionation of conditioned medium to study the spontaneous export of FGF2 (Seelenmeyer et al., 2008). Based on these experiments, we conclude that FGF1 is exported from the surface of membrane protrusions in stressed cells, and FGF1 subsequently partitions as a soluble protein into the medium. It is possible that the high curvature of the cell membrane in protrusions facilitates the transmembrane movement of PS along with its associated FGF1 to the external surface of the bleb. Bleb formation requires the dissociation between the plasma membrane and underlying subplasmalemmal actin cytoskeleton (Charras et al., 2009; Fackler and Grosse, 2008). The retraction of cell protrusions relies on the local increase of ezrin, a protein responsible for the attachment of cell membrane to filamentous actin (Arpin et al., 2011). Our experiments with inducible expression of CA ezrin demonstrate the importance of blebbing for stress-induced export of FGF1.
The experiments with U937 cells provided additional support for the role of blebbing and PS externalization in FGF1 export. Indeed, although heat shock, which does not influence U937 cell shape, failed to induce the release of FGF1 from these cells, phorbol ester-induced differentiation caused both the formation of cell protrusions and massive FGF1 export from U937 cells. In addition, the differentiation of U937 cells was accompanied by PS externalization. Both FGF1 release and PS exposure in differentiated U937 cells depended on intracellular calcium.
PLSCR1 has been widely assumed to mediate the cell surface exposure of PS through its capacity to mediate Ca2+ activated transbilayer movement of phospholipids in reconstituted membrane vesicles, thereby promoting collapse of transbilayer lipid asymmetry (Sims and Wiedmer, 2001). Interestingly, PLSCR1, being a signal peptide-less protein (like FGF1), is secreted through a lipid raft-dependent nonclassical pathway (Merregaert et al., 2010). We performed a series of experiments to assess the potential role of PLSCR1 in the stress-induced export of FGF1 from differentiated U937 cells, which express this protein at higher levels than do undifferentiated cells. These results indicate that PLSCR1 is not required for FGF1 secretion from U937 cells. Although a strong correlation exists between FGF1 export and PLSCR1 expression in U937 cells, PLSCR1 has been shown to be neither sufficient nor necessary for PS externalization in live cells (Zhou et al., 2002), and it has other functions besides phospholipid translocation (Ben-Efraim et al., 2004; Chen et al., 2010). Thus, the acidic phospholipid transporter(s) involved in the nonclassical secretion of FGF1 have yet to be elucidated. In addition, the mechanistic similarity between stress- induced FGF1 export from fibroblast and differentiation-related FGF1 release from macrophages is also a subject of further studies. Both processes exhibit correlation between FGF1 secretion and PS exposure. However, unlike stressed fibroblasts (Landriscina et al., 2001a), FGF1 export from differentiated macrophages was resistant to copper chelation by tetrathiomolybdate.
In conclusion, bleb formation and PS externalization appear to be causally related to stress- induced FGF1 export. Although the exact molecular mechanism of stress-induced FGF1 translocation has yet to be understood, the finding that pharmacological inhibition of PS externalization also inhibits FGF1 release suggests that the two processes are intertwined. Potentially, inhibitors of PS export, or PS-targeting antibodies such as bavituximab, could impede FGF1 release and angiogenesis in tumors. Interestingly, the recent study by Riedl et al. (Riedl et al., 2011) demonstrates a strong correlation between the non-apoptotic externalization of PS and malignancy of melanomas.
Supplementary Material
Acknowledgments
Contract grant sponsor 1: NIH; Contract grant numbers: RR15555 (Project 4), R01 HL35627 Contract grant sponsor 2: Maine Cancer Foundation
We are very grateful to Monique Arpin (Institut Curie, Paris, France) for providing the ezrin constructs. We thank Peregrine Pharmaceutical, Tustin, CA for providing bavituximab. We are deeply thankful to Alan Schroit (University of Texas Southwestern Medical Center, Dallas) for the constructive discussion of our results. The study has been supported by a Maine Cancer Foundation grant to IP and NIH grants P20 RR15555to Robert Friesel (Project 4 IP) and HL35627 to IP. PJS was supported by NIH grants HL036946 and HL063819. In this work, we used the services of the Flow Cytometry Core supported by NIH grant P20 RR181789 (Don Wojchowski, PI), and Protein, Nucleic Acid and Cell Imaging Core, and Recombinant Viral Vector Core supported by grant P20 RR15555 (Robert Friesel, PI).
Abbreviations
- CA
constitutively active
- FGF1
fibroblast growth factor 1
- PLSCR1
phospholipid scramblase 1
- PS
phosphatidylserine
References
- Al-Nedawi K, Meehan B, Rak J. Microvesicles: messengers and mediators of tumor progression. Cell Cycle. 2009;8(13):2014–2018. doi: 10.4161/cc.8.13.8988. [DOI] [PubMed] [Google Scholar]
- Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol. 1993;120(1):129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananyeva NM, Tijurmin AV, Berliner JA, Chisolm GM, Liau G, Winkles JA, Haudenschild CC. Oxidized LDL mediates the release of fibroblast growth factor-1. ArteriosclerThrombVascBiol. 1997;17:445–453. doi: 10.1161/01.atv.17.3.445. [DOI] [PubMed] [Google Scholar]
- Anderheggen B, Jassoy C, Waldmann-Laue M, Forster T, Wadle A, Doering T. Taurine improves epidermal barrier properties stressed by surfactants-a role for osmolytes in barrier homeostasis. J Cosmet Sci. 2006;57(1):1–10. [PubMed] [Google Scholar]
- Arpin M, Chirivino D, Naba A, Zwaenepoel I. Emerging role for ERM proteins in cell adhesion and migration. Cell Adh Migr. 2011;5(2):199–206. doi: 10.4161/cam.5.2.15081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Efraim I, Zhou Q, Wiedmer T, Gerace L, Sims PJ. Phospholipid scramblase 1 is imported into the nucleus by a receptor-mediated pathway and interacts with DNA. Biochemistry. 2004;43(12):3518–3526. doi: 10.1021/bi0356911. [DOI] [PubMed] [Google Scholar]
- Charras GT, Mitchison TJ, Mahadevan L. Animal cell hydraulics. J Cell Sci. 2009;122(Pt 18):3233–3241. doi: 10.1242/jcs.049262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CW, Sowden M, Zhao Q, Wiedmer T, Sims PJ. Nuclear phospholipid scramblase 1 prolongs the mitotic expansion of granulocyte precursors during G-CSF-induced granulopoiesis. J Leukoc Biol. 2010;90(2):221–233. doi: 10.1189/jlb.0111006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey K, Amaya E. FGF signalling: diverse roles during early vertebrate embryogenesis. Development. 2010;137(22):3731–3742. doi: 10.1242/dev.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte M, Kolev V, Kacer D, Mouta-Bellum C, Soldi R, Graziani I, Kirov A, Friesel R, Liaw L, Small D, Verdi J, Maciag T, Prudovsky I. Novel cross-talk between three cardiovascular regulators: thrombin cleavage fragment of Jagged1 induces fibroblast growth factor 1 expression and release. Mol Biol Cell. 2008;19(11):4863–4874. doi: 10.1091/mbc.E07-12-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engling A, Backhaus R, Stegmayer C, Zehe C, Seelenmeyer C, Kehlenbach A, Schwappach B, Wegehingel S, Nickel W. Biosynthetic FGF-2 is targeted to non-lipid raft microdomains following translocation to the extracellular surface of CHO cells. J Cell Sci. 2002;115(Pt 18):3619–3631. doi: 10.1242/jcs.00036. [DOI] [PubMed] [Google Scholar]
- Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181(6):879–884. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairn GD, Schieber NL, Ariotti N, Murphy S, Kuerschner L, Webb RI, Grinstein S, Parton RG. High-resolution mapping reveals topologically distinct cellular pools of phosphatidylserine. J Cell Biol. 2011;194(2):257–275. doi: 10.1083/jcb.201012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fievet B, Louvard D, Arpin M. ERM proteins in epithelial cell organization and functions. Biochim Biophys Acta. 2007;1773(5):653–660. doi: 10.1016/j.bbamcr.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Graziani I, Bagala C, Duarte M, Soldi R, Kolev V, Tarantini F, Kumar TK, Doyle A, Neivandt D, Yu C, Maciag T, Prudovsky I. Release of FGF1 and p40 synaptotagmin 1 correlates with their membrane destabilizing ability. Biochem Biophys Res Commun. 2006 doi: 10.1016/j.bbrc.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve AG, Rabouille C. Golgi bypass: skirting around the heart of classical secretion. Cold Spring Harb Perspect Biol. 2011;3(4) doi: 10.1101/cshperspect.a005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix A, Westbroek W, Bracke M, De Wever O. An ex(o)citing machinery for invasive tumor growth. Cancer Res. 2010;70(23):9533–9537. doi: 10.1158/0008-5472.CAN-10-3248. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Nelson CM, Yamazaki K, Takebe K, Przybylo J, Madden B, Radisky DC. Non-classical export of epimorphin and its adhesion to {alpha}v-integrin in regulation of epithelial morphogenesis. J Cell Sci. 2007;120(Pt 12):2032–2043. doi: 10.1242/jcs.006247. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhao Q, Zhou CX, Gu ZM, Li D, Xu HZ, Sims PJ, Zhao KW, Chen GQ. Antileukemic roles of human phospholipid scramblase 1 gene, evidence from inducible PLSCR1-expressing leukemic cells. Oncogene. 2006;25(50):6618–6627. doi: 10.1038/sj.onc.1209677. [DOI] [PubMed] [Google Scholar]
- Jackson A, Friedman S, Zhan X, Engleka KA, Forough R, Maciag T. Heat shock induces the release of fibroblast growth factor 1 from NIH 3T3 cells. Proc Natl Acad Sci U S A. 1992;89(22):10691–10695. doi: 10.1073/pnas.89.22.10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JB, Schepers TM, Dean WL, Sonnenfeld G, McLeish KR. Role of intracellular calcium concentration and protein kinase C activation in IFN-gamma stimulation of U937 cells. J Immunol. 1990;144(11):4305–4311. [PubMed] [Google Scholar]
- Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009;9(5):639–651. doi: 10.2174/156800909789057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landriscina M, Bagala C, Mandinova A, Soldi R, Micucci I, Bellum S, Prudovsky I, Maciag T. Copper induces the assembly of a multiprotein aggregate implicated in the release of fibroblast growth factor 1 in response to stress. J Biol Chem. 2001a;276(27):25549–25557. doi: 10.1074/jbc.M102925200. [DOI] [PubMed] [Google Scholar]
- Landriscina M, Soldi R, Bagala C, Micucci I, Bellum S, Tarantini F, Prudovsky I, Maciag T. S100A13 participates in the release of fibroblast growth factor 1 in response to heat shock in vitro. J Biol Chem. 2001b;276(25):22544–22552. doi: 10.1074/jbc.M100546200. [DOI] [PubMed] [Google Scholar]
- Lang PA, Kempe DS, Akel A, Klarl BA, Eisele K, Podolski M, Hermle T, Niemoeller OM, Attanasio P, Huber SM, Wieder T, Lang F, Duranton C. Inhibition of erythrocyte “apoptosis” by catecholamines. Naunyn Schmiedebergs Arch Pharmacol. 2005;372(3):228–235. doi: 10.1007/s00210-005-0009-2. [DOI] [PubMed] [Google Scholar]
- LaVallee TM, Tarantini F, Gamble S, Carreira CM, Jackson A, Maciag T. Synaptotagmin-1 is required for fibroblast growth factor-1 release. J Biol Chem. 1998;273(35):22217–22223. doi: 10.1074/jbc.273.35.22217. [DOI] [PubMed] [Google Scholar]
- Lenoir G, Williamson P, Holthuis JC. On the origin of lipid asymmetry: the flip side of ion transport. Curr Opin Chem Biol. 2007;11(6):654–661. doi: 10.1016/j.cbpa.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Luster TA, He J, Huang X, Maiti SN, Schroit AJ, de Groot PG, Thorpe PE. Plasma protein beta-2- glycoprotein 1 mediates interaction between the anti-tumor monoclonal antibody 3G4 and anionic phospholipids on endothelial cells. J Biol Chem. 2006;281(40):29863–29871. doi: 10.1074/jbc.M605252200. [DOI] [PubMed] [Google Scholar]
- Mandinova A, Soldi R, Graziani I, Bagala C, Bellum S, Landriscina M, Tarantini F, Prudovsky I, Maciag T. S100A13 mediates the copper-dependent stress-induced release of IL-1{alpha} from both human U937 and murine NIH 3T3 cells. J Cell Sci. 2003;116(Pt 13):2687–2696. doi: 10.1242/jcs.00471. [DOI] [PubMed] [Google Scholar]
- Marconescu A, Thorpe PE. Coincident exposure of phosphatidylethanolamine and anionic phospholipids on the surface of irradiated cells. Biochim Biophys Acta. 2008;1778(10):2217–2224. doi: 10.1016/j.bbamem.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Merregaert J, Van Langen J, Hansen U, Ponsaerts P, El Ghalbzouri A, Steenackers E, Van Ostade X, Sercu S. Phospholipid scramblase 1 is secreted by a lipid raft-dependent pathway and interacts with the extracellular matrix protein 1 in the dermal epidermal junction zone of human skin. J Biol Chem. 2010;285(48):37823–37837. doi: 10.1074/jbc.M110.136408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirnikjoo B, Balasubramanian K, Schroit AJ. Suicidal membrane repair regulates phosphatidylserine externalization during apoptosis. J Biol Chem. 2009;284(34):22512–22516. doi: 10.1074/jbc.C109.022913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouta Carreira C, Landriscina M, Bellum S, Prudovsky I, Maciag T. The comparative release of FGF1 by hypoxia and temperature stress. Growth Factors. 2001;18(4):277–285. doi: 10.3109/08977190109029116. [DOI] [PubMed] [Google Scholar]
- Nanjundan M, Sun J, Zhao J, Zhou Q, Sims PJ, Wiedmer T. Plasma membrane phospholipid scramblase 1 promotes EGF-dependent activation of c-Src through the epidermal growth factor receptor. J Biol Chem. 2003;278(39):37413–37418. doi: 10.1074/jbc.M306182200. [DOI] [PubMed] [Google Scholar]
- Neary PM, Hallihan P, Wang JH, Pfirrmann RW, Bouchier-Hayes DJ, Redmond HP. The evolving role of taurolidine in cancer therapy. Ann Surg Oncol. 2010;17(4):1135–1143. doi: 10.1245/s10434-009-0867-9. [DOI] [PubMed] [Google Scholar]
- Nickel W, Seedorf M. Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu Rev Cell Dev Biol. 2008;24:287–308. doi: 10.1146/annurev.cellbio.24.110707.175320. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2(3) doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudovsky I, Bagala C, Tarantini F, Mandinova A, Soldi R, Bellum S, Maciag T. The intracellular translocation of the components of the fibroblast growth factor 1 release complex precedes their assembly prior to export. J Cell Biol. 2002;158(2):201–208. doi: 10.1083/jcb.200203084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudovsky I, Tarantini F, Landriscina M, Neivandt D, Soldi R, Kirov A, Small D, Kathir KM, Rajalingam D, Kumar TK. Secretion without Golgi. J Cell Biochem. 2008;103(5):1327–1343. doi: 10.1002/jcb.21513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedl S, Rinner B, Asslaber M, Schaider H, Walzer S, Novak A, Lohner K, Zweytick D. In search of a novel target -Phosphatidylserine exposed by non-apoptotic tumor cells and metastases of malignancies with poor treatment efficacy. Biochim Biophys Acta. 2011;1808(11):2638–2645. doi: 10.1016/j.bbamem.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier F, Camoin-Jau L, Anfosso F, Sampol J, Dignat-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. J Cell Mol Med. 2009;13(3):454–471. doi: 10.1111/j.1582-4934.2008.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelenmeyer C, Stegmayer C, Nickel W. Unconventional secretion of fibroblast growth factor 2 and galectin-1 does not require shedding of plasma membrane-derived vesicles. FEBS Lett. 2008;582(9):1362–1368. doi: 10.1016/j.febslet.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Seigneuret M, Devaux PF. ATP-dependent asymmetric distribution of spin-labeled phospholipids in the erythrocyte membrane: relation to shape changes. Proc Natl Acad Sci U S A. 1984;81(12):3751–3755. doi: 10.1073/pnas.81.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JT, Opalenik SR, Wehby JN, Mahesh VK, Jackson A, Tarantini F, Maciag T, Thompson JA. Serum-starvation induces the extracellular appearance of FGF-1. Biochim Biophys Acta. 1996;1312(1):27–38. doi: 10.1016/0167-4889(96)00013-4. [DOI] [PubMed] [Google Scholar]
- Sims PJ, Wiedmer T. Unraveling the mysteries of phospholipid scrambling. Thromb Haemost. 2001;86(1):266–275. [PubMed] [Google Scholar]
- Soldi R, Mandinova A, Venkataraman K, Hla T, Vadas M, Pitson S, Duarte M, Graziani I, Kolev V, Kacer D, Kirov A, Maciag T, Prudovsky I. Sphingosine kinase 1 is a critical component of the copper-dependent FGF1 export pathway. Exp Cell Res. 2007;313(15):3308–3318. doi: 10.1016/j.yexcr.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahelin RV, Hwang JH, Kim JH, Park ZY, Johnson KR, Obeid LM, Cho W. The mechanism of membrane targeting of human sphingosine kinase 1. J Biol Chem. 2005;280(52):43030–43038. doi: 10.1074/jbc.M507574200. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Davletov BA, Berghuis AM, Sudhof TC, Sprang SR. Structure of the first C2 domain of synaptotagmin I: a novel Ca2+/phospholipid-binding fold. Cell. 1995;80(6):929–938. doi: 10.1016/0092-8674(95)90296-1. [DOI] [PubMed] [Google Scholar]
- Tarantini F, Gamble S, Jackson A, Maciag T. The cysteine residue responsible for the release of fibroblast growth factor-1 residues in a domain independent of the domain for phosphatidylserine binding. J Biol Chem. 1995;270(49):29039–29042. doi: 10.1074/jbc.270.49.29039. [DOI] [PubMed] [Google Scholar]
- van den Bogert C, Dontje BH, Holtrop M, Melis TE, Romijn JC, van Dongen JW, Kroon AM. Arrest of the proliferation of renal and prostate carcinomas of human origin by inhibition of mitochondrial protein synthesis. Cancer Res. 1986;46(7):3283–3289. [PubMed] [Google Scholar]
- Vance JE, Steenbergen R. Metabolism and functions of phosphatidylserine. Prog Lipid Res. 2005;44(4):207–234. doi: 10.1016/j.plipres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Yamori Y, Taguchi T, Hamada A, Kunimasa K, Mori H, Mori M. Taurine in health and diseases: consistent evidence from experimental and epidemiological studies. J Biomed Sci. 2010;17(Suppl 1):S6. doi: 10.1186/1423-0127-17-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao KW, Li X, Zhao Q, Huang Y, Li D, Peng ZG, Shen WZ, Zhao J, Zhou Q, Chen Z, Sims PJ, Wiedmer T, Chen GQ. Protein kinase Cdelta mediates retinoic acid and phorbol myristate acetate- induced phospholipid scramblase 1 gene expression: its role in leukemic cell differentiation. Blood. 2004;104(12):3731–3738. doi: 10.1182/blood-2004-04-1630. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Zhao J, Wiedmer T, Sims PJ. Normal hemostasis but defective hematopoietic response to growth factors in mice deficient in phospholipid scramblase 1. Blood. 2002;99(11):4030–4038. doi: 10.1182/blood-2001-12-0271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.