Abstract
Objective
Serum adiponectin protects against incident ischemic heart disease (IHD). However, in patients with existing IHD, higher adiponectin levels are paradoxically associated with worse outcomes. We investigated this paradox by evaluating the relationship between adiponectin and cardiovascular events in patients with existing IHD.
Methods
We measured total serum adiponectin and cardiac disease severity by stress echocardiography in 981 outpatients with stable IHD who were recruited for the Heart and Soul Study between September 2000 and December 2002. Subsequent heart failure hospitalizations, myocardial infarction, and death were recorded.
Results
During an average of 7.1 years of follow-up, patients with adiponectin levels in the highest quartile were more likely than those in the lowest quartile to be hospitalized for heart failure (23% vs. 13%; demographics-adjusted hazard ratio (HR) 1.63, 95% confidence interval (CI) 1.04–2.56, p=0.03) or die (49% vs. 31%; HR 1.67, 95% CI 1.24–2.26, p<0.008), but not more likely to have a myocardial infarction (12% vs. 17%; HR 0.64, 95% CI 0.38–1.06, p=0.08). The combined outcome of myocardial infarction, heart failure, or death occurred in 56% (136/245) of participants in the highest quartile of adiponectin vs. 38% (94/246) of participants in the lowest quartile (HR 1.54, 95% CI 1.31–2.21, p<0.002). Adjustment for left ventricular ejection fraction, diastolic dysfunction, inducible ischemia, C-reactive protein, and NT-proBNP attenuated the association between higher adiponectin and increased risk of subsequent events (HR 1.43, 95% CI 0.98–2.09 p=0.06).
Conclusions
Higher concentrations of adiponectin were associated with heart failure and mortality among patients with existing IHD.
Keywords: adiponectin, ischemic heart disease, outcomes, reverse epidemiology
1. Introduction
Adiponectin is an abundant serum protein that is secreted primarily from adipose tissue [1], with concentrations that are inversely associated with obesity [2]. In animal and in vitro models, adiponectin exerts insulin-sensitizing, anti-inflammatory and anti-atherosclerotic effects [1]. Likewise, in humans low adiponectin levels, such as those seen in obesity, are associated with low HDL [3], hypertension [4], insulin resistance [2, 3], and diabetes [2]. Given its connections with cardiovascular disease risk factors, recent population studies have investigated the association of adiponectin with incident cardiovascular events. In patients without previously diagnosed cardiovascular disease, higher levels of adiponectin are thought to be protective against disease [5–7], although this relationship is not consistently present in women [6, 8], minorities [9], or the elderly [10].
Despite adiponectin being associated with a favorable cardiovascular risk profile, higher levels of adiponectin have paradoxically been associated with inducible ischemia [11], worse outcomes among patients with acute coronary syndrome [12], and worse outcomes among patients with existing heart failure [10]. The underlying mechanisms of the association between adiponectin and severity of disease in individuals with existing cardiovascular disease are not well understood. In the present study, we further investigated this apparent paradox by evaluating the association between adiponectin and cardiovascular disease outcomes in a cohort of patients with stable ischemic heart disease (IHD).
2. Methods
2.1. Participants
The Heart and Soul Study is a prospective cohort study designed to investigate the effects of psychosocial factors on health outcomes in patients with stable IHD. Methods have been previously described [13]. Patients were eligible if they had at least 1 of the following: history of myocardial infarction, angiographic evidence of ≥50% stenosis in ≥1 coronary vessels, evidence of exercise-induced ischemia by treadmill ECG or stress nuclear perfusion imaging, or a history of coronary revascularization. Patients were excluded if they were unable to walk one block, had an acute coronary syndrome within the previous six months, or were likely to move out of the area within three years. Between September 2000 and December 2002, 1024 subjects were recruited from 12 outpatient clinics in the San Francisco Bay Area, including 549 (54%) with a history of myocardial infarction, 237 (23%) with a history of revascularization but not myocardial infarction, and 238 (23%) with a diagnosis of coronary disease that was documented by their physician, based on a positive angiogram or treadmill test in over 98% of cases. All participants completed a full-day study including medical history, extensive questionnaires, and an exercise treadmill test with baseline and stress echocardiograms. 12-h fasting serum samples were obtained in the morning prior to stress test and frozen at −80°C. Of 1024 participants, 39 were excluded from this analysis because frozen serum was not available to perform the adiponectin assay, and 4 were excluded due to lack of complete outcome data, resulting in 981 participants for this analysis. Institutional Review Boards at each site approved this study protocol. All participants provided written informed consent.
2.2. Measurement of adiponectin
Total serum adiponectin level was determined by immunoassay of thawed fasting serum samples (Linco, Millipore, St. Charles, MO). Each sample was assayed in duplicate, and adiponectin level was calculated as the average of two measurements. The lowest detectable measurement for adiponectin was 145.4pg/mL. The inter-assay coefficient of variation for this multiplexed immunoassay was 14.2–21.8%, and the intra-assay coefficient of variation was 1.4–7.9%. No significant cross-reactivity was observed within the panel [11].
2.3. Outcome ascertainment
Annual telephone interviews were conducted with participants or their proxy to inquire about interval hospitalization or death. For any reported event, medical records, electrocardiograms, death certificates, autopsy, and coroner’s reports were obtained. Each event was adjudicated by 2 independent and blinded reviewers. In the event of disagreement, the adjudicators conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator.
The primary outcome was a composite of myocardial infarction, heart failure, or death from any cause. Secondary outcomes were the individual components of myocardial infarction, heart failure, and death from any cause. Myocardial infarction was defined using standard diagnostic criteria [14]. Heart failure was defined as hospitalization or emergency department visit for heart failure. Death was verified by death certificates.
2.4. Other patient characteristics
Demographic characteristics, medical history, and smoking status were assessed by self-report questionnaire. We measured weight and height and calculated the body mass index (BMI) (kg/m2). Participants were asked to bring their medication bottles to the study appointment, and research personnel recorded all current medications. Medications were categorized using Epocrates Rx (San Mateo, CA). Total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, insulin, glucose, glycosylated hemoglobin, creatinine, and high sensitivity C-reactive protein (CRP) were determined from 12-h fasting serum samples. Insulin levels were measured with a Linco Multiplex immunoassay (Millipore, St. Charles, MO). Levels of the amino terminal fragment of the prohormone brain-type natriuretic peptide (NT-proBNP) were determined using Roche Diagnostics Elecsys NT-proBNP electrochemiluminescence immunoassay (Elecsys proBNP, Roche Diagnostics, Indianapolis, IN). Estimated glomerular filtration rate (eGFR) was calculated using the abbreviated Modification of Diet in Renal Disease (MDRD) Study equation [15].
Participants underwent symptom-limited exercise stress testing according to a standard Bruce protocol (those unable to complete the standard protocol were converted to a manual protocol) with continuous 12-lead electrocardiogram monitoring. Prior to exercise, participants underwent complete resting two-dimensional echocardiograms with all standard views using an Acuson Sequoia ultrasound system (Siemens Medical Solutions, Mountain View, CA) with a 3.5-MHz transducer and Doppler ultrasound examination. Standard two-dimensional parasternal short-axis and apical two- and four-chamber views were obtained during held inspiration and were used to calculate the left ventricular ejection fraction [16]. Diastolic dysfunction was defined as pseudonormal or restrictive filling on mitral inflow [13]. At peak exercise, precordial long- and short-axis and apical two- and four-chamber views were obtained to assess for wall motion abnormalities. We defined exercise-induced ischemia as the presence of one or more new wall motion abnormality at peak exercise that was not present at rest [11]. A single experienced cardiologist (NBS), who was blinded to the results of the adiponectin assays and clinical histories, interpreted all echocardiograms.
2.5. Statistical Analysis
Participants were divided into quartiles on the basis of serum adiponectin level. Baseline participant characteristics across quartiles were compared using analysis of variance (ANOVA) for continuous variables and Χ2 test for dichotomous variables. We compared the frequency of outcomes across quartiles using the Χ2 test. We compared rates of the primary outcome as well as myocardial infarction, heart failure, and death between quartile IV vs. quartile I in multivariate Cox regression models adjusted for baseline demographics (age, sex, race), clinical risk factors (diabetes, systolic blood pressure, diastolic blood pressure, eGFR, beta-blocker use, aspirin use, and statin use), metabolic markers (BMI, hemoglobin A1c, insulin, glucose, total cholesterol, HDL, and triglycerides), and measures of cardiac disease severity (LV ejection fraction, diastolic dysfunction, inducible ischemia, log C-reactive protein, log-NT-proBNP). We further examined risk associated with the continuous value of adiponectin level after log-transformation to normalize the distribution, and expressed risk per standard deviation (SD) increase (0.8µg/mL) of log adiponectin using the same multivariate Cox regression models. We tested for interactions between adiponectin and age, sex, race, BMI, diabetes, serum creatinine, and left ventricular ejection fraction. Analyses were performed using Statistical Analysis Software (version 9.2; SAS Institute Inc, Cary, NC).
3. Results
Among 981 participants, the median adiponectin level was 21.3 µg/mL (interquartile range 12.6–35.6 µg/mL). Compared to those participants in the lowest quartile, participants in the highest quartile were older, less likely to be male, and more likely to be white (Table 1). While no significant difference was noted with regard to prevalence of hypertension, myocardial infarction, or heart failure, participants with higher adiponectin were less likely to have diabetes mellitus. With regard to medication use, participants in the highest quartile were less likely to be taking aspirin, beta-blocker, or statin. Participants in the highest quartile had lower BMI, higher HDL cholesterol, lower non-HDL cholesterol, lower triglycerides, lower levels of insulin and fasting glucose. Higher adiponectin was associated with worse renal function as determined by lower eGFR. There was no significant difference in serum CRP. Participants in the highest quartile of adiponectin had lower LV ejection fraction, more diastolic dysfunction, greater prevalence of inducible ischemia, and higher NT-proBNP than participants in the lowest quartile.
TABLE 1.
Baseline Characteristics of 981 Participants by Quartile of Adiponectin.
| Quartile of adiponectin |
|||||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| Adiponectin level (µg/mL) | 1.2 – 12.6 | 12.6 – 21.3 | 21.3 – 35.6 | 35.6 – 121 | |
| N in each quartile | 246 | 245 | 245 | 245 | P value |
| Demographic characteristics | |||||
| Age (yr) | 64.0±10.5 | 64.9±10.2 | 67.6±10.9 | 70.5±11.2 | <.0001 |
| Male sex | 219(89%) | 203(83%) | 192(78%) | 186(76%) | 0.001 |
| White race | 125(51%) | 133(54%) | 155(63%) | 178(73%) | <.0001 |
| Current smoking | 49(20%) | 55(23%) | 46(19%) | 43(18%) | 0.52 |
| Medical history | |||||
| Hypertension | 185(75%) | 164(67%) | 175(72%) | 167(68%) | 0.21 |
| Myocardial infarction | 141(58%) | 126(52%) | 127(52%) | 131(53%) | 0.55 |
| Congestive heart failure | 41(17%) | 44(18%) | 42(17%) | 46(19%) | 0.92 |
| Diabetes mellitus | 88(36%) | 71(29%) | 51(21%) | 49(20%) | <.0001 |
| Current medication use | |||||
| Beta-blocker | 169(69%) | 135(55%) | 147(60%) | 112(46%) | <.0001 |
| Aspirin | 212(86%) | 183(75%) | 190(78%) | 174(71%) | 0.0005 |
| Statin | 178(72%) | 150(61%) | 155(63%) | 148(60%) | 0.02 |
| ACE inhibitor or ARB | 139(57%) | 117(48%) | 120(49%) | 129(53%) | 0.2 |
| Metabolic variables | |||||
| Body mass index (kg/m2) | 29.7±5.7 | 29.1±5.2 | 28.3±5.3 | 26.6±4.7 | <.0001 |
| HDL cholesterol (mg/dL) | 39.2±10.2 | 42.5±10.9 | 46.7±12.5 | 54.3±16.8 | <.0001 |
| Non-HDL cholesterol (mg/dL) | 132.4±36.1 | 142.5±49.1 | 129.7±40.2 | 123.2±37.4 | <.0001 |
| Triglycerides (mg/dL) | 179.9±177.8 | 160.5±144.1 | 118.5±69.1 | 105.2±77.6 | <.0001 |
| Serum insulin (pg/mL) | 466.4±502.5 | 397.0±662.9 | 358.4±313.3 | 289.3±247.2 | 0.0003 |
| Fasting glucose (mg/dL) | 127.5±40.0 | 121.2±45.3 | 116.8±38.3 | 114.5±47.1 | 0.004 |
| Glycosylated hemoglobin (%) | 6.1±1.2 | 6.0±1.3 | 5.9±1.1 | 5.9±1.1 | 0.07 |
| eGFR | 79±22 | 79±23 | 75±25 | 71±22 | <.0001 |
| Cardiac function | |||||
| LV ejection fraction (%) | 61.9±9.6 | 62.7±8.3 | 62.2±9.6 | 60.2±10.7 | 0.03 |
| Diastolic dysfunction | 20(9%) | 16(7%) | 28(13%) | 42(20%) | 0.0004 |
| Inducible ischemia | 43(18%) | 52(23%) | 48(22%) | 74(33%) | 0.002 |
| Log C-reactive protein | 0.8±1.3 | 0.6±1.3 | 0.8±1.3 | 0.6±1.4 | 0.08 |
| Log NT-proBNP | 4.8±1.3 | 5.0±1.2 | 5.3±1.3 | 5.9±1.3 | <.0001 |
Values are expressed as mean +/− standard deviation or number ( % within quartile ).
Abbreviations: ACE: angiotensin converting enzyme; ARB: angiotensin receptor blocker; HDL: high-density lipoprotein; eGFR: estimated glomerular filtration rate; LV: left ventricular; NT-proBNP: amino terminal fragment of the prohormone of brain-type natriuretic peptide.
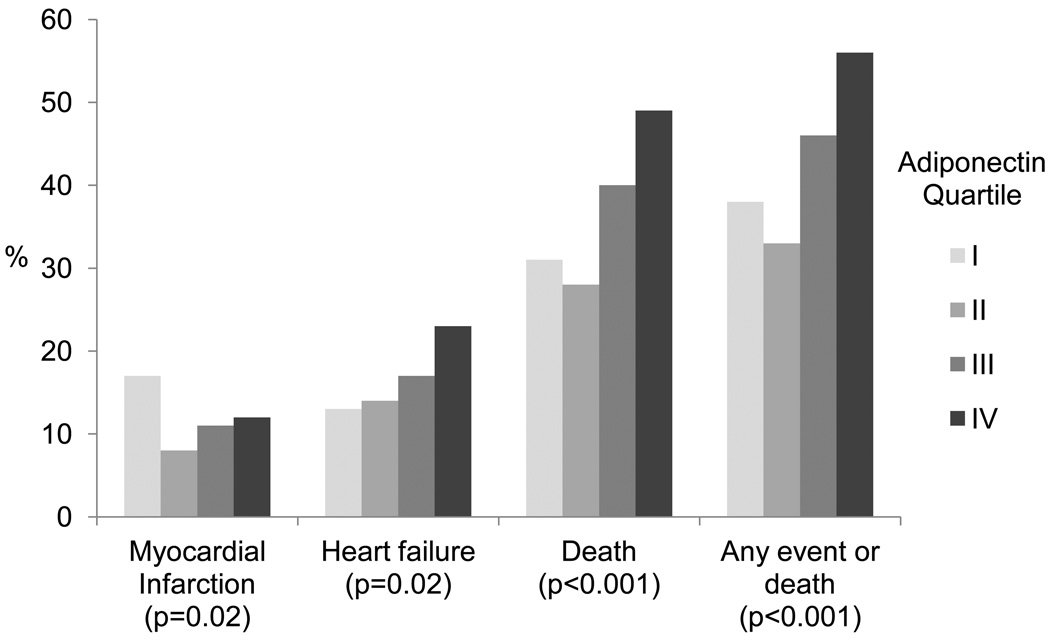
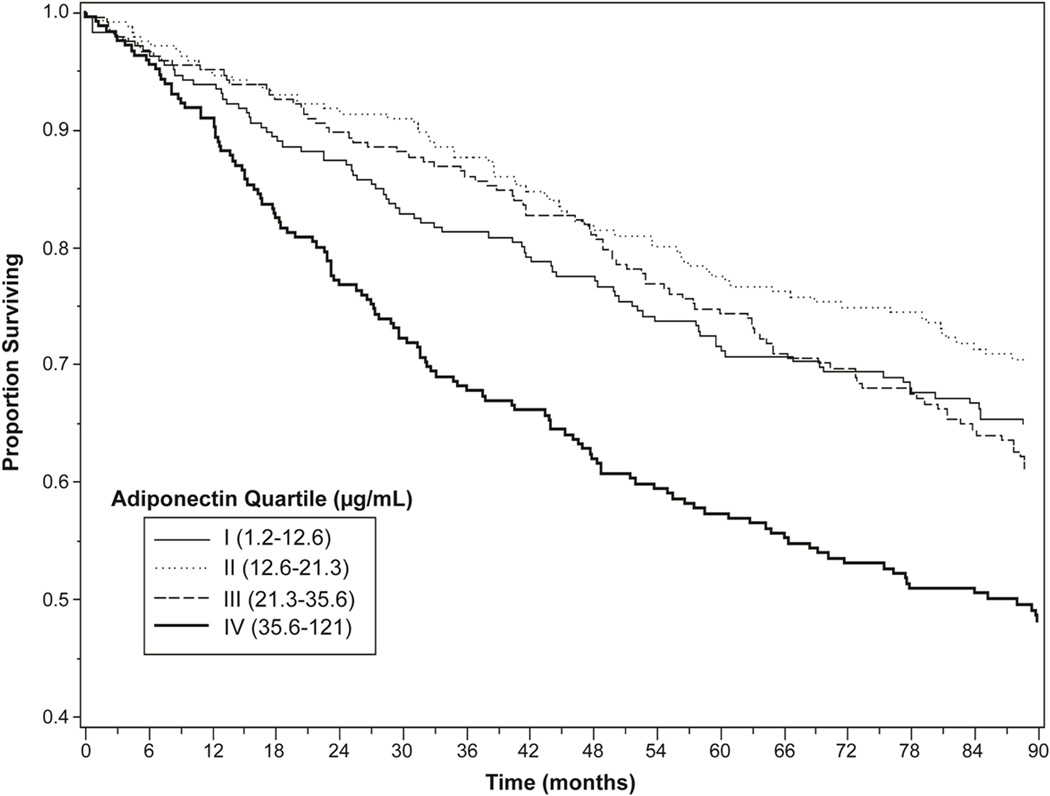
During an average of 7.1 years of follow-up, there were 123 myocardial infarctions, 173 heart failure hospitalizations, and 375 deaths from any cause. The combined outcome of myocardial infarction, heart failure, or death occurred in 440 participants. Participants in the highest quartile of adiponectin were more likely than those in the lowest quartile to be hospitalized for heart failure or die, but less likely to have a myocardial infarction (Figure 1). When evaluated as a combined outcome, participants in the highest quartile of adiponectin were more likely to experience myocardial infarction, heart failure, or death (56% vs. 38%, unadjusted hazard ratio 1.70, 95% confidence interval 1.31–2.21, p <0.0001) (Figure 2). Hospitalization for heart failure occurred in 23% (56/245) of participants with adiponectin levels in the highest quartile vs. 13% (33/246) of those in the lowest quartile (hazard ratio 1.63, 95% confidence interval 1.04–2.56, p=0.03, adjusted for demographics). Similarly, 49% (121/245) of participants in the highest quartile of adiponectin died vs. 31% (76/246) of participants in the lowest quartile (hazard ratio 1.67, 95% CI 1.24–2.26, p < 0.008, adjusted for demographics). However, myocardial infarction occurred in 12% (29/245) of participants with adiponectin levels in the highest quartile vs. 17% (41/246) of those in the lowest quartile (hazard ratio 0.64, 95% confidence interval 0.38–1.06, p=0.08, adjusted for demographics).
Figure 1. Adverse Cardiovascular Outcomes by Quartile of Adiponectin.
Any event or death is defined as myocardial infarction, heart failure, or death.
Figure 2. Survival Free of Cardiovascular Events or Death by Adiponectin Quartile.
Cardiovascular events or death includes myocardial infarction, heart failure, or death from any cause. P<0.0001 for difference between Quartile I and Quartile IV.
To examine the influence of baseline characteristics on differences in outcomes between the highest and lowest quartiles of adiponectin, multivariate Cox proportional hazards models were applied for the outcomes of myocardial infarction, heart failure, all-cause mortality, and the combined outcome of myocardial infarction, heart failure, or death (Table 2). After adjusting for demographics (age, sex, race), clinical risk factors (diabetes, eGFR, beta-blocker use, aspirin use, and statin use), and metabolic markers (BMI, hemoglobin A1c, insulin, glucose, non-HDL cholesterol, HDL cholesterol, and triglycerides), the participants in the highest quartile of adiponectin had higher hazard rates of heart failure (hazard ratio 2.11, 95% confidence interval 1.27–3.51, p = 0.004), death (hazard ratio 2.09, 95% confidence interval 1.50–2.93, p < 0.0001), and any event or death (hazard ratio 1.85, 95% confidence interval 1.36–2.51, p < 0.0001) compared to participants in the lowest quartile of adiponectin. After further adjustment for baseline cardiac disease severity (LV ejection fraction as a continuous variable, diastolic dysfunction, inducible ischemia on stress echocardiography, log CRP, and log NT-proBNP) the association of adiponectin with any event or death was attenuated.
TABLE 2.
Association of Adiponectin Quartile with Adverse Cardiovascular Outcomes After Adjusting for Prognostic Markers.
| HR (95%CI) quartile IV vs. I of adiponectin |
||||||||
|---|---|---|---|---|---|---|---|---|
| Myocardial Infarction |
P value |
Heart failure | P value |
Death | P value |
Any event or death* |
P value |
|
| Model 1a | 0.64 (0.38–1.06) | 0.08 | 1.63 (1.04–2.56) | 0.03 | 1.67 (1.24–2.26) | 0.008 | 1.54 (1.31–2.21) | 0.002 |
| Model 2b | 0.71 (0.43–1.20) | 0.2 | 1.62 (1.02–2.56) | 0.04 | 1.62 (1.19–2.20) | 0.002 | 1.51 (1.14–2.00) | 0.004 |
| Model 3c | 0.82 (0.46–1.46) | 0.51 | 2.11 (1.27–3.51) | 0.004 | 2.09 (1.50–2.93) | <.0001 | 1.85 (1.36–2.51) | <.0001 |
| Model 4d | 0.78 (0.39–1.55) | 0.48 | 0.99 (0.48–2.02) | 0.97 | 1.77 (1.12–2.67) | 0.007 | 1.43 (0.98–2.09) | 0.06 |
Any event or death is defined as myocardial infarction, heart failure, or death.
Model 1 adjusts for Demographics (age, sex, race).
Model 2 adjusts for Model 1 + Clinical Risk Factors (diabetes, eGFR, beta-blocker, aspirin, statin).
Model 3 adjusts for Model 2 + Metabolic Markers (BMI, Hemogloblin A1c, insulin, glucose, non-HDL cholesterol, HDL, triglycerides).
Model 4 adjusts for Model 3 + measures of baseline cardiac disease severity (LV ejection fraction, diastolic dysfunction, inducible ischemia, log CRP, log NT-proBNP).
Abbreviations: eGFR,estimated glomerular filtration rate; BMI, body mass index; HDL, high-density lipoprotein; LV, left ventricular; CRP, C-reactive protein; NT-proBNP, amino terminal fragment of the rohormone of braintype natriuretic peptide.
When serum adiponectin level was analyzed as a continuous variable, each SD increase in log adiponectin (0.8 µg/mL) was associated with an increased rate of the composite of myocardial infarction, heart failure, and death (Table 3). This association persisted after adjustment for demographics, clinical risk factors, and metabolic markers, but was no longer statistically significant after adjustment for measures of baseline cardiac disease severity. We found no evidence that the association between adiponectin and cardiovascular events varied by age, sex, race, BMI, diabetes, serum creatinine, or left ventricular ejection fraction (all P values for interaction > 0.05).
TABLE 3.
Association of Adiponectin with Adverse Cardiovascular Outcomes After Adjusting for Prognostic Markers.
| Log adiponectin per 1-SD increase |
||
|---|---|---|
| HR (95% CI) | P value | |
| Model 1a | 1.20 (1.08–1.33) | 0.0005 |
| Model 2b | 1.18 (1.07–1.31) | 0.001 |
| Model 3c | 1.27 (1.14–1.43) | <.0001 |
| Model 4d | 1.11 (0.96–1.27) | 0.16 |
Adverse cardiovascular outcomes defined as myocardial infarction, heart failure, or death.
Model 1 adjusts for Demographics (age, sex, race).
Model 2 adjusts for Model 1 + Clinical Risk Factors (diabetes, eGFR, beta-blocker, aspirin, statin).
Model 3 adjusts for Model 2 + Metabolic Markers (BMI, Hemogloblin A1c, insulin, glucose, non-HDL cholesterol, HDL, triglycerides).
Model 4 adjusts for Model 3 + measures of baseline cardiac disease severity (LV ejection fraction, diastolic dysfunction, inducible ischemia, log CRP, log NT-proBNP).
Abbreviations: eGFR,estimated glomerular filtration rate; BMI, body mass index; HDL, high-density lipoprotein; LV, left ventricular; CRP, C-reactive protein; NT-proBNP, amino terminal fragment of the prohormone of brain-type natriuretic peptide.
4. Discussion
In a cohort of patients with stable IHD, we found that higher adiponectin concentrations were associated with lower BMI, lower non-HDL cholesterol, higher HDL cholesterol, and less diabetes. Despite this favorable metabolic profile, higher adiponectin concentrations were also associated with worse cardiovascular outcomes, particularly heart failure and death. Remarkably, nearly half of participants with adiponectin levels in the highest quartile died during the 7 years of follow-up. After adjustment for worse baseline cardiac disease severity, the association between higher adiponectin levels and adverse cardiovascular events was no longer statistically significant. These findings provide further evidence for an “adiponectin paradox” in which higher levels of adiponectin may be secreted as a protective or compensatory response to worse cardiovascular disease.
Previous studies in patients with existing cardiovascular disease also found that despite having a more favorable traditional cardiovascular risk profile, patients with higher adiponectin had worse cardiovascular outcomes [17–20]. In a population study including both individuals with and without cardiovascular disease, higher adiponectin was protective in those without cardiovascular disease and predictive of worse outcomes in those with existing disease [17]. Similarly, studies of patients with coronary disease found that higher concentrations of adiponectin were associated with a higher risk of future cardiovascular events [12, 18, 19]. Our study extends these findings to a population of outpatients with stable IHD. In addition, our study provides insight into the potential mechanisms for this association by demonstrating worse baseline cardiovascular disease severity in patients with higher adiponectin concentrations. We found not only that higher adiponectin was associated with worse baseline cardiac dysfunction, but also that the association between adiponectin and heart failure, myocardial infarction, or death was attenuated by adjusting for markers of baseline cardiac dysfunction. This suggests that the association between adiponectin and worse cardiovascular outcomes may be related to worse baseline cardiovascular function in those with higher adiponectin.
Of note, we found that the association between adiponectin and adverse cardiovascular events was limited to heart failure and death and did not extend to myocardial infarction. This is in contrast to a study of subjects with coronary disease by Cavusoglu et al in which adiponectin was associated with greater risk of myocardial infarction [18]. A potential explanation for these findings may be differences in the populations studied. Whereas our study includes only subjects with stable IHD, Cavusoglu et al studied a heterogenous population of subjects referred for cardiac catheterization, with a majority of participants referred for acute coronary syndrome, which may have different effects on adiponectin concentrations than stable IHD. More similar to our population of patients, Maiolino et al studied subjects undergoing cardiac catheterization for chest pain or suspected coronary artery disease with a majority of subjects having stable IHD, and found that adiponectin was not associated with greater risk of myocardial infarction, but was associated with greater risk of cardiovascular mortality [19]. While adiponectin may have a neutral association with future myocardial infarction in patients with stable IHD, its primary relationship with worse cardiovascular outcomes appears to be in the realm of myocardial dysfunction. In past studies of patients with heart failure, higher adiponectin was associated with heart failure and death [10, 20].
The apparent paradox of adiponectin, a protein known to have insulin-sensitizing, anti-inflammatory, and anti-atherogenic properties [1], being associated with worse cardiovascular outcomes has been referred to as “reverse epidemiology” [21] or index event bias [22]. Reverse epidemiology, where the risk factors for a disease identified in a healthy population are unexpectedly inversely associated with outcomes in a population with the disease, has been described in several other diseases, including heart failure [23]. It remains unknown whether the reversal of associations is related to alterations in causal pathways, timing differences amongst multiple risk factors, survival bias, or unknown mechanisms. In recurrence risk research, participants selected on the basis of having had an event will likely have a different distribution and interrelatedness of risk factors than the general population, and the effects of this index event bias can unpredictably influence findings [22].
The mechanisms behind the phenomenon of reverse epidemiology for adiponectin and heart disease are not known. One hypothesis is that while adiponectin protects against the development of disease, once disease is established, adiponectin concentrations are elevated as a counter-regulatory response to try to protect against further inflammation and atherosclerosis [18]. In addition to its vascular effects, adiponectin has been noted to have several direct effects on the heart. On a cellular level, adiponectin has been noted to block apoptosis of cardiac myocytes in in vitro models, including models of hypoxia and reoxygenation injury [1]. On an anatomic level, higher levels of adiponectin have been associated with greater formation of coronary collaterals in patients with at least one occluded coronary artery [24]. Recent evidence suggests that adiponectin may even be released from the heart in patients with heart failure [25].
One potential mechanism for increasing adiponectin levels in patients with greater severity of cardiac disease may be stimulation of adiponectin production by natriuretic peptides [26]. Natriuretic peptides and adiponectin are correlated in patients with heart failure [20]. In vitro, natriuretic peptides enhance the expression and secretion of adiponectin from adipocytes in a dose-dependent fashion [26]. In addition, it has been demonstrated that infusion of atrial natriuretic peptide in patients with heart failure results in increased adiponectin levels compared with a saline infusion control group [26]. Despite the ability of adiponectin to favorably influence traditional cardiac risk factors and directly impact the heart, its compensatory effects may not be sufficient to overcome the burden of existing disease. Indeed, our results support the theory that worse baseline cardiac disease explains part of the association between adiponectin and heart failure. Those with greater disease severity may have greater secretion of adiponectin to attempt to compensate for the effects of IHD, implicating adiponectin as a marker of disease severity.
Another potential explanation for the association of elevated adiponectin with worse outcomes is adiponectin resistance [27]. Animal models support the phenomenon of adiponectin resistance at the level of adiponectin receptor gene expression [28]. In human studies of heart failure patients, compared to normal controls, the heart failure patients exhibit functional adiponectin resistance, with increased adiponectin expression and decreased expression of the predominant adiponectin receptor in skeletal muscle [29]. Van Berendoncks et al [29] found that the relative overexpression of adiponectin and underexpression of the adiponectin receptor in heart failure patients could be reversed with exercise training, which suggests a future area of study for treating adiponectin-associated adverse cardiovascular outcomes.
Several limitations should be considered in the interpretation of these results. Our study population was predominantly male, and sex-specific differences in adiponectin limit generalizability to females [6, 8, 17]. Most participants had IHD documented by history of prior myocardial infarction or revascularization, but 23% of participants were included based on angiogram or positive stress test. Due to the imperfect specificity of stress testing, our study may have included some individuals without significant obstructive coronary artery disease. The adiponectin immunoassay used in this study measured total adiponectin, rather than high-molecular weight adiponectin. In some studies, high-molecular weight adiponectin was shown to be a more predictive marker for cardiovascular events [30]. Additionally, due to the observational nature of the study, we cannot exclude the effects of confounding from variables not represented in our models.
In summary, we found that higher adiponectin concentrations are associated with death and heart failure in a cohort of patients with stable IHD, and that this association is related to greater baseline cardiac disease severity. This contributes to the weight of evidence demonstrating that adiponectin, considered to be protective against incident IHD, is paradoxically associated with worse outcomes in those with existing cardiovascular disease. With the observation that the association between adiponectin and outcomes is related to worse baseline cardiac disease, we provide additional insight into the possibility that adiponectin is elevated as a compensatory response to IHD. Still, the mechanisms by which cardiovascular disease and adiponectin influence each other remain incompletely understood and merit further study.
Acknowledgments
Alexis Beatty is supported by Award Number F32HL110518 from the National Heart, Lung, and Blood Institute. Mary Zhang was supported by a fellowship from the University of California, San Francisco (Medical Student Research Training Program). The Heart and Soul Study was supported by grants from the Department of Veterans Affairs (Epidemiology Merit Review Program), the National Heart, Lung and Blood Institute (R01 HL079235), the Robert Wood Johnson Foundation (Generalist Physician Faculty Scholars Program), the American Federation for Aging Research (Paul Bees on Faculty Scholars in Aging Research Program), the Ischemia Research and Education Foundation, and Nancy Kirwan Heart Research Fund. None of these funding sources had any role in the collection of data, interpretation of results, or preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ouchi N, Shibata R, Walsh K. Cardioprotection by adiponectin. Trends Cardiovasc Med. 2006;16:141–146. doi: 10.1016/j.tcm.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto Y, Hirose H, Saito I, et al. Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci (Lond) 2002;103:137–142. doi: 10.1042/cs1030137. [DOI] [PubMed] [Google Scholar]
- 4.Adamczak M, Wiecek A, Funahashi T, et al. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens. 2003;16:72–75. doi: 10.1016/s0895-7061(02)03197-7. [DOI] [PubMed] [Google Scholar]
- 5.Pischon T, Girman CJ, Hotamisligil GS, et al. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 6.Ai M, Otokozawa S, Asztalos BF, et al. Adiponectin: An independent risk factor for coronary heart disease in men in the Framingham offspring Study. Atherosclerosis. 2011;217:543–548. doi: 10.1016/j.atherosclerosis.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frystyk J, Berne C, Berglund L, et al. Serum adiponectin is a predictor of coronary heart disease: a population-based 10-year follow-up study in elderly men. J Clin Endocrinol Metab. 2007;92:571–576. doi: 10.1210/jc.2006-1067. [DOI] [PubMed] [Google Scholar]
- 8.Lawlor DA, Davey Smith G, Ebrahim S, et al. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677–5683. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 9.Kanaya AM, Wassel Fyr C, Vittinghoff E, et al. Serum adiponectin and coronary heart disease risk in older Black and White Americans. J Clin Endocrinol Metab. 2006;91:5044–5050. doi: 10.1210/jc.2006-0107. [DOI] [PubMed] [Google Scholar]
- 10.Wannamethee SG, Whincup PH, Lennon L, et al. Circulating adiponectin levels and mortality in elderly men with and without cardiovascular disease and heart failure. Arch Intern Med. 2007;167:1510–1517. doi: 10.1001/archinte.167.14.1510. [DOI] [PubMed] [Google Scholar]
- 11.Zhang MH, Spies C, Ali S, et al. Adiponectin and inducible ischemia in patients with stable coronary heart disease: data from the Heart and Soul study. Atherosclerosis. 2009;205:233–238. doi: 10.1016/j.atherosclerosis.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson SR, Sabatine MS, Wiviott SD, et al. Assessment of adiponectin and the risk of recurrent cardiovascular events in patients presenting with an acute coronary syndrome: Observations from the Pravastatin Or atorVastatin Evaluation and Infection Trial-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Am Heart J. 2011;161:1147–1155. doi: 10.1016/j.ahj.2011.02.014. e1141. [DOI] [PubMed] [Google Scholar]
- 13.Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luepker RV. Case Definitions for Acute Coronary Heart Disease in Epidemiology and Clinical Research Studies: A Statement From the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 16.Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 17.Dekker JM, Funahashi T, Nijpels G, et al. Prognostic value of adiponectin for cardiovascular disease and mortality. J Clin Endocrinol Metab. 2008;93:1489–1496. doi: 10.1210/jc.2007-1436. [DOI] [PubMed] [Google Scholar]
- 18.Cavusoglu E, Ruwende C, Chopra V, et al. Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J. 2006;27:2300–2309. doi: 10.1093/eurheartj/ehl153. [DOI] [PubMed] [Google Scholar]
- 19.Maiolino G, Cesari M, Sticchi D, et al. Plasma adiponectin for prediction of cardiovascular events and mortality in high-risk patients. J Clin Endocrinol Metab. 2008;93:3333–3340. doi: 10.1210/jc.2007-2405. [DOI] [PubMed] [Google Scholar]
- 20.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 21.Rathmann W, Herder C. Adiponectin and cardiovascular mortality: evidence for "reverse epidemiology". Horm Metab Res. 2007;39:1–2. doi: 10.1055/s-2007-958630. [DOI] [PubMed] [Google Scholar]
- 22.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA. 2011;305:822–823. doi: 10.1001/jama.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalantar-Zadeh K, Block G, Horwich T, et al. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–1444. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 24.Soydinc S, Davutoglu V, Sari I. High serum levels of adiponectin improve coronary collateral development in patients with coronary artery disease. Tohoku J Exp Med. 2007;211:347–352. doi: 10.1620/tjem.211.347. [DOI] [PubMed] [Google Scholar]
- 25.Takano H, Obata JE, Kodama Y, et al. Adiponectin is released from the heart in patients with heart failure. Int J Cardiol. 2009;132:221–226. doi: 10.1016/j.ijcard.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 26.Tsukamoto O, Fujita M, Kato M, et al. Natriuretic peptides enhance the production of adiponectin in human adipocytes and in patients with chronic heart failure. J Am Coll Cardiol. 2009;53:2070–2077. doi: 10.1016/j.jacc.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 27.Kintscher U. Does adiponectin resistance exist in chronic heart failure? Eur Heart J. 2007;28:1676–1677. doi: 10.1093/eurheartj/ehm233. [DOI] [PubMed] [Google Scholar]
- 28.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 29.Van Berendoncks AM, Garnier A, Beckers P, et al. Exercise training reverses adiponectin resistance in skeletal muscle of patients with chronic heart failure. Heart. 2011;97:1403–1409. doi: 10.1136/hrt.2011.226373. [DOI] [PubMed] [Google Scholar]
- 30.von Eynatten M, Humpert PM, Bluemm A, et al. High-molecular weight adiponectin is independently associated with the extent of coronary artery disease in men. Atherosclerosis. 2008;199:123–128. doi: 10.1016/j.atherosclerosis.2007.10.002. [DOI] [PubMed] [Google Scholar]