Abstract
We assessed the relationship of insulin resistance with cognitive decline and brain atrophy over two years in early Alzheimer’s disease (AD, n=48) and nondemented controls (n=61). Intravenous glucose tolerance tests were conducted at baseline to determine insulin area-under-the-curve (AUC). A standard battery of cognitive tasks and MRI were conducted at baseline and 2-year follow-up. In nondemented controls, higher baseline insulin AUC was associated with 2-year decline in global cognitive performance (beta=−0.36, p=0.005). In early AD, however, higher insulin AUC was associated with less decline in global cognitive performance (beta=0.26, p=0.06), slower global brain atrophy (beta=0.40, p=0.01) and less regional atrophy in the bilateral hippocampi and cingulate cortices. While insulin resistance is associated with cognitive decline in nondemented aging, higher peripheral insulin may have AD-specific benefits or insulin signaling may be affected by systemic physiologic changes associated with AD.
1. Introduction
While insulin is best-known for its peripheral role in stimulating cellular glucose uptake, its neuromodulatory role in the CNS is increasingly recognized. Insulin receptors are expressed in all regions of the brain, with a high density of receptors in the hippocampus, entorhinal cortex, and olfactory bulb.[1, 2] CNS insulin has a minor role in promoting glucose uptake in glial cells and specific neural circuits, including the hippocampal circuit.[3] An extensive amount of data suggests the cascade of signals triggered by the activated insulin receptor promotes synaptogenesis and synaptic remodeling.[4, 5] Insulin signaling also influences neuronal survival by protecting against apoptosis, hypoxic stress, and nitric oxide toxicity.[6–8]
While insulin is an important neurotrophic and neuroprotective factor in the context of an intact insulin signaling system, dysfunction in the insulin signaling cascade, such as in diabetes mellitus, is associated with negative health outcomes including dementia and Alzheimer’s Disease (AD). Epidemiologic studies of older adults have found that type 2 diabetes is associated with an increased risk of subsequent development of cognitive decline, dementia and AD.[9, 10] The risks are not confined to those with overt diabetes: individuals with prediabetic states of insulin resistance,[11] the metabolic syndrome [11, 12], and hyperinsulinemia [13, 14] are also at an increased risk of developing dementia. Dysfunctional insulin signaling is also associated with structural brain changes including whole-brain[15] and medial temporal atrophy[16].
In addition to broad epidemiological associations, insulin signaling abnormalities are observed in AD and mechanistically related to AD neuropathology. Individuals with AD have lower levels of insulin in the cerebrospinal fluid[17] and autopsy studies have demonstrated fewer insulin receptors and downstream signaling activity in the AD brain compared to normal controls.[18] Through its actions on GSK3α and GSK3β, insulin influences the metabolism of beta-amyloid peptides[19] and the phosporylation of Tau.[20, 21] Clinical data in humans suggests that insulin may enhance clearance of Aβ from the CNS.[22–24] Accumulating experimental data suggest that Aβ triggers downregulation of insulin receptors in neurons, an effect that is reversed by insulin, suggesting a protective role of insulin in AD pathogenesis.[25] Exogenously administered insulin has also been shown to improve cognitive performance in AD.[26–29] The cognitive benefits of insulin and its apparent influence on AD-related neuropathology has driven research into new therapeutic approaches, such as the use of insulin sensitizers to enhance insulin signaling[30] and the administration of intranasal insulin to raise insulin levels in the CNS.[31]
As AD and type 2 diabetes mellitus are two of the most common diseases in older adults it is increasingly important to more precisely define the role of insulin in brain aging and AD. We previously examined the relationship of insulin response with cognitive and imaging data in a cross-sectional study of non-diabetic individuals with AD. Contrary to our original hypothesis, higher insulin levels were associated with higher brain volumes, better global cognitive performance, and lower dementia severity in the AD cohort.[32] Our data[32][32][32][32] and others [33–36] suggest a relationship reversal in which insulin-mediated physiology may impact the AD brain differently than the non-AD brain, or alternatively that AD affects peripheral and perhaps central insulin-mediated physiology. Understanding the specific role of insulin in AD may benefit therapeutic strategies and biomarker development. Thus, we performed a 2-year longitudinal, observational study of 109 non-diabetic individuals with and without AD to assess the relationship of insulin-mediated glucoregulation with cognitive and imaging markers reflective of AD progression. We were particularly interested in assessing if a differential relationship existed across the AD and nondemented controls given our prior findings suggesting that insulin was associated with brain volume and cognition in AD but not nondemented controls.
2. Methods
2.1 Sample and Recruitment
Data used in these analyses came from participants enrolled in the longitudinal Brain Aging Project at the University of Kansas Alzheimer and Memory Program who had baseline intravenous glucose tolerance tests and longitudinal cognitive evaluations (baseline and 2-years later). Nondemented older controls (n=61) were without evidence of functional cognitive decline (Clinical Dementia Rating [CDR] 0) at both time points. AD participants met criteria for AD[37] in the very mild (CDR 0.5, n=40) or mild (CDR 1, n=8) stages at baseline and demonstrated persistent cognitive and functional impairments 2 years later (CDR >or=0.5). Informed consent was obtained on all participants prior to enrollment into the study.
AD participants were recruited largely by media appeals and from a referral-based memory clinic. All nondemented participants were recruited by self-referral in response to media coverage and word of mouth. Study exclusions at baseline are previously described[32] and include diabetes mellitus (clinical diagnosis, use of an anti-diabetic agent, or 2-hour post-load serum glucose >199) and unstable ischemic heart disease within the last 2 years.
2.2 Clinical Assessment
The clinical assessment was conducted at baseline and two years later, as previously described.[32] Dementia severity was determined using the CDR[38] and change in the CDR sum of box score was used as a measure of progression in dementia severity. A trained psychometrician administered a standard psychometric battery[32] that included the Wechsler Memory Scale [WMS]–Revised Logical Memory I and II, Free and Cued Selective Reminding Task, Boston Naming Test (15 item), WMS III Digit Span Forwards and Backwards, Wechsler Adult Intelligence Scale [WAIS] letter–number sequencing, Trailmaking A and B, Verbal Fluency (animals, fruits, and vegetables), Stroop Color-Word Test, and WAIS Block Design. Cognitive performance scores were converted to z-scores based on the mean and standard deviation of 82 nondemented participants and the overall mean served as a composite measure of global cognitive performance. Cognitive decline was defined as change in the global cognition composite from baseline to 2-year follow-up evaluation.
2.3 Intravenous Glucose Tolerance Test
A 14-sample intravenous glucose tolerance test (IVGTT) was performed at 8:30AM after a 12-hour overnight fast. An intravenous glucose bolus of 0.3 g/kg body weight was delivered and blood samples were drawn over three hours.[32] The total areas-under-the-curve (AUC) for insulin and glucose were determined by the trapezoidal rule. Glucose and insulin AUCs served as the primary measures and secondary measures included fasting and two-hour post-load insulin and glucose. No participants met criteria for the diagnosis of diabetes at baseline (two-hour post-load glucose level ≥200 mg/DL). Serum insulin was measured by radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX) with a sensitivity of 1.3mU/l.
2.4 Neuroimaging
Structural MRI was obtained using a Siemens 3.0 Tesla Allegra scanner. T1-weighted anatomic images were acquired to provide detailed gross anatomy with high gray-white matter contrast (MP-RAGE; 1×1×1mm3 voxels; TR=2500ms, TE=4.38ms, TI=1100ms, FOV 256×256mm2 with 18% oversample, flip angle=8 degrees). Fluid attenuated inversion recovery (FLAIR) images were used for white matter lesion assessments (Ti=2500, TR=10,000, TE=81.0, flip angle=180 degrees, slice thickness=4mm with 0 gap). Baseline MRI was missing for 5 nondemented and 3 AD participants (refused, unable, or inadequate imaging data) and follow-up MRI data was missing on 4 nondemented and 6 AD participants; thus, longitudinal imaging analyses were performed on 52 nondemented and 39 AD participants. Participants without complete imaging data were not different from their overall cohort in key measures of age, education, global cognitive performance, insulin and glucose measures with one exception: the 9 nondemented participants were slightly more educated than the overall nondemented cohort (18.6 years vs 16.3 years, p=0.03).
Baseline brain volumes were determined using the VBM5 toolbox in MATLAB 7.1 (MathWorks, Natick, MA). Segmentation volumes for total gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) were used to calculate baseline normalized whole-brain volume (% total intracranial volume). Global whole brain atrophy over 2-years was assessed using Structural Image Evaluation Normalization of Atrophy (SIENA)[39] which assesses local shifts in brain edges to estimate percent of brain volume change (PBVC), where negative numbers represent atrophy.
Longitudinal regional voxel-based analyses were performed using SPM8 algorithms (Wellcome Department of Cognitive Neurology, London) in MATLAB 7.2 on Linux. First, the two-year follow-up MRI was rigidly registered onto the subject’s baseline image followed by high dimensional deformation warp resulting in a field map of change (determinant of the Jacobian matrix) at each voxel. The baseline gray matter segment and Jacobian determinants were multiplied voxel-by-voxel to form a map of gray matter change. Next, Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL)[40] was used to create a customized template of the atrophy maps through a nonlinear image registration procedure iteratively matching the images to a template generated from their own mean. This DARTEL template and flow fields from the template creation were normalized to Montreal Neurological Institute (MNI) space. The flow fields and parameters from this normalization step were applied to gray matter atrophy maps. To preserve the original tissue volumes, the normalized gray matter atrophy images were modulated and smoothed with an 8mm isotropic Gaussian kernal to accommodate inexact spatial normalization.
The normalized, modulated, and smoothed gray matter change maps for each individual were then analyzed in SPM8. A General Linear Model (GLM) multiple regression analysis was used to examine linear relationships of insulin AUC and longitudinal regional gray matter atrophy within diagnosis groups, including age and gender as confounding variables. Voxels are reported with reference to MNI space. For all analyses, results were considered significant at p<0.001 uncorrected for multiple comparisons, with clusters exceeding an extent threshold of 100 voxels and Z>3.0.
2.5 Covariates
We examined several important covariates that may potentially mediate a relationship of insulin with brain atrophy or cognitive decline including Apolipoprotein E4 carrier status, history of hypertension (reported medical history of hypertension requiring treatment), and body mass index (BMI; kg body weight/height in meters2), and physical activity level using the Physical Activity Scale in the Elderly. Additionally, white matter lesions were manually traced on baseline FLAIR images as a measure of vascular related brain injury using Medical Image Processing, Analysis, and Visualization (Johns Hopkins University) by a single trained rater and expressed as volume in mm3. Reliability of white matter assessments were assessed with 10 blinded repeat assessments inserted randomly into the rater’s workflow. Intra-rater reliability was excellent (Intraclass Correlation Coefficient=0.995). Baseline cognitive performance was used as an additional covariate for analyses assessing 2-year rates of cognitive decline. Baseline brain volume was used as an additional covariate for analyses assessing 2-year rates of brain atrophy.
2.6 Statistical Analyses
Analyses were conducted using SPSS 17.0 (SPSS Inc., Chicago, IL). Continuous variables were summarized by means and standard deviations while categorical variables were summarized by frequency and percent. Student’s t-test was used to compare continuous demographic and imaging variables in early AD and nondemented groups. A chi square test was used to compare categorical variables.
Linear regression was used to assess the relationship of insulin and glucose values with two-year rates of cognitive decline and whole brain atrophy. All analyses were conducted within diagnostic groups (early AD and nondemented subjects separately) as opposed to the entire cohort combined. All imaging analyses were adjusted for age and sex to control for their known influence on brain size and atrophy rates. All cognitive analyses were controlled for age, sex, and education given each variables known influence on cognitive performance. In secondary analyses, we also assessed the role of additional potential covariates by adding them singly to the regression model. We did not fully-adjust for all potential covariates given the large number of covariates and relatively small sample size. These additional covariates included education, hypertension history, APOE4 carrier status, physical activity, BMI, white matter lesion burden, baseline brain volume (atrophy analyses), and baseline cognitive performance (cognitive performance analyses). Univariate general linear model was used to examine for the presence of group (AD vs. nondemented) × insulin/glucose values in predicting cognitive decline and brain atrophy.
3. Results
3.1 Sample Characteristics (Table 1)
Table 1.
Sample Characteristics
| Nondemented (n=61) | Alzheimer’s Disease (n=48) | P Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age, years | 73.3 (6.7) | 75.2 (6.3) | 0.13 |
| Mean Follow Up, years | 2.0 (0.3) | 2.0 (0.3) | 0.89 |
| Education, years | 16.6 (2.8) | 15.5 (2.9) | 0.04 |
| Female – % (n) | 54.1 (33) | 56.3 (27) | 0.82 |
| ApoE4 Carrier, % (n) | 29.5 (18) | 56.3 (27) | 0.01 |
| Body Mass Index | 26.4 (3.9) | 26.1 (3.9) | 0.75 |
| Hypertension, % (n) | 23.0 (14) | 43.8 (21) | 0.02 |
| Baseline MMSE | 29.5 (0.8) | 26.1 (3.1) | <0.001 |
| MMSE 2-Year Change | −0.3 (1.1) | −3.3 (5.3) | <0.001 |
| Baseline Global Cognition – z score | 0.15 (0.49) | −1.50 (0.93) | <0.001 |
| Global Cognition 2-Year Change | −0.04 (0.34) | −0.59 (0.64) | <0.001 |
| CDR Sum of Boxes | 0.0 (0.1) | 2.9 (1.2) | <0.001 |
| CDR Sum of Boxes Change | 0.0 (0.1) | 2.1 (3.2) | <0.001 |
| Insulin (3-hour AUC) | 2403 (1273) | 2910 (1609) | 0.07 |
| Fasting Insulin (uU/mL) | 7.2 (4.6) | 8.4 (5.4) | 0.19 |
| 2-hour Insulin (uU/mL) | 7.5 (4.4) | 10.4 (7.8) | 0.03 |
| Glucose (3-hour AUC) | 20808 (5516) | 22911 (3803) | 0.03 |
| Fasting Glucose (mg/dL) | 97.7 (9.0) | 100.4 (12.6) | 0.19 |
| 2-hour Glucose (mg/dL) | 92.8 (12.4) | 96.8 (19.0) | 0.19 |
| Baseline Brain Volume (%TICV) | 70.3 (3.3) | 67.0 (3.4) | <0.001 |
| Brain Atrophy (% Brain Volume Loss) | −0.89 (1.22) | −2.80 (2.42) | <0.001 |
| White Matter Lesion Burden (mm3) | 11943 (14230) | 12871 (15105) | 0.76 |
All data are means (SD) unless otherwise noted. ApoE4: Apolipoprotein E4 allele. AUC: area under the curve. TICV: total intracranial volume.
The AD and nondemented groups were comparable in age, education, and sex distribution. The nondemented cohort had minimal cognitive change over two years with a mean change of −0.05 standard deviations (range +0.7 to −1.4) on the global cognitive index (composite of 12 standard tests). Individuals with AD had lower baseline cognitive performance and brain volumes and demonstrated greater rates of cognitive decline and brain atrophy than the nondemented control group. Modest elevations or trends were observed for glucose and insulin values in AD compared to nondemented participants. At 2-year follow up, three AD and one nondemented participant had developed diabetes and were treated with oral agents. Excluding these participants from the analyses did not alter the following results (data not shown).
3.2 Nondemented Aging Cohort
3.2.1 Cognitive Decline (Table 2)
Table 2.
Relationship of Insulin and Glucose Measures with 2-year Rates of Cognitive Decline and Brain Atrophy
| Nondemented | Alzheimer’s Disease | |||
|---|---|---|---|---|
| Cognitive Decline | Brain Atrophy | Cognitive Decline | Brain Atrophy | |
| Insulin AUC | −0.36** | −0.04 | 0.26 (p=0.06) | 0.40* |
| Fasting Insulin | −0.28* | −0.05 | 0.16 | 0.36* |
| 2hr Insulin | −0.41** | −0.05 | 0.35* | 0.47** |
| Glucose AUC | −0.18 | 0.08 | 0.31* | 0.35* |
| Fasting Glucose | −0.26* | −0.03 | 0.21 | 0.24 |
| 2hr Glucose | −0.25* | −0.03 | 0.19 | 0.20 |
Data represent standardized betas describing the relationship between glucose and insulin variables (predictor variable) with 2-year rates of cognitive decline and brain atrophy (dependent variable) in nondemented and early AD cohorts. Cognitive analyses are controlled for age, sex, and education. Brain atrophy analyses are controlled for age and sex.
p<0.05
p<0.01
In nondemented participants, insulin AUC was associated with decline in global cognition when controlling for age, sex, and education (beta=−0.35, p=0.006) with greater rates of global cognitive decline associated with higher insulin AUC levels (Figure 1). Controlling for additional individual covariates of interest (baseline cognitive performance, hypertension history, APOE4 carrier status, physical activity, BMI, white matter lesion burden) did not change the results. The secondary insulin response variables of fasting insulin, 2-hour insulin, fasting glucose, and 2-hour glucose levels were also inversely associated with cognitive decline.
Figure 1. Relationship of Insulin and Cognitive Decline in Nondemented Controls.
Insulin area under the curve over a three-hour intravenous glucose tolerance test is associated with cognitive decline over two years in older adults without dementia, with increasing insulin levels associated with greater decline in cognition. Cognitive measures are corrected for age, sex, and education and presented in the figure as standardized values.
In individual cognitive tasks, insulin AUC was associated with decline in memory performance (Selective Reminding Task; beta=−0.31, p=0.02), Trailmaking A task (beta=−0.26, p=0.05), and a trend to greater decline in verbal fluency (beta=−0.22, p=0.08).
3.2.2 Brain Atrophy
Insulin AUC was not associated with brain atrophy (beta=−0.04, p=0.76). In regional atrophy analyses, insulin was not related to positive or negative change in brain volume.
3.3 Alzheimer’s Disease Cohort
3.3.1 Cognitive Decline
In the AD cohort, a trend was observed relating insulin AUC with cognitive decline (beta=0.26, p=0.06) controlling for age, sex, and education, with higher insulin AUC associated with less decline over two years. This relationship of insulin with cognitive decline was significantly different from that observed in nondemented individuals (group × insulin interaction p=0.007). In analyses of secondary variables of insulin response, 2-hour insulin (beta=0.35, p=0.01), and glucose AUC (beta=0.31, p=0.04; group × Glucose AUC interaction, p<0.001) were also associated with cognitive decline.
In individual cognitive tasks, insulin AUC was only related to 2-year change in verbal fluency (beta=0.29, p=0.05).
3.3.2 Brain Atrophy
In the AD group, insulin AUC was associated with brain atrophy when controlling for age and sex (beta=0.39, p=0.01), with higher insulin AUC associated with less brain atrophy (figure 2 and table 2). Controlling for additional individual covariates of interest (education, baseline brain volume, hypertension history, ApoE4 carrier status, physical activity, BMI, white matter lesion burden) did not attenuate this relationship. A significant group × insulin AUC interaction was present in predicting brain atrophy (p=0.02) demonstrating the relationship between insulin and brain atrophy was different across AD and nondemented groups. Secondary measures of fasting insulin, 2-hour insulin, and glucose AUC were similarly related to brain atrophy (table 2).
Figure 2. Relationship of Insulin and Whole Brain Atrophy in Early Alzheimer’s Disease.
Insulin area-under-the-curve in response to a three hour intravenous glucose tolerance test is associated with brain atrophy in individuals with early Alzheimer’s disease, with increasing insulin levels associated with less brain atrophy. Atrophy measures are corrected for age and sex and presented in the figure as standardized values (thus, positive values do not represent increased brain volume over two years). Brain volume declined in 37 AD participants, was unchanged in one, and increased in one (0.2%) over the two year study.
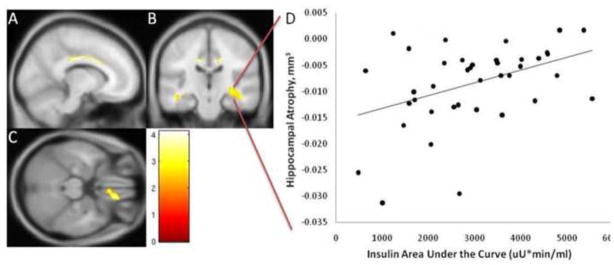
In regional brain atrophy analyses, higher insulin AUC was associated with less atrophy in limbic regions, including the bilateral hippocampi and cingulate gyri, left middle temporal gyrus, right parahippocampal gyrus and right orbitofrontal gyrus. Higher insulin AUC was associated with greater atrophy of the right inferior frontal cortex, inferior parietal, superior and temporal cortices and the putamen (Table 3).
Table 3.
Areas of gray matter volume change in patients with Alzheimer’s Disease and Nondemented Elderly correlated with Higher and Lower Insulin AUC over the follow-up period
| Peak | k | Z | P | ||||
|---|---|---|---|---|---|---|---|
| AUCi | Region | Coordinate (mm) | Value | ||||
| Pos | Talairach | x | y | z | uncorrected | ||
| R Cingulate Gyrus | 15 | −3 | 38 | 680 | 3.92 | <.001 | |
| L Cingulate Gyrus | −11 | −14 | 35 | 809 | 3.67 | <.001 | |
| L Middle Temporal Gyrus/Hippocampus | −46 | −20 | −16 | 224 | 3.48 | <.001 | |
| R Orbitofrontal Gyrus | 13 | 36 | −24 | 683 | 3.29 | 0.001 | |
| R Hippocampus | −24 | −14 | −13 | 394 | 3.28 | 0.001 | |
| R Hippocampus/Parahippocampal Gyrus | −44 | −15 | −17 | 1113 | 3.25 | 0.001 | |
| Neg | L Inferior Frontal Gyrus | −50 | 30 | 10 | 372 | 4.09 | <.001 |
| L Superior Temporal Gyrus | −53 | 12 | −4 | 183 | 3.62 | <.001 | |
| L Inferior Parietal Lobe | −41 | −47 | 40 | 201 | 3.61 | <.001 | |
| R Superior Temporal Pole | 65 | 8 | −6 | 315 | 3.50 | <.001 | |
| R Putamen | 29 | 10 | −3 | 141 | 3.33 | <.001 | |
4. Discussion
Our findings suggest important differences in the relationship of peripheral insulin levels with cognitive changes and brain atrophy in AD and normal aging. In cognitively normal individuals without diabetes, higher insulin levels in response to a glucose challenge were associated with increased rates of age-related cognitive decline. In contrast, there is a distinct reversal of this relationship in individuals with early AD, where higher insulin and glucose levels were associated with slower cognitive decline. This finding was strengthened by our longitudinal imaging data which demonstrated higher insulin and glucose levels in the AD cohort were associated with lower rates of whole brain atrophy and regional atrophy in areas affected by AD neuropathology such as the hippocampus and cingulate cortices. These group-specific relationships suggest insulin signaling may affect brain health in a disease-specific manner or that insulin signaling may be affected by changes in AD brain physiology.
Our findings in older adults without dementia are consistent with several studies suggesting that insulin resistance has detrimental long-term cognitive effects.[11, 13, 14] Our cognitively normal cohort was carefully screened for the presence of dementia and mild cognitive impairment twice over two years by a clinician using a standard and validated clinical evaluation sensitive to detecting the earliest clinical changes of AD.[38] Glucose and insulin levels were inversely associated with age-related cognitive change over two years, with higher insulin and glucose levels associated with greater cognitive decline even when controlling for important covariates including age, sex, hypertension, physical activity level, and BMI. These findings are in line with prior studies suggesting that insulin resistance, even in nondiabetics, is associated with cognitive impairment and risk of cognitive decline[41] and suggest that even mild variations along a continuum of insulin resistance may be cognitively relevant in older adults without dementia. Previously hypothesized mechanisms through which insulin resistance may influence cognition include vascular injury, brain insulin resistance, glucose toxicity, and advanced glycation end products.[42]
The negative association between higher insulin levels and cognitive change, however, was not observed in our AD cohort. In contrast, we observed that higher levels of insulin and glucose were associated with slower cognitive decline over two years in the AD cohort. This finding is consistent with our cross-sectional data from a subset of individuals included in this longitudinal study.[32] This finding may also be relevant to two clinical studies suggesting AD subjects with type II diabetes, which is often accompanied by hyperinsulinemia, had lower rates of cognitive decline than non-diabetic AD subjects.[33, 34] Notably, our observation was present despite the absence of anti-diabetic therapies, a mechanism previously hypothesized to explain the unexpected slower clinical decline observed in diabetics with AD.[33] Moreover, our longitudinal neuroimaging data lends additional convergent evidence to these clinical findings. We found that higher insulin levels in AD were associated with less whole brain volume loss over the two-year study. We also used an optimized voxel-based 3-dimensional longitudinal analysis method and found less atrophy in relation to higher insulin levels in regions prominently affected by AD neuropathology, including the hippocampus and cingulate cortices. Our imaging results suggest lower AD neuropathological burden could exist in those with higher insulin levels and converge with autopsy data demonstrating lower AD-related pathology in diabetics compared to nondiabetics.[35, 36]
The mechanisms underlying these relationships in our early AD cohort remain unclear but emerging data suggests an important and beneficial role for insulin in neurodegenerative disease. Insulin is a neuroprotective[21] and neurotrophic[43] factor that protects neurons from amyloid-beta related toxicity.[25] Individuals with AD have lower insulin levels in the cerebrospinal fluid[17] and reduced insulin signaling in the brain[18] compared to those without dementia. Early results of clinical trials designed to increase CNS levels of insulin suggest intranasal insulin has memory benefits and alters peripheral amyloid levels.[26] It is thus possible that increased peripheral insulin levels commonly observed in AD, and in our AD cohort, may protect against or compensate for low CNS insulin or aberrant CNS insulin signaling.
It is important to consider the increasingly recognized heterogeneity in the underlying neuropathological substrate responsible for the AD dementia syndrome, in particular the role of vascular injury. For instance, individuals with the AD clinical syndrome and diabetes have more extensive vascular pathology than those with AD alone.[36, 44] Thus, it is feasible that our cohort of AD participants with higher peripheral insulin levels may also have more heterogeneous underlying pathologies (i.e., AD and cerebrovascular disease). This possibility is relevant to our findings as AD with diabetes may be associated with slower clinical decline than those with pure AD[45] while cerebrovascular disease influences the earlier clinical expression of AD at lower levels of neuropathological burden[46, 47] Nevertheless, we attempted to account for the potential confounding influence of vascular disease by excluding individuals with a history of stroke or coronary artery disease and controlling for a measure of vascular burden (white matter lesion burden) reducing the likelihood of this possibility.
While our data could indicate a role for insulin signaling in the development and progression of AD, the possibility of “reverse causation” should be considered. Amyloid deposition has been reported in the brain and pancreas in AD[48] and reduced insulin secretion is associated with increased AD risk.[49] Additionally, AD-related pathological brain changes occur in regions regulating metabolism and appetite[50] that may result in systemic alterations in insulin levels. AD pathological changes are associated with declining body mass index and weight loss years prior to the clinical onset of AD symptoms.[51] Thus, systemic metabolic changes may be the result of AD brain changes.
As the participants in this study were without diabetes or clinical evidence of significant cerebrovascular or coronary artery disease the results may not be generalizable to a broader population. The lack of diabetics or individuals with more severe insulin resistance does not allow us to directly test for the presence of a U-shaped risk curve as suggested by prior studies linking both low and high insulin levels with cognitive outcomes.[49, 52] Additionally, participants with AD were in the earliest clinical stages of the disease, with most (83%) being in the very mild (CDR 0.5) stage. Many of these participants would meet criteria for MCI, although our methods are accurate in identifying individuals in the earliest clinical stages of AD[53] and our cohort progressively declined over two years on clinical, cognitive, and imaging measures as expected for an early AD cohort. While insulin and glucose were associated with cognitive decline in cognitively normal individuals, there was no association with brain atrophy. This may be related to the reduced variance of brain atrophy in normal aging compared to that observed in AD thus reducing our power to resolve a possible association.
In summary, measures of insulin resistance were associated with cognitive change over two years in our control cohort, as expected, but not in those with AD. In fact, an elevated insulin response was associated with better long term cognitive and brain structural outcomes over two years in individuals with AD. This relationship reversal suggests insulin-mediated physiology may impact the AD brain differently than the non-AD brain, or that AD affects peripheral and perhaps central insulin-mediated physiology. Understanding the specific role of insulin and insulin signaling in the AD brain may benefit AD therapeutic development efforts or contribute to biomarker development and help define an AD-specific peripheral phenotype.
Figure 3. Insulin and Regional Brain Volume in Early Alzheimer’s Disease.
Insulin area-under-the-curve was related to regional atrophy in limbic regions (identified by yellow, reflecting Z scores > 3.25, k>100, p<.001 uncorrected). Results are displayed overlaid on the A) sagittal, B) coronal, and C) axial views of a standard, spatially normalized MRI. The relationship between insulin and regional atrophy in the cingulate cortex is visible in A and B, the medial temporal cortex in B, and the orbitofrontal cortex in C. The anatomical location and description of brain regions is in Table 3. D) The scatterplot demonstrates the relationship of insulin and gray matter volume atrophy in the largest cluster (right hippocampus/parahippocampal gyrus) in individuals with early AD.
Highlights.
Peripheral insulin, cognitive change, and brain atrophy in aging and Alzheimer’s.
Higher insulin in aging is associated with greater 2-year decline in cognition.
Higher insulin in AD is associated with less cognitive decline and brain atrophy.
Acknowledgments
This study was supported by grants R03 AG026374 and R21 AG029615 from the National Institutes of Aging and K23NS058252 from the National Institute on Neurological Disorders and Stroke. The University of Kansas General Clinical Research Center (M01RR023940) provided essential space, expertise, and nursing support. The Hoglund Brain Imaging Center is supported by grant C76 HF00201 and generous support from Forrest & Sally Hoglund.
Footnotes
None of the authors has any financial conflict of interests to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Unger JW, Livingston JN, Moss AM. Insulin receptors in the central nervous system: Localization, signalling mechanisms and functional aspects. Progress in Neurobiology. 1991;36:343–362. doi: 10.1016/0301-0082(91)90015-s. [DOI] [PubMed] [Google Scholar]
- 2.Adamo M, Raizada MK, LeRoith D. Insulin and insulin-like growth factor receptors in the nervous system. Molecular Neurobiology. 1989;3:71–100. doi: 10.1007/BF02935589. [DOI] [PubMed] [Google Scholar]
- 3.van der Heide LP, Ramakers GMJ, Smidt MP. Insulin signaling in the central nervous system: Learning to survive. Progress in Neurobiology. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Chiu SL, Chen CM, Cline HT. Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron. 2008;58:708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao WQ, Townsend M. Insulin resistance and amyloidogenesis as common molecular foundation for type 2 diabetes and Alzheimer’s disease. Biochim Biophys Acta. 2009;1792:482–496. doi: 10.1016/j.bbadis.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzaki TMH, Mitsuda N, Namikawa K, Kiyama H, Miyake S, Tohyama M. Activation of Akt Kinase Inhibits Apoptosis and Changes in Bcl-2 and Bax Expression Induced by Nitric Oxide in Primary Hippocampal Neurons. Journal of Neurochemistry. 1999;73:2037–2046. [PubMed] [Google Scholar]
- 7.Yamaguchi A, Tamatani M, Matsuzaki H, Namikawa K, Kiyama H, Vitek MP, Mitsuda N, Tohyama M. Akt Activation Protects Hippocampal Neurons from Apoptosis by Inhibiting Transcriptional Activity of p53. 2001;276:5256–5264. doi: 10.1074/jbc.M008552200. [DOI] [PubMed] [Google Scholar]
- 8.Philpott KL, McCarthy MJ, Klippel A, Rubin LL. Activated Phosphatidylinositol 3-Kinase and Akt Kinase Promote Survival of Superior Cervical Neurons. 1997;139:809–815. doi: 10.1083/jcb.139.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ott A, Stolk RP, van Harskamp F, Pols HAP, Hofman A, Breteler MMB. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- 10.Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O’Brien PC, Palumbo PJ. Risk of dementia among persons with diabetes mellitus: a population- based cohort study. American Journal of Epidemiology. 1997;145:301–308. doi: 10.1093/oxfordjournals.aje.a009106. [DOI] [PubMed] [Google Scholar]
- 11.Xu W, Qiu C, Winblad B, Fratiglioni L. The effect of borderline diabetes on the risk of dementia and Alzheimer’s disease. Diabetes. 2007;56:211–216. doi: 10.2337/db06-0879. [DOI] [PubMed] [Google Scholar]
- 12.Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, Tylavsky FA, Newman AB. The Metabolic Syndrome, Inflammation, and Risk of Cognitive Decline. JAMA: The Journal of the American Medical Association. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 13.Peila R, Rodriguez BL, White LR, Launer LJ. Fasting insulin and incident dementia in an elderly population of Japanese-American men. Neurology. 2004;63:228–233. doi: 10.1212/01.wnl.0000129989.28404.9b. [DOI] [PubMed] [Google Scholar]
- 14.Luchsinger JA, Tang MX, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63:1187–1192. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 15.Araki Y, Nomura M, Tanaka H, Yamamoto H, Yamamoto T, Tsukaguchi I, Nakamura H. MRI of the brain in diabetes mellitus. Neuroradiology. 1994;36:101–103. doi: 10.1007/BF00588069. [DOI] [PubMed] [Google Scholar]
- 16.den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia. 2003;46:1604–1610. doi: 10.1007/s00125-003-1235-0. [DOI] [PubMed] [Google Scholar]
- 17.Craft S, Peskind E, Schwartz MW, Schellenberg GD, Raskind M, Porte D., Jr Cerebrospinal fluid and plasma insulin levels in Alzheimer’s disease: relationship to severity of dementia and apolipoprotein E genotype. Neurology. 1998;50:164–168. doi: 10.1212/wnl.50.1.164. [DOI] [PubMed] [Google Scholar]
- 18.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? Journal of Alzheimer’s Disease. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 19.Phiel CJ, Wilson CA, Lee VMY, Klein PS. GSK-3[alpha] regulates production of Alzheimer’s disease amyloid-[beta] peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- 20.Schubert M, Brazil DP, Burks DJ, Kushner JA, Ye J, Flint CL, Farhang-Fallah J, Dikkes P, Warot XM, Rio C, Corfas G, White MF. Insulin Receptor Substrate-2 Deficiency Impairs Brain Growth and Promotes Tau Phosphorylation. Journal of Neuroscience. 2003;23:7084–7092. doi: 10.1523/JNEUROSCI.23-18-07084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Kustermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Bruning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proceedings of the National Academy of Sciences. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gasparini L, Gouras GK, Wang R, Gross RS, Flint Beal M, Greengard P, Xu H. Stimulation of [beta]-amyloid precursor protein trafficking by insulin reduces intraneuronal [beta]-amyloid and requires mitogen-activated protein kinase signaling. Journal of Neuroscience. 2001;21:2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-[Beta;] levels. Nature Medicine. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 24.Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, Schwartz MW, Plymate S, Craft S. Insulin increases CSF A{beta}42 levels in normal older adults. Neurology. 2003;60:1899–1903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- 25.De Felice FG, Vieira MN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao WQ, Ferreira ST, Klein WL. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci U S A. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reger MA, Watson GS, Frey WH, 2nd, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiology of Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Craft S, Asthana S, Schellenberg G, Baker L, Cherrier M, Boyt AA, Martins RN, Raskind M, Peskind E, Plymate S. Insulin effects on glucose metabolism, memory, and plasma amyloid precursor protein in Alzheimer’s disease differ according to apolipoprotein-E genotype. Annals of the New York Academy of Sciences. 2000;903:222–228. doi: 10.1111/j.1749-6632.2000.tb06371.x. [DOI] [PubMed] [Google Scholar]
- 28.Craft S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Disease & Associated Disorders. 2006;20:298–301. doi: 10.1097/01.wad.0000213866.86934.7e. [DOI] [PubMed] [Google Scholar]
- 29.Strachan MW. Insulin and cognitive function in humans: experimental data and therapeutic considerations. Biochemical Society Transactions. 2005;33:1037–1040. doi: 10.1042/BST20051037. [DOI] [PubMed] [Google Scholar]
- 30.Landreth G. PPARgamma agonists as new therapeutic agents for the treatment of Alzheimer’s disease.[comment] Experimental Neurology. 2006;199:245–248. doi: 10.1016/j.expneurol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JC, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 32.Burns JM, Donnelly JE, Anderson HS, Mayo MS, Spencer-Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D, Hansen D, Brooks WM. Peripheral insulin and brain structure in early Alzheimer disease. Neurology. 2007;69:1094–1104. doi: 10.1212/01.wnl.0000276952.91704.af. [DOI] [PubMed] [Google Scholar]
- 33.Mielke MM, Zandi PP, Sjogren M, Gustafson D, Ostling S, Steen B, Skoog I. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64:1689–1695. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- 34.Sanz C, Andrieu S, Sinclair A, Hanaire H, Vellas B. Diabetes is associated with a slower rate of cognitive decline in Alzheimer disease. Neurology. 2009;73:1359–1366. doi: 10.1212/WNL.0b013e3181bd80e9. [DOI] [PubMed] [Google Scholar]
- 35.Beeri MS, Silverman JM, Davis KL, Marin D, Grossman HZ, Schmeidler J, Purohit DP, Perl DP, Davidson M, Mohs RC, Haroutunian V. Type 2 Diabetes Is Negatively Associated With Alzheimer’s Disease Neuropathology. J Gerontol A Biol Sci Med Sci. 2005;60:471–475. doi: 10.1093/gerona/60.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonnen JA, Larson EB, Brickell K, Crane PK, Woltjer R, Montine TJ, Craft S. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66:315–322. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 38.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412b–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- 40.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 42.Geroldi C, Frisoni GB, Paolisso G, Bandinelli S, Lamponi M, Abbatecola AM, Zanetti O, Guralnik JM, Ferrucci L. Insulin Resistance in Cognitive Impairment: The InCHIANTI Study. Archives of Neurology. 2005;62:1067–1072. doi: 10.1001/archneur.62.7.1067. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka M, Sawada M, Yoshida S, Hanaoka F, Marunouchi T. Insulin prevents apoptosis of external granular layer neurons in rat cerebellar slice cultures. Neuroscience Letters. 1995;199:37–40. doi: 10.1016/0304-3940(95)12009-s. [DOI] [PubMed] [Google Scholar]
- 44.Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia. Neurology. 75:1195–1202. doi: 10.1212/WNL.0b013e3181f4d7f8. [DOI] [PubMed] [Google Scholar]
- 45.Bruandet A, Richard F, Bombois S, Maurage CA, Deramecourt V, Lebert F, Amouyel P, Pasquier F. Alzheimer disease with cerebrovascular disease and vascular dementia: clinical features and course compared with Alzheimer disease. Journal of Neurology, Neurosurgery & Psychiatry. 2009;80:133–139. doi: 10.1136/jnnp.2007.137851. [DOI] [PubMed] [Google Scholar]
- 46.Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. Journal of the American Medical Association. 1997;277:813–817. [PubMed] [Google Scholar]
- 47.Burns JM, Church JA, Johnson DK, Xiong C, Marcus D, Fotenos AF, Snyder AZ, Morris JC, Buckner RL. White Matter Lesions Are Prevalent but Differentially Related With Cognition in Aging and Early Alzheimer Disease. Arch Neurol. 2005;62:1870–1876. doi: 10.1001/archneur.62.12.1870. [DOI] [PubMed] [Google Scholar]
- 48.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased Risk of Type 2 Diabetes in Alzheimer Disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 49.Ronnemaa E, Zethelius B, Sundelof J, Sundstrom J, Degerman-Gunnarsson M, Berne C, Lannfelt L, Kilander L. Impaired insulin secretion increases the risk of Alzheimer disease. Neurology. 2008;71:1065–1071. doi: 10.1212/01.wnl.0000310646.32212.3a. [DOI] [PubMed] [Google Scholar]
- 50.Loskutova N, Honea RA, Brooks WM, Burns JM. Reduced limbic and hypothalamic volumes correlate with bone density in early Alzheimer’s disease. J Alzheimers Dis. 2010;20:313–322. doi: 10.3233/JAD-2010-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson DK, Wilkins CH, Morris JC. Accelerated Weight Loss May Precede Diagnosis in Alzheimer Disease. Arch Neurol. 2006;63:1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 52.Peila R, Rodriguez BL, Launer LJ. Type 2 Diabetes, APOE Gene, and the Risk for Dementia and Related Pathologies: The Honolulu-Asia Aging Study. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 53.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild Cognitive Impairment Represents Early-Stage Alzheimer Disease. Archives of Neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]