Abstract
The gonadotropin-releasing hormone (GnRH) neuron is the pivotal control center in a tightly regulated reproductive axis. The release of GnRH controls estradiol production by the ovary, and estradiol acts at the hypothalamus to regulate GnRH release. However, the mechanisms of estradiol feedback are just beginning to be understood. We have previously shown that estradiol administered to the female mouse modulates sodium currents in fluorescently-labeled GnRH neurons. In the current studies, estradiol (1 nM) was applied directly, for 16–24 hours, to hypothalamic cultures from young or aged female ovariectomized mice. The direct application of estradiol modulated a tetrodotoxin-sensitive sodium current in isolated GnRH neurons from both young and aged animals. Estradiol, and the specific estrogen receptor-β agonist DPN, decreased current amplitude measured in the morning (AM), but had no effect on afternoon currents. These compounds also decreased the rise and decay slope of the current response, increased the width of the current, and increased action potential width in AM recordings. In addition, estradiol decreased the amplitude of the depolarizing afterpotential (DAP); this effect was not time-of-day dependent. The ER-β agonist DPN did not mimic the effect of estradiol on DAPs, and the modulation of DAPs by estradiol was no longer present in cells from postreproductive animals. These results indicate that estradiol can affect the physiology of GnRH neurons via multiple pathways that are differentially regulated during the transition to reproductive senescence, suggesting that estradiol regulation of GnRH neuronal output is modulated during the aging process.
Keywords: GnRH, estradiol, sodium current, aging, depolarizing afterpotential
I. Introduction
Reproduction in mammals is critically dependent upon the appropriate neurosecretion of gonadotropin-releasing hormone (GnRH). The coordinated release of GnRH at the median eminence is directly responsible for the release of gonadotropic hormones from the anterior pituitary, which then regulate the production of gametes and hormones from the gonads. Gonadal hormones act at the hypothalamus to regulate GnRH release, but mechanisms of hormone regulation of GnRH synthesis and release are poorly understood (Kelly and Ronnekleiv, 2008). In females, the steroid hormone estradiol provides feedback to regulate the production and secretion of GnRH (Hrabovszky et al., 2001, Legan and Tsai, 2003, Petersen et al., 2003, Hu et al., 2008). A number of studies now suggest that estradiol may act directly on GnRH neurons (Abraham et al., 2003, Abe et al., 2008, Hu et al., 2008, Chu et al., 2009). However, a direct action of estradiol in isolated, native adult GnRH neurons has not yet been demonstrated.
In recent years, it has become clear that a number of changes are occurring in the hypothalamus (Wise et al., 2002) during reproductive aging. The transition to reproductive senescence is characterized by a decreased LH pulse frequency (Hall et al., 2000, Rubin, 2000, Wise et al., 2002), variable cycle length (Wise et al., 2002), and an attenuated LH response (Wise et al., 2002) to estradiol. Our study of adult, isolated GnRH neurons has revealed a number of changes with animal aging (Wang et al., 2008). Isolated GnRH neurons from postreproductive mice fire in characteristic bursts of activity, as seen in neurons from younger animals, but the frequency and patterning of activity is reduced with increased age. The amplitude of the depolarizing afterpotential (DAP) following a 200-ms current pulse is significantly attenuated in neurons from middle aged and old animals. We have recently demonstrated that the sodium current underlying the action potential, the transient sodium current, and the persistent sodium current are significantly reduced in GnRH neurons from young animals chronically treated with estradiol (Wang et al., 2010). However, the effect of in vivo estradiol on sodium currents is blunted in GnRH neurons from aged mice (Wang et al., 2010), suggesting that estradiol regulation of GnRH neurons is attenuated in postreproductive animals.
Voltage-dependent sodium channels play a key role in governing cell excitability (Raman and Bean, 1997, 1999, Fry et al., 2007) and repetitive (Kiss, 2008) and spontaneous (Raman and Bean, 1997) firing. A tetrodotoxin-sensitive sodium current also underlies the DAP (Chu and Moenter, 2006, Wang and Kuehl-Kovarik, 2010) that has been shown to contribute to bursting activity in GnRH neurons (Kuehl-Kovarik et al., 2005). Aging appears to modulate not only DAP amplitude, but the effect of in vivo estradiol on sodium currents in GnRH neurons. The current study was undertaken to examine the direct effect of estradiol on sodium currents and depolarizing afterpotentials in GnRH neurons, and the role of aging in the modulation of these effects.
2. Results
2.1 Estradiol directly modulates sodium current density in GnRH neurons
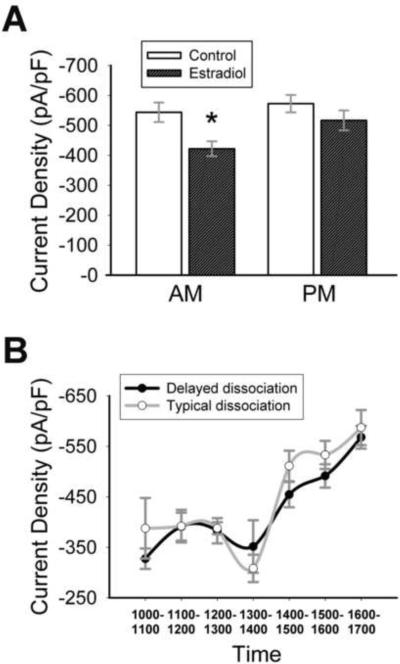
To analyze the ion currents flowing during action potentials, a particularly useful experimental protocol is the action potential clamp, performed in isolated cell bodies [Figure 1 A–C; see (Bean, 2007) for review]. Using this protocol, we have shown that in vivo estradiol attenuates sodium current amplitude (Wang et al., 2010) in adult GnRH neurons. To determine if estradiol can directly regulate sodium currents, 17β-estradiol (1nM) was applied to cultures of GnRH neurons isolated from young adult OVX animals. Ethanol (vehicle; final concentration = 0.05%) was applied to control neurons. All recordings were performed after ≥16 hours in estradiol. Current was converted to current density (current/cell capacitance) to take cell size into account. Capacitance did not vary between estradiol-treated and control neurons (estradiol = 11.1±0.4 pF, control = 11.3±0.4 pF; p = 0.74, n = 30–50). There was no difference in total current density between the two groups (p = 0.17, n = 30–50). However, an interesting pattern was observed (Figure 1D). When recordings were divided into AM (1000–1400 h) and PM (1400–1700 h) groups, there was a significant effect of time on treatment (Two-way ANOVA: F(1,58) = 16.6; p < 0.001, n = 13–17). Currents from estradiol-treated cells were significantly smaller than control in the AM (Figure 1D, 2A (a); p < 0.001, n = 13–17), but were not different than control currents in the PM (p = 0.10, n = 15–17). Estradiol significantly increased currents in PM cultures (Figure 2A (b), 2C; p < 0.001, n = 17), as compared to AM cultures, while currents in control cultures were unchanged (p = 0.78, n = 13–17). Currents from control treatments were significantly reduced in recordings performed two days after dissociation, so only data from day one were analyzed. Capacitance (AM control = 10.8±0.55 pF; AM estradiol = 10.6±0.49 pF; PM control = 11.9±0.56 pF; PM estradiol = 11.7±0.72 pF; p ≥ 0.2, n = 15–25) and input resistance (AM control = 1.8±0.14 GΩ; AM estradiol = 1.7±0.16 GΩ; PM control = 1.8±0.11 GΩ; PM estradiol = 1.5±0.19 GΩ; p ≥ 0.2, n = 15–25) were unaffected by time or hormone treatment. When currents were examined on an hour-by-hour basis (Figure 2B; one-way ANOVA; n = 3–8 cells from multiple animals), sodium currents in estradiol-treated neurons remained constant in the AM (1000–1200 h, p > 0.93), then briefly decreased before dramatically increasing between 1300–1400 hours (Figure 2 B, C; p < 0.001, n = 5–6). On this hourly basis, currents in estradiol-treated cells from 1100–1300 hours were significantly smaller than currents recorded at 1500–1600 hours (Figure 2B; F(6,33) = 9.08; p < 0.003, n = 3–8; One-way ANOVA). Currents in control cells were much more variable (Figure 2B). A reduced concentration of estradiol (0.1 nM) had no effect on AM current density (mean amplitude = −543.7±66.7 pA/pF; p = 0.77 compared to control, n = 7).
Figure 1.
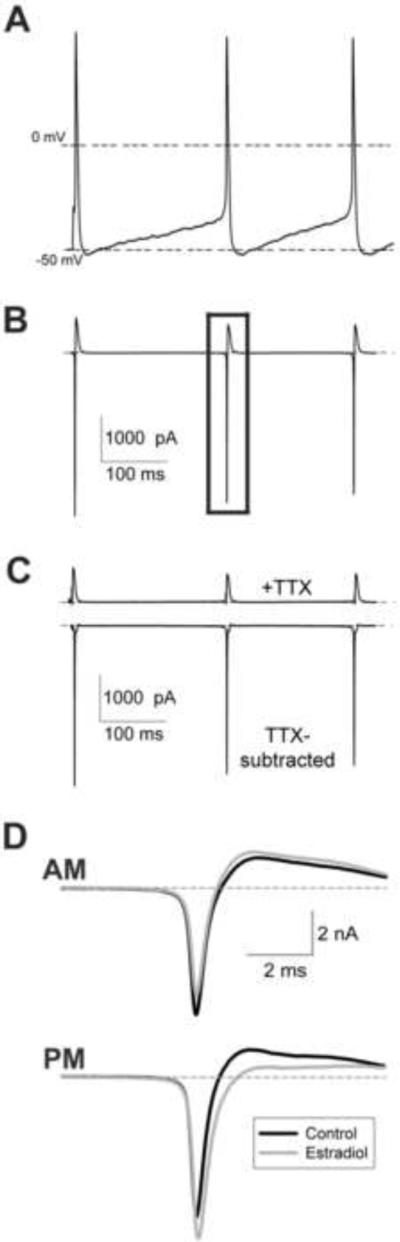
Action potential-evoked sodium currents in GnRH neurons. (A) Action potential command waveform, consisting of multiple previously recorded action potentials in GnRH neurons. The first spike is evoked, the following spikes are spontaneous. (B) Representative current response to action potential voltage command. The current demarked by the rectangle was used for all analyses. (C) Representative current traces after tetrodotxin (TTX; 500 nM) application (upper panel) and subtracted TTX-sensitive sodium currents (lower panel). (D) Representative traces (average of 10 records) from isolated neurons incubated in ACSF (control; black) or 1 nM estradiol (gray). Recordings were taken before (AM; upper panel) or after (PM; lower panel) 1400 h. Note the reduction in current amplitude by estradiol in AM recordings. Cells were of similar capacitance (12–13 pF).
Figure 2.
Estradiol directly modulates sodium current amplitude in GnRH neurons. (A) Currents in estradiol-treated cells (dark) are significantly reduced in the AM group compared to control (white) or the PM group. a: significantly different from control cells, p<0.001; b: significantly different from AM estradiol-treated cells, p < 0.001; n = 13–17. (B) Sodium current density in estradiol-treated cells (closed circles) dramatically increases after 1300 hours (*: p < 0.001 when compared to 1300 h, n = 5–6; a: p < 0.003 when compared to 1100, 1200, or 1300 h, n = 3–8). (C) Representative sodium currents (from cells with comparable capacitances) recorded at 1315 h (black) and 1445 h (gray) from two estradiol-treated neurons on the same day. Note the dramatic difference in current amplitude before and after the 1400 h time point.
2.2 The effects of estradiol are not affected by time in culture
Although it is clear that time of day modulates the effect of estradiol on sodium current density, whether this was due to actual time of recording, or time that the neurons were exposed to estradiol, was unknown. Therefore, nine young OVX females were subjected to a 12:12 time reversal for 10 days (12:12 D:L). Mice were sacrificed in the dark at 2300 h in their subjective day (1100 hours). Under these conditions, estradiol had an overall significant effect on sodium current density (Figure 3A; Two-way ANOVA: F(1,88) = 8.03, p = 0.006, n = 18–26) and there was no interaction between time and treatment (p = 0.30). However, sodium currents in estradiol-treated cells were significantly reduced in the AM compared to PM currents (p = 0.03, n = 20–25).
Figure 3.
Estradiol directly modulates AM sodium currents in GnRH neurons from time-reversed animals. (A) Mean AM current density in estradiol-treated neurons (dark) is significantly reduced when compared to control (white; *, p = 0.006) or PM currents (p = 0.03; n = 20–25). (B) Delaying the time of sacrifice and dissociation for three hours (black) does not shift the overall curve, and AM currents are significantly smaller than PM currents (p = 0.003, 1500–1600 h vs 1000–1200 h, n = 8–19). The typical dissociation curve (white; from Figure 2) is provided for comparison.
Although there is no time-treatment interaction in cells from time-reversed animals, results suggest that the effects of estradiol are not strictly diurnal. This did not rule out the possibility that sensitivity to estradiol in GnRH neurons oscillates over a 12-hour period. To determine if the effect of estradiol on sodium currents is related to the time that estradiol is applied in culture instead of time of day, the time of sacrifice, dissociation, and estradiol application was delayed for three hours in six animals. Next day recordings were performed as usual. In AM recordings, estradiol significantly reduced sodium currents as compared to PM currents (p < 0.001, n = 38–57). When the average current density over time in cells from the delayed dissociation was compared to earlier data (Figure 3B), it was clear that the significant increase in sodium current density was not delayed, and AM currents (1000, 1100, 1200 h) were significantly smaller than PM currents (1500, 1600 h; One-way ANOVA: F(6,83) = 8.52; p = 0.003, n = 8–19).
2.3 The AM effects of estradiol are mediated through ERβ
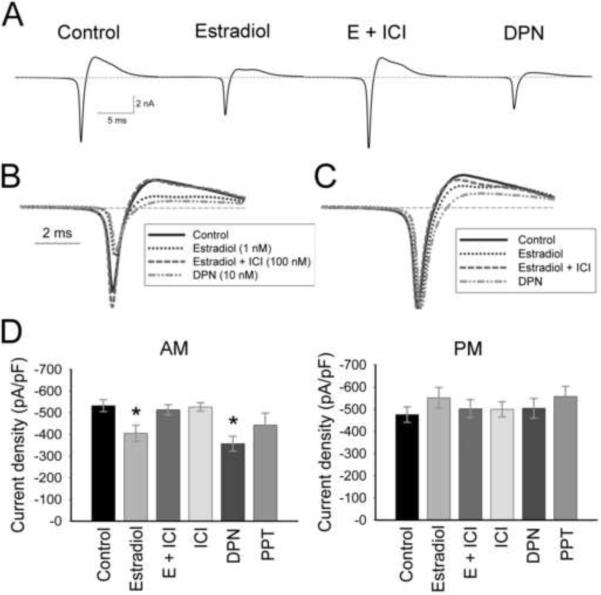
To determine if estradiol was acting through a classical estrogen receptor (ER), the ER antagonist, ICI 182,780 (ICI; 100 nM), the ERα-specific agonist PPT (10 nM), or the ERβ-specific agonist DPN (10 nM) was added to hypothalamic cultures at the time of dissociation (Figure 4; Table 1). There was, again, a significant effect of time on treatment (Figure 4D; Table I; Two-way ANOVA: F(5, 171) = 3.2, p = 0.008). Within the AM group, only estradiol (E) or DPN attenuated sodium currents (Figure 4A, B, D; Table I; p ≤ 0.03, n = 10–25). The addition of ICI (E + ICI) completely blocked the attenuation of sodium current amplitude seen with estradiol alone (Figure 4A, B, D; E compared to E + ICI, p = 0.03, n = 12–15), while ICI alone had no effect on sodium currents (Figure 4D). Sodium currents in cells treated with PPT were not significantly reduced (Figure 4D; Table I; p = 0.12, n = 10–25). While currents within PM recordings were not significantly modulated by any treatment, estradiol or DPN significantly increased currents in PM recordings when compared to AM recordings (p ≤ 0.02, n = 10–15).
Figure 4.
The effects of estradiol on AM sodium current amplitude are mediated through ERβ. (A) Representative AM traces (average of 10 records) from cells of similar capacitance (11–12 pF) incubated in ACSF (control), 1 nM estradiol, 1 nM estradiol + 100 nM ICI 182,780 (E + ICI), or 10 nM DPN. Traces are overlaid in B, and normalized to peak current in C. Note the shift in time of peak current in estradiol- and DPN-treated cells compared to currents in control cells. (D) Mean current amplitude in AM recordings is significantly reduced in estradiol-treated and DPN-treated neurons (*, p ≤ 0.03 compared to control, E + ICI, or ICI). The ERα agonist PPT did not significantly reduce AM currents (p = 0.12). No estrogen receptor agonists or antagonists modulated currents from PM recordings (p = 0.67). n = 25 for control cells, 10–15 for treated cells.
Table I.
Effect of estradiol (1 nM) or ER agonists (10 nM) on sodium currents and action potentials in isolated GnRH neurons from young female mice.
| Property | Young Control | Young Estradiol | Young DPN | Young PPT | ||||
|---|---|---|---|---|---|---|---|---|
| AM | PM | AM | PM | AM | PM | AM | PM | |
| Sodium Currents: | ||||||||
| Amplitude (pA/pF) | −532.58±28.0 n = 25 |
−475.18±24.0 n = 24 |
−405.57±36.5a n = 15 |
−551.0±13.0b n = 13 |
−357.10±34.6a n = 12 |
−503.66±44.4b n = 10 |
−443.11±55.5 n = 10 |
−556.60±45.9 n = 10 |
| Rise slope (V/ms) (maximum) | −21.43±1.8 n = 21 |
−19.50±1.9 n = 22 |
−14.30±1.6a n = 11 |
−20.48±2.9 n = 10 |
−15.12±2.4a n = 12 |
−21.40±3.11 n = 10 |
−22.30±3.9 n = 7 |
−30.61±6.9 n = 7 |
| Decay slope (V/ms) (maximum) | 12.92±1.1 n = 21 |
12.49±1.0 n = 22 |
8.59±1.0a n = 11 |
13.27±1.9 n = 10 |
9.42±1.2a n = 12 |
13.63±1.9 n = 10 |
12.26±2.3 n = 7 |
19.11±4.3 n = 7 |
| Width (1/2 max; ms) | 0.47±0.006 n = 21 |
0.49±0.02 n = 22 |
0.49±0.02 n = 11 |
0.48±0.02 n = 10 |
0.50±0.01a n = 12 |
0.44±0.007b,c n = 10 |
0.48±0.01 n = 7 |
0.47±0.008 n = 7 |
| Action Potentials: | ||||||||
| Amplitude (mV) | 128.6±1.07 n = 17 |
126.8±2.39 n = 14 |
125.4±3.42 n = 8 |
129.1±1.17 n = 9 |
126.2±2.0 n = 9 |
126.5±1.67 n = 7 |
131.9±0.9 n = 4 |
125.4±1.5 n = 3 |
| Width (1/2 max; ms) | 1.55±0.07 n = 17 |
1.53±0.07 n = 14 |
1.64±0.13 n = 8 |
1.50±0.07 n = 9 |
1.72±0.12d n = 9 |
1.46±0.05 n = 7 |
1.65±0.08 n = 4 |
1.53±0.15 n = 3 |
| AHP Amplitude (mV) | −4.65±0.55 n = 17 |
−4.48±0.54 n = 14 |
−4.35±0.44 n = 8 |
−3.82±0.46 n = 9 |
−3.82±1.05 n = 9 |
−3.20±0.82 n = 7 |
−4.46±0.09 n = 4 |
−4.30±0.27 n = 3 |
| DAP Amplitude (mV) | 4.68±0.26 n = 23 |
4.66±0.23 n = 16 |
3.51±0.41a n = 11 |
4.07±0.53 n = 10 |
4.79±0.68 n = 9 |
3.51±0.51c n = 7 |
6.27±0.62e n = 4 |
4.19±0.28 n = 3 |
Significantly different from AM control (p<0.05).
Significantly different from same-treatment AM (p<0.05).
Significantly different from PM control (p<0.05).
Significantly different from same-treatment PM (p<0.05).
Significantly larger than AM control or estradiol DAP (p<0.05).
2.4 Estradiol and DPN affect kinetics in AM recordings
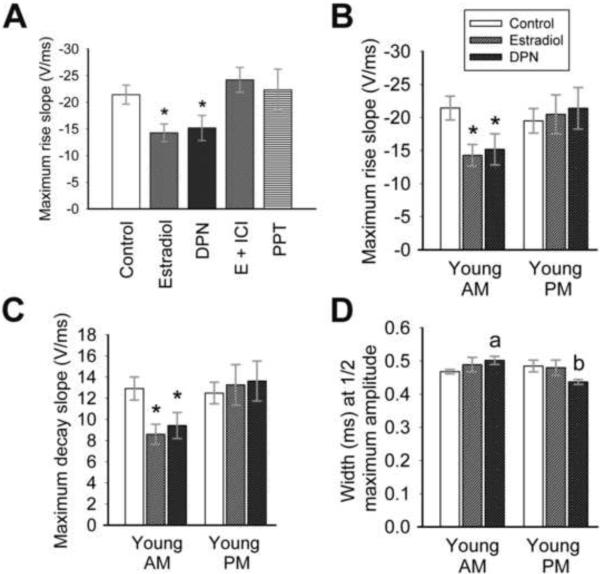
The kinetics of sodium current rise and decay appear to be affected by estradiol and DPN in AM recordings (see Figure 4C). Therefore, the sodium current underlying the action potential was analyzed for rise and decay slope, maximum rise and decay slope, rise and decay time, and width at half maximum amplitude (Figure 5). Both estradiol (1 nM) and DPN (10 nM) significantly decreased maximum rise slope in AM recordings (Figure 5 A, B; Table I; One-way ANOVA: F(5,73) = 3.29; p = 0.01, n = 10–21) from cells isolated from young OVX animals. No differences were seen between treatments in PM recordings (Figure 5B; Table I; One-way ANOVA: F(5,75) = 1.30; p = 0.28, n = 7–22), nor were any AM to PM measurements statistically significant. Similarly, maximum decay slope (Figure 5C; Table I; One-way ANOVA: F(2,43) = 4.38; p = 0.02, n = 11–21), rise slope (One-way ANOVA: F(2,43) = 3.20; p < 0.05, n = 11–21), and decay slope (One-way ANOVA: F(2,43) = 4.97; p = 0.01, n = 11–21) were significantly decreased by estradiol and DPN in AM recordings from young OVX animals. Estradiol did not modulate overall rise time and decay time.
Figure 5.
Estradiol and DPN modulate sodium current kinetics in AM recordings. (A) Estradiol (1 nM) or DPN (10 nM) significantly reduces the maximum rise slope of sodium currents evoked by an action potential protocol in AM recordings (*, p = 0.01 vs. other treatments) in cells isolated from young animals. The Erα agonist PPT has no effect. The maximum rise and decay slopes (B and C) are significantly decreased by estradiol or DPN in AM recordings (*, p < 0.05) only. No significant differences are noted in PM recordings. (D) The width of the sodium current at half-maximum amplitude is increased significantly by DPN in AM recordings in cells from young animals (a, p = 0.01 compared to control AM), while the width is significantly reduced by DPN in PM recordings (b, p = 0.04 compared to control PM, p < 0.001 compared to DPN AM). n = 21–22 for control cells, 10–15 for treated cells. The legend in (B) applies to graphs B – D.
The width of the sodium current response at half maximum amplitude (width) did not appear to be significantly modulated by estradiol (Figure 5D) in recordings from young OVX animals. However, DPN significantly increased the width in AM recordings (Figure 5D (a); Table I; p = 0.01, n = 12–21) and decreased width in PM recordings (Figure 5D (b); Table I; p = 0.04 compared to control PM and p < 0.001 compared to DPN AM; n = 10–22). These results suggest that hormone, mediated through the ERβ receptor, increases the width of the sodium current response in GnRH neurons.
2.5 DPN significantly affects action potential width
Estradiol clearly affects the sodium current underlying the action potential. Therefore, the amplitude of the action potential, afterhyperpolarization potential (AHP) and depolarizing afterpotential (DAP) were analyzed, as were action potential kinetics. No treatments modulated the amplitude of the action potential or AHP (Table I). Kinetics were not significantly modulated by estradiol or DPN, although hormone consistently reduced maximum slope (both rise and decay) in AM recordings from young animals (Figure 6B, C). Consistent with the action of DPN on sodium current width, the width of the action potential at half maximum amplitude was significantly increased in AM recordings when compared to PM recordings in DPN-treated cells (Figure 6A, see 5D; Table I; p = 0.03, n = 7–9), indicating that the width of the sodium current does impact action potential width, and both are modulated by DPN.
Figure 6.

An ERβ agonist significantly affects action potentials. (A) There is a trend towards an increased action potential width in the AM, and a decreased width in the PM, with incubation in either estradiol or 10 nM DPN (*, p = 0.03 compared to PM DPN). (B,C) There is a trend towards decreased maximum slopes in the AM with estradiol or DPN treatment. n = 14–17 for control cells, 7–9 for treated cells. The legend in (A) applies to all graphs.
2.6 Estradiol reduces both AM and PM depolarizing afterpotentials
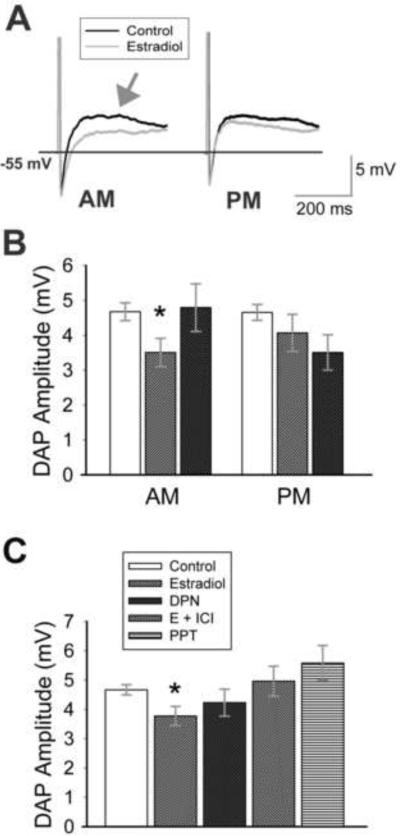
The depolarizing afterpotential (DAP) plays a role in bursting in GnRH neurons (Kuehl-Kovarik et al., 2005). The DAP that follows a burst of action potentials has been shown to be modulated by age (Wang et al., 2008), but is not modulated by in vivo estradiol. However, in vitro estradiol significantly reduced DAP amplitude in AM recordings (Figure 7 A, B; Table I; p = 0.02, n = 11–23). Unlike sodium current amplitude, DAP amplitude was reduced (nonsignificantly) in PM recordings as well as AM recordings (Figure 7B; Table I), and there was no difference in DAP amplitude between AM and PM estradiol-treated cells (p = 0.41, n = 10–11). Therefore, results (AM and PM) were combined to demonstrate a significant reduction in DAP amplitude with estradiol application (Figure 7C; p = 0.01, n = 21–39) that was not modulated by time of day. Interestingly, the effect of estradiol on DAP amplitude does not appear to be mediated through ERβ, as DPN had no consistent effect on the amplitude of the DAP (Figure 7 B, C; p = 0.19, n = 16–39). Although ICI appeared to eliminate the effect of estradiol on DAP amplitude (Fig 7C; p < 0.05, E + ICI vs. estradiol, n = 14–21), neither DPN nor PPT (Figure 7 B, C; Table I) clearly replicated the effect of estradiol. These results suggest that multiple ERs modulate the direct effects of estradiol on GnRH neurons; not all are time-sensitive.
Figure 7.
Estradiol reduces depolarizing afterpotential amplitude. (A) Representative traces (average of 5 records) of action potentials (cropped) and resultant depolarizing afterpotentials (DAPs; arrow) recorded from cells isolated from young adult animals. Cells were incubated in ACSF (control; black) or 1 nM estradiol (gray). Recordings were taken before (AM) or after (PM) 1400 h. (B) Estradiol treatment results in a significant decrease in DAP amplitude in AM recordings (*, p = 0.02 compared to control, n = 11–23) and also reduces DAP amplitude in PM recordings. When AM and PM data are combined (C), DAP amplitude is significantly decreased by estradiol (*, p < 0.05 compared to control, E+ICI, or PPT, n = 14–39, except PPT = 7) in young animals. The ERβ agonist DPN does not significantly decrease overall DAP amplitude (p = 0.19; n = 16), and has no effect in AM recordings. The legend in (C) applies to all graphs.
2.7 GnRH neurons from aged animals respond to estradiol
In vivo estradiol reduces sodium current density only in young, reproductive animals; sodium currents are not significantly attenuated in peripostreproductive and aged animals (Wang et al., 2010). To determine if in vitro estradiol attenuated the sodium current underlying the action potential in GnRH neurons from aged animals, AM recordings were performed in cells isolated from 13-month old, postreproductive OVX animals. Interestingly, 1 nM estradiol significantly reduced sodium current density in neurons from aged animals (Figure 8A; p = 0.02, n = 9–10). Thus, estradiol directly affects GnRH neurons from both young and aged mice, although longer-term, in vivo effects are lost in aged mice. The effect of estradiol on the kinetics of the inward current, and the corresponding action potential, was examined in recordings from old animals. Although similar in pattern to young animals, the decrease in maximum rise slope (old control, −22.13±2.6 V/ms; old estradiol, −18.66±2.3 V/ms; p = 0.33) and maximum decay slope (old control, 13.01±1.3 V/ms; old estradiol, 11.02±1.0 V/ms; p = 0.25, n = 9–10) with estradiol application in cells from aged animals was nonsignificant. However, estradiol evoked a significant increase in sodium current width in AM recordings from cells isolated from aged animals (Figure 8B, p < 0.001, n = 9–10), similar to results seen with DPN treatment in cells from young animals. The increase in sodium current width was reflected by a nonsignificant increase in action potential width at half-maximum amplitude with estradiol exposure (old control, 1.64±0.09 ms; old estradiol, 1.78±0.09 ms; p = 0.29, n = 6–8).
Figure 8.
Estradiol directly modulates sodium currents, but not DAPs, in GnRH neurons from aged animals. (A) Mean AM current density is significantly decreased by estradiol treatment in GnRH neurons from aged animals (*, p = 0.02, n = 9–10). Upper traces represent overlaid currents (average of 10 records, 11–12 pF) from a control or estradiol-treated neuron isolated from a 13-month old (aged) animal. (B) The width at half-maximum sodium current amplitude is significantly increased by estradiol treatment in aged animals (*, p < 0.001; n = 9–10). A similar result is seen in young animals (Figure 5D). (C) Estradiol application to cells from aged animals has no effect on DAP amplitude in AM recordings (n = 6–8). The legend in (A) applies to all graphs.
The DAP that follows a burst of action potentials has been shown to be modulated by age (Wang et al., 2008), but the amplitude of the DAP following a single action potential is not age-dependent. The DAP is not modulated by in vivo estradiol (Wang et al., 2008), yet in vitro estradiol modulates DAP amplitude in GnRH neurons from young animals. Interestingly, although 1 nM estradiol directly modulates inward sodium currents in aged GnRH neurons, estradiol did not reduce DAP amplitude in cells from aged animals (Figure 8C; p = 0.75, n = 6–8). Thus, some effects of estradiol are lost in GnRH neurons from aged animals, while others are maintained.
3. Discussion
These studies demonstrate that estradiol (1 nM) applied directly to adult, native GnRH neurons attenuates the sodium current underlying the action potential in cells from both young and aged mice. The ERβ agonist DPN (10 nM) mimics the actions of estradiol, while addition of the ER antagonist ICI 182,780 completely abolishes modulation, indicating that the effects of estradiol on these sodium currents are mediated via an ERβ-dependent pathway. In addition to modulating sodium current amplitude, estradiol and/or DPN decrease the rise and decay slope of the response, which is reflected in a widened current response and increased action potential width. The amplitude of the depolarizing afterpotential is also reduced by 1 nM estradiol in young mice. Notably, aging significantly attenuates the effect of estradiol on the DAP, although estradiol modulates the sodium current underlying the action potential at all ages studied.
Interestingly, estradiol only attenuates sodium currents in AM recordings; altering the time of dissociation or estradiol application does not modify this effect. Circadian regulation during a “critical period” is suspected to enable the LH surge and resultant ovulation (de la Iglesia and Schwartz, 2006). In addition, core clock proteins, hallmarks of suprachiasmatic “clock” neurons, have been detected in GnRH-eGFP neurons (Hickok and Tischkau, 2009). Clock proteins have been shown to oscillate in a time-of-day dependent manner (Hickok and Tischkau, 2009), and regulate pulsatile GnRH secretion from GT1 cells (Chappell, 2005). Time-dependent effects of in vivo estradiol have been demonstrated for firing activity (Chu and Moenter, 2006), calcium currents (Sun et al., 2010) and calcium channel mRNA expression in GnRH neurons (Zhang et al., 2009). Sodium currents appear to be modulated by estradiol in a time-dependent manner. However, the modulation of sodium currents is not abolished or reversed in time-reversed mice. Thus, attenuation, although cyclic, is apparent at least twice in a 24 hour period. Our findings suggest that, in isolated GnRH neurons, the modulation of sodium currents by estradiol is independent of the critical period that dictates the LH surge, but could be under the control of an endogenous clock. In the intact animal, GnRH neurons are subjected to multiple inputs relaying information about circadian rhythms, nutritional status, and environmental surroundings that are no longer present in isolated neurons. It appears that estradiol interacts with both endogenous and exogenous regulatory mechanisms to directly influence GnRH neuronal function.
Because estradiol increases sodium current width, it appears that sodium current kinetics underlie the increase in action potential width, although a contributory modulation of potassium currents cannot be ruled out. Both rise slope and decay slope of the sodium current are decreased by direct estradiol; an increased width would indicate that a slowed decay predominates. We have previously shown that in vivo estradiol prolongs the inactivation time constant of the transient sodium current (Wang et al., 2010), suggesting that estradiol modulates the inactivation of sodium channels to increase action potential width. Altered action potential kinetics could modulate bursting or other aspects of firing. Action potential amplitude is not modulated by estradiol; spike broadening could therefore allow an increased influx of calcium (Wheeler et al., 1996), resulting in the modulation of numerous cellular events. The physiological impact of slowed sodium kinetics and spike broadening by direct estradiol in AM recordings is not clear at this time.
In vivo estradiol attenuates sodium currents and modulates the kinetics of the transient sodium current in GnRH neurons. However, the in vivo effect of estradiol is blunted in postreproductive animals. In vivo estradiol does not attenuate DAPs (Wang et al., 2008), but in vitro estradiol attenuates the both the sodium current and the depolarizing afterpotential in GnRH neurons. These results suggest that a modified concentration of estradiol or hormone metabolism alters the outcome of in vivo estradiol exposure. The effect of in vitro estradiol on DAPs is quite distinct from sodium current modulation. The attenuation of DAP amplitude by estradiol is not time-dependent, as is the modulation of sodium currents. The ERβ agonist DPN has no overall effect on DAPs. Interestingly, estradiol affects sodium currents in neurons from both young and aged animals, but has no effect on the DAP in cells from aged animals. A decrease in DAP amplitude by estradiol could produce a blunted response to excitatory inputs and reduced spike patterning, resulting in smaller, irregular discharges. This inhibitory effect of estradiol appears to be lost in neurons from old animals. It is apparent from these studies that multiple estrogen receptors and modulatory pathways are involved in the attenuation of currents by estradiol; some appear sensitive to an endogenous clock or time-dependent mechanism, others are affected by the aging process. Estradiol (10 nM) has been shown to directly and rapidly increase DAP amplitude in recordings from GnRH neurons in the slice preparation (Chu et al., 2009). The reasons for these differences are unclear, but could be due to the isolated preparation, the duration of estradiol application, or the concentration of estradiol used.
Although glutamate receptors were blocked in our preparation, we did not block GABAergic transmission, or other, slower acting transmitters such as VIP. Thus, we cannot say with absolute certainty that the action of estradiol was directly on GnRH neurons in our culture paradigm. However, the cellular environment in any given dish is quite heterogeneous, both between dishes and between animals. Yet the effect of estradiol on sodium currents is consistent, suggesting that the effect of estradiol is direct. There is mounting evidence that estradiol affects GnRH neurons both directly and indirectly (Hu et al., 2008, Chu et al., 2009, Moenter et al., 2009, Noel et al., 2009, Sun et al., 2010), that it can decrease or increase firing frequency (Hu et al., 2008, Chu et al., 2009), and that it can act through classical (Chu et al., 2009, Sun et al., 2010) and nonclassical (Noel et al., 2009, Sun et al., 2010) receptors. Our results suggest that multiple pathways and receptor types are involved in the modulation of action potentials and DAPs. Because estradiol was applied in a chronic fashion, we cannot yet speculate on how estradiol is modulating sodium channel expression and function. Future experiments will include the discrete, rapid application of estradiol to isolated GnRH neurons to determine if the effect of estradiol on sodium currents is a rapid, membrane event or mediated through slower genomic mechanisms.
Currently, we know that there are many aspects to hormone regulation of the reproductive axis. A number of studies now suggest that estradiol acts directly on GnRH neurons (Abraham et al., 2003, Abe et al., 2008, Hu et al., 2008, Chu et al., 2009, Sun et al., 2010, Zhang et al., 2010, Kenealy et al., 2011). We are the first to demonstrate a direct action of estradiol in isolated, native adult GnRH neurons. In addition, our data demonstrate that estradiol acts through multiple regulatory pathways; some pathways are lost in neurons from aged animals. Thus, the modulation of GnRH neuronal function by estradiol appears to be modified by aging or the transition to a postreproductive state. The transition to reproductive senescence is characterized by hypothalamic changes, including an attenuated LH response to estradiol (Hall et al., 2000, Rubin, 2000, Wise et al., 2002). Our studies suggest that modulation of direct estradiol regulation of GnRH neurons in aged animals could contribute to hypothalamic changes seen in the transition to reproductive senescence.
4. Experimental procedures
Animals
Adult virgin female GnRH-eGFP transgenic mice (Suter et al., 2000) were used for all experiments. Animals were used at 3 months of age (young, reproductive) or 13 months of age (old, postreproductive). Animals were maintained in a colony at the University of Missouri, under a 12 hour light:dark (L:D) cycle (lights on 0700 hours CST). Some animals were time reversed (D:L 12:12) 10 days prior to use. All animal experimentation was conducted in accord with accepted standards of humane animal care, and the University of Missouri Animal Care and Use Committee approved all procedures. All mice were bilaterally ovariectomized (OVX) under isoflurane anesthesia. Banamine (0.025 μg/10 g body weight) was administered pre-operatively as an analgesic. Mice were sacrificed 7–10 days after ovariectomy. A total of 48 mice were used. At least four mice were used for each experimental group, and cells were divided between 2–3 treatments per mouse. Two to four recordings were performed in the AM or PM from each treatment.
Neuronal Dissociation and culture
Cells were acutely isolated as described previously (Wang et al., 2008). Briefly, brains sliced at 400 μm in low-calcium artificial cerebrospinal fluid (ACSF; in mM: 124 NaCl, 3 KCl, 2 MgSO4, 1.25 NaH2PO4, 26 NaHCO3, 10 dextrose, 0.1 CaCl2, pH 7.4; bubbled with 95% O2, 5% CO2). Mice were sacrificed at the same time each day (1100 h). Isolated regions were enzymatically treated with proteinase K (Sigma, St. Louis, MO; 0.2 mg/ml) followed by trypsin (Sigma; 1 mg/ml) at 30°C for 22 minutes. Neurons were spherical, with no or rudimentary processes after 24 hours in culture. Slices were triturated with flame-polished Pasteur pipettes and plated. Cells were incubated at 37°C in phenol-free Neurobasal-A/B-27 (Life Technologies, Inc., Carlsbad, CA). The glutamate receptor blockers APV (1 mM) and NBQX (10 μM) were added at the time of dissociation.
Cells were incubated overnight in the following compounds: 1) ACSF + vehicle (ethanol, final concentration 0.05%); 2) 1 nM 17β-estradiol (E; Sigma); 3) ICI 182,780 (ICI; Tocris, Ellisville, MO), an antagonist of “classical” (ERα and ERβ) estrogen receptors, 100 nM; 4) 1 nM E + 100 nM ICI; 5) DPN, a selective ERβ agonist, 10 nM (Tocris); or 6) PPT, a selective ERα agonist, 10 nM (Tocris). Two to three treatments were applied to separate cultures obtained from each mouse.
Electrophysiology
Electrophysiology was performed 16–24 hours post-dissociation. Treatments were shuffled in the AM (before 1400 hours CST) and PM (after 1400 hours CST) to ensure that time, within AM or PM, did not affect results. Fluorescent cells were viewed on a Nikon Diaphot-TMD inverted microscope with a GFP filter. Cells were perfused with normal-calcium ACSF (2.5 mM CaCl2) at 22°C. No hormone was present at the time of recording. Whole-cell recordings were obtained with thin-walled borosilicate glass micropipettes (World Precision Instruments; 1.5–3 MΩ) filled with a potassium gluconate intracellular solution (in mM: 120 potassium gluconate, 1 CaCl2, 1 MgCl2, 10 HEPES, 1 NaCl, 5 EGTA, 2 ATP, 0.2 GTP, pH 7.2–7.4). All recordings were performed using a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, CA), digitized with a Digidata 1322A (Molecular Devices), and stored on a Dell computer (Dell, Round Rock, TX) using pClamp 9.2 software.
Whole-cell configuration was initiated only upon the establishment of a GΩ seal. Only stable recordings with a series resistance of less than 10 MΩ was included. Series resistance was compensated 70–80%. Data were digitized at 20 kHz and low-pass filtered at 4 kHz. To evoke the ionic current underlying the action potential, a pre-recorded spike train was used as the voltage command. Leak current was corrected with P/4 subtraction. All amplitude measurements were taken in reference to the prepulse baseline.
Whole-cell current-clamp recordings were initiated to examine action potential responses and resulting depolarizing afterpotentials [DAPs; (Kuehl-Kovarik et al., 2002, Kuehl-Kovarik et al., 2005, Chu and Moenter, 2006)] in cells isolated from animals at different ages under varying hormone treatments. Cells were hyperpolarized to −55 mV to prevent spontaneous activity. Depolarizing current pulses were delivered at 3.5 ms, 200–220 pA, to evoke a single action potential. All amplitude measurements were taken in reference to the prepulse baseline.
Data Analysis
Data were acquired with pClamp 9.2 (Molecular Devices) and analyzed with Clampfit and Sigmaplot (Systat Software, Inc, Chicago, IL) software. Each cell was analyzed as an individual data point. Current density was calculated as current/capacitance (pA/pF). Comparisons between time-of-day and hormone treatments were made with one- and two-way ANOVAs (multiple groups) and unpaired t-tests (two groups) or Mann-Whitney rank sum tests for nonparametric data (two groups). An alpha level of 0.05 was used to determine significance. All values are expressed as mean ± SEM.
Highlights
In vitro estradiol directly attenuated sodium currents in GnRH neurons from young and aged animals.
Estradiol modulation of sodium current was time-dependent, and mediated through ERβ.
Estradiol directly attenuated the DAP; modulation was not time-dependent or mediated through ERβ.
The DAP was not modulated in neurons from aged animals.
These results suggest that estradiol regulation of GnRH neuronal output is modulated during aging.
Acknowledgements
These studies were supported by NIH AG023139 to M.C.K.-K.
Abbreviations
- ACSF
artificial cerebrospinal fluid
- DAP
depolarizing afterpotential
- DPN
diarylpropionitrile; a highly potent estrogen receptor-β agonist
- E
estradiol
- ER
estrogen receptor
- eGFP
enhanced green fluorescent protein
- GnRH
gonadotropin-releasing hormone
- ICI
ICI 182,780; an antagonist of classical estrogen receptors
- LH
luteinizing hormone
- OVX
ovariectomized
- PPT
4,4', 4” –(4-propyl-[1H]-pyrazole-1,3,3-triyl)trisphenol; a potent estrogen receptor-α agonist
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Classification: Systems Neuroscience and Behavior
References
- Abe H, Keen KL, Terasawa E. Rapid action of estrogens on intracellular calcium oscillations in primate luteinizing hormone-releasing hormone-1 neurons. Endocrinology. 2008;149:1155–1162. doi: 10.1210/en.2007-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Chappell PE. Clocks and the black box: circadian influences on gonadotropin-releasing hormone secretion. J Neuroendocrinol. 2005;17:119–130. doi: 10.1111/j.1365-2826.2005.01270.x. [DOI] [PubMed] [Google Scholar]
- Chu Z, Andrade J, Shupnik MA, Moenter SM. Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. J Neurosci. 2009;29:5616–5627. doi: 10.1523/JNEUROSCI.0352-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z, Moenter SM. Physiologic regulation of a tetrodotoxin-sensitive sodium influx that mediates a slow afterdepolarization potential in gonadotropin-releasing hormone neurons: possible implications for the central regulation of fertility. J Neurosci. 2006;26:11961–11973. doi: 10.1523/JNEUROSCI.3171-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147:1148–1153. doi: 10.1210/en.2005-1311. [DOI] [PubMed] [Google Scholar]
- Fry M, Boegle AK, Maue RA. Differentiated pattern of sodium channel expression in dissociated Purkinje neurons maintained in long-term culture. J Neurochem. 2007;101:737–748. doi: 10.1111/j.1471-4159.2007.04470.x. [DOI] [PubMed] [Google Scholar]
- Hall JE, Lavoie HB, Marsh EE, Martin KA. Decrease in gonadotropin-releasing hormone (GnRH) pulse frequency with aging in postmenopausal women. J Clin Endocrinol Metab. 2000;85:1794–1800. doi: 10.1210/jcem.85.5.6612. [DOI] [PubMed] [Google Scholar]
- Hickok JR, Tischkau SA. In vivo circadian rhythms in gonadotropin-releasing hormone neurons. Neuroendocrinology. 2009;91:110–120. doi: 10.1159/000243163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Hu L, Gustofson RL, Feng H, Leung PK, Mores N, Krsmanovic LZ, Catt KJ. Converse regulatory functions of estrogen receptor-alpha and -beta subtypes expressed in hypothalamic gonadotropin-releasing hormone neurons. Mol Endocrinol. 2008;22:2250–2259. doi: 10.1210/me.2008-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Ronnekleiv OK, Terasawa E. STX, a Novel Nonsteroidal Estrogenic Compound, Induces Rapid Action in Primate GnRH Neuronal Calcium Dynamics and Peptide Release. Endocrinology. 2011;152:3182–91. doi: 10.1210/en.2011-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss T. Persistent Na-channels: origin and function. A review. Acta Biol Hung. 2008;59(Suppl):1–12. doi: 10.1556/ABiol.59.2008.Suppl.1. [DOI] [PubMed] [Google Scholar]
- Kuehl-Kovarik MC, Partin KM, Handa RJ, Dudek FE. Spike-dependent depolarizing afterpotentials contribute to endogenous bursting in gonadotropin releasing hormone neurons. Neuroscience. 2005;134:295–300. doi: 10.1016/j.neuroscience.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM. Episodic bursting activity and response to excitatory amino acids in acutely dissociated gonadotropin-releasing hormone neurons genetically targeted with green fluorescent protein. J Neurosci. 2002;22:2313–2322. doi: 10.1523/JNEUROSCI.22-06-02313.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan SJ, Tsai HW. Oestrogen receptor-alpha and -beta immunoreactivity in gonadotropin-releasing hormone neurones after ovariectomy and chronic exposure to oestradiol. J Neuroendocrinol. 2003;15:1164–1170. doi: 10.1111/j.1365-2826.2003.01115.x. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Chu Z, Christian CA. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones. J Neuroendocrinol. 2009;21:327–333. doi: 10.1111/j.1365-2826.2009.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G-Protein Coupled Receptor 30 (GPR30) in Rapid Action of Estrogen in Primate LHRH Neurons. Mol Endocrinol. 2009;21:316–21. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J Neurosci. 1999;19:1663–1674. doi: 10.1523/JNEUROSCI.19-05-01663.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS. Hypothalamic alterations and reproductive aging in female rats: evidence of altered luteinizing hormone-releasing hormone neuronal function. Biol Reprod. 2000;63:968–976. doi: 10.1095/biolreprod63.4.968. [DOI] [PubMed] [Google Scholar]
- Sun J, Chu Z, Moenter SM. Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2010;30:3912–3923. doi: 10.1523/JNEUROSCI.6256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM. Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology. 2000;141:412–419. doi: 10.1210/endo.141.1.7279. [DOI] [PubMed] [Google Scholar]
- Wang Y, Garro M, Dantzler HA, Taylor JA, Kline DD, Kuehl-Kovarik MC. Age affects spontaneous activity and depolarizing afterpotentials in isolated gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:4938–4947. doi: 10.1210/en.2008-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Garro M, Kuehl-Kovarik MC. Estradiol attenuates multiple tetrodotoxin-sensitive sodium currents in isolated gonadotropin-releasing hormone neurons. Brain Res. 2010;1345:137–45. doi: 10.1016/j.brainres.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kuehl-Kovarik MC. Flufenamic acid modulates multiple currents in gonadotropin-releasing hormone neurons. Brain Res. 2010;1353:94–105. doi: 10.1016/j.brainres.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Changes in action potential duration alter reliance of excitatory synaptic transmission on multiple types of Ca2+ channels in rat hippocampus. J Neurosci. 1996;16:2226–2237. doi: 10.1523/JNEUROSCI.16-07-02226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Smith MJ, Dubal DB, Wilson ME, Rau SW, Cashion AB, Bottner M, Rosewell KL. Neuroendocrine modulation and repercussions of female reproductive aging. Recent Prog Horm Res. 2002;57:235–256. doi: 10.1210/rp.57.1.235. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rick EA, Kelly MJ, Ronnekleiv OK. 17Beta-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009;29:10552–10562. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Kelly MJ, Ronnekleiv OK. 17Beta-estradiol rapidly increases K(ATP) activity in GnRH via a protein kinase signaling pathway. Endocrinology. 2010;151:4477–4484. doi: 10.1210/en.2010-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]