Abstract
We have recently demonstrated that visuospatial working memory performance predicts the rate of motor skill learning, particularly during the early phase of visuomotor adaptation. Here, we follow up these correlational findings with direct manipulations of working memory resources to determine the impact on visuomotor adaptation, a form of motor learning. We conducted two separate experiments. In the first one, we used a resource depletion strategy to investigate whether the rate of early visuomotor adaptation would be negatively affected by fatigue of spatial working memory resources. In the second study, we employed a dual n-back task training paradigm that has been shown to result in transfer effects [1] over five weeks to determine whether training-related improvements would boost the rate of early visuomotor adaptation. The depletion of spatial working memory resources negatively affected the rate of early visuomotor adaptation. However, enhancing working memory capacity via training did not lead to improved rates of visuomotor adaptation, suggesting that working memory capacity may not be the factor limiting maximal rate of visuomotor adaptation in young adults. These findings are discussed from a resource limitation / capacity framework with respect to current views of motor learning.
Keywords: working memory, visuomotor adaptation, resource depletion, cognitive training
1. Introduction
Visuomotor adaptation paradigms, in which individuals modify movements in response to a systematic alteration of visual inputs, have been used extensively to study adaptive learning [2–7]. Real world examples of visuomotor adaptation include learning to control movements of a mouse to manipulate a cursor on a computer screen, or learning to use robotic tools for arthroscopic surgery. While visuomotor adaptation clearly involves sensorimotor processes, cognitive processes such as attention and spatial working memory are also thought to play a role [8–12].
For example, Eversheim and Bock [8] employed a dual-task paradigm and found that a spatial cognitive task interfered with the early stages (first several minutes) of sensorimotor adaptation. We recently provided further support for the role of spatial cognition in sensorimotor adaptation. We found that individual differences in spatial working memory performance were predictive of the rate of early, but not late, sensorimotor adaptation (Anguera et al., 2010). In addition, participants recruited the right dorsolateral prefrontal cortex (DLPFC) and bilateral inferior parietal lobules (IPL) during spatial working memory performance and the early phase of visuomotor adaptation. This led to our proposal that motor error information is loaded into spatial working memory and used to update the mapping between visual and motor space [12, 13]. While these findings provide some support that spatial working memory is engaged during the early stages of visuomotor adaptation, the correlational nature of the design leaves room for other interpretations.
The resource depletion framework offers a more direct way to probe the relationship between spatial working memory and visuomotor adaptation, given the view that higher cognitive processes are resource limited and can be temporarily depleted [cf. 14, 15–18]. For example, this within-subject approach was recently adopted by Persson and colleagues [18] who fatigued three distinct interference resolution processes for 18 minutes and then examined subsequent performance on tasks that relied upon different interference resolution mechanisms. These authors reported that subsequent performance was only affected when the new task called upon the same resources as those that were initially depleted, with no deleterious effects observed during tasks utilizing different cognitive resources. Here, we rely upon a similar approach for Experiment 1: selectively fatigue spatial working memory with intensive task performance of a spatial working memory task, and then evaluate performance on tasks that engage either related or unrelated cognitive processes. Given our recent findings regarding the role of spatial working memory during the early adaptation period, we hypothesized that the depletion of spatial working memory resources would lead to a reduced rate of early adaptation. Such a finding would not only provide more direct support for the role of spatial working memory in visuomotor adaptation, but it would also imply that the rate of visuomotor adaptation is dependent on the cognitive status of an individual.
Similar to the resource depletion approach, a resource augmentation approach via training would also provide evidence for a necessary role of spatial working memory in visuomotor adaptation. Generalization of benefits from cognitive training to a non-trained task requires that the two tasks rely upon overlapping cognitive processes and brain regions [19]. Several studies have shown that working memory training can lead to benefits on non-trained working memory tasks that span beyond a single training session [20]. In addition, cognitive training on a dual n-back task, which loads heavily on working memory requirements, has been shown to positively transfer across multiple domains, including fluid intelligence (Gf) and working memory [1, 21, 22]. Given that Gf is associated with problem solving & learning [23], as well as working memory and metacognition [24, 25], training on the same dual n-back task may generalize to improvements in rate of visuomotor adaptation. We evaluated this hypothesis in Experiment 2.
Thus, in the present study, we conducted two separate experiments to further probe the contributions of spatial working memory to visuomotor adaptation. The first experiment used a resource depletion approach targeting spatial working memory, while the second experiment consisted of n-back training targeting working memory over several weeks. In both studies we evaluated the impact on a subsequent visuomotor adaptation task, hypothesizing that spatial working memory depletion would reduce the rate of visuomotor adaptation while working memory augmentation would speed the rate of visuomotor adaptation.
2. Experiment 1 – Spatial Working Memory Resource Depletion
2.1 Methods
2.1.1 Participants
Twenty-eight right-handed participants (19.0 ± 1.1 yrs; 19 men) were recruited from the University of Michigan student population and were paid $10/hour for their participation. Data for two participants were corrupted during data collection, while an additional three performed the card rotation task more than 2.5 SD below the group mean leading to their exclusion, leaving 23 participants (16 males) for data analysis. Each participant signed an IRB-approved informed consent document and filled out a health history questionnaire prior to the experiment.
2.1.2 Experimental Setup and Procedure
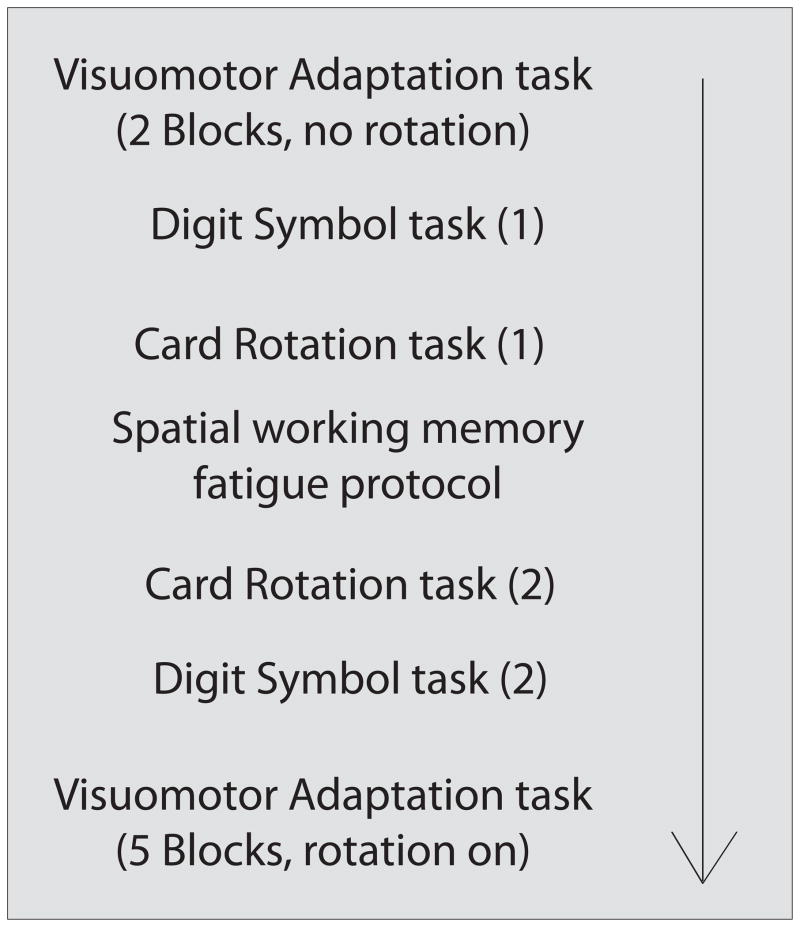
The experiment was performed in a dimly lit room, with stimuli presented on a 15-in. monitor at a 60cm viewing distance. Tasks were presented in a systematic order (Figure 1) to test the pre-post effects of the spatial working memory depletion paradigm. Participants performed the following neuropsychological assessments: (1) mental rotation and spatial relation abilities were tested using Thurston s card rotation (2-D) task [26]; (2) sensorimotor processing speed was determined by the digit – symbol substitution task from WAIS-R [27]. The first (of 2) page of the card rotation task and the original version of the digit symbol task were administered prior to the spatial working memory task. Following the spatial working memory task, the second page of the card rotation task and a second version of the digit symbol task (where the digit symbol mappings were changed) were administered to evaluate potential fatigue effects on spatial working memory and speed of processing. The entire testing period lasted approximately 50 minutes.
Figure 1.
Schematic depiction of the basic design of Experiment 1.
The spatial working memory fatigue protocol was administered using custom E-prime 1.1 software (Psychology Software Tools, Pittsburgh). The task, modeled after the one employed by Reuter-Lorenz et al. [28] and Anguera et al. [12], required participants to memorize a three item target set (three solid circles) in a 500 ms period. Following presentation of the target set, participants saw a blank screen for 3000 ms (retention interval, RI). During this period, they were instructed to mentally 'connect the dots' of the target set, and then mentally rotate this shape by 30° clockwise. Following the RI, participants were given 3000 ms to decide whether the subsequently presented probe set of open circles formed the same configuration as the target set that they had mentally rotated. This was followed by a 1000ms inter-trial interval before the presentation of the subsequent target set. Participants performed 14 blocks (10 trials/block), taking a break of a self-determined duration (approximately 30–60sec) between each block of trials. 70% of the trials were 'match' trials in which the probe set was rotated 30° clockwise. The remaining 'non-match' trials had two of the three probe circles displaced by 1.1cm (hard), 1.5cm (medium), and 1.9cm (easy) from the original target dot configuration. The entire fatigue protocol lasted approximately 20 minutes.
For the visuomotor adaptation task, task presentation and response collection were accomplished with custom LabVIEW 6.1 software (National Instruments, Austin, TX). Targets (0.8 cm in diameter) appeared for 4 sec in one of four locations: 4.8 cm to the right, left, above, or below the centrally located home position (0.8 cm in diameter). Participants controlled a cursor with a dual potentiometer joystick with their thumb and index finger, making small wrist and finger movements to control the joystick, with real-time feedback displayed as a cursor on the projection screen. Participants were asked to move the cursor into the target circle as quickly and accurately as possible, and to maintain the cursor within the circle until the target disappeared. Upon target disappearance, they were told to release the spring-loaded joystick handle so that it would re-center for the subsequent trial. The next trial began 1 sec later, resulting in an inter-trial interval (from one target presentation to the next) of 5 sec. Participants performed 7 blocks (B) of the task (24 trials per block), with the first two experimental blocks (B1-B2) performed under normal visual feedback conditions, whereas the subsequent 5 blocks (B3–B7; adaptation period) were performed with visual feedback rotated 30° clockwise about the center of the screen.
2.1.3 Behavioral Data Processing
For both the card rotation and digit symbol tasks, a difference score of the number correct minus incorrect was calculated. For the spatial working memory task, the response time and percentage of correct responses were calculated for each block. To evaluate fatigue-related effects, we calculated a difference score based upon the mean performance across the 1st 6 blocks versus the subsequent 8 blocks. This division was based on our previous work [12], in which the entire spatial working memory task lasted 6 blocks,1 whereas here we examined how performance would decline in the subsequent 8 blocks. For the visuomotor adaptation task, the x and y coordinates from the joystick were recorded at a rate of 100 Hz. These data were analyzed off-line using custom LabVIEW 6.1 software to track behavioral changes with learning. The data were first filtered with a dual low-pass Butterworth digital filter, using a cutoff frequency of 10 Hz. The resultant joystick path was calculated by computing the square root of the sum of the squared x and y coordinate data at each time point. The tangential velocity profile was then calculated through differentiation of the resultant position data. Movement onset and offset were computed through the application of Teasdale, Bard, Fleury, Young, and Proteau s [29] optimal algorithm to the velocity profile for each movement. Learning was assessed by measuring direction error (DE), which is the angle between a straight line from the start to the target position and the position at peak velocity. We utilized the linear slope across the first three adaptation blocks for each subject to measure the rate of early learning based on our previous work with this task [12]. Each participant s slope, along with their spatial working memory accuracy difference score, were entered into a one-tailed Pearson s correlation analysis (due to the directionally specific nature of our hypothesis) to evaluate the effect of spatial working memory resource depletion on the rate of early visuomotor adaptation.
2.2 Results
Table 1 shows the group mean and standard deviation for performance on each of the neuropsychological tests, the spatial working memory task, and the visuomotor adaptation task. While there was no significant correlation between the change in card performance and the change in spatial working memory accuracy (r= .19, p> .35), we did observe a significant correlation between spatial working memory accuracy over the 1st 6 blocks with pre card performance (r= .40, p< .05), unlike pre digit symbol performance (r= .33, p> .10). In addition there was also a significant correlation between post card performance and the mean accuracy across the final 8 spatial working memory blocks (r= .50, p< .02). Importantly, the spatial working memory fatigue protocol did not lead to a difference in digit symbol performance (t(1,22)= .35, p> .70); however, participants did show a significant reduction in card rotation performance following the fatigue protocol (t(1,22)= 2.48, p< .05). Together these effects suggest that the fatigue protocol specifically affected spatial working memory resources, rather than exerting a general affect on cognitive resources (as measured by speed of processing) or producing generalized fatigue.
Table 1.
Group mean (M) and standard deviation (SD) for each behavioral measure.
| Task | Units | M | SD |
|---|---|---|---|
| Pre-Card rotation | # of correct-incorrect cards (3min) | 68.1* | 11.0 |
| Post-Card rotation | - | 63.8 | 10.0 |
| Pre-Digit symbol | # of correct symbols (2min) | 90.4 | 12.6 |
| Post-Digit symbol | - | 91.0 | 15.1 |
| Spatial working memory | |||
| 1st 6 blocks: Spatial rotation accuracy | % correct | 78%** | 8.7% |
| Final 8 blocks: Spatial rotation accuracy | - | 71% | 9.0% |
| 1st 6 blocks: Spatial rotation RT | ms | 1260 | 182 |
| Final 8 blocks: Spatial rotation RT | - | 1216 | 160 |
| Visuomotor adaptation | |||
| DE (early) | linear slope | 3.6 | 2.8 |
p< .05,
p< .001 between pre/post-test and 1st 6/Final 8.
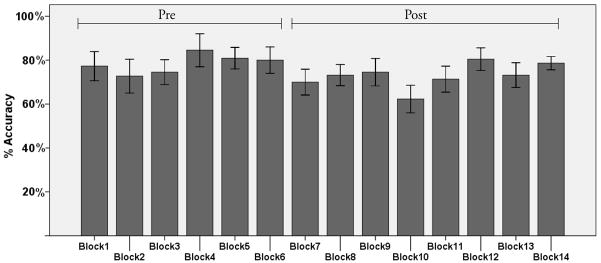
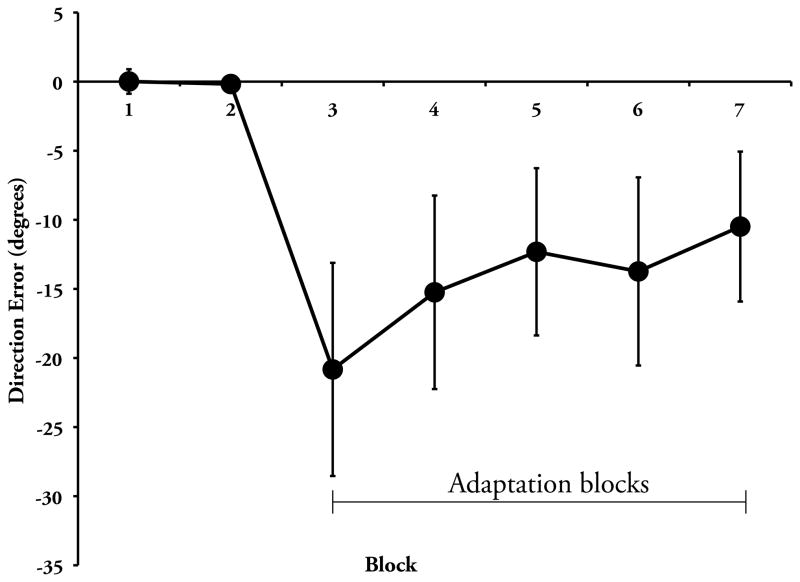
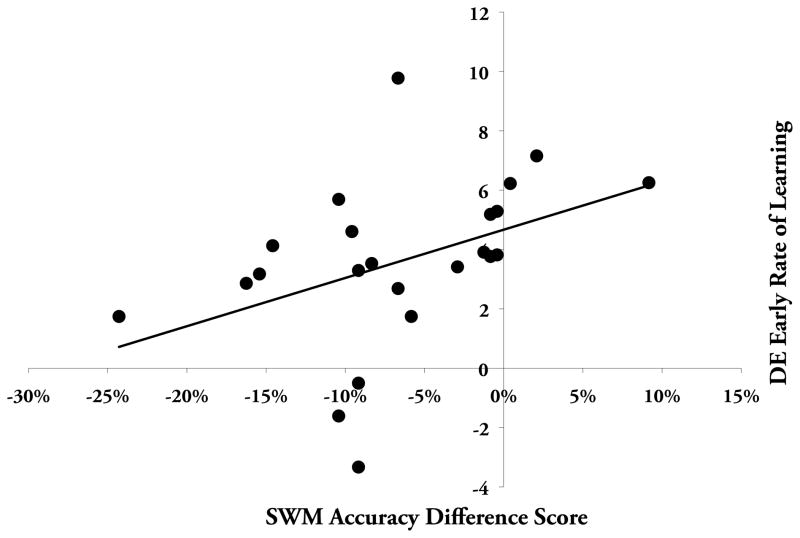
The change in spatial working memory accuracy and the effects on visuomotor adaptation are consistent with this interpretation. Spatial working memory accuracy was significantly reduced following the initial 6 blocks (t(1,22)= 3.70, p< .001; see Figure 2) of the working memory task, while RT did not change (t(1,22)= 1.21, p> .20), supporting the notion that a speed/accuracy trade-off does not underlie the change in task accuracy. Figure 3 illustrates performance by block for visuomotor adaptation. There was no significant correlation between scores on the pre fatigue blocks and the rate of visuomotor adaptation (r= .34, p> .10), suggesting that individual working memory capacity prior to the fatigue protocol was not associated with the rate of adaptation. While we did not see a correlation between DE rate of learning and card rotation task performance using pre (r= −.25, p> .20), post (r= −.14, p> .50), or a difference score (pre - post; r= .16, p> .40), there was a significant correlation between the change in spatial working memory accuracy and the subsequent rate of visuomotor adaptation, as measured by the slope of DE (Figure 4; r=.42, p< .05)2. This finding supports the idea that the depletion of spatial working memory resources negatively affected the rate of early visuomotor adaptation.3
Figure 2.
Spatial working memory performance across each block (group mean ± SE). The spatial working memory fatigue index was calculated by subtracting mean performance across blocks 1–6 from mean performance across blocks 7–14.
Figure 3.
Visuomotor adaptation task performance for participants who underwent spatial working memory resource depletion (group mean ± SD). Blocks 1 and 2 were performed under veridical visual feedback, whereas Blocks 3–7 were performed under 30° clockwise rotation of the display about the center of the screen.
Figure 4.
Correlation between the rate of early adaptation and spatial working memory accuracy difference score assessing the fatiguing of spatial working memory resources (r= −.42, p= .023).
Experiment 2 – Augmenting Working Memory Via Training
2.3 Methods
2.3.1 Participants
Sixty-nine individuals from the University of Michigan and greater Ann Arbor community (21.0 ±3.0 yrs; 35 males) were recruited and paid for their participation. None of these individuals participated in Experiment 1. Participants were randomly assigned to one of two training groups: an n-back training group or a knowledge trainer control group (for more details see 2.3.2 Experimental Setup and Procedure). Four participants withdrew from the study during or after the pre-test (i.e. they did not start training), nine participants dropped out during training (3 from the experimental group, and 6 from the control group), and data for eleven participants were corrupted during data collection for the visuomotor adaptation task while another participant performed the card rotation task more than 2.5 SD below the group mean at pre-test leading to their exclusion. This resulted in a total of 44 participants for our analyses (22 participants in each intervention group; 24 males total). Each participant signed an IRB-approved informed consent document prior to the study, and was paid $150 upon training completion.
2.3.2 Experimental Setup and Procedure
The study was conducted over the course of five weeks in a computer lab at the University of Michigan. Participants completed two days of pre-testing as part of a larger study. Of relevance here, the test battery included a working memory assessment using an n-back task (n= 3 and 4) with abstract shapes [22], an automated operation span task [30], as well as the card rotation task and the digit – symbol substitution task from Experiment 1, and finally, a visuomotor adaptation task. These assessments were then repeated after five weeks of training.
Training
Participants came into the lab 4 to 5 days per week (average = 4.5 days) for approximately 25 minutes of training per session. Before each training session, participants were asked “How motivated are you for today s training session?” using a 1 ( not at all ) to 9 ( extremely ) scale. Similarly, following each training session participants were asked how engaged they were (“How seriously did you train in today s training session?”) using the aforementioned scale. The dual-task intervention (dual n-back; NB) was comparable to the one reported by Jaeggi and colleagues [22]. Participants wore headphones while sitting in front of a computer screen focusing on a central fixation cross. Two sets of stimuli were presented simultaneously every 3 seconds. One set consisted of single letters presented auditorily through the headphones, while the other set consisted of blue squares on a black background displayed in different spatial locations on the computer screen. The goal was to decide if for each modality (auditory or visual) the current stimulus matched the stimulus presented n stimuli back. Participants responded via key press whenever the presented letter matched the one that appeared n stimuli back in the sequence, and another key whenever the currently presented square was at the same position as the one n stimuli back in the series. No responses were required for non-targets. The value of n was always the same for visual and auditory stimuli. The value of n varied between blocks of trials and was adjusted based on an individual s performance level. Thus, as an individual improved his or her performance, the task also became more challenging and n increased by one, or if performance declined then n decreased by one. The task was therefore adaptive in difficulty in an effort to keep it both challenging and engaging for participants throughout the course of training.
The control group trained using a custom-made crystallized intelligence trainer (Knowledge Trainer [KT]) by answering general knowledge, vocabulary, and trivia questions. The KT was fashioned after the dual-task training task such that new questions were presented daily to engage and challenge participants. Questions that were answered incorrectly during a particular session were presented again during the next session. Questions were presented in a multiple-choice format using the same response keys as the n-back training task. They covered a variety of topics including vocabulary, geography, taxonomy, and pop culture. The correct answer was provided after participants responded, and periodically “fun facts” related to the questions were presented to keep participants engaged with the task.
Pre- and Post-Testing
The visual single n-back baseline assessment consisted of 3- and 4-back levels (3 runs at 20+n trials each; 6 targets) using random shapes as stimuli [22]. The operation span task requires participants to recall a sequence of unrelated letters in the correct order in addition to solving a series of simple mathematical equations. We presented three sets of stimuli per set size (i.e., the number of letters to be recalled), and the set sizes ranged from 3 to 7 [30]. The visuomotor adaptation task was presented to participants on the computer using custom Presentation 14.5 software (Neurobehavioral Systems, Albany, CA). The task itself, including target presentation and instruction, was identical to that in Experiment 1 with the exception of the joystick used to make responses (Logitech Extreme 3D joystick (Fremont, CA)). Participants controlled a cursor using a standard gaming joystick placed on the desk in front of them and held the joystick using their whole hand. Participants performed 14 blocks (B) of the task (24 trials per block): one practice block under normal feedback conditions whose data was not included in the analysis, one baseline block (B1) again under veridical feedback, followed by 10 adaptation blocks (B2-B11) with visual feedback rotated 30° clockwise about the center of the screen, and finally two more blocks (B12-B13) again under veridical feedback conditions to test for after-effects of the rotation.
2.3.3 Behavioral Data Processing
A difference score of the number correct minus incorrect was calculated for the card rotation task and the digit symbol task, as well as for the 3- and 4-back tasks. For the operation span task, we used the total number of letters recalled correctly [30]. We analyzed the visuomotor adaptation data off-line using custom MATLAB software (The MathWorks, Inc., Natick, MA) and the same analysis procedures as in Experiment 1. Training improvements on the n-back tasks, operation span, card rotation and the visuomotor adaptation tasks were calculated using difference scores between pre- and post-test performance. As described in Experiment 1, the rate of early adaptation in the visuomotor adaptation task was used to measure performance on this task, in addition to direction error at each period (baseline, adaptation, after-effects)
2.4 Results
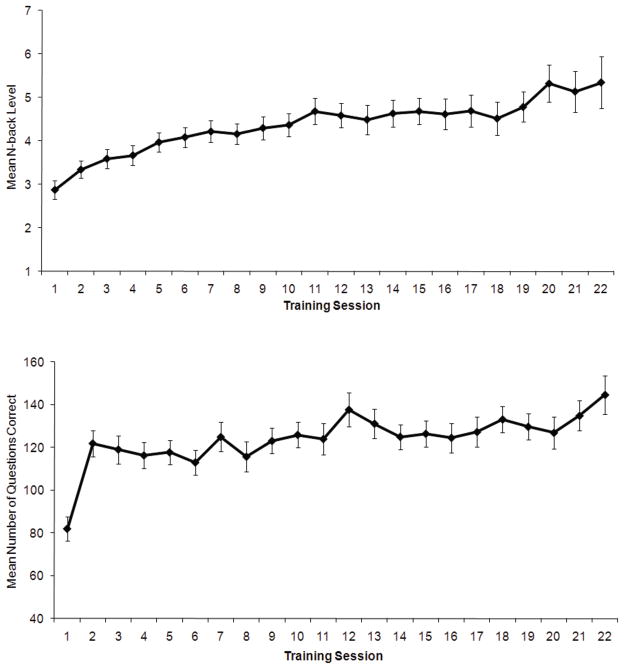
There was no difference in the number of training sessions completed by the NB (21.2 sessions) and the KT groups (22.4 sessions; t(1,42)= −1.40, p> .15). Mean performance over the course of training for the NB group is presented in Figure 5A (average level of n reached during each session), while mean performance for the KT group quantified by the average number of questions answered correctly in each training session is presented in Figure 5B. There was a significant linear performance increase in both groups over the course of the training (NB: y = .09x + 3.34; R2 = .90; P < .001; KT: y = 1.21x + 109.56; R2 = .56; P < .001)4. A group (KT, NB) by session (1st two training sessions, final two training sessions) ANOVA revealed a main effect of motivational decline across the training period (F(1,42)= 5.70, p< .05), a main effect of group showing that the NB group was less motivated than the KT group (F(1,42)= 8.74, p< .01), but no group by session motivational decline (F(1,42)= .005, p> .90). However, a separate group by session ANOVA examining engagement during each training session revealed only a main effect of session (F(1,42)= 21.05, p< .001), without a group difference (F(1,42)= 1.76, p> .15) or group by session interaction (F(1,42)= .20, p> .65), suggesting the level of engagement changed equivalently between groups across the training period.
Figure 5.
Figure 5A. Mean performance over the course of training for the NB group (average level of n reached during each session). 5B: Mean performance over the course of training for the KT group (average number of questions answered correctly in each training session).
Table 2 shows the group means and standard deviation for pre- and post-test performance on working memory using the abstract shape n-back task (n= 3 and 4), operation span, the card rotation task, the digit-symbol substitution task, and the visuomotor adaptation task. A MANOVA with all the cognitive measures as dependent variables was significant (F(5,37)= 4.23, p< .05) showing more transfer overall for the NB group5. Follow-up univariate ANOVAs revealed significant intervention effects for the 3-back (F(1,41)= 4.68, p< .05), 4-back (F(1,41)= 4.70, p< .05), and operation span tasks (F(1,42)= 3.90, p< .05). There were no intervention effects for the digit symbol substitution task (F(1,42)=.23, p>.60) and card rotation task (F(1,42)=.53, p>.40). These results suggest that NB training transfers to tasks that engage similar resources as the training task.
Table 2.
Group mean and standard deviation on cognitive and motor measures pre and post training.
| Pre-Test | Post-Test | Effect Size (Cohen’s d) | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Dual n-back training group (NB) | |||||
| Card Rotation | 52.6 | 21.8 | 62.7 | 16.4 | .52 |
| Digit Symbol Substitution | 69.2 | 8.2 | 76.0 | 7.5 | .86 |
| 3 Back* | .43 | .28 | .74 | .31 | 1.05 |
| 4 Back* | .33 | .25 | .63 | .31 | 1.06 |
| Operation Span* | 40.08 | 16.22 | 51.00 | 14.25 | .71 |
| Visuomotor Adaptation (DE) | 4.95 | 3.62 | 2.71 | 3.93 | -.59 |
| Knowledge training group (KT) | |||||
| Card Rotation | 63.8 | 13.0 | 69.6 | 12.2 | .46 |
| Digit Symbol Substitution | 71.2 | 10.9 | 75.8 | 13.0 | .38 |
| 3 Back* | .46 | .24 | .60 | .35 | .47 |
| 4 Back* | .38 | .24 | .47 | .35 | .30 |
| Operation Span* | 44.48 | 14.50 | 46.87 | 16.29 | .15 |
| Visuomotor Adaptation (DE) | 3.57 | 2.71 | 2.87 | 3.65 | −.22 |
(Card Rotation: # correct-incorrect cards, 2 pages, 6 min; Digit-Symbol: # of symbols, 2 min; 3- & 4-back: accuracy percentage, hits-false alarms; Operation Span: Score; Visuomotor Adaptation DE: linear slope; Significant Session Effects,
p<.05)
Despite the improvement on the training task and transfer to other working memory tasks, there was no group (NB vs. KT) by test-session (pre vs. post) interaction for the rate of early learning (i.e. slope) on the visuomotor adaptation task (F(1,42)= .001, p> .90; Figure 6), nor was there a main effect of test session for the rate of early learning, suggesting an absence of test-retest benefits (F(1,42)= .74, p> .35). Furthermore, neither group showed a correlation between the rate of early learning and slope across the post-adaptation blocks (r< .32, p> .15 for each comparison), with no significant difference in these correlations (z= .14, p> .85).
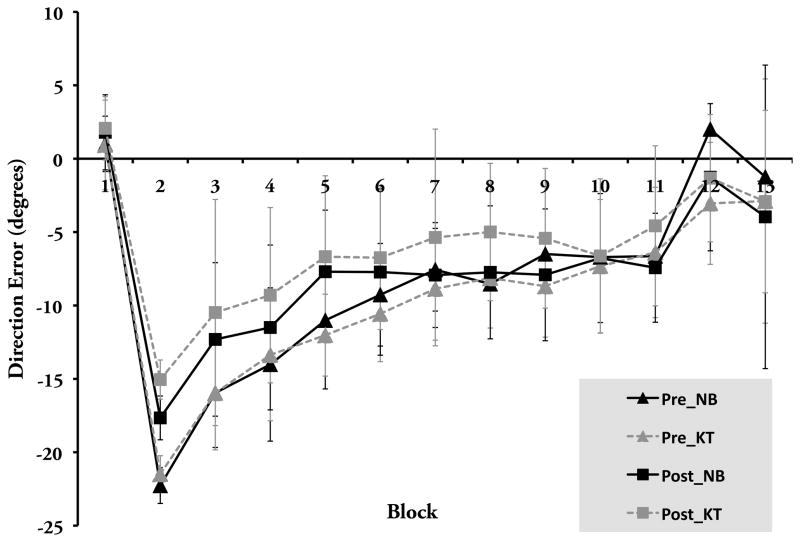
Figure 6.
Visuomotor adaptation task performance for training participants (group mean ± SD). Blocks 1 (baseline) and 12–13 (after-effects) were performed under veridical visual feedback, whereas Blocks 2–11 (adaptation) were performed under 30° clockwise rotation of the display about the center of the screen. Pre refers to performance prior to training, Post refers to performance afterwards. NB: n-back task training group, KT: knowledge training group.
Curiously, baseline performance was worse following the training (F(1,42)= 18.00, p< .001); however, the group by test-session interaction was not significant (F(1,42)= .39, p> .50), indicating this effect was not the result of NB training per se. For the adaptation blocks, there was a session (pre vs. post) by block (10) interaction (F(9,378)= 4.92, p< .001), suggesting an overall improvement in performance over these blocks following training. However, a session (pre vs. post) by block (10) by group ANOVA did not reveal a significant interaction (F(9,378)= 1.11, p> .35) suggesting the training did not have a differential between group effect. Furthermore, block-to-block repeated contrasts of the aforementioned interaction also showed an asymptotic pattern of performance (F(1,43)< 2.30, p> .13 for all block comparisons), providing additional evidence that the NB training was no more effective than the KT training protocol. Finally, for the after-effect blocks a group (2) by session (2) by washout block (2) ANOVA failed to reach significance (F(1,42)= 1.22, p> .25), supporting the idea again that there was no differential training benefit observed.
The change in motivation and engagement across the experiment was not related to any change in the rate of early adaptation, as there was no correlation between the difference in the rate of early learning (pre-post) and either the change in motivation (r= -.04, p> .80) or engagement (r= .07, p> .60). Thus, though an intensive NB training paradigm improved performance on a variety of WM measures, there was no beneficial transfer of these effects to the visuomotor adaptation task. Moreover, motivation declines do not appear to be the cause of this lack of transfer.
3. Discussion
In two experiments we evaluated the effects of spatial working memory depletion and potential augmentation via training on the rate of sensorimotor adaptation. The findings from the first experiment provide new evidence supporting the role of spatial working memory during the early stages of visuomotor adaptation: working memory resource depletion was associated with reduced rates of adaptation. However, working memory augmentation via training did not transfer to improvements in the rate of visuomotor adaptation, suggesting that spatial working memory capacity may not be the factor limiting maximal rates of visuomotor adaptation in young adults. These findings are discussed further below with respect to motor learning viewed from a resource limitation / capacity framework.
3.1 Spatial working memory depletion and visuomotor adaptation
Similar to the approach taken by Persson and colleagues [18], the depletion of spatial working memory resources resulting from intensive task performance was validated by the decline in card rotation performance (a measure of spatial working memory that also correlated with the spatial working memory depletion task). One alternative explanation regarding these findings is that the fatigue effect was not due to the spatial working memory protocol per se, but more general fatigue over time. While an inherent limitation of the present study is the absence of a control group performing a non-fatiguing task to directly exclude this possibility, the depletion effect did show specificity to spatial working memory faculties, as digit symbol substitution task performance (a measure of processing speed based on central executive cognitive processing) was unchanged following the fatigue protocol. These findings are consistent with those reported by Persson and colleagues [18], as their 20 minute within-subject depletion protocol led to specific performance impairments in related cognitive domains while leaving those unrelated intact.
Most pertinent to the present study, larger changes in spatial working memory performance across the fatigue protocol were correlated with slower rates of subsequent visuomotor adaptation. While the strength of the observed correlation was modest, it is in line with the hypotheses put forth here regarding the specificity of the resource depletion approach. Furthermore, this result extends our previous findings that show spatial working memory is associated with the rate of visuomotor adaptation, and that the two tasks share overlapping neural resources [12]. More specifically, we previously observed the engagement of the right DLPFC and bilateral IPLs during both the early adaptation period and spatial working memory task, suggesting the use of SWM processes to resolve the mental rotation aspects of visuomotor adaptation [12]. The current work provides more direct evidence regarding the role of spatial working memory processes during the early stages of visuomotor adaptation.
The present findings are congruent with a limited-resource model of executive function, similar to the proposal put forth by Persson et al. [18]: higher cognitive processes are resource limited and can be temporarily depleted, affecting performance on subsequent tasks that rely upon these depleted faculties. While the resource depletion approach was previously evidenced by studies examining the suppression of stereotypes, negative attitudes, and self-regulation [32, 33] as well as interference resolution [18], we have now extended its use to the study of motor learning dependent upon spatial working memory processes. We have proposed that motor error information loaded into spatial working memory may be used to update the mapping between visual and motor space for subsequent actions [12, 13]. A participant in this paradigm must recall the original, veridical visuomotor map, mentally rotate this map with their spatial working memory resources, and then use this updated mapping on subsequent movements. In the current experiment, the depletion of spatial working memory resources may have negatively affected this process, resulting in slower visuomotor adaptation. Thus, the rate of visuomotor adaptation is dependent on an individual s moment-to-moment cognitive status, including spatial working memory resource availability.
3.2 Augmentation of (spatial) working memory and visuomotor adaptation
Dual n-back training did not have a beneficial effect on visuomotor adaptation performance; more specifically, the rate of early visuomotor adaptation did not benefit from cognitive training. The lack of transfer to visuomotor adaptation performance was unexpected, given that the neural correlates associated with Gf are at prefrontal and parietal regions [24] comparable to regions we have previously reported to be active for both spatial working memory and visuomotor adaptation tasks [12]. However, transfer to a non-learned task following training is believed to be limited by the amount of direct neural overlap between the underlying processes that mediate both tasks [19]. While previous work has shown that the right DLPFC is activated during this n-back task[34], how activity changes at this and other neural regions engaged during this particular n-back task following training have not been measured. Therefore, the degree of overlap between neural regions engaged for this task and the visuomotor adaptation task is unknown.
Instead, these findings appear to be more consistent with the idea that participants had already reached their respective capacities regarding the allocation and/or engagement of spatial working memory resources for motor learning improvements. Although working memory training had an effect on a measure of general working memory capacity (operation span), it did not lead to a significant improvement in performance of the card rotation task. This suggests the possibility that either the visuospatial processes needed for card rotation were not sufficiently taxed with NB training, or that this training did not generalize its benefits to mental rotation abilities. Indeed, the n-back training task did not involve a spatial transformation component as in the card rotation and spatial working memory fatigue protocol, which is a limitation of the training paradigm in trying to directly manipulate spatial working memory. Our previous work has shown that the association between visuomotor adaptation and spatial working memory is best evidenced by the involvement of a comparable mental rotation effect (in this case, 2-dimensional mental rotations for the card rotation task and spatial working memory task), as performance of a 3-dimensional mental rotation task did not show a significant correlation [12]. One important aspect regarding the design of the present study is that the visuomotor adaptation evaluation prior to/following training was one of a number of tasks that participants completed in the course of a greater study testing the breadth of transfer following the n-back working memory training. That is, the evaluation of visuomotor adaptation following this type of training was a part of a larger, prescribed training study which prevented us from incorporating aspects of mental rotation in a more direct fashion, which we acknowledge is an inherent limitation of the present study.
However, this particular cognitive training paradigm has shown broad transfer abilities to both near and distant (i.e. visuospatial) domains such as matrix reasoning [1, 31] that suggested the possibility of boosting these spatial working memory resources. This interpretation agrees with the modest gains observed on most of the cognitive measures tested after training, unlike the visuomotor adaptation task. Thus, training-related gains in visuomotor adaptation performance would not materialize in this setting given that these participants were already at capacity with respect to working memory faculties, and the breadth of the training did not generalize to 2-dimensional mental rotation abilities. However, the observed pattern of results is not unexpected if one considers that spatial working memory capacity may not be the only potential limiting factor regarding the rate of visuomotor adaptation.
There are several other processes that contribute to the underlying mechanisms engaged during visuomotor adaptation, such as spatial attention [8, 10], error detection [35, 36], and motor planning [37, 38], among others. The possibility exists that the modulation of any of these processes would also lead to changes in the rate of visuomotor adaptation. Future studies that manipulate these other components could extend the present findings and provide a more thorough characterization of visuomotor adaptation processes.
Another potential reason for the absence of training transfer to visuomotor adaptation gains may involve the role of explicit cognitive strategies. The transfer of training gains to an unlearned task has been shown to be facilitated by the use of explicit strategies [39]. This notion has been exploited by several previous cognitive training studies [40–43], as practice alone does not always lead to the development of effective performance strategies [44]. Indeed, our recent work has demonstrated that an explicit strategy can provide rapid performance improvements during sensorimotor adaptation [7]. While implicit aspects of sensorimotor adaptation have been suggested to override explicit strategies [38], recent work has demonstrated that certain strategic processes may be able to work in concert with the implicit effects of sensorimotor adaptation [45]. Cognitive instruction that matches the adaptation process may facilitate visuomotor adaptation following n-back training; however, in the present study cognitive benefits that generalized from training were not directly transformed into an explicit approach for visuomotor adaptation. Thus, in summary, the integration of the observed cognitive training benefits for visuomotor adaptation may have been precluded in part by: i) participants reliance on spatial working memory during visuomotor adaptation already being at a ceiling level, and / or ii) the implicit nature of visuomotor adaptation.
A final caveat regarding the cognitive training findings involves the amount of effort put forth by participants during the post-visuomotor adaptation testing. Motivation has been shown to play a critical role as evidenced through both behavioral and neural measures during n-back tasks [46–48], engaging a distributed network comprising the DLPFC, the superior parietal lobule, and precentral regions. Most related to the present study, Jaeggi and colleagues [46] suggested that the activation patterns observed at these regions were affected when participants were not trying to achieve their best performance. In this case, the load-dependent activations were believed to represent a more global network that involved motivation [49, 50] with respect to strategic task processing. In the present study, both motivation and engagement declined across training for each group, with the NB group showing overall less motivation than the KT group (p< .05). However, the equivalent decline in training engagement suggests that while NB participants arrived at training with less motivation, they still gave a similar effort as the KT group during training. There is evidence for this in the present study when comparing pre- and post-baseline performance, as movements under veridical feedback should not have differed or become worse following training. Likewise, there was no correlation between the change in motivation and change in the rate of learning from pre to post. In addition, despite declines in motivation and engagement, NB participants still exhibited training related gains and also showed transfer to other measures of working memory.
3.2 Conclusions
The present findings extend the correlational nature of our previous work [12] as the fatiguing of spatial working memory resources affected the rate of visuomotor adaptation. A 5-week cognitive training intervention targeting working memory resources had no effect on the rate of visuomotor adaptation, suggesting that spatial working memory resources i) were already at capacity prior to training and ii) may not be the rate-limiting factor of visuomotor adaptation in young adults. In summary, these findings suggest that augmenting the rate of sensorimotor adaptation not only requires the direct engagement of spatial working memory resources, but may also benefit from the incorporation of explicit strategies in a manner that is congruent with the adaptation process.
Highlights.
We examined the manipulation of working memory resources on visuomotor adaptation.
The depletion of spatial working memory resources perturbed the rate of adaptation.
The enhancement of working memory resources had no effect on visuomotor adaptation.
Working memory may not be the rate-limiting factor of adaptation in young adults.
Acknowledgments
This work was supported by the National Institutes of Health [AG024106 (RS)], the UM NIH Claude D. Pepper Center Human Subjects and Assessment Core (NIH AG024824) and a Michigan Center for Advancing Safe Transportation throughout the Lifespan Research Excellence Program Grant # DTRT07-G-0058. JAB is supported by the National Institutes of Health (NIA T32 AG000114, R. Miller, PI). JAB and RDS are members of The LIFE Course: Evolutionary and Ontogenetic Dynamics; JAA is a LIFE alumnus.
Footnotes
Across these 1st 6 blocks, both accuracy and response time were comparable to the performance observed in Anguera et al. (2010), where 6 blocks were used as well.
This effect was significant using either a 1 or 2 tailed approach.
One may argue that a better construction of such a difference score would be to take either i) the 1st 3– final 3 spatial working memory blocks or ii) the 1st 2 – final 2. In either case, the selection of these alternative difference scores led to the same significant effects result reported above (r= .43 and .44, respectively).
As seen in Figure 4B, performance during the first session was lower than the other training sessions. However, the exclusion of this session still results in a significant linear increase: y = .89x + 114.99; R2 = .64; P < .001.
One participant failed to complete the single n-back task, thus the MANOVA had (5,37) degrees of freedom.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6829–33. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bock O. Adaptation of aimed arm movements to sensorimotor discordance: evidence for direction-independent gain control. Behavioural brain research. 1992;51:41–50. doi: 10.1016/s0166-4328(05)80310-9. [DOI] [PubMed] [Google Scholar]
- 3.Ghilardi M, Ghez C, Dhawan V, Moeller J, Mentis M, Nakamura T, et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871:127–45. doi: 10.1016/s0006-8993(00)02365-9. [DOI] [PubMed] [Google Scholar]
- 4.Pine ZM, Krakauer JW, Gordon J, Ghez C. Learning of scaling factors and reference axes for reaching movements. Neuroreport. 1996;7:2357–61. doi: 10.1097/00001756-199610020-00016. [DOI] [PubMed] [Google Scholar]
- 5.Seidler RD, Noll DC, Chintalapati P. Bilateral basal ganglia activation associated with sensorimotor adaptation. Experimental brain research. Experimentelle Hirnforschung. 2006;175:544–55. doi: 10.1007/s00221-006-0571-y. [DOI] [PubMed] [Google Scholar]
- 6.Welch RB, Choe CS, Heinrich DR. Evidence for a three-component model of prism adaptation. Journal of experimental psychology. 1974;103:700–5. doi: 10.1037/h0037152. [DOI] [PubMed] [Google Scholar]
- 7.Benson BL, Anguera JA, Seidler RD. A spatial explicit strategy reduces error but interferes with sensorimotor adaptation. Journal of neurophysiology. 2011 doi: 10.1152/jn.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eversheim U, Bock O. Evidence for processing stages in skill acquisition: a dual-task study. Learn Mem. 2001;8:183–9. doi: 10.1101/lm.39301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNay EC, Willingham DB. Deficit in learning of a motor skill requiring strategy, but not of perceptuomotor recalibration, with aging. Learn Mem. 1998;4:411–20. doi: 10.1101/lm.4.5.411. [DOI] [PubMed] [Google Scholar]
- 10.Taylor JA, Thoroughman KA. Divided attention impairs human motor adaptation but not feedback control. Journal of neurophysiology. 2007;98:317–26. doi: 10.1152/jn.01070.2006. [DOI] [PubMed] [Google Scholar]
- 11.Taylor JA, Thoroughman KA. Motor adaptation scaled by the difficulty of a secondary cognitive task. PLoS ONE. 2008;3:e2485. doi: 10.1371/journal.pone.0002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Contributions of spatial working memory to visuomotor learning. Journal of cognitive neuroscience. 2010;22:1917–30. doi: 10.1162/jocn.2009.21351. [DOI] [PubMed] [Google Scholar]
- 13.Seidler RD, Benson BL, Boyden NB, Kwak Y. Skill acquisition. In: Oschner K, Kosslyn S, editors. Invited review for the Oxford Handbook of Cognitive Neurosciencein press. [Google Scholar]
- 14.Engle RW, Conway ARA, Tuholski SW, Shisler RJ. A Resource Account of Inhibition. Psychological Science. 1995;6:122–5. [Google Scholar]
- 15.Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin. 2000;126:247–59. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- 16.Parasuraman R. In: The attentive brain. Parasuraman R, editor. Cambridge: MIT Press; 1998. [Google Scholar]
- 17.Vohs KD, Heatherton TF. Self-regulatory failure: a resource-depletion approach. Psychol Sci. 2000;11:249–54. doi: 10.1111/1467-9280.00250. [DOI] [PubMed] [Google Scholar]
- 18.Persson J, Welsh KM, Jonides J, Reuter-Lorenz PA. Cognitive fatigue of executive processes: interaction between interference resolution tasks. Neuropsychologia. 2007;45:1571–9. doi: 10.1016/j.neuropsychologia.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dahlin E, Neely AS, Larsson A, Backman L, Nyberg L. Transfer of learning after updating training mediated by the striatum. Science. 2008;320:1510–2. doi: 10.1126/science.1155466. [DOI] [PubMed] [Google Scholar]
- 20.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nature neuroscience. 2004;7:75–9. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- 21.Chein JM, Morrison AB. Expanding the mind's workspace: training and transfer effects with a complex working memory span task. Psychonomic Bulletin & Review. 2010;17:193–9. doi: 10.3758/PBR.17.2.193. [DOI] [PubMed] [Google Scholar]
- 22.Jaeggi SM, Studer-Luethi B, Buschkuehl M, Su YF, Jonides J, Perrig WJ. The Relationship Between N-back Performance and Matrix Reasoning - Implications for Training and Transfer. Intelligence. 2010;38:625–35. [Google Scholar]
- 23.Carpenter PA, Just MA, Shell P. What one intelligence test measures: a theoretical account of the processing in the Raven Progressive Matrices Test. Psychological review. 1990;97:404–31. [PubMed] [Google Scholar]
- 24.Gray JR, Chabris CF, Braver TS. Neural mechanisms of general fluid intelligence. Nat Neurosci. 2003;6:316–22. doi: 10.1038/nn1014. [DOI] [PubMed] [Google Scholar]
- 25.Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nature reviews. 2004;5:471–82. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- 26.Ekstrome R, French J, Harman H. Manual for kit of factor referenced cognitive tests. Princeton, New Jersey: Educational Testing Service; 1976. [Google Scholar]
- 27.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 28.Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. Journal of cognitive neuroscience. 2000;12:174–87. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- 29.Teasdale N, Bard C, Fleury M, Young DE, Proteau L. Determining movement onsets from temporal series. J Mot Behav. 1993;25:97–106. doi: 10.1080/00222895.1993.9941644. [DOI] [PubMed] [Google Scholar]
- 30.Unsworth N, Heitz RP, Schrock JC, Engle RW. An automated version of the operation span task. Behav Res Methods. 2005;37:498–505. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]
- 31.Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18:394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- 32.Richeson JA, Baird AA, Gordon HL, Heatherton TF, Wyland CL, Trawalter S, et al. An fMRI investigation of the impact of interracial contact on executive function. Nature neuroscience. 2003;6:1323–8. doi: 10.1038/nn1156. [DOI] [PubMed] [Google Scholar]
- 33.Vohs KD, Schmeichel BJ. Self-regulation and the extended now: controlling the self alters the subjective experience of time. Journal of personality and social psychology. 2003;85:217–30. doi: 10.1037/0022-3514.85.2.217. [DOI] [PubMed] [Google Scholar]
- 34.Jaeggi SM, Buschkuehl M, Etienne A, Ozdoba C, Perrig WJ, Nirkko AC. On how high performers keep cool brains in situations of cognitive overload. Cogn Affect Behav Neurosci. 2007;7:75–89. doi: 10.3758/cabn.7.2.75. [DOI] [PubMed] [Google Scholar]
- 35.Anguera JA, Seidler RD, Gehring WJ. Changes in performance monitoring during sensorimotor adaptation. Journal of neurophysiology. 2009;102:1868–79. doi: 10.1152/jn.00063.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinder MR, Riek S, Tresilian JR, de Rugy A, Carson RG. Real-time error detection but not error correction drives automatic visuomotor adaptation. Experimental brain research. Experimentelle Hirnforschung. 2010;201:191–207. doi: 10.1007/s00221-009-2025-9. [DOI] [PubMed] [Google Scholar]
- 37.Kawato M. Internal models for motor control and trajectory planning. Current opinion in neurobiology. 1999;9:718–27. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- 38.Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci. 2006;26:3642–5. doi: 10.1523/JNEUROSCI.5317-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahncke HW, Connor BB, Appelman J, Ahsanuddin ON, Hardy JL, Wood RA, et al. Memory enhancement in healthy older adults using a brain plasticity-based training program: a randomized, controlled study. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12523–8. doi: 10.1073/pnas.0605194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baron A, Mattila WR. Response slowing of older adults: effects of time-limit contingencies on single- and dual-task performances. Psychology and aging. 1989;4:66–72. doi: 10.1037//0882-7974.4.1.66. [DOI] [PubMed] [Google Scholar]
- 41.Bherer L, Kramer AF, Peterson MS, Colcombe S, Erickson K, Becic E. Transfer effects in task-set cost and dual-task cost after dual-task training in older and younger adults: further evidence for cognitive plasticity in attentional control in late adulthood. Experimental aging research. 2008;34:188–219. doi: 10.1080/03610730802070068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kramer AF, Larish JF, Strayer DL. Training for Attentional Control in Dual-Task Settings - a Comparison of Young and Old Adults. Journal of Experimental Psychology-Applied. 1995;1:50–76. [Google Scholar]
- 43.Verhaeghen P, Marcoen A, Goossens L. Improving Memory Performance in the Aged through Mnemonic Training - a Meta-Analytic Study. Psychology and aging. 1992;7:242–51. doi: 10.1037//0882-7974.7.2.242. [DOI] [PubMed] [Google Scholar]
- 44.Maquestiaux F, Hartley AA, Bertsch J. Can practice overcome age-related differences in the psychological refractory period effect? Psychology and aging. 2004;19:649–67. doi: 10.1037/0882-7974.19.4.649. [DOI] [PubMed] [Google Scholar]
- 45.Taylor JA, Ivry RB. Flexible Cognitive Strategies during Motor Learning. PLoS computational biology. 7:e1001096. doi: 10.1371/journal.pcbi.1001096. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaeggi SM, Seewer R, Nirkko AC, Eckstein D, Schroth G, Groner R, et al. Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. NeuroImage. 2003;19:210–25. doi: 10.1016/s1053-8119(03)00098-3. [DOI] [PubMed] [Google Scholar]
- 47.Pochon JB, Levy R, Fossati P, Lehericy S, Poline JB, Pillon B, et al. The neural system that bridges reward and cognition in humans: An fMRI study. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:5669–74. doi: 10.1073/pnas.082111099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:10081–6. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Brain research. 1996;5:175–81. doi: 10.1016/s0926-6410(96)00054-7. [DOI] [PubMed] [Google Scholar]
- 50.Ingvar DH. The will of the brain: cerebral correlates of willful acts. Journal of theoretical biology. 1994;171:7–12. doi: 10.1006/jtbi.1994.1206. [DOI] [PubMed] [Google Scholar]