Abstract
In adult male rats, vasopressin (AVP) facilitates social recognition via activation of V1a receptors within the lateral septum. Much less is known about how AVP affects social recognition in adult females or in juvenile animals of either sex. We found that administration of the specific V1a receptor antagonist (CH2)5Tyr(Me)AVP into the lateral septum of adult rats impaired, whereas AVP extended, social discrimination in both sexes. In juveniles, however, we detected a sex difference, such that males but not females showed social discrimination. Interestingly, administration of the V1a receptor antagonist to juveniles (either intracerebroventricularly or locally in the lateral septum) did not prevent social discrimination, but instead significantly decreased the investigation of a novel as opposed to a familiar animal in both sexes, with stronger effects in males. V1a receptors were found to be abundantly expressed in the lateral septum with higher binding density in females than in males at both ages. These findings demonstrate that activation of V1a receptors in the septum is important for social recognition in both sexes, and that the roles of septal V1a receptors in social recognition change during development.
Keywords: septum, social discrimination, social memory, male, female, V1a receptor
INTRODUCTION
Social recognition reflects the ability to distinguish familiar from unfamiliar individuals and is essential for effective and appropriate social interaction and communication. In both adult male rats and mice, the neuropeptide arginine vasopressin (AVP) facilitates social recognition via activation of the V1a receptor (V1aR) in the lateral septum. For example, septal infusions of AVP improve social recognition in male rats, whereas infusions of AVP V1aR antagonists impair it (Dantzer et al., 1988; Engelmann and Landgraf, 1994; Everts and Koolhaas, 1997, 1999). In addition, over-expression of V1aR in the lateral septum using viral vector-mediated gene transfer extends social recognition in male rats (Landgraf et al., 2003). Finally, male mice lacking the V1aR show impaired social recognition (Bielsky et al., 2004; but see also Wersinger et al., 2007), which was normalized by viral vector-mediated V1aR expression in the lateral septum (Bielsky et al., 2005a).
Several findings suggest that septal AVP may be more important for social recognition in males than in female rats. Although the role of the septal V1aR has never been directly tested in females, V1aR antagonists injected peripherally or intracerebroventricularly do not impair social recognition in adult females (Bluthe & Dantzer, 1990; Engelmann et al., 1998). This may be related to sex differences in septal AVP innervation. The lateral septum receives most of its AVP innervation from the bed nucleus of the stria terminalis (BNST) and the medial amygdala (MeA) (De Vries & Buijs, 1983; Caffe et al., 1987). Male rats typically have more AVP-expressing cells in the BNST and MeA and denser AVP neuronal fibers in the lateral septum (De Vries et al., 1981, Van Leeuwen et al., 1985; Miller et al., 1989). This sex difference in septal AVP fiber density becomes evident by the second week of life and appears even more pronounced in 5-week-old juveniles than in adults (De Vries et al., 1981). This suggests that AVP may be more important for male social recognition than for female social recognition in juveniles as well as adults. As AVP fiber density in the lateral septum and AVP mRNA levels in the BNST and MeA are lower in juveniles than in adults (De Vries et al., 1981; Szot & Dorsa 1993), we also hypothesized that AVP is more important for the regulation of social recognition in adult rats than in juveniles. We used the social discrimination paradigm (Engelmann et al., 1995; Lukas et al., 2011) to measure the effects of blockade of V1aR (central or in the lateral septum) or administration of AVP (in the lateral septum) on social discrimination abilities of juvenile and adult rats of both sexes. We demonstrate here that the V1aR in the lateral septum is involved in social recognition in sex- and age-specific ways.
MATERIAL AND METHODS
Animals
Wistar rats were obtained from Charles River (Raleigh, NC) and maintained under standard laboratory conditions (12 h light/dark cycle, lights off at 14:00 h, 22°C, 50% humidity, food and water ad libitum). Rats were housed in same-sex groups of 2 (adults) or 4 (juveniles) in standard rat cages (48 × 27 × 20 cm). The experiments were conducted in accordance with the guidelines of the NIH and approved by the University of Massachusetts Institutional Animal Care and Use Committee.
Cannulation
After one week of daily handling to familiarize rats with the injection procedure, juvenile (33 days of age) and adult (12 weeks of age) males and females were anesthetized with isoflurane (Butler Schein Animal Health, Dublin, OH) and mounted on a stereotaxic frame with the tooth bar set at −4.5 mm. Guide cannulae (21 gauge for lateral ventricle, 22 gauge for lateral septum; Plastics One, Roanoke, VA) were implanted stereotaxically 2 mm dorsal to the targets, which for injections into the lateral ventricle (juveniles only) were 1.0 mm caudal to bregma, - 1.6 mm lateral to the midline, and 2.0 mm ventral to the surface of the skull and for injections into the lateral septum 0.4 (juvenile) or 0.3 (adult) mm caudal to bregma, −1.0 mm lateral to the midline, 3.6 (juvenile) or 4.0 (adult) mm ventral to the surface of the skull. For septal injections, cannulae were implanted under an angle of 10° from the midsagittal plane to avoid damage to the sagittal sinus. Cannulae were fixed to the skull with two stainless steel screws and Cerebond adhesive and closed with a dummy cannula (Plastics One, Roanoke, VA). After surgery, rats were individually housed in standard rat cages (48 × 27 × 20 cm). Histology was used to check proper placement of the cannulae.
Social discrimination test
The ability of rats to discriminate between a previously encountered (familiar) and a novel rat was tested according to Lukas et al. (2011). A 3-week-old sex-matched stimulus rat was introduced into the home-cage of the experimental rat for 4 min. After an interval of 1 h (Experiments 1 and 2), or 2 or 3 h (Experiment 3), the now familiar rat was reintroduced along with a novel 3-week-old sex-matched rat for 4 min. Tests were performed at the end of the light phase between 10:00 h and 12:00 h. All tests were videotaped and the time spent investigating the stimulus rats (sniffing the anogenital and head/neck regions) was measured by a researcher blind to the treatment condition using JWatcher (http://galliform.psy.mq.edu.au/jwatcher/). The percentage of time investigating the novel rat (time investigating novel rat/time investigating familiar + novel rat × 100) was measured. A subject showed social discrimination when the percentage of time the subject spent investigating the novel rat differed significantly from chance level (50 %). The absolute time investigating the familiar + novel rat was measured to verify that treatments did not alter social investigation behavior.
Experimental procedures
Two days after cannulation, rats were exposed to the social discrimination test in their home cage. To determine the effects of pharmacological manipulation of the AVP system on the formation of social memory, rats received an injection immediately after exposure to the stimulus animal. The injection was given into the lateral ventricle (Exp. 1) or lateral septum (Exp. 2 and 3) via an injector cannula extending 2 mm beyond the guide cannula and connected via polyethylene tubing to a Hamilton syringe. The injector cannula was kept in place for 30 s following injection to allow for tissue uptake and was then replaced by a dummy cannula.
Experiment 1: Effects of central blockade of V1aR on social discrimination in juveniles
Juveniles received an injection into the lateral ventricle with either Ringer’s solution (vehicle) (pH 7.4; 5 µl Ringer; males n=10; females n=10) or the specific V1aR antagonist d(CH2)5Tyr(Me)AVP (V1aR-A) (0.75 µg/5 µl Ringer; males n=10; females n=10) and tested for social recognition 1 h later. The interval period of 1 h was chosen because juvenile male rats showed successful social discrimination at this interval period (Lukas et al., 2011).
Experiment 2: Effects of blockade of V1aR in the lateral septum on social discrimination in juveniles and adults
Rats received an injection into the lateral septum with either Ringer’s solution (vehicle) (0.5 µl Ringer; male juveniles n=7; female juveniles n=8; male adults n=8; female adults n=7) or V1aR-A (10 ng/0.5 µl Ringer; male juveniles n=8; female juveniles n=7; male adults n=8; female adults n=9) and tested for social recognition 1 h later. The interval period of 1 h was chosen because juvenile and adult male rats showed successful social discrimination at this interval period (Lukas et al., 2011).
Experiment 3: Effects of AVP in the lateral septum on social discrimination in juveniles and adults
Rats received an injection into the lateral septum with either Ringer’s solution (vehicle) (0.5 µl Ringer; male juveniles n=10; female juveniles n=9; male adults n=9; female adults n=7) or AVP (200 pg/0.5 µl Ringer; male juveniles n=7; female juveniles n=6; male adults n=9; female adults n=6). Pilot studies demonstrated a lack of social discrimination after an interval of 2 h (juvenile males and females, and adult males) or 3 h (adult females). Accordingly, rats were tested for extended effects of AVP on social recognition after an interval of 2 h (juvenile males and females and adult males) or 3 h (adult females).
Experiment 4: V1aR binding density in the lateral septum
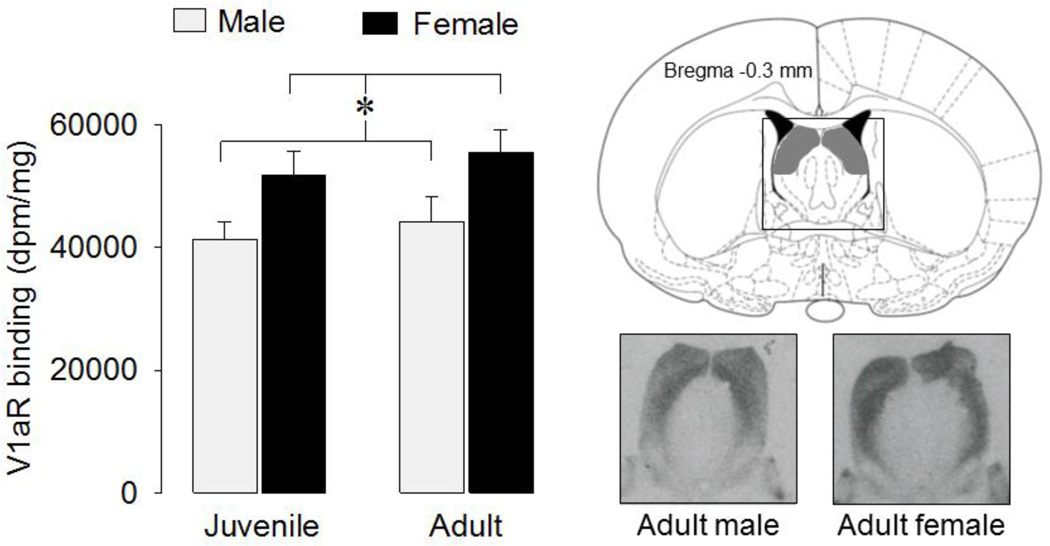
To test for sex and age differences in V1aR binding in the lateral septum, a different set of juvenile males (n=8), juvenile females (n=6), adult males (n=6) and adult females (n=6) were killed with CO2 and quickly decapitated. Brains were removed and quickly frozen in pre-chilled n-methyl butane on dry ice, and stored at −20°C. Brains were cut with a cryostat into 16 µm coronal sections that were thaw-mounted on slides and then stored at −80°C. Receptor autoradiography was performed according to Lukas et al. (2010) using a linear V1aR antagonist [125I]-d(CH2)5Tyr(Me)AVP (Perkin Elmer, USA) as tracer. Briefly, the slides were thawed and dried at room temperature followed by a short fixation in paraformaldehyde (0.1%). The slides were washed two times in 50 mM Tris (pH 7.4), exposed to tracer buffer (50 pM tracer, 50mM Tris, 10mM MgCl2, 0.01% BSA) for 60 min, and washed four times in Tris + 10mMMgCl2. The slides were then shortly dipped in pure water, air-dried, exposed to Biomax MR films (Kodak), and developed after eight days. The optical density of V1aR was measured using ImageJ (NIH, http://rsb.info.nih.gov/ij/). Receptor density was calculated per rat by taking the mean of bilateral measurements of four to six brain sections containing the lateral septum at bregma −0.1 ± 0.3 mm (Paxinos & Watson, 1998). At that level, V1aR binding density in the lateral septum is sharply delineated by the ventricles laterally and by the myelinated fibers of the fornix and fimbriae medially and of the corpus callosum dorsally. These features are also easily recognized in unstained sections, which we used to verify location. V1aR binding density was measured in the area bordered by the corpus callosum dorsally, the fornix fimbriae medially, the ventricles laterally, and a horizontal line that connected the lateral septum at its widest point ventrally was outlined (see shaded area in Fig. 4). After subtraction of tissue background, the data were converted to disintegrations per minute/milligram (dpm/mg) tissue using a [125I] standard microscale (American Radiolabeled Chemicals Inc, St. Louis, MO).
Figure 4. V1aR binding densities in the lateral septum.
Females showed higher V1aR binding density than males (*: p < 0.01, two-way ANOVA). Bars indicate means + SEM. The shaded area in the diagram indicates the area of the lateral septum where V1aR binding density was measured. Images represent coronal sections of the lateral septum showing V1aR binding in the lateral septum of an adult male and adult female.
Statistics
Total social investigation time (time investigating the familiar + novel rat in sec) was analyzed using a two-way ANOVA (sex × treatment; Experiment 1) or a three-way ANOVA (age × sex × treatment; Experiments 2 and 3). Social discrimination was measured by testing whether the time subjects spent investigating the novel rat differed from chance level (50 %) using one sample t-tests. Differences in the percentage of investigation time that subjects spent investigating the novel rat among groups were analyzed using a two-way ANOVA (sex × treatment) for Experiment 1 or a three-way ANOVA (age × sex × treatment) for Experiments 2 and 3. For Experiment 4, V1aR binding density was analyzed using a two-way ANOVA (sex × age). In case three-way interactions were found (Exp. 2), two-way ANOVAs were run to examine the nature of these interactions. Where appropriate, Bonferroni post-hoc tests or one-way ANOVAs were used to test for differences among groups. For all tests the software package SPSS (PASW Statistics 18) was used. Data are presented as mean ± SEM. Significance was set at p < 0.05.
RESULTS
Experiment 1: Effects of central blockade of V1aR on social discrimination in juveniles
Social investigation time
There was no significant effect of treatment or sex on total social investigation time. Vehicle and V1a-A-treated males investigated stimulus animals for 29.5 ± 2.7 and 28.3 ± 3.0 seconds, respectively, while vehicle and V1A-treated females investigated stimulus animals for 25.5 ± 4.2 and 25.2 ± 2.0 seconds.
Social discrimination
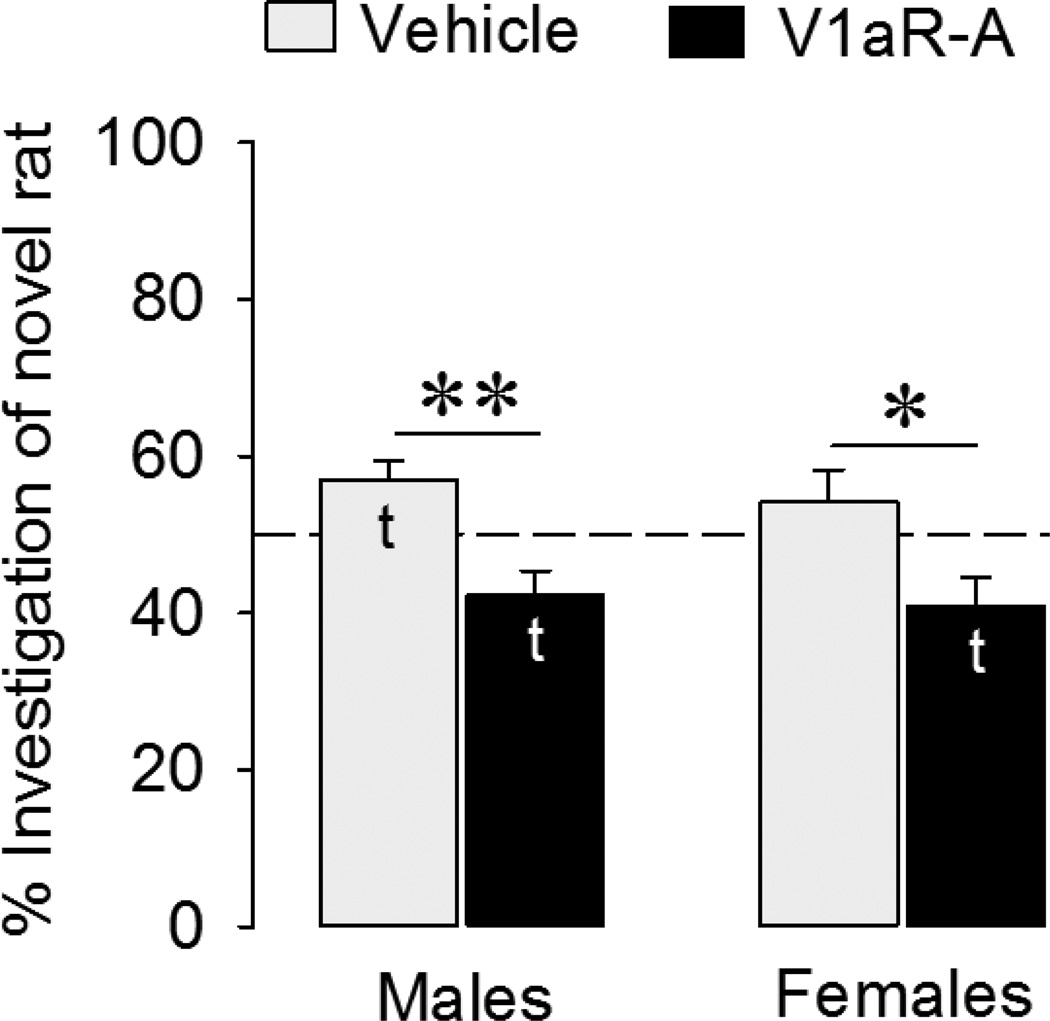
Vehicle-treated juvenile males spent more time investigating novel versus familiar stimulus animals than predicted by chance (t = 2.81, p < 0.05). However, juvenile females did not show social discrimination (t = 0.98, p = 0.35) (Fig. 1). In V1aR-A treated animals, both sexes actually investigated novel stimulus animals less than predicted by chance (t = −2.47, p < 0.05 in males; t = −2.55, p < 0.05 in females) (Fig. 1). In other words, blockade of V1aR resulted in a preference to investigate familiar as opposed to novel conspecifics. The two-way ANOVA indicated a main effect of treatment (F(1,36) = 16.8, p < 0.001). When the data were tested independently by sex, this effect of V1aR was significant in males (F(1,18)=13.5, p < 0.005, one-way ANOVA) as well as females (F(1,18)=5.75, p < 0.05, one-way ANOVA). No main effects of sex or sex by treatment interaction were found. Because we show here that blockade of V1aR does not block social discrimination in juveniles, as it has been reported to do in adults, we compared the effects of septal injections in juvenile and adult rats side by side in the following experiments.
Figure 1. Effects of intracerebroventricular injections of a V1aR antagonist (V1aR-A) on social discrimination in juveniles.
Social discrimination was tested after an interval of 1 h and is expressed as the percentage time spent investigating the novel versus the familiar rat. Dashed line indicates chance level. Bars indicate means + SEM. t: significantly different from chance level (p < 0.05, one sample t-test). V1aR-A treatment reduced the percentage of time spent with novel rats (p < 0.001; two-way ANOVA); Follow-up one-way ANOVAs indicated that this effect was significant in both males and females (*: p < 0.05; **: p < 0.005).
Experiment 2: Effects of blockade of V1aR in the lateral septum on social discrimination in juveniles and adults
Social investigation time
V1aR-A injections in the lateral septum did not significantly change total social investigation time in any of the groups. However, a significant age × sex interaction (F(1,54) = 16.5, p < 0.001) was found. A post-hoc test demonstrated that adult males showed longer social investigation times than adult females (which is consistent with previous reports: Bluthe & Dantzer, 1990). Adult males show longer investigation times than juvenile animals, which do not show a sex difference in investigation time (Table 1).
Table 1. Total social investigation times (sec) in rats exposed to the social discrimination test.
Total social investigation time was calculated as total time investigating the familiar and novel rat. V1aR-A (Exp. 2) or AVP (Exp. 3) injections into the lateral septum had no effect on total social investigation time in any of the groups. However, total social investigation times were significantly higher in adult males than in any other group, irrespective of the treatment.
| Juvenile | Adult | ||
|---|---|---|---|
| Exp. 2 | |||
| Male | Vehicle | 30.6 ± 1.8 | 71.3 ± 6.7** |
| V1aR-A | 34.4 ± 5.1 | 60.6 ± 6.4** | |
| Female | Vehicle | 18.4 ± 2.3 | 25.8 ± 3.8 |
| V1aR-A | 23.2 ± 2.2 | 29.2 ± 4.1 | |
| Exp.3 | |||
| Male | Vehicle | 32.5 ± 3.7 | 62.9 ± 7.2** |
| AVP | 39.4 ± 6.8 | 58.4 ± 3.3* | |
| Female | Vehicle | 33.9 ± 4.0 | 21.4 ± 2.6 |
| AVP | 22.8 ± 4.8 | 23.8 ± 3.7 | |
All groups, n=7–10. Data represent means (total social investigation time of familiar + novel rat in sec) ± SEM. A significant age × sex interaction was found in Exp. 2 (F(1,54) = 16.5, p < 0.001) as well as Exp. 3 (F(1,55) = 19.0, p<0.001). Post-hoc tests indicated that adult males spent more time investigating conspecifics than any other group and this effect was also found when tested separately by treatment:
p < 0.01 vs. adult females and juvenile males,
p < 0.001 vs. adult females and juvenile males.
Social discrimination
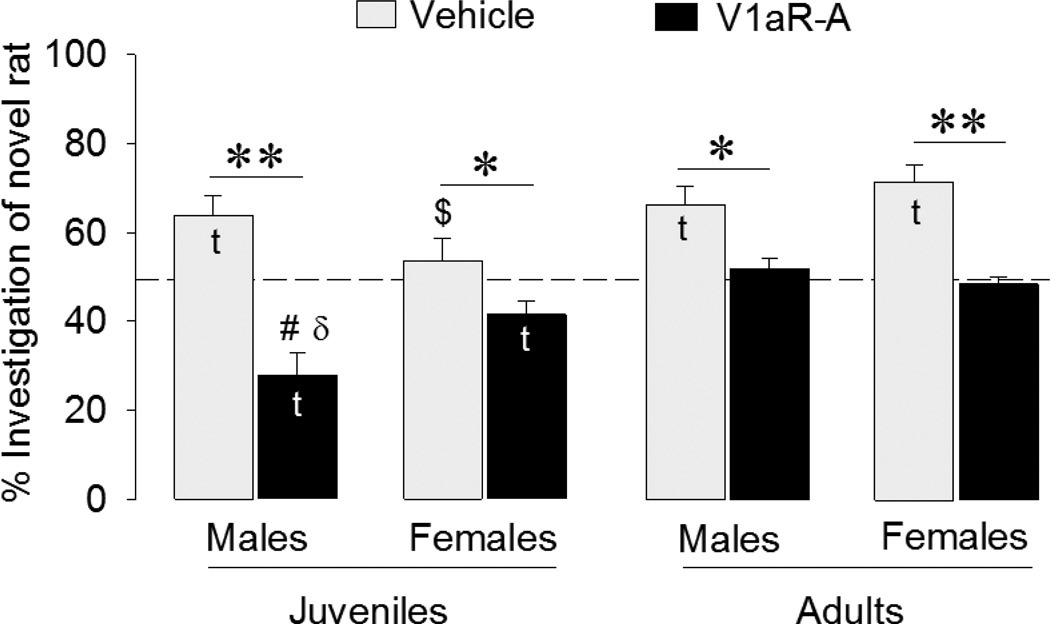
As in Experiment 1, vehicle-treated juvenile males spent a larger percentage of time investigating the novel animal (t = −3.47, p < 0.01), whereas females did not show a preference (t = 0.69, p = 0.51) (Fig. 2). Once again, V1aR-A treated animals investigated the novel animal less than predicted by chance (t = −4.37, p < 0.005 in males; t = −2.77, p < 0.05 in females) (Fig. 2) and thus preferred to investigate familiar as opposed to novel conspecifics. Adult animals showed different social preferences than did the juveniles. Confirming previous studies mentioned in the Introduction, vehicle-treated adult animals spent a larger percentage of time investigating the novel than the familiar animal (t = 4.13, p <0.005 in males; t = 5.60, p < 0.005 in females). We also confirmed that V1aR-A-treatment blocked social discrimination in adult males, with investigation time of the novel animal not differing from chance (t = 0.78, p = 0.46). Unexpectedly, we found the same to be true for adult females (t = −0.85, p = 0.42) (Fig. 2). These data suggest several interactions between treatment, age, and sex, which we confirmed in a subsequent three-way ANOVA, which indicated a significant interaction between age × sex × treatment (F(1,54) = 8.60, p < 0.01). In general, we found that V1aR-A treatment reduced the percentage of time rats spent investigating the novel animal compared to vehicle treatment (F(1,54) = 60.3, p < 0.001). Bonferroni post-hoc tests indicated that this was true for all sex/age groups (p < 0.05 in juvenile females and adult males; p < 0.001 in juvenile males and adult females). We also found that, overall, juveniles showed less preference for novel animals than did adults (F(1,54) = 22.4, p < 0.001). This effect was caused predominantly by the V1aR-A-treated juvenile males spending significantly less time investigating the novel animal than did their adult counterparts (p < 0.001, Bonferroni post-hoc test) as well as by vehicle-treated juvenile females spending significantly less time investigating the novel animal than did their adult counterparts (p < 0.01, Bonferroni post-hoc test). Finally, to further investigate the nature of the three-way interaction, we ran two-way ANOVAs testing the effects of sex and treatment independently for the two age groups. This showed a significant sex × treatment in juveniles (F(1,26) = 6.56, p < 0.05), but not in adults. This interaction was mainly caused by the more robust reversal in social preference after V1aR-A treatment in male than in female juveniles. A Bonferroni post-hoc test confirmed that V1a-A-treated juvenile males spent a significantly lower percentage of time investigating the novel animal than did their female counterparts (p < 0.05).
Figure 2. Effects of septal injections of a V1aR antagonist (V1aR-A) on social discrimination in juveniles and adults.
Social discrimination was tested after an interval of 1 h and is expressed as the percentage time spent investigating the novel versus familiar rat. Dashed line indicates chance level. Bars indicate means + SEM. t: significantly different from chance level (p < 0.05, one sample t-test). V1aR-A treatment reduced the percentage of time spent with novel rats (p < 0.001, three-way ANOVA). Bonferroni post hoc test confirmed this effect for all groups (*: p < 0.05; **: p < 0.001). #: different from V1aR-A-treated adult males (p < 0.001, Bonferroni post-hoc test). $: different from vehicle-treated adult females (p < 0.01, Bonferroni post-hoc test). δ: significantly different from V1a-A-treated juvenile females (p < 0.05 Bonferroni post-hoc test).
Experiment 3: Effects of local application of AVP in the lateral septum on social discrimination in juveniles and adults
Social investigation time
AVP treatment had no significant effect on total social investigation time. Similar to Experiment 2, a significant age × sex interaction (F(1,55) = 19.0, p < 0.001) was found with adult males showing significantly longer social investigation times than adult females and juvenile males. This effect was found irrespective of treatment (p < 0.001 in vehicle-treated animals; p < 0.01 in AVP-treated animals) (Table 1).
Social discrimination
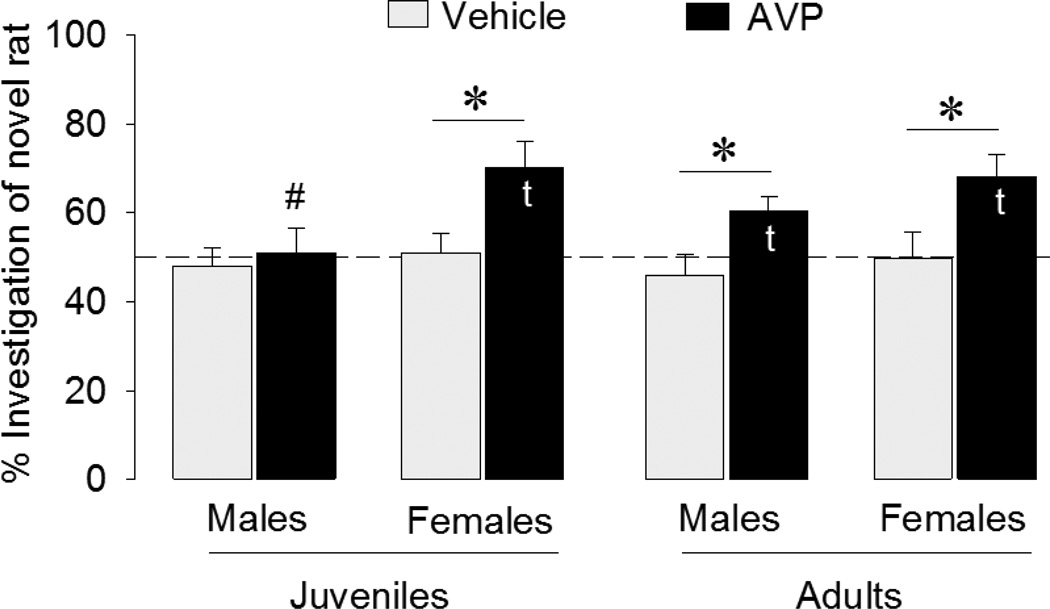
As expected, none of the vehicle-treated groups showed social discrimination (t = −0.50, p = 0.63 in juvenile males; t = 0.24, p = 0.82 in juvenile females; t = −0.87, p = 0.41 in adult males; t = −0.06, p = 0.95 in adult females) (Fig. 3). Although the same was true for AVP-treated male juveniles (t = 0.16, p = 0.88), all other AVP-treated groups showed social preference, spending more time investigating novel versus familiar stimulus animals than predicted by chance (t = 3.47, p < 0.05 in juvenile females; t = 3.04, p < 0.05 in adult males; t = 3.62, p < 0.05 in adult females). These differences were reflected in a main effect of treatment (F(1,55) = 16.1, p < 0.001) in the three-way ANOVA. Separate one-way ANOVA’s indicated that AVP significantly increased social preference in juvenile females (F(1, 13) = 7.18, p < 0.05), adult males (F(1, 16) = 6.22, p < 0.05), adult females (F(1, 11) = 5.27, p < 0.05), but not in juvenile males (F(1, 15) = 0.19, p = 0.67), which may have contributed to a main effect of sex (F(1,55) = 6.2, p < 0.05). When running separate one-way ANOVA for each age and treatment group, it was found that this sex difference was significant in AVP-treated juveniles only (F(1, 11) = 5.71, p < 0.05). However, there were no main effects of age or any interaction effects. The lack of an AVP effect in juvenile males only may therefore have been caused by chance.
Figure 3. Effects of septal injections of AVP on social discrimination in juveniles and adults.
Rats were tested for extended effects of AVP on social recognition after an interval of 2 h (juvenile males and females and adult males) or 3 h (adult females) as pilot studies demonstrated a lack of social discrimination after these interval periods. Social discrimination is expressed as the percentage time spent investigating the novel versus familiar rat. Dashed line indicates chance level. Bars indicate means + SEM. t: significantly different from chance level (p < 0.05, one sample t-test). *: AVP significantly increased the percentage of time spent investigating the novel animals in all groups except juvenile males (p < 0.05, one-way ANOVAs). #: AVP-treated juvenile males spent a significantly lower percentage of time investigating the novel animal than did their female counterparts (p < 0.05, one-way ANOVA).
Experiment 4: V1aR binding density in the lateral septum
V1aR binding density was significantly higher in the lateral septum of females than of males (F(1,22) = 8.81, p < 0.01) (Fig. 4).
DISCUSSION
In social discrimination tests, adult rats typically investigate novel conspecifics more than familiar ones (Engelmann et al., 1995). In this study, we demonstrate that blockade of V1aR in the lateral septum prevents this effect, while septal injections of AVP extend social discrimination in adult males and females. Interestingly, in juveniles, blockade of V1aR (regardless of whether the antagonist was injected intracerebroventricularly or locally in the lateral septum) actually resulted in a preference to investigate familiar as opposed to novel conspecifics. This effect was significantly stronger in males than in females. Moreover, septal AVP injections in juveniles facilitated social discrimination in females, but not in males. Together, these findings demonstrate sex- and age-specific modulations of social recognition by the septal AVP system.
We confirmed our previous findings (Lukas et al., 2011) demonstrating that the social discrimination test can be used reliably in 5-week-old juvenile males. However, juvenile females failed to show a difference in time investigating familiar versus novel stimulus animals. Yet, after intracerebroventricular as well as septal injections of the specific V1aR antagonist d(CH2)5Tyr(Me)AVP, juvenile males as well as females spent significantly more time investigating familiar compared to novel rats. This suggests that V1aR blockade induced a preference for spending more time with familiar conspecifics in juveniles. It is unclear whether the lack of social discrimination displayed by control females reflects an absence of social recognition or just a lack of preference. Shorter interexposure intervals (e.g. 30 min instead of 1 h) or a procedure like the habituation-dishabituation test, in which habituation to a repeatedly presented conspecific and dishabituation to a novel conspecific is measured (Gheusi et al., 1994), might indicate whether a lack of social discrimination represents a lack of social recognition.
The sex difference in social discrimination was seen in juveniles only, suggesting sex-related changes in the development of the neural circuitry underlying social recognition. One of the systems most likely involved, in this regard, is septal AVP innervation. Septal injections of AVP facilitate social recognition in adult male rats (Dantzer et al.,1988, Engelmann & Landgraf, 1994; Landgraf et al., 2003). Moreover, upregulation of septal V1aR using viral vectors facilitate social recognition in adult male rats and V1aR knockout adult male mice (Landgraf et al., 2003, Bielsky et al., 2005a), whereas blocking V1aR in the lateral septum or knocking down septal V1aR expression impairs social recognition in adult male rats (Dantzer et al., 1988, Landgraf et al., 1995; Everts & Koolhaas, 1997). Levels of AVP fiber innervation in the lateral septum and AVP mRNA expression in the BNST and MeA, which are the main sources of septal AVP innervation (De Vries & Buijs, 1983; Caffe et al., 1987), are higher in males than in females (De Vries et al., 1981; Szot & Dorsa, 1993). The sex difference in AVP fiber innervation in the lateral septum is even more profound in juveniles than in adults (De Vries et al., 1981). The lower levels of AVP in juvenile females led us to hypothesize that AVP is less important for social discrimination in juvenile females than in their male counterparts. However, we found that intracerebroventricular as well as septal injections of the V1aR antagonist affected social discrimination in juvenile males as well as females. This suggests that despite low AVP peptide levels, septal V1aR modulates social discrimination in juvenile females. This is further supported by the finding that both male and female juvenile rats show abundant septal V1aR binding densities. We now propose that low septal AVP fiber density may underlie the lack of social discrimination displayed by vehicle-treated juvenile females.
The sex difference in septal AVP innervation in juvenile rats may contribute to the sex differences in the behavioral effects seen in this study. For example, the change in social preference after V1aR antagonist injections into the lateral septum was significantly larger in juvenile males than in juvenile females. This may be expected if AVP innervation in the lateral septum is more involved in social discrimination in males than in females. A similar situation may occur in mice, where a knockout of the V1aR gene decreases anxiety-related behaviors in males, but not in females (Bielsky et al., 2004, 2005b), a difference that has been suggested to be related to the sexually dimorphic AVP innervation of the brain (Bielsky et al., 2005b). The lower levels of AVP in juvenile female rats may explain why we found that AVP injections intothe lateral septum facilitated social discrimination in females but not in males. In males, the higher endogenous levels of AVP may have stimulated social discrimination to near maximum, which would make additional AVP injections less effective. In addition, the higher levels of V1aR binding in the lateral septum of female ats found in the current study may further have potentiated AVP effects in females.
Our data do not suggest a difference in the importance of septal V1aR for social discrimination in adult males and females. We confirm previous studies (Dantzer et al., 1988; Engelmann & Landgraf, 1994; Landgraf et al., 2003) by showing that V1aR blockade in the lateral septum impairs social discrimination in adult male rats. However, we show for the first time that this effect is seen in adult females as well. This contrasts with previous studies using peripheral injections of a centrally acting AVP antagonist (Bluthe & Dantzer, 1990) or intracerebroventricular injections of the specific V1aR antagonist d(CH2)5Tyr(Me)AVP) (Engelmann et al., 1998) that failed to produce this effect in adult females. As these studies did not target the lateral septum specifically, perhaps V1aR activation in other brain regions has opposing effects on social discrimination, at least in adult females, which may cancel each other out. This would be in line with an emerging concept of brain region-specific effects of AVP on subsets of social behavior, which might explain the involvement of AVP in a variety of social behaviors. For example, we recently demonstrated that AVP released in the lateral septum correlated positively with intermale aggression, while AVP released in the BNST correlates negatively with intermale aggression (Veenema et al., 2010). This is in line with the finding that application of AVP into the lateral septum stimulates (Koolhaas et al., 1991; Veenema et al., 2010), whereas AVP in the BNST reduces (Veenema et al., 2010) intermale aggression under certain circumstances.
In accord with previous studies (Dantzer et al., 1988; Bluthe & Dantzer, 1990; Engelmann & Landgraf, 1994; Landgraf et al., 2003), we confirm that AVP extends social discrimination in both adult males and females. In conjunction with the V1aR antagonist data, these findings suggest that the septal AVP system is important for the regulation of social recognition in both males and females, which counters our initial hypothesis. However, it should be noted that we only used a single dose of the V1aR antagonist (10 ng/0.5 µl Ringer) and of AVP (200 pg/0.5 µl Ringer). It is possible, therefore, that different doses of the V1aR antagonist and / or AVP yield sex differences in social discrimination behavior. Nevertheless, our present findings in females add to a growing body of evidence supporting a role of AVP in the regulation of social behavior in females. For example, AVP has been shown to be essential for female social behaviors, including maternal care and maternal aggression (Bosch & Neumann, 2008). Moreover, intranasally applied AVP alters perception of facial expression in women and men, albeit in different ways (Thompson et al., 2006). The current notion that AVP is important for male but not for female social behavior should therefore be reevaluated.
In conclusion, we demonstrate a robust sex- and age-specific regulation of social recognition by the septal AVP system. Injections of a V1aR antagonist in the lateral septum causes juvenile rats to spend more time with a familiar rat, while it blocks social discrimination in adult rats. Juvenile female, but not male, rats show improved social discrimination after AVP administration in the lateral septum. To our knowledge this is the first study comparing juvenile males and females in the effects of manipulations of the AVP system on social behavior.
HIGHLIGHTS.
> Septal vasopressin V1a receptor blockade impairs social discrimination in adult rats. > The effect is similar in males and females. > Septal V1a receptor blockade does not impair social discrimination in juveniles. > V1a receptor-treated juveniles investigate familiar rats more than novel ones. > Role of septal V1a receptors in social recognition changes during development
ACKNOWLEDGEMENTS
We would like to thank Dr. Maurice Manning for providing the V1aR antagonist, Chido Kativhu and Caroline Smith for technical assistance, and Dr. Nancy Forger for critically reading the manuscript. This research was funded by the German Research Foundation VE453/4-1 to AHV and NSF Grant IBN 9421658 and NIH Grant RO1 MH047538 to GJD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005a;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Young LJ. Sexual dimorphism in the vasopressin system: lack of an altered behavioral phenotype in female V1a receptor knockout mice. Behav Brain Res. 2005b;164:132–136. doi: 10.1016/j.bbr.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Hu SB, Szegda KL, Westphal H, Young LJ. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29:483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Dantzer R. Social recognition does not involve vasopressinergic neurotransmission in female rats. Brain Res. 1990;535:301–304. doi: 10.1016/0006-8993(90)91613-l. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams' trait anxiety. Proc Natl Acad Sci U S A. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffé AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261:237–252. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- Dantzer R, Koob GF, Bluthé RM, Le Moal M. Septal vasopressin modulates social memory in male rats. Brain Res. 1988;457:143–147. doi: 10.1016/0006-8993(88)90066-2. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273:307–317. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain--presence of a sex difference in the lateral septum. Brain Res. 1981;218:67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Bluthé RM, Goodalla G, Dantzer R. Social and individual recognition in rodents: Methodological aspects and neurobiological bases. Behavioural Processes. 1994;33:59–87. doi: 10.1016/0376-6357(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol Behav. 1994;55:145–149. doi: 10.1016/0031-9384(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Wotjak CT, Landgraf R. Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav Brain Res. 1998;90:89–94. doi: 10.1016/s0166-4328(97)00084-3. [DOI] [PubMed] [Google Scholar]
- Everts HG, Koolhaas JM. Lateral septal vasopressin in rats: role in social and object recognition? Brain Res. 1997;760:1–7. doi: 10.1016/s0006-8993(97)00269-2. [DOI] [PubMed] [Google Scholar]
- Everts HG, Koolhaas JM. Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behav Brain Res. 1999;99:7–16. doi: 10.1016/s0166-4328(98)00004-7. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Moor E, Hiemstra Y, Bohus B. The testosterone-dependent vasopressinergic neurons in the medial amygdala and lateral septum: involvement in social behaviour of male rats. In: Jard S, Jamison R, editors. vasopressin. Paris/London: INSERM/John Libbey; 1991. pp. 213–219. [Google Scholar]
- Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, Terwilliger EF, Niwa M, Wigger A, Young LJ. Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur J Neurosci. 2003;18:403–411. doi: 10.1046/j.1460-9568.2003.02750.x. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Neumann ID, Veenema AH. Maternal separation interferes with developmental changes in brain vasopressin and oxytocin receptor binding in male rats. Neuropharmacology. 2010;58:78–87. doi: 10.1016/j.neuropharm.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Lukas M, Bredewold R, Landgraf R, Neumann ID, Veenema AH. Early life stress impairs social recognition due to a blunted response of vasopressin release within the septum of adult male rats. Psychoneuroendocrinology. 2011;36:843–853. doi: 10.1016/j.psyneuen.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Miller MA, Vician L, Clifton DK, Dorsa DM. Sex differences in vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization. Peptides. 1989;10:615–619. doi: 10.1016/0196-9781(89)90152-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edition. San Diego: Academic Press; 1998. [Google Scholar]
- Szot P, Dorsa DM. Differential timing and sexual dimorphism in the expression of the vasopressin gene in the developing rat brain. Dev Brain Res. 1993;73:177–183. doi: 10.1016/0165-3806(93)90136-x. [DOI] [PubMed] [Google Scholar]
- Thompson RR, George K, Walton JC, Orr SP, Benson J. Sex-specific influences of vasopressin on human social communication. Proc Natl Acad Sci U S A. 2006;103:7889–7894. doi: 10.1073/pnas.0600406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leeuwen FW, Caffe AR, De Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325:391–394. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, Neumann ID. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm Behav. 2010;58:273–281. doi: 10.1016/j.yhbeh.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS., 3rd Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 2007;6:540–551. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]