Abstract
The goal of this research is to identify the neural response to rewarding food cues before and after eating in overweight/obese (OB) and normal-weight (NW) adults. Based on the previous literature, we expected greater differential activation to food cues vs. objects for OB compared to NW participants both prior to eating and after consumption of a typical lunch. Twenty-two overweight/obese (11 male) and 16 normal-weight (6 male) individuals participated in a functional magnetic resonance imaging task examining neural response to visual cues of high- and low-calorie foods before and after eating. The OB group demonstrated increased neural response to high- and low-calorie foods after eating in comparison to the NW participants in frontal, temporal, and limbic regions. In addition, greater activation in corticolimbic regions (lateral OFC, caudate, anterior cingulate) to high-calorie food cues was evident in OB vs. NW participants after eating. These findings suggest that for OB individuals, high-calorie food cues show sustained response in brain regions implicated in reward and addiction even after eating. Moreover, food cues did not elicit similar brain response after eating in the NW group suggesting that neural activity in response to food cues diminishes with reduced hunger for these individuals.
Keywords: fMRI, obesity, reward system, corticolimbic, OFC, high-calorie food
Food intake in humans is influenced by a variety of factors above and beyond homeostatic control. Availability, sensory cues (e.g., aroma, visual appeal, taste), and pleasure are factors that influence what and how much humans eat even after being satiated. Given the present state of obesity in America and worldwide, understanding how these factors influence food intake has become essential for health, welfare, and economic reasons (Rigby, Kumanyika, & James, 2004). Within the past decade, animal models of food motivation have been supplemented by noninvasive human investigations of the food appetitive and reward system. Both disordered (Dimitropoulos & Schultz, 2008; Farooqi et al., 2007) and neurotypical populations (Gautier et al., 2000; Goldstone et al., 2009; Killgore et al., 2003; LaBar et al., 2001; Stoeckel et al., 2008; Tataranni et al., 1999; Wang, Volkow, Thanos, & Fowler, 2004) have been examined using neuroimaging techniques designed to further understand the neural mechanisms involved during hunger and satiety and how they relate to obesity and disordered eating.
Research to date indicates that visual food cues activate food motivation and reward neural circuitry (e.g., prefrontal cortex [PFC], orbitofrontal cortex [OFC], amygdala, dorsal and ventral striatum, hypothalamus, insula) when hungry, and that high-calorie food cues elicit greater response in these regions relative to low-calorie food images (Killgore et al., 2003; LaBar et al., 2001; Stoeckel et al., 2008; Wang et al., 2004). Neural response to visual food cues in reward regions is seen in both normal-weight and obese individuals and across various fasting durations. The effect of food cues on neural response after satiety has also been examined, albeit less frequently, with varying results across studies. For example, research has indicated that normal-weight individuals show decreased activation to food cues after eating. LaBar et al. (2001) found that pictures of food presented during functional magnetic resonance imaging (fMRI) elicited greater activation in the amygdala, parahippocampal gyrus, and right fusiform gyrus when participants were hungry as compared to when they were satiated by a meal of their choice. In addition, Goldstone and colleagues (2009) reported no significant differential activation in appetitive and reward regions to high vs. low calorie foods after eating breakfast. In contrast, research with overweight and obese individuals suggests food cues continue to elicit neural response after eating. Specifically, Martin and colleagues (2010) found brain activity was greater in response to food vs. object cues in the medial prefrontal cortex, caudate, superior frontal gyrus, and hippocampus after obese participants ingested a 500-calorie meal. Research using other modalities (e.g., eye-tracking of food cues) is consistent with fMRI work indicating retained salience of food cues after ingesting a liquid meal among obese individuals (Castellanos et al., 2009).
Direct comparisons of obese to normal-weight individuals have also shown differential response to food cues associated with weight status (Bruce et al., 2010; Geliebter et al., 2006; Martin et al., 2010; Rothemund et al., 2007; Stoeckel et al., 2008). Collectively, studies indicate that obese individuals show greater activation to food cues in comparison to normal-weight participants in multiple brain regions, including reward system regions. Greater activation to food vs. object cues among obese participants compared to controls has been seen in the PFC, OFC, anterior cingulate, insula, amygdala, and striatum during hunger (Stoeckel et al., 2008), in the PFC, caudate, hippocampus, and temporal lobe immediately after eating (Martin et al., 2010), and in the striatum, insula, hippocampus, and parietal lobe in a neutral appetitive state (neither hungry or satiated) (Rothemund et al., 2007). In addition, differential activation to food types (high-calorie, low-calorie, binge foods) has been examined between obese and normal-weight individuals after fasting and during a neutral appetitive state. For example, obese individuals show greater response to high vs. low-calorie cues than those with normal-weight in regions such as the putamen (Rothemund et al., 2007), lateral OFC, medial PFC, insula, striatum, and amygdala (Stoeckel et al., 2008). There is some evidence of greater neuronal response to food cues among normal-weight compared to overweight/obese groups such as in the medial PFC (Stoeckel et al., 2008) and temporal regions (Martin et al., 2010), but the majority of reported results on direct comparisons between normal-weight and obese/overweight groups indicate greater activation to food cues among overweight/obese individuals.
To date, much of the food-related neuroimaging literature has utilized long periods of caloric deprivation for examining neural response during hunger (e.g., 8–36hrs; Gautier et al., 2000; Gautier et al., 2001; Goldstone et al., 2009; Karhunen, Lappalainen, Vanninen, Kuikka, & Uusitupa, 1997; LaBar et al., 2001; Stoeckel et al., 2008; Tataranni et al., 1999) with some exceptions (Killgore et al., 2003; Martin et al., 2010). It is unclear whether the duration of fasting affects neural responsivity since studies vary dramatically in both imaging protocol, prescan procedures, and statistical methods (e.g., small volume corrections to uncorrected whole brain analyses) where stringent criteria or region of interest (ROI) analyses may affect results reporting. The goal of the work presented here is to extend existing research by identifying the neural response to rewarding foods during the normative caloric deprivation that occurs between meals. Our aim was to examine a more naturalistic hunger and satiation that occurs during the course of a typical day in westernized society. Specifically, we aimed to examine neural responsiveness elicited by high-calorie food cues in normal-weight and overweight/obese individuals before and after eating. Based on the previous literature, we expected greater differential activation to food cues vs. objects for overweight/obese compared to normal-weight participants both prior to eating and after consumption of a typical lunch. We were most interested in the neural response specific to high- and low-calorie food cues after eating as this literature is lacking and we feel it may illuminate the continued impact of highly desirable food after eating. We hypothesized that rewarding (high-calorie) foods would elicit greater neural responsiveness in multiple brain regions, including the corticolimbic reward system (OFC, anterior cingulate, insula, ventral striatum, and amygdala; Berthoud & Morrison, 2008; Kringelbach, 2004), even after ingestion of a 750-calorie meal for a sample of overweight and obese participants (hereafter referred to as obese) in comparison to normal-weight participants. In contrast, based on the previous literature we expected normal-weight participants to show less neural response across brain regions, including corticolimbic regions, to food cues (regardless of calorie type) in comparison to obese participants before and after eating.
Methods
Participants
Twenty-two obese (OB) [BMI mean(SD): 31.6 (4.5)] and 16 normal-weight (NW) individuals participated in this research (see Table 1 for group characteristics). These individuals were recruited from advertisements to the Case Western Reserve University community. Participants were in good health, had normal to corrected-normal vision, and were eligible for MRI scanning (i.e., free of ferromagnetic implants). Individuals who reported a history of psychiatric or neurological problems, significant weight loss or gain in the past 6 months, or head injury with loss of consciousness were not eligible to participate. All participants gave informed written consent and were financially compensated for their participation. This research was approved by the University Hospitals Case Medical Center Institutional Review Board for Human Investigation.
Table 1.
Participant Characteristics
| Normal-Weight (n = 16) |
Obese (n = 22) |
statistic, p-value | |
|---|---|---|---|
| Age | 24.6 (4.2) | 24.8 (6.7) | t=−.11, p=.92 |
| BMI* | 22.7 (1.4) | 31.6 (4.5) | t=−7.62, p<.001 |
| #(%) male | 6(37.5%) | 11(50%) | X=.59, p=.52 |
| % right handed | 94% | 82% | X=5.31 , p=.15 |
| Food Preference | |||
| Low Calorie | 3.79(.38) | 3.78(.45) | t=.05, p=.96 |
| High Calorie | 3.83(.41) | 3.74(.62) | t=.53, p=.60 |
For age, BMI, and food preference, values are presented as mean (SD). BMI indicates body mass index, based on height and weight obtained during testing.
p < .001.
Procedure
Participants were scanned between 12 and 2pm consecutively for a premeal and postmeal scan. As part of a larger project comparing normal-weight and overweight/obese individuals to individuals with a rare disorder (Prader-Willi syndrome; PWS), scanning was constrained by the study parameters regarding individuals with PWS. Thus, scanning on separate days (and as a result, counterbalancing premeal and postmeal state) was not feasible. Participants were asked to eat a light breakfast before 8:00am prior to their appointment on the day of their scans and to refrain from eating until the experimental procedure was completed. Fifteen participants in each group reported eating breakfast [fasting hours- OB: 6.2(.68) range=5–8hrs, NW: 5.6(1.1) range=3–7hrs, t=−1.79, p = .08]. Participant report of breakfast content was recorded and estimated for caloric intake; this did not differ between groups (OB: 372.1(190) calories; NW: 270(135) calories, t=−1.6, p = .12, n=15 per group). Eight participants (OB: n=7; NW: n=1) reported not eating breakfast as they typically do not eat breakfast. To determine whether the participants who consumed breakfast differed from those that did not, the premeal scan fMRI data was compared between the two groups (p < .05, uncorrected). The two groups failed to differ in their response to food cues on any contrasts of interest (e.g., high-calorie vs. low-calorie). The groups also did not differ on hunger ratings before and after the premeal scan (hunger before scan: t=.43, p=.67; after premeal scan: t=.39, p=.69) or lunch calories consumed (t=.41 p=.68). Further confirmation was provided by conducting the fMRI analyses with only participants who ate breakfast (n=15 per group) and key findings remained the same. Therefore, all analyses reported hereafter disregard breakfast consumption status.
Prior to scanning, participants underwent neuropsychological testing (as part of a larger study not reported here) and training on the functional tasks. Height, weight, and a food preference assessment were also obtained during this time. The food preference assessment was administered to obtain a measure of high- and low-calorie food preference for each participant. The assessment required the participants to rate photograph flash cards of 74 foods (7” × 6”; PCI Educational Publishing, 2000) that included desserts, meats, fruits, vegetables, snacks, breads, and pastas on a 5-point Likert scale from ‘dislike’ to ‘like’. The photographs for the food preference assessment were different from the images used in the fMRI task. High-calorie (e.g., cakes, cookies, potato chips, hot dogs) and low-calorie (e.g., fruits and vegetables) food preference ratings did not differ within or between groups (see Table 1).
Following the premeal scan, participants were given a meal prepared by the Dahms Clinical Research Unit at University Hospitals standardized to provide approximately 750 calories and consisting of a sandwich (choice of turkey, roast beef, or vegetarian), carton of milk, a serving of fruit, and either a side of a vegetable or cottage cheese. Menu choices were balanced for macronutrient content. Participants were instructed to eat to satiation and any remaining food was weighed to estimate the number of calories consumed. The postmeal scan typically began within 30 minutes of meal termination. Immediately before and after premeal and postmeal scans, participants answered the question, ‘How hungry are you right now?’ on a scale ranging from 0–8 with 0 being ‘not hungry at all’ to 8 - ‘extremely hungry’. It should be noted that while participants were instructed to eat until satiated, a direct measure of satiation was not administered but was indirectly inferred by change in hunger status.
fMRI task design
Changes in blood oxygen level-dependent (BOLD) contrast were measured in a block design perceptual discrimination task. Participants indicated by a button press whether side-by-side color images of high-calorie food (e.g., cake, doughnuts, potato chips, fries), low-calorie food (fresh vegetables or fruits), or objects (furniture) were the “same” or “different” object. Images were modified for consistent size, brightness, and resolution. Each image was presented only once during the fMRI procedure. The same/different task parameters were selected to ensure participants were attending to the stimuli. Images were presented in blocks corresponding with the 3 image types: high calorie foods, low-calorie foods, and furniture. This paradigm has previously been shown to activate the lateral OFC, insula, hypothalamus, thalamus, and amygdala in response to food cues (Dimitropoulos & Schultz, 2008). All functional runs were composed of 8 blocks (21 seconds each, with a 14-second rest between blocks), with 6 image pairs per block. Stimulus duration was set at 2250 ms and the interstimulus interval (ISI) at 1250 ms. Each run presented blocks of furniture, high-calorie foods, and low-calorie foods in a counterbalanced order. Two functional runs were presented during each scanning session (pre-meal and post-meal).
fMRI Data Acquisition
All scanning was conducted at the Case Center for Imaging Research. Imaging data was acquired on a 4.0T Bruker MedSpec MR scanner using an 8-channel phase array trasmitt receive head coil. Head motion was minimized by placement of foam padding around the head. Functional images were acquired using a gradient-echo single-shot echo-planar sequence over 35 contiguous axial slices aligned parallel to AC-PC plane with an inplane resolution of 3.4 X 3.4 X 3 mm (TR = 1950, TE = 22 ms, flip angle = 90 degrees). BOLD activation data was acquired during two runs (5:01 minutes, 157 EPI volumes/measurements) per MRI session. The visual stimuli were back-projected onto a translucent screen placed near the end of the MRI scanner and viewed through a mirror mounted on the head coil. 2D T1-weighted structural images (TR = 300, TE = 2.47ms, FOV = 256, matrix = 256 × 256, flip angle = 60 degrees, NEX = 2), 3mm thick, positioned in the same plane and slice locations as the echo-planar data for in-plane registration and a high resolution 3D structural volume (3D MPRAGE, contiguous, sagittal acquisition, 176 slice select partitions, each with 1 mm isotropic voxels, TR = 2500, TE = 3.52ms, TI = 1100, FOV = 256, matrix = 256 × 256, flip angle = 12 degrees, NEX = 1) were collected during the initial (premeal) session.
fMRI Data Preprocessing and Analysis
Image processing, analyses, and tests of statistical significance were performed using Brainvoyager QX (Brain Innovation, Maastricht, The Netherlands; Goebel, Esposito, & Formisano, 2006). Preprocessing steps included trilinear three dimensional motion correction, spatial smoothing using a Gaussian filter with a full-width half-maximum value of 7 mm, and linear trend removal. Motion correction parameters were added to the design matrix and motion >2 mm along any axis (x, y, or z) resulted in the discard of that data (<1% discarded for this sample). Data for each individual was aligned with high-resolution 2D and 3D anatomical images for display and localization. The individual data sets underwent piecewise linear transformation into a proportional 3D grid defined by Talairach and Tournoux (1988) and were coregistered with the high-resolution 3D data set and resampled to 3 mm3 voxels. The normalized data sets were entered into a second level analysis in which functional activation was examined using a random effects general linear model (GLM) analysis for the pre-meal scans and for the post-meal scans. For each of the time periods (pre/post-meal) the following contrasts were compared between the obese and normal-weight subjects: high-calorie foods, low-calorie foods, all food (high- and low-calorie combined) and objects. Resulting statistical maps were corrected for multiple comparisons, using cluster-based threshold correction (based on Monte Carlo simulations performed within Brain Voyager). An initial threshold p-value of p < .01 and a minimum contiguous cluster correction applied to each contrast map ranging from 7–12 voxels (189–324 mm3) provided a family-wise correction of p < .05.
The between-group interaction analysis of group (OB vs. NW) by condition contrast (food vs. object; high-calorie vs. low calorie; high-calorie vs. object; low-calorie vs. object) was performed for each hunger state. To visualize the interaction effects, post-hoc analyses were performed on clusters with the most distinct differences across group and condition and for clusters in the corticolimbic reward systems (OFC, anterior cingulate, insula, ventral striatum, and amygdala). Specifically, for post-hoc analyses, the magnitude of activation of the BOLD signal (beta values) were extracted for each subject. SPSS (Version 17; SPSS, Inc; Chicago, IL) was used to perform the post-hoc analyses (t-tests) and to confirm the Brain Voyager findings. Upon extraction, beta contrasts were computed for each calorie condition vs. nonfood objects during each hunger state (high calorie – object, premeal state; low-calorie – object, premeal state; high calorie – object, postmeal state; low-calorie – object, postmeal state). Post hoc paired Student t-tests were then performed to identify differences between high and low contrasts for each meal state separately for each region within each group.
Results
Behavioral Data
Hunger
Ratings on the hunger scale prior to each scan session differed significantly between premeal and postmeal conditions, with participants in both groups indicating greater hunger before premeal scan session: premeal scan- OB mean(SD)=4.72(1.5), NW=4.59(1.5); postmeal scan- OB=.45(.85) NW=.44(.81). Groups did not differ on hunger status at premeal (t=−.266, p=.79) or postmeal scans (t=−.06, p=.95). This data indicates food manipulation was effective, with both groups reporting decreased hunger from premeal to postmeal sessions.
Task Accuracy
Task accuracy during the functional runs (same/different task) was greater than 90% for scan session: premeal mean percentage = 97.3(.03); postmeal = 99.0(.02), for food and nonfood conditions: overall food = 93.8(2.9); overall nonfood = 94.5(1.7) (t=−1.42, p=.16), and for each group: OB = 99.1(.02), NW = .97.8(.02). Accuracy between groups did not differ (t=−1.68, p=.11).
Lunch calories consumed
On average, OB participants consumed 591 calories (SD = 68.4) and NW participants consumed 607 calories (SD=116.1), t=.91, p=.37. Of the 750 calories provided in the meal, items most likely to remain uneaten included portions of condiments (mayonnaise and/or mustard) and the vegetable side dish.
fMRI Data
Premeal response: group × condition interaction
To examine group differences in the premeal condition, the following contrasts were examined: OB > NW [(i) food > object, (ii) high-calorie > low-calorie, (iii) high-calorie > object, (iv) low-calorie > object], NW > OB [(v) food > object, (vi) high-calorie > low-calorie, (vii) high-calorie > object, (viii) low-calorie > object].
In the premeal condition, the obese group showed significantly greater activity than the normal- weight group to food vs. object and to high-calorie vs. object stimuli in primarily prefrontal cortical areas including the bilateral anterior prefrontal cortex (aPFC) (x, y, z = 23, 58, 0; −34, 63, 2). OB showed greater activation than NW to low-calorie vs. object contrasts in the aPFC as well as the superior frontal gyrus (BA6; −3, 11, 60) and cerebellum (47, −57, −33). In contrast, the NW group showed greater activity than the OB group in food vs. object conditions primarily in more posterior regions including parietal (−46, 0, 7), mid-cingulate (−14, −9, 42;−23, −26, 44) and temporal lobe (−34, −1, −28; −43, −30, 17). All significant between-group activation regions (p < .05, corrected) are included in Table 2.
Table 2.
Brain regions that differed by group and visual cue contrast during premeal and postmeal scans
| Premeal | Postmeal | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak Voxel | Peak Voxel | ||||||||||
| Brain Region (Hemisphere) | x | y | z | Cluster Size* |
t | x | y | z | Cluster Size* |
t | |
| OB > NW | Food vs. Object | ||||||||||
| Anterior PFC/BA 10 (R) | 23 | 58 | 0 | 237 | 3.11 | ||||||
| Anterior PFC/BA 10 (L) | −34 | 63 | 2 | 679 | 3.63 | ||||||
| DLPFC/BA 9 (R) | 0 | 53 | 21 | 695 | 3.40 | ||||||
| OFC/BA 47 (R) | 29 | 25 | −9 | 214 | 3.16 | ||||||
| Superior Frontal Gyrus/BA 6 (R) | 17 | 15 | 48 | 427 | 3.36 | ||||||
| Posterior Cingulate/BA 30 (R) | 18 | −46 | 0 | 756 | 4.64 | ||||||
| Temporal/Entorhinal Cortex/BA 34 (R) | 29 | 6 | −9 | 204 | 3.25 | ||||||
| Superior Temporal Gyrus/BA 38 (R) | 44 | 8 | −11 | 428 | 3.40 | ||||||
| Cerebellum-Anterior Lobe (L) | −10 | −44 | −10 | 414 | 3.89 | ||||||
| High Calorie vs. Low Calorie | |||||||||||
| No regions survived threshold | |||||||||||
| High Calorie vs. Object | |||||||||||
| Anterior PFC/BA 10 (L) | −33 | 63 | 0 | 256 | 3.53 | ||||||
| PFC/BA 8 (R) | 4 | 23 | 51 | 587 | 3.32 | ||||||
| Medial Frontal Gyrus/BA 6 (R) | 2 | 47 | 37 | 691 | 3.26 | ||||||
| OFC/BA 47 (R) | 32 | 29 | −3 | 399 | 3.34 | ||||||
| Anterior Cingulate/BA 25 (L) | −4 | 16 | −15 | 281 | 4.09 | ||||||
| Caudate (R) | 8 | 7 | 14 | 378 | 3.11 | ||||||
| Hippocampus (R) | 27 | −35 | −2 | 222 | 3.95 | ||||||
| Low Calorie vs. Object | |||||||||||
| Anterior PFC/BA 10 (R) | 42 | 59 | 12 | 200 | 3.22 | ||||||
| Anterior PFC/BA 10 (L) | −36 | 60 | 5 | 442 | 3.33 | −16 | 59 | 3 | 618 | 3.80 | |
| Superior Frontal Gyrus/BA 6 (L/R) | −3 | 11 | 60 | 409 | 3.27 | 20 | 15 | 47 | 205 | 3.40 | |
| Cerebellum-Posterior Tonsil (R) | 47 | −52 | −33 | 360 | 4.17 | ||||||
| DLPFC/BA 9 (R) | 0 | 52 | 24 | 338 | 3.02 | ||||||
| Posterior Cingulate/BA 30 (R) | 21 | −48 | 3 | 490 | 3.82 | ||||||
| Caudate (L) | −2 | 22 | 3 | 533 | 3.47 | ||||||
| Ant.Temporal Lobe/BA 38 (R) | 45 | 4 | −13 | 284 | 3.50 | ||||||
| Ant.Temporal Lobe/BA 38 (L) | −50 | 18 | −13 | 465 | 3.60 | ||||||
| Temporal Supramarginal Gyrus/BA 40 (L) | −57 | −50 | 20 | 234 | 3.02 | ||||||
| Mid Temporal Gyrus/BA 39 (R) | 53 | −63 | 24 | 481 | 3.25 | ||||||
| NW > OB | Food vs. Object | ||||||||||
| DLPFC/BA 9 (L) | −29 | 28 | 35 | 255 | 2.99 | ||||||
| Precentral Gyrus/BA 44 (L) | −46 | 0 | 7 | 267 | 3.14 | ||||||
| Cingulate/BA 31 (L) | −23 | −26 | 44 | 343 | 3.80 | ||||||
| High Calorie vs. Low Calorie | |||||||||||
| Postcentral Gyrus/BA 43 (L) | −55 | −12 | 15 | 612 | 3.92 | ||||||
| Insula/BA 13 (L) | −40 | −2 | 15 | 635 | 3.46 | ||||||
| Parahippocampal Gyrus/BA28 (L) | −23 | −12 | −15 | 559 | 3.91 | ||||||
| Cerebellum-Posterior Tonsil (R) | 45 | −50 | −34 | 522 | 4.10 | ||||||
| Cerebellum-Posterior Lobe (L) | −16 | −65 | −19 | 393 | 3.56 | ||||||
| High Calorie vs. Object | |||||||||||
| PFC/BA 8 (L) | −31 | 30 | 39 | 490 | 3.14 | ||||||
| Insula/BA 13 (L) | −40 | −1 | 10 | 961 | 3.83 | ||||||
| Cingulate/BA 24 (L) | −14 | −9 | 42 | 538 | 3.45 | ||||||
| Mid Temporal Gyrus/BA 21 (L) | −34 | −1 | −28 | 321 | 3.90 | ||||||
| Superior Temporal Gyrus/BA 41 (L) | −43 | −30 | 17 | 417 | 3.25 | ||||||
| Postcentral Gyrus/BA 43 (L) | −54 | −18 | 17 | 573 | 3.46 | ||||||
| Low Calorie vs. Object | |||||||||||
| No regions survived threshold | |||||||||||
OB = obese, NW = normal-weight, BA = Brodmann area, PFC = prefrontal cortex, DLPFC = dorsolateral prefrontal cortex, OFC = orbitofrontal cortex, Ant. = anterior. x, y, z = coordinates in Talairach space. t statistic for peak voxel. All results are whole brain p < .05, corrected.
Cluster size is reported in mm3.
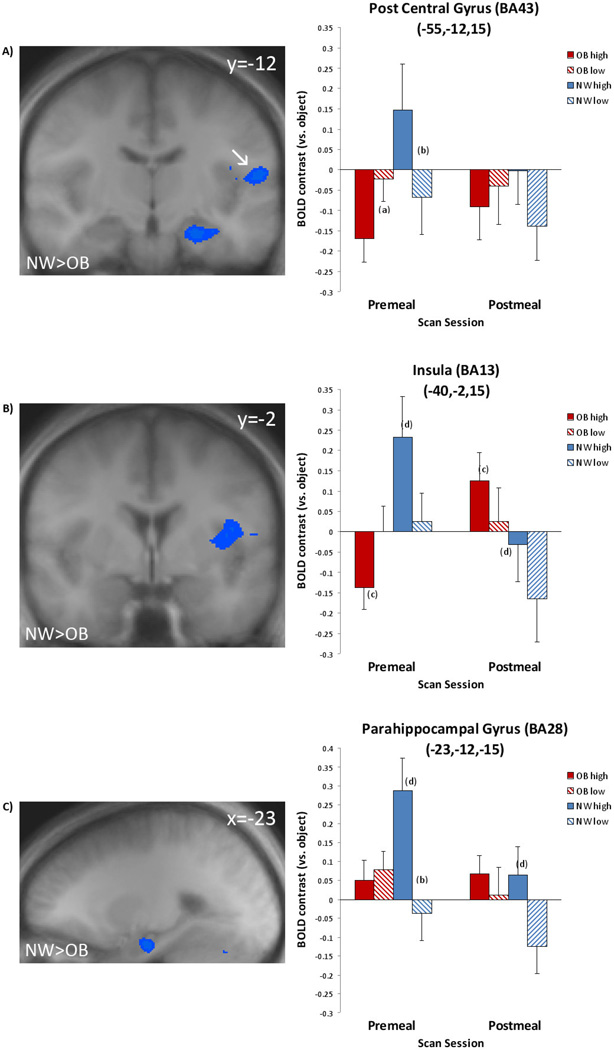
Neural response in normal-weight participants showed greater distinction between high- vs. low-calorie foods relative to the obese participants. During premeal, the OB group did not show greater responses to high- vs. low-calorie foods than the NW group. In contrast, the NW group showed greater response to high- vs. low-calorie food cues than the OB in the left hemisphere postcentral gyrus (BA43;−55, −12, 15), insula (−40, −2, 15), parahippocampal gyrus (−23, −12, −15) (see Table 2/Figure 1) and bilaterally in the cerebellum (45, −50, −34; −16, −65, −19).
Figure 1.
Normal-Weight vs. obese. Left: Pre-meal scan results. Increased normal-weight group activation to high-calorie vs. low-calorie food during premeal condition in A) postcentral gyrus/BA43, B) insula/BA13, and C) parahippocampal gyrus/BA28. Significant activation to high-calorie foods (p < .05, corrected) is shown in shades of blue. Coordinates are given in standard Talairach space. Right and left are reversed by radiologic convention. Right: Magnitude of average activation for high calorie and low calorie foods at premeal and postmeal. Beta values reflect BOLD contrast averaged across voxels in region for calorie type relative to objects. BA=Brodmann Area, NW=normal-weight, OB=obese, OB high=obese group, high-calorie food cue, OB low=obese group, low-calorie food cue, NW high=normal-weight group, high-calorie food cue, NW low=normal-weight group, low-calorie food cue. a–dindicate significan t differences (p < .05) in post-hoc t-tests as follows: aobese group, high- vs. low-calorie, premeal condition, normal-weight group, high- vs. low-calorie, premeal condition, cobese group, high- vs. low-calorie, postmeal condition, normal-weight group, high-calorie, premeal vs. postmeal condition.
Post-hoc analyses
Post-hoc analyses were conducted on significant regions in the NW>OB high-vs. low-calorie contrast to confirm the BV findings and illuminate within-group differences. In addition to corticolimbic regions (insula), other regions were selected because the high- vs. low-calorie contrast showed the most significant differences between groups. Cerebellum findings were excluded from post-hoc analyses because activation was seen in this region in response to low-calorie vs. object cues in the OB > NW contrast (see Table 2). For NW participants during the premeal scan, greater response was elicited to high-calorie food cues in comparison to low-calorie food cues in the postcentral gyrus (BA43; p < .05; Figure 1a). Response also differed significantly for the OB participants (p < .05) with high-calorie foods eliciting greater deactivation in the postcentral gyrus than low-calorie foods during the premeal scan. For the parahippocampal gyrus (BA28), response was significantly greater (p < .05) to high-calorie cues than low-calorie cues during the premeal scan for NW participants (Figure 1b). In addition, in NW participants, parahippocampal activation significantly decreased (p < .05) from premeal to postmeal scans in response to high-calorie food cues (Figure 1b). High-calorie food cues elicited differential response in the insula by meal state for both groups (Figure 1c). For NW participants, activation was significantly greater (p < .05) in response to high-calorie cues than low-calorie cues during the premeal scan. In contrast, for OB participants, high-calorie cues elicited greater response in the insula than low-calorie cues during the postmeal scan (p < .05).
Postmeal response: group × condition interaction
To examine group differences in the postmeal condition, the following contrasts were examined: OB > NW [(i) food > object, (ii) high-calorie > low-calorie, (iii) high-calorie > object, (iv) low-calorie > object], NW > OB [(v) food > object, (vi) high-calorie > low-calorie, (vii) high-calorie > object, (viii) low-calorie > object].
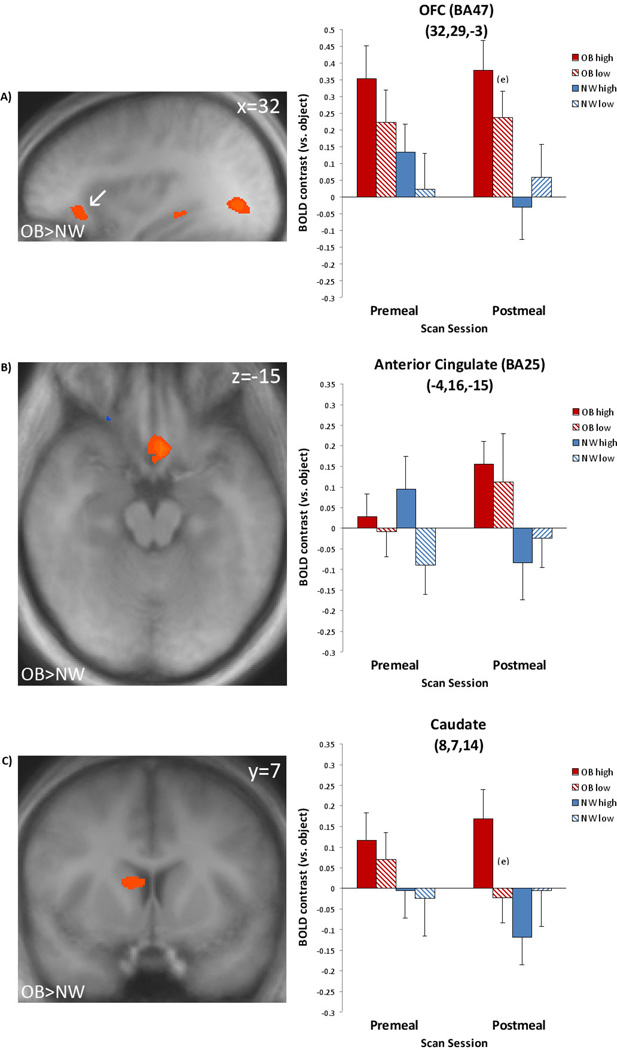
In the postmeal state, the obese group showed greater response compared to the normal-weight group to food vs. object contrasts in multiple regions, including frontal areas [dorsolateral PFC (BA9;0, 53, 21), lateral OFC (BA47;29, 25, −9), and superior frontal gyrus (BA6;17, 15, 48)], as well as temporal and more posterior regions such as the posterior cingulate (18, −46, 0) and entorhinal cortex (29, 6, −9). Greater response was shown among OB compared to NW participants for the high-calorie vs. object contrast in several regions that are part of the corticolimbic reward systems: lateral OFC (32, 29, −3), anterior cingulate (−4, 16, −15), caudate (8, 7, 14) (see Table 2; Figure 2), and other frontal regions including PFC (BA8;4, 23, 51), and medial frontal gyrus(BA6;2, 47, 37). The low-calorie vs. object contrast yielded greater response among the OB than NW participants in frontal areas [aPFC (−16, 59, 3), dorsolateral PFC (0, 52, 24) and superior frontal gyrus (BA6;−3, 11, 60)], temporal lobe regions [anterior temporal lobe (45, 4, −13; −50, 18, −13), temporal supramarginal gyrus (BA40;−57, −50, 20), and mid temporal gyrus (53, −63, 24)], caudate (−2, 22, 3) and posterior cingulate (21, −48, 3). The NW group did not show greater response than the OB group in any contrast during postmeal state. In addition, like the premeal state, the OB group did not show greater response than the NW group to the high-calorie vs. low-calorie contrast. See Table 2 for all between-group activation regions that reached significance (p < .05, corrected).
Figure 2.
Obese vs. Normal-Weight. Left: Post-meal scan results. Increased obese group activation to high-calorie vs. object cues during postmeal condition in A) lateral OFC/BA47, B) anterior cingulate/BA25, and C) Caudate. Significant activation to high-calorie food cues (p < .05, corrected) is shown in shades of red. Coordinates are given in standard Talairach space. Right and left are reversed by radiologic convention. Right: Magnitude of average activation for high calorie and low calorie foods at premeal and postmeal. Beta values reflect BOLD contrast averaged across voxels in region for calorie type relative to objects. BA=Brodmann Area, NW=normal-weight, OB=obese, OB high=obese group, high-calorie food cue, OB low=obese group, low-calorie food cue, NW high=normal-weight group, high-calorie food cue, NW low=normal-weight group, low-calorie food cue. esignificant difference (p < .05) in post-hoc t-tests : obese group, high- vs. low-calorie, postmeal condition.
Post-hoc analyses
Significant corticolimbic regions in the OB>NW high- vs. nonfood contrast were chosen for post-hoc analyses to confirm the BV findings and illuminate within-group differences (see Figure 2). For OB participants during the postmeal scan, high-calorie food cues elicited greater response in the lateral OFC (BA47; p < .05) than low-calorie cues (Figure 2a). Similarly, response in the caudate also differed significantly for the OB participants (p < .05) with high-calorie foods eliciting greater activation than low-calorie foods during the postmeal scan (Figure 2c).
Discussion
This study used fMRI to examine differences in neural response to food cues between obese and normal-weight individuals before and after eating. Our data extends the food neuroimaging literature by providing evidence of greater activation to food cues (both high- and low-calorie types) after eating among obese in comparison to normal-weight individuals. Prefrontal and corticolimbic regions including the OFC, caudate, and anterior cingulate showed significantly greater response to high-calorie food cues vs. objects after eating in obese participants in comparison to the normal-weight group. These brain regions have been implicated in hedonic response, reward processing, and addiction. Findings are of particular interest because participants ate a sizeable meal and reported decreased hunger immediately prior to scanning, thus indicating the continued impact of high-calorie food cues on brain reward circuitry after food consumption for obese participants. In addition, food cues did not elicit similar brain response after eating in normal-weight individuals suggesting the neural activity in response to food cues is diminished with reduced hunger.
Premeal response
Our findings show increased anterior prefrontal cortex activation among obese compared to normal-weight participants in response to the combined food condition and both types of food cues separately. However, we also found that across contrast type (e.g., high-calorie vs. object, etc.) normal-weight individuals showed greater activation in multiple regions in comparison to the obese group, with the exception of response to low-calorie foods. In fact, for the high- vs. low-calorie contrast, groups differed dramatically as the normal-weight group exhibiting greater activation in the insula, postcentral gyrus, parahippocampal gyrus and cerebellum and the obese group did not show greater differential activation to high- vs. low-calorie cues in any region in comparison to the normal-weight group.
At first glance, these findings were somewhat surprising and unexpected based on the previous literature. Several studies have shown greater activation to food cues for obese vs. normal-weights during fasting and particularly for high- vs. low-calorie cues (Martin et al., 2010; Stoeckel et al., 2008) and thus, we predicted similar findings. However, there are two points of interest in the present findings. First, there is greater activation in anterior prefrontal regions of the brain in the obese group compared to the normal-weight group for the premeal food and high-calorie vs. objects contrasts. Previous research has shown greater response of the PFC to food cues in those with disordered eating in comparison to a normal-weight group (Holsen et al., 2006); and it has been implicated in addiction, engaging in cue-induced activation in response to alcohol-associated images in alcoholics (George et al., 2001; Grusser et al., 2004). Second, for the normal-weight group, the low-calorie food cues do not appear to engage neural systems similarly to high-calorie cues as shown by the significant difference between high- and low-calorie activations for this group. Post-hoc examination of the beta values in the insula, post central gyrus and parahippocampal gyrus results (Figure 1) show that the group differences are primarily driven by increased activation in these regions to high-calorie foods in the normal-weight group, and in the case of the post central gyrus and insula regions, also a deactivation to high-calorie foods for the obese group. These regions play a role in the sensory processing of taste and olfaction. The insula has been consistently shown to activate to visual food cues and primate research has demonstrated the primary taste cortex is located within the insula (Pritchard, Macaluso, & Eslinger, 1999). The postcentral gyrus (BA43) has been implicated in taste perception (located within the somatosensory region closest to the tongue) and food cues have been previously shown to activate this region (Frank et al., 2010; Haase, Green, & Murphy,2011; Killgore et al., 2003; Wang et al., 2004). Similarly, although the parahippocampal gyrus is best known for memory encoding and retrieval, it appears to be involved in processing visual food cues as it has repeatedly been shown to differentially respond to food vs. object cues in previous research (Berthoud, 2002; Bragulat et al., 2010; Haase et al., 2011; Killgore et al., 2003; LaBar et al., 2001; Tataranni et al., 1999). In addition, stimulation of the parahippocampal gyrus has been found to increase autonomic and endocrine effects such as gastric secretion (Halgren,1982). Low-calorie foods appear to elicit greater neural response than we expected for the obese group which is indicated by the high vs. low-calorie contrast results (where no significant activations are seen compared to normal-weight) and the significant low-calorie vs. object findings.
Postmeal response
In contrast to the premeal condition, the postmeal results indicate greater activation to high- and low-calorie food cues among obese compared with normal-weight participants. Food vs. object, high-calorie vs. object, or low-calorie vs. object contrasts were shown to elicit activation in frontal, temporal, and more posterior regions. As expected, normal-weight participants did not show greater activation in any regions than obese participants during the postmeal task. However, there was no significant group effect for the high- vs. low-calorie conditions. The obese group exhibited less differential activation to high vs. low calorie foods than we predicted, showing greater activation to both the high vs. object and low vs. object contrasts.
Our primary findings indicate increased activation to high-calorie foods (vs. object) after eating in obese individuals. Right hemisphere frontal regions (lateral OFC, PFC/BA8 medial frontal gyrus/BA6) showed greater response to high-calorie foods in the obese group. Prefrontal regions (BA6,8) have been previously shown to respond to food cues in obese and normal-weight samples and specifically to high-calorie foods while hungry (Rothemund et al., 2007; Stoeckel et al., 2008). The lateral OFC plays an important role in food-related neural circuitry and responds preferentially to high-calorie food cues (Goldstone et al., 2009; Rothemund et al., 2007; Stoeckel et al., 2008). Primate research has demonstrated connections to the primary taste cortex in the insula and the hypothalamus, and identified that the secondary taste cortex is located in the lateral OFC (Baylis, Rolls, & Baylis, 1995; Rolls, 1999). Activation of the lateral OFC has been shown to be positively correlated with an individual’s subjective ratings of pleasantness of food indicating that highly rewarding foods may activate this area more so than less desirable foods (Kringelbach, O’Doherty, Rolls, & Andrews, 2003). Our findings indicate the OFC region does not decrease response after eating in obese individuals (see Figure 2). Similar activation of the OFC was not seen in the normal-weight comparison group. The lateral OFC has also been shown to be modulated by hunger with decreased neuronal firing after satiation of a particular taste (Critchley & Rolls, 1996). It is interesting that the meal used to attain satiety in this study did not include high fat/sweet foods. If neurons in the lateral OFC are subject to food-specific satiety, in that satiation to one particular food does not reduce firing in response to another food type (Critchley & Rolls, 1996), this may support the continued OFC activity seen in response to high-calorie foods after eating among obese participants.
The anterior cingulate also showed a differentiated response between groups after eating, with greater response among the obese group to high-calorie vs. object. Previous findings indicate the ACC shows greater activation to high- vs. low-calorie foods while hungry and smaller decreases in signal change after eating in obese individuals compared to controls (Bruce et al., 2010; Stoeckel et al., 2008). The ACC has been involved in food motivation, activating in response to fat and sucrose administration (De Araujo & Rolls, 2004), and exhibiting increased activation to drug-related cues among addicts (Volkow, Fowler, Wang, Swanson, & Telang, 2007). Recent research has also shown severity of food addiction positively correlates with activation in the ACC during anticipation of palatable food (Gearhardt et al., 2011). In addition, high-calorie food vs. object cues elicited greater response in the caudate region in the obese group. Unlike previous research using PET indicating decreased activation in the caudate and putamen following a liquid meal (Gautier et al., 2000), our findings indicate continued activation of the striatum to high-calorie foods. This is consistent with evidence from the animal literature indicating that neurons distributed through the nucleus accumbens, caudate, and putamen mediate the hedonic impact of high sugar/fat content foods (Kelley et al., 2005).
Summary and Conclusions
Our findings demonstrate that obese and normal-weight individuals differ substantially in their brain response to food cues, particularly after eating. While hungry, obese individuals show greater response to both food cue types in anterior prefrontal regions implicated in addiction. In contrast, during premeal, normal-weight individuals show a clear preferential response to high- vs. low-calorie cues in regions involved in sensory processing-a difference that is not observed postmeal. After eating, the impact of high-calorie foods is apparent among obese participants as high-calorie cues continue to elicit activation in brain areas involved in reward processing and taste even after reported decreased hunger. Moreover, low-calorie foods also elicit greater neural response after eating among obese compared to normal-weight participants highlighting the continued responsiveness to these types of food cues among obese individuals and the decreased activation among those with normal-weight. These findings are particularly interesting given that the majority of participants underwent normative caloric deprivation prior to eating lunch making these findings generalizable to natural fasting/eating cycles.
This study has several limitations. First, due to the constraints on data collection as part of a larger project, we were unable to counterbalance fasting and postmeal state across individuals. While this is not ideal and findings should be replicated with counterbalanced procedures, both short and longer duration (1–14 days) test-retest fMRI studies have shown good test-retest reliability in sensorimotor tasks (Friedman et al., 2008) and in striatal response during alcohol cue reactivity tasks (Schacht et al., 2011). The lack of this counterbalance renders within-group before and after meal comparison difficult to interpret, though, and is hence not the primary focus here. The lack of counterbalance across meal states is minimized in the between-group results, since both groups are matched in scanning procedure. In future studies, counterbalancing would allow more complete analysis of the within-group between-time modulation of food response. Second, the inclusion of both males and females in this sample may have unknown effects on the data set as reward functioning in women has been demonstrated to vary depending on stage of menstrual cycle (Dreher et al., 2007), a factor not taken into account in this sample given the demands of the larger project. It should be noted that participants did not have a preference to a specific food type based on the food preference assessment; this may be a result of administering the task directly prior to the fasting scan, which may reflect increased palatability during hunger. However, just because one might rate a food highly, it does not necessarily mean they would prefer it to another palatable food if given the choice (e.g., author A.D. loves carrots but if given the choice of ice cream or carrots, ice cream will always win). A measure of food preference decision-making may yield more discriminating results on high vs. low-calorie preference. Despite the behavioral ratings, both obese and normal-weight participants show differential brain activation by calorie type. In addition, future studies should replicate these findings with the inclusion of better measures of satiety. Although hunger ratings were assessed at four time points (before and after each scan) and showed decreased hunger after eating, direct ratings of satiety were not obtained. We indirectly inferred satiety by change in hunger status. Finally, we did not limit this sample to right-handed participants since as a part of the larger project these participants were being compared to a rare population in which we could not select on handedness criteria. While this study is not without its limitations,these findings provide preliminary evidence among obese for sustained response to food cues in reward-related brain regions even after eating, when compared to response in normal-weight controls. Future work should expand on these findings by examining the degree to which dieting and eating habits affect neural response to food cues.
Participants in this study indicated only moderate hunger prior to the fasting scan. Even those who skipped breakfast indicated only moderate hunger prior to scanning. Much of the previous research has focused on examining neural response after a prolonged, atypical fast. Our findings are of interest because extreme hunger is not necessary to elicit neural response to food cues. In fact, understanding how neural systems respond during more typical hunger may give us critical insight into the mechanisms behind overeating. It is interesting to note that neural response to food cues did not differ between those who did and those who did not consume breakfast. This may indicate that for individuals who typically skip breakfast, reward response to food cues is not fundamentally different from those who consume breakfast. Also of interest is the fact that the majority of participants who skipped breakfast were obese; this may indicate poorer dietary intake as research has shown that eating breakfast is related to healthier eating habits and reduced total daily food intake (de Castro, 2007; Leidy & Racki, 2010).
We have shown here that for obese individuals, high-calorie food cues show sustained response in brain regions implicated in reward and addiction even after ingestion of a sizeable meal. This continued hedonic response after a high caloric load may be critical to understanding overeating behavior. Future work directed at the extent to which the addition of a high-calorie sweet/savory food to a meal curbs neural response in reward systems for obese individuals is warranted given the current findings.
Functional MRI was used to examine brain response to food before and after eating
Obese showed greater brain response to food cues after eating than normal-weight
Increased response of OFC, caudate, and anterior cingulate after eating in obese
Corticolimbic response after eating implies continued salience of high-calorie food
Activity in response to food cues in normal weight diminishes with reduced hunger
Acknowledgments
This work was supported by grants RO3HD058766-01 and UL1 RR024989 from the National Institutes of Health, and the ACES Opportunity Grant from the National Science Foundation. We thank the Case Center for Imaging Research, Jack Jesberger, Brian Fishman, and Angela Ferranti and Kelly Kanya for their research assistance; to Jennifer Urbano Blackford and Elinora Price for their helpful comments on the manuscript; and to all individuals who participated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest.
References
- Baylis LL, Rolls ET, Baylis GC. Afferent connections of the caudolateral orbitofrontal cortex taste area of the primate. Neuroscience. 1995;64(3):801–812. doi: 10.1016/0306-4522(94)00449-f. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Multiple neural systems controlling food intake and body weight. Neuroscience and Biobehavioral Reviews. 2002;26(4):393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Morrison C. The brain, appetite, and obesity. Annual Review of Psychology. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- Bragulat V, Dzemidzic M, Bruno C, Cox CA, Talavage T, Considine RV, et al. Food-related odor probes of brain reward circuits during hunger: A pilot FMRI study. Obesity (Silver Spring, Md.) 2010;18(8):1566–1571. doi: 10.1038/oby.2010.57. [DOI] [PubMed] [Google Scholar]
- Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International Journal of Obesity (2005) 2010;34(10):1494–1500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K, et al. Obese adults have visual attention bias for food cue images: Evidence for altered reward system function. International Journal of Obesity (2005) 2009;33(9):1063–1073. doi: 10.1038/ijo.2009.138. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Rolls ET. Hunger and satiety modify the responses of olfactory and visual neurons in the primate orbitofrontal cortex. Journal of Neurophysiology. 1996;75(4):1673–1686. doi: 10.1152/jn.1996.75.4.1673. [DOI] [PubMed] [Google Scholar]
- De Araujo IE, Rolls ET. Representation in the human brain of food texture and oral fat. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2004;24(12):3086–3093. doi: 10.1523/JNEUROSCI.0130-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro JM. The time of day and the proportions of macronutrients eaten are related to total daily food intake. The British Journal of Nutrition. 2007;98(5):1077–1083. doi: 10.1017/S0007114507754296. [DOI] [PubMed] [Google Scholar]
- Dimitropoulos A, Schultz RT. Food-related neural circuitry in Prader-Willi syndrome: Response to high- versus low-calorie foods. Journal of Autism and Developmental Disorders. 2008;38(9):1642–1653. doi: 10.1007/s10803-008-0546-x. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(7):2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science (New York, N.Y.) 2007;317(5843):1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Laharnar N, Kullmann S, Veit R, Canova C, Hegner YL, et al. Processing of food pictures: Influence of hunger, gender and calorie content. Brain Research. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Friedman L, Stern H, Brown GG, Mathalon DH, Turner J, Glover GH, et al. Test-retest and between-site reliability in a multicenter fMRI study. Human Brain Mapping. 2008;29:958–972. doi: 10.1002/hbm.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, et al. Differential brain responses to satiation in obese and lean men. Diabetes. 2000;49(5):838–846. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- Gautier JF, Del Parigi A, Chen K, Salbe AD, Bandy D, Pratley RE, et al. Effect of satiation on brain activity in obese and lean women. Obesity Research. 2001;9(11):676–684. doi: 10.1038/oby.2001.92. [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Archives of General Psychiatry. 2011;68(8):808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of General Psychiatry. 2001;58(4):345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Ladell T, Logan M, Schneider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46(1):31–35. doi: 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with Brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping. 2006;27:392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone AP, de Hernandez CG, Beaver JD, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems towards high-calorie foods. The European Journal of Neuroscience. 2009;30(8):1625–1635. doi: 10.1111/j.1460-9568.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, et al. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology. 2004;175(3):296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Halgren E. Mental phenomena induced by stimulation in the limbic system. Human Neurobiology. 1982;1(4):251–260. [PubMed] [Google Scholar]
- Haase L, Green E, Murphy C. Males and females show differential brain activation to taste when hungry and sated in gustatory and reward areas. Appetite. 2011;57(2):421–434. doi: 10.1016/j.appet.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Brooks WM, Butler MG, Thompson TI, Ahluwalia JS, et al. Neural mechanisms underlying hyperphagia in prader-willi syndrome. Obesity (Silver Spring, Md.) 2006;14(6):1028–1037. doi: 10.1038/oby.2006.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karhunen LJ, Lappalainen RI, Vanninen EJ, Kuikka JT, Uusitupa MI. Regional cerebral blood flow during food exposure in obese and normal-weight women. Brain: A Journal of Neurology. 1997;120 (Pt 9)(Pt 9):1675–1684. doi: 10.1093/brain/120.9.1675. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiology & behavior. 2005;86(5):773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high- versus low-calorie foods. NeuroImage. 2003;19(4):1381–1394. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. Food for thought: Hedonic experience beyond homeostasis in the human brain. Neuroscience. 2004;126(4):807–819. doi: 10.1016/j.neuroscience.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral Cortex (New York, N.Y.: 1991) 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Parrish TB, Kim YH, Nobre AC, Mesulam MM. Hunger selectively modulates corticolimbic activation to food stimuli in humans. Behavioral Neuroscience. 2001;115(2):493–500. doi: 10.1037/0735-7044.115.2.493. [DOI] [PubMed] [Google Scholar]
- Leidy HJ, Racki EM. The addition of a protein-rich breakfast and its effects on acute appetite control and food intake in 'breakfast-skipping' adolescents. International Journal of Obesity (2005) 2010;34(7):1125–1133. doi: 10.1038/ijo.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring, Md.) 2010;18(2):254–260. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- Pritchard TC, Macaluso DA, Eslinger PJ. Taste perception in patients with insular cortex lesions. Behavioral Neuroscience. 1999;113(4):663–671. [PubMed] [Google Scholar]
- Rigby NJ, Kumanyika S, James WP. Confronting the epidemic: The need for global solutions. Journal of Public Health Policy. 2004;25(3–4):418–434. doi: 10.1057/palgrave.jphp.3190040. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The brain and emotion. New York: Oxford University Press; 1999. [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37(2):410–421. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Schacht JP, Anton RF, Randall PK, Li X, Henderson S, Myrick H. Stability of fMRI striatal response to alcohol cues: A hierarchical linear modeling approach. NeuroImage. 2011;56:61–68. doi: 10.1016/j.neuroimage.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41(2):636–647. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar steriotaxic atlas of the human brain. 3-dimensional proportional system: An approach to cerebral imaging. New York: Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(8):4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: Results of imaging studies and treatment implications. Archives of Neurology. 2007;64(11):1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: A concept review. Journal of Addictive Diseases. 2004;23(3):39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]