Abstract
Testosterone (T) administration is associated with increased satellite cell number and skeletal muscle hypertrophy, although there is considerable heterogeneity in the response of different skeletal muscle groups to T in vivo. We investigated the effects of T on the growth and differentiation of satellite cells isolated from levator ani (LA) and gastrocnemius (gastroc) muscles. T up regulated follistatin (Fst) expression, but down regulated the mRNA and protein expression of a number of genes in the transforming growth factor-beta (TGF-β)-signaling pathway. Inhibition of Fst expression by small interfering RNA (siRNA) inhibited myogenic differentiation and blocked the pro-myogenic effects of T. Treatment of satellite cells with T or Fst up regulated the expression of Pax7 and PCNA, and increased their proliferation. T and Fst blocked TGF-β induced inhibition of growth and myogenic differentiation and down regulated TGF-β-dependent transcriptome in both LA and gastroc cells. We conclude that T stimulation of satellite cell proliferation and myogenic differentiation are associated with up regulation of Fst and inhibition of TGF-β-signaling.
Keywords: Transforming growth factor-β, follistatin, myostatin, myosin heavy chain II
1. Introduction
Testosterone is an important mediator of body composition (Bhasin et. al., 2007; Bhasin et. al., 1997a; Bhasin et. al., 1997b; Bhasin et. al., 2003; Sattler et. al., 2009), but the molecular mechanisms by which testosterone increases muscle mass are incompletely understood. We have demonstrated previously that testosterone induces myogenic differentiation of mesenchymal multipotent stem cells through androgen receptor (AR)/β-catenin/T-cell factor 4 (TCF-4) pathway that up-regulates follistatin (Fst) via non-canonical Wnt signaling (Singh et. al., 2009). Fst is an extra-cellular protein, which inhibits mainly activins and selected members of TGF-β family including myostatin and GDF11. Although it is reported that Fst does not block the action of TGF-β’s themselves directly (Bradley et. al., 2008; Rodino-Klapac et. al., 2009), in a recent report it was demonstrated that it neutralizes TGF-β1’s activity and protein expression via Smad2 phosphorylation (Zhu et. al. 2011). Several lines of evidence suggest that follistatin plays an important role in the regulation of skeletal muscle mass. Transgenic mice over-expressing full length Fst have increased muscle mass (Lee et. al. 2001), whereas Fst knockout mice die soon after birth with insufficient muscle development and skeletal abnormalities (Matzuk et. al., 1995). Similarly, Fst over-expression in the skeletal muscle is associated with muscle hypertrophy in the rat and trout (Gilson et. al., 2009; Medeiros et. al., 2009). However, it is not clear whether Fst is endogenously expressed in the satellite cells, and whether and how it mediates androgen action on the satellite cell differentiation and proliferation. Our primary aim was to test the hypothesis that the promyogenic and proliferative effects of T are mediated by inhibition of TGF β signaling through Fst.
T administration induces skeletal muscle fiber hypertrophy and increases the number of satellite cells in healthy young (Sinha-Hikim et. al., 2003) and older men (Sinha-Hikim et. al., 2006). However, there is substantial heterogeneity in the anabolic response of various skeletal muscle groups to androgen administration observed mainly in vivo (Hawke and Garry, 2001; Schmalbruch and Hellhammer, 1977). For instance, LA muscle group is highly responsive to androgen withdrawal as well as to androgen supplementation (Ho et. al., 2004). In contrast, the gastroc muscle group shows substantially lesser response to androgen administration than LA. The reasons for these differences in the magnitude of anabolic response of LA and gastroc to T treatment are not understood. Our secondary aim was to determine whether differences in Fst expression or in the ability of T to induce Fst expression in satellite cells from different muscle groups play a role in mediating the differential response to androgen administration.
To test these hypotheses, we utilized primary cultures of satellite cells isolated from skeletal muscle groups that show either high (LA) or low (gastroc) androgen responsiveness. We investigated the effects of testosterone on the expression of Fst and TGF-β/BMP signaling pathway genes in satellite cells derived from the high and low responder muscles maintained in either the differentiation or proliferative conditions. We assessed whether testosterone blocks the effects of TGF-β on satellite cell proliferation and differentiation. To elucidate the intermediate role of Fst during testosterone’s actions, we used small inhibitory RNAs (siRNA) to block Fst expression in satellite cells isolated from both wild type and Fst over-expressing F66 male mice. We show here that testosterone promotes the proliferation as well as the myogenic differentiation of satellite cells through induction of Fst and inhibition of TGF-β signaling and action. We also show that although the satellite cells isolated from LA and gastroc differ significantly in their basal expression levels of AR and Fst, satellite cells from both groups show significant increase in their myogenic differentiation in response to testosterone administration.
2. Materials and Methods
2.1. Cell Culture
Satellite cell primary cultures were isolated as previously described (Rando and Blau, 1994). Briefly, LA and gastroc muscles were excised from 2–3 month old C57/BL6 male mice. We also isolated satellite cells from LA muscle from 2–3 month old follistatin over-expressing F66 male mice (kind gift from Dr. Se-Jin Lee, Johns Hopkins University). Each muscle was minced and it underwent enzymatic digestion at 37°C in 0.2% collagenase solution for 1 hour. Myofibers were purified from interstitial cells and tendons by a series of trituration, sedimentation, and washings. Myofiber fragments were passed through a 40μm cell strainer, resuspended in DMEM medium containing 10% FBS and 1% antibiotic solution (penicillin/streptomycin) and plated in culture dish. Cells were allowed to adhere for 4 hours to remove fibroblasts that readily adhere to plastic. The primary myoblasts which remained in suspension were transferred onto collagen-coated plates and cultured in growth medium (GM) containing 20% FBS, 10% horse serum, 1% chick embryo extract, and 1% antibiotic solution. Myogenic differentiation was induced in these cells by allowing them to differentiate in differentiation medium (DM) containing DMEM, 1% horse serum and 1% antibiotic solution.
2.2. Single and double-labeling immunodetection
Satellite cells grown in proliferation medium on eight-well collagen coated chamber slides were fixed in 4% paraformaldehyde. For AR immunolabeling, cells were blocked with normal goat serum and incubated with1:100 dilutions of rabbit anti-AR antibody followed by incubation with fluorescein anti-rabbit secondary antibody. For double immunolabeling, cells were blocked with 10% horse serum and incubated with 1:100 dilution of anti-Pax7 (Developmental Biology, Hybridoma Bank) antibody. Cells were further incubated with 1:200 dilution of anti-mouse secondary antibody conjugated with Texas-red. Cells were blocked with 10% goat serum and subsequent reaction was carried out by incubating the cells with 1:10,000 dilution of rabbit anti-Fst antibody (kind gift, Dr. Alan Schneyer, Pioneer Valley Life Science Institute, MA) followed by incubation with 1:200 dilution of streptavidin-fluorescein isothiocyanate (Vector Laboratories, Burlingame, CA). Cells were further incubated with 1:200 anti-rabbit biotinylated secondary antibody. The slides were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI) and mounted in Prolong anti-fade solution (Molecular Probes, Eugene, OR) (Singh et. al., 2006; Singh et. al., 2009).
2.3. Detection of myosin heavy chain expression by immunocytochemistry
Satellite cells grown in myogenic differentiation medium on four-well collagen coated chamber slides were fixed in 4% paraformaldehyde, quenched with H2O2, blocked with normal horse serum, and incubated with anti-MHC II antibody (MF20; Hybridoma Bank) at 1:100. Detection was based on a secondary biotinylated secondary antibody (1:200), followed by the addition of the streptavidin-horseradish peroxidase ABC complex (1:100), Vectastain (Elite ABC System; Vector Laboratories, Burlingame, CA) were counterstained with Meyer’s hematoxylin and 3,3′-diaminobenzidine and H2O2 solution (Sigma). Cells solution (Sigma) and mounted in prolong fade (Molecular Probes, Eugene, OR) (Singh et. al., 2003; Singh et. al., 2009).
2.4. Western blot analysis
Cell lysates (50–100 μg) in M-PER lysis buffer (Bio-Rad Hercules, CA) were subjected to western blot analyses by 7.5–12% SDS-PAGE, using 1:3000 dilution of anti-Fst (kind gift from Dr. Alan Schneyer, Pioneer Valley Life Science Institute, MA); 1:500 dilution of anti-pSmad2/3 (Santa Cruz Biotech, CA) and 1:5000 dilutions of anti-GAPDH (Chemicon, Temecula, CA) antibodies. The washed filters were incubated with 1:2000 dilution of secondary antibodies linked to horseradish peroxidase. Immunoreactive bands were visualized by using the ECL detection system (Amersham Biosciences, Piscataway, NJ).
2.5. Inhibition of Fst in satellite cell primary cultures by small inhibitory RNA
Follistatin levels were down-regulated in primary cultures of satellite cells isolated from LA muscle from C57BL6J and Fst over-expressing F66 mice using Fst small inhibitory RNA (siRNA). Mouse Fst gene was targeted by using ON-TARGET plus SMART pool siRNA which consists of four siRNA sequences-siRNA1 5′UAAAGAAACGUGUGAGAAC3′, siRNA2 5′GACUACAGCUUUCCUAUCU3′, siRNA3 5′GAACAGUACCUUUGUGGAA3′, siRNA4 5′GAGGGAAAGUGUAUCACAA3′ (Dharmacon, Lafayette, CO). These siRNA were used at 100nM concentrations with standard transfection protocol using lipofectamine 2000 (Invitrogen, Carlsbad, CA). As a control we used 100nM random siRNA. We were able to get about 75–80% inhibition of Fst gene expression. MHC II protein expression and phenotypic analysis of cells in various groups was analyzed after allowing the cells to differentiate under differentiating conditions for 4 days either in presence of absence of testosterone.
2.6. Quantitating mRNA expression by Real-Time Quantitative PCR
Total RNA was extracted by using Trizol reagent, and equal amounts (2 μg) of RNA were reverse transcribed using RNA High Capacity cDNA kit (Applied Biosystems, Foster City, CA). The Power Sybr Green PCR master mix was used with 7500 fast real-time PCR system (Applied Biosystems). We used the following primer pairs: AR (187 bp, forward 5′CAGGAGGAAGGAGAAAACTCCA3′; reverse 5′ATTGACAAGGCAGCAAAGGAATC3′), FST (Qiagen, PRM04451E), ACVR2 (Qiagen, PRM04452A), MHC II (119 bp, forward 5′GGACCCACTGAACGAGACC 3′; reverse 5′ TGGCAGCTCCACCACTACTT3′), PAX7 (132 bp, forward 5′TCTCCAAGATTCTGTGCCGAT3′; reverse 5′CGGGGTTCTCTCTCTTATACTCC3′); and SMAD7 (67 bp, forward 5′GAATCTTACGGGAAGATCAACCC3′; reverse 5′CGCAGAGTCGGATAAGGTG 3′); CD44 (106 bp, forward 5′TCTGCCATCTAGCACTAAGAGC3′; reverse 5′GTCTGGGTATTGAAAGGTGTAGC3′); SMAD2 (71 bp, forward 5′ACAGCAAATACGGTAGATCAGTC3′) BMP7 (64 bp, forward 5′CCTGTCCATCTTAGGTTGCC3′; reverse 5′CGCCGAATTATGCTTTCCCTG3′), TGF-β1 (64bp, forward 5′AGCTGGTGAAACGGAAGCG3′; reverse 5′GCGAGCCTTAGTTTGGACAGG3′) TGF-βR2 (135 bp, forward 5′GACTGTCCACTTGCGACAAC3′; reverse 5′GGCAAACCGTCTCCAGAGTAA3′) Samples of 25 ng cDNA were analyzed in quadruplicate in parallel with GAPDH (152 bp, forward 5′ATCACTGCCACCCAGAAGACT3′; reverse 5′CATGCCAGTGAGCTTCCCGTT3′) controls. The experimental mRNA starting quantities were calculated from the standard curves and averaged using 7500 software v1.4 (Singh et. al, 2003; Singh et. al., 2006; Singh et. al., 2009).
2.7. PCR Array analysis
Aliquots of total cellular RNA isolated with Trizol reagent from LA and gastroc satellite cells undergoing myogenic differentiation for 48 h with or without T (100 nM) treatment were subjected to RT2 profiler PCR Array (TGFβ/BMP signaling pathway, PAMM-035C-12; SA Bioscience, Frederick, MD) analysis. We also performed PCR Array analysis using RNA samples isolated from LA satellite cells from Fst over-expressing F66 as well as from C57BL6J mice. In some cases, LA satellite cells were treated with random siRNA and Fst siRNA alone or in combination with testosterone (100nM) for 48 hrs. This PCR Array contains pre-dispensed primer sets for the specified genes into a 96-well PCR plate designed to study the expression profiles of 84 genes related to TGF-β mediated signal transduction. Raw data were analyzed using PCR Array Data Analysis, and fold changes in relative gene expression were presented after background correction and normalization with a house-keeping gene (Artaza et. al., 2008) using ΔΔCt method following the manufacturer’s instruction (SA Bioscience, Frederick, MD).
2.8. Image analysis
MHC II immunostaining was quantified by image analysis using Image Pro 4.01 software (Media Cybernetics, Silver Spring, MD), coupled to a Leica DMLB microscope/VCC video camera. We analyzed total area of immunopositive cells per field from at least 20 fields per treatment group. Three different replicates per group were performed, and data are represented as mean ± SD (Singh et. al, 2003; Singh et. al., 2006; Singh et. al., 2009).
2.9. Statistical analysis
Data are presented as mean ± SD., and between-group differences were analyzed using ANOVA. If the overall ANOVA revealed significant differences, then pair-wise comparisons between groups were performed by Newman-Keuls multiple comparison test. All comparisons were two-tailed, and P values <0.05 were considered statistically significant. The experiments were repeated at least three times, and data from representative experiments are shown.
3. Results
3.1. Characterization of satellite cells isolated from high and low testosterone responder muscles
Satellite cells were isolated from LA and gastroc muscle groups of 2–3 month old C57BL6J male mice and maintained in primary cultures using previously described methods (Rando and Blau, 1994). The cells were immunostained using anti-Pax7 antibody, and the number of Pax7+ cells was expressed as a percent of total number of cells per field. We did not find a significant difference in the percent of Pax7 (91±2 in LA vs. 85±4 in gastroc) positive cells between the cultures derived from the LA and gastroc muscle groups (Fig 1A, left panel). The basal levels of AR expression in these cultures were analyzed by immunofluorescence using anti-AR antibody. In both LA and gastroc satellite cell, AR immunoreactivity was detected in a majority of cells (Fig 1A, right panel).
Fig. 1.

(A) Left panel: Determination of percentage purity of satellite cell population from LA and gastroc primary cultures. Isolation of satellite cell primary cultures from levator-ani (LA) and gastrocnemius (gastroc) muscles from C57BL6J male mice and their characterization by Pax7 immunofluorescence analysis. Total number of Pax7 positive cells was counted per field and percentage positive cells were determined by counting total number of cells present under bright field (BF). A total of 15 different fields were analyzed. Right panel: Expression of AR and Fst in satellite cell primary cultures. (B) LA and gastroc satellite cells grown on 4-well chamber slides were fixed with 4% paraformaldehyde (PFA) and single or double immunofluorescence staining was performed using either anti-Fst or anti-Pax7 antibodies. Nuclei were stained with DAPI (blue). (C) Real-time quantitative analysis of basal AR, FST, PAX7 as well as CD44 and SMAD2 gene expression in LA and gastroc satellite cells (*, p≤ 0.05 and **, p≤ 0.01). Each experiment was repeated 3 times.
3.2. Expression of Fst and Pax7 in primary cultures of satellite cells from the LA and gastroc muscles
We analyzed the expression of Fst in satellite cell primary cultures isolated from both LA and gastroc cells by performing double immunofluorescence analysis using anti-Fst and anti-Pax7 antibodies. While Pax7 was expressed exclusively in the nucleus, Fst was expressed mostly in the cytoplasm in both these cells (Fig. 1B). Also, Fst (green) was expressed exclusively in Pax7+ (red) cells, suggesting that Fst is indeed expressed in satellite cells isolated from both LA and gastroc muscles (Fig, 1B). We also compared the gene expression of AR, PAX7 and FST as well as some non-specific genes such as CD44 and SMAD2 expressed in LA and gastroc satellite cell primary cultures. The expression levels of AR (3.4±0.3 fold), FST (2.4± 0.25 fold), and PAX7 (1.9± 0.4 fold) mRNA were significantly higher in LA than in gastroc satellite cells as analyzed by quantitative real-time PCR analysis (Fig. 1C). On the other hand, mRNA expression levels of CD44 and SMAD2 did not differ significantly between gastroc and LA satellite cells (Fig. 1C).
3.3. Testosterone treatment up regulates Fst expression in LA and gastroc satellite cell primary cultures
We have previously demonstrated that testosterone up regulates Fst expression in mouse mesenchymal pluripotent C3H10T1/2 and 3T3-L1 cells during their differentiation (Singh et. al. 2006; Singh et. al. 2009). Since satellite cells are major contributors to the overall muscle mass, we tested whether these cells respond to testosterone treatment by up regulating Fst expression, and there is a difference in the response of satellite cells based on the abundance of AR in these cells. We treated LA and gastroc cells with optimal concentration of testosterone (100 nM, based on our preliminary experiments) for different time points (0–72hrs), and analyzed the protein expression of Fst by Western blot analysis. Fst expression levels were significantly increased in both cultures after T treatment (LA: 2.55±0.42 fold, gastroc: 2.3±0.3 fold) after 72 hours (Fig. 2A). Fst expression levels did not change significantly in untreated control cells derived from either LA or gastroc maintained in growth conditions (data not shown). In order to further test the role of Fst during myogenic differentiation, we treated satellite cells with recombinant mouse Fst (0.5 μg/ml; F288 from R& D System) and allowed the cells to grow for 4 days. We found that Fst significantly up regulated the area of MHC II+ cells in both LA (2.51 ±0.4 fold) and gastroc satellite cells (2.1±0.1 fold) (Fig. 2B).
Fig. 2.
(A) Effect of testosterone treatment on Fst expression in LA and gastroc satellite cell primary cultures. Cells were treated with 100nM testosterone for different time points (0–72hrs). Fst and GAPDH protein expression was analyzed by Western blot analysis (top panel) and quantitative densitometric analysis (bottom panel). (B) Effect of Fst (0.5μg/ml) on myosin heavy chain (MHC II) expression in primary cultures of LA and gastroc muscles. Cells were allowed to differentiate in 6-well plates in satellite cell differentiation medium (DM) (growth medium containing 2% horse serum) either in presence or absence of Fst for 4 days. Cells were fixed with 4% PFA and immunocytochemical analysis was performed using anti-MHC II antibody. Image analysis was performed by averaging values obtained from 20 different fields. Each experiment was performed 3 times (*, p≤ 0.05 and **, p≤ 0.01).
3.4. Testosterone induces myogenic differentiation of satellite cells and anti-Fst antibody block testosterone-induced increase in MHC II expression
Primary cultures of satellite cells isolated from LA and gastroc muscles were treated with anti-Fst antibody alone, T (100 nM) or T plus anti-Fst antibody and allowed to differentiate under myogenic conditions for 4 days. Under basal conditions, area of MHC II+ cells in LA-derived cultures was significantly higher (2.48±0.24 fold) compared to the gastroc-derived cultures (Fig. 3A–B) and anti-Fst antibody treatment significantly inhibited the basal myogenic differentiation (LA: 66±8.2%; gastroc: 62.2±6.8%). T treatment of these cells led to significant increase (LA: 2.6±0.42 fold; gastroc: 2.5±0.3 fold) in the area of MHC II+ cells in both satellite cell cultures (Fig. 3A–B). Simultaneous treatment of testosterone-treated cells with neutralizing concentrations of anti-Fst antibody (0.2 μg/ml) significantly blocked testosterone-induced increase in the area of MHC II+ cells (LA: 56.2±3.8%; gastroc: 53.4±4.2%) (Fig. 3A–B). This suggests that Fst may play an intermediate role during testosterone-induced induction of MHC II expression in LA and gastroc satellite cell primary cultures under myogenic differentiation conditions.
Fig. 3.

(A) Effect of testosterone and anti-Fst antibody on myosin heavy chain (MHC II) expression in primary cultures of LA and gastroc muscles. Cells (2 × 105) were allowed to differentiate in 6-well plates in satellite cell differentiation medium (DM) (growth medium containing 2% horse serum) in control medium (Con), anti-Fst antibody (anti-Fst ab), and either 100nM testosterone (T) alone or in combination with anti-Fst antibody (T+anti-Fst ab) for 4 days. Cells were fixed with 4% PFA and immunocytochemical analysis was performed using anti-MHC II antibody. (B) Image analysis was performed by averaging values obtained from 20 different fields. Each experiment was performed 3 times (*, p≤ 0.05 and **, p≤ 0.01).
3.5. Knock down of follistatin by small interfering RNA (siRNA) inhibits testosterone’s effect on MHC II expression
To further elucidate the intermediate role of Fst in T-mediated induction of myogenic differentiation, we performed siRNA-mediated knock-down of Fst in primary cultures of satellite cells isolated from LA muscle of C57BL6J male mice (Fig. 4A–C) as well from Fst over-expressing male F66 mice (Fig. 4D). We utilized ON-TARGET plus SMART pool Fst siRNA (siFst), which consists of four siRNA sequences directed against mouse Fst. Non-specific random siRNA (siRan), was used as a control for transfection. In satellite cell primary cultures derived from LA muscle of wild type mice, T treatment significantly increased Fst expression (2.3± 0.4-fold) in siRNA transfected cells. Transfection with siFst significantly knocked down Fst protein (77± 6.7 %) and gene expression (81±4.4%) in LA satellite cells (Fig. 4A, left and middle panels respectively). Simultaneous treatment of these cells with T (100 nM) did not significantly affect Fst expression under these conditions (Fig. 4A, middle panel). We also analyzed whether siRNA-mediated inhibition of Fst impairs the ability of satellite cells to differentiate into multinucleated myotubes, and their response to testosterone treatment. We found significant up regulation of MHC II mRNA (2.5± 0.2 fold) (Fig. 4A, right panel) and protein (1.74±0.3 fold) expression after T treatment in satellite cells transfected with random siRNA as analyzed by quantitative real-time PCR and quantitative image analysis of MHC II positive cells respectively (Fig. 4B,C). Transfection of these cells with Fst siRNA pool was associated with a significant down regulation of the basal expression of MHC II mRNA (82±3.6%) (Fig. 4A, right panel) and protein (74 ±2.8 %) (Fig. 4B, C). Also, transfection of Fst siRNA in Fst over-expressing LA satellite cells significantly down-regulated MHC II protein expression (75±3.45%) (Fig. 4D). Furthermore, testosterone treatment was unable to increase MHC II expression in cells that were transfected with Fst siRNA pool (Fig. 4D). These data indicate that Fst plays an important role in mediating testosterone’s effect on myogenic differentiation of satellite cells.
Fig. 4.
(A) Left panel: Western blot analysis of LA satellite cells treated with random (siRan) and Fst small interfering RNA (siFst). Middle and right panels: Real-time quantitative PCR analysis of the effect of Fst siRNA-mediated inhibition on Fst and MHC II gene expression. Random siRNA (siRan) and Fst siRNA (siFst) transfected cells were treated for 16 hrs and subsequently maintained in differentiation medium (DM) for 4 days with or without T (100nM). Total cellular RNA was isolated, Fst and MHC II mRNA expression was analyzed by RT and real-time PCR and data was presented after normalizing with GAPDH. Three different replicates were performed, and data are represented as mean ± SD. (B) Satellite cells were transfected with random siRNA and Fst siRNA and allowed to differentiate in DM with or without T (100nM) as described above. Cells were fixed with 4% PFA, immunocytochemical analysis was performed using anti-MHC II antibody and a representative picture is shown. (C) Image analysis was performed by averaging MHC II area per field using 20 different fields for each treatment group (**, p≤ 0.01). (D) Fst siRNA-mediated inhibition of Fst protein expression in LA satellite cells isolated from F66 (Fst-over expressing mice) and response of testosterone treatment on MHC II expression. Experiments were performed as described in Fig. 4B using satellite cells from F66 male mice. MCH II expression was analyzed after various treatments. A representative picture is shown in the top panel. Quantitative image analysis is shown in the bottom panel (**, p≤ 0.01). Each experiment was repeated 3 times.
3.6. Testosterone and Fst blocks TGF-β induced inhibition of MHC II expression and induction of Smad2/3 phosphorylation in satellite cells from LA and gastroc muscle groups
TGF-β is reported to inhibit the myogenic differentiation of satellite cells. Since testosterone treatment of satellite cells significantly induced Fst expression in these cells, we determined whether testosterone and Fst antagonize the inhibitory effects of TGF-β in these cells. Accordingly, we tested the effect of TGF-β (5 ng/ml) alone or in combination of T (100 nM) or Fst (0.5 μg/ml) on the myogenic differentiation of satellite cells isolated from both LA and gastroc muscles. TGF-β treatment significantly inhibited the formation of MHC II positive myotubes, and down regulated MHC II protein (LA: 60.6 ±6.7 %; gastroc: 34.2 ± 4.2 % in gastroc) (Fig. 5A–B) and mRNA (LA: 59.4±6.8%; gastroc: 54.6±4.4%) (Fig. 5C) and SMAD7 mRNA (LA: 43.2±7.2%; gastroc: 48.6±5.2%) expression in satellite cells maintained in differentiation conditions (Fig. 5C). On the other hand, T (100 nM) up regulated MHC II protein (LA: 2.05 ±0.4 fold; gastroc: 2.2 ±0.8 fold) (Fig. 5A-B) and gene (LA: 2.9±0.8 fold; gastroc: 2.1±0.3 fold) as well as SMAD 7 (LA: 2.3 ±0.2 fold, gastroc: 1.7 ±0.34 fold) mRNA expression (Fig. 5C). Simultaneous treatment of TGF-β-treated LA and gastroc satellite cells with T (100 nM) antagonized the inhibitory effects of TGF-β effects on myogenesis by significant induction of MHC II protein (LA: 3.7±0.9 fold; gastroc: 4.4±1.1 fold) (Fig. 5A–B) and gene expression (LA: 4.0±0.4 fold; gastroc: 3.4±0.6 fold) compared to TGF-β treated group alone (Fig. 5C). TGF-β plus Fst treatment also significantly up regulated MHC II protein (LA: 4.3±0.8 fold; gastroc: 3.8±0.9 fold) compared to the TGF-β treated group alone (Fig. 5A–B). SMAD7 expression was also up regulated (LA: 2.2±.3 fold; gastroc: 2.4±0.2 fold) after TGF-β plus T treatment compared to TGF-β alone (Fig. 5C). Both T and Fst treatment was able to inhibit TGF-β induced phosphorylation of Smad2/3 in differentiating LA and gastroc satellite cells after 4 days (Fig. 5D).
Fig. 5.


(A–B) Effect of testosterone and Fst on TGF-β-induced inhibition of myogenic differentiation in satellite cells. LA and gastroc satellite cells were allowed to differentiate in 6-well plate in DM after various treatments for 4 days. Cells were fixed with 4% PFA and immunocytochemical analysis was performed using anti-MHC II antibody (top panel). Bottom panel, Image analysis was performed by averaging values obtained from 20 different fields (**, p≤ 0.01). (C) Real-time quantitative PCR analysis was performed using total cellular RNA from LA and gastroc satellite cell primary cultures after various treatments and mRNA expression of MHC II and SMAD7 was analyzed. Three different replicates were performed, and data are represented as mean ± SD. Each experiment was repeated 3 times (*, p≤ 0.05 and **, p≤ 0.01). (D). Effect of T and Fst on pSmad2 in TGF-β treated satellite cells. LA and gastroc satellite cells were treated either with TGF- β (5ng/ml) alone or in combination of T (100nM) or Fst (0.5μg/ml) under differentiation conditions for 4 days. 40μg total protein was analyzed by western blot analysis using either anti-pSmad2/3 or anti-GAPDH antibody.
3.7. Testosterone inhibits TGF-β/BMP signaling pathway genes in satellite cells derived from LA and gastroc muscle groups
As stimulation of satellite cell differentiation by testosterone was associated with significant induction of Fst, which interacts and inhibits the signaling of activins and some other TGF-β super-family members, we analyzed the expression of various genes involved in TGF-β/BMP signaling pathway using RNA samples from LA and gastroc satellite cells treated with and without T (100 nM) for 48 hours in myogenic differentiation medium. T treatment was associated with alterations in the expression of several key genes in the TGF-β signaling pathway, including cyclin-dependent kinase inhibitor p21, BMP7, integrin beta 7 (Itgb7), Fst, transforming growth factor beta receptor II (Tgfbr2), serpine-1 (PAI-1), Gdf6 and Smurf1 (Smad specific E3 ubiquitin ligase) in cells from both muscles (Table 1–2); while some non-overlapping changes were found in LA and gastroc-derived satellite cells. Some targets of TGF-β signaling such as collagen type1aα (Col1aα), BMP2/4, noggin, activin receptor 2a (Acvr2a), among others, were significantly changed only in LA-derived satellite cells (Table 1), while Amh (anti-Mullerian hormone), GDF2,-3, and −7, nodal, Smad3 and Inhbb (inhibin beta-β) were significantly changed only in the gastroc-derived satellite cells (Table 2). Noggin, an antagonist of BMP signaling, has recently been demonstrated to be up-regulated during satellite cell differentiation (Ono et. al., 2011). Our data demonstrate that although there are some cell type-specific effects of T, it significantly increased Fst expression. Testosterone treatment was associated with an overall inhibition of TGF-β/BMP pathway genes in both LA and gastroc-derived satellite cells.
Table 1.
PCR Array analysis of the effect of T on TGF-β/BMP signaling pathway genes in LA satellite cell primary culture grown in DM after 48 hrs. Experiment was performed in triplicates using total RNA obtained from three different set of experiments (p ≤ 0.05 were considered significant)
| Gene | Gene Bank | Description | T vs. Con LA | P value |
|---|---|---|---|---|
| Acvr2a | NM_007396 | Activin receptor IIA | −1.64 | 0.01 |
| Bmp2 | NM_007553 | Bone morphogenetic protein 2 | −1.63 | 0.02 |
| Bmp4 | NM_007554 | Bone morphogenetic protein 4 | −1.86 | 0.0001 |
| Bmp7 | NM_007557 | Bone morphogenetic protein 7 | 2.28 | 0.0015 |
| cdkn1a | NM_007669 | Cyclin-dependent kinase inhibitor 1 A (p21) | −1.45 | 0.028 |
| Chrd | NM_009893 | Chordin | 2.49 | 0.007 |
| Col 1a1 | NM_007742 | Collagen. type I. alpha 1 | −1.87 | 0.016 |
| Fst | MM_008046 | Follistatin | 2.50 | 0.005 |
| Gdf1 | NM_008107 | Growth differentiation factor 1 | 1.22 | 0.04 |
| Gdf2 | NM_019506 | Growth differentiation factor 2 | 1.97 | 0.049 |
| Gdf3 | MM_008108 | Growth differentiation factor 3 | 1.47 | 0.039 |
| Gdf5 | NM_008109 | Growth differentiation factor 5 | −1.22 | 0.02 |
| Gdf6 | NM_013526 | Growth differentiation factor 6 | −1.52 | 0.08 |
| Gdf7 | NM_013527 | Growth differentiation factor 7 | 1.37 | 0.5 |
| IGF1 | NM_010512 | Insulin like growth factor 1 | −1.17 | 0.6 |
| Itgb7 | NM_013566 | Insulin beta 7 | 3.09 | 0.5 |
| Myc | NM_010849 | Myelocytomatosis oncogene* | 1.71 | 0.02 |
| Nog | NM_008711 | Noggin | 6.73 | 0.04 |
| Serpine1 | NM_008871 | Serine peptidase inhibitor member 1 | −1.44 | 0.03 |
| Smurf1 | MM_029438 | SMAD specific E3 ubiquitin protoin ligase 1 | −1.99 | 0.01 |
| Tdgf1 | NM_011562 | Teratocarcinoma-derived growth factor 1 | 4.02 | 0.026 |
| Tgfb1 | NM_011577 | Transforming growth factor. beta 1 | −1.42 | 0.01 |
| Tgfbr2 | NM_009371 | Transforming growth factor. beta receptor II | −1.48 | 0.04 |
Table 2.
PCR Array analysis of the effect of T on TGF-β/BMP signaling pathway genes in gastroc satellite cell primary culture grown in DM after 48 hrs. Experiment was performed in triplicates using total RNA obtained from three different set of experiments (p ≤ 0.05 were considered significant)
| Gene | Gene Bank | Description | T vs. Con Gas | p value |
|---|---|---|---|---|
| Amh | NM_007445 | Anti-Mullerian hormone | −1.9 | 0.0003 |
| Bmp7 | NM_007557 | Bone morphogenetic protein 7 | 1.6 | 0.001 |
| Cdkn1a | NM_007669 | Cyclin-dependent Kinase inhibitor 1A (p21) | −1.7 | 0.001 |
| Fst | NM_008046 | Follistatin | 2.1 | 0.01 |
| Gdf1 | NM_008107 | Growth differentiation factor 1 | −1.5 | 0.01 |
| Gdf2 | NM_019506 | Growth differentiation factor 2 | −2.2 | 0.00003 |
| Gdf3 | NM_008108 | Growth differentiation factor 3 | −2.9 | 0.0001 |
| Gdf6 | NM_013526 | Growth differentiation factor 6 | −1.8 | 0.00002 |
| Gdf7 | MM_013527 | Growth differentiation factor 7 | −2.8 | 0.004 |
| IGF1 | NM_010512 | Insulin like growth factor 1 | −1.12 | 0.6 |
| Inhbb | NM_008381 | Inhibin beta-B | −5.1 | 0.002 |
| Itgb7 | NM_013566 | integrin beta 7 | 1.7 | 0.04 |
| Nodal | NM_013611 | Nodal | −3.1 | 0.05 |
| Serpine1 | NM_008871 | Serine peptidase inhibitor member 1 | −1.7 | 0.003 |
| Smad3 | NM_016769 | MAD homolog 3 (Drosophila) | 1.8 | 0.004 |
| Smurf1 | NM_029438 | SMAD specific E3 ubiquitin protoin ligase 1 | −1.8 | 0.009 |
| Tdgf1 | NM_011562 | Teratocarcinema-derived growth factor 1 | −8.2 | 0.014 |
| Tgfbr2 | NM_009371 | Transforming growth factor. beta receptor 11 | −2.7 | 0.009 |
3.8. Testosterone mediated inhibition of TGF-β/BMP signaling pathway genes are dependent on follistatin expression
To further test our central hypothesis that testosterone-induced inhibition of TGF- β/BMP signaling is mediated at least in part by Fst, we performed PCR Array analysis from LA satellite cells isolated from the following groups-i) F66 mice; ii) wild type C57BL6J mice and transfected with either Fst siRNA or random siRNA and iii) wild type mice and transfected with Fst siRNA with and without T (100 nM). While F66 LA satellite cells expressed significantly higher levels of Fst mRNA compared to that from the wild type mice, we found significant down-regulation of several genes including BMP4 and 5, Bmpr2, GDF1, 2, 6, and 7 as well as Tgfb1 and Tgfbr1, 2 and 3, suggesting that increased Fst expression was associated with an overall reduced expression of TGF-β/BMP family member genes (Table 3). Fst siRNA-mediated inhibition of Fst was associated with a significant induction of Acvr2a, BMP1, 4 and 5, GDF6 and 7 as well as TGF-β1, 2 and 3 (Table 4). Finally, testosterone treatment of LA satellite cells transfected with the Fst siRNA was not associated with the expected inhibition of TGF-β/BMP signaling pathway genes. A small sub-set of genes were significantly affected by testosterone treatment, although the fold differences were not remarkable (Table 5). We performed quantitative real-time PCR analysis with a small subset of genes in various groups to validate the findings of our PCR Array analysis (Fig. 6) and found similar trend in their gene expression. Our combined PCR Array data therefore, support the hypothesis that inhibition of TGF-β signaling pathway by testosterone is mediated at least in part by Fst.
Table 3.
PCR Array analysis of the effect of Fst over-expression (F66 mice) on TGF-β/BMP signaling pathway genes in LA satellite cell primary culture grown in DM after 48 hrs. Experiment was performed in triplicates using total RNA obtained from three different set of experiments (p ≤ 0.05 were considered significant)
| Gene | Gene Bank | Description | F66 vs WT | p value |
|---|---|---|---|---|
| Acvr2a | NM_007394 | Activin A receptor. type 1 | −2.50 | 0.10 |
| Bmp1 | Mm. 27757 | Bone morphogenetic protein 1 | 3.81 | 0.002 |
| Bmp2 | Mm. 103205 | Bone morphogenetic protein 2 | −8.06 | 0.2 |
| Bmp4 | Mm 6813 | Bone morphogenetic protein 4 | −9.72 | 0.002 |
| Bmp5 | NM_007555 | Bone morphogenetic protein 5 | −3.87 | 0.001 |
| Bmp7 | NM_007557 | Bone morphogenetic protein 7 | 2.26 | 0.008 |
| Bmpr1a | NM_009758 | Bone morphogenetic protein receptor, type 1A | 8.48 | 0.001 |
| Bmpr1b | NM_007560 | Bone morphogenetic protein receptor, type 1B | −1.92 | 0.03 |
| Bmpr2 | NM_007561 | Bone morphogenic protein receptor, type II (serine/threonine Kinase) | −9.82 | 0.06 |
| FKBP | NM_016863 | FK506 binding protein 1b | 2.38 | 0.001 |
| Fst | NM_008046 | Follistatin | 3.89 | 0.000 |
| Gdf 1 | NM_008107 | Growth differentiation factor 1 | −8.52 | 0.07 |
| Gdf2 | NM_019506 | Growth differentiation factor 2 | −5.49 | 0.001 |
| Gdf6 | NM_013526 | Growth differentiation factor 6 | −1.60 | 0.04 |
| Gdf7 | NM_013527 | Growth differentiation factor 7 | −3.87 | 0.3 |
| Igf1 | NM_010512 | Insulin-like growth factor 1 | −1.74 | 0.14 |
| Myc | NM_010849 | Myelocytomatosis oncogene | 1.46 | 0.017 |
| Serpine1 | NM_008871 | Serine (or cysteine) peptidase inhibitor, clade E. member 1 | −1.96 | 0.2 |
| Smurf1 | NM_029438 | SMAD specific E3 ubiquitin protein ligase 1 | −2.84 | 0.03 |
| Tgfb1 | NM_011577 | Transforming growth factor, beta 1 | −4.9 | 0.001 |
| Tgfb2 | NM_009367 | Transforming growth factor, beta 2 | −5.2 | 0.05 |
| Tgfb3 | NM_009368 | Transforming growth factor, beta 3 | −6.1 | 0.07 |
| Tgfbr1 | NM_009370 | Transforming growth factor, beta receptor 1 | −1.6 | 0.09 |
| Tgfbr2 | NM_009371 | Transforming growth factor, beta receptor II | −4.6 | 0.03 |
| Tgfbr3 | NM_011578 | Transforming growth factor, beta receptor III | −5.6 | 0.005 |
Table 4.
PCR Array analysis of the effect of Fst knock down on TGF-β/BMP signaling pathway genes in LA satellite cell primary culture grown in DM after 48 hrs. Experiment was performed in triplicates using total RNA obtained from three different set of experiments (p ≤ 0.05 were considered significant)
| Gene | Gene Bank | Description | siFst vs WT | p value |
|---|---|---|---|---|
| Acvr2a | NM_007396 | Activin receptor IIA | 4.54 | 0.01 |
| Bmp1 | NM_009755 | Bone morphogenetic protein 1 | 1.89 | 0.01 |
| Bmp4 | NM_007553 | Bone morphogenetic protein 4 | 1.78 | 0.38 |
| Bmp5 | NM_007554 | Bone morphogenetic protein 5 | 2.21 | 0.08 |
| Chrd | NM_009893 | Chordin | −1.78 | 0.03 |
| Fst | NM_008046 | Follistatin | −2.16 | 0.002 |
| Gdf6 | NM_013526 | Growth differentiation factor 6 | 2.36 | 0.01 |
| Gdf7 | NM_013527 | Growth differentiation factor 7 | 2.98 | 0.04 |
| Igf1 | NM_010512 | Insulin-like growth factor 1 | 1.99 | 0.08 |
| Inhbb | NM_008381 | Inhibin beta-B | 2.63 | 0.01 |
| Nog | NM_008711 | Noggin | −2.91 | 0.01 |
| Serpine1 | NM_008871 | Serine (or cysteine) peptidase inhibitor, clade E. member 1 | 3.38 | 0.003 |
| Smurf1 | NM_029438 | SMAD specific E3 ubiquitin protein ligase 1 | 1.96 | 0.01 |
| Tgfb1 | NM_011577 | Transforming growth factor, beta 1 | 2.14 | 0.02 |
| Tgfb2 | NM_009367 | Transforming growth factor, beta 2 | 4.42 | 0.005 |
| Tgfb3 | NM_009368 | Transforming growth factor, beta 3 | 4.94 | 0.05 |
Table 5.
PCR Array analysis of the effect of T (100nM) treatment in Fst siRNA transfected LA satellite cells on TGF-β/BMP signaling pathway genes grown in DM after 48 hrs. Experiment was performed in triplicates using total RNA obtained from three different set of experiments (p ≤ 0.05 were considered significant). Each PCR Array data was performed three times and data are presented as mean ±SD
| Gene | Gene Bank | Description | siIFst+T vs siFst | p value |
|---|---|---|---|---|
| Bmp1 | NM_009755 | Bone morphogenetic protein 1 | 1.58 | 0.05 |
| Bmp4 | NM_007553 | Bone morphogenetic protein 4 | −1.28 | 0.001 |
| Chrd | NM_0090893 | Chordin | −1.35 | 0.03 |
| Fst | NM_008046 | Follistatin | 1.32 | 0.6 |
| Gdf5 | NM_008109 | Growth differentiation factor 5 | 1.67 | 0.002 |
| Gdf7 | NM_013527 | Growth differentiation factor 7 | 1.58 | 0.001 |
| Igf1 | NM_010512 | Insulin-like growth factor 1 | 1.39 | 0.4 |
| Tgfb1 | NM_011577 | Transforming growth factor, beta 1 | 1.66 | 0.0013 |
| Tgfb3 | NM_009368 | Transforming growth factor, beta 3 | 1.63 | 0.0015 |
Fig. 6.

Validation of PCR Array gene expression data by quantitative real-time PCR analysis. Real-time quantitative PCR analysis was performed in samples used (A: LA; B: Gastroc and C: LA from F66 mice)for PCR Array analysis (Table 1–3) using specified primers. Three different replicates were performed, and data are represented as mean ± SD. Each experiment was repeated 3 times (*, p≤ 0.05 and **, p≤ 0.01).
3.9. Effect of testosterone, TGF-β and Fst treatment on PAX7 and PCNA gene expression and proliferation of satellite cells
We determined the effects of T (100 nM), TGF-β (5 ng/ml) and Fst (0.5 μg/ml) treatment on PAX7 and PCNA gene expression as well as on the proliferation of LA and gastroc-derived satellite cells under growth (non-differentiating) conditions. Testosterone treated cells were also subjected to co-incubation with anti-Fst antibody (0.2 μg/ml) to test whether the proliferative effects of T was mediated via induction of Fst. The basal expression of PAX7 (57.3±5.2 %), AR (66.8±2.45) and PCNA (48.4±3.8 %) was lower in gastroc satellite cells compared to those derived from LA (Figure 7A). Treatment of these cells with T (100 nM) was associated with a significant increase in PAX7 (LA:89.2±5.1%; gastroc: 90.7±3.1%), AR (LA:131±27.8%; gastroc:141±7.6%) and PCNA (LA:209±17.2:%; gastroc: 78.8±12.3%) mRNA expression (Fig. 7A). While TGF-β treatment of these satellite cells significantly down regulated PAX7 (LA: 61.6±5.2%; gastroc: 48±2.1%) as well as PCNA (LA: 69.4±5.2%; gastroc: 52.3±4.4 %) mRNA expression, Fst treatment significantly increased PAX7 (LA: 60.4±11.2%; gastroc: 73.3±6.5%) and PCNA mRNA (LA: 72.2±8.4 %; gastroc: 96.4±11.6%) (Fig. 7B, right panel).
Fig. 7.

(A) Testosterone treatment promotes PAX7, PCNA and AR gene expression in satellite cells. LA and gastroc satellite cell primary cultures growing under proliferation conditions were treated with T (100nM) for 48 hrs and quantitative real-time PCR analysis was performed using PAX7, PCNA and AR gene specific primers. Data was presented after normalizing with GAPDH. Three different replicates were performed, and data are represented as mean ± SD. (B) Regulation of PAX7 and PCNA gene expression by TGF-β (5μg/ml) (left panel) and recombinant Fst (0.5μg/ml) (right panel) in LA and gastroc satellite cells. Cells were treated for 48 hrs and quantitative real-time PCR analysis was performed using PAX7 and PCNA gene specific primers. Data was presented after normalizing with GAPDH. Three different replicates were performed, and data are represented as mean ± SD (*p<0.01). (C) Effect of testosterone, TGF-β and Fst LA (left panel) and Gastroc (right panel) on satellite cell proliferation. 1.5 × 105 cells were plated on a T25 flask and treated with medium alone (Con) or with T (100nM), T (100nM) + anti-Fst antibody (0.2μg/ml), TGF-β (5ng/ml) alone or in combination with T or Fst or Fst (0.5μg/ml) alone and allowed to proliferate. Cells were counted after 48 hrs. Three different replicates were performed, and data are represented as mean ± SD (*, p≤ 0.05 **, p≤ 0.01). Each experiment was repeated 4 times.
Both T and Fst treatment significantly increased satellite cell proliferation in both LA (con vs. T, 48.2 ±4.4%; con vs. Fst 51 ±3.5%) and gastroc (con vs. T, 24.7 ± 1.8%; con vs. Fst, 37.5 ±5.2%) satellite cells. T-induced proliferation of both these cells was significantly inhibited in the presence of anti-Fst antibody (0.2 μg/ml) (LA: 21.4±3.3%; gastroc: 16±2.8 fold). Incubation of cells with IgG or anti-Fst antibody alone did not significantly change the proliferation of either of these cells (data not shown). TGF-β (5 ng/ml) treatment of LA-derived satellite cells significantly inhibited satellite cell proliferation (32±5.4%), and simultaneous treatment of TGF-β treated cells with T led to a significant increase in cell proliferation (56.5±5.2%) compared to the TGF- β group (Fig. 7C). Similarly, we observed a significant increase in cell proliferation in TGF- β plus Fst treatment group (23.2±4.4%) compared to the TGF- β group alone in LA-derived satellite cells (Fig. 7C). On the other hand, the effect of TGF-β either alone or in combination with T on the proliferation of gastroc-derived satellite cells did not reach significant levels when compared to the control group (Fig. 7C). The apparent disagreement of our cell proliferation data with that of the real-time PCR data for PAX7 and PCNA after TGF-β treatment in gastroc may be due to the differences in the sensitivities of two methods.
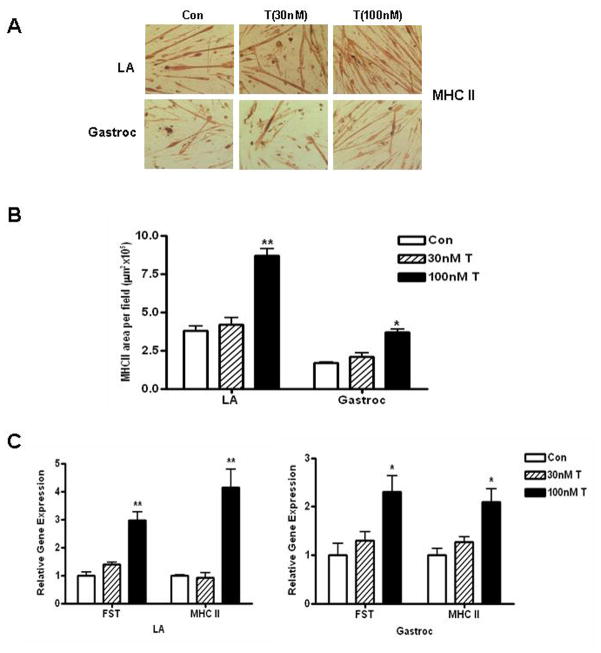
4.0. Dose-dependent effects of T on myogenic differentiation
In order to test whether satellite cells from LA and gastroc may differ in their sensitivity to different doses of testosterone, we treated these cells with varying concentrations (0, 30 and 100 nM) of T and allowed them to differentiate for 4 days under differentiation conditions. While we found that both LA and gastroc cells respond to the highest dose of T (100 nM), there was no effect of lower concentrations of T (30 nM) either on MHC II protein (Fig. 8A–B) or on mRNA expression of Fst or MHC II (Fig. 8C). However, there was significant increase in Fst and MHC II gene expression with higher concentration of T (100 nM) as observed in our previous experiments (Fig. 8C).
Fig. 8.
Dose-response of T treatment on myogenic differentiation and gene expression profile. (A) LA and gastroc satellite cells were allowed to differentiate in 6-well plate in DM after treatment with different doses of T (0–100nM) for 4 days. Cells were fixed with 4% PFA and immunocytochemical analysis was performed using anti-MHC II antibody (top panel). Bottom panel, Image analysis was performed by averaging values obtained from 20 different fields. (B) Quantitative real-time PCR analysis of FST and MHC II gene expression in RNA samples obtained after 4 days of treatment with different doses of T. Experiments were repeated 3 times and data are presented as mean ± SD (*, p≤ 0.05 **, p≤ 0.01).
4. Discussion
We have utilized primary cultures of satellite cells isolated from two different mouse muscle groups that express high and low levels of AR, as a model to investigate the molecular mechanism of T action, and to investigate the intermediate role of Fst. Several lines of evidence provided in this manuscript support the hypothesis that Fst plays an important role in mediating the effects of T on satellite cells isolated from LA and gastroc muscles. First, Fst gene and protein expression were significantly up regulated as early as 24–48 hours following testosterone treatment. Second, pharmacological inhibition of Fst by neutralizing concentrations of anti-Fst antibody led to a significant inhibition of T-induced increase in the area of MHC II+ cells under myogenic differentiation conditions as well as inhibited testosterone-induced increase in satellite cell proliferation. Furthermore, siRNA-mediated inhibition of Fst significantly attenuated T-induced increase in MHC II gene and protein expression, and testosterone’s effects on TGF-β associated transcriptome. In our study, we find that SMAD7 gene was significantly down regulated after TGF-β treatment for 4 days in both LA and gastroc satellite cells. Smad7 is reported to inhibit TGF-β signaling in a variety of conditions (Hayashi et. al. 1997; Zhou et. al. 2011), although in some cases it was identified as an early responsive gene after TGF-β treatment (Jungert et. al. 2006). Using LA satellite cells isolated from Fst-over-expressing F66 male mice (Fig. 4D) we also found that Fst knock-down by siRNA led to inhibition of T effects on myogenic differentiation. Collectively, these findings point to an important role of Fst in mediating the effects of testosterone on myogenic differentiation.
TGF-β levels are found to be significantly higher in old or injured myofibres and satellite cells (Carlson et. al., 2009). It has also been reported that attenuation of TGF-β signaling in old and injured muscle restored generation of satellite cells and muscle repair (Allen and Boxhorn, 1987; Carlson et. al., 2009). TGF-β1 induces apoptosis of many cell types including satellite cells (Li et. al. 2009) and inhibits their myogenic differentiation (Hawke et. al. 2001). We show here that testosterone blocks TGF-β signaling and action in satellite cell primary cultures in association with the induction of follistatin expression. Testosterone administration blocked the effects of TGF-β on the growth and differentiation of satellite cells in primary cultures maintained in growth or differentiation conditions, respectively. Furthermore, testosterone and Fst both inhibited TGF-β-induced Smad2/3 phosphorylation. The PCR array data provide additional evidence that testosterone down regulates TGF-β-dependent gene expression. We show that several important TGF-β/BMP signaling pathway genes are modulated by T treatment and this inhibition of TGF-β signaling by testosterone is significantly abolished in satellite cells transfected with Fst siRNA. In satellite cell primary cultures, T is a potent inhibitor of key TGF-β-pathway genes, including Acvr2a, serpine1 (PAI-1), Smad specific E3 ubiquitin ligase 1 (Smurf1), nodal, Tgfbr2, and CDK inhibitor p21, while it up regulates Fst, Myc, BMP7, noggin and chordin, among others. Using siRNA-pool developed against Fst and neutralizing anti-Fst antibodies, we show that Fst is essential for mediating the inhibitory effects of testosterone on TGF-β action. AR activation by testosterone up regulates Fst, which cross-communicates the intracellular signal to the TGF-β signaling pathway. We, therefore, conclude that T-induced inhibition of TGF-β signaling and induction of myogenic differentiation and proliferation is mediated via regulation of endogenous Fst levels.
A growing body of evidence suggests that Fst is an important regulator of skeletal muscle mass (Bradley et. al., 2008; Gilson et. al., 2009; Li et. al., 2009; Medeiros et. al., 2009). A genetic study using a transgenic mice model in which Fst gene was over-expressed under the control of muscle-specific myosin light chain promoter showed a significant increase in muscle mass (Lee et. al., 2001). The hyper-expression of Fst in the rainbow trout resulted in increased muscling (Medeiros et. al., 2009). A series of in vitro studies have also demonstrated that recombinant Fst increased myogenesis as well as satellite cell proliferation (Li et. al., 2009; Medeiros et. al., 2009). On the other hand, the loss-of-function mutant mice created by a targeted deletion of the FST gene, resulted in death of the pups within hours of birth due to musculoskeletal defects (Matzuk et. al., 1995). These studies suggest that manipulation of FST expression could provide an important means to counteract muscle loss associated with various conditions including sarcopenia, cancer, and HIV-related cachexia. The data presented here suggest that Fst also is an important mediator of the skeletal muscle response to pro-myogenic anabolic stimuli, such as androgens.
The mechanisms by which testosterone regulates Fst expression in satellite cells need further investigation. Fst is a down-stream target of AR/β-catenin activation in mesenchymal multipotent C3H10T1/2 cells (Singh et. al., 2009). Upon androgen binding to AR, AR/β-catenin associates with TCF-4, which then leads to the cascade of events that result in Fst up regulation and myogenic differentiation (Singh et. al., 2009). As Fst promoter has a TCF-4 binding site (CTTTGAT) at position -223 to -217 from the transcriptional start site, these findings suggest plausible molecular mechanisms by which testosterone regulates Fst expression through Wnt/β-catenin.
Our experimental approach has some notable advantages and limitations. The use of highly enriched populations of primary satellite cells rather than muscle-derived (e.g., C2C12) or other cell lines is a strength. The use of diverse approaches, including the use of siRNAs as well as neutralizing antibodies to block Fst expression or action lends strength to the inferences drawn from these experiments. We used a pool of multiple siRNAs to minimize the chances of off-target nonspecific effects and to effectively block Fst expression. Testosterone concentrations used in our experiments are within the range of concentrations that have been found in athletes and in some clinical trials (Bhasin et., al., 1996; Bhasin et. al., 2001). We recognize that no in vitro model can fully replicate the complexities of the whole organism. Also, there is some inherent artificiality in the culture media; small differences in medium composition and culture conditions across laboratories can contribute to quantitative or even qualitative differences in the observed response. The important but poorly understood effects of neural and vascular adaptations (Godinho et. al., 1987; Nnodim 2001), and of the cell-cell interactions that are prevalent in vivo, but which are not recapitulated in vitro models, necessarily constrain the inferences that can be drawn from in vitro models.
We found that satellite cells derived from LA express substantially higher levels of AR and Fst under basal conditions than those derived from the gastroc. However, in spite of these differences in the basal expression levels of AR and Fst, satellite cells from both muscle groups responded robustly to T and Fst treatment by up-regulation of myogenic differentiation. Thus, in sharp contrast to the quantitative differences in the responsiveness of the LA and gastroc muscle groups to testosterone administration in an intact mouse, the satellite cells derived from these two muscle groups respond well in vitro to testosterone administration by undergoing growth or myogenic differentiation depending upon the culture conditions. It is possible that in vivo differences in the neural or vascular inputs or cell-cell interactions may contribute to the observed differences in androgen responsiveness of LA and gastroc muscle groups in the mouse (Godinho et. al., 1987; Nnodim 2001). Indeed, experimental denervation renders the LA muscle in the rodents substantially less responsive to changes in androgen concentrations than a fully innervated LA muscle (Godinho et. al., 1987). We also cannot exclude the possibility that culture conditions including the composition of the growth and differentiation media and matrix, which undoubtedly differ from the endogenous cellular milieu in more ways than we understand - may alter the responsiveness of satellite cells to testosterone in vitro. These caveats notwithstanding, it is apparent that the satellite cells even from the gastroc retain the ability to undergo growth or differentiation in response to testosterone under appropriate conditions. The mechanisms that contribute to differences in the response of LA and gastroc muscles observed in vivo, cannot be fully explained by the in vitro studies and need further investigation, as they have important therapeutic implications.
Acknowledgments
The authors would like to thank Dr. Alan Schneyer (Pioneer Valley Life Science Institute, MA) for anti-Fst antibody and Dr. Se-Jin Lee (Johns Hopkins University, MA) for his generous gift of Fst over-expressing F66 male mice. This work was supported by National Institute of Aging Grant SC1AG033407 (R.S), and in part by 1SC1CA165865-01A1 (S.P), 5U54CA 143931, and Charles Drew University MSI endowment sub-award (5S21MD000103, RS).
Footnotes
Disclosures
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Melissa Braga, Email: melissabraga@cdrewu.edu.
Shalender Bhasin, Email: Shalender.Bhasin@bmc.org.
Ravi Jasuja, Email: jasuja@bu.edu.
Shehla Pervin, Email: shehlapervin@cdrewu.edu.
Rajan Singh, Email: rajansingh@mednet.ucla.edu.
References
- Allen RE, Boxhorn LK. Inhibition of skeletal muscle satellite cell differentiation by transforming growth factor-beta. J Cell Physiol. 1987;133(3):567–572. doi: 10.1002/jcp.1041330319. [DOI] [PubMed] [Google Scholar]
- Artaza JN, Singh R, Ferrini MG, Braga M, Tsao J, Gonzalez-Cadavid NF. Myostatin promotes a fibrotic phenotypic switch in multipotent C3H 10T1/2 cells without affecting their differentiation into myofibroblasts. J Endocrinol. 2008;196:235–249. doi: 10.1677/JOE-07-0408. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Parker RA, Sattler F, Haubrich R, Alston B, Umbleja T, Shikuma CM. AIDS Clinical Trials Group Protocol A5079 Study Team. Effects of testosterone supplementation on whole body and regional fat mass and distribution in human immunodeficiency virus-infected men with abdominal obesity. J Clin Endocrinol Metab. 2007;92:1049–1057. doi: 10.1210/jc.2006-2060. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiological doses of testosterone on muscle size and strength in normal men. New Eng J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Storer TW, Berman N, Yarasheski KE, Clevenger B, Phillips J, Lee WP, Bunnell TJ, Casaburi R. Testosterone replacement increases fat-free mass and muscle size in hypogonadal men. J Clin Endocrinol Metab. 1997a;82:407–413. doi: 10.1210/jcem.82.2.3733. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Taylor WE, Singh R, Artaza J, Sinha-Hikim I, Jasuja R, Choi H, Gonzalez-Cadavid NF. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A Biol Sci Med Sci. 2003;58:M1103–110. doi: 10.1093/gerona/58.12.m1103. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Tenover JS. Age-associated sarcopenia--issues in the use of testosterone as an anabolic agent in older men. J Clin Endocrinol Metab. 1997b;82:1659–1660. doi: 10.1210/jcem.82.6.4061. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheki KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Storer TW. Androgen effects on body composition. Growth Horm IGF Res. 2003;(Suppl A):S63–71. doi: 10.1016/s1096-6374(03)00058-3. [DOI] [PubMed] [Google Scholar]
- Bradley L, Yaworsky PJ, Walsh FS. Myostatin as a therapeutic target for musculoskeletal disease. Cell Mol Life Sci. 2008;65:2119–2124. doi: 10.1007/s00018-008-8077-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson ME, Conboy MJ, Hsu M, Barchas L, Jeong J, Agrawal A, Mikels AJ, Agrawal S, Schaffer DV, Conboy IM. Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8:676–689. doi: 10.1111/j.1474-9726.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson H, Schakman O, Kalista S, Lause P, Tsuchida K, Thissen JP. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am J Physiol Endocrinol Metab. 2009;297:E157–164. doi: 10.1152/ajpendo.00193.2009. [DOI] [PubMed] [Google Scholar]
- Godinho RO, Lima-Landman MT, Souccar C, Lapa AJ. Trophic control of cholinesterase activity in a testosterone-dependent muscle of the rat; effects of castration and denervation. Exp Neurol. 1987;96:558–568. doi: 10.1016/0014-4886(87)90218-4. [DOI] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA, Jr, Wrana JL, Falb D. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89(7):1165–73. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Ho MH, Bhatia NN, Bhasin S. Anabolic effects of androgens on muscles of female pelvic floor and lower urinary tract. Curr Opin Obstet Gynecol. 2004;16:405–409. doi: 10.1097/00001703-200410000-00009. [DOI] [PubMed] [Google Scholar]
- Jungert K, Buck A, Buchholz M, Wagner M, Adler G, Gress TM, Ellenrieder V. Smad-Sp1 complexes mediate TGFbeta-induced early transcription of oncogenic Smad7 in pancreatic cancer cells. Carcinogenesis. 2006;27(12):2392–401. doi: 10.1093/carcin/bgl078. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, McFarland DC, Velleman SG. Transforming growth factor-beta1-induced satellite cell apoptosis in chickens is associated with beta1 integrin-mediated focal adhesion kinase activation. Poult Sci. 2009;88:1725–1734. doi: 10.3382/ps.2008-00534. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- Medeiros EF, Phelps MP, Fuentes FD, Bradley TM. Overexpression of follistatin in trout stimulates increased muscling. Am J Physiol Regul Integr Comp Physiol. 2009;297:R235–242. doi: 10.1152/ajpregu.91020.2008. [DOI] [PubMed] [Google Scholar]
- Nnodim JO. Testosterone mediates satellite cell activation in denervated rat levator ani muscle. Anat Rec. 2001;263:19–24. doi: 10.1002/ar.1072. [DOI] [PubMed] [Google Scholar]
- Ono Y, Calbaheu F, Morgan JE, Katagiri T, Amthor H, Zammit PS. BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death Differ. 2011;18:222–234. doi: 10.1038/cdd.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodino-Klapac LR, Haidet AM, Kota J, Handy C, Kaspar BK, Mendell JR. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve. 2009;39:283–296. doi: 10.1002/mus.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler FR, Castaneda-Sceppa C, Binder EF, Schroeder ET, Wang Y, Bhasin S, Kawakubo M, Stewart Y, Yarasheski KE, Ulloor J, Colletti P, Roubenoff R, Azen SP. Testosterone and growth hormone improve body composition and muscle performance in older men. J Clin Endocrinol Metab. 2009;94:1991–2001. doi: 10.1210/jc.2008-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H, Hellhammer U. The number of nuclei in adult rat muscles with special reference to satellite cells. Anat Rec. 1997;189:169–175. doi: 10.1002/ar.1091890204. [DOI] [PubMed] [Google Scholar]
- Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, Bhasin S. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology. 2006;147:141–154. doi: 10.1210/en.2004-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- Singh R, Bhasin S, Braga M, Artaza JN, Pervin S, Taylor WE, Krishnan V, Sinha SK, Rajavashisth TB, Jasuja R. Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/ beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology. 2009;150(3):1259–1268. doi: 10.1210/en.2008-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab. 2006;91:3024–3033. doi: 10.1210/jc.2006-0357. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab. 2003;285:E197–205. doi: 10.1152/ajpendo.00370.2002. [DOI] [PubMed] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, McMahon C, Bhagat T, Alencar C, Yu Y, Fazzari M, Sohal D, Heuck C, Gundabolu K, Ng C, Mo Y, Shen W, Wickrema A, Kong G, Friedman E, Sokol L, Mantzaris I, Pellagatti A, Boultwood J, Platanias LC, Steidl U, Yan L, Yingling JM, Lahn MM, List A, Bitzer M, Verma A. Reduced SMAD7 leads to overactivation of TGF-beta signaling in MDS that can be reversed by a specific inhibitor of TGF-beta receptor I kinase. Cancer Res. 2011;71(3):955–63. doi: 10.1158/0008-5472.CAN-10-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Li Y, Lu A, Gharaibeh B, Ma J, Kobayashi T, Quintero AJ, Huard J. Follistatin improves skeletal muscle healing after injury and disease through an interaction with muscle regeneration, angiogenesis, and fibrosis. Am J Pathol. 2011;179(2):915–30. doi: 10.1016/j.ajpath.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]