Abstract
The effects of chronic cocaine dependence on cortical inhibitory/excitatory processes are not well characterized. Employing transcranial magnetic stimulation measures of motor cortical excitability, we have previously reported an elevation of motor threshold (MT) suggesting reduced excitability and an increased long-interval intracortical facilitation (LICF) suggesting increased excitability. In the current study, we used an expanded battery of TMS cortical excitability measures to further examine motor cortex excitability in a larger sample of well-characterized and closely monitored for drug use, abstinent cocaine-dependent subjects (N=52) and healthy controls (N=42). Furthermore, coil-to-cortex distance was assessed in a subsample of both groups. We verified that long-interval intracortical facilitation (LICF), possibly representing glutamatergic cortical neurotransmission, was significantly increased in cocaine-dependent patients. Significantly longer cortical silent periods (CSP) and elevated MT were also observed while there was no significant abnormality in long-interval intracortical inhibition (LICI). Increased LICF and CSP duration suggest increased cortical excitability and increased inhibition, respectively, of different neurotransmitter systems in cocaine-dependent patients. Increased MT might reflect an adaptation to those effects of cocaine abuse that enhance cortical excitability. Overall, the data point to the complex nature of chronic cocaine dependence on the balance of cortical inhibitory/excitatory mechanisms.
Keywords: transcranial magnetic stimulation, cocaine dependence, motor threshold, paired-pulse stimulation, cortical silent period, cortical excitability
Introduction
The effects of chronic cocaine use on cortical excitability in humans are not well characterized. Understanding of the chronic effects of cocaine dependence on cortical inhibitory and excitatory mechanisms is likely to be important not only for understanding the disorder itself, but also for the development of treatment and rehabilitation programs. Transcranial magnetic stimulation (TMS) provides a noninvasive and safe method to examine cortical excitation and inhibition in humans (Barr et al., 2008; Ziemann and Hallett, 2007). The current study optimized TMS assessments to shed new light on paucities in the literature.
When a TMS pulse of sufficient strength is delivered to the motor cortex, the corticospinal pathway is activated and the muscles represented by the stimulated region twitch. The threshold intensity of the magnetic pulse necessary to induce a motor response (motor threshold or MT) is thought to reflect voltage-gated sodium channel conductivity and hence membrane excitability in cortical pyramidal neurons (Ziemann et al., 1996a). Drugs that block voltage-gated ion channels, but lack significant action on neurotransmitter function, increase MT (Ziemann et al., 1996a). Glutamatergic facilitation and GABA(B) receptor-mediated inhibition can be studied in human motor cortex by long-interval paired-pulse TMS protocols (McDonnell et al., 2006; Nakamura et al., 1997). Long-interval intracortical facilitation (LICF) at an interstimulus interval of 25-30 ms most likely reflects glutamatergic mechanisms (Nakamura et al., 1997), similar to paired-pulse facilitation of excitatory postsynaptic currents mediated by glutamatergic receptors in animal preparations (Clark et al., 1994). Long-interval intracortical inhibition (LICI) at an interstimulus interval of 50-150 ms is enhanced by baclofen, a specific GABA(B) receptor agonist, and therefore reflects GABA(B) receptor-mediated cortical inhibition (McDonnell et al, 2006).
Another widely used TMS-based cortical excitability measure is the cortical silent period (CSP). This silent period represents a transient absence or significant decrement of EMG activity during sustained voluntary muscle contraction and can be induced by TMS over the motor cortex area. Evidence suggests that the CSP duration reflects motor cortical inhibition possibly mediated by GABA(B) receptors (Siebner et al., 1998). A significant correlation between the P50 suppression and CSP has been shown in healthy individuals (Moeller et al., 2007).
Using single-pulse TMS, we have previously observed that MT is significantly elevated in abstinent cocaine-dependent subjects as compared to age and gender matched healthy controls (Boutros et al., 2001; 2005). This finding does not appear to concur with evidence for increased excitability (Lason, 2001) and decreased inhibition (Fein et al., 1996) in cocaine abusing individuals. Subsequently, using a paired-stimulus protocol, we demonstrated exaggerated motor cortical excitability in a relatively small sample of abstinent chronic cocaine users (Sundaresan et al., 2007). The enhanced long-interval intracortical facilitation (LICF) most likely reflects increased glutamatergic neurotransmission.
In the current study, we used an expanded battery of TMS cortical excitability measures in a relatively large sample of well-characterized and closely monitored for drug-use abstinent cocaine-dependent subjects. We hypothesized that either increased LICF and/or decreased LICI, as markers of enhanced cortical excitability, will be detected. An important new feature of the current study was the assessment of coil-to-cortex distance (CCD) in a subsample of both subject groups.
Materials and methods
52 cocaine-dependent individuals (48 men; mean age: 42 years, range 21-57 years) and 42 healthy controls (34 men; mean age: 41 years, range 23-57 years) were examined. The study was approved by Wayne State University Human Investigations Committee. Based on a Structured Clinical Interview for DSM-IV all cocaine-dependent subjects met criteria for dependence on cocaine but without meeting criteria for dependence on other substances. Subjects were excluded if they had a history of non-cocaine related Axis-I psychiatric disorders. Additionally, subjects with known Axis-II diagnoses were excluded based on available medical records. None of the subjects were receiving any CNS-active medications. All cocaine-dependent subjects were free of cocaine use for at least three weeks (mean abstinence duration: 4.13±2.64 months). None of the subjects had a history of any neurological disorders. All cocaine-dependent subjects were recruited from treatment and/or long-term rehabilitation programs which routinely employ random urine drug screens and were able to verify the duration of abstinence obtained as self-report from the subjects. A urine screen was obtained from all subjects in the morning of the study. None of the subjects exhibited outward evidence of stress (i.e., perspiring or agitation), as stress could have a possible effect on laboratory measurements (White et al., 2005). All subjects completed the procedure without complaints or side effects.
The Cocaine Experience Questionnaire (CEQ)(Satel and Edell, 1991) was administered by trained raters in order to determine whether a subject had experienced paranoid symptoms during cocaine use. An analog scale (range 0-6) assessed the psychotic experience itself ranging from minimally distressing to intolerable and terrifying. Scores of 5 or 6 on this sub-scale indicate a frankly psychotic experience. Insight, behavioral severity and behavioral response to CIP were assessed using CEQ.
TMS testing was divided over two sessions, one session in each of two separate days, being not more than one week apart. One hemisphere was measured in one session, and the other hemisphere in the other session, with the order of hemispheres randomized. Motor responses were recorded from the right and left first dorsal interosseus (FDI) muscles, using surface electromyogram (EMG) electrodes (bandpass filter 1Hz-2kHz, sampling rate 5kHz). Motor evoked potential (MEP) amplitudes were measured peak-to-peak. A figure-of-8, hand-held coil was used to deliver monophasic magnetic pulses generated by a Magstim magnetic stimulator. TMS was applied to the left and right motor cortex as guided by the ten/twenty EEG placement position of C3 and C4, respectively; the optimal site for stimulation of the FDI was determined using a search procedure (Rossini et al., 1999).
Motor thresholds
Starting at a stimulus intensity that was below the MT, single pulses were delivered. The strength of the stimulus was incremented by 5% until a motor response was obtained. Subsequently, 1% increments/decrements were used for the final determination of resting motor threshold (RMT), which was defined as the lowest stimulus intensity (in % of maximum stimulator output) that elicited at least five small MEPs (greater than 50 μV) out of ten consecutive stimulations and is given as a percentage of the maximum output of the magnetic stimulator.
Active motor thresholds (AMT) were obtained after RMTs were determined. Subjects were asked to use their index finger and thumb to press a lever of a dynamometer to achieve a level of tension 10% of maximum voluntary contraction. Stimulus intensity was then lowered from RMT by 1% increments until the AMT was determined in a similar fashion to RMT. AMT was defined as the lowest stimulus intensity that generated a MEP > 200 μV in at least five out of ten trials.
Cortical Silent period
Subjects were instructed to exert a steady voluntary isometric muscle contraction of the target muscle at 10% maximum effort and to maintain the contraction until told to relax. Ten pulses each at six different intensities (100,110,120,130,140,150% of AMT) were delivered and the conditional averages of the single-trial peak-to-peak MEP amplitudes were used for analysis. The CSP duration was measured in milliseconds in the EMG trace from the onset of the MEP to the end of this period defined as the first point when background EMG was restored to 50% of pre-stimulus EMG amplitude. We followed an automated procedure for CSP determination (Daskalakis et al., 2003), which included squaring of the EMG signal (for enhancement and rectification) and a modified threshold criterion for the CSP offset of 50% of the pre-stimulus EMG level (instead of 25% used in Daskalakis et al., 2003). The EMG pre-stimulus analysis period contained 200 samples (40 ms).
The steadiness of baseline EMG preceding the motor evoked potential (MEP) was assessed by calculating the coefficient of variation of consecutive EMG data points (SD/mean x100). The mean MEP amplitude at each intensity was calculated from the responses to ten stimuli.
Paired-pulse measures
Short-interval intracortical inhibition (SICI) and intracortical facilitation (ICF) were tested in the relaxed FDI. The intensity of the conditioning stimulus was 80% of RMT (subthreshold) followed by a suprathreshold test stimulus with intensity of 120% of RMT. Three conditions (10 repeats each) were randomly presented. In the control condition, the test stimulus was given alone. In the other two conditions, the conditioning stimulus was given prior to the test stimulus at ISIs of 3 and 10 ms. SICI and ICF were expressed as the percentage of the mean conditioned MEP amplitude to the mean unconditioned MEP amplitude (Kujirai et al., 1993; Ziemann et al., 1996c).
LICF and LICI were tested in the relaxed FDI using a long ISI paired-pulse TMS protocol (McDonnell et al., 2006; Nakamura et al., 1997). The intensity of both stimuli was 120% of RMT (suprathreshold). Four conditions (10 repeats each) were randomly presented. In the control condition, the test stimulus was given alone. In the other three conditions, the conditioning stimulus was given prior to the test stimulus at ISIs of 30, 50, or 100 ms. LICF and LICI were expressed as the percentage of the mean conditioned MEP amplitude to the mean unconditioned MEP amplitude.
MEP intensity curves
Two different paradigms were used and the FDI muscle was at rest for both. In the first paradigm, stimulus intensity was changed in 10% steps of RMT between 100-150% of RMT. In the second paradigm, TMS was applied at 10% steps between 50-100% of the maximum stimulator output. Five stimuli were delivered at each intensity and the mean MEP amplitude at each intensity was calculated.
MRI coil-cortex measurements
One potential significant confound with using TMS as a cortical excitability probe is the effect of the distance from the coil to the motor cortex (Kozel et al., 2000; Herbsman et al. 2009); relatively small changes in CCD can result in significant differences in the strength of stimuli necessary to induce a criterion MEP (Nahas et al., 2001). To control for this potentially important confound, the distance from the center edge of fiducials (Vitamin-E tablets placed on the cap at the point of stimulation) to the nearest cortex was measured in the coronal plane (mm) of anatomical magnetic resonance (MR) images using OsiriX software.
Statistics
Differences between the motor thresholds (RMT and AMT) of the Control group and the Cocaine group were assessed using a three-way analysis of variance (ANOVA) with GROUP as the between-group effect, and SIDE (hemisphere of stimulation: left/right) and condition (resting/active) as within-group effects. Short-interval paired-pulse measures were compared between the two groups using a three-way ANOVA with GROUP as the between-group effect, and SIDE (hemisphere of stimulation: left/right) and ISI as within-group effects. Based on a strong a priori hypothesis from our previous results (LICF at ISI of 30 ms was significantly increased in abstinent cocaine-dependent subjects as compared to controls (Sundaresan et al., 2007)), LICF values at ISI of 30 ms were compared between the two groups using a two-way ANOVA with GROUP as the between-group effect, and SIDE (left/right) as an within-group effect. A three-way ANOVA was carried out for LICI measures as well. CSP and MEP intensity curves measures were also compared between the two groups using a three-way ANOVA with GROUP as the between-group effect, and SIDE (left/ right) and stimulus INTENSITY as within-group effects.
Upon significant main effects or their interactions, post-hoc analyses were performed using two-tailed t-tests for independent samples to compare TMS measures between groups. Regarding the CCD measurements, a two-tailed independent samples t-test was used to test the null hypothesis of no significant difference between groups. Effect sizes (Cohen’s d) for the significant findings are also provided.
Relationships between the observed abnormalities of motor cortical excitability and clinical features of the patients from CEQ (i.e., abstinence duration, insight, behavioral severity, behavioral response to CIP) were assessed using Pearson’s and Spearman’s correlation two-tailed tests. Correction for multiple correlations was performed using Bonferroni’s method.
For the full methodology we refer the reader to the supplemental data section.
Results
Comparison of CCD between the Cocaine group and the Control group (assessed in a subsample of twelve subjects per each group) supports the null hypothesis of no significant difference in mean distances measured in both groups (Cocaine group: 18.1±2.1 mm; Control group: 17.9±1.6 mm; p=0.71).
Motor thresholds
RMT and AMT for both hemispheres are provided in Table 1 for Control and Cocaine groups. A three-way ANOVA with GROUP as the between-group effect, and SIDE (left/right) and condition (resting/active) as within-group effects, showed a significant effect of GROUP (F(1,62)=6.04, p=0.017) and condition (F(1,62)=137.08, p<0.0001). There were no significant effects of SIDE (F(1,62)=0.58, p=0.81) and no significant SIDE-by-GROUP (F(1,62)=0.76, p=0.38), condition-by-GROUP (F(1,62)=0.008, p=0.93), SIDE-by-condition (F(1,62)=0.004, p=0.95) and SIDE-by-condition-by-GROUP (F(1,62)=0.52, p=0.47) interactions. Post-hoc two-tailed t-tests for independent samples showed significant differences between the MT (RMT and AMT) of the Control and Cocaine groups. Specifically, the RMT in Cocaine group was significantly higher than the Control group on the left hemisphere (t77=2.17, p=0.033), and showed a trend towards significance on the right hemisphere (t77=1.85, p=0.069). The AMT in the Cocaine group was significantly higher compared to the Control group on the right hemisphere (t79=2.09, p=0.039), but no significant difference was found on the left side (p=0.2).
Table 1.
Motor thresholds (expressed as % of the maximum machine output).
| LEFT | RIGHT | ||||||
|---|---|---|---|---|---|---|---|
| Group | N | Mean | Std. deviation |
N | Mean | Std. deviation |
|
| RMT | HEALTHY | 36 | 50.25 | 8.35 | 36 | 49.83 | 7.47 |
| PATIENT | 43 | 54.44 * | 8.71 | 43 | 53.34 | 9.15 | |
| AMT | HEALTHY | 37 | 44.72 | 8.50 | 38 | 43.50 | 6.29 |
| PATIENT | 43 | 47.01 | 7.74 | 43 | 46.91 * | 8.08 | |
p<05
SICI and ICF
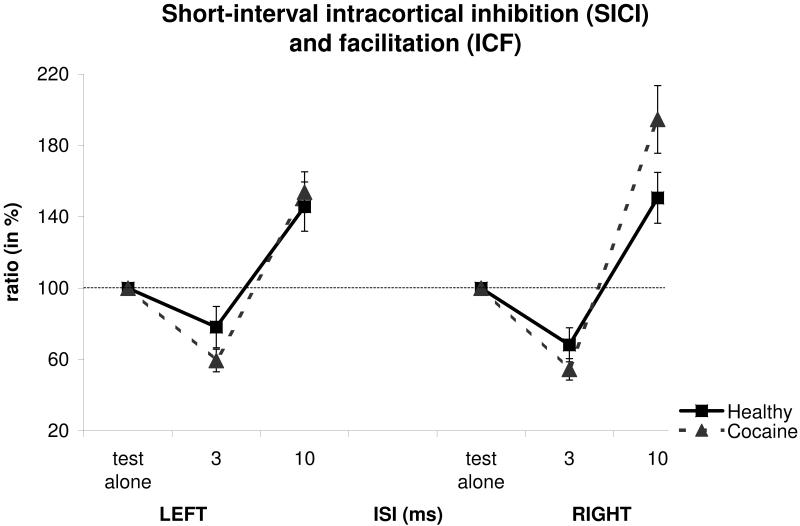
Measures taken with short-interval paired-pulse TMS are provided in Figure 1. A three-way ANOVA with GROUP as the between-group effect, SIDE (left/right) and ISI (3, 10 ms) as within-group effects, showed no significant effect of GROUP (F(1,70)=0.33, p=0.56) and SIDE (F(1,70)=0.38, p=0.54). On the other hand, there was a significant effect of ISI (F(1,70)=119.39, p<0.0001). In addition, no significant ISI-by-GROUP (F(1,70)=3.87, p=0.05), SIDE-by-GROUP (F(1,70)=1.10, p=0.29), SIDE-by-ISI (F(1,70)=0.27, p=0.59) and SIDE-by-ISI-by-GROUP (F(1,70)=0.03, p=0.86) interactions were found.
Figure 1.
Mean short-interval paired-pulse transcranial magnetic stimulation (TMS) measures from abstinent cocaine-dependent and healthy individuals (expressed as % ratios of the mean conditioned motor evoked potential (MEP) to the mean unconditioned MEP). Short-interval intracortical inhibition (SICI) and facilitation (ICF) are assessed at interstimulus intervals (ISIs) of 3 and 10 ms, respectively. Ratios smaller or larger than 100% represent inhibition or facilitation, respectively. Error bars represent SEM (standard error of the mean). ISI values on the x-axis are expressed in milliseconds.
LICF and LICI
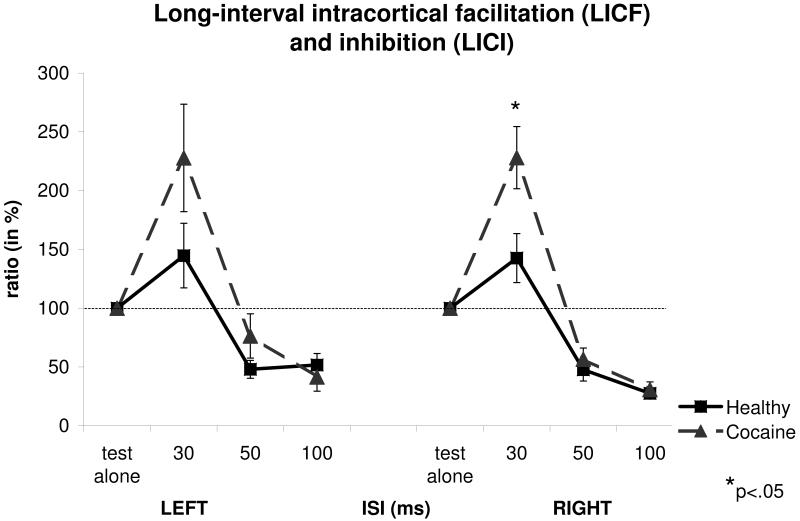
Measures taken with long-interval paired-pulse TMS are provided in Figure 2. For LICF at ISI of 30ms, a two-way ANOVA with GROUP as the between-group effect, and SIDE (left/right) as within-group effect, showed a significant effect of GROUP (F(1,67)=4.74, p=0.03) and no significant effects of SIDE (F(1,67)=0.36, p=0.54) and SIDE-by-GROUP interaction (F(1,67)=0.74, p=0.39). Post-hoc analyses using the two-tailed t-test for independent samples, showed a significant increase of LICF in the Cocaine group compared to the Control group at ISI=30ms on the right hemisphere (t81=2.47, p=0.015, Cohen’s d=0.55). No significant differences were observed between the two groups for the amplitude of the unconditioned (test alone) MEP for this measure (mean 1.43±1.15mV for the cocaine group vs. 1.16±0.79mV for the control group; t81=1.21, p=0.23). This is an important negative finding because LICF and LICI vary with the amplitude of the unconditioned MEP (Sanger et al., 2001).
Figure 2.
Mean long-interval paired-pulse transcranial magnetic stimulation (TMS) measures from abstinent cocaine-dependent and healthy individuals (expressed as % ratios of the mean conditioned motor evoked potential (MEP) to the mean unconditioned MEP). Long-interval intracortical facilitation (LICF) and inhibition (LICI) are assessed at interstimulus intervals (ISIs) of 30 and 50 / 100 ms, respectively. Ratios larger or smaller than 100% represent facilitation and inhibition, respectively. Error bars represent SEM (standard error of the mean). Inter-stimulus interval (ISI) values on the x-axis are expressed in milliseconds.
For LICI measures, a three-way ANOVA with GROUP as the between-group effect, SIDE (left/right) and ISI (50 and 100ms) as within-group effects, showed no significant effects of GROUP (F(1,69)=0.033, p=0.85) and SIDE (F(1,69)=3.14, p=0.08). On the other hand, there was a significant effect of ISI (F(1,69)=5.31, p=0.024). In addition, no significant SIDE-by-GROUP (F(1,69)<0.001, p=0.98), ISI-by-GROUP (F(1,69)=1.26, p=0.26), SIDE-by-ISI (F(1,69)=0.87, p=0.35) and SIDE-by-ISI-by-GROUP (F(1,69)=1.17, p=0.28), interactions were found.
The absolute sizes of the unconditioned MEPs in the paired pulse experiments in the Cocaine vs. Control groups are provided in Table 2. Two-tailed independent samples t-tests showed no significant differences between groups for these unconditioned MEPs.
Table 2.
Mean amplitudes of the unconditioned motor evoked potentials (MEPs) in the paired pulse experiments (expressed in mV).
| LEFT | RIGHT | ||||||
|---|---|---|---|---|---|---|---|
| Group | N | Mean | Std. deviation |
N | Mean | Std. deviation |
|
|
SICI-
ICF |
HEALTHY | 37 | 1.52 | 1.72 | 40 | 1.25 | 0.89 |
| PATIENT | 43 | 1.79 | 1.31 | 45 | 1.73 | 1.35 | |
|
LICF-
LICI |
HEALTHY | 38 | 0.97 | 0.81 | 39 | 1.16 | 0.79 |
| PATIENT | 41 | 1.36 | 1.04 | 44 | 1.43 | 0.17 | |
Cortical silent period duration
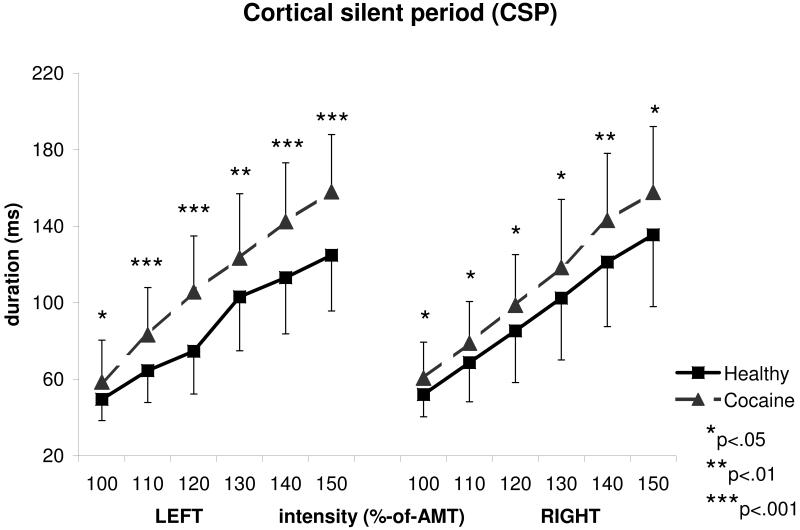
Figure 3 provides the CSP durations obtained from the left and right hemisphere in Cocaine patients vs. Controls. A three-way ANOVA with GROUP as the between-group effect, SIDE (left/right) and INTENSITY (100, 110, 120, 130, 140, 150% of AMT) as within-group effects, showed a significant effect of GROUP (F(1,54)=12.97, p=0.001), INTENSITY (F(5,270)=350.44, p<0.0001) and INTENSITY-by-GROUP interaction (F(5,270)=4.36, p=0.007). There was no significant effect of SIDE (F(1,54)=2.82, p=0.099), nor significant interactions for SIDE-by-GROUP (F(1,54)=0.54, p=0.46), SIDE-by-INTENSITY (F(5,270)=1.81, p=0.11) or SIDE-by-INTENSITY-by-GROUP (F(5,270)=1.44, p=0.21). Post-hoc analyses using the two-tailed t-test for independent samples, showed a significantly longer CSP in the Cocaine group compared to the Control group on the left hemisphere at intensities of 100%AMT (t83=2.34, p=0.022), 110%AMT (t81=4.01, p=0.0001, Cohen’s d=0.89), 120%AMT (t81=4.95, p<0.0001), 130%AMT (t81=2.97, p=0.004), 140%AMT (t59=3.79, p=0.0003), 150%AMT (t59=4.37, p<0.0001), and on the right hemisphere at intensities of 100%AMT (t82=2.5, p=0.014), 110%AMT (t82=2.19, p=0.031, Cohen’s d=0.48), 120%AMT (t82=2.29, p=0.025), 130%AMT (t82=2.14, p=0.035), 140%AMT (t69=2.69, p=0.009), and 150%AMT (t69=2.59, p=0.012).
Figure 3.
Mean cortical silent period (CSP) duration measures from abstinent cocaine-dependent and healthy individuals expressed in milliseconds. Longer CSP duration values represent increased inhibition. Intensities of stimulation are shown in percentages of AMT (active motor threshold). Error bars represent standard deviations.
Using two-tailed independent samples t-tests, no significant differences in mean amplitudes (Table 1, supplemental) and steadiness of the baseline EMG (Table 2, supplemental) in the CSP procedure were found between groups. As a disproportionate number of male subjects in the two groups could also influence the results, a comparison between CSP scores of female and male subjects for the healthy group were conducted, but revealed no significant differences at all intensity levels (independent samples t-test, p<0.05).
MEP intensity curves
RMT-based measures
A three-way ANOVA, with GROUP as the between-group effect, SIDE and INTENSITY as within-group effects, showed no significant effect of GROUP (F(1,54)=3.11, p=0.084) or SIDE (F(1,54)=0.001, p=0.97) (Figure 1a, supplemental). A significant effect of INTENSITY (F(5,270)=65.47, p<0.0001), but no significant INTENSITY-by-GROUP (F(5,270)=0.98, p=0.43) and SIDE-by-INTENSITY-by-GROUP (F(5,270)=0.407, p=0.84) interactions were observed.
Machine-output based measures
A three-way ANOVA, with GROUP as the between-group effect, SIDE and INTENSITY as within-group effects, showed no significant effect of GROUP (F(1,43)=0.85, p=0.36) and no significant effect of SIDE (F(1,43)=0.142, p=0.71) (Figure 2b, supplemental). A significant effect of INTENSITY (F(5,215)=55.78, p<0.0001), but no significant INTENSITY-by-GROUP (F(5,215)=0.42, p=0.83) and SIDE-by-INTENSITY-by-GROUP (F(5,215)=0.97, p=0.44) interactions were observed.
Relationships of TMS measures with clinical features
Cortical silent period (CSP) duration in abstinent cocaine-dependent subjects showed a negative correlation with abstinence duration (right hemisphere’s CSP-140%AMT: Pearson’s r=−0.38; p=0.11, corrected). Left hemisphere’s motor cortex CSP-140%AMT measure showed a significant, strong positive correlation with behavioral severity (Spearman’s r=0.64; p=0.004, corrected) and behavioral response to cocaine-induced psychosis (CIP) [Spearman’s r=0.65; p=0.004, corrected]. Paired-pulse TMS-based facilitation and inhibition measures and motor thresholds didn’t show any significant association with clinical measures such as abstinence duration, insight, behavioral severity and behavioral response to CIP.
Discussion
This study was carried out in the largest population of abstinent cocaine-dependent individuals assessed with a wide array of TMS excitability measures to date. Several important controls were employed to buttress data interpretation, e.g., assuring equality of CCD between groups and closely monitoring drug use prior to testing. The findings reveal a complex picture of abnormalities of the balance of excitation-inhibition in motor cortex of abstinent chronic cocaine-dependent individuals that concurs with, and extends, existing literature.
Consistent with our preliminary report (Sundaresan et al., 2007), we found an abnormally increased LICF (at ISI=30ms) in chronic cocaine users which likely signifies enhanced glutamatergic excitability through NMDA ionotropic receptors (Clark et al., 1994; Nakamura et al., 1997), as chronic cocaine has been shown to enhance ionotropic glutamatergic activity (Hemby et al., 2005). Glutamatergic mechanisms underlie several clinical aspects of cocaine dependence including withdrawal and modulation of glutamatergic transmission has been promising in the treatment of cocaine-addicted individuals (Dackis and O’Brien, 2003; Uys and LaLumiere, 2008).
LICI measures on the other hand are mostly considered to reflect a GABA(B)-mediated inhibition (McDonnell et al., 2006). No significant group difference was shown for LICI at ISI of 100 ms at which LICI can be tested without overlap with the preceding LICF (Nakamura et al., 1997; Sanger et al., 2001). This finding is in apparent discord with preclinical studies suggesting that GABA(B) receptor-mediated transmission is persistently down-regulated (Kushner and Unterwald, 2001; Frankowska et al., 2008) and such a down-regulation would be expected to reduce LICI. This interpretation supports claims that that GABA(B) receptor agonists may be useful in the treatment of cocaine addiction (Brebner et al., 2002; Roberts, 2005; Sofuoglu and Kosten, 2005). However, we did not observe a decrease in LICI in cocaine-abusing humans. Thus, it is likely that LICI is regulated by additional other neurotransmitter systems that remain intact with cocaine abuse.
The most interesting finding in the current study is the prolongation of CSP duration in cocaine-dependent patients which was significant at all tested intensities in the motor cortex of both hemispheres. While GABA(B) receptors may be involved in both CSP and LICI, dissociations between CSP and LICI have been described previously (Berardelli et al., 1996), suggesting that CSP and LICI do not measure the same (albeit overlapping) processes. It should also be noted that recently the CSP was shown to positively correlate with LICI (Farzan et al., 2010) in a sample of healthy individuals. Cortical dopamine is known to be altered in cocaine-abusing humans (Volkow et al., 1999; Martinez et al., 2011). The CSP elicited by TMS of the motor cortex is also altered in neuropsychiatric disorders and modulated by dopamine. For examples, L-DOPA and the dopamine receptor agonist pergolide prolong the CSP in normal subjects, an effect that is reduced by atypical antipsychotics (Acler et al., 2009; Ziemann et al., 1996b; Fitzgerald et al., 2002). However, the CSP is prolonged by the atypical antipsychotic clozapine (Daskalakis et al., 2008a; Liu et al., 2008). A significant prolongation of the TMS-induced CSP in subjects with history of cocaine-dependence may reflect an increase in the availability of cortical dopamine under chronic cocaine withdrawal due to a decrease in dopamine metabolism as has been indicated in rat frontal cortex (Karoum et al., 1994). Other factors also may play a possible role in explaining why CSP could dissociate from LICI. For instance, CSP is measured in the active muscle (LICI at rest) and it strongly depends on motor attention, instruction set and regularity of pre-stimulus EMG (Conte et al., 2007; Mathis et al., 1998; Nilsson et al., 1997). In this regard, the steadiness of pre-stimulus EMG in our study was quantitatively assessed between patients and controls and no difference was found between groups.
Our data conform to previously reported findings of elevated MT in abstinent cocaine users and we proposed that the elevated RMT may reflect a maladaptation to the cocaine-induced enhancement in cortical excitability (Boutros et al., 2001, 2005; Sundaresan et al., 2007). RMT reflects voltage-gated sodium channel conductivity and neuronal membrane stability (Ziemann et al., 1996a) and an elevation in RMT may be a consequence of reduced sodium conductance and a reduction in cortical activity. Preclinical reports also support a decreased membrane excitability and suppression of auditory-evoked activity (Nasif et al., 2005a, Jimenez-Rivera and Waterhouse, 1991).
The observed abnormalities of motor cortical excitability related to clinical features of the patients. For higher stimulation intensities, we found a negative association between the CSP duration and the abstinence duration. We also found a strong positive correlation of CSP duration with behavioral severity and behavioral response to cocaine-induced psychosis (CIP). These findings imply that the abnormally longer CSP tend to normalize in the course of cocaine abstinence but this would have to be proven in a longitudinal study design.
It is relevant to stress that most individuals in this study were tested after three to six months of abstinence. There is a wealth of literature showing that the duration of withdrawal dictates the brain state, therefore also cortical excitability (Hammer et al., 1993; Thompson et al., 2004). It is also important to distinguish between “basal” brain state, and the state evoked by drug-relevant stimuli (e.g., cocaine-associated cues or cocaine itself). For example, activity of cortical neurons is decreased during cocaine abstinence, but increased in response to excitatory stimuli, including re-exposure to cocaine or other cocaine-like drugs (Kufahl et al., 2005; Volkow et al., 2003; Volkow et al., 2004). Similar alterations in cortical excitability are found in withdrawn rats after chronic exposure to this drug (Ford et al., 2009; Nasif et al., 2005a, 2005b). Thus, the outcomes observed in the current study most likely reflect the overall hypo-active basal state associated with withdrawal from chronic cocaine abuse rather than the vulnerability to cocaine addiction.
It is possible that CSP prolongation may also partially explain the elevated MT and even the LICF findings (the former may be related to simply having excessive inhibition dampens cortical responses, the latter may be compensatory). In terms of generalizability of the results, a limitation of this study is that cortical excitability was assessed only in motor cortex; future studies should incorporate other sensitive brain regions (e.g. dorsolateral prefrontal cortex)(Daskalakis et al., 2008b; Farzan et al., 2009).
As conclusion, the effects of chronic cocaine-dependence on cortical functions are undoubtedly complex and in need of further probing. A preclinical, translational research study verifying the neurotransmitter mechanisms for each of the outcome measures described here would be highly informative.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Acler M, Fiaschi A, Manganotti P. Long-term levodopa administration in chronic stroke patients. A clinical and neurophysiologic single-blind placebo-controlled cross-over pilot study. Restorative Neurology and Neuroscience. 2009;27(4):277–83. doi: 10.3233/RNN-2009-0477. [DOI] [PubMed] [Google Scholar]
- 2.Barr MS, Fitzgerald PB, Farzan F, George TP, Daskalakis ZJ. Transcranial magnetic stimulation to understand the pathophysiology and treatment of substance use disorders. Current Drug Abuse Reviews. 2008;1:328–339. doi: 10.2174/1874473710801030328. [DOI] [PubMed] [Google Scholar]
- 3.Berardelli A, Rona S, Inghilleri M, Manfredi M. Cortical inhibition in Parkinson’s disease. A study with paired magnetic stimulation. Brain. 1996;119:71–77. doi: 10.1093/brain/119.1.71. [DOI] [PubMed] [Google Scholar]
- 4.Boutros NN, Lisanby SH, Tokuno H, Torello MW, Campbell D, Berman R, et al. Elevated motor threshold in drug-free, cocaine-dependent patients assessed with transcranial magnetic stimulation. Biological Psychiatry. 2001;49(4):369–73. doi: 10.1016/s0006-3223(00)00948-3. [DOI] [PubMed] [Google Scholar]
- 5.Boutros NN, Lisanby SH, McClain-Furmanski D, Oliwa G, Gooding D, Kosten T. Cortical excitability in cocaine-dependent patients: A replication and extension of TMS findings. Journal of Psychiatric Research. 2005;39:295–302. doi: 10.1016/j.jpsychires.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Brebner K, Childress AR, Roberts DC. A potential role for GABA B agonists in the treatment of psychostimulant addiction. Alcohol & Alcoholism. 2002;37:478–484. doi: 10.1093/alcalc/37.5.478. [DOI] [PubMed] [Google Scholar]
- 7.Clark KA, Randall AD, Collingridge GL. A comparison of paired-pulse facilitation of AMPA and NMDA receptor-mediated excitatory postsynaptic currents in the hippocampus. Experimental Brain Research. 1994;101:272–278. doi: 10.1007/BF00228747. [DOI] [PubMed] [Google Scholar]
- 8.Conte A, Gilio F, Iezzi E, Frasca V, Inghilleri M, Berardelli A. Attention influences the excitability of cortical motor areas in healthy humans. Experimental Brain Research. 2007;182(1):109–17. doi: 10.1007/s00221-007-0975-3. [DOI] [PubMed] [Google Scholar]
- 9.Dackis C, O’Brien C. Glutamatergic agents for cocaine dependence. Annals of the New York Academy of Sciences. 2008;1003:328–45. doi: 10.1196/annals.1300.021. [DOI] [PubMed] [Google Scholar]
- 10.Daskalakis ZJ, Molnar GF, Christensen BK, Sailer A, Fitzgerald PB, Chen R. An automated method to determine the transcranial magnetic stimulation-induced contralateral silent period. Clinical Neurophysiology. 2003;114(5):938–44. doi: 10.1016/s1388-2457(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 11.Daskalakis ZJ, Christensen BK, Fitzgerald PB, Moller B, Fountain SI, Chen R. Increased cortical inhibition in persons with schizophrenia treated with clozapine. Journal of Psychopharmacology. 2008a;22(2):203–9. doi: 10.1177/0269881107084002. [DOI] [PubMed] [Google Scholar]
- 12.Daskalakis ZJ, Farzan F, Barr MS, Maller JJ, Chen R, Fitzgerald PB. Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a TMS-EEG study. Neuropsychopharmacology. 2008b;33(12):2860–9. doi: 10.1038/npp.2008.22. [DOI] [PubMed] [Google Scholar]
- 13.Farzan F, Barr MS, Wong W, Chen R, Fitzgerald PB, Daskalakis ZJ. Suppression of gamma-oscillations in the dorsolateral prefrontal cortex following long interval cortical inhibition: a TMS-EEG study. Neuropsychopharmacology. 2009;6:1543–51. doi: 10.1038/npp.2008.211. [DOI] [PubMed] [Google Scholar]
- 14.Farzan F, Barr MS, Levinson AJ, Chen R, Wong W, Fitzgerald PB, et al. Reliability of long-interval cortical inhibition in healthy human subjects: a TMS-EEG study. Journal of Neurophysiology. 2010;104(3):1339–46. doi: 10.1152/jn.00279.2010. [DOI] [PubMed] [Google Scholar]
- 15.Fein G, Biggins C, MacKay S. Cocaine abusers have reduced auditory P50 amplitude and suppression compared to both normal controls and alcoholics. Biological Psychiatry. 1996;39:955–965. doi: 10.1016/0006-3223(95)00299-5. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald PB, Brown TL, Daskalakis ZJ, Kulkarni J. A transcranial magnetic stimulation study of the effects of olanzapine and risperidone on motor cortical excitability in patients with schizophrenia. Psychopharmacology (Berlin) 2002;162(1):74–81. doi: 10.1007/s00213-002-1068-4. [DOI] [PubMed] [Google Scholar]
- 17.Ford KA, Wolf ME, Hu XT. Plasticity of L-type Ca2+ channels after cocaine withdrawal. Synapse. 2009;63(8):690–7. doi: 10.1002/syn.20651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frankowska M, Wydra K, Faron-Górecka A, Zaniewska M, Kuśmider M, Dziedzicka-Wasylewska M, et al. Neuroadaptive changes in the rat brain GABA(B) receptors after withdrawal from cocaine self-administration. European Journal of Pharmacology. 2008;599(1-3):64. doi: 10.1016/j.ejphar.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Hammer RP, Jr, Pires WS, Markou A, Koob GF. Withdrawal following cocaine self-administration decreases regional cerebral metabolic rate in critical brain reward regions. Synapse. 1993;14(1):73–80. doi: 10.1002/syn.890140110. [DOI] [PubMed] [Google Scholar]
- 20.Hemby SE, Tang W, Muly EC, Kuhar MJ, Howell L, Mash DC. Cocaine-induced alterations in nucleus accumbens ionotropic glutamate receptor subunits in human and non-human primates. Journal of Neurochemistry. 2005;95(6):1785–93. doi: 10.1111/j.1471-4159.2005.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbsman T, Forster L, Molnar C, Dougherty R, Christie D, Koola J, et al. Motor threshold in transcranial magnetic stimulation: the impact of white matter fiber orientation and skull-to-cortex distance. Human Brain Mapping. 2009;30(7):2044–55. doi: 10.1002/hbm.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jimenez-Rivera CA, Waterhouse BD. Effects of systemically and locally applied cocaine on cerebrocortical neuron responsiveness to afferent synaptic inputs and glutamate. Brain Research. 1991;546:287–296. doi: 10.1016/0006-8993(91)91493-k. [DOI] [PubMed] [Google Scholar]
- 23.Karoum F, Egan MF, Wyatt RJ. Selective reduction in dopamine turnover in the rat frontal cortex and hypothalamus during withdrawal from repeated cocaine exposure. European Journal of Pharmacology. 1994;254(1-2):127–32. doi: 10.1016/0014-2999(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 24.Kozel FA, Nahas Z, deBrux C, Molloy M, Lorberbaum J, Bohning D, et al. “Coil-Cortex Distance relates to Age, Motor Threshold, and Antidepressant Response to Repetitive Transcranial Magnetic Stimulation”. The Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:376–384. doi: 10.1176/jnp.12.3.376. [DOI] [PubMed] [Google Scholar]
- 25.Kufahl PR, Li Z, Risinger RC, Rainey CJ, Wu G, Bloom AS, et al. Neural responses to acute cocaine administration in the human brain detected by fMRI. Neuroimage. 2005;28(4):904–14. doi: 10.1016/j.neuroimage.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 26.Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, et al. Corticocortical inhibition in human motor cortex. The Journal of Physiology. 1993;471:501–19. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kushner SA, Unterwald EM. Chronic cocaine administration decreases the functional coupling of GABA(B) receptors in the rat ventral tegmental area as measured by baclofen-stimulated 35S-GTPgammaS binding. Life Sciences. 2001;69(9):1093–102. doi: 10.1016/s0024-3205(01)01203-6. [DOI] [PubMed] [Google Scholar]
- 28.Lason W. Neurochemical and pharmacological aspects of cocaine-induced seizures. Polish Journal of Pharmacology. 2001;53:57–60. [PubMed] [Google Scholar]
- 29.Liu SK, Fitzgerald PB, Daigle M, Chen R, Daskalakis ZJ. The relationship between cortical inhibition, antipsychotic treatment, and the symptoms of schizophrenia. Biological Psychiatry. 2009;65(6):503–9. doi: 10.1016/j.biopsych.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. Imaging Dopamine Transmission in Cocaine Dependence: Link Between Neurochemistry and Response to Treatment. American Journal of Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathis J, de Quervain D, Hess CW. Dependence of the transcranially induced silent period on the ‘instruction set’ and the individual reaction time. Electroencephalography and Clinical Neurophysiology. 1998;109(5):426–35. doi: 10.1016/s0924-980x(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 32.McDonnell MN, Orekhov Y, Ziemann U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Experimental Brain Research. 2006;173:86–93. doi: 10.1007/s00221-006-0365-2. [DOI] [PubMed] [Google Scholar]
- 33.Möller B, Light GA, Fitzgerald PB, Snyder JS, Chen R, Daskalakis ZJ. Relationship between P50 suppression and the cortical silent period. Neuroreport. 2007;18(14):1503–6. doi: 10.1097/WNR.0b013e3282ef6a29. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. Journal of Physiology (London) 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nahas Z, Teneback CC, Kozel A, Speer AM, DeBrux C, Molloy M, et al. Brain effects of TMS delivered over prefrontal cortex in depressed adults: role of stimulation frequency and coil-cortex distance. The Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13(4):459–70. doi: 10.1176/jnp.13.4.459. [DOI] [PubMed] [Google Scholar]
- 36.Nasif FJ, Sidiropoulou K, Hu XT, White FJ. Repeated cocaine administration increases membrane excitability of pyramidal neurons in the rat medial prefrontal cortex. Journal of Pharmacology and Experimental Therapeutics. 2005a;312(3):1305–13. doi: 10.1124/jpet.104.075184. [DOI] [PubMed] [Google Scholar]
- 37.Nasif FJ, Hu XT, White FJ. Repeated cocaine administration increases voltage-sensitive calcium currents in response to membrane depolarization in medial prefrontal cortex pyramidal neurons. Journal of Neuroscience. 2005b;25(14):3674–9. doi: 10.1523/JNEUROSCI.0010-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nilsson J, Panizza M, Arieti P. Computer-aided determination of the silent period. Journal of Clinical Neurophysiology. 1997;14(2):136–43. doi: 10.1097/00004691-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Roberts DC. Preclinical evidence for GABAB agonists as a pharmacotherapy for cocaine addiction. Physiology and Behavior. 2005;86:18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Rossini PM, Berardelli A, Deuschl G, Hallett M, Maertens de, Noordhout A, Paulus W, et al. Applications of magnetic cortical stimulation: The International Federation of Clinical Neurophysiology. Electroencephalography and Clinical Neurophysiology Supplement. 1999;52:171–85. [PubMed] [Google Scholar]
- 41.Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. Journal of Physiology (London) 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Satel SL, Edell WS. Cocaine-induced paranoia and psychosis proneness. American Journal of Psychiatry. 1991;148:1708–1711. doi: 10.1176/ajp.148.12.1708. [DOI] [PubMed] [Google Scholar]
- 43.Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle and Nerve. 1998;21(9):1209–12. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 44.Sofuoglu M, Kosten TR. Novel approaches to the treatment of cocaine addiction. CNS Drugs. 2005;19:13–25. doi: 10.2165/00023210-200519010-00002. [DOI] [PubMed] [Google Scholar]
- 45.Sundaresan K, Ziemann U, Stanley J, Boutros NN. Cortical inhibition and excitation in abstinent cocaine-dependent patients; A transcranial magnetic stimulation study. NeuroReport. 2007;18(3):289–292. doi: 10.1097/WNR.0b013e3280143cf0. [DOI] [PubMed] [Google Scholar]
- 46.Thompson AM, Swant J, Gosnell BA, Wagner JJ. Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience. 2004;127(1):177–85. doi: 10.1016/j.neuroscience.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Uys JD, LaLumiere RT. Glutamate: the new frontier in pharmacotherapy for cocaine addiction. CNS and Neurological Disorders - Drug Targets. 2008;7(5):482–91. doi: 10.2174/187152708786927868. [DOI] [PubMed] [Google Scholar]
- 48.Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. Journal of Psychopharmacology. 1999;13(4):337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- 49.Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, et al. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. Journal of Neuroscience. 2003;23(36):11461–8. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 51.White PM, Kanazawa A, Yee CM. Gender and suppression of mid-latency ERP components during stress. Psychophysiology. 2005;42:720–725. doi: 10.1111/j.1469-8986.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 52.Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Annals of Neurology. 1996a;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- 53.Ziemann U, Bruns D, Paulus W. Enhancement of human motor cortex inhibition by the dopamine receptor agonist pergolide: evidence from transcranial magnetic stimulation. Neuroscience Letters. 1996b;208(3):187–90. doi: 10.1016/0304-3940(96)12575-1. [DOI] [PubMed] [Google Scholar]
- 54.Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. The Journal of Physiology. 1996c;496:873–81. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziemann U, Hallett M. Basic neurophysiological studies with transcranial magnetic stimulation. In: George MS, Belmaker RH, editors. Transcranial magnetic stimulation in clinical psychiatry. American Psychiatric Publishing, Inc.; Washington, D.C.: 2007. pp. 59–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.