Abstract
Background
Manganese (Mn) is a naturally occurring element and an essential nutrient for humans and animals. However, exposure to high levels of Mn may cause neurotoxic effects. The pathological mechanisms associated with Mn neurotoxicity are poorly understood, but several reports have established it is mediated, at least in part, by oxidative stress.
Objectives
The present study was undertaken to test the hypothesis that a decrease in acetylcholinesterase (AChE) activity mediates Mn-induced neurotoxicity.
Methods
Groups of 6 rats received 4 or 8 intraperitoneal (i.p.) injections of 25 mg MnCl2/kg/day, every 48 hours. Twenty-four hours after the last injection, brain AChE activity and the levels of F2-isoprostanes (F2-IsoPs) and F4-neuroprostanes (F4-NPs) (biomarkers of oxidative stress), as well as prostaglandin E2 (PGE2) (biomarker of neuroinflammation) were analyzed.
Results
The results showed that after either 4 or 8 Mn doses, brain AChE activity was significantly decreased (p<0.05), to 60 ± 16 % and 55 ± 13 % of control levels, respectively. Both treated groups exhibited clear signs of neurobehavioral toxicity, characterized by a significant (p<0.001) decrease in ambulation and rearings in open-field. Furthermore, Mn treatment caused a significant increase (p<0.05) in brain F2-IsoPs and PGE2 levels, but only after 8 doses. In rats treated with 4 Mn doses, a significant increase (p<0.05) in brain F4-NPs levels was found. To evaluate cellular responses to oxidative stress, we assessed brain nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) and Mn-superoxide dismutase (Mn-SOD, SOD2) protein expression levels. A significant increase in Mn-SOD protein expression (p<0.05) and a trend towards increased Nrf2 protein expression was noted in rat brains after 4 Mn doses vs. the control group, but the expression of these proteins was decreased after 8 Mn doses. Taken together, these results suggest that the inhibitory effect of Mn on AChE activity promotes increased neuronal oxidative stress and neuroinflammatory biomarkers.
Keywords: Manganese neurotoxicity, acetylcholinesterase, F2-isoprostanes, prostaglandin E2, Mn-SOD, rat brain
1. INTRODUCTION
Manganese (Mn) is a transition metal that is essential for normal cell growth and development (Au et al., 2008). Mn acts as a cofactor for a variety of metalloenzymes, such as the mitochondrial protein superoxide-dismutase (Mn-SOD), a critical enzyme in attenuating oxidative stress (Stallings et al., 1991), arginase, which is responsible for urea production in the liver, pyruvate carboxylase, an essential enzyme in gluconeogenesis (Crossgrove and Zheng, 2004), as well as glutamine synthetase, an astrocyte-specific enzyme that converts glutamate into glutamine (Aschner and Gannon, 1994; Burton and Guilarte, 2009). Additional Mn-dependent enzymes include oxidoreductases, transferases, hydrolases, lyases, isomerases and ligases (Aschner and Aschner, 2005). Clinical manifestations of Mn deficiency include seizures, retarded growth, skeletal abnormalities and impaired reproductive function (Roth and Garrick, 2003) and Mn deficiency during development is also associated with convulsive disorders (Aschner and Gannon, 1994).
At the other end of the spectrum, excessive exposure to Mn may cause neurotoxicity (McMillan, 1999; Aschner et al., 2005) characterized by a frequently irreversible parkinsonian-like syndrome, which includes fixed gaze, bradykinesia, postural difficulties, rigidity, tremor, dystonia and decreased mental status (Levy and Nassetta, 2003). Neurotoxic effects resulting from excessive Mn exposure were first described by Couper in 1837 in Scottish laborers grinding Mn black oxide in a chemical industry (Couper, 1837; Meyer-Baron et al., 2009). Presently, occupational exposure occurs in miners, welders and workers in the ferromanganese-alloy industry and the manufacturing of dry cell batteries (Takeda, 2003; Jankovic, 2005; Burton and Guilarte, 2009). Mn neurotoxicity may also result from parenteral nutrition therapy, especially in patients with liver disease or immature hepatic function, such as the premature neonate (Erikson et al., 2007). In contrast to numerous reports describing Mn toxicity following inhalation exposure in humans, there are relatively few reports on manganism arising from water or dietary sources (Dorman et al., 2001). Kawamura and coworkers (1941) documented outbreaks of manganism in Japan and Greece, respectively, due to consumption of well-water contaminated with extremely high levels of Mn (1.8 to 14 mg Mn/l). Wasserman and coworkers (2006 (2011) also reported adverse impact of Mn exposure associated with water consumption on child developmental outcomes in Bangladesh. In another cross-sectional study, neurological symptoms and elevated hair Mn levels were reported in elderly individuals residing in an area in Greece for more than 10 years who consumed drinking water with Mn levels of 1.8 – 2.3 mg/l (Kondakis et al., 1989). Toxicological oral studies have been performed in rodents, also demonstrating biochemical changes in the brain following administration of 1 mg MnCl2.4H2O/ml in drinking water (approximately 40 mg Mn/kg-day) for over two years (Lai et al., 1981, 1982).
Manganism is linked to increased brain Mn levels primarily in brain regions known also to be iron (Fe)-rich, namely, caudate, putamen, globus pallidus, substantia nigra and subthalamic nuclei. These regions are collectively referred to as the basal ganglia (Erikson and Aschner, 2003). Notably, cholinergic neurons projecting to the striatum synapse with nigral-dopaminergic (DAergic) and gamma-aminobutyric acid (GABA) GABAergic neurons in the striatum (Moore and Wuerthele, 1979).
Studies in humans and animals indicate that excessive Mn exposure is associated with behavioral changes and altered cognitive function (Rohling and Demakis, 2007; Bouchard, et al., 2008). Mn-induced cortical cholinergic dysfunction is compatible with these cognitive de cits, as well as with the dementia observed later on in the clinical course of manganism (Finkelstein et al., 2007). Significant inhibition of acetylcholinesterase (AChE) activity was observed following lengthy periods of exposure to Mn in adult (Sitaramayya et al., 1974; Martinez and Bonilla, 1981) and neonatal (Deskin et al., 1981; Lai et al., 1984) rat brain. The inhibition of AChE prevents the hydrolysis of acetylcholine (ACh), leading to accumulation of ACh in the synaptic cleft and overstimulation of muscarinic and nicotinic ACh receptors. Decreased activity of AChE, the enzyme responsible for ACh hydrolysis, was shown to be associated with increased oxidative and nitrosative stress (Milatovic et al., 2006), corroborating other reports on the ability of Mn to increase oxidative stress (Donaldson et al., 1981; Donaldson, 1984; Donaldson and Labella, 1984; Ali et al., 1995; Hamai and Bondy, 2004).
In several experimental pathological conditions, the increased AChE activity is also associated with oxidative stress. Thomé et al (2011) reported that AChE activity and lipid peroxidation were increased in rats’ brain in an experimental model of exposure to aged and diluted sidestream smoke (ADSS). Rosemberg et al. (2010) also reported that ethanol exposure increased AChE activity and lipoperoxidation in zebrafish brain. A study by Schallreuter and Elwary (2007) showed that epidermal AChE is a target to H2O2-mediated oxidation of methionine and tryptophan residues. Enzyme kinetics revealed concentration dependent activation/deactivation of AChE by H2O2.
Excessive ROS production induces the oxidation of membrane polyunsaturated fatty acids, yielding a multitude of lipid peroxidation products (Halliwell and Gutteridge, 1990). Two families of such products are the F2-isoprostanes (F2-IsoPs) and F4-neuroprostanes (F4-NPs). F2-IsoPs are a group of arachidonic acid (AA)-derived prostanoid isomers generated from the free radical-initiated peroxidation of AA independent of cyclooxygenase (COX) (Musiek and Morrow, 2005; Milatovic and Aschner, 2009; Roberts and Milne, 2009). F2-IsoPs are isomeric to COX-derived prostaglandin (PG) F2α (Greco and Minghetti, 2004). Analogously, auto-oxidation of the omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) generates an array of IsoP-like compounds termed neuroprostanes (NPs) (Musiek et al, 2005). Unlike the fatty acid AA, DHA is highly concentrated in neuronal membranes, reflecting F4-NPs oxidative damage to neuronal membranes (Roberts et al., 1998; Reich et al., 2000, 2001). Therefore, the measurement of F4-NPs provides a more selective index of neuronal oxidative damage, while the measurement of F2-IsoPs provides an index of global brain oxidative damage, integrating stress levels both in glial and neuronal cells (Arneson and Roberts, 2007). ROS generation is also associated with inflammatory responses and release of inflammatory mediators including prostaglandins, which are derivatives of AA oxidation catalysed by the oxidation of COX-2 (Milatovic et al, 2009). Herein, we assessed the effect of Mn on neuroinflammation by measuring prostaglandin E2 (PGE2) levels.
NF-E2-related factor 2 (Nrf2) is a leucine zipper transcription factor belonging to the cap ‘n’ collar family; it is involved in the induction of genes encoding antioxidant proteins, including those in the GSH family. Given the ability of Mn to induce oxidative stress, we determined its protein expression levels in response to Mn treatment. We also analyzed the expression of Mn-SOD, a mitochondria-specific anti-oxidant enzyme, given that Mn preferentially accumulates in this organelle, where it disrupts oxidative phosphorylation and increases ROS generation (Gunter et al., 2006).
Given the above, the present study was conducted to investigate the hypothesis that the in vivo modulatory effect of Mn on AChE activity is associated with oxidative damage, neuroinflammation and the up regulation of oxidative stress defense enzymes (Mn-SOD) and transcription factors (Nrf2).
2. EXPERIMENTAL PROCEDURE
2.1. Materials
Manganese chloride tetrahydrate (MnCl2.4H2O; 99.99%), Mn standard for Graphite Furnace Atomic Absorption Spectrometry (GFAAS), acetyltiocholine (ATCh) iodide, hydrogen peroxide (H2O2, 30%) and 5-5′-dithiobis(2-nitrobenzoic acid) (DTNB) were purchased from Sigma Aldrich (St. Louis, MO). 15-F2α-IsoP-d4 (internal standard for F2-IsoPs that contains four deuterium atoms), PGE2 internal standard and prostaglandin F2, E2 and D2 methyl esters were purchased from Cayman Chemicals (Ann Arbor, MI). Nitric acid (HNO3; 65%) and magnesium matrix modifier for GFAAS were purchased from Merck (Darmstadt, Germany). Albumin standard was purchased from Pierce (Rockford, USA). Rabbit polyclonal antibody Nrf2 (sc-722) was purchased from Santa Cruz Biotechnology (California, USA). Rabbit polyclonal antibody Mn-SOD was purchased from Stressgen Biotechnologies Corporation (San Diego, CA). Monoclonal anti-β-actin antibody produced in mouse was purchased from Sigma Aldrich (St. Louis, MO). Goat primary antibody to rabbit IgG horseradish peroxidase (HRP) was purchased from Abcam (San Francisco, CA, USA) and goat primary anti-mouse IgG HRP labeled secondary antibody was purchased from KPL (MD, USA). The chemiluminescent kit was purchased by Pierce Biotechnology. The HyBlot CL autoradiography film was purchased from Denville Scientific Inc., Metuchen, NJ. Bio-Rad western-blot assay reagents were used (Bio-Rad Laboratories, Hercules, CA).
2.2. Animals and Treatment
Six-week-old male Wistar rats (180±15 g), Specific Pathogen Free, were obtained from Charles River Laboratories (Barcelona, Spain) and maintained under standard environmental conditions. The animals were housed in a room, with 12-12h light/dark cycle, 50–70% humidity at 24°C. All experiments were performed in accordance with the Guiding Principles for the University’s Care and Use of Animals, University of Lisbon, Portugal. The rats were randomly divided into 3 groups as follows: (A) control (saline solution-treated) with 4 and 8 doses (n=6 each), (B) Mn 4 doses (25 mg/kg body weight, as MnCl2.4H2O, n=6), and (C) Mn 8 doses (25 mg/kg body weight, as MnCl2.4H2O, n=6).
All rats received intraperitoneal (i.p.) injections on alternate days. The schedule of Mn injections over time permits the accumulation of detectable brain Mn levels, while lessening the effects of acute exposure in other organs. This methodology is based on the long half-life of Mn in the brain (51–74 days) relative to its short half-life in visceral organs such as the heart and the liver (Silva and Bock, 2008).
Mn speciation is another important factor determinant for toxicity. Mn2+ is the predominant form in biological systems. The valence state of Mn may also influence its disposition in the body. Roels et al. (1997) showed that more soluble forms of Mn (e.g., MnCl2 vs MnO2) given to three-month-old rats given via intratracheal instillation are more readily delivered to the brain.
The animals were euthanized with pentobarbital (20 mg/kg), 24 hours (h) after the last dose followed by cervical dislocation. The cerebral hemispheres were immediately removed and stored at −80°C until biochemical analyses and chemical determinations.
2.3. Behavioral Assays
Locomotor activity was evaluated in an open-field apparatus. Animals were individually placed at the center of the open-field apparatus (60 cm × 90 cm × 30 cm, divided into six equal squares). Spontaneous ambulation (number of segments crossed with the four paws) and rearing (exploratory activity expressed by the number of rearings on the hind limbs) were recorded for 5 min (Markel et al., 1989; Marreilha dos Santos et al., 2011).
2.4. Determination of brain AChE activity
AChE (EC 3.1.1.7) activity was determined by the method of Ellman et al (1961), which is based on the formation of the yellow 5-thio-2-nitrobenzoate anion produced in the reaction between 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) and thiocholine, after the AChE-mediated hydrolysis of ATCh. Samples were weighed (100 mg of the brain homogenate) and 1.8 ml of 0.1 M buffer sodium phosphate dibasic anhydrous/sodium phosphate monobasic pH 7.4 and 0.2 ml of 5% Triton were added to each tube. Briefly, the reaction was initiated after the addition of 0.05 ml of DTNB and 0.05 ml of ATCh iodide, which was used as substrate. The final concentrations of DTNB and substrate were 0.33 mM and 1.56 mM, respectively. The reaction was followed spectrophotometrically by the increase of absorbance at 412 nm. The activity was expressed as nanomole of ATCh hydrolyzed per minute per milligram protein. An aliquot of the homogenate was used for protein determination. Total protein was measured with the method of Bradford (1976). Bovine serum albumin was used as the standard. While the DTNB method has some limitations and is not fully specific, it has been widely used in assays analogous to those described herein (Cederblad et al., 1986).
2.5. Quantification of PGE2 in brain
PGE2 was measured by a stable isotope dilution gas chromatographic/negative ion chemical ionization-mass spectrometric assay. [4H2]-PGE2 (2 ng) and [4H2]-PGD2 (4.25 ng) were added to brain homogenates after extraction with an indomethacin 10−6M methanolic solution. The sample was then acidified to pH 3 and extracted on a C18 Sep-Pak cartridge. PGE2 was eluted with a mixture of ethyl acetate:heptane and evaporated under a stream of N2. PGE2 in methoxylamine solution was extracted with ethyl acetate and dried under a N2 stream. The pentafluorobenzyl esters were formed by incubation with pentafluorobenzyl bromide (10% in acetonitrile) and diisoproplyethanolamine. The pentafluorobenzyl esters were purified by thin layer chromatography (TLC), converted to O-methyloxime pentafluorobenzyl ester trimethylsilyl derivatives after incubation with N,O-bis(trimethylsilyl)-trifluoroacetamide and dimethylformamide and finally, PGE2 was dissolved in undecane that dried over a bed of calcium hydride. Gas chromatographic/negative ion chemical ionization-mass spectrometric analysis was performed as described previously with the M-181 ions for PGE2 (m/z 526) and the [4H2]-PGE2 as internal standard (m/z 528) (Milatovic et al, 2009).
2.6. Quantification of F2-IsoPs and F4-NPs in brain
Total F2-IsoPs and F4-NPs were determined with a stable isotope dilution method with detection by gas chromatography (GC)/mass spectrometry (MS) and selective ion monitoring. Briefly, brain samples were homogenized in Folch solution and the phospholipids were first extracted from the tissue sample and submitted to alkaline hydrolysis to release the F2-IsoPs and F4-NPs esterified in membranes. The brain homogenates were acidified to pH 3, followed by the addition of 0.1 ng of 15- F2α-IsoP-d4 internal standard. F2-IsoPs and F4-NPs were subsequently purified by C18 and silica Sep-Pak extraction and by thin layer chromatography (TLC). For the quantification of F4-NPs instead of scraping 1 cm below and 1 cm above where PGF2α methyl ester migrates on TLC for analysis of F2-IsoPs, the area scraped was extended to 3 cm above where PGF2 methyl ester migrates. F2-IsoPs and F4-NPs were then converted to a trimethylsilyl ether derivative, and quantified by negative ion chemical ionization mass spectrometry. For quantification purposes of F2-IsoPs we compared the integrated peak area of derivatized F2-IsoPs (m/z 569) with the area of the deuterated internal standard peak (m/z 573). Quantification of F4-NPs levels was achieved through single ion monitorization (SIM) and comparing the area of the appropriate peaks in the m/z 593 SIM chromatogram of the F4-NPs to that of the single peak of the internal standard in the m/z 593 SIM chromatogram (Roberts et al., 1998; Musiek and Morrow, 2005; Arneson and Roberts, 2007; Milatovic and Aschner, 2009).
2.7. Western Blot Analysis
Rat brain homogenates were prepared in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors [4-(2-aminoethyl)benzenesulfonyl fluoride, pepstatinA, E-64, bestatin, leupeptin and aprotinin]. All of the samples were heated for 5 min at 95°C and centrifuged at 13.000 × g for 0.5 min to remove insoluble materials. Equal amounts of protein were loaded in the SDS-PAGE gel and the protein concentrations were determined with the Bradford method (1976). After electrophoresis, the protein was transferred onto a nitrocellulose membrane blocked with 5% non-fat milk, and incubated overnight with a primary antibody. Nrf2 primary antibody (0.1 mg/ml) and Mn-SOD primary antibody (2.5 × 10−5 mg/ml) were applied overnight at room temperature, after which immunoreactivity was detected with HRP-conjugated secondary antibody. Protein–antibody complexes were detected with an enhanced chemiluminescent kit (Pierce Biotechnology, Rockford, IL) and exposed to HyBlot CL autoradiography film (Denville Scientific Inc., Metuchen, NJ). Molecular weights of Nrf2, Mn-SOD and β-actin proteins were approximately 68, 25, and 42 kDa, respectively. Individual blot densities were quantified by AlphaeaseFC 4.0. The individual blot densities of Mn-SOD and Nrf2 proteins were normalized to the loading control β-actin.
2.8. Brain Mn Analysis
Brain Mn concentrations were determined by GFAAS, using a PerkinElmer Analyst 700 atomic absorption spectrometer equipped with an HGA Graphite Furnace and a programmable sample dispenser (AS 800 Auto Sampler) and WinLab 32 for atomic absorption software. Prior to GFAAS analysis, brain samples were digested with an oxidizing acid mixture of 4:1 (v/v) 65% suprapure nitric acid:hydrogen peroxide, using a microwave-assisted acid digestion. Mg (NO3)26H2O (0.84 mol/l) was used as a chemical modifier.
2.9. Statistical Analysis
The data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test, except for the evaluation of the changes in body weight, where the statistical analyse of significance was carried by unpaired t-test. All analyses were carried out with GraphPad Prism 4.02 software for Windows (GraphPad Software, San Diego, CA, USA). Results were considered statistically significant at values of p<0.05. The AlphaEaseFC 4.0 software was used to calculate optical the density, and Prism 4.0 (GraphPad, San Diego, CA, USA) was used to calculate and plot the results.
3. RESULTS
3.1. Bodyweight
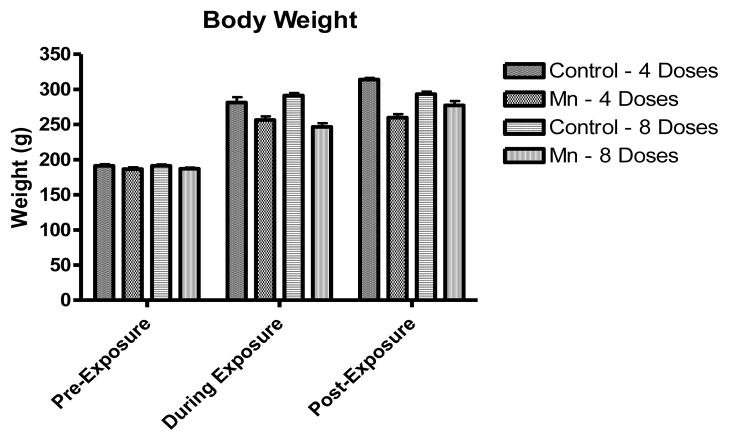
Bodyweight was assessed at different time-points during the experimental period. Fig.1 shows the rats’ bodyweights at three different time-points: pre-exposure (immediately before the first Mn dose), during exposure (the middle of the study) and post-exposure (at the end of the study). As shown in Fig.1, a decrease in bodyweight gain was inherent to the Mn-treated groups (either 4 or 8 doses of MnCl2 25 mg/kg every 48 h) compared to controls, but the effect was statistically indistinguishable from the controls (p<0.05).
Figure 1.
3.2. Locomotor activity assessment
The locomotor activity was assessed by the open-field test. Two parameters, ambulation (horizontal activity) and rearing (vertical activity) were analyzed at two different time points, before the beginning of the experiment and immediately prior to the animals’ sacrifice. Fig. 2 illustrates the effect of Mn treatment on locomotor activity. Mn treatment caused a significant decrease (p<0.001) in ambulation (Fig. 2A) and the number of rearings (Fig. 2B) in the open-field (at both Mn doses) compared with the control group. Neither of these parameters was statistically different among the 4 and 8 dose Mn treated groups.
Figure 2.
3.3. Brain Mn concentrations
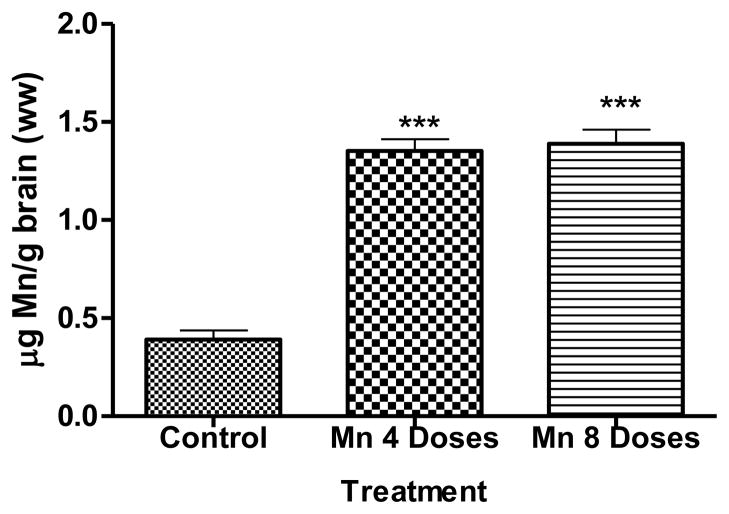
Brain Mn concentrations are shown in Fig. 3. Significantly higher (p<0.001) brain Mn concentrations were observed both in the 4 and 8 dose Mn treated groups compared with the control group. Mn accumulation in the brain did not increase in a dose-dependent manner and Mn concentrations in the 4 dose Mn treatment group were indistinguishable from those in the 8 dose Mn treatment group.
Figure 3.
3.4. Brain AChE activity
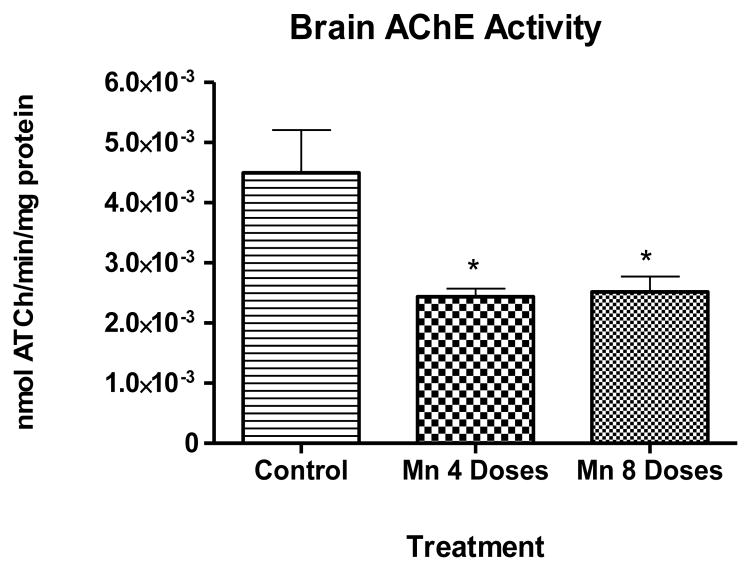
Four doses of Mn caused a significant decrease (p<0.05) in brain AChE activity 24 h after the last injection (Fig. 4). Brain AChE activity was statistically indistinguishable between the two Mn treatment groups (4 or 8 doses).
Figure 4.
3.5. F2-IsoPs and F4-NPs levels in brain
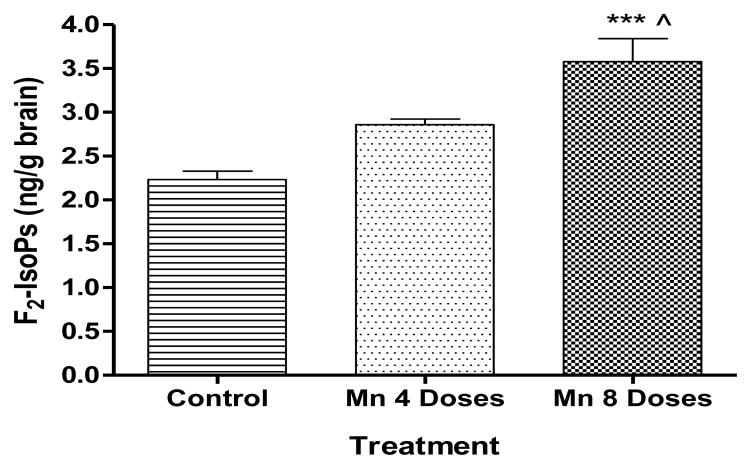
We tested the ability of Mn to induce oxidative stress in rats’ brains by measuring F2-IsoPs and F4-NeuroPs, lipid peroxidation biomarkers of oxidative injury. Fig. 5 shows a trend towards an increase in F2-IsoPs levels in the 4 dose Mn treated group, but the effect was statistically indistinguishable from the control group. In contrast, treatment with 8 Mn doses caused a significant increase (p<0.001) in F2-IsoPs levels compared with the control and the 4 doses Mn treated group (p < 0.05).
Figure 5.
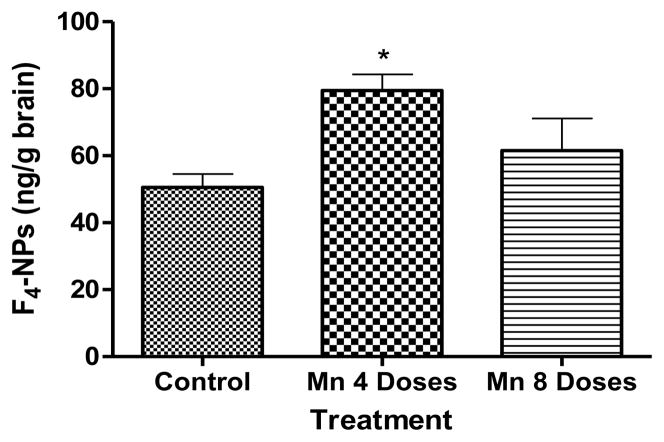
F4-NPs levels in the brain of rats treated with either 4 or 8 Mn doses caused an increase in F4-NPs levels vs. the control group. However, this effect was only statistically significant (p<0.05) for the 4 dose Mn treated group (Fig. 6).
Figure 6.
3.6. PGE2 levels in brain
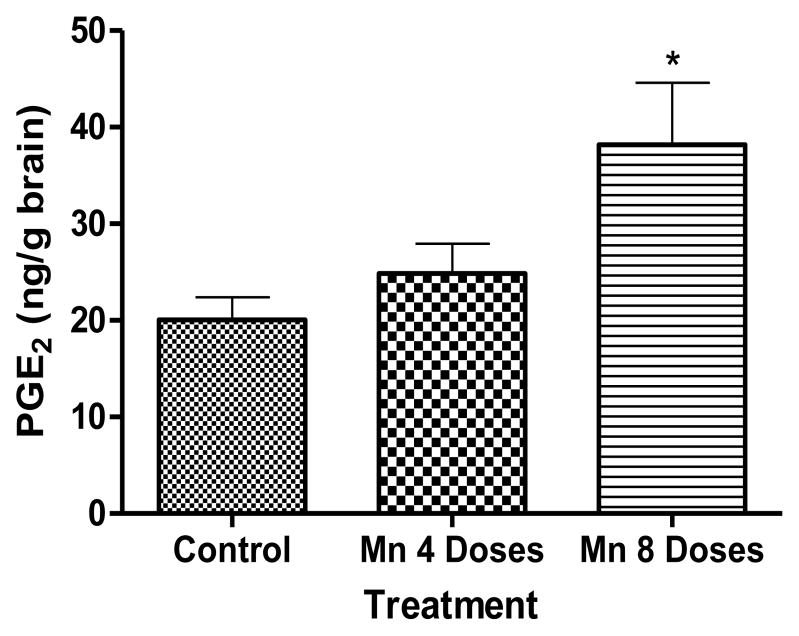
As shown in Fig. 7, Mn induced a dose- and time-dependent increase in PGE2 levels, but the effect was statistically significant (p<0.01) only in the 8 dose Mn treated group vs. the control group.
Figure 7.
3.7. Mn-SOD and Nrf2 expression in brain
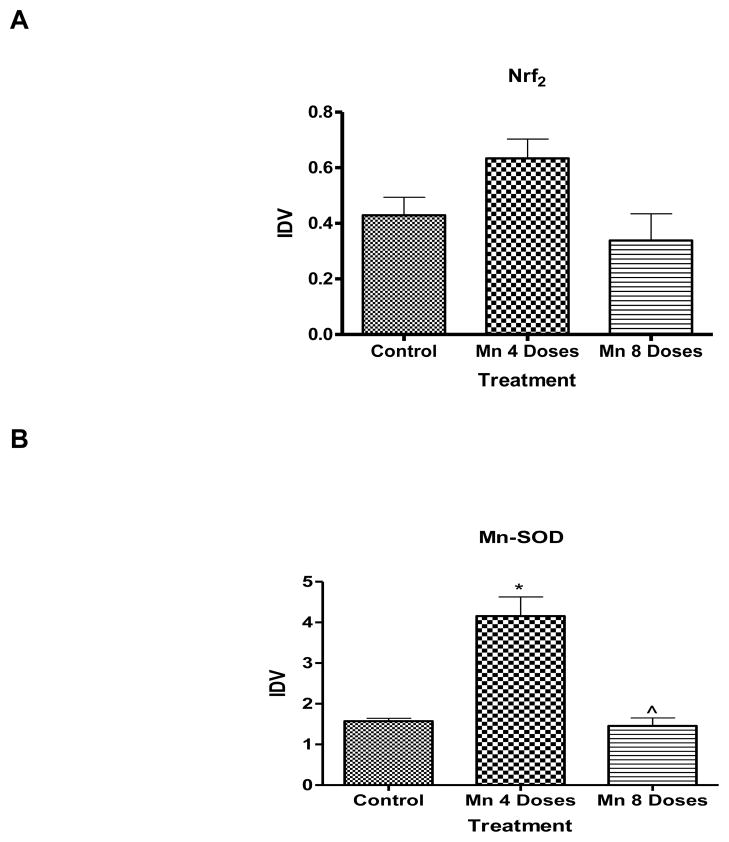
Mn significantly enhanced (p<0.05) brain Mn-SOD protein expression after 4 doses compared with the control group (Fig. 8B); after 8 doses the Mn-SOD levels were statistically indistinguishable from the control group. Either 4 or 8 dose Mn treatment did not result in statistically significant change in brain Nrf2 protein expression compared with the control group (Fig. 8A).
Figure 8.
4. DISCUSSION
Although Mn is an essential element which is present at low concentrations in food, water and air, exposure to excessive levels of this metal may lead to elevated brain Mn accumulation and adverse neurological effects (Crossgrove and Zheng, 2004; Aschner et al., 2005; Lu et al., 2005). The neurotoxic events of Mn are mediated, at least in part, via interference with central cholinergic systems. Finkelstein and co-workers (2007) hypothesized that the Parkinson’s-like movement disorder inherent to excessive exposure to Mn results from imbalance between the DAergic and cholinergic systems of the basal ganglia. The delicate functional neurochemical balance is disrupted upon damage to both dopamine (DA) and cholinergic systems, caused either directly or indirectly via GABAergic and glutamatergic interneurons. While most research on Mn toxicity has focused on perturbations in the DAergic and GABAergic system, evidence exists in support of a direct effect of the DAergic system on striatal cholinergic function. Striatal cholinergic function is inhibited by nigrostriatal activity, since DAergic receptor blockers enhance ACh release and lower striatal concentrations of DA (Stadler et al., 1973). Previous in vivo studies show that Mn alters the activity of enzymes involved in cholinergic transmission, such as AChE (Deskin et al., 1981; Lai et al., 1984; Liapi et al., 2008) and choline acetyltransferase (Lai et al., 1981, 1984; Martinez and Bonilla, 1981). AChE is the degradative enzyme of ACh and thereby responsible for the termination of cholinergic response in muscarinic and nicotinic brain ACh receptors (Milatovic et al., 2006). Although none of the studies in which the activity of AChE was measured in brain of rats exposed acutely or chronically to Mn developed hypercholinergic signs, it was demonstrated that AChE activity is modulated by Mn exposure (see below).
Exposure to excessive levels of Mn may cause a variety of neurotoxic effects that involve (among others) alterations in biomarkers of oxidative stress (Taylor et al., 2006; Erikson et al., 2007). Liapi et al (2008) investigated the effects of short-term exposure to Mn on the adult rat brain antioxidant status and the activities of AChE, noting that AChE activity was significantly increased in the Mn exposed group. Herein, we have used an in vivo model to test the hypothesis that Mn’s effect on AChE activity contributes to oxidative stress injury in the rat’s brain by measuring F4-NPs and F2-IsoPs levels. Our results show that after 4 or 8 dose Mn treatment, brain AChE activity significantly decreased (p<0.05) to 60 ± 16 % and 55 ± 13 % of control levels, respectively (Fig.4).
The results are contradictory to those of Liapi et al. (2008) and in agreement with Deskin et al. (1981), Lai et al. (1981, 1982), Martinez and Bonilla (1981) and Sitaramayya et al. (1974). Deskin et al (1981) reported that AChE activity was significantly reduced in the striatum of rats treated with MnCl2 (20 μg/g body weight/day), administered by oral gavage from birth to day 24 post-partum. Sitaramayya et al (1974) reported a significant decrease of AChE activity in the cerebral cortex, cerebellum and the remaining cerebral tissue in rats treated with MnCl2 inoculated intraperitoneally for 120 days. In contrast, Liapi et al. (2008) reported a significant increase of the activity of AChE in rats’ whole brains treated daily with MnCl2 50 mg/Kg for 7 days by the i.p. route. In the study by Lai et al. (1982), the distribution of AChE activity was assessed in various brain regions in the immediate postnatal period and found that the AChE activity was highest in the striatum, lowest in the cerebellum.
Whilst none of the studies in which the activity of AChE was measured in the brain of rats exposed acutely or chronically to Mn developed hypercholinergic signs, there are several reports of seizures in patients receiving parenteral nutrition with hypermanganesemia (Fitzgerald et al., 1999; Komaki et al., 1999; Hsieh et al., 2007). Although cases of convulsions associated with Mn intoxication are a rare presentation of Mn intoxication, there is no satisfactory explanation for these symptoms. Seizures are clinical signs associated with hyperstimulation by ACh (Harris, 2006) and both seizures and convulsions are considered typical sequalae of systemic application of sublethal doses of AChE inhibitors (Milatovic et al., 2006).
Exposure to excessive Mn levels is associated with motor disturbances (Calne et al., 1994; Pal et al., 1999). Behavioral analysis on spontaneous activity of both ambulation and rearing showed a significant decrease (p<0.001) following Mn administration vs. the control group (Figures 2A and 2B, respectively). This is consistent with previous studies where rats exposed to MnCl2 showed a decrease in spontaneous activity in open-field (Bonilla, 1984; Nachtman et al., 1986; Salehi et al., 2003; Torrente et al., 2005; Tapin et al., 2006; Vezér et al., 2007, Marreilha dos Santos et al., 2011). Our results may be explained by the accumulation of Mn in the striatum, which is a region involved in motor coordination that is under neuronal regulation of DA, ACh, GABA and glutamate (Moore and Wuerthele, 1979; Carlsson and Carlsson, 1990). Chronic exposure to Mn can lead to a movement disorder due to preferential Mn accumulation in the globus pallidus and other basal ganglia nuclei (Fitsanakis et al., 2008). Consistent with previous studies (Gianutsos et al., 1985), the Mn exposed groups showed a significant increase (p<0.05) in brain Mn concentrations (Fig. 3) compared with the control group. Studies both in primates and rodents suggest that homeostatic control of brain Mn concentrations is tight, since even in conditions of high exposures, levels of brain Mn increase only several fold (3–4) (Erikson et al., 2004).
Bodyweight was measured several times along the experiment axis, establishing a trend for diminished weight-gain in the Mn-treated group as compared to the control group (Fig. 1). Similar results have been described in other studies (Torrente et al., 2005; Vezér et al., 2007). This suggests that it is unlikely that malnutrition plays a significant role in the genesis of the neurochemical changes observed in this rat model.
F2-IsoPs are a group of AA-derived prostanoid isomers generated from the free radical-initiated peroxidation of AA independent of COX (Musiek and Morrow, 2005; Milatovic and Aschner, 2009; Roberts and Milne, 2009). Analogously, auto-oxidation of the DHA generates F4-NPs (Musiek et al., 2005). Analysis of cerebral biomarkers of oxidative damage showed Mn induced dose-dependent increase in brain F2-IsoPs levels (Fig.5). These results corroborate a recent study in mice (Milatovic et al., 2009) that showed a dose- and time-dependent increase in F2-IsoPs levels in the brain after MnCl2 exposure. Nevertheless, a dose-dependent increase in Mn-induced F4-NPs generation was absent. Fig. 6 shows that only the 4 dose Mn treated rats caused a significant increase of F4-NPs concentration, 24 h following the last injection. Our results show that F4-NPs are earlier biomarkers of Mn-induced oxidative injury than F2-IsoPs. This may be related to the fact that DHA is more susceptible to peroxidation than AA, due to its higher degree of unsaturation (six double bonds versus four, respectively) (Greco and Minghetti, 2004). In the brain, DHA is preferentially distributed in gray matter, where it accounts for about one third of the total fatty acids of neuron phospholipids (Greco and Minghetti, 2004). AA is relatively evenly distributed in brain with similar concentrations in gray and white matter, as well as within glia and neurons. Thus, F2-IsoPs quantification is a general reflection of oxidative damage in the whole brain, whilst F4-NeuroPs in particular to gray matter (Reich et al., 2000, 2001). Accordingly, the quantification of NPs may provide a more selective and sensitive measure of oxidant injury than F2-IsoPs. Nevertheless, since F4-NPs levels didn’t increase in a dose-dependent manner, likely due to its degradation and/or elimination from the brain, consideration should be given to the suitability of this biomarker in evaluating neuronal damage when used as a single biomarker of oxidative stress.
As an additional indicator of Mn neurotoxicity, we have evaluated the neuroinflammatory response by measuring PGE2 levels. We observed a significant increase in PGE2 levels in rat brain only after 8 doses with MnCl2 (Fig.7). These data corroborates a previous in vivo study (Milatovic et al., 2009) showing that MnCl2 exposure caused a time- and dose-dependent increase in PGE2 levels in mice brains. ROS generation is also associated with inflammatory responses and release of inflammatory mediators, including prostaglandins (Wang et al., 2004; Milatovic et al., 2009). These findings suggest that biomakers of neuroinflammation may not be as sensitive as the biomarkers of oxidative stress in evaluating brain injury mediated by Mn.
Free radicals generated by oxidative stress cause damage that can contribute to numerous chronic diseases. Mammalian cells respond to this damage by increased transcription of cytoprotective phase II genes, which are regulated by Nrf2 (Kang et al., 2010). There are multiple genes regulated by Nrf2: phase II detoxification enzymes, as well as antioxidant proteins including glutathione-S-transferases, NAD(P)H:quinone oxidoreductase-1, γ-glutamylcysteine synthase, ferritin, and heme oxygenase-1 (Chen and Kunsch, 2004). Under stressful conditions, Nrf2 translocates into the nucleus and binds to the antioxidant response element (ARE), activating defensive gene expression (Kaspar and Jaiswal, 2011). The western blot analysis showed a significant increase of Mn-SOD protein expression and increased tendency towards increased Nrf2 expression after 4 Mn doses, reflecting an adaptive response to increased oxidative stress (Fig. 8A and B). Notably, we observed a decrease in cellular defense mechanisms associated with oxidative stress upon 8 Mn doses (demonstrating protein expression levels analogous to controls). Morello et al. (2007) also observed a reduction in the expression of Mn-SOD after chronic Mn treatment. Interestingly, a reduction in mitochondrial Mn-SOD has been reported to exacerbate glutamate toxicity in cultured mouse cortical neurons (Li et al., 1998). Changes in Mn-containing proteins have been observed in many neurodegenerative diseases, including Alzheimer’s disease, amyotrophic lateral sclerosis and Parkinsonian-like syndrome (Yin et al., 2008). Mn-SOD is critical in preventing or limiting apoptosis and necrosis resulting from cellular damage caused by ROS (Frankel et al., 2000; Raha and Robinson, 2000; Kitzawa et al., 2002). In addition, Mn-SOD, high intra-cellular catalase and glutathione levels offsets the apoptosis triggered by ROS (Simon et al., 2000).
The decrease in both proteins’ expression, approaching control levels after 8 doses Mn treatment suggests this effect may predispose the cells to Mn-induced oxidative stress, which can compromise cell survival. Fujimura et al. (1999) found that Mn-SOD blocks cytosolic release of mitochondrial cytochrome c after ischemia, a critical step in the apoptotic process. As the previous study shows that Mn-SOD expression is a critical protein in the prevention of apoptosis process, at high levels of exposure to Mn, the cell is prone to apoptosis.
Consistent with the analogous Mn brain levels in animals exposed to 4 or 8 Mn injections (probably owing to saturation of transport mechanisms), AChE activity was also indistinguishable between the two groups. The F2-IsoPs and PGE2 are late biomarkers of the neurotoxic effect of Mn, since the anti-oxidant defenses (Mn-SOD and Nrf2) are challenged earlier in the time course to prevent the deleterious effects to the brain. Thus, it is not surprising that we found a poor correlation between F2-IsoPs and AChE activity levels (r2 = 0.642 only for the 8 doses). The effect of Mn on AChE activity contributes to the oxidative stress, consistent with the increase in SOD-Mn levels.
The present study shows that AChE inhibition in rats’ brain, may lead to oxidative events and eventually disrupt the balance between ACh and DA in the cholinergic-dopaminergic system (Finkenstein et al., 2007). ACh is thought to act directly on DAergic terminals, possibly via collaterals, because cholinergically-induced release of DA occurs in the presence of tetrodotoxin, a neurotoxin that prevents the generation of action potentials (Moore and Wuerthele, 1979). ACh releases DA from isolated perfused striata (Besson et al., 1969) from striatal slices and from striata in vivo (Giorguieff et al., 1976), while intraventricular injections of atropine increase striatal concentrations of homovanillic acid (Moore and Wuerthele, 1979). Since Mn’s effects are region-specific, future studies should be addressed on DA and ACh balance in various brain areas in order to clarify the neurochemical/pathophysiological mechanisms associated with its neurotoxicity.
In summary, the present study determined whether Mn effects on AChE activity play a role in oxidative stress and neurotoxicity. Our results indicate that Mn inhibits AChE concomitant with significant changes in the levels of biomarkers of oxidative stress (F2-IsoPs and F4-NPs). We posit that AChE is a target of Mn in the central nervous system that may trigger or contribute to the development of oxidative stress. These results suggest that AChE activity may serve as an early biomarker of Mn neurotoxicity and risk assessment inherent populations exposed to this metal. AChE is not a marker of cholinergic function, since it is not a specific enzyme of the central cholinergic neurons. AChE is localized also within the nigrostriatal DAergic neurons (Butcher, 1977). Therefore, the utility of AChE activity as an early biomarker of Mn neurotoxicity should be further addressed in biomonitoring Mn neurotoxicity.
We posit that.
Acetylcholinesterase (AChE) is a target of Mn in the central nervous system
Mn inhibits AChE, representing a novel mechanistic finding for its mode of action
AChE inhibition may trigger or contribute to the development of oxidative stress
Excess Mn can trigger the release of inflammatory mediators
AChE activity may serve as an early biomarker of Mn neurotoxicity
Acknowledgments
This study was funded by FCT (Foundation for Science and Technology of Portugal; SFRH/BD/64128/2009), by i-Med.UL, Faculty of Pharmacy, University of Lisbon, and a grant from the National Institute of Environmental Health Sciences ES R01 10563 (MA). I would like to thank Prof. Daiana Silva Ávila and Dr. M. Sidoryk-Wegrzynowicz for their support at Vanderbilt University.
Footnotes
Conflict of interest statement
None of the authors has any financial interest or conflict of interest related to the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ali S, Duhart H, Newport G, Lipe G, Slikker W. Manganese-induced reactive oxygen species: comparison between Mn+2 and Mn+3. Neurodegeneration. 1995;4(3):329–334. doi: 10.1016/1055-8330(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 2.Arneson K, Roberts J. Measurement of products of docosahexaenoic acid peroxidation, neuroprostanes, and neurofurans. Methods Enzymol. 2007;433:127–143. doi: 10.1016/S0076-6879(07)33007-3. [DOI] [PubMed] [Google Scholar]
- 3.Aschner M, Gannon M. Manganese (Mn) transport across the rat blood-brain barrier: saturable and transferring-dependent transport mechanisms. Brain Res Bull. 1994;33(3):345–349. doi: 10.1016/0361-9230(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 4.Aschner J, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aschner M, Erikson K, Dorman D. Manganese dosimetry: species differences and implications for neurotoxicity. Crit Rev Toxicol. 2005;35:1–32. doi: 10.1080/10408440590905920. [DOI] [PubMed] [Google Scholar]
- 6.Au C, Benedetto A, Aschner M. Manganese transport in eukaryotes: the role of DMT1. NeuroToxicology. 2008;29(4):569–576. doi: 10.1016/j.neuro.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besson M, Cheramy A, Feltz P, Glowinski J. Release of newly synthesized dopamine from dopamine-containing terminals in the striatum of the rat. Proc Nat Acad Sci USA. 1969;62:741– 748. doi: 10.1073/pnas.62.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonilla E. Chronic manganese intake induces changes in the motor activity of rats. Exp Neurol. 1984;84:696–700. doi: 10.1016/0014-4886(84)90216-4. [DOI] [PubMed] [Google Scholar]
- 9.Bouchard M, Mergler D, Baldwin M, Panisset M. Manganese cumulative exposure and symptoms: a follow-up study of alloy workers. NeuroToxicology. 2008;29(4):577–583. doi: 10.1016/j.neuro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–252. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 11.Burton N, Guilarte T. Manganese neurotoxicity: lessons learned from longitudinal studies in nonhuman primates. Environ Health Perspect. 2009;117(3):325–332. doi: 10.1289/ehp.0800035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butcher L. Nature and mechanisms of cholinergic monoaminergic interactions in the brain. Life Sci. 1977;21:1207–1226. doi: 10.1016/0024-3205(77)90001-7. [DOI] [PubMed] [Google Scholar]
- 13.Calne D, Chu N, Huang C, Lu C, Olanow W. Manganism and idiopathic parkinsonism: similarities and differences. Neurology. 1994;44:1583–1586. doi: 10.1212/wnl.44.9.1583. [DOI] [PubMed] [Google Scholar]
- 14.Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic system within the basal ganglia—implications for schizophrenia and Parkinson’s disease. Trends Neurosci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- 15.Cederblad G, Harper P, Lindgren K. Spectrophotometry of carnitine in biological fluids and tissue with a cobas bio centrifugal analyser. Clin Chem. 1986;32(2):342–46. [PubMed] [Google Scholar]
- 16.Chen X, Kunsch C. Induction of cytoprotective genes through Nrf2 antioxidant response element pathway: a new therapeutic approach for the treatment of inflammatory diseases. Curr Pharm Des. 2004;10:879–91. doi: 10.2174/1381612043452901. [DOI] [PubMed] [Google Scholar]
- 17.Couper J. On the effects of black oxide of manganese which inhaled into the lungs. Br Ann Med Pharm. 1837;1:41–2. [Google Scholar]
- 18.Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR in Biomed. 2004;17:544–553. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deskin R, Bursian S, Edens F. The effect of chronic manganese administration on some neurochemical and physiological variables in neonatal rats. Gen Pharmacol. 1981;12:279–280. doi: 10.1016/0306-3623(81)90058-6. [DOI] [PubMed] [Google Scholar]
- 20.Donaldson J, LaBella F, Gesser D. Enhanced autoxidation of dopamine as a possible basis of manganese neurotoxicity. NeuroToxicology. 1981;2(1):53–64. [PubMed] [Google Scholar]
- 21.Donaldson J. Involvement of manganese in physiological and biochemical processes: an overview. NeuroToxicology. 1984;5(1):1–3. [PubMed] [Google Scholar]
- 22.Donaldson J, Labella F. The effects of manganese on the cholinergic receptor in vivo and in vitro may be mediated through modulation of free radicals. NeuroToxicology. 1984;5(1):105–112. [PubMed] [Google Scholar]
- 23.Dorman D, Struve M, James R, McManus B, Marshall M, Wong B. Influence of dietary manganese on the pharmacokinetics of inhaled manganese sulfate in male CD rats. Toxicol Sci. 2001;60:242–251. doi: 10.1093/toxsci/60.2.242. [DOI] [PubMed] [Google Scholar]
- 24.Ellman G, Courtney K, Andres V, Feather-Stone R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 25.Erikson K, Aschner M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int. 2003;43:475–480. doi: 10.1016/s0197-0186(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 26.Erikson K, Dobson A, Dorman D, Aschner M. Manganese exposure and induced oxidative stress in rat brain. Sci Total Environ. 2004;334–335:409–416. doi: 10.1016/j.scitotenv.2004.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erikson K, Thompson K, Aschner J, Aschner M. Manganese neurotoxicity: a focus on the neonate. Pharmacol Ther. 2007;113(2):369–377. doi: 10.1016/j.pharmthera.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkelstein Y, Milatovic D, Aschner M. Modulation of cholinergic systems by manganese. NeuroToxicology. 2007;28:1003–1014. doi: 10.1016/j.neuro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Fitzgerald K, Mikalunas V, Rubin H, McCarthey R, Vanagunas A, Craig R. Hypermanganesemia in patients receiving total parenteral nutrition. J Parenter Enteral Nutr. 1999;23:333–336. doi: 10.1177/0148607199023006333. [DOI] [PubMed] [Google Scholar]
- 30.Fitsanakis V, Zhang N, Anderson J, Erikson K, Avison M, Gore J, Aschner M. Measuring brain manganese and iron accumulation in rats following 14 weeks of low-dose manganese treatment using atomic absorption spectroscopy and magnetic resonance imaging. Toxicol Sci. 2008;103(1):116–124. doi: 10.1093/toxsci/kfn019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frankel D, Mehindate K, Schipper H. Role of heme oxygenase-1 in the regulation of manganese superoxide dismutase gene expression in oxidatively-challenged astroglia. J Cell Physiol. 2000;185:80–86. doi: 10.1002/1097-4652(200010)185:1<80::AID-JCP7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 32.Fujimura M, Morita-Fujimura Y, Kawase M, Copin J, Calagui B, Epstein C, Chan P. Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome C and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci. 1999;19(9):3414–3422. doi: 10.1523/JNEUROSCI.19-09-03414.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gianutsos G, Seltzer M, Saymeh R, Wu M, Michel R. Brain manganese accumulation following systemic administration of different forms. Arch Toxicol. 1985;57(4):272–275. doi: 10.1007/BF00324791. [DOI] [PubMed] [Google Scholar]
- 34.Giorguieff M, Lefloc’h M, Westfall T, Glowinski J, Besson M. Nicotinic effect of acetylcholine on the release of newly synthesized [aH]dopamine in rat striatal slices and cat caudate nucleus. Brain Res. 1976;106(1):117–131. doi: 10.1016/0006-8993(76)90077-9. [DOI] [PubMed] [Google Scholar]
- 35.Greco A, Minghetti L. Isoprostanes as biomarkers and mediators of oxidative injury in infant and adult central nervous system diseases. Curr Neurovasc Res. 2004;1(4):341–54. doi: 10.2174/1567202043362036. [DOI] [PubMed] [Google Scholar]
- 36.Gunter T, Gavin C, Aschner M, Gunter K. Speciation of manganese in cells and mitochondria: a search for the proximal cause of manganese neurotoxicity. NeuroToxicology. 2006;27(5):765–776. doi: 10.1016/j.neuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Halliwell B, Gutteridge J. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 38.Hamai D, Bondy S. Oxidative basis of manganese neurotoxicity. Ann N Y Acad Sci. 2004;1012:129–141. doi: 10.1196/annals.1306.010. [DOI] [PubMed] [Google Scholar]
- 39.Harris C. The Toxicology Handbook for Clinicians. Mosby Elsevier Inc; Philadelphia: 2006. [Google Scholar]
- 40.Hsieh C, Liang J, Peng S, Lee W. Seizure associated with total parenteral nutrition – related hypermanganesemia. Pediatr Neurol. 2007;36(3):181–183. doi: 10.1016/j.pediatrneurol.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Jankovic J. Searching for a relationship between manganese and welding and Parkinson’s disease. Neurology. 2005;64:2021–2028. doi: 10.1212/01.WNL.0000166916.40902.63. [DOI] [PubMed] [Google Scholar]
- 42.Kang H, Hong Y, Kim H, Bae I. CR6-interacting factor 1 (Crif1) regulates NF-E2-related factor-2 (Nrf2) protein stability by proteasome-mediated degradation. J Biol Chem. 2010;285(28):21258–21268. doi: 10.1074/jbc.M109.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawamura R, Ikuta H, Fukuzumi S, Yamada R, Tsubaki S, Kodama T, Kurata S. Intoxication by manganese in well water. Kitasato Arch Exp Med. 1941;18:145–169. (abstract) [Google Scholar]
- 44.Kaspar J, Jaiswal A. Tyrosine phosphorylation controls nuclear export of Fyn, allowing Nrf2 activation of cytoprotective gene expression. FASEB J. 2011;25:1076–1087. doi: 10.1096/fj.10-171553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitazawa M, Wagner J, Kirby M, Anantharam V, Kanthasamy A. Oxidative stress and mitochondrial-mediated apoptosis in dopaminergic cells exposed to methylcyclopentadienyl manganese tricarbonyl. J Pharmacol Exp Ther. 2002;302:26–35. doi: 10.1124/jpet.302.1.26. [DOI] [PubMed] [Google Scholar]
- 46.Komaki H, Maisawa S, Sugai K, Kobayashi Y, Hashimoto T. Tremor and seizures associated with chronic manganese intoxication. Brain Dev. 1999;21(2):122–124. doi: 10.1016/s0387-7604(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 47.Kondakis X, Makris N, Leotsinidis M, Prinou M, Papapetropoulos T. Possible health effects of high manganese concentration in drinking water. Arch Environ Health. 1989;44:175–178. doi: 10.1080/00039896.1989.9935883. [DOI] [PubMed] [Google Scholar]
- 48.Lai J, Leung T, Lim L. Brain regional distribution of glutamic acid decarboxylase, choline acetyltransferase, and acetylcholinesterase in the rat: effects of chronic manganese chloride administration after two years. J Neurochem. 1981;36:1443–1448. doi: 10.1111/j.1471-4159.1981.tb00585.x. [DOI] [PubMed] [Google Scholar]
- 49.Lai J, Leung T, Lim L. The ontogeny of acetylcholinesterase activities in rat brain regions and the effect of chronic treatment with manganese chloride. J Neurochem. 1982;39(6):1767–1769. doi: 10.1111/j.1471-4159.1982.tb08019.x. [DOI] [PubMed] [Google Scholar]
- 50.Lai J, Leung T, Lim L. Differences in neurotoxic effects of manganese during development and aging: some observations on brain regional neurotransmitter and non-neurotransmitter metabolism in a developmental rat model of chronic manganese encephalopathy. NeuroToxicology. 1984;5(1):37–47. [PubMed] [Google Scholar]
- 51.Levy B, Nassetta W. Neurologic effects of manganese in humans. Int J Occup Environ Health. 2003;9:153–163. doi: 10.1179/oeh.2003.9.2.153. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Copin L, Reola L, Calagui B, Gobbel G, Chen S, Sato S, Epstein C, Chan P. Reduced mitochondrial manganese-superoxide dismutase activity exacerbates glutamate toxicity in cultured mouse cortical neurons. Brain Res. 1998;814:164–170. doi: 10.1016/s0006-8993(98)01082-8. [DOI] [PubMed] [Google Scholar]
- 53.Liapi C, Zarros A, Galanopoulou P, Theocharis S, Skandali N, Al-Humadi H, Anifantaki F, Gkrouzman E, Mellios Z, Tsakiris S. Effects of short-term exposure to manganese on the adult rat brain antioxidant status and the activities of acetylcholinesterase, (Na, K)-ATPase and Mg-ATPase: modulation by L-cysteine. Basic Clin Pharmacol Toxicol. 2008;103(2):171–175. doi: 10.1111/j.1742-7843.2008.00281.x. [DOI] [PubMed] [Google Scholar]
- 54.Lu L, Zhang LL, Li GJ, Guo W, Liang W, Zheng W. Alteration of serum concentrations of manganese, iron, ferritin, and transferrin receptor following exposure to welding fumes among career welders. NeuroToxicology. 2005;26(2):257–265. doi: 10.1016/j.neuro.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Markel A, Galaktionov Y, Efimov V. Factor analysis of rat behavior in an open field test. Neurosci Behav Physiol. 1989;19 (4):279–286. doi: 10.1007/BF01236015. [DOI] [PubMed] [Google Scholar]
- 56.Marreilha dos Santos AP, Lopes Santos M, Batoréu MC, Aschner M. Prolactin is a peripheral marker of manganese neurotoxicity. Brain Res. 2011;1382:282–90. doi: 10.1016/j.brainres.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez H, Bonilla E. Water intake and brain choline-acetyltransferase and acetylcholinesterase activities in manganese treated rats. Neurobehav Toxicol Teratol. 1981;3(3):277–280. [PubMed] [Google Scholar]
- 58.McMillan D. A brief history of the neurobehavioral toxicity of manganese: some unanswered questions. NeuroToxicology. 1999;20:499–507. [PubMed] [Google Scholar]
- 59.Meyer-Baron M, Knapp G, Schaper M, Van Thriel C. Performance alterations associated with occupational exposure to manganese – a meta-analysis. NeuroToxicology. 2009;30:487–496. doi: 10.1016/j.neuro.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Milatovic D, Gupta R, Aschner M. Anticholinesterase toxicity and oxidative stress. Sci World J. 2006;6:295–310. doi: 10.1100/tsw.2006.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milatovic D, Aschner M. Measurement of isoprostanes as markers of oxidative stress in neuronal tissue. Curr Protoc Toxicol. 2009;39:12.14.1–12.14.12. doi: 10.1002/0471140856.tx1214s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Milatovic D, Zaja-Milatovic S, Gupta R, Aschner M. Oxidative stress and neurodegeneration in manganese-induced neurotoxicity. Toxicol Appl Pharmacol. 2009;240(2):219–225. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moore K, Wuerthele S. Regulation of nigrostriatal and tuberoinfundibular-hypophyseal dopaminergic neurons. Prog Neurobiol. 1979;13(3):325–359. doi: 10.1016/0301-0082(79)90019-4. [DOI] [PubMed] [Google Scholar]
- 64.Morello M, Zatta P, Zambenedetti P, Martorana A, D’Angelo V, Melchiorri G, Bernardi G, Sancesario G. Manganese intoxication decreases the expression of manganoproteins in the rat basal ganglia: an immunohistochemical study, Brain Res. Bull. 2007;74:406–415. doi: 10.1016/j.brainresbull.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 65.Musiek E, Morrow J. Quantification of F2-Isoprostanes by gas chromatography/mass spectrometry as a measure of oxidant stress. Curr Protoc Toxicol. 2005;17:17.6. doi: 10.1002/0471140856.tx1706s24. [DOI] [PubMed] [Google Scholar]
- 66.Musiek E, Yin H, Milne G, Morrow J. Recent advances in the biochemistry and clinical relevance of the isoprostane pathway. Lipids. 2005;40(10):987–994. doi: 10.1007/s11745-005-1460-7. [DOI] [PubMed] [Google Scholar]
- 67.Nachtman J, Tubben R, Commisaris R. Behavioral effects of chronic manganese administration in rats: locomotor activity studies. Neurobehav Toxicol Teratol. 1986;8:711–715. [PubMed] [Google Scholar]
- 68.Pal P, Samii A, Calne D. Manganese neurotoxicity: a review of clinical features, imaging and pathology. NeuroToxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- 69.Raha S, Robinson B. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 70.Reich E, Zackert W, Brame C, Chen Y, Roberts L, Hachey D, Montine T, Morrow J. Formation of novel D-ring and E-ring isoprostane-like compounds (D4/E4-neuroprostanes) in vivo from docosahexaenoic acid. Biochemistry. 2000;39(9):2376–2383. doi: 10.1021/bi992000l. [DOI] [PubMed] [Google Scholar]
- 71.Reich E, Markesbery W, Roberts L, Swift L, Morrow J, Montine T. Brain regional quantification of F-ring and D/E-ring isoprostanes and neuroprostanes in Alzheimer’s disease. Am J Pathol. 2001;158:293–297. doi: 10.1016/S0002-9440(10)63968-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roberts L, Montine T, Markesbery W, Tapper A, Hardy P, Chemtob S, Dettbarn W, Morrow J. Formation of isoprostane-like compounds (neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 1998;273(22):13605–13612. doi: 10.1074/jbc.273.22.13605. [DOI] [PubMed] [Google Scholar]
- 73.Roberts L, Milne G. Isoprostanes. J Lipid Res. 2009;50:S219–S223. doi: 10.1194/jlr.R800037-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roels H, Meiers G, Delos M, Ortega I, Lauwerys R, Buchet J, Lison D. Influence of the route of administration and the chemical form (MnCl2, MnO2) on the absorption and cerebral distribution of manganese in rats. Arch Toxicol. 1997;71:223–230. doi: 10.1007/s002040050380. [DOI] [PubMed] [Google Scholar]
- 75.Rohling M, Demakis G. Potential neuropsychological profiles in welders occupationally exposed to manganese: an examination of effect size patterns meta-analysis and manganese exposure. J Clin Exp Neuropsychol. 2007;29 (8):813–822. doi: 10.1080/13803390601087072. [DOI] [PubMed] [Google Scholar]
- 76.Rosemberg D, Rocha R, Rico E, Zanotto-Filho A, Dias R, Bogo M, Bonon C, Moreira J, Klamt F, Souza D. Taurine prevents enhancement of acetylcholinesterase activity induced by acute ethanol exposure and decreases the level of markers of oxidative stress in zebrafish brain. Neuroscience. 2010;171 (3):683–92. doi: 10.1016/j.neuroscience.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 77.Roth J, Garrick M. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem Pharmacol. 2003;66:1–13. doi: 10.1016/s0006-2952(03)00145-x. [DOI] [PubMed] [Google Scholar]
- 78.Salehi F, Krewski D, Mergler D, Normandin L, Kennedy G, Philippe S, Zayed J. Bioaccumulation and locomotor effects of manganese phosphate/sulphate mixture in Sprague–Dawley rats following subchronic (90 days) inhalation exposure. Toxicol Appl Pharmacol. 2003;191(3):264–271. doi: 10.1016/s0041-008x(03)00238-2. [DOI] [PubMed] [Google Scholar]
- 79.Schallreuter K, Elwary S. Hydrogen peroxide regulates the cholinergic signal in a concentration dependent manner. Life Sci. 2007;80 (24–25):2221–26. doi: 10.1016/j.lfs.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 80.Silva A, Bock N. Manganese-enhanced MRI: an exceptional tool in translational neuroimaging. Schizophr Bull. 2008;34(4):595–604. doi: 10.1093/schbul/sbn056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simon H, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–18. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 82.Sitaramayya A, Nagar N, Chandra S. Effect of manganese on enzymes in rat brain. Acta Pharmac Tox. 1974;35:185–190. doi: 10.1111/j.1600-0773.1974.tb00737.x. (abstract) [DOI] [PubMed] [Google Scholar]
- 83.Stadler H, Lloyd K, Gadea-Ciria M, Bartholini G. Enhanced striatal acetylcholine release by chlorpromazine and its reversal by apomorphine. Brain Res. 1973;55(2):476–480. doi: 10.1016/0006-8993(73)90317-x. [DOI] [PubMed] [Google Scholar]
- 84.Stallings W, Metzger A, Pattridge K, Fee J, Ludwig M. Structure-function relationships in iron and manganese superoxide dismutases. Free Radic Res Commun. 1991;12–13:259–268. doi: 10.3109/10715769109145794. [DOI] [PubMed] [Google Scholar]
- 85.Takeda A. Manganese action in brain function. Brain Res Rev. 2003;41:79–87. doi: 10.1016/s0165-0173(02)00234-5. [DOI] [PubMed] [Google Scholar]
- 86.Tapin D, Kennedy G, Lambert J, Zayed J. Bioaccumulation and locomotor effects of manganese sulphate in Sprague–Dawley rats following subchronic (90 days) inhalation exposure. Toxicol Appl Pharmacol. 2006;211(2):166–74. doi: 10.1016/j.taap.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 87.Taylor M, Erikson K, Dobson A, Fitsanakis V, Dorman D, Aschner M. Effects of inhaled manganese on biomarkers of oxidative stress in the rat brain. NeuroToxicology. 2006;27(5):788–797. doi: 10.1016/j.neuro.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 88.Thomé G, Spanevello R, Mazzanti A, Fiorenza A, Duarte M, da Luz S, Pereira M, Morsch V, Schetinger M, Mazzanti C. Vitamin E decreased the activity of acetylcholinesterase and level of lipid peroxidation in brain of rats exposed to aged and diluted sidestream smoke. Nicotine Tob Res Set. 2011;6 doi: 10.1093/ntr/ntr154. [epub] [DOI] [PubMed] [Google Scholar]
- 89.Torrente M, Colomina M, Domingo J. Behavioral effects of adult rats concurrently exposed to high doses of oral manganese and restraint stress. Toxicology. 2005;211:59–69. doi: 10.1016/j.tox.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 90.Wang T, Qin L, Liu B, Liu Y, Wilson B, Eling T, Langenbach R, Taniura S, Hong J. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J Neurochem. 2004;88:939–947. doi: 10.1046/j.1471-4159.2003.02242.x. [DOI] [PubMed] [Google Scholar]
- 91.Wasserman G, Liu X, Parvez F, Factor-Litvak P, Ahsan H, Levy D, Kline J, Van Geen A, Mey J, Slavkovich V, Sidique A, Islam T, Graziano J. Arsenic and manganese exposure and children’s intellectual function. NeuroToxicology. 2011;32:450–457. doi: 10.1016/j.neuro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wasserman G, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, Kline J, Van Green A, Slavkovich V, Lolacono N, Chen Z, Zheng Y, Graziano J. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114(1):124–9. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vezér T, Kurunczi A, Náray M, Papp A, Nagymajtényi L. Behavioral effects of subchronic inorganic manganese exposure in rats. Am J Ind Med. 2007;50(11):841–852. doi: 10.1002/ajim.20485. [DOI] [PubMed] [Google Scholar]
- 94.Yin Z, Aschner J, dos Santos AP, Aschner M. Mitochondrial-dependent manganese neurotoxicity in rat primary astrocyte cultures. Brain Res. 2008;1203:1–11. doi: 10.1016/j.brainres.2008.01.079. [DOI] [PMC free article] [PubMed] [Google Scholar]