Abstract
Polycystic ovary syndrome (PCOS) is an endocrine disorder that affects 5–8% of reproductive age women. The primary features of PCOS are hyperandrogenemia, chronic anovulation and infertility. It has been suggested that defects in ovarian steroid metabolism contribute to the follicular growth arrest and abnormal production of ovarian steroid hormones that are characteristic of PCOS. 2-methoxyestradiol (2-ME) is formed by the action of catechol-O-methyltransferase (COMT) on 2-hydroxyestradiol. COMT expression is increased in the follicles and ovarian stroma of women with PCOS. Moreover, 2-ME decreases granulosa cell proliferation and steroidogenesis, raising the possibility that ovarian dysfunction associated with PCOS is due, in part, to increased synthesis of 2-ME resulting from increased COMT activity. Four single-nucleotide polymorphisms (SNPs) (rs6269, rs4633, rs4818, rs4680) in the COMT gene characterize haplotypes, which are associated with large variations in COMT enzymatic activity. The aim of this study was to determine whether individual COMT SNPs and the COMT haplotypes are associated with PCOS using a family-based test of association and linkage. Additionally, we examined the relationships between COMT SNPs and haplotypes with quantitative variables usually assessed in the evaluation of women with PCOS. There were no significant correlations between genotype and total testosterone, non-SHBG bound testosterone and BMI. However, we found that the prolactin level in women with PCOS varied significantly with COMT haplotype, and suggest that this association reflects a genetic factor influencing the stress response. Our findings suggest that common variants and haplotypes of the COMT gene are not major contributors to risk for PCOS, but that COMT genotype may influence prolactin levels.
Keywords: Catechol-O-Methyltransferase, COMT, polycystic ovary syndrome, prolactin5
1. INTRODUCTION
Polycystic ovary syndrome (PCOS), which affects 5–8% of reproductive-age women, is characterized by chronic anovulation due to follicular growth arrest and hyperandrogenism of ovarian origin (Knochenhauer et al., 1998; Azziz et al., 2004). PCOS is considered to be a complex disorder with multiple genes, as well as, social and environmental factors contributing to disease (Legro et al., 1998; Urbanek et al., 1999).
Salih et al. (Salih et al., 2007; 2008) suggested that one possible mechanism for follicular growth arrest in PCOS is increased production of the estrogen metabolite, 2-methoxyestradiol (2-ME), which inhibits granulosa cell proliferation and also reduces steroidogenesis (Spicer and Hammond, 1988;1989). These authors demonstrated increased expression of catechol-O-methyltransferase (COMT), the enzyme that forms 2-ME from the catechol estrogen, 2-hydroxyestradiol in follicular tissue and ovarian stroma in women with PCOS. Salih et al. hypothesized that increased ovarian expression of COMT and the subsequent increase in 2-ME production leads to ovulatory dysfunction.
Allelic variation in the COMT gene has been associated with differences in COMT enzyme activity, which is involved in the metabolism of catecholamines, including dopamine, epinephrine, norepinephrine, as well as catechol estrogens (Mannisto and Kaakkola, 1999; Nackley et al., 2006). Past research has largely focused on a single nucleotide polymorphism (SNP), rs4680. The minor allele of rs4680 causes a valine to methionine substitution (Val158Met), which has been associated with a 4-fold decrease in COMT activity in the homozygous state (Mannisto and Kaakkola, 2000). Recently, haplotypes characterized by three additional SNPs (rs6269, rs4633, and rs4818) have been identified, and these haplotypes have been shown to account for larger variation in COMT activity (Nackley et al., 2006). Nackley et al. (2006) demonstrated in a mammalian expression system that COMT haplotypes could be ordered in a progression of enzyme activity with the ACCG haplotype showing a 18–25 fold decrease in activity and the ATCA haplotype showing a 2.5–3 fold decrease in activity compared to the GCGG high activity haplotype (Nackley et al., 2006). Thus, allelic variation in the COMT gene could influence the level of ovarian 2-ME production and, based on the work of Salih et al., (2007; 2008) contribute to risk of PCOS.
In the present study, we employed a candidate gene approach to investigate whether allelic variation in the COMT gene is associated and linked with PCOS using a family-based method, the transmission disequilibrium test (TDT). We also tested for associations between COMT SNPs and haplotypes and quantitative parameters that we evaluated in the women with PCOS, including total testosterone, non-SHBG bound testosterone, and BMI. We also evaluated prolactin levels in the subjects to rule out the possibility that the anovulation that characterizes PCOS was caused by hyperprolactinemia (Filho et al., 2007), creating an opportunity to examine the relationship between prolactin levels and COMT SNPs and haplotypes.
2. SUBJECTS AND METHODS
2.1 Diagnosis of PCOS and phenotyping
Diagnostic criteria for PCOS have been described in detail elsewhere (Legro et al., 1998; Urbanek et al., 1999). All women included in this study (family-based analysis and quantitative trait analysis) were considered affected with PCOS if they had elevated total testosterone (T) (greater than 58 ng/dl) or non-SHBG bound testosterone (uT) (greater that 15 ng/dl) and six or fewer menses per year. Women with clinical or biochemical evidence of non-classical 21-hydroxylase deficiency, thyroid disease, androgen-secreting tumors, or prolactin level greater than 30 ng/ml were excluded (Dunaif et al., 1996). Women included in the study were either evaluated on site at one of four study centers (Brigham and Women’s Hospital, Boston Massachusetts; Pennsylvania State University College of Medicine, Hershey Pennsylvania; University of Pennsylvania State University, Philadelphia Pennsylvania; Northwestern University Feinberg School of Medicine, Chicago Illinois) or off site at a local hospital as previously described (Dunaif et al., 1996).
Fasting venous blood samples were collected between 0800 and 1000 h for measurement of serum T, and uT at a central laboratory (Penn State Hershey Medical Center) as previously described (Legro et al., 1998; Urbanek et al., 1999). Prolactin was measured at one of three locations: Brigham and Women’s Hospital (n = 131), Northwestern University Feinberg School of Medicine (n = 132), or the Hershey Medical Center core laboratory (n = 435). At Brigham and Women’s Hospital prolactin was measured using the Bayer, Technicon Immuno 1 System, according to the manufacturer’s protocol (Bayer Corp.). At Northwestern University, prolactin was measured using the Access Immunoassay Systems, according to the manufacturer’s protocol (Beckman Coulter). At the Hershey Medical Center core laboratory, prolactin was measured using a solid-phase immunoradiometric assay (IRMA) from Siemens Healthcare Diagnostics. All assays, were standardized to the WHO 3rd International Standard for Prolactin 84/500. Inter-assay coefficients of variation (CV) were <10% for all assays across the dynamic range of 0.25–200 ng/ml. The precision of all three assays ranged from 3.3–7.0%. T and uT levels were determined as previously described (Legro et al., 1998).
This study was approved by the institutional review boards of the University of Pennsylvania, Pennsylvania State University College of Medicine, Brigham and Women’s Hospital, Northwestern University, the University of Alabama at Birmingham and Cedars-Sinai Medical Center. All subjects provided written informed consent for participation in this study.
2.2 SNP genotyping
Allelic discrimination for four COMT SNPs was performed using predesigned TaqMan Genotyping assays C___2538746_1 for SNP rs6269, C___2538747_20 for SNP rs4633, C___2538750_10 for SNP rs4818, C__25746809_50 for SNP rs4680 (Applied Biosystems). Allelic PCR products were analyzed using the Applied Biosystems 7900 HT Sequence Detection System and SDS version 2.2 software. Genotypes were auto-called with the quality value set at 0.95. Three individuals from the Centre d’Etude du Polymorphisme Humain collection were genotyped on each 96-well plate, and no discrepancies were observed; genotypes were all in Hardy-Weinberg equilibrium.
Error-checking of genotypes in the family material was performed with Merlin software (version 1.1.2; http://www.sph.umich.edu/csg/abecasis/merlin/index.html) and families with one or more Mendelian discrepancies for a marker were excluded in the analysis of that marker.
2.3 PCOS family-based study
Four COMT SNPs were genotyped in 440 families with PCOS: 46 multiplex families (two or more affected daughters and parents) and 394 simplex families (one affected daughter and parents). The total number of offspring with PCOS was 489. The self-identified ethnicities of probands in the families were: 87% white, 4% Hispanic, 1% black and 7% other or unknown.
Linkage and association between SNPs and PCOS was tested with the TDT (Spielman et al., 1993).
Genetic Power Calculator software (http://pngu.mgh.harvard.edu/~purcell/gpc/) (Purcell et al., 2003) was used to determine that with the sample size there was approximately 80% power (P = 0.05) to detect a relative risk ratio of 3.0.
2.4 Quantitative trait analysis of women with PCOS
The quantitative TDT (QTDT) program (version 2.6.0) in Merlin was used to test for association between the SNPs and haplotypes and quantitative measures (prolactin, body mass index (BMI), non-SHBG bound testosterone (uT), total testosterone (T) using the orthogonal association model and the environmental, polygenic, and additive variance components (http://www.sph.umich.edu/csg/abecasis/QTDT/) (Abecasis et al., 2000) in the family study. We corrected for multiple testing using Bonferroni adjustment based on testing of 4 SNPs: the adjusted p-value of 0.0125 corresponded to a nominal P=0.05.
A separate quantitative trait analysis was performed with a total of 698 unrelated women diagnosed with PCOS were studied, which included the PCOS women from the family-based analysis with the exception that women with ethnicities known to be other than white (Hispanic, Black, or other) were excluded from the study. The self-identified ethnicities of subjects were 561 White and 137 unknown. The ethnic profile of the general patient population in the study areas is greater than 85% white with the remaining ethnicities (Hispanic, Black, and other) representing the minority of cases. Therefore, the 698 subjects were composed of whites and individuals of unknown ethnicity, who were assumed to be mostly white in accordance with the general population.
Linear regression in PLINK was used to test for associations between each of the 4 SNPs and quantitative measures. Inter-SNP linkage disequilibrium calculations for COMT were performed in PLINK (Hill et al., 2011). Haplotype frequencies were generated in PLINK based on the four COMT SNPs, with a haplotype exclusion threshold of < 0.01. Linear regression (lm) in PLINK was used to test for associations between COMT haplotype and prolactin level. In this analysis each haplotype was included as a separate predictor in the lm model and coded as an additive term (1) vs. all other haplotypes (0). Associations were further investigated in PLINK to condition by covariates.
Based on published haplotype-specific COMT activity level and work showing that ordering haplotypes according to reported activity provided functionally relevant information (Hill et al., 2011), we also coded COMT haplotypes to reflect enzymatic activity. Haplotypes were assigned to subjects in PLINK and then imputed into R for analysis. Haplotypes were assigned based on the most likely phase reconstructed haplotypes generated by the expectation-maximization algorithm implemented in PLINK. R was used for the activity analysis because it allowed for the activity term to be a factor with six progressive levels. COMT haplotypes were sequentially ordered 1 through 6 where 1 was ACCG/ACCG, 2 was ACCG/ATCA, 3 was ACCG/GCGG, 4 was ATCA/ATCA, 5 was ATCA/GCGG, and 6 was GCGG/GCGG. The activity of ACCG/GCGG was predicted to be at the midpoint of GCGG and ACCG (half of the 18–25 fold difference reported by Nackley et al. (2006), whereas ATCA/ATCA was predicted to be closer to GCGG/ATCA because the ATCA haplotype only shows 2.5–3 fold decreased activity [10]. Linear regression in R was used to test for an association between increasing enzyme activity and prolactin level. P-value and Beta coefficients were calculated after adjusting for age, body mass index, unbound testosterone, and total testosterone.
3. RESULTS
3.1 COMT SNPs and haplotypes are not associated with PCOS in a family-based test
Haplotype analysis showed the four COMT SNPs (rs6269, rs4633, rs4818, and rs4680) to be in very high linkage disequilibrium (LD), with D’ ≥ 0.972 for each pair wise analysis. Three main haplotypes of COMT were identified in the study population: ACCG, ATCA, and GCGG. The haplotype frequencies were 0.101, 0.503, and 0.369, respectively. This haplotype structure and the haplotype frequencies are in agreement with previously published data [10,16]. A fourth haplotype, GCCG, was identified by PLINK, but only represented 1% of the COMT alleles. Since the GCCG haplotype was at the exclusion threshold for haplotype frequencies, it was removed from analysis.
In the family-based analysis, no significant association was found between any of the four SNPs or the three haplotypes and PCOS in the TDT analysis (Table 2).
Table 2.
Family-based linkage and association between COMT polymorphisms and PCOS and quantitative measures associated with PCOS.
| TDT-X2 | Quantative TDT-X2 | |||||
|---|---|---|---|---|---|---|
| Allele | PCOS | prolactin | BMI | uT | T | |
| rs6269 | A*:G | 2.16 | 4.48** | 0.06 | 0.37 | 0.44 |
| rs4633 | T*:C | 1.23 | 1.19 | 1.51 | 0.1 | 0.35 |
| rs4818 | C*:G | 2.50 | 4.87§ | 0.43 | 0.44 | 0.6 |
| rs4680 | A:G* | 1.38 | 1.71 | 2.48 | 0.07 | 0.34 |
| haplotype | ACCG | 0.11 | 2.45 | 2.01 | 0.1 | 0.01 |
| ATCA | 1.85 | 1.52 | 1.55 | 0.21 | 0.53 | |
| GCGG | 1.93 | 5.10¶ | 0.19 | 0.32 | 0.45 | |
SNP allele which is over-transmitted in the TDT; major allele in parental population in bold.
nominal P-values: ** =0.034,
0.027,
0.024.
3.2 Assessment of quantitative traits and allelic variation in the COMT
No significant association was identified in the family-based study between COMT SNPs and haplotypes and any of the other quantitative traits, except prolactin. QTDT analysis suggested a nominally significant association between prolactin levels and two SNPs (rs6269 and rs4818), and with the GCGG haplotype, although none of these associations were significant after correction for multiple testing.
We carried out a separate quantitative trait analysis with a larger cohort of PCOS women (White or unknown ethnicity), which included the PCOS subjects from the family-based study. These additional women were initially enrolled for the family-based study but could not be included because parental DNA was not available. Clinical characteristics for all the subjects are presented in Table 1. Consistent with diagnostic criteria, the mean total and free testosterone levels of the women were elevated at 79.5 ng/ul and 26.4 ng/ul, respectively. The average plasma prolactin level of women was 8.4 ng/ml, with values ranging from 0.6 to 25.0 ng/ml. Mean prolactin level was not significantly different between assay sites at an alpha level of 0.05. Women with PCOS were obese with a mean BMI of 35.6 (kg/m2). Allele frequencies and haplotype frequencies were not significantly different between the three recruitment sites at an alpha level of 0.05. No differences in either genotype frequency or prolactin levels between sites justified combining all subjects into a single study population for further analysis.
Table 1.
Clinical and metabolic characteristics of women with PCOS (N=698)
| Characteristic | Mean ± SE (S.I.) | Range |
|---|---|---|
| Prolactin (ng/ml) | 8.4 ± 0.1 (365.2 pmol/L) | 0.6 – 25.0 |
| Age (years) | 28.4 ± 0.2 | 13 – 53 |
| BMI (kg/m2) | 35.6 ± 0.3 | 16.5 – 64.5 |
| T (ng/dl) | 79.5 ± 1.2 (2.8 nmol/L) | 28 – 337 |
| uT (ng/dl) | 26.4 ± 0.6 (0.9 nmol/L) | 1.29 – 211 |
SE, standard error; S.I., International System of Units; BMI, body mass index; T, total testosterone; uT, unbound testosterone.
We found no significant associations between COMT individual SNPs or haplotypes with T, uT, and BMI in the PCOS women. However there were significant associations between COMT SNPs and haplotypes and prolactin levels, consistent with the observation of a nominally significant association from the QTDT analysis. This significant association remained after adjusting for age BMI, T, and uT as covariates. After adjusting for these covariates, rs6269, rs4818, and the haplotypes ACCG and GCGG all remained nominally significantly associated with prolactin level (P = 0.021, P = 0.011, P < 0.0001, and P = 0.005, respectively), and the haplotype association was significant after correction for multiple testing (Table 3). The ACCG haplotype, which is correlated with low COMT activity [10], was associated with higher prolactin levels (β = 1.490), and the GCGG haplotype, which is associated with high COMT activity (9), was associated with lower neither individual SNPs nor haplotypes are associated prolactin levels (β = −0.619).
Table 3.
Linear regression analysis for association between prolactin and COMT in women with PCOS.
| COMT | Frequency | β-Estimate | P-value | β- Estimateadjusted |
P-value adjusted |
|
|---|---|---|---|---|---|---|
| SNP | rs6269 | 0.384 (G) | −0.524 | 0.017 | −0.505 | 0.021 |
| rs4633 | 0.490 (C) | 0.091 | 0.670 | 0.077 | 0.717 | |
| rs4818 | 0.375 (G) | −0.585 | 0.009 | −0.567 | 0.011 | |
| rs4680 | 0.485 (G) | 0.054 | 0.804 | 0.055 | 0.799 | |
| Haplotype | ACCG | 0.101 | 1.519 | > 0.0001 | 1.490 | >0.0001 |
| ATCA | 0.503 | −0.046 | 0.829 | −0.045 | 0.834 | |
| GCGG | 0.369 | −0.628 | 0.005 | −0.619 | 0.005 | |
SNP frequency (minor allele). COMT haplotype SNP order: rs6269, rs4633, rs4818, rs4680. adjusted, P-value and Beta coefficients after adjusting for age, body mass index, unbound testosterone, and total testosterone. SNP, single nucleotide polymorphism.
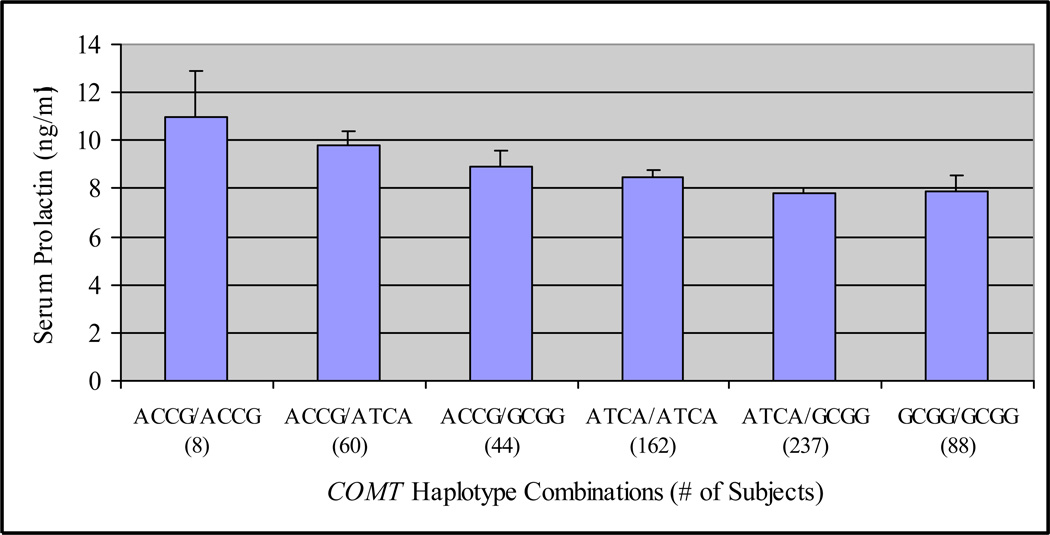
Figure 1 reveals declining mean prolactin levels in women with PCOS as a function of haplotypes progressing from those associated with low to high COMT enzymatic activity as described by Nackley et al. (2006). This relationship was tested in the current study, and results of a linear regression model that included a term to reflect enzymatic activity of the COMT haplotype (I,e., coded as an ordinal variable), age, BMI, T, and uT are shown in Table 4. A significant negative relationship between increasing enzymatic activity related to COMT haplotype and prolactin was observed (P = 0.0002, β = −0.525). Prolactin levels also significantly decreased with age (P = 0.028, β = −0.065).
Figure 1.
Mean (± SE) serum prolactin according to ascending COMT enzymatic activity level in women with PCOS. COMT relative activity was ordered, based on the two COMT haplotypes of each subject, from 1 (low activity) to 6 (high activity) in accordance with reported information on enzyme activity (Nackley et al., 2006). Ordered COMT haplotypes: 1=ACCG/ACCG, 2=ACCG/ATCA, 3= ACCG/GCGG, 4= ATCA/ATCA, 5=ATCA/GCGG, 6=GCGG/GCGG. Mean prolactin level decreased linearly from low to high activity (P < 0.0002).
Table 4.
Linear regression model of prolactin level in women with PCOS including COMT haplotype specified according to reported enzymatic activity.
| Term | Estimate (S.E.) | P-value |
|---|---|---|
| COMT haplotypes | −0.525 (0.140) | 0.0002 |
| Age (years) | −0.065 0(0.029) | 0.028 |
| BMI (kg/m2) | −0.037 (0.020) | 0.063 |
| Total testosterone (ng/dl) | −0.007 (0.007) | 0.314 |
| Non-SHBG bound T (ng/dl) | 0.018 (0.015) | 0.253 |
| Intercept | 14.066 (1.201) | <0.0001 |
COMT haplotype SNP order: rs6269, rs4633, rs4818, rs4680. Ordered COMT haplotypes: 1=ACCG/ACCG, 2=ACCG/ATCA, 3= ACCG/GCGG, 4= ATCA/ATCA, 5=ATCA/GCGG, 6=GCGG/GCGG. Haplotypes were ordered from 1 (low activity) to 6 (high activity) in accordance with reported information on enzyme activity (9). Haplotypes showed decreasing prolactin level as haplotypes moved from low to high activity alleles. S.E., standard error; BMI, body mass index.
4. DISCUSSION
2-ME is known to be produced in the ovarian follicle and to influence granulosa cell metabolism, proliferation and angiogenesis (Spicer and Hammond, 1988; 1989; Basini et al., 2006; 2007; Eichenlaub-Ritter et al., 2007; Shang et al., 2001; Spicer et al., 1987). Studies on ovarian tissue in the context of PCOS suggest that increased local production of 2-ME could contribute to the development of the characteristic morphological ovarian abnormality of follicular maturation arrest. The aim of this study was to determine if allelic variation in COMT is associated with PCOS and PCOS-related quantitative traits, based on the hypothesis that the resulting differences in COMT activity alter ovarian production of 2-ME, which influences follicular growth and function. Our family-based analysis of association and linkage to the four COMT individual SNPs and the haplotypes, did not support the hypothesis since there was no evidence for significant association. However, these negative findings are not definitive since the sample size in our study had a power to detect a relative risk ratio of 3.0, and, therefore, significant associations with a relative ratio below 3.0 may indeed exist. In addition, genetic variants that were not evaluated in our study might contribute to PCOS risk. Finally, genetic variation is not the only possible explanation for the findings of Salih et al. (2007; 2008). The increased COMT expression in the PCOS ovary could have resulted from differences in COMT protein turnover, or the increased volume of stromal tissue expressing COMT in the PCOS ovary.
We also examined quantitative traits related to PCOS and found no associations of the individual COMT SNPs and haplotypes with T, uT or BMI. However, a significant association was found with prolactin levels. We included prolactin in our quantitative trait analysis because COMT metabolizes multiple catecholamines that participate in the regulation of prolactin, but it is not clear how variation in this enzyme affects prolactin level in humans. One of the main catecholamines that is metabolized by COMT is dopamine, the primary inhibitor of prolactin secretion. We, therefore, expected that haplotypes associated with decreased COMT activity would be associated with decreased prolactin level. However, our results show that haplotypes linked with decreased COMT activity are associated with increased serum prolactin. However, COMT inhibitors administered to humans cause inconsistent, or no change in prolactin levels (Keranen et al., 1996), which suggests that prolactin secretion is not directly regulated by COMT metabolism of dopamine.
Estrogen also influences prolactin by stimulating secretion, but the association between COMT and prolactin appears to be independent of this relationship. Women with PCOS have increased extraglandular aromatization of estrogen. After adjusting for parameters linked to extraglandular estrogen production, the associations remained significant and the estimates were relatively unchanged. This suggests that the observed associations are unlikely to be related to increased estrogen. COMT also metabolizes catechol-estrogens by converting 2-hydroxyestrogens (2-OH) to 2-methoxyestrogens (2-ME). Both 2-OH and 2-ME are biologically active, but neither has been shown to effect prolactin level in humans (Lamberts and Oosterom, 1983; Merriam et al., 1983). Thus, it is unlikely that the association between COMT haplotype and prolactin level reflects alterations in estrogen metabolism. Prolactin levels are negatively correlated with age (Yasui-Furukori et al., 2008), but our analysis compensated for any effect of age on the associations with COMT genotypes.
With COMT action on dopamine and estrogen metabolism not likely to account for the relationship between COMT haplotypes and prolactin level, central catecholamine metabolism is left as a potential mechanism by which COMT might be influencing prolactin levels. Monoamine oxidase inhibitors (MAOI), which increase central catecholamines, have been shown to increase prolactin levels (Torre and Falorni, 2007). These findings suggest that decreased COMT activity mimics the action of MAOIs, and that extra-hypothalamic regulation of the HPA axis is important in determining serum prolactin level in PCOS.
It is of note that COMT genotype has been associated with the endocrine response to psychological stress (Alexander et al., 2011; Jabbi et al., 2007; Walder et al., 2010). Homozygosity for the minor allele (G) of rs4680, which reduced COMT activity, has been correlated with increased cortisol secretion in several studies. Prolactin secretion is also increased by stress (Torre and Falorni, 2007).. In our study, homozygosity for the low COMT activity ACCG haplotype (where rs4680 is the 4th SNP in the sequence) is associated with the highest prolactin levels. This observation, taken together with the evidence noted above for COMT involvement in modulating the hypothalamic-pituitary-adrenal axis, is consistent with the notion that variation in the COMT gene and COMT activity have a role in governing the endocrine response to stress.
There are several caveats surrounding our findings of an association between COMT haplotypes, prolactin and PCOS. First, prolactin assays were performed as a screening test in subject phenotyping to exclude subjects whose amenorrhea might be caused by hyperprolactinemia (Filho et al., 2007; Murdoch et al., 1986; Schmidt et al., 2011). Consequently, individuals who had abnormally elevated prolactin levels would have been excluded. There is no known pathophysiological link between prolactin and PCOS, so the significant association between COMT haplotypes and prolactin do not indicate a direct or indirect role for COMT in PCOS. Second, prolactin assays were performed at three different sites rather than a central laboratory. Thus, assay variation might confound our findings. However, when incorporated into our analysis, the assay site did not affect the significant associations, mitigating concerns about site-to-site assay variability. Third, it is not known whether the associations between COMT haplotypes and prolactin are specific to PCOS, or whether the association is relevant to neuroendocrine function in the general population.
In conclusion, the allelic variants of COMT examined in this study do not have a major influence on risk of PCOS, but they do appear to have a relationship to prolactin levels in women with PCOS.
Highlights.
Examination of single nucleotide polymorphisms and haplotypes in the catechol-O-methyltransferase (COMT) gene, which encodes an enzyme that is involved in estrogen and monoamine metabolism, revealed that allelic variation is not a major risk factor for polycystic ovary syndrome (PCOS).
Quantitative trait analysis demonstrated that COMT haplotypes were significantly associated with prolactin levels in PCOS women, but not with total terstosterone, non-SHBG-bound testosterone and BMI.
COMT haplotypes associated with low COMT activity were associated with higher prolactin levels.
COMT involvement in central catecholamine metabolism may explain the haplotype relationships with prolactin levels.
ACKNOWLEDGEMENTS
We thank Wendy Ankener (Department of Genetics, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania 19104) for performing the genotype assays used in this study. We thank and acknowledge the women and their families who participated in the study. We also thank the study coordinators (B. Sheetz, S. Ward, and J. Schindler) and the General Clinical Research Center nursing staff at Brigham and Women’s Hospital, Northwestern University, and Pennsylvania State University.
This research was supported in part by the Eunice Kennedy Shriver NICHD/NIH through the cooperative agreement U54-HD03449 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (J.F.S., R.S.L.); P50 HD44405 (A.D.); RR10732 and C06 RR016499 (to Pennsylvania State University General Clinical Research Center (GCRC)); M01 RR00048 (to Northwestern University GCRC); Pennsylvania Department of Health using Tobacco Settlement Funds (RSL- SAP 41-000-26343) and UL1RR031990 (to the Virginia Commonwealth University Center on Clinical and Translational Research)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abecasis GR, Cardon LR, Cookson WO. A general test of association for quantitative traits in nuclear families. Am. J. Hum. Genet. 2000;66:279–292. doi: 10.1086/302698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander N, Osinsky R, Mueller E, Schmitz A, Guenthert S, Kuepper Y, Hennig J. Genetic variants within the dopaminergic system interact to modulate endocrine stress reactivity and recovery. Behav. Brain Res. 2011;216:53–58. doi: 10.1016/j.bbr.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- Basini G, Bussolati S, Santini SE, Bianchi F, Careri M, Mangia A, Musci M, Grasselli F. Antiangiogenesis in swine ovarian follicle: a potential role for 2-methoxyestradiol. Steroids. 2007;72:660–665. doi: 10.1016/j.steroids.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Basini G, Santini SE, Grasselli F. 2-Methoxyestradiol inhibits superoxide anion generation while it enhances superoxide dismutase activity in swine granulosa cells. Ann. NY. Acad. Sci. 2006;1091:34–40. doi: 10.1196/annals.1378.052. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Scott D, Finegood D, Quintana B, Whitcomb R. The insulin-sensitizing agent troglitazone improves metabolic and reproductive abnormalities in the polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 1996;81:3299–3306. doi: 10.1210/jcem.81.9.8784087. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Winterscheidt U, Vogt E, Shen Y, Tinneberg HR, Sorensen R. 2-methoxyestradiol induces spindle aberrations, chromosome congression failure, and nondisjunction in mouse oocytes. Biol. Reprod. 2007;76:784–793. doi: 10.1095/biolreprod.106.055111. [DOI] [PubMed] [Google Scholar]
- Filho RB, Domingues L, Naves L, Ferraz E, Alves A, Casulari LA. Polycystic ovary syndrome and hyperprolactinemia are distinct entities. Gynecol Endocrinol. 2007;23(5):267–272. doi: 10.1080/09513590701297708. [DOI] [PubMed] [Google Scholar]
- Hill LD, York TP, Kusanovic JP, Gomez R, Eaves LJ, Romero R, Strauss JF., 3rd Epistasis between COMT and MTHFR in maternal-fetal dyads increases risk for preeclampsia. PLoS One. 2011;6:e16681. doi: 10.1371/journal.pone.0016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M, Kema IP, van der Pompe G, te Meerman GJ, Ormel J, den Boer JA. Catechol-o-methyltransferase polymorphism and susceptibility to major depressive disorder modulates psychological stress response. Psychiatr. Genet. 2007;217:183–193. doi: 10.1097/YPG.0b013e32808374df. [DOI] [PubMed] [Google Scholar]
- Keranen T, Gordin A, Koulu M, Scheinin M, Antila S, Sundberg S, Wikberg T. COMT inhibition by entacapone does not affect growth hormone or prolactin secretion in healthy volunteers. J. Neural Transm. 1996;103:729–736. doi: 10.1007/BF01271232. [DOI] [PubMed] [Google Scholar]
- Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J. Clin. Endocrinol. Metab. 1998;83:3078–3082. doi: 10.1210/jcem.83.9.5090. [DOI] [PubMed] [Google Scholar]
- Lamberts SW, Oosterom R. Absence of a suppressive effect of 2-hydroxyestrone on hyperprolactinemia in patients with prolactinomas before and after estradiol administration. J. Clin. Endocrinol .Metab. 1983;56:230–233. doi: 10.1210/jcem-56-2-230. [DOI] [PubMed] [Google Scholar]
- Legro RS, Driscol ID, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol. Rev. 1999;51:593–628. [PubMed] [Google Scholar]
- Merriam GR, Pfeiffer DG, Loriaux DL, Lipsett MB. Catechol estrogens and the control of gonadotropin and prolactin secretion in man. J. Steroid Biochem. 1983;19:619–625. doi: 10.1016/0022-4731(83)90227-3. [DOI] [PubMed] [Google Scholar]
- Murdoch AP, Dunlop W, Kendall-Taylor P. Studies of prolactin secretion in polycystic ovary syndrome. Clin. Endocrinol. (Oxf) 1986;24:165–175. doi: 10.1111/j.1365-2265.1986.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–1933. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Purcell S, Cherny SS, Sham PC. Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics. 2003;19:149–150. doi: 10.1093/bioinformatics/19.1.149. [DOI] [PubMed] [Google Scholar]
- Salih SM, Jamaluddin M, Salama SA, Fadl AA, Nagamani M, Al-Hendy A. Regulation of catechol O-methyltransferase expression in granulosa cells: a potential role for follicular arrest in polycystic ovary syndrome. Fertil. Steril. 2008;89:1414–1421. doi: 10.1016/j.fertnstert.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Salih S, Xu X, Veenstra TD, Duleba AJ, Fouad H, Nagamani M, Al-Hendy A. Lower levels of urinary 2-hydroxyestrogens in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2007;92:3285–3291. doi: 10.1210/jc.2006-2719. [DOI] [PubMed] [Google Scholar]
- Shang W, Konidari I, Schomberg DW. 2-Methoxyestradiol, an endogenous estradiol metabolite, differentially inhibits granulosa and endothelial cell mitosis: a potential follicular antiangiogenic regulator. Biol. Reprod. 2001;65:622–627. doi: 10.1095/biolreprod65.2.622. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Brännström M, Landin-Wilhelmsen K, Dahlgren E. Reproductive hormone levels and anthropometry in postmenopausal women with polycystic ovary syndrome (PCOS): a 21-year follow-up study of women diagnosed with PCOS around 50 years ago and their age-matched controls. J. Clin. Endocrinol. Metab. 2011;96:2178–2185. doi: 10.1210/jc.2010-2959. [DOI] [PubMed] [Google Scholar]
- Spicer LJ, Hammond JM. Catecholestrogens inhibit proliferation and DNA synthesis of porcine granulosa cells in vitro: comparison with estradiol, 5 alpha-dihydrotestosterone, gonadotropins and catecholamines. Mol. Cell. Endocrinol. 1989;64:119–126. doi: 10.1016/0303-7207(89)90072-5. [DOI] [PubMed] [Google Scholar]
- Spicer LJ, Hammond JM. Comparative effects of androgens and catecholestrogens on progesterone production by porcine granulosa cells. Mol. Cell. Endocrinol. 1988;56:211–217. doi: 10.1016/0303-7207(88)90063-9. [DOI] [PubMed] [Google Scholar]
- Spicer LJ, Walega MA, Hammond JM. Metabolism of [3H]2-hydroxyestradiol by cultured porcine granulosa cells: evidence for the presence of a catechol-O-methyltransferase pathway and a direct stimulatory effect of 2-methoxyestradiol on progesterone production. Biol. Reprod. 1987;36:562–571. doi: 10.1095/biolreprod36.3.562. [DOI] [PubMed] [Google Scholar]
- Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM) Am. J. Hum .Genet. 1993;52:506–516. [PMC free article] [PubMed] [Google Scholar]
- Torre DL, Falorni A. Pharmacological causes of hyperprolactinemia. Ther. Clin. Risk Manag. 2007;3:929–951. [PMC free article] [PubMed] [Google Scholar]
- Urbanek M, Legro RS, Driscoll DA, Azziz R, Ehrmann DA, Norman RJ, Strauss JF, 3rd, Spielman RS, Dunaif A. Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc. Natl. Acad. Sci. U. S. A. 1999;96:8573–8585. doi: 10.1073/pnas.96.15.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder DJ, Trotman HD, Cubells JF, Brasfield J, Tang YL, Walker EF. Catechol-O-methyltransferase modulation of cortisol secretion in psychiatrically at-risk and healthy adolescents. Psychiatr .Genet. 2010;20:166–170. doi: 10.1097/YPG.0b013e32833a1ff3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui-Furukori N, Saito M, Tsuchimine S, Nakagami T, Sato Y, Sugawara N, Kaneko S. Association between dopamine-related polymorphisms and plasma concentrations of prolactin during risperidone treatment in schizophrenic patients. Prog Neuropsychopharmacol Biol. Psychiatry. 2008;32:1491–1495. doi: 10.1016/j.pnpbp.2008.05.006. [DOI] [PubMed] [Google Scholar]