Abstract
Efforts to develop selective agonists for dopamine D1-like receptors led to the discovery of dihydrexidine and doxanthrine, two bioisosteric β-phenyldopamine-type full agonist ligands that display selectivity and potency at D1-like receptors. We report herein an improved methodology for the synthesis of substituted chromanoisoquinolines (doxanthrine derivatives) and the evaluation of several new compounds for their ability to bind to D1- and D2-like receptors. Identical pendant phenyl ring substitutions on the dihydrexidine and doxanthrine templates surprisingly led to different effects on D1-like receptor binding, suggesting important differences between the interactions of these ligands with the D1 receptor. We propose, based on the biological results and molecular modeling studies, that slight conformational differences between the tetralin and chroman-based compounds lead to a shift in the location of the pendant ring substituents within the receptor.
Keywords: dopamine D1receptor, agonist, dihydrexidine, doxanthrine
1. Introduction
Central dopamine neurotransmission has evolved to mediate important sensory processing functions such as novelty detection, attention, memory formation, and coding of rewarding stimuli [1,2]. Executive and volitional functions such as reward seeking, behavioral reinforcement, and motor control are likewise mediated by dopaminergic pathways [3]. Furthermore, dopaminergic systems are involved in the manifestation of CNS pathologies including Parkinson's disease [4], schizophrenia [5],_ENREF_5 and substance abuse [6].
We have been interested for more than two decades in investigating the physiological role of the dopamine D1 receptor by developing selective ligands for the activation of this receptor subtype. Structure-activity relationship studies of dopamine analogs have enabled the recognition of a “β-phenyldopamine pharmacophore” [7,8] as one template for the development of compounds displaying preference for the activation of D1-like receptors over D2-like receptors. This predictive model has enabled the discovery of several molecules that have shown significant potency and selectivity for dopamine D1-like receptors [9-12]. The prototype of this type of compound is dihydrexidine 1, (DHX, Figure 1.) [13], a hexahydrobenzo[a]phenanthridine developed as a conformationally-restricted molecule that incorporates the β-phenyldopamine pharmacophore, and is a potent dopamine D1 full agonist with ten-fold higher affinity for D1-like receptors over D2-like receptors [9]. This compound demonstrated significant therapeutic potential in an in vivo model of Parkinson's disease in African green monkeys [14] and helped to establish the importance of D1 receptor activation in the control of motor function. Dihydrexidine also has been shown to increase blood flow in the prefrontal cortex [15], and improve working memory in aged monkeys [16].
Figure 1.
Structures of β-phenyldopamine-based dopamine D1 receptor agonists.
Given the success that has been achieved with the development of bioisosteres of certain dopamine agonists, and in view of the results of Horn et al. [17], who reported a ten-fold loss of D2 affinity by introduction of an oxygen atom into the structure of the D2 agonist 6,7-ADTN, it was conjectured that a similar modification of the structure of DHX might yield a ligand with enhanced D1-selectivity. This line of reasoning led to the discovery of doxanthrine, (+)-2, (DOX, Figure 1.), a chromanoisoquinoline bioisostere of 1 that displays a greater than 300-fold selectivity for D1-like receptors over D2-like receptors, with higher efficacy than dopamine for activation of the D1 receptor[18].
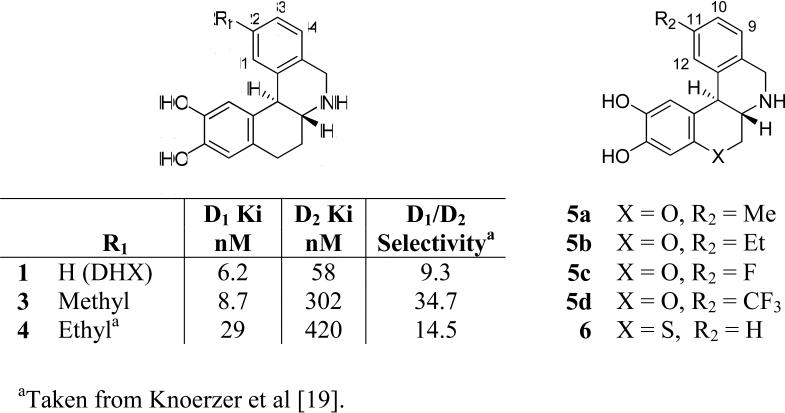
To develop structure-activity relationships of compounds derived from the new chromanoisoquinoline template, we reviewed modifications of the hexahydrobenzo[a]phenanthridine ring system that had resulted in compounds with improved biological properties. In the DHX series, substitution on the β-phenyl ring at the 2-position with methyl and ethyl groups gave ligands with increased D1 selectivity as a result of decreased D2 affinity [19] (Figure 2). We hypothesized that compounds with analogous substitution at the 11-position of 2 would display D1-selectivity greater than the parent compound. We also wished to investigate the effects of electronegative substituents on the β-phenyl ring of doxanthrine, which had not been chemically accessible in the DHX series. To pursue these directions, we prepared compounds bearing methyl, ethyl, fluoro, and trifluoromethyl substituents at the 11-position of the chromanoisoquinoline template (compounds 5a-d, Figure 2) and assessed their affinities at D1 and D2 receptors. In addition, because the chroman oxygen of DOX appears to be instrumental in conferring D1 selectivity to the ligand, we prepared and tested the sulfur-containing thiochromanoisoquinoline 6. The differences in polarity and size of the sulfur atom in relation to the methylene group of DHX and the oxygen atom of DOX will allow a more complete survey of the nature of the receptor residue(s) that are situated in proximity to this important position when the ligand is bound to the D1 receptor. The results of these studies are reported herein.
Figure 2.
Structures and dopamine receptor affinities of substituted hexahydrobenzo[a]phenanthridines and proposed analogues.
2. Results and Discussion
2.1. Chemistry
The synthesis of compounds 5a-d (Scheme 1) was based on a variation of the methodology employed to prepare doxanthrine, 2 [18]. The key step of this synthesis involves a Michael-type addition of an aryl metal reagent to nitrochromene 11 (Scheme 1).
Scheme 1.
Retrosynthetic analysis for the preparation of compounds 5a-d and 6.
Starting from commercially available sesamol 7, aldehyde 10 was prepared in very modest yield by Friedel-Crafts-type formylation using dichloromethyl methyl ether and SnCl4 (Scheme 2). Unfortunately, direct conversion of sesamol into the phenolic benzaldehyde 10 was accompanied by the formation of large amounts of insoluble, deep-blue-colored xanthylium ion 8, which typically precipitated out of the dichloromethane reaction medium.
Scheme 2.
Synthesis of nitrochromene 11 and xanthylium side product 8.
Xanthylium ion8 appears to form from a chloromethoxymethyl intermediate under the formylation conditions, which is sufficiently reactive, particularly in the presence of Lewis acids, to be attacked by the electron rich aromatic ring of another molecule of sesamol. This heterodimerization product is then poised to undergo intramolecular attack by the phenolic hydroxy to yield a hemiacetal, which can aromatize to yield a xanthydrol ether. Aided by the electron donating oxygen atoms that flank the aromatic rings, the xanthydrol ether species will readily eliminate methanol, forming a resonance-stabilized xanthylium ion, 8, which displays coloration and solubility properties consistent with similar compounds reported previously in the literature [20,21].
To avoid formation of this xanthylium side-product, sesamol was acetylated prior to Friedel-Crafts formylation. This simple procedure allowed formation of aldehyde 10 in excellent yield and essentially free from the dimerization by-product. The desired nitrochromene 11 was then obtained in one step by treatment of 10 with nitroethylene generated in situ [22,23]. Extremely slow addition of 2-nitroethanol ensured minimization of base-catalyzed polymerization of nitroethylene, which was nevertheless a significant side reaction. Using this methodology, it was possible to prepare relatively large amounts of 11.
Nitrochromene 11 is known to undergo Michael-type addition of aryl-metal reagents to provide exclusively trans adducts [18]. Thus, we anticipated that an appropriately substituted aryl-metal reagent would allow access to 11-substituted doxanthrine analogs 5a-d. In the original synthesis of DOX 2, a Grignard reagent was employed. That approach, however, suffered severe limitations because of the requirement for ortho/para substituted aryl halides that are not readily available or easily synthesized. We therefore reasoned that para-substituted aryloxazolines 13a-d (Scheme 3), which are easily accessible from commercially available para--substituted benzoic acids, could be ortho-lithiated, and after treatment with nitrochromene 11, would allow exclusive production of the appropriately substituted trans-adducts 14a-d. The oxazoline moiety of these intermediates was anticipated to undergo facile acid hydrolysis to yield the carboxylic acids, which would allow eventual formation of the isoquinoline ring of the final products. Thus, we reasoned that para-substituted aryloxazoline reagents would function as versatile synthons in the preparation of a variety of substituted isoquinolines 17a-d.
Scheme 3.
Synthesis of chromanoisoquinolines 5a-d.
The completion of the synthesis of compounds 5a-d is shown in Scheme 3. Treatment of nitrochromene 11 with organolithium reagents prepared by ortho-lithiation of aryloxazolines 13a-d, gave, as anticipated, exclusively the trans adducts 14a-d. Mild treatment of compounds 14a-d with aqueous HCl gave the amine salts 15a-d as products of partial hydrolysis of the oxazoline ring. Reduction of the nitro groups of these compounds with powdered zinc in acetic acid led to intermediate amine salts that upon basification and gentle heating readily cyclized to form insoluble lactams 16a-d, which crystallized upon formation. These lactams were then reduced by treatment with borane in THF, and the resulting amines 17a-d were demethylated with BCl3 to yield the catecholamine hydrochloride target compounds 5a-d.
To prepare the thiochromanoisoquinoline analog 6 we employed a strategy parallel to the synthesis of 2 [18], but employing the nitrothiochromene 20 as the Michael acceptor for the addition of aryl-Grignard reagent 21. The transformations that led to 6 are shown in Scheme 4.
Scheme 4.
Synthesis of thiochromanoisoquinoline 6.
To prepare nitrothiochromene 20, we relied on the Newman-Kwart rearrangement [24] of phenyl-O-thiocarbamate 18 into phenyl-S-thiocarbamate 19. Thus, we prepared 18 from the previously synthesized sesamaldehyde 10, and heated it in diphenyl ether at 250 ºC for 15 min to effect the rearrangement, obtaining modest yields of 19. Basic hydrolysis of 19 gave a thiophenol that proved to be relatively unstable. By immediate treatment of the hydrolysate mixture with 2-nitroethanol, phthalic anhydride, and dibutylamine, we were able to prepare good quantities of nitrothiochromene 20, isolated as bright orange crystals. The methodology followed in the subsequent steps was basically identical to that employed in the synthesis of 2 [18].
2.2. Pharmacology
The affinities of compounds 5a-d and 6 for dopamine D1-like and D2-like receptors were evaluated in a radioligand displacement assay using porcine striatal tissue preparations. Those results are shown in Table 1. Standard ligands for D1- and D2-like receptors, SCH23390 and chlorpromazine, were included in the assays for comparison.
Table 1.
Binding affinity of new compounds at D1- and D2-like receptors in porcine striatal tissue.
| Binding in porcine striatal homogenatesa | |||
|---|---|---|---|
| Ligand | D1-like Ki (nM) | D2-like Ki (nM) | Binding selectivity (D2-like/D1-like) |
| DOX (2) | 26 ± 4.5 | 1700 ± 190 | 80 ± 19 |
| 11-Me-DOX (5a) | 150 ± 25 | 6800 ± 860 | 55 ± 13 |
| 11-Et-DOX (5b) | 180 ± 34 | 6400 ± 900 | 49 ± 14 |
| 11-F-DOX (5c) | 110 ± 18 | 740 ± 110 | 8.5 ± 2.1 |
| 11-CF3-DOX (5d) | 570 ± 87 | 6900 ± 980 | 14 ± 3.1 |
| 6 | 200 ± 41 | 2500 ± 410 | 15 ± 5.6 |
| SCH23390 | 0.46 ± 0.08 | NA | NA |
| Chlorpromazine | NA | 7.6 ± 2 | NA |
All results shown are the mean ± SEM for 4-11 independent experiments.
3. Conclusions
Competition binding assays using porcine striatal preparations revealed that all four of the 11-substituted analogs 5a-d had significantly lower affinity at D1-like receptors than their structural predecessor 2, DOX. The methyl substituted compound 5a had a nearly six-fold decrease in D1 affinity (D1 Ki = 150 nM) compared to 2 (D1 Ki = 26 nM). Given that introduction of a methyl group at the analogous 2-position of DHX (compound 3) did not significantly reduce the affinity of the ligand in comparison to DHX, the loss of affinity for compound 5a was unexpected. Extension of the hydrophobic bulk to an ethyl group (compound 5b) incurred an even greater loss of D1 affinity (D1 Ki = 185 nM) compared to 2. Although the ethyl substituted analog of DHX (compound 4) also displayed affinity lower than the unsubstituted compound [19], its chroman isostere 5b clearly shows a more dramatic negative effect of the ethyl substituent on affinity.
The disparity between the effects brought about by introduction of identical alkyl substitutions at the 11-position of doxanthrine (2) and the analogous 2-position of dihydrexidine, 1 must reflect critical differences between the interactions of these two isosteric ligands within the D1 dopamine receptor. The D1 binding affinities of 1 and 2 reflect very similar ΔG° values for their interaction with the D1 receptor, revealing that, from a thermodynamic point of view, the net effect of replacing the 8-methylene of 1 with an oxygen atom was insignificant. When one considers the potential for solvation of the chroman oxygen of 2 and its analogs in an aqueous environment, and thus, the desolvation energy that must be overcome upon binding of 2 to the D1 receptor, the similar D1 binding affinities of 1 and 2 can most easily be explained by invoking the formation of energetically favorable interactions between 2 and some element(s) of the D1 binding site that can compensate for the energetically unfavorable desolvation process. Aside from inductive effects on the amine group, it appears likely that the chroman oxygen of DOX and its analogs may be engaged in a specific interaction when bound to the D1 receptor. Replacement of the chroman oxygen of DOX with a sulfur atom resulted in a nearly eight-fold decrease in D1 affinity.
After performing docking, molecular dynamics, and energy minimization of both methyl-substituted derivatives, 3 and 5a, in our previously constructed “activated” model of the D1 receptor [25], a slight conformational difference was observed between the DOX and DHX analogs, with the pendant phenyl ring deviating slightly more from the catechol plane in 5a than in 3. This change forces the DOX analog to lean more into the space occupied by TM6 (and TM7) than the DHX analog, toward residues at the extracellular end of TM6.
It is postulated that these relatively minor conformational differences can be tolerated by the D1 receptor for the parent ligands, DHX (2) and DOX (5), but that they result in differing tolerance for substituents at the 2- or 11-positions of the pendant phenyl rings, respectively. This effect is most evident in the case of the methyl-substituted compounds, where the DHX analogue largely retains its affinity but the DOX-derived structure does not. Unfortunately, our molecular modeling studies have not allowed us to identify what can only be a subtle structural basis for the difference, but very recent mutagenesis experiments of the D1 dopamine receptor in our laboratory have shown that residues in extracellular loop 2 (EL2), particularly L190, differentially affect the affinity of 3 and 5a (to be published elsewhere). Specifically, mutant L190A had significantly increased affinity for 5a, but not for 3, suggesting a reduced steric interaction and/or increased hydrophobic interaction between the 11-alkyl substituent of 5a and the smaller methyl side chain of alanine. Unfortunately, the state of the art in modeling extracellular loops in GPCRs is not very advanced, which may explain our inability to identify ligand-receptor interactions that can account for our results, and reinforcing our observation that the differences in the binding poses of the two series of ligands must be subtle.
Substitution with fluoro and trifluoromethyl groups gave sharp decreases in D1 affinity compared with the unsubstituted ligand 2. The effect was more prominent with the bulkier trifluoromethyl group (5d), which gave a 22-fold decrease in D1 affinity compared to 2, and an almost four-fold decrease compared to the methyl substituted compound 5a. These comparisons suggest that both steric and electronic factors may account for the decreased affinity of compound 5d. In general, electron-withdrawing groups were especially detrimental to affinity, suggesting that aromatic stacking interactions may not be the driving force behind binding in this accessory region [26]. _ENREF_22
In summary, development of a novel methodology for the production of variously substituted trans-fused chromanoisoquinolines has allowed comparison of the receptor-binding properties of bioisosteres and revealed important differences between the ligand/receptor complexes of DHX 1 and DOX 2 with the D1 dopamine receptor. Explanations for the divergent SAR of the two series appear likely to be based on subtle conformational differences caused by the presence of the heteroatom at the 4-position of 2, resulting in differential interactions with residues within the ligand binding site.
4. Experimental
4.1. Chemistry
All reagents were commercially available (Aldrich) and were used without further purification unless otherwise indicated. Dry THF and diethyl ether were obtained by distillation immediately before use from benzophenone-sodium under nitrogen. Column chromatography was carried out using silica gel 60 (230-400 mesh). J.T. Baker flexible thin layer chromatography sheets (silica gel IB2-F) were used to monitor reactions. Melting points were determined using a Thomas-Hoover apparatus and are uncorrected. H1-NMR spectra were recorded using a 300 MHz Bruker ARX-300 NMR spectrometer. Chemical shifts are reported in δ values ppm relative to an internal reference (0.03%, v/v) of tetramethylsilane (TMS) in CDCl3, except where noted. Chemical ionization mass spectra (CIMS) using isobutane as a carrier gas were obtained with a Finnigan 4000 spectrometer. Elemental analyses were performed by the Purdue University Microanalysis Laboratory. All the reactions were carried out under an inert atmosphere of argon.
4.2. Synthesis
4.2.1. 9H-Bis([1,3]dioxolo[4,5-b:4',5'-i])xanthylium chloride (8)
In a dry two-neck flask and under an inert atmosphere, sesamol (50.98 g, 369 mmol) was dissolved in 600 mL of CH2Cl2. Into this flask 52 mL (448 mmol) of SnCl4 were added and the solution was cooled to 0 °C. Cl2CHOCH3 (35 mL, 387.5 mmol) was then added dropwise and the mixture was warmed to room temperature and stirred for 3 h. The mixture was allowed to settle and the solvent was removed by decanting, keeping a positive pressure of nitrogen through one of the flask necks. Into the flask were then added 200 mL of CH2Cl2, and the mixture was vigorously stirred for 20 min. The solvent was again removed by decanting and 200 mL of CH2Cl2 were added. The mixture was filtered using a Buchner funnel and the solids rinsed with CH2Cl2. The collected solids were placed under vacuum to yield 51.8 g (46%) of a blue-green solid. 1H NMR (acetone-d6): δ 5.85 (4H, OCH2O), 6.11 (1H, CH), 6.32 (2H, ArH), 6.45 (2H, ArH); EIMS: m/z 269 (M+, 100); HRMS Calculated for C15H11O6 (xanthylium-H2O solvate) 287.0556, found 287.0166.
4.2.2. 5-Hydroxybenzo[1,3]dioxole acetate (9)
In a two-neck flask fitted with a mechanical stirrer, a suspension of 26 g (1.086 mol) of NaH in 300 mL of dry THF was cooled and stirred on an ice bath. A solution of 75 g (0.543 mol) of sesamol in 300 mL of dry THF was added dropwise over 1 h. After the addition, 205 mL (2.174 mol) of acetic anhydride were added, resulting in a thick suspension. This mixture was stirred for 3 h at RT. The reaction mixture was then cooled to 0 °C and quenched by slow careful addition of 2N HCl. Water (300 mL) was then added and the solution was extracted with CH2Cl2 (3 × 50 mL). These extracts were washed once with 50 mL of water, 50 mL of brine, and were then dried over MgSO4. The mixture was filtered and the solvents removed by rotary evaporation. Kugelrohr distillation at 80 °C and 0.1 torr gave 94.89 g (97%) of the product as a clear oil. This material had properties identical to those reported previously for this compound [27].
4.2.3. 6-Hydroxybenzo[1,3]dioxole-5-carboxaldehyde (10)
In a two-neck flask, 40 g of 9 (0.222 mol) were dissolved in 500 mL CH2Cl2 and the solution cooled to 0 °C. Through a dropping funnel, 52 mL (0.444 mol) of SnCl4 were added dropwise, followed by slow addition of 22 mL (0.244 mol) of Cl2CHOCH3, causing the formation of a precipitate. The reaction was stirred for 2 h and was then poured over ice. The mixture was partitioned and the organic layer was washed with of 2M HCl (3 × 20 mL), and then with water (2 × 50 mL). The organic solution was dried over Na2SO4, filtered, and the solvent removed by rotary evaporation to afford a solid, which was triturated under cold MeOH, filtered, and dried to yield 34.38 g (93.2% ) of 10 as a tan solid: mp 120-121 °C (Lit. [28] mp 125-126). 1H NMR (CDCl3): δ 9.63 (s, 1H, CHO), 6.87 (s, 1H, ArH), 6.47 (s, 1, ArH), 6.02 (s, 2H, ArOCH2O), 1.54 (s, 1H, ArOH). CIMS: m/z 167 (M+H+, 100). Anal. (C8H6O4) C, H.
4.2.4 3-Nitro-2H-6,7-methylenedioxychromene (11)
In a three-necked 1 L flask equipped with a Dean Stark trap, condenser, and mechanical stirring, 34.38 g (0.207 mol) of sesamaldehyde 10 were dissolved in 500 mL of toluene containing 17.5 mL of di-n-butylamine (0.104 mmol) and 61.3 g (0.414 mol) of phthalic anhydride. This mixture was vigorously stirred while heating at reflux. Through a cannula, 32.65 mL (0.455 mol) of 2-nitroethanol were added into the reaction flask over 4.5 days using a syringe pump to maintain a rate of 0.3 mL/hr. The reaction was stirred at reflux for 12 more hours, and then cooled to RT. The contents were filtered through Celite, and the mixture was washed with 2 N NaOH (4 × 300 mL), brine (100 mL), and then dried over MgSO4. After filtration, the organic solution was concentrated under reduced pressure and the crude concentrate was passed through a short column of silica to remove dark polar impurities. The solvent was removed under reduced pressure, and the residual red powder was triturated under 2:1 hexanes/EtOAc. The solid was collected by filtration and dried under vacuum to yield 35.56 g (77.7%) of pure nitrochromene 11 as a bright red powder: mp 139 °C; 1H NMR (CDCl3): δ 5.20 (s, 2H, ArOCH2); 6.02 (s, 2H, OCH2O); 6.49 (s, 1H, ArH); 6.69 (s, 1H, ArH); 7.75 (s, 1H, ArCH). CIMS: m/z 222 (MH+, 100). Anal. (C10H7NO5) C, H, N.
4.2.5. (±)-Trans-4-[2-(4,4-dimethyloxazolin-2-yl)-5-methylphenyl]-6,7-methylenedioxy-3-nitrochroman (14a)
Toluyloxazoline 13a [29] (3.85 g, 20.34 mmol) was dissolved in 40 mL of dry THF and stirred at -45 °C in a dry ice/chlorobenzene bath. Using a syringe, 8.14 mL (20.34 mmol) of a 2.5 M solution of nBuLi in hexanes was slowly added, and the solution turned bright orange. The reaction was stirred for 1 h and was then cannulated into a flask containing 3.0 g (13.56 mmol) of nitrochromene 11 dissolved in 200 mL of dry THF previously cooled to -78° C. This mixture was allowed to warm to room temperature over one hour, and was then quenched with an aqueous solution of saturated NH4Cl. The mixture was extracted with CH2Cl2 (4 × 30 mL), the pooled organic extracts were rinsed with water (50 mL), and then brine (20 mL). The extracts were dried over MgSO4, filtered, and the solvent removed under reduced pressure to yield a dark oil. This oil was dissolved in 40 mL of MeOH, whereupon immediate crystallization occurred. The crystallizing mixture was then kept at 0 °C overnight, filtered, and the collected solids rinsed with cold methanol to yield 3.27 g of pure product as cream-colored crystals. A second crop was obtained from MeOH to yield a total of 3.52 g (65.5%) of the product mp 172-175 °C. 1H-NMR (CDCl3): δ 7.85 (d, 1H, ArH); 7.14 (d, 1H, ArH); 6.79 (s, 1H, ArH); 6.45 (s, 1H, ArH); 6.32 (s, 1H,ArH); 5.92-5.86 (m, 2H, OCH2); 4.21 (br, 1H, ArCHAr); 4.93 (br, 1H, CHNO2); 4.65-4.59 (m, 1H, OCH2); 4.15-4.11 (dd, 1H, Jgem = 11.7 Hz, Jvic = 2.4 Hz); 4.06 (s, 2H, oxazoline CH2); 2.28 (s, 3H, CH3); 1.32-1.3 (2s, 6H, 2CH3). CIMS: m/z 411 (M+H+, 100). Anal. (C22H22N2O6) C, H, N.
4.2.6. (±)-Trans-2,3-methylenedioxy-11-methyl-6a,12b-dihydro-6H-chromeno[3,4-c]isoquinolin-8-one (16a)
One gram of nitro-oxazoline 14a was dissolved in 60 mL THF and 20 mL of a 2N aqueous HCl solution were added. This reaction was stirred for 48 h, and then the solution was concentrated under reduced pressure to one-half of its initial volume. The solution was then extracted with EtOAc (3 × 30 mL), the extracts were washed once with water (10 mL), dried over MgSO4, filtered, and the solvent removed under reduced pressure to yield 940 mg of the hydrochloride salt 15a as a tan solid that was used without further purification. Hydrochloride 15a (2.9 g, 6.237 mmol) was dissolved in 50 mL CH3COOH, 5 g of Zn powder were added, and the mixture was stirred under an inert atmosphere for 3 h. The reaction was filtered through Celite, and the zinc salts on the filter were rinsed with THF. The filtrate was concentrated under reduced pressure and 10 mL of MeOH were added to the residue. This solution was then basified to pH 9 by slow addition of concentrated aqueous ammonia. This mixture was warmed to 50° C and stirred for 1 hr. After cooling, the solid was collected by filtration, washed on the filter with cold MeOH, and dried to yield 1.45 g (67%) of fine, white, cotton-like needles: mp >300 °C (dec). 1H-NMR (CDCl3): δ 7.98 (d, 1H, ArH); 7.46 (s, 1H, ArH); 6.99 (s, 1H, ArH); 6.51 (s, 1H, NH); 6.00-5.96 (2d, 2H, OCH2O); 4.30-4.26 (dd, 1H, OCH2, Jgem = 9.1 Hz, Jvic = 3.6 Hz); 4.21 (d, 1H, ArCHAr, Jtrans = 11.1 Hz); 3.95 (t, 1H, OCH2, Jgem = 9.1 Hz); 3.89-3.86 (dd, 1H, CHN, Jtrans = 11.1 Hz, Jvic = 3.6 Hz); 2.41 (s, 3H, CH3). EIMS: m/z 309 (M+, 100). Anal. (C18H15NO4) C, H, N.
4.2.7. (±)-Trans-2,3-methylenedioxy-11-methyl-6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]-isoquinoline (17a)
Lactam 16a (1.3 g, 4.28 mmol) was suspended in 200 mL of dry THF and stirred at reflux. Into this flask, 21.4 mL (21.4 mmol) of a 1M BH3 solution in THF were added and the reaction was stirred at reflux for 30 h. The mixture was then cooled to 0 °C and water was added carefully to quench the reaction. The mixture was concentrated under reduced pressure to about one-third of its original volume and 100 mL of water were added. The mixture was then extracted with EtOAc (2 × 15 mL) and the extracts dried over MgSO4. The drying agent was then removed by filtration and the filtrate concentrated under reduced pressure to afford a solid that was dissolved in 30 mL of a 2 M solution of HCl in ethanol. This solution was stirred at 70 °C for 40 min and then cooled to 0 °C overnight to induce crystallization. The crystals were collected by filtration to obtain 1.06 g of the HCl salt of 17a. A second batch was obtained by crystallization from EtOH to obtain an additional 0.13 g. The combined crops of this salt were suspended in MeOH, and ammonia was added to pH 9. Water was added, the suspension was extracted with CH2Cl2, the extracts dried over MgSO4, filtered, and concentrated under reduced pressure to yield 1.05 g (85%) of the amine. An analytical sample was obtained by recrystallization from MeOH: mp: 97-99 °C. 1H-NMR (MeOD): δ 7.26-7.23 (m, 2H, 2ArH); 7.16-7.13 (d, 1H, ArH); 6.86 (s, 1H, ArH); 6.48 (s, 1H, ArH); 5.91-5.88 (2d, 2H, OCH2O); 4.46-4.39 (m, 3H, OCH2, CH2N); 4.22 (d, 1H, ArHAr, Jtrans = 11.4 Hz); 4.03 (t 1H, OCH2, Jgem = 10.5 Hz); 2.30 (s, 3H, CH3). EIMS: m/z 295 (M+, 100). Anal. (C18H17NO3) C, H, N.
4.2.8. (±)-Trans-11-methyl-2,3-dihydroxy-6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]isoquinoline hydrobromide (5a)
Amine 17a (300 mg, 1.02 mmol) was dissolved in 15 mL of CH2Cl2 under an inert atmosphere and the solution was cooled to -78 °C. Into this flask, 8.1 mL (8.1 mmol) of 1M BBr3 in CH2CL2 were added through a syringe. The solution was allowed to warm to 0 °C and was stirred for 4 h. To quench the reaction, 10 mL of dry MeOH were added and the solution was stirred for another hour. The solvents were removed under reduced pressure and 10 mL of MeOH were again added. The solvents were then removed to yield a yellow film, which was dissolved in 0.5 mL of isopropanol and stored at -15° C until crystals appeared (60 days). The solvents were then removed under reduced pressure to yield a yellow solid that was triturated under cold EtOH and filtered to provide 221 mg (60%) of the product, pure by NMR: mp: 195-200 °C dec. 1H-NMR (D2O): δ 7.30 (d, 1H, ArH); 7.28 (s, 1H, ArH); 7.22 (d, 1H, ArH); 6.99 (s, 1H, ArH); 6.52 (s, 1H, ArH); 4.56-4.51 (dd, 1H, OCH2, Jgem = 10.2 Hz, Jvic = 4.5 Hz); 4.51-4.42 (2d, 2H, CH2N); 4.22 (d, 1H, ArCHAr, Jtrans = 11.7 Hz); 4.13 (t, 1H, OCH2); 3.24 (dt, 1H, CHN, Jtrans = 11.7 Hz, Jvic = 4.5 Hz). ESIMS: m/z 283 (M+, 100). High res. ESIMS for C17H19NO3 (M+H+): calc. 284.1287, found 284.1289.
4.2.9. 2-(4-Ethylphenyl)-4,4-dimethyloxazoline (13b)
A solution of 2-amino-2-methylpropanol (12.76 mL, 133.1 mmol) in 100 mL CH2Cl2 was cooled to 0 °C. Into this flask, 4-ethylbenzoyl chloride (11.22 g, 66.59 mmol) was introduced dropwise. This mixture was stirred for 4 h, then 10.2 mL (139.8 mmol) of SOCl2 were introduced dropwise. The mixture was allowed to warm to room temperature overnight. The solvents were removed by rotary evaporation, and water (50 mL) was added. The aqueous mixture was washed twice with 15 mL CH2Cl2, and then basified with aqueous ammonia. The cloudy mixture was extracted with CH2Cl2 (3 × 30 mL), and the pooled organic extracts were washed once with 20 mL of water and dried over MgSO4. The mixture was then filtered and the solvents removed under reduced pressure to yield 7.5 g (55%) of oxazoline 13b as a colorless oil. 1H NMR (CDCl3): δ 7.93 (d, 2H, ArH); 7.25 (d, 2H, ArH); 4.18 (s, 2H, OCH2); 2.68 (q, 2H, CH2); 1.43 (s, 6H, 2CH3); 1.22 (t, 3H, CH3).
4.2.10. (±)-Trans-4-[2-(4,4-dimethyl-oxazolin-2-yl)-5-ethylphenyl]-6,7-methylenedioxy-3-nitrochroman (14b)
Under dry nitrogen, 7.50 g (36.89 mmol) of oxazoline 13b were dissolved in 15 mL of dry THF, and cooled to -78 °C in a dry ice/acetone bath. Into this flask, 15 mL (37.5 mmol) of a 2.5M solution of nBuLi in hexanes were introduced through a syringe. The mixture was stirred for 4 h, then a solution of 5.44 g (34.59 mmol) of nitrochromene 11 in 40 mL of dry THF, previously to cooled to -78 °C, was introduced through a cannula. The reaction was removed from the dry-ice bath and stirred for 30 min, and then was quenched with a saturated solution of NH4Cl. The mixture was then extracted with 3 × 30 mL CH2Cl2, washed once with water, once with brine, and dried over MgSO4. The mixture was then filtered and the filtrate was concentrated by rotary evaporation to yield a dark oil. This oil was then dissolved in hot EtOH and left to cool overnight in a freezer. The resulting crystals were collected by filtration and air-dried to yield 5.29 g of the product as yellowish crystals. A second crop provided an additional 0.35 g (50.6% total yield): mp 138° C. 1H NMR (CDCl3): δ 7.85 (dd, 1H, ArH); 7.17 (d, 1H, ArH); 6.80 (s, 1H, ArH); 6.45 (s, 1H, ArH); 6.33 (s, 1H, ArH); 5.92-5.86 (2d, 2H, OCH2O); 5.66 (br, 1H, ArCHAr); 4.95 (br, 1H, CHNO2); 4.66-4.60 (m, 1H, OCH2); 4.14-4.05 (m, 3H, OCH2, oxazoline CH2); 2.60 (q, 2H, ArCH2); 1.29 (2s, 6H, 2CH3); 1.12 (t, 3H, CH3). ESIMS: m/z (rel. intensity) 424 (M+, 100). Anal. (C23H24NO2) C, H, N.
4.2.11. (±)-Trans-2,3-methylenedioxy-11-ethyl-6a,12b-dihydro-6H-chromeno[3,4-c]isoquinolin-8-one (16b)
In a 100 mL flask, 500 mg (1.18 mmol) of nitro-oxazoline 14b were dissolved in 20 mL of THF and 20 mL 2 M HCl and stirred overnight at room temperature. The solution was concentrated under reduced pressure to one-half of its original volume and extracted with EtOAc (4 × 15 mL). The extracts were washed once with brine, dried over MgSO4, filtered, and the solvents removed by rotary evaporation to yield 516 mg of the crude HCl salt 15b as a tan foam. This salt was dissolved in a mixture of 50 mL CH3COOH and 10 mL of THF. Powdered zinc metal (2 g) was added and the suspension was stirred overnight. The mixture was then filtered and the zinc salts on the filter were rinsed with CH3COOH. The filtrate was concentrated by rotary evaporation, the residue was dissolved in 20 mL EtOH, and then basified with ammonia. This mixture was stirred at 50 °C for 4 h and was then cooled overnight. The resulting crystals were collected by filtration, rinsed on the filter with cold EtOH, and then dried under vacuum to yield 0.318 g (83.5%) of lactam 16b as a white fluffy product: mp >240 °C. 1H NMR (CDCl3): δ 8.01 (d, 1H, ArH); 7.47 (s, 1H, ArH); 7.26 (m, 1H, ArH); 6.99 (s, 1H, ArH); 6.87 (s, 1H, NH); 6.51 (s, 1H, ArH); 6.00-5.96 (2d, 2H, OCH2O); 4.35-4.31 (dd, 1H, OCH2, Jgem = 9.1 Hz, Jvic = 3.1 Hz); 4.25-4.21 (d, 1H, ArCHAr, Jtrans = 11.4 Hz); 3.99-3.93 (dd, 1H, OCH2, Jgem = 9.1 Hz, Jvic = 11.1 Hz); 3.91-3.82 (dt, 1H, CHN, Jtrans = 11.1 Hz, Jvic = 3.1 Hz); 2.74-2.67 (q, 2H, ArCH2); 1.29-1.22 (t, 3H, CH3). EIMS: m/z 323 (M+, 100). Anal. (C19H17NO4) C, H, N.
4.2.12. (±)-Trans-2,3-methylenedioxy-11-ethyl-6a,12b-tetrahydro-6H-chromeno[3,4-c]isoquinoline hydrochloride (17b)
In a 500 mL flask, 1.97 g (6.09 mmol) of lactam 16b was suspended in 200 mL of dry THF. Into this suspension, 61 mL (60.87 mmol) of a 1 M solution of BH3 in THF were introduced, and the reaction was stirred and heated at reflux for 48 h. The clear solution was then reduced to about one-third volume by rotary evaporation, cooled to 0 °C, and quenched with water. This mixture was extracted with 3 × 30 mL CH2Cl2, and the organic extracts were washed once with 10 mL water and once with brine. The pooled extracts were then dried over MgSO4, filtered, and the solvents removed by rotary evaporation to yield a viscous oil. This oil was stirred for 4 h at 40 ° C with 25 mL of 2 M HCl in EtOH, whereupon the product crystallized. The crystals were collected by filtration and dried to yield 1.97 g (94%) of HCl salt 17b as a white powder: mp >240 °C. 1H NMR (DMSO-d6): δ 10.07 (br, 2H, +NH2); 7.36 (d, 1H, ArH); 7.22 (br, 2H, 2ArH); 6.98 (s, 1H, ArH); 6.67 (s, 1H, ArH); 6.05 (2d, 2H, OCH2O); 4.49-4.31 (m, 4H, OCH2, CH2N, ArCHAr); 4.16 (dd, 1H, OCH2, Jgem = Jvic = 10.5 Hz); 3.16 (dt, 1H, CHN, Jtrans = Jvic = 10.8 Hz, Jvic2 = 4.5 Hz); 2.61 (q, 2H, ArCH2); 1.16 (t, 3H, CH3). EIMS: m/z 309 (M+, 100). Anal. (C19H19NO3) C, H, N.
4.2.13. (±)-Trans-2,3-dihydroxy-11-ethyl-6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]isoquinoline hydrobromide (5b)
In a 50 mL flask, 150 mg (0.485 mmol) of amine 17b (free base) was dissolved in 10 mL CH2Cl2. Under an inert, dry atmosphere, this solution was cooled to -78 °C and 2 mL (2 mmol) of a 1M solution of BBr3 in CH2Cl2 were added. The flask was then placed in an ice-water bath, stirred overnight at 0 °C, and then 5 mL of dry MeOH were added. The reaction flask was allowed to warm to RT and the solvents were removed by rotary evaporation. Dry MeOH (5 mL) was added to the residue and the solvents were removed under reduced pressure. Again, 5 mL of MeOH were added and the solvents were removed to yield a yellow foam that was pure by NMR, and was crystallized from EtOAc/EtOH to yield 19 mg (12%) of 5b as light yellow powder: mp 188-203 °C dec. 1H NMR (D2O): δ 7.37 (d, 1H, ArH); 7.36 (s, 1H, ArH); 7.31 (d, 1H, ArH); 7.05 (s, 1H, ArH); 6.59 (s, 1H, ArH); 4.61-4.56 (dd, 1H, OCH2, Jvic = 4.2 Hz); 4.55-4.43 (2d, 2H, CH2N); 4.32-4.28 (d, 1H, ArCHAr, Jtrans = 11.4 Hz); 4.22-4.15 (dd, 1H, OCH2, Jgem = Jvic = 10.8 Hz); 3.36-3.26 (dt, 1H, CHN, Jtrans = 11.4 Hz, 4.72-4.52, Jvic = 4.2 Hz); 2.66-2.63 (q, 2H, ArCH2); 1.18 (t, 3H, CH3) ESIMS: m/z 297 (M+, 100). High res. ESIMS for C18H21NO3 (M+H+): calc. 297.1365, found 297.1369.
4.2.14. (±)-Trans-4-[2-(4,4-dimethyloxazolin-2-yl)-5-fluorophenyl]-6,7-methylenedioxy-3-nitrochroman (14c)
Under a dry N2 atmosphere, 1.747 g (9.04 mmol) of oxazoline 13c[30] were dissolved in 15 mL of dry THF, and cooled to -45 °C in a dry ice/chlorobenzene bath. Into this flask, 3.6 mL (9.04 mmol) of a 2M solution of nBuLi in hexanes were introduced through a syringe. The mixture was stirred for 1.25 h and then a solution of 1.0 g (4.52 mmol) of nitrochromene 11 in 40 mL of dry THF, previously cooled to -78 °C, was introduced through a cannula. The reaction was removed from the dry ice bath and stirred for 30 min, and then 20 mL of a saturated solution of NH4Cl were added. The mixture was extracted with CH2Cl2 (3 × 30 mL), washed once with water, and once with brine. The organic extracts were dried over MgSO4, filtered, and the solvents removed by rotary evaporation to yield a crude oil. This oil was then dissolved in hot MeOH, whereupon immediate crystallization occurred. The mixture was cooled, and the crystals were collected and dried to afford 1.22 g of the product as yellowish crystals. A second crop yielded 0.06 g of crystals (68% total yield): mp 183 °C. 1H NMR (CDCl3): δ 7.85 (dd, 1H, ArH); 7.08-7.01 m, 1H, ArH); 6.75 (dd, 1H, ArH); 6.48 (s, 1H, ArH); 6.35 (s, 1H, ArH); 5.95-5.91 (2d, 2H, OCH2O); 5.75 (s, 1H, ArCHAr); 4.98 (br, 1H, CHNO2); 4.72-4.66 (m, 1H, OCH2); 4.10 (dd, 1H, OCH2, Jgem = 12.6 Hz); 4.06 (s, 2H, oxazoline CH2); 1.31 (d, 6H, 2CH3). ESIMS: m/z 415 (M+, 100). Anal. (C21H19FN2O6) C, H, N.
4.2.15. (±)-Trans-2,3-methylenedioxy-11-fluoro-6a,12b-dihydro-6H-chromeno[3,4-c]isoquinolin-8-one (16c)
A solution of 500 mg (1.21 mmol) of oxazoline 14c were dissolved in 10 mL of THF and 10 mL 2M HCl. This solution was stirred for 4 h at 60 °C, allowed to cool, and extracted four times with 15 mL EtOAc. The extracts were washed once with brine. Upon standing, the organic layer produced crystals, which were filtered and rinsed with EtOAc to yield 549 mg (97%) of HCl salt 15c. These crystals were then dissolved in 15 mL CH3COOH, and 0.5 g of powdered zinc metal were added. This suspension was stirred overnight and then filtered, rinsing the zinc salts on the filter with CH3COOH. The filtrate was then concentrated by rotary evaporation. The residue was dissolved in 5 mL EtOH and then basified with ammonia to produce crystallization. This suspension was heated at 50 °C and stirred for 4 h, then cooled and stirred overnight. The crystals were collected by filtration, and rinsed on the filter with EtOAc/EtOH. Drying under vacuum afforded 265 mg (72.3%) of lactam 16c as a white fluffy product: mp >240 °C. 1H NMR (DMSO-d6): δ 7.95 (dd, 1H, ArH); 7.29 (m, 2H, 2ArH); 7.17 (s, 1H, ArH); 6.60 (s, 1H, ArH); 6.02 (d, 2H, OCH2O); 4.31 (dd, 1H, OCH2, Jgem = 9.9 Hz, Jvic = 3.6 Hz); 4.25 (d, 1H, ArCHAr, Jtrans = 11.4 Hz); 3.87 (dd, 1H, Jgem = Jtrans = 10.5 Hz); 3.67 (dt, 1H, CHN, Jtrans = 11.4 Hz, Jvic = 3.9 Hz). EIMS: m/z 313 (M+, 100). Anal. (C17H12FNO4) C, H, N.
4.2.16. (±)-Trans-2,3-methylenedioxy-11-fluoro-6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]isoquinoline (17c)
In a 250 mL flask, 1.3 g (4.86 mmol) of lactam 16c were suspended in 40 mL of dry THF. Into this suspension, 20 mL (20 mmol) of a 1M solution of BH3 in THF were introduced, and the solution was stirred at reflux overnight. The clear solution was then cooled to 0 °C, quenched with water, and reduced to one-third of its volume by rotary evaporation. This mixture was extracted with CH2Cl2 (3 × 15 mL), and the extracts were washed once with 10 mL water and once with brine. This solution was then dried over MgSO4, filtered, and the filtrate was concentrated by rotary evaporation to yield a viscous oil. This oil was stirred for 45 min with 10 mL of a 2 M solution of HCl in EtOH, to produce a white precipitate. The mixture was filtered and the collected solids dried to yield 1.05 g of the HCl salt as a white powder. Water (30 mL) was added to the filtrates and the mixture was washed with 20 mL of ether, basified with NH4OH and extracted with CH2Cl2 (10 mL × 3). The extracts were dried over MgSO4, filtered and the solvent removed under reduced pressure. The residue was then crystallized from cold EtOH to yield an additional 170 mg of the free base as white crystals (88.7% total yield): mp 132-135 °C. 1H NMR (CDCl3): δ 7.21 (dd, 1H, ArH); 7.14 (dd, 1H, ArH); 6.95 (dt, 1H, ArH); 6.89 (s, 1H, ArH); 6.52 (s, 1H, ArH); 5.95 (s, 2H, OCH2O); 4.31 (dd, 1H, OCH2, Jgem = 10.2 Hz, Jvic = 5.1 Hz); 4.05 (s, 2H, ArCH2N); 3.87 (d, 1H, ArCHAr, Jtrans = 10.8 Hz); 3.88 (t, 1H, OCH2, Jvic = 10.2 Hz); 3.03-2.94 (dt, 1H, CHN, Jtrans = 10.8 Hz). EIMS: m/z 299 (M+, 100). High res. EIMS for: C17H15FNO3 calc. 299.2964, found 299.2966.
4.2.17. (±)-Trans-2,3-dihydroxy-11-fluoro-6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]isoquinoline hydrochloride (5c)
In a 50 mL flask, 100 mg (0.334 mmol) of amine 17c was dissolved in 10 mL CH2Cl2. The solution was cooled to -78 °C and 1.33 mL (1.33 mmol) of a 1 M solution of BCl3 in CH2Cl2 were added. The reaction flask was then placed into an ice-water bath, stirred for 6 h, and then 5 mL of dry MeOH were added. The reaction flask was allowed to warm to RT and the solvents were removed by rotary evaporation. Another 5 mL of MeOH were added and the solution was concentrated again by rotary evaporation, leaving a yellow foam that was crystallized from EtOAc/EtOH to yield 31 mg (28%) of 5c as a light yellow powder: mp 181-200 °C dec. 1H NMR (DMSO-d6): δ 10.18 (br, 2, +NH2); 9.43 (s, 1H, OH); 9.10 (s, 1H, OH); 7.77 (t, 1H, ArH); 7.46 (m, 2H, 2ArH); 7.07 (s, 1H, ArH); 6.63 (s, 1H, ArH); 4.72-4.52 (m, 4H, ArCHAr, ArCH2N, OCH2,); 4.32 (t, 1H, OCH2); 3.36 (dt, 1H, CHN, Jtrans = 11.1 Hz). ESIMS: m/z 288 (M+, 100). High res. ESIMS for (M +): C16H16FNO3 calc. 288.1036, found 288.1036.
4.2.18. 2-(4-trifluoromethylphenyl)-4,4-dimethyloxazoline (13d)
This compound was prepared using the methodology of Meyers, et al. [31] and had properties identical to those reported previously [32]. Its NMR spectrum has not been previously reported. 1H NMR (CDCl3): δ 8.03 (d, 2H, ArH); 7.63 (d, 2H, ArH); 4.12 (s, 2H, OCH2); 1.47 (s, 6H, 2CH3); 1.22 (t, 3H, CH3).
4.2.19. (±)-Trans-4-[2-(4,4-dimethyl-oxazolin-2-yl)-5-trifluoromethylphenyl]-6,7-methylenedioxy-3-nitrochroman (14d)
Under a dry atmosphere, 2.20 g (9.04 mmol) of oxazoline 13d were dissolved in 35 mL of dry THF and cooled to -78 °C. Into this flask, 3.8 mL (9.5 mmol) of a 2M solution of nBuLi in hexanes were introduced through a syringe. The mixture was stirred for 1 hr, at which point a solution of 1 g (4.52 mmol) of nitrochromene 11 in 150 mL of dry THF, previously cooled to -78 °C, was introduced through a cannula. The reaction was removed from the dry-ice bath and stirred for 30 min, and was then quenched by adding 10 mL of a saturated solution of NH4Cl. The solution was then extracted with 3 × 50 mL CH2Cl2, the extracts washed once with water, once with brine, dried over MgSO4, filtered, and the filtrate was then concentrated by rotary evaporation to yield a crude oil. This oil was dissolved in MeOH and cooled to RT overnight. The resulting crystals were collected by filtration and dried to yield 1.45 g (69%) of the product as yellow crystals: mp 176° C. 1H NMR (CDCl3): δ 8.07 (d, 1H, ArH); 7.63 (d, 1H, ArH); 7.30 (s, 1H, ArH); 6.50 (s, 1H, ArH); 6.30 (s, 1H, ArH); 5.96-5.91 (2d, 2H, OCH2O); 5.76 (s, 1H, ArCHAr); 4.96 (br, 1H, CHNO2); 4.72-4.62 (m, 1H, OCH2); 4.12-4.08 (s, m, 3H, oxazoline CH2, OCH2); 1.39-1.33 (m, 6H, 2CH3). ESIMS: m/z 464 (M+, 100). Anal. (C22H19F3N2O6) C, H, N.
4.2.20. (±)-Trans-2,3-methylenedioxy-11-trifluoromethyl-6a,12b-dihydro-6H-chromeno[3,4-c]-isoquinolin-8-one (16d)
In a 250 mL flask, 2.57 g (5.56 mmol) of oxazoline 14d were dissolved in a mixture of 40 mL of THF and 40 mL 2M HCl. This solution was stirred overnight, 100 mL of water were added, and the mixture was then extracted with 4 × 30 mL CH2Cl2. The pooled organic extracts were washed twice with 10 mL of water and dried over MgSO4. The mixture was filtered, and the filtrate was concentrated by rotary evaporation to afford 2.57 g (89.5%) of a yellow foam that could be crystallized from iPrOH, but was used without further purification. In a 250 mL flask, 2.79 g of HCl salt 15d were dissolved in 150 mL of CH3COOH. Powdered Zn (1 g) was added and the suspension was stirred overnight. The reaction was filtered, the solids on the filter were rinsed with CH3COOH, and the filtrate was reduced to dryness under vacuum. The residue was dissolved in 15 mL EtOH, and ammonia was added to basify (pH 10) the mixture. This solution was cooled to 0 °C overnight. The resulting solid was collected by filtration, washed on the filter with cold EtOH, and then dried under vacuum to yield 1.77 g (90.6%) of lactam 16d as a white, fluffy product: mp >240° C. 1H NMR (DMSO-d6): δ 8.85 (s, 1, NH); 8.10 (d, 1H, ArH); 7.82 (d, 1H, ArH); 7.79 (s, 1H, ArH); 7.19 (s, 1H, ArH); 6.63 (s, 1H, ArH); 6.04 (2d, 2H, OCH2O, Jgem = 14.1 Hz); 4.33 (d, 1H, ArCHAr, Jtrans = 11.7 Hz); 4.32 (dd, 1H, OCH2, Jgem = 10.2 Hz, Jvic = 3.6 Hz); 3.89 (t, 1H, OCH2, Jgem = 10.2 Hz), 3.73 (dt, 1H, NCH, Jtrans = 11.7 Hz, Jvic = 3.6 Hz). ESIMS: m/z 363 (M+, 100). Anal. (C18H12F3NO4) C, H, N.
4.2.21. (±)-Trans-2,3-methylenedioxy-11-trifluoromethyl-6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]isoquinoline hydrochloride (17d)
In a 250 mL flask, 1.765 g (4.86 mmol) of lactam 16d were suspended in 50 mL of dry THF. Into this suspension, 24.3 mL (24.3 mmol) of a 1 M solution of BH3 in THF were introduced, and the reaction was heated at reflux and stirred overnight. The clear solution was then reduced to about one-third of its volume by rotary evaporation, cooled to 0 °C, and diluted with H2O. This mixture was extracted with 3 × 25 mL CH2Cl2, and the extracts were washed once with 10 mL water and once with brine. This solution was then dried over MgSO4, filtered, and the solvents removed by rotary evaporation to yield a viscous oil. A 2M solution of HCl in EtOH (10 mL) was added to this oil, and the mixture was stirred for 45 min to produce, after 10 min, a white precipitate. The suspension was cooled to 0 °C for 2 h and filtered. The solid on the filter was rinsed with cold EtOH and dried to afford 1.57 g (84%) of the HCl salt as a white powder: mp >240° C. 1H NMR (CDCl3): δ 7.78 (d, 1, ArH); 7.71 (d, 2H, ArH); 7.63 (s, 1H, ArH); 7.02 (s, 1H, ArH); 6.69 (s, 1H, ArH); 6.05 (2d, 2H, OCH2O); 4.61-4.44 (m, 4H, OCH2, NCH2, ArCHAr); 4.18 (t, 1H, OCH2); 3.26 (dt, 1H, J1 = 10.8 Hz, J2 = 3.9 Hz). ESIMS: m/z 349 (M+, 100). Anal. (C18H14F3NO3) C, H, N.
4.2.22. (±)-Trans-2,3-dihydroxy-11-trifluoromethyl-6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]isoquinoline hydrochloride (5d)
In a 50 mL flask, 702 mg (2.01 mmol) of amine 17d was dissolved in 20 mL CH2Cl2 under a dry N2 atmosphere. The flask was cooled to -78 °C and 8 mL (8 mmol) of a 1 M solution of BCl3 in CH2Cl2 were added. The reaction flask was then placed in an ice-water bath, and stirred for 4.5 h, at which time 4 mL of dry MeOH were added. The reaction was allowed to warm to RT and the solvents were removed by rotary evaporation. Addition of 5 mL of dry MeOH and concentration by rotary evaporation was repeated twice more, to leave a tan-orange foam, which was crystallized from CH3CN/MeOH to afford a light orange powder: mp >187 °C dec. 1H NMR (DMSO-d6): δ 10.03 (br, 2, +NH2); 9.25 (s, 1H, OH); 8.92 (s, 1H, OH); 7.77 (d, 1H, ArH); 7.71 (s, 1H, ArH); 7.69 (d, 1H, ArH); 6.81 (s, 1H, ArH); 6.41 (s, 1H, ArH); 4.59-4.45 (2d, 2H, ArCH2N, Jgem = 15.1 Hz); 4.18 (t, 1H, OCH2); 3.26 (dt, 1, CHN, Jtrans = 10.8 Hz, Jvic = 3.6 Hz). ESIMS: m/z (rel. intensity) 337 (M+, 100). High res. ESIMS for C17H16F3NO3 (M+H+): calc. 338.1004, found 338.1000.
4.2.23. 6-(O-Dimethylthiocarbamoyl)-1,3-benzodioxole (18)
Under N2, NaH (0.32 g, 13.25 mmol) was slurried with stirring in 20 mL of dry THF at -78 °C. A solution of sesamaldehyde 10 (2 g, 12.05 mmol) dissolved in 50 mL of dry THF was then added dropwise to the suspension, followed by a solution of 1.638 g (13.25 mmol) of dimethylthiocarbamoyl chloride dissolved in 15 mL of dry THF. The reaction was allowed to warm to RT, was stirred for 12 h, and was then carefully quenched with water and extracted with CH2Cl2 (3 × 15 mL). The pooled extracts were washed with brine, dried over MgSO4, filtered, and the solvent removed under reduced pressure to afford an orange solid, which was recrystallized from EtOH to yield 2.30 g (75%) of pure thiocarbamate 18 as light-brown flakes: mp 137-140 °C. 1H NMR (CDCl3): δ 9.89 (s, 1H, CHO); 7.31 (s, 1H, ArH); 6.60 (s, 1H, ArH); 6.10 (s, 2H, OCH2O); 3.48 (s, 3H, CH3); 3.41 (s, 3H, CH3). CIMS: 254 (M+H+, 100). Anal. (C11H11NO4S) C, H, N.
4.2.24. 6-(S-Dimethylthiocarbamoyl)-1,3-benzodioxole (19)
In a single-necked flask attached to a condenser, and under an inert atmosphere, 2.27 g (8.96 mmol) of thiocarbamate 18 were dissolved in 35 mL of diphenyl ether. This solution was heated at 250 °C for 15 min and then immediately cooled to RT with an ice bath. Hexanes (100 mL) was added to the solution, which was stored overnight at 0 °C to cause precipitation. The solid was collected by filtration, washed repeatedly on the filter with hexanes, and purified by column chromatography to yield 1.27 g (56%) of the product as a brown solid. This compound was used without further characterization.
4.2.25. 3-Nitro-2H-6,7-methylenedioxythiochromene (20)
In a single-neck flask, 2.96 g of thiocarbamate 19 (11.70 mmol) were dissolved in 400 of mL of MeOH and heated to 60 °C. Next, 35 mL of a 2M solution of NaOH in MeOH were added, and the solution was heated at reflux for 2.5 h until TLC indicated complete hydrolysis. The mixture was acidified to pH 7 with 2 M HCl in EtOH. The solvents were then removed under reduced pressure, avoiding exposure to air. Toluene (400 mL) was added to the mixture, followed by 1 mL (5.85 mmol) of dibutylamine, 3.46 g (23.39 mmol) of phthalic anhydride, and 0.3 mL (4.19 mmol) of 2-nitroethanol. This mixture was heated at reflux for 3 h, at which time addition of 2-nitroethanol was begun at a rate of about 1 drop every 15 minutes, for a total of 1.6 mL (22.33 mmol) of the reagent added over 8 h. The reaction was stirred at reflux overnight and was then cooled and filtered through Celite. The filtrate was extracted several times with 2 M NaOH, washed once with water, dried over Na2SO4, filtered, and reduced to dryness by rotary evaporation. The crude nitrochromene was purified by column chromatography to yield 1.78 g (64%) of a bright red powder: mp: 128-131 °C. 1H NMR (CDCl3): δ 7.82 (s, 1H, ArCH); 6.82 (s, 1H, ArH); 6.81 (s, 1H, ArH); 6.03 (s, 2H, OCH2O); 4.07 (s, 2H, OCH2). EIMS: 237 (M+, 100). High res. EIMS for C10H8NO4S (M+): calc. 237.0096, found 237.0099.
4.2.26. (±)-Trans-2,3-methylenedioxy-6a,12b-dihydro-6H-thiochromeno[3,4-c]isoquinoline (22)
In a dry flask, 2.99 g (12.30 mmol) of 2-(2-bromophenyl)-1,3-dioxane were dissolved in 25 mL of dry THF and 597 mg (26.61 mmol) of magnesium powder were added, along with 1 drop of 1,2-dibromoethane. This solution was heated at reflux and stirred for 1 h until formation of the Grignard reagent was complete. The mixture was then cooled to 0 °C and, through a dropping funnel, a solution of 972 mg (4.10 mmol) of nitrothiochromene 20 in 35 mL of dry THF were added dropwise over 0.5 h. The reaction was stirred for 10 min and was then quenched with a conc solution of NH4Cl. The mixture was then filtered and extracted several times with EtOAc. The combined organic extracts were washed once with water, once with brine, dried over MgSO4, and filtered. The filtrate was reduced to dryness to yield an oil that was purified using silica gel flash column chromatography, eluting with a 4:1 mixture of hexanes:EtOAc to provide 867 mg (52.7%) of pure 22 as a white powder: mp 154 °C. 1H NMR (CDCl3): δ 7.22 (m, 1H, ArH); 7.27-7.24 (m, 2H, ArH); 6.60 (s, 1H, ArH); 6.48 (s, 1H, ArH); 5.85 (s, 2H, OCH2O); 5.80 (d, 1H, ArCHAr, J = 3.3 Hz); 5.62 (s, 1H, OCHO); 5.28-5.25 (m, 1H, CHNO2); 4.27-4.18 (m, 2H, OCH2C); 3.98-3.90 (m, 2H, OCH2C); 3.43 (m, 2H, SCH2); 2.26-2.20 (m, 1H, CCH2C); 1.40 (bd, 1H, CCH2C). EIMS: 401 (M+, 100). Anal. (C20H19NO6S) C, H, N.
4.2.27. (±)-Trans-2,3-methylenedioxy-6a,7,8,12b-tetrahydro-6H-thiochromeno[3,4-c]isoquinoline (24)
Into a solution of 550 mg (1.37 mmol) of compound 22 in 10 mL of THF and 30 mL CH3COOH were added 2 g of powdered zinc. This suspension was vigorously stirred for 6 h at 70 °C and was then cooled to RT, filtered, and the filtrate was concentrated under reduced pressure. To the residue was added 30 mL of 2 M ethanolic HCl and this solution was stirred for 30 min. The solvents were removed once again and 30 mL of a 2 M solution of NaOH and 30 mL of CH2Cl2 were added. This biphasic mixture was stirred overnight. The organic layer was then separated, washed with water, dried over Na2SO4, filtered, and the solvent removed to leave 418 mg of a light brown solid (23), which was used without further purification.
To a solution of 400 mg of the crude imine 23 (1.356 mmol) in 10 mL of dry THF and 30 mL of EtOH was added 85 mg of NaCNBH3 (1.36 mmol) followed by dropwise addition of 4 mL of a 2M solution of HCl in EtOH over 10 minutes. The suspension was stirred for 4 h and was then reduced under vacuum to about one-third of its original volume. Into this suspension, 10 mL of a 2M solution of NaOH were added and the mixture was extracted with CH2Cl2 (3 × 10 mL). The pooled organic extracts were washed once with water, dried over Na2SO4, filtered, and the solvent was removed. The resulting residue was purified by column chromatography, eluting with EtOAc to yield 316 mg (78%) of amine 24 as a tan solid. An analytical sample was prepared by crystallizing the HCl from EtOH/Et2O: mp >260 °C dec. 1H NMR (DMSO-d6): δ 10.11 (bs, 1H, +NH); 9.69 (bs, 1H, +NH); 7.49-7.40 (m, 4H, ArH); 7.07 (s, 1H, ArH); 6.48 (s, 1H, ArH); 6.00 (d, 2H, OCH2O); 4.41 (bs, 2H, ArCH2N); 4.27 (d, 1H, ArCHAr); 3.45-3.42 (m, 2H, SCH2, CHN); 3.11 (t, 1H, SCH2). High res. ESIMS for C17H16NO2S (M+H+): calc. 298.0902, found 298.0904.
4.2.28. (±)-2,3-Dihydroxy-6a,7,8,12b-tetrahydro-6H-thiochromeno[3,4-c]isoquinoline hydro-chloride (6)
A solution of 300 mg (1.01 mmol) of amine 24 in 8 mL of CH2Cl2 was placed into a flask, cooled to -78 °C, and 4 mL (4.0 mmol) of a 1M solution of BBr3 in CH2Cl2 were added through a syringe. This solution was allowed to warm to 0 °C in an ice bath and was stirred for 3 h. Dry EtOH (5 mL) was then added to quench the reaction, and the solution was stirred for 30 min at RT. The solvents were removed by rotary evaporation, and 5 mL of dry EtOH were again added. Removal of the solvent yielded a white powder that was collected by filtration to afford a total of 104 mg (34%) of pure catecholamine 6 as a tan powder, in two crops: mp 288-294 °C dec. 1H NMR (DMSO-d6): δ 10.03 (bs, 2H, +NH2); 9.18 (s, 1H, ArOH); 9.04 (s, 1H, ArOH); 7.49-7.38 (m, 4H, 4ArH); 6.79 (s, 1H, ArH); 6.49 (s, 1H, ArH); 4.43 (t, 2H, ArCH2N); 4.26-4.22 (d, 1H, ArCHAr, Jtrans = 10.5 Hz); 3.45-3.35 (m, 1H, NCH2); 3.28-3.19 (m, 1H, SCH2); 3.10-3.03 (t, 1H, SCH2, J = 10.5 Hz). ESIMS: 286 (M+H+, 100). Anal. (C16H16ClNO2S) C, H, N.
4.3. Pharmacology Methods
4.3.1. Materials
[3H]spiperone (95 Ci/mmol) and [3H]SCH-23390 (81 Ci/mmol) were purchased from Amersham Biosciences (Piscataway, NJ, USA). [3H]Cyclic AMP (30 Ci/mmol) was purchased from PerkinElmer (Boston, MA, USA). Chlorpromazine, SCH-23390, butaclamol, and most other reagents were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA).
4.3.2. Competition Binding Experiments
Porcine striatal tissue was obtained fresh from the Purdue Butcher Block and prepared as previously described [18]. Briefly, striatal tissue was homogenized using a potter-type homogenizer, suspended in homogenization buffer (20 mM Hepes, 0.32 M sucrose, pH 7.4), and centrifuged at 1,000 × g for 10 minutes at 4 °C. The pellet (P1) was discarded and the supernatant was centrifuged at 30,000 × g for 10 min at 4 °C. The resulting pellet (P2) was resuspended in 50 mM Tris buffer (pH 7.4) by briefly using a Kinematica homogenizer, and then centrifuged at 30,000 × g for 30 min at 4 °C. This pellet was resuspended again in 50 mM Tris buffer, dispensed into 1 ml aliquots, and centrifuged for 10 min. at 13,000 × g and 4 °C. A BCA protein assay was used to determine the final protein concentration of the pellets. Supernatant was aspirated and pellets were frozen at -80° C until use.
Radioligand binding assays were performed as previously described [18], with only minor modifications. Pellets were resuspended (1 mL/mg) in receptor binding buffer (50 mM Hepes, 4 mM MgCl2, pH 7.4) and 75 μg protein was used per assay tube. Receptor isotherms were performed with [3H]SCH-23390 and [3H]spiperone to determine Bmax and Kd for D1-like and D2-like receptor sites, respectively (760 fmol/mg and 0.44 nM for [3H]SCH-23390, and 250 fmol/mg and 0.075 nM for [3H]spiperone). All D2-like receptor binding assays were performed in the presence of 50 nM ketanserin to mask 5-HT2A sites. Nonspecific binding was defined with 5 μM butaclamol. Drug dilutions for competitive binding experiments were made in receptor binding buffer and added to assay tubes containing 75 μg protein and either 1 nM [3H]SCH-23390 or 0.15 nM [3H]spiperone. All binding experiments were incubated for 30 min at 37 °C, and were terminated by filtration with ice cold wash buffer using a 96-well Packard Filtermate cell harvester. After the samples were dried, 30 μL of Packard Microscint O was added to each well. Radioactivity was counted using a Packard Topcount scintillation counter.
A versatile new synthesis for substituted chromanoisoquinolines
Parallel substitutions of two bioisosteric ligands give differing affinities
Very closely-related structures must bind differently to the D1 dopamine receptor
Acknowledgments
This work was supported by NIH grants MH MH42705 (DEN), MH60973 (VJW), and GM085604 (MAL)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 2.Wooten GF, Trugman JM. The dopamine motor system. Mov. Disord. 1989;4(Suppl 1):S38–S47. doi: 10.1002/mds.870040506. [DOI] [PubMed] [Google Scholar]
- 3.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr. Opin. Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 4.Mailman R, Huang X, Nichols DE. Parkinson's disease and D1 dopamine receptors. Curr. Opin. Investig. Drugs. 2001;2:1582–1591. [PubMed] [Google Scholar]
- 5.Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr. Bull. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 7.Riggs RM, McKenzie AT, Byrn SR, Nichols DE, Foreman MM, Truex LL. Effect of beta-alkyl substitution on D-1 dopamine agonist activity: absolute configuration of beta-methyldopamine. J. Med. Chem. 1987;30:1914–1918. doi: 10.1021/jm00393a039. [DOI] [PubMed] [Google Scholar]
- 8.Zhang A, Neumeyer JL, Baldessarini RJ. Recent Progress in Development of Dopamine Receptor Subtype-Selective Agents: Potential Therapeutics for Neurological and Psychiatric Disorders. Chem. Rev. 2007;107:274–302. doi: 10.1021/cr050263h. [DOI] [PubMed] [Google Scholar]
- 9.Brewster WK, Nichols DE, Riggs RM, Mottola DM, Lovenberg TW, Lewis MH, Mailman RB. trans-10,11-dihydroxy-5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridine: a highly potent selective dopamine D1 full agonist. J. Med. Chem. 1990;33:1756–1764. doi: 10.1021/jm00168a034. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh D, Snyder SE, Watts VJ, Mailman RB, Nichols DE. 9-Dihydroxy-2,3,7,11b-tetrahydro-1H-naph[1,2,3-de]isoquinoline: a potent full dopamine D1 agonist containing a rigid-beta-phenyldopamine pharmacophore. J. Med. Chem. 1996;39:549–555. doi: 10.1021/jm950707+. [DOI] [PubMed] [Google Scholar]
- 11.Grubbs RA, Lewis MM, Owens-Vance C, Gay EA, Jassen AK, Mailman RB, Nichols DE. 8,9-dihydroxy-1,2,3,11b-tetrahydrochromeno[4,3,2,-de]isoquinoline (dinoxyline), a high affinity and potent agonist at all dopamine receptor isoforms. Bioorg. Med. Chem. 2004;12:1403–1412. doi: 10.1016/j.bmc.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Michaelides MR, Hong Y, DiDomenico S, Jr, Asin KE, Britton DR, Lin CW, Williams M, Shiosaki K. (5aR,11bS)-4,5,5a,6,7,11b-hexahydro-2-propyl-3-thia-5-azacyclopent-1-ena[c]-phenanthrene-9,10-diol (A-86929): a potent and selective dopamine D1 agonist that maintains behavioral efficacy following repeated administration and characterization of its diacetyl prodrug (ABT-431) J. Med. Chem. 1995;38:3445–3447. doi: 10.1021/jm00018a002. [DOI] [PubMed] [Google Scholar]
- 13.Salmi P, Isacson R, Kull B. Dihydrexidine--the first full dopamine D1 receptor agonist. CNS. Drug Rev. 2004;10:230–242. doi: 10.1111/j.1527-3458.2004.tb00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor JR, Lawrence MS, Redmond DE, Jr., Elsworth JD, Roth RH, Nichols DE, Mailman RB. Dihydrexidine, a full dopamine D1 agonist, reduces MPTP-induced parkinsonism in monkeys. Eur. J. Pharmacol. 1991;199:389–391. doi: 10.1016/0014-2999(91)90508-n. [DOI] [PubMed] [Google Scholar]
- 15.Mu Q, Johnson K, Morgan PS, Grenesko EL, Molnar CE, Anderson B, Nahas Z, Kozel FA, Kose S, Knable M, Fernandes P, Nichols DE, Mailman RB, George MS. A single 20 mg dose of the full D1 dopamine agonist dihydrexidine (DAR-0100) increases prefrontal perfusion in schizophrenia. Schizophr. Res. 2007;94:332–341. doi: 10.1016/j.schres.2007.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- 17.Horn AS, Grol CJ, Dijkstra D, Mulder AH. Facile syntheses of potent dopaminergic agonists and their effect on neurotransmitter release. J. Med. Chem. 1978;21:825–828. doi: 10.1021/jm00206a023. [DOI] [PubMed] [Google Scholar]
- 18.Cueva JP, Giorgioni G, Grubbs RA, Chemel BR, Watts VJ, Nichols DE. trans-2,3-dihydroxy-6a,7,8,12b-tetrahydro-6H-chromeno[3,4-c]isoquinoline: synthesis, resolution, and preliminary pharmacological characterization of a new dopamine D1 receptor full agonist. J. Med. Chem. 2006;49:6848–6857. doi: 10.1021/jm0604979. [DOI] [PubMed] [Google Scholar]
- 19.Knoerzer TA, Watts VJ, Nichols DE, Mailman RB. Synthesis and biological evaluation of a series of substituted benzo[a]phenanthridines as agonists at D1 and D2 dopamine receptors. J. Med. Chem. 1995;38:3062–3070. doi: 10.1021/jm00016a009. [DOI] [PubMed] [Google Scholar]
- 20.McClelland RA, Banait N, Steenken S. Electrophilic reactions of xanthylium carbocations produced by flash-photolysis of 9-xanthenols. J. Am. Chem. Soc. 1989;111:2929–2935. [Google Scholar]
- 21.Millls OS, Mooney NJ, Robinson PM, Watt CIF, Box BG. Preparation and properties of some crown-ethers incorporating stable carbocations. J. Chem. Soc. Perk. T. 1995;2:697–706. [Google Scholar]
- 22.Alneirabeyeh M, Reynaud D, Podona T, Ou L, Perdicakis C, Coudert G, Guillaumet G, Pichat L, Gharib A, Sarda N. Methoxy and hydroxy derivatives of 3,4-dihydro-3-(di-normal-propylamino)-2H-1-benzopyrans - new synthesis and dopaminergic activity. Eur. J. Med. Chem. 1991;26:497–504. [Google Scholar]
- 23.Dauzone D, Royer R. A convenient one-pot synthesis of 2-unsubstituted 3-nitro-2H-chromenes. Synthesis-Stuttgart. 1984 [Google Scholar]
- 24.Beaulieu F, Snieckus V. Directed ortho-metalation of O-aryl and O-pyridyl thiocarbamates - a versatile synthetic method for substituted phenol into thiophenol conversion. Synthesis-Stuttgart. 1992:112–118. [Google Scholar]
- 25.Bonner LA, Laban U, Chemel BR, Juncosa JI, Lill MA, Watts VJ, Nichols DE. Mapping the catechol binding site in dopamine D(1) receptors: synthesis and evaluation of two parallel series of bicyclic dopamine analogues. ChemMedChem. 2011 doi: 10.1002/cmdc.201100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter CA, Lawson KR, Perkins J, Urch CJ. Aromatic interactions. J. Chem. Soc. Perk. T. 2001;2:651–669. [Google Scholar]
- 27.Pirrung MC, Fallon L, Zhu J, Lee YR. Photochemically removable silyl protecting groups. J. Am. Chem. Soc. 2001:3638–3643. doi: 10.1021/ja002370t. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez de Peredo A, Leonce S, Monneret C, Dauzone D. Synthesis and biological evaluation of flavonones and flavones related to podophyllotoxin. Chem. Pharm. Bull. 1998;46:79–83. doi: 10.1248/cpb.46.79. [DOI] [PubMed] [Google Scholar]
- 29.Mallory FB, Butler KE, Evans AC, Brondyke EJ, Mallory CW, Yang C, Ellenstein A. Phenacenes: a family of graphite ribbons. 2. Syntheses of some [7]phenacenes and an [11]phenacene by stilbene-like photocyclizations. J. Am. Chem. Soc. 1997;119:2119–2124. [Google Scholar]
- 30.Newman MS, Kannan R. Syntheses of 8-fluorobenzo[a]pyrenes and 9-fluorobenzo[a]pyrenes and 9-fluoro-7,12-dimethybenz[a]anthracenes and 10-fluoro-7,12-dimethylbenz[a]anthracenes. J. Org. Chem. 1979;44:3388–3390. [Google Scholar]
- 31.Meyers AI, Mihelich ED, Nolen RL. Oxazolines. X. Synthesis of gamma-butyrolactones. J. Org. Chem. 1974;39:2783–2787. [Google Scholar]
- 32.Newman MS, Veeraraghavan S. Syntheses of 9-(trifluoromethyl)- and 10-(trifluoromethyl)-7,12-dimethylbenz[a]anthracenes. J. Org. Chem. 1983;48:3246–3248. [Google Scholar]