Abstract
Introduction
The G-protein coupled muscarinic acetylcholine receptors, widely expressed in the CNS, have been implicated in Fragile × Syndrome (F×S). Recent studies have reported an overactive signaling through the muscarinic receptors in the Fmr1KO mouse model. Hence, it was hypothesized that reducing muscarinic signaling might modulate behavioral phenotypes in the Fmr1KO mice. Pharmacological studies from our lab have provided evidence for this hypothesis, with subtype-preferring muscarinic M1 and M4 receptor antagonists modulating select behaviors in the Fmr1KO mice. Since the pharmacological antagonists were not highly specific, we investigated the specific role of M4 receptors in the Fmr1KO mouse model, using a genetic approach.
Methods
We created a double mutant heterozygous for the M4 receptor gene and hemizygous for the Fmr1 gene and examined the mutants on various behaviors. Each animal was tested on a behavior battery comprising of open-field activity (activity), light-dark (anxiety), marble burying (perseverative behavior), prepulse inhibition (sensorimotor gating), rotarod (motor coordination), passive avoidance (learning and memory) and hotplate (analgesia). Animals were also tested on the audiogenic seizure protocol and testis weights were measured.
Results
Reduction of M4 receptor expression in the heterozygotes completely rescued the analgesic response and partly rescued the acoustic startle response phenotype in the Fmr1KO mice. However, no modulation was observed in a number of behaviors including learning and memory, activity, perseverative behavior and audiogenic seizures.
Conclusion
Reducing M4 receptor signaling altered only select behavioral phenotypes in the Fmr1KO mouse model, suggesting that other targets are involved in the modulation of fragile × behaviors.
Keywords: Fragile × syndrome, muscarinic, M4 receptors, analgesic response, behavior, sensorimotor gating
1. Introduction
Fragile × syndrome (F×S) is the most common inherited cause of intellectual disability and in individuals with the full mutation (i.e. those with over 200 CGG repeats in the 5’ untranslated region of the Fmr1 gene) a wide range of behavioral abnormalities are manifested including hyperactivity, obsessive-compulsive behavior, aggression, self-injurious behavior, alterations in sensorimotor gating, cognitive impairments, abnormal social behavior, and autism [1]. An Fmr1KO mouse model was created [2] that recapitulates some of the phenotypes seen in patients including hyperactivity, altered social interaction, susceptibility to audiogenic seizures and performance impairments on some assays of learning and memory [3–11]. Recent studies have elucidated a potential role for the muscarinic cholinergic receptors (mAChR) in F×S [12,13]. The muscarinic agonist, carbachol has been shown to cause alterations in the synaptic response in the subiculum of the Fmr1KO mice [12]. Also, enhanced carbachol M1-mediated LTD has been reported in the Fmr1KO mice [13]. Increased expression of the downstream targets of FMRP, such as EF1a and CaMKII, following muscarinic activation by carbachol was observed in WT but not in the Fmr1KO mice, indicating the presence of an overactive muscarinic signaling in the KO mice [13]. Of the five molecularly distinct mAChR subtypes (M1–M5) [14] expressed throughout the brain, M1 and M4 receptors show high expression in the brain with distinct patterns. The M4 receptor has been shown to be predominantly expressed in the striatum, which is part of the extrapyramidal network involved in the coordination of movement and also in the cortex, hippocampus and basal ganglia [15–21]. Data from M4 knockout mice [19] suggested a role for the M4 receptors in regulating sensorimotor gating [22], cognitive processes [23], locomotor activity [19] and analgesic response [24], all of which were altered in the Fmr1KO mouse model [2,3,5,6,25–28]. Based on the findings from a variety of sources (e.g. electrophysiological, biochemical, and behavioral) we hypothesized that reduction of M4 activity might modulate F×S behavior. Recently, our lab reported that the M4 receptor-preferring antagonist tropicamide modulated perseverative behavior, learning and memory and audiogenic seizures (AGS) in WT and Fmr1KO mice [29]. In a separate study we also reported that the M1 receptor-preferring antagonist dicyclomine modulated select fragile × phenotypes including perseverative behavior and AGS in the Fmr1KO mouse model [30]. Taken together these recent pharmacological reports strengthen the hypothesis that reducing the activity of muscarinic receptors, in particular the M4 and M1 subtypes could potentially modulate phenotypes in Fmr1KO mice.
The goal of the present study was to further understand the specific role of M4 receptors in modulating the F×S phenotypes using a genetic strategy rather than a pharmacological approach. Importantly, since we have shown that numerous Fmr1KO behavioral phenotypes were dependent on genetic background [28], and thus influenced by dominant genetic modifiers, the current study also addressed the potential that the M4 receptor gene might be one of those dominant modifiers. Therefore, in the current study, we used a genetic approach to reduce M4 receptor expression by 50% in the presence of the Fmr1 mutation to further investigate the role of M4 receptor as a modulator of Fmr1KO behaviors. Additionally, although pharmacological modulation of M4 receptor signaling altered F×S phenotypes [29], the potential non-specific effects of the pharmacological agents used can be avoided using the receptor-specific genetic approach. Results from the current study demonstrated that genetically reducing M4 receptor expression resulted in modulation of select fragile × behaviors. The double mutants, heterozygous for the M4 gene and hemizygous for the Fmr1 gene showed a complete reversal of the analgesic phenotype and a partial rescue of the acoustic startle response phenotype in the Fmr1 KO mice.
2. Materials and Methods
2.1. Animals
Subjects for this study were generated by crossing heterozygous Fmr1 female (Fmr1+/−) (C57BL/6J background backcrossed for 14–17 generations) and heterozygous M4 (M4 +/−) male mice (Gomeza et al., 1999) (backcrossed for over 10 generations to C57BL/6NTac mice). Therefore, it is important to note that the current mice are on a genetic background that is ‘F1’ between B6J and B6NTac, and although there should be few differences compared to our routine ‘pure’ B6J background (e.g. [30]) the current mice are not genetically the same as those Fmr1KO on a B6J background. The resultant progeny consisted of 4 genotypes: Wild type (WT), M4 heterozygotes (M4 Het), Fmr1 hemizygous knockout (Fmr1KO) and the double mutant heterozygous for M4 and hemizygous for Fmr1 (DM). The total number of animals used in the study includes 8 wildtypes, 15 M4 heterozygotes, 13 Fmr1KOs and 15 double mutants. Although using strict littermate helps control for between-litter variability, we were not necessarily able to obtain all the four genotypes in each litter. So we tested 2–3 litters together (in batches) to account for the variability. The animals were 2–3 months of age, except those used in the audiogenic seizure study, which were 20–22 days of age. Only male mice were used in this study. Mice were housed 2–5 per cage with access to food and water ad libitum. They were maintained in a 12 hr light: dark cycle. Prior to testing all the animals were transferred to the testing room followed by a 30-minute acclimatization period. The animal care and behavioral testing procedures were approved by the Baylor College of Medicine Animal Care and Use Committee and followed NIH guidelines.
2.2. Behavior testing assignment
All the animals went through a standard testing battery comprising of open-field activity (OFA) (day 1 and 2), Light dark (LD), marble-burying (MB), prepulse inhibition (PPI), rotarod, passive avoidance (PA) and hot plate. This testing battery enables us to test a wide range of behavioral assay and minimizes the number of animals used in the study [31,32]. However, for the audiogenic seizures we used a separate set of mice.
2.3. PCR techniques
Standard PCR techniques were used to genotype the animals [33,19]. For the WT allele of the Fmr1 gene we used the following primers: Fmr1_S1 (5’-GTG GTT AGC TAA AGT GAG GAT GAT-3’) and Fmr1_S2 (5’- CAG GTT TGT TGG GAT TAA CAG ATC- 3’) and the expected band size was 527bp. For the KO allele of the Fmr1 gene we used the following primers: Fmr1_S1 and Fmr1_N2 (5’-TGG GCT CTA TGG CTT CTG A- 3’) and the band size was 501 bp. The following protocol was used for genotype the Fmr1 locus: 94°C for 2 minutes, 94°C for 30 seconds followed by 58°C for 30 seconds and 72°C for 30 seconds. This cycle was repeated 30 times. Final extension was done at 72 for 10 minutes. The WT and KO PCRs were run separately using the same conditions. For the WT allele of the M4 gene the following primers were used: M4-A (5'-GGA GAA GAA GGC CAA GAC TCT GG- 3’) and M4-B (5'-GGC AGT CAC ACA TTC ACT GCC TG-3’) and the expected band size was 367 bp. For the KO allele of the M4 gene the following primers were used: M4-B and NEO-1 (5'-CAG CTC ATT CCT CCC ACT CAT GAT-3’) and the expected band size was 480 bp. The following protocol was used to genotype the M4 locus: 94°C for 10 minutes, 94°C for 30 seconds followed by 30 seconds of annealing at 60°C and 72°C for one minute. This cycle was repeated 30 times and the final extension was done at 72°C for 10 minutes. The WT and the KO reactions were run separately using the same protocol.
2.4. Behavior experiments
2.4.1. Open-field activity
Total distance travelled in an open field was used as a measure of activity. The testing chamber comprised of a plexiglass arena (40 × 40 × 30 cm) that was brightly lit with overhead lights (~ 800 lux). Recordings were made using the automated versamax animal activity monitoring system (Accuscan Instruments, Columbus OH). Throughout testing a white background noise was present (55 dB). Each test session lasted for about 30 minutes and the total distance travelled and the number of vertical rearing responses were recorded. We also measured anxiety-like behavior defined by the ratio of center distance, which is the distance travelled by the animal in the center (22.5 × 22.5 cm) of the chamber, to the total distance traveled. The same test was repeated on the second day to measure the response to a novel environment.
2.4.2. Light dark
Light- dark exploration assay was used to measure anxiety-like responses. The testing chamber comprised of a rectangular plexiglass chamber divided in to two unequal compartments, separately by a partition with an opening (8.5 cm × 5 cm) large enough to allow the animal to transition between the compartments. The large chamber was brightly lit (≈800 lux), transparent and uncovered (30 cm × 21 cm × 21 cm). The small chamber was made of black Plexiglas and cover with a lid (15 cm × 21 cm × 21 cm). At the start of the experiment the animals were placed in the light chamber and the number of transitions between the chambers (all four paws inside a chamber) over a 10 minute period was recorded. White noise was present throughout testing.
2.4.3. Marble Burying
Perseverative behavior was measured using the marble-burying (MB) assay [34]. A standard mouse cage filled with approximately 10 cm of SANI-CHIP bedding was used as the test chamber. Twenty black marbles (15 mm) were arranged in the testing chamber in a 4×5 matrix, equidistant from each other. Each animal was allowed to explore the chamber for 20 minutes and at the end of the testing period the number of marbles buried was determined. We defined buried as more than 50% of the surface being covered by the bedding. White noise (55 dB) was present throughout testing.
2.4.4. Prepulse Inhibition of the acoustic startle response
The acoustic startle responses and prepulse inhibition were used to assess sensorimotor gating using the SR-Lab System (San Diego Instruments, San Diego, CA, USA) as previously described [33]. The apparatus comprised of a sound attenuating chamber, which contained a cylindrical tube where the animal was placed during testing. Each animal was acclimated to a background white noise of 70 dB for about 5 minutes prior to the test session. Each test session consisted of 48 trials comprising of 6 blocks of eight trial types each presented in a pseudo random order. Each block had a “No stimulus” trial used to measure baseline movement where no sound was presented, a “startle only” trial comprised of a 40 ms, 120 dB sound burst, a “prepulse only” trials (74, 78 or 82 dB) comprising of three different 20 ms prepulses and finally the “prepulse inhibition” trials composed of the presentation of one of the three prepulse sounds, 100ms prior to the startle stimulus. The inter-trial interval ranged from 10 s– 20 s, and the startle response was recorded every 1 ms for 65 ms following the onset of the startle stimulus. Percent PPI of the startle response was calculated as follows: 100–[(response to acoustic prepulse plus startle stimulus trials/startle response alone trials) × 100].
2.4.5. Rotarod
Motor coordination and skill learning were investigated by assessing the ability of the animal to walk on a rotating rod (Ugo Basile, Comerio, VA, Italy). We used a 2-day protocol consisting of 4 trials per day. Each trial lasted for 5 minutes with the rod accelerating at a speed of 4–40 rpm in 5 minutes. The time spent walking on the rod was recorded. Intertrial interval was 10–15 minutes.
2.4.6. Passive Avoidance
Learning and memory performance was assessed using a passive-avoidance assay. A modular shuttle box (Med Associates Inc., St. Albans, VT, USA) containing two chambers, one illuminated with bright light and the other covered with black felt, separated from each other by a barrier containing a door was used for training and testing. The first day is the training day which began with the animal being placed in the light chamber and allowed to explore the chamber for 10 seconds and then the door to the dark chamber was opened. As soon as the animal entered the dark chamber with all its 4 paws, the door was shut and a mild foot shock (0.75 mA, 2 s) was delivered. Following the foot shock the animal was allowed to stay in the dark chamber for an additional 10 seconds and returned to its’ home cage. The latency to enter the dark side was measured. Memory for this aversive event was tested the following day. The test animal was again placed in the light chamber and allowed to explore the chamber for 10 seconds after which the door to the dark chamber is raised and the animal was allowed to enter the dark chamber. The latency to enter the dark chamber was measured and this was used as the index for learning and memory. Maximum time of 300 s was given to each animal and then the animal was returned to the home cage.
2.4.7. Hot plate
Analgesic response to a thermal stimulus was measured using a hot plate analgesia meter (Columbus instruments, Columbus, OH, USA). Each animal was placed on the hot plate (55° C) and the latency (maximum 45 s) to first hind limb response (i.e. hind paw shake, jumping and hind paw licking) was measured.
2.4.8. Audiogenic seizures
Susceptibility to audiogenic seizure was measured in 19–21 days old mice in a sound attenuated chamber illuminated with bright light using a priming protocol adopted by our lab [35]. Two mini personal alarms (SKU 49-728, RadioShack, Fort Worth, TX, USA) emitting 140 dB sound stimulus were attached to the underside of two clear plexiglass lids used to cover the testing chamber, that comprised of a standard mouse cage. The protocol consists of a one-minute silent period, which was followed by two minutes of loud sound (alarm on), then another minute of silence followed by two minutes of loud sound. Based on previous studies in the lab we did not observe seizures in the KO mice with a single presentation of sound stimulus and hence we have used a priming protocol that involves 2 sound presentations. Both wild running and clonic tonic seizures were recorded and percentage of animals that exhibited seizures were calculated as follows: (total number of animals seized/total number of animals) × 100
2.5. Testis weight measurement
Testis weight was measured from animals that had completed the behavior battery. In order to control for the body weight, we calculated testis: body weight ratio.
2.6. Statistics
For each behavioral measure except audiogenic seizures, one-way ANOVA were used to analyze the data. Follow-up comparisons were made using Least Significant Difference (LSD) test. For Audiogenic seizures, we used the Fischer exact probability test (one tailed) to analyze the percentage of seizures. The level of significance was at p<0.05. Data were analyzed using the SPSS statistical software (SPSS, Chicago, IL).
3. Results
The goal of this study was to assess the modulation of Fmr1KO behaviors by reducing the amount of M4 receptor protein by 50%. Here we defined modification by changes in the double mutant (DM; heterozygous for the M4 receptor gene and hemizygous null for the Fmr1 gene) in comparison with the Fmr1KO phenotype. A rescue was defined by significant differences between the DM and the Fmr1KO and no significant difference between DM and the WT. A partial rescue was when the DM was neither different from the WT nor from the Fmr1KO. No modulation was when the DM resembled the Fmr1KO phenotype. All the follow-up analyses comparing the genotypes were performed only when the one-way ANOVA revealed a significant overall effect of genotype.
3.1. Behavioral modifications
We observed modifications in only select Fmr1KO behaviors and hence in this section we present behavioral data from all the assays in which we observed a modification of Fmr1KO phenotype followed by assays that showed a lack of modulation of the Fmr1KO phenotype.
3.1.1. Modulation of thermal sensitivity by the M4 receptors
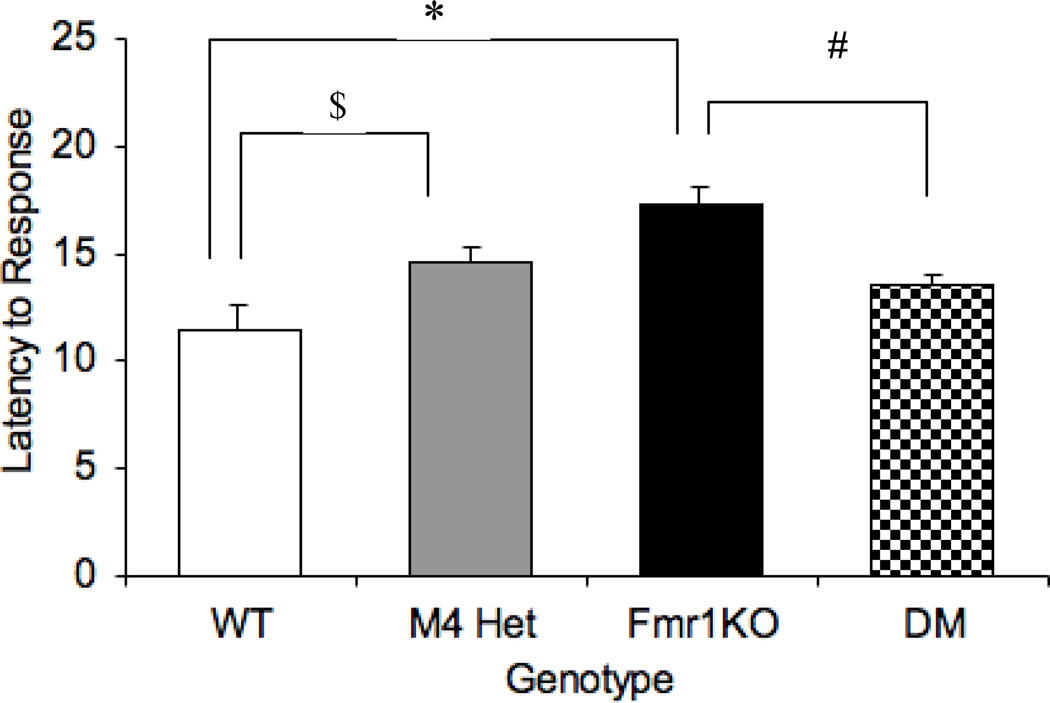
M4 receptors have been implicated in mediating analgesic response [36]. In this study we examined thermal sensitivity using the hot-plate assay as a measure of analgesic response. One-way ANOVA revealed an overall main effect of genotype [F (3,47)= 8.35; P= 0.0001]. The Fmr1KO mice showed a decreased sensitivity to the thermal stimulus indicated by an increased latency to respond to the stimulus (p< 0.001) (Figure 1). Previous studies have reported an absence of a hot plate phenotype in the M4 KO mice under basal conditions [24]. But the M4 heterozygotes in our study showed an increased latency compared to the WT (p<0.05). However, the M4 mutation in combination with the Fmr1 mutation, rescued the increased latency phenotype seen in the Fmr1KO mice. The latency to respond to the thermal stimulus in the double mutant mice were comparable to the WT (p=0.09) and significantly different from the Fmr1KO (p<0.01) indicating a reversal of the phenotype, suggesting a potential interaction between these pathways for this type of response.
Fig 1.
Modification of analgesic response by the M4 receptor in the Fmr1KO mice. Latency to hind limb response in the hot plate assay across different genotypes. # p<0.01 compared to the Fmr1KO and $ p<0.05 compared to the WT (n = 8–14).
3.1.2. Modulation of the acoustic startle response by the M4 receptors
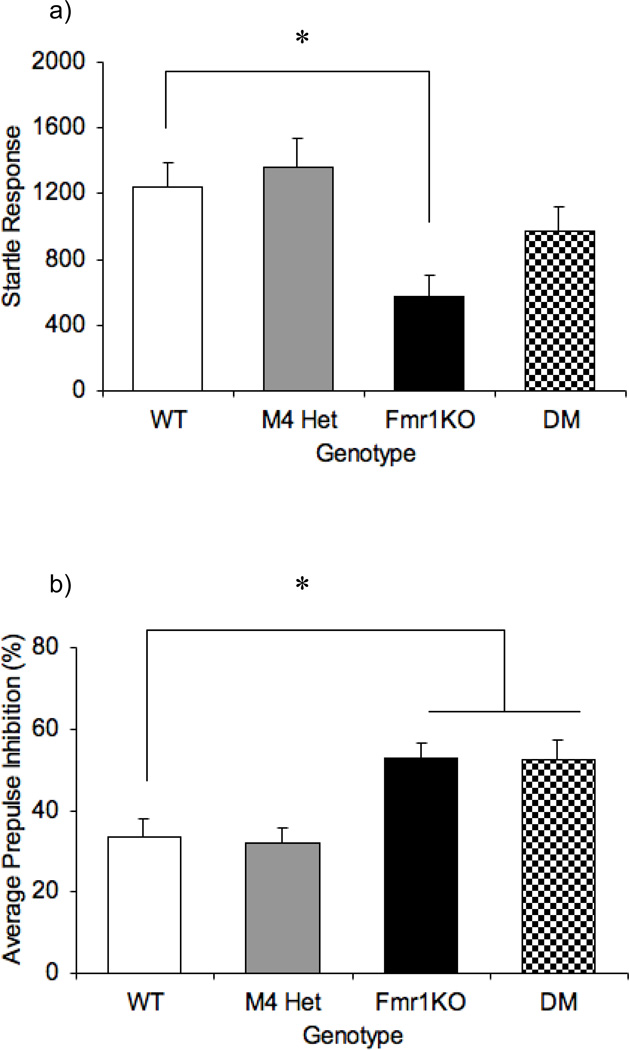
The prepulse inhibition (PPI) test was used to assess sensorimotor gating impairments. Previous studies have reported a decreased startle response and increased PPI in the Fmr1KO mice [3, 8, 28, 33]. Similarly, the M4 receptor has also been shown to be a key player in controlling acoustic startle response [22]. In this latter study, the M4 KO mice showed increased startle response, but normal PPI compared to the WT mice [22]. Analysis on the startle response data revealed an overall effect of genotype [F (3,47)= 4.8; P= 0.005]. The M4 heterozygotes displayed a normal startle response (p= 0.653). Also, previous studies have shown that the Fmr1KO mice showed a decreased startle response phenotype [3, 8, 28, 33]. Consistent with the literature the Fmr1KO mice in our study also showed decreased startle response compared to the WT mice (p=0.012). The startle response in the DM mice was not significantly different from the WT (P= 0.28), and trending to be significantly different from the Fmr1KO (P=0.072), suggesting a partial rescue of the startle response phenotype (Fig.2a). Thus the M4 mutation appears to act as a dominant modifier modulating the acoustic startle response phenotype in the Fmr1KO mice.
Fig 2.
Modification of acoustic startle response by the M4 receptor in the Fmr1KO mice. Bars indicate mean ± SEM for acoustic startle response to the 120dB startle stimulus (a), average percentage inhibition of the startle response (b) across the four genotypes. * p<0.05 compared to the WT (n=8–14).
Interestingly, the PPI analysis also showed an overall effect of genotype [F (3,47)= 6.6, P=0.001] (Fig.2b) with the Fm1KO having an increased PPI (p= 0.01), which is consistent with the literature [28,33]. Similarly, the DM mice had increased PPI (p= 0.01) that was not different from the Fmr1KO response (p=0.958). PPI measurements in the M4 heterozygotes were comparable to the data obtained with WT mice (p= 0.838). These results demonstrate that although M4 receptors regulate startle in Fmr1KO mice, the reduction in M4 receptor expression in the M4 heterozygotes might not be sufficient to alter sensorimotor gating.
3.1.3. Modulation of activity by the M4 receptors
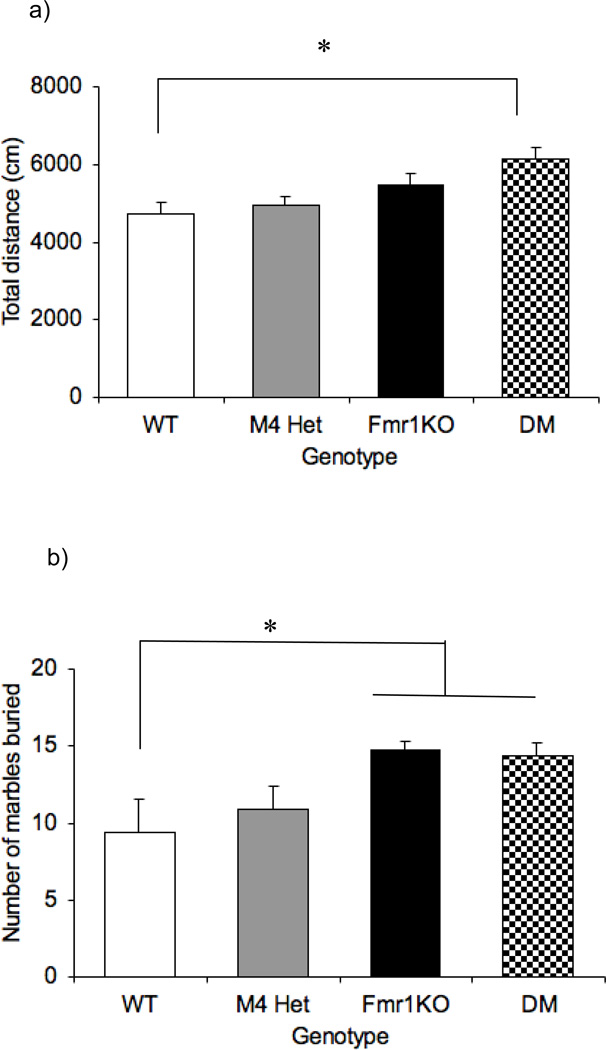
Figure 3 illustrates total distance travelled in an open field as a measure of activity in the four genotypes of animals. There was an overall effect of genotype [F(3,47)=4.5; p= 0.007]. Follow-up analysis revealed that neither Fmr1KO mice (p=0.126) nor M4 heterozygotes (p= 0.621) were differentially active relative to the WT littermates. However the DM mice showed a significant increase (p= 0.003) in the total distance travelled compared to the WT suggesting a synergistic effect on activity between M4 and Fmr1. Also, habituation to the novel environment was assessed by measuring total distance travelled in the same open field on the following day. All the genotypes showed a decrease in activity on the open field suggesting similar habitation to the environment (data not shown).
Fig 3.
Effect of reducing M4 protein levels on activity and perseverative behavior. Bars represent ± SEM for total distance in the open field as a measure of activity (a) and number of marbles buried as a measure of perseverative behavior (b) across the four genotypes. *p<0.01 compared to the WT (n=8–14).
3.1.4. Absence of modulation of the learning and memory phenotype
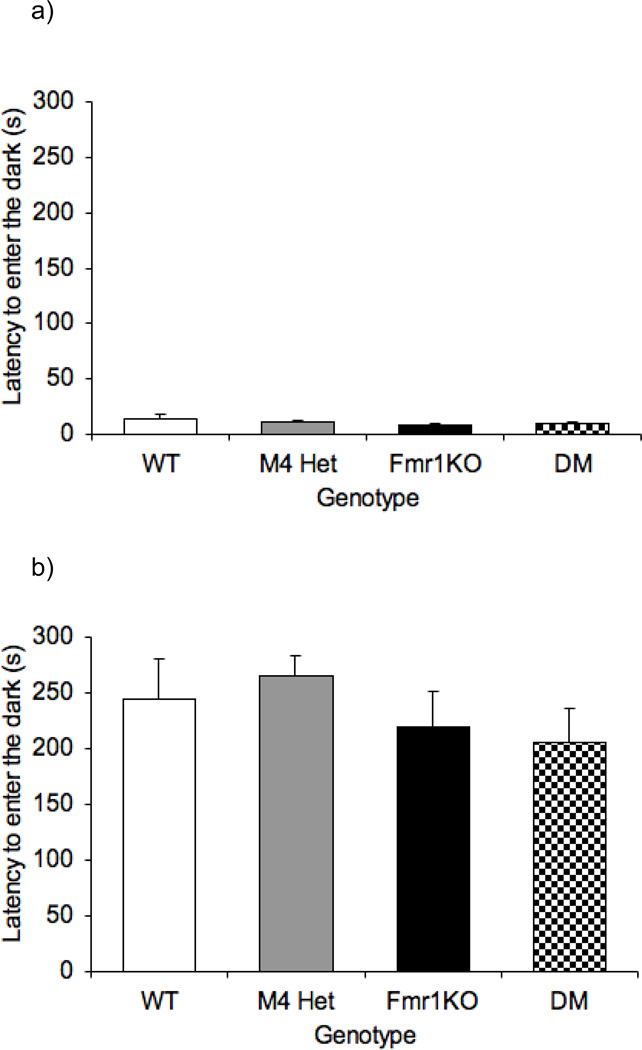
The passive avoidance test was used to assess learning and memory performance. The passive avoidance phenotype in the Fmr1KO mice has been inconsistent with some reports suggesting an impairment [26,30] while others reporting no effect [2,29]. Figure 4a and 4b illustrate the latency to enter the dark in the passive avoidance test across all the genotypes on day 1 and day 2 respectively. One-way ANOVA revealed no overall effect of genotype on the latency to enter the dark on day 1 [F(3,47)= 0.948 p=0.425] or day 2 [F(3,47)= 0.992; p=0.405]. The day 2 data clearly indicate a lack of interaction between the M4 receptor and Fmr1.
Fig 4.
Absence of modification of the learning and memory phenotype in the Fmr1KO mice. Bars indicate mean ± SEM for latency to enter the dark chamber in the passive avoidance assay on day 1 (a) and day 2(b) across four genotypes (n=8–14)
3.1.5. Absence of modulation of perseverative behavior, anxiety-like responses, motor behavior, or audiogenic seizures
Figure 4b illustrates marble burying across the different genotypes. One-way ANOVA revealed an overall effect of genotype [F(3,47)= 30.96; p=0.013] and follow-up tests showed that the Fmr1KO and DM mice buried significantly more marbles compared to the WT (p< 0.01). The M4 heterozygotes buried similar number of marbles as the WT (p=0.426).
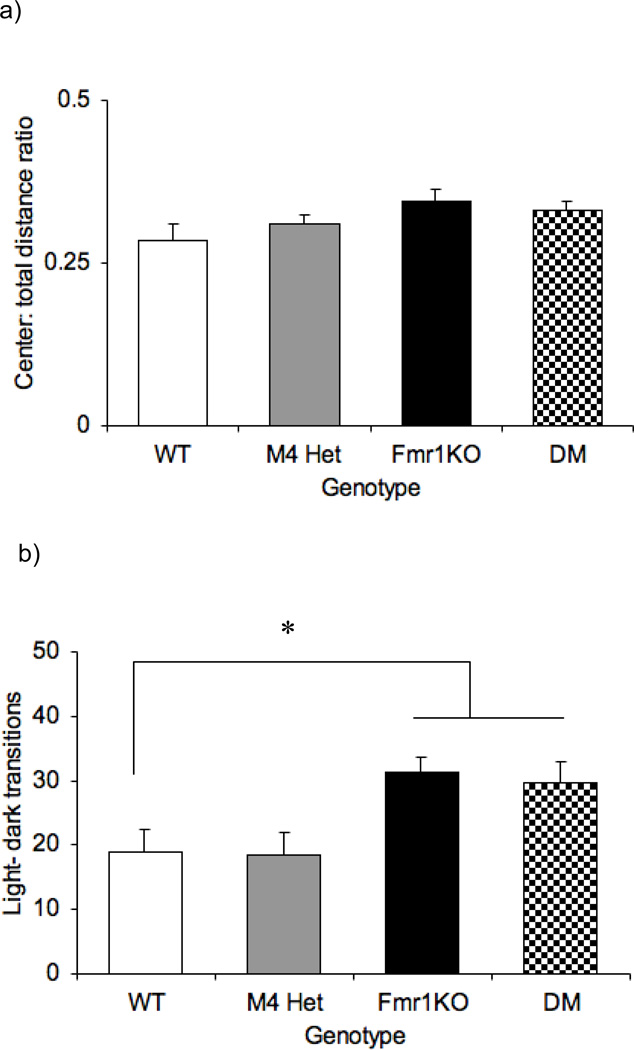
Anxiety-like traits were measured by the center: total distance ratio in an open field and number of transitions in the light dark assay (Fig 5a). One-way ANOVA revealed an absence of an overall effect of genotype [F (3,47)= 1.967; p= 0.132] in the center: total distance ratio. However, the number of transitions in the LD assay revealed an overall effect of genotype [F (3,47)= 4.551; p=0.007]. The Fmr1KO (p<0.05) and the DM (p<0.05) mice were both significantly different from the WT mice (Fig 5b) indicating a lack of modulation of the Fmr1KO phenotype by the M4 receptors.
Fig 5.
No modulation of anxiety like responses by the M4 receptor. Bars represent ± SEM for the center: total distance traveled in an open field, an index of anxiety response (a) and number of light-dark transitions in the light-dark assay (b). * p<0.05 compared to the WT (n=8–14)
Motor coordination was assessed using the rotarod assay. Figure 6 illustrates the average time spend over a period of 2 days on a rotating rod. One-way ANOVA revealed no overall effect of genotype [F (3,47)=0.822; P=0.488]. The lack of a motor coordination phenotype in the Fmr1KO mice is consistent with a previous study from our lab [28].
Fig 6.
Effect of reducing M4 protein levels on motor coordination assessed by rotarod assay. Bars indicate ± SEM for the average time spent on the rotating rod across the four genotypes (n=8–14).
Figure 7 illustrates the percentage of audiogenic seizures across various genotypes. Fmr1KO mice have been shown to be susceptible to audiogenic seizures [3, 7, 10, 30, 37]. Consistent with the literature, the Fmr1KO mice showed a significantly increased percentage of seizures compared to the WT mice (P<0.05). However, the DM mice were similar to the Fmr1KO mice (p=0.5) and did not modulate the Fmr1KO phenotype.
Fig 7.
No modulation of AGS by the M4 receptors. Bars represent value ± SEM for percentage of seizures across genotypes. *p<0.05 compared to the WT
3.2. Absence of modulation of testis weight in the Fmr1KO mice
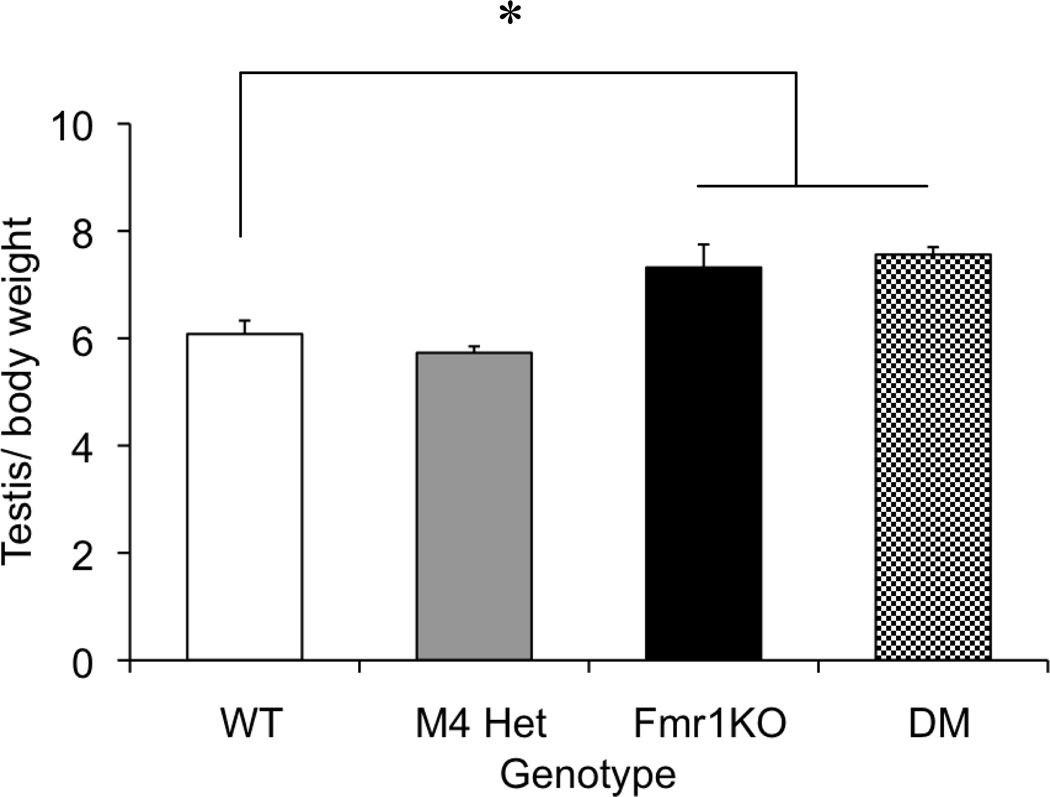
One of the most consistent morphologic phenotypes in the Fmr1KO mice is machroorchidism [2], which has been shown to be caused by increased proliferation of the sertoli cells [38]. Muscarinic receptors are also expressed in the testis and have been shown to regulate sertoli cell proliferation [39]. Hence we investigated the effect of reducing the M4 receptor levels by 50% on testis weight in the Fmr1KO mice. Testis weights were measured from animals that completed the behavior battery (10–12 weeks). In order to control for variations in body weight, a ratio of testis weight: body weight was analyzed. One-way ANOVA revealed an overall main effect of genotype [F(3,33)=13.9; p<0.001], with the Fmr1 KO showing an increased testis weight compared to the WT mice (p< 0.01) (Figure 8). The M4 heterozygotes were not significantly different from the WT mice (p=0.375). In the double mutants, reduction in M4 receptor expression did not alter the Fmr1KO phenotype. The double mutants were significantly different from the WT (p=0.001) but comparable to the KO (p=0.475) mice suggesting that 50% reduction of M4 receptor expression did not modulate the testicular phenotype in the Fmr1KO mice.
Fig 8.
No modulation of the macroorchidism phenotype in the Fmr1KO mice. Bars represent values ± SEM for testis: body weight measurements across four genotypes. *p<0.05 compared to the WT (n=8–14)
4. Discussion
The main objective of this study was to determine whether reducing the muscarinic M4 receptor levels by creating a double mutant heterozygous for M4 and hemizygous null for the Fmr1 gene, modulates fragile × phenotypes in Fmr1KO mice. In the double mutants, we observed modulation of select behaviors including the hotplate-analgesia response and the acoustic startle response in the Fmr1KO mice, suggesting that the M4 receptor positively modulates some of the Fmr1 phenotypes. However, we did not observe any modulation of a number of other behaviors including PPI, anxiety-related responses, learning and memory and audiogenic seizures suggesting that other factors are involved in modulating these processes.
4.1. M4 receptors mediated modulation of analgesic response and sensorimotor gating in the Fmr1KO mice
Overall, the analgesic response in the Fmr1KO mice assessed by the hot-plate assay is clearly influenced by the genetic background. Spencer et al. [28] have shown that in the C57BL/6J background there was a trend towards a significant analgesic response in the hot plate assay, whereas in the D2.B6-F1 hybrid background there seems to be a robust analgesic effect seen by an increase in the latency to respond to the thermal stimulus [28]. However, in general, studies involving the B6 mice have shown an absence of the pain-sensitivity phenotype in the Fmr1KO mice, using the hot plate and tail flick assays [40, 27]. In F×S patients, self-injurious behavior has been reported which is thought to be due to altered pain sensitivity [41–43]. Also, FMRP is highly expressed in the dorsal root ganglion, a region that is involved in pain processing [27]. In our study the Fmr1KO mice showed a robust analgesic response seen by a significant increase in the latency to respond in the hot-plate assay. Muscarinic receptors have also been implicated in analgesic response in rodents (reviewed by [36]). Similar to FMRP, the M4 receptors have also been found to be present in the dorsal root ganglia of the rat spinal cord, which is essential for pain transmission [44]. Tropicamide, an M4 receptor-preferring antagonist, reduces the antinociceptive effects of LXM-10, an acetylcholine receptor agonist [45]. Also, the analgesic responses mediated by the muscarinic agonists CMI-936 and CMI-1145 were reduced in M4 KO mice, indicating a role for the M4 receptors in pain sensitivity [24]. In this study, the M4 heterozygous mice also showed an increased latency to respond compared to the WT. Interestingly, reducing M4 receptor expression in combination with the Fmr1 mutation reversed the analgesic response seen in the Fmr1KO mice, suggesting an interaction between the pathways. These findings are intriguing and other types of pain sensitivity assessments in these mutants will be necessary in order to thoroughly understand modulation of pain processing in the Fmr1KO mice by the M4 receptors.
Prepulse inhibition is one of the most robust phenotypes observed in the Fmr1KO mice. Several studies have reported an increased prepulse inhibition response and decreased startle in the Fmr1KO mice suggesting a role for FMRP in sensorimotor gating [3,6,8,28,33]. Interestingly, cholinergic receptors also play an important role in sensorimotor gating with evidence from lesion studies involving the cholinergic brain stem nuclei (pedunculopontine and laterodorsal tegmental nuclei) showing defects in PPI [46, 47]. However, despite having increased startle the M4 KO mice have normal prepulse inhibition [22]. Also, pharmacological antagonism using the M4 preferring antagonist tropicamide resulted in decreased PPI in mice [48, 30] and an increased startle response [48]. In the current study, the Fmr1KO mice showed decreased startle response consistent with the literature. In the current study the startle response of M4 heterozygotes was comparable to WT mice; however, the reduction in M4 receptor expression in the presence of Fmr1 mutation partially modulated the reduced startle in the Fmr1 KO mice. These data demonstrate that M4 acts as a dominant modifier of the Fmr1 startle response phenotype. We do not observe any modulation of the increased PPI response in Fmr1KO mice by the presence of a M4 heterozygous mutation, suggesting that the mechanism underlying the PPI phenotype in the Fmr1KO mice might not involve M4 receptors.
4.2. M4 receptor mediated modulation of learning and memory in the Fmr1KO mice
Several studies have implicated muscarinic receptors in learning and memory processes [see reviews 49,50,51,52]. However, previous studies have reported no impairment in the passive avoidance assay in the M4 KO mice [23]. In our lab we have shown that the M4 receptor-preferring tropicamide enhances learning and memory in the passive avoidance assay in the WT and in the Fmr1KO mice [29]. Fmr1KO mice exhibit learning and memory defects [2,5,25], but the passive avoidance phenotype in the Fmr1KO mice has been inconsistent with some reports suggesting an impairment [26,30] while others reporting no effect [2,29]. In our current study, we did not observe an Fmr1KO phenotype, and the DM mice also did not show any modulation. Since we have shown that genetic background significantly impacts the behavior of Fmr1KO mice [28], it is possible that the lack of an impairment seen in the present study resulted in part from the fact that the genetic background of the present mice was an F1 between B6J (Fmr1 female heterozygotes) and B6NTac (M4 male heterozygotes) instead of the pure B6J that we used previously. It is also possible that in this present study the decrease in pain sensitivity seen in the Fmr1KO mice might contribute in part to the lack of phenotype in the passive avoidance assay. Investigating other types of learning and memory assays might help us better understand the potential for modulation of learning and memory process in the Fmr1KO by changes in M4 receptors.
4.3. Synergistic effect of the M4 receptors on locomotor activity in the Fmr1KO mice
The Fmr1KO mice have been shown to exhibit a hyperactivity phenotype [2,25,26,28]. Similarly, the M4 KO mice showed hyperactivity in an open field [19,23]. Tropicamide, an M4 receptor-preferring antagonist, has been shown to increase activity in the WT and in the Fmr1KO mice [30]. In the present study, neither the Fmr1 mutation nor the M4 mutation led to significant hyperactivity, but when combined in the DM mice a synergistic effect was observed, suggesting that the M4 receptors and the FMRP pathway either directly or indirectly interact with each other to increase locomotor activity. Along with other factors, the balance between the cholinergic and the dopaminergic neurotransmission is essential for maintaining normal motor activity [53]. In the M4 KO mice, activation of the D1 receptors by a D1 agonist further enhanced the locomotor responses compared to the WT mice, suggesting that the M4 receptor acts as an inhibitor of the D1 mediated locomotor response in the striatum [19]. Also, FMRP has been shown to be important to mediate dopamine-induced locomotor response in the Fmr1KO mice [54]. Hence, the interaction between the M4 receptor and FMRP might be mediated through the dopaminergic system, which could play an important role in this synergistic enhancement in hyperactivity. However further investigations are required to understand the mechanism.
4.4. Absence of modulation of fragile × behaviors
Decreased anxiety-like responses have been reported in the Fmr1KO mice, however this has been shown to be dependent on the genetic background [28]. Little is known about the role of M4 receptors in mediating anxiety-related responses. In the current study, we observed an absence of an anxiety-like phenotype in the Fmr1KO mice in an open field. However, in the light-dark assay we observed an Fmr1KO phenotype that was not modulated in the DM mice suggesting that M4 is not an important modifier in regulating anxiety-like responses in the Fmr1KO mice. Similarly, perseverative behavior in Fmr1 KO mice measured by number of marbles buried has been shown to be strain dependent [28]. Recent work from our lab has shown that the M4 receptor-preferring antagonist tropicamide reduced marble burying in WT and in Fmr1KO mice [29]. In the present study, we observed an Fmr1KO phenotype with the KO mice burying more marbles than the WT. However the DM mice failed to modulate the Fmr1KO phenotype, suggesting that reducing M4 receptor expression by 50% is not sufficient in modulating perseverative behavior in Fmr1KO mice. Muscarinic M4 receptors are highly expressed in the striatum and have been shown to be important for coordinating motor responses [19,55]. However, in our study we failed to observe an overall effect of genotype suggesting that the M4 receptor might not play a role in modulating motor coordination. Similarly, we failed to observe a modulation of the AGS phenotype in the Fmr1KO mice suggesting that 50% reduction in M4 receptor expression does not modulate the seizure phenotype.
4.5. Absence of modulation of machroorchidism by the M4 receptors in the Fmr1KO mice
One of the most robust anatomical phenotypes in the Fmr1KO is macroorchidism [2], which has been shown to be due to the increased proliferation of sertoli cells in the Fmr1KO mice [38]. Interestingly, muscarinic receptors have been shown to be important for sertoli cell proliferation [39,56]. All five mAChR subtypes (M1–M5) are expressed in the rat testis [57,58]. In our study, we observed an increase in the testis: body weight ratio in Fmr1 KO mice, but reducing M4 receptor expression in the DM mice failed to rescue this macroorchidism phenotype.
Previously, we had used a pharmacological approach to reduce M4 receptor activity in the Fmr1KO mice and observed modulation of perseverative behavior, learning and memory and AGS [29]. Both the genetic and pharmacological approaches have advantages and disadvantages. While the M4 antagonist tropicamide showed five fold selective affinity to M4 receptors, studies have shown that the antagonist binds other muscarinic receptors with lower affinity relative to M4 [59]. In contrast, the major advantage of the genetic approach is that it offers receptor selectivity by targeting M4 receptor and investigates specific roles for M4 receptor in modulating fragile × behaviors. Our recent study evaluating the effect of tropicamide on Fmr1 KO behavior [29] utilized a single injection of the antagonist; therefore the observed modulation of Fmr1 KO and WT behavior was due to the acute effect of the antagonist at the time of testing. However, the genetic mutation reflects a change that is present throughout lifetime and the modulation observed reflects a chronic change in the receptor level and activity. Although the M4 knockout mice do not exhibit embryonic lethality, the M4 receptors are expressed during embryonic development and absence of M4 receptors throughout development, might result in developmental changes in the double mutants (M4.Het.Fmr1KO) that could also account for the discrepancy between the genetic and pharmacological approaches. Also, the stress associated with the injection itself could add variability to the behaviors assessed by the pharmacological study. Regardless of the approach the reduction of M4 receptor clearly modulates select fragile × behaviors.
In summary, the present study has shown that a reduction in M4 receptor expression modified select behaviors in the Frm1KO mice. However, in a number of behaviors reduced M4 receptor expression failed to modulate Fmr1KO phenotypes. Some of the possible reasons for this lack of modification are as follows: 1. The M4 receptors might not be important in mediating these particular behavioral responses 2. Immunoprecipitation studies using the M4 subtype specific antisera showed a significant reduction in the expression of functional M4 receptors in the M4 heterozygotes mice (Gomeza et al., 1999). However, reduction of M4 protein levels in the heterozygotes in the DM mice might not be sufficient to produce the modulation. As mentioned above, one aspect of the current study was to determine if the M4 gene was a dominant modifier of Fmr1KO phenotypes, therefore, we limited our present study to examining DM mice carrying only one copy of the M4 receptor gene. Thus, future studies will be necessary to determine if M4 null mutants have a different impact on an Fmr1KO background. 3. There might be compensation through other muscarinic receptors including the M1 subtype, which is highly expressed in the brain.
In conclusion, our study suggests that reducing M4 receptor expression positively modulates select behaviors in Fmr1 KO mice including analgesic response and acoustic startle response, supporting our previous findings that the M4 receptor represents a potential therapeutic target. More studies involving other behaviors and electrophysiological and biochemical studies will help to understand the mechanism through which the M4 receptor modulates the behavior of the Fmr1KO mice. Nevertheless this study provides further evidence for the involvement of the cholinergic system in F×S.
Research Highlights.
M4 receptor modulates select fragile × behaviors
Genetic reduction of M4 rescued the analgesic response
Genetic reduction of M4 partly rescued the acoustic startle response
M4 receptors are a potential therapeutic target for Fragile × Syndrome
Acknowlegments
This research was supported by the Baylor Fragile × Center and the Baylor College of Medicine Intellectual and Developmental Disabilities Research Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Surabi Veeraragavan, Email: veerarag@bcm.edu.
Deanna Graham, Email: deannag@bcm.edu.
Nghiem Bui, Email: nbui@bcm.edu.
Lisa A Yuva-Paylor, Email: lisay@bcm.edu.
Jürgen Wess, Email: jwess@helix.nih.gov.
Richard Paylor, Email: rpaylor@bcm.edu.
References
- 1.Hagerman RJ, Hagerman PJ. Fragile × syndrome: Diagnosis, treatment, and research. 3rd ed. Baltimore: The Johns Hopkins University Press; 2002. [Google Scholar]
- 2.Bakker CE, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, et al. Fmr1 knockout mice: A model to study fragile × mental retardation. Cell. 1994;78(1):23–33. [PubMed] [Google Scholar]
- 3.Chen L, Toth M. Fragile × mice develop sensory hyperreactivity to auditory stimuli. Neuroscience. 2001;103(4):1043–1050. doi: 10.1016/s0306-4522(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 4.Dobkin C, Rabe A, Dumas R, El Idrissi A, Haubenstock H, Brown WT. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. Neuroscience. 2000;100(2):423–429. doi: 10.1016/s0306-4522(00)00292-x. [DOI] [PubMed] [Google Scholar]
- 5.D'Hooge R, Nagels G, Franck F, Bakker CE, Reyniers E, Storm K, et al. Mildly impaired water maze performance in male Fmr1 knockout mice. Neuroscience. 1997;76(2):367–376. doi: 10.1016/s0306-4522(96)00224-2. [DOI] [PubMed] [Google Scholar]
- 6.Frankland PW, Wang Y, Rosner B, Shimizu T, Balleine BW, Dykens EM, et al. Sensorimotor gating abnormalities in young males with fragile × syndrome and Fmr1-knockout mice. Mol Psychiatry. 2004;9(4):417–425. doi: 10.1038/sj.mp.4001432. [DOI] [PubMed] [Google Scholar]
- 7.Musumeci SA, Bosco P, Calabrese G, Bakker C, De Sarro GB, Elia M, et al. Audiogenic seizures susceptibility in transgenic mice with fragile × syndrome. Epilepsia. 2000;41(1):19–23. doi: 10.1111/j.1528-1157.2000.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen DM, Derber WJ, McClellan DA, Crnic LS. Alterations in the auditory startle response in Fmr1 targeted mutant mouse models of fragile × syndrome. Brain Res. 2002;927(1):8–17. doi: 10.1016/s0006-8993(01)03309-1. [DOI] [PubMed] [Google Scholar]
- 9.Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile × syndrome. Genes Brain Behav. 2005;4(7):420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 10.Yan QJ, Asafo-Adjei PK, Arnold HM, Brown RE, Bauchwitz RP. A phenotypic and molecular characterization of the fmr1-tm1Cgr Fragile × mouse. Genes Brain Behav. 2004;3(6):337–359. doi: 10.1111/j.1601-183X.2004.00087.x. [DOI] [PubMed] [Google Scholar]
- 11.Spencer CM, Graham DF, Yuva-Paylor LA, Nelson DL, Paylor R. Social behavior in Fmr1 knockout mice carrying a human FMR1 transgene. Behav Neurosci. 2008;122(3):710–715. doi: 10.1037/0735-7044.122.3.710. [DOI] [PubMed] [Google Scholar]
- 12.D'Antuono M, Merlo D, Avoli M. Involvement of cholinergic and gabaergic systems in the fragile × knockout mice. Neuroscience. 2003;119(1):9–13. doi: 10.1016/s0306-4522(03)00103-9. [DOI] [PubMed] [Google Scholar]
- 13.Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile × syndrome mental retardation. J Neurosci. 2007;27(43):11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caulfield MP, Birdsall NJ. International Union of Pharmacology XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50(2):279–290. [PubMed] [Google Scholar]
- 15.Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci. 1991;11(10):3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AI. Immunological localization of M1–M5 muscarinic acetycholine receptors in peripheral tissues and brains. Life Sciences. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, et al. Development of antisera selective for M4 and M5 muscarinic cholinergic receptors: distribution of M4 and M5 receptors in rat brain. Mol Pharmacol. 1993;43:149–157. [PubMed] [Google Scholar]
- 18.Wolfe BB, Yasuda RP. Development of selective antisera for muscarinic cholinergic receptor subtypes. Ann N Y Acad Sci. 1995;10:186–193. doi: 10.1111/j.1749-6632.1995.tb17474.x. [DOI] [PubMed] [Google Scholar]
- 19.Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brondkin J, et al. Enhancement of D1 dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc. Natl. Acad. Sci. 1999;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santiago MP, Potter LT. Biotinylated m4-toxin demonstrates more M4 muscarinic receptor protein on direct than indirect striatal projection neurons. Brain Res. 2001;894:12–20. doi: 10.1016/s0006-8993(00)03170-x. [DOI] [PubMed] [Google Scholar]
- 21.Oki T, Takagi Y, Inagaki S, Taketo MM, Manabe T, Matsui M, Yamada S. Quantitative analysis of binding parameters of [3H]N-methylscopolamine in central nervous system of muscarinic acetylcholine receptor knockout mice. Brain Res Mol Brain Res. 2005;133:6–11. doi: 10.1016/j.molbrainres.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Thomsen M, Wess J, Fulton BS, Fink-Jensen A, Caine SB. Modulation of prepulse inhibition through both M(1) and M (4) muscarinic receptors in mice. Psychopharmacology. 2010;208(3):401–416. doi: 10.1007/s00213-009-1740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzavara ET, Bymaster FP, Felder CC, Wade M, Gomeza J, Wess J, et al. Dysregulated hippocampal acetylcholine neurotransmission and impaired cognition in M2, M4 and M2/M4 muscarinic receptor knockout mice. Mol Psychiatry. 2003;8:673–679. doi: 10.1038/sj.mp.4001270. [DOI] [PubMed] [Google Scholar]
- 24.Duttaroy A, Gomeza J, Gan JW, Siddiqui N, Basile AS, Harman WD, et al. Evaluation of Muscarinic Agonist-Induced Analgesia in Muscarinic Acetylcholine Receptor Knockout Mice. Mol Pharmacol. 2002;62(5):1084–1093. doi: 10.1124/mol.62.5.1084. [DOI] [PubMed] [Google Scholar]
- 25.Peier AM, McIlwain KL, Kenneson A, Warren ST, Paylor R, Nelson DL. (Over)correction of FMR1 deficiency with YAC transgenics: behavioral and physical features. Hum Mol Genet. 2000;9(8):1145–1159. doi: 10.1093/hmg/9.8.1145. [DOI] [PubMed] [Google Scholar]
- 26.Qin M, Kang J, Smith CB. Increased rates of cerebral glucose metabolism in a mouse model of fragile × mental retardation. Proc Natl Acad Sci USA. 2002;99(24):15758–15763. doi: 10.1073/pnas.242377399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, et al. Decreased nociceptive sensitization in mice lacking the fragile × mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci. 2007;27(51):13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, et al. Modifying behavioral phenotypes in Fmr1 KO mice: Genetic background differences reveal autistic-like responses. Autism Research. 2011;4(1):40–56. doi: 10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veeraragavan S, Bui N, Perkins JR, Paylor LA, Paylor R. The modulation of fragile × behaviors by the muscarinic M4 antagonist, tropicamide. Behav Neurosci. 2011 doi: 10.1037/a0025202. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veeraragavan S, Bui N, Perkins JR, Yuva-Paylor LA, Carpenter RL, Paylor R. Modulation of behavioral phenotypes by a muscarinic M1 antagonist in a mouse model of fragile × syndrome. Psychopharmacology. 2011;217(1):143–151. doi: 10.1007/s00213-011-2276-6. [DOI] [PubMed] [Google Scholar]
- 31.McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: Effects of training history. Physiol Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- 32.Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87(1):95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Paylor R, Yuva-Paylor LA, Nelson DL, Spencer CM. Reversal of sensorimotorgating abnormalities in Fmr1 knockout mice carrying a human FMR1 transgene. Behav Neurosci. 2008;122:1371–1377. doi: 10.1037/a0013047. [DOI] [PubMed] [Google Scholar]
- 34.Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–373. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan QJ, Rammal M, Tranfaglia M, Bauchwitz RP. Suppression of two major fragile × syndrome mouse model phenotypes by the mGluR5 antagonist MPEP. Neuropharmacology. 2005;49:1053–1066. doi: 10.1016/j.neuropharm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Wess J, Duttaroy A, Gomeza J, Zhang W, Yamada M, Felder CC, et al. Muscarinic receptor subtypes mediating central and peripheral antinociception studied with muscarinic receptor knockout mice: A review. Life Sci. 2003;72:2047–2054. doi: 10.1016/s0024-3205(03)00082-1. [DOI] [PubMed] [Google Scholar]
- 37.Thomas AM, Bui N, Graham D, Perkins JR, Yuva-Paylor LA, Paylor R. Genetic reduction of group 1 metabotropic glutamate receptors alters select behaviors in a mouse model of Fragile × syndrome. Behav Brain Res. 2011;223:310–321. doi: 10.1016/j.bbr.2011.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slegtenhorst-Eegdeman KE, de Rooij DG, Verhoef-Post M, van de Kant HJ, Bakker CE, Oostra BA, et al. Macroorchidism in FMR1 knockout mice is caused by increased Sertoli cell proliferation during testicular development. Endocrinology. 1998;139(1):156–162. doi: 10.1210/endo.139.1.5706. [DOI] [PubMed] [Google Scholar]
- 39.Lucas TF, Avellar MC, Porto CS. Effects of carbachol on rat Sertoli cell proliferation and muscarinic acetylcholine receptors regulation: an in vitro study. Life Sci. 2004;75(14):1761–1773. doi: 10.1016/j.lfs.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Zhao MG, Toyoda H, Ko SW, Ding HK, Wu LJ, Zhuo M. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile × syndrome. J Neurosci. 2005;25:7385–7392. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Symons FJ, Clark RD, Hatton DD, Skinner M, Bailey DB., Jr Self-injurious behavior in young boys with fragile × syndrome. Am J Med Genet A. 2003;118A:115–121. doi: 10.1002/ajmg.a.10078. [DOI] [PubMed] [Google Scholar]
- 42.Symons FJ, Danov SE. A prospective clinical analysis of pain behavior and self-injurious behavior. Pain. 2005;117(3):473–477. doi: 10.1016/j.pain.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Symons FJ, Byiers BJ, Raspa M, Bishop E, Bailey DB. Self-injurious behavior and fragile × syndrome: findings from the national fragile × survey. Am J Intellect Dev Disabil. 2011;115(6):473–481. doi: 10.1352/1944-7558-115.6.473. [DOI] [PubMed] [Google Scholar]
- 44.Bernardini N, Levey AI, Augusti-Tocco G. Rat dorsal root ganglia express m1–m4 muscarinic receptor proteins. J Peripher Nerv Syst. 1999;4(3–4):222–232. [PubMed] [Google Scholar]
- 45.Xiong Y, Zhao X, Sun Q, Li R, Li C, Ye J. Antinociceptive mechanism of the spirocyclopiperazinium compound LXM-10 in mice and rats. Pharmacol Biochem Behav. 2010;95(2):192–197. doi: 10.1016/j.pbb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Swerdlow NR, Geyer MA. Prepulse inhibition of acoustic startle in rats after lesions of the pedunculopontine tegmental nucleus. Behav Neurosci. 1993;107(1):104–117. doi: 10.1037//0735-7044.107.1.104. [DOI] [PubMed] [Google Scholar]
- 47.Jones CK, Shannon HE. Bilateral lesions of the laterodorsal tegmental nucleus disrupt prepulse inhibition of acoustic startle reflex in rats. Schizophren Res. 1998;29:199. doi: 10.1016/j.pbb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Ukai M, Okuda A, Mamiya T. Effects of anticholinergic drugs selective for muscarinic receptor subtypes on prepulse inhibition in mice. Eur J Pharmacol. 2004;492(2–3):183–187. doi: 10.1016/j.ejphar.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 49.Levey AI. Muscarinic acetylcholine receptor expression in memory circuits: implications for treatment of Alzheimer disease. Proc Natl Acad Sci U S A. 1996;93(24):13541–13546. doi: 10.1073/pnas.93.24.13541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem. 2003;80(3):178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 51.Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16(6):710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasselmo ME, Sarter M. Modes and Models of Forebrain Cholinergic Neuromodulation of Cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17(6):228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 54.Wang H, Wu LJ, Kim SS, Lee FJ, Gong B, Toyoda H, et al. FMRP acts as a key messenger for dopamine modulation in the forebrain. Neuron. 2008;59(4):634–647. doi: 10.1016/j.neuron.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 55.Betz AJ, McLaughlin PJ, Burgos M, Weber SM, Salamone JD. The muscarinic receptor antagonist tropicamide suppresses tremulous jaw movements in a rodent model of parkinsonian tremor: possible role of M4 receptors. Psychopharmacology (Berl) 2007;194(3):347–359. doi: 10.1007/s00213-007-0844-6. [DOI] [PubMed] [Google Scholar]
- 56.Davenport CW, Heindel JJ. Cholinergic inhibition of cAMP accumulation in Sertoli cells cultured from immature hamsters. J Androl. 1987;8(5):307–313. doi: 10.1002/j.1939-4640.1987.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 57.Palmero S, Bardi G, Coniglio L, Falugi C. Presence and localization of molecules related to the cholinergic system in developing rat testis. Eur J Histochem. 1999;43(4):277–283. [PubMed] [Google Scholar]
- 58.Borges MO, Abreu ML, Porto CS, Avellar MC. Characterization of muscarinic acetylcholine receptor in rat Sertoli cells. Endocrinology. 2001;142(11):4701–4710. doi: 10.1210/endo.142.11.8465. [DOI] [PubMed] [Google Scholar]
- 59.Lazareno S, Buckley NJ, Roberts F. Characterization of muscarinic m4 binding sites in rabbit lung, chicken heart and NG108-15 cells. Mol Pharmacol. 1990;38:805–815. [PubMed] [Google Scholar]