Abstract
Neuroticism is associated with greater susceptibility to the adverse effects of stress and greater exposure to the stressors associated with acculturation in U.S. born Mexican Americans. Neuroticism and acculturation have been associated with injury to crucial stress response systems and are known risk factors for certain mood and anxiety disorders. The purpose of the current study was to examine the effects of neuroticism, and acculturation on the cortisol awakening response (CAR) in healthy Mexican-American adults. Salivary cortisol samples were collected at awakening and 30, 45, and 60 minutes thereafter, on two consecutive weekdays from 59 healthy Mexican American adult males (26) and females (33), ages 18 to 38 years. Participants were assessed for level of neuroticism and acculturation. Data were analyzed using a mixed effects regression model with repeated measures at four time points. Results showed a significant Neuroticism × Acculturation × Time interaction. The CAR was virtually eliminated in highly acculturated Mexican Americans with greater Anglo orientation and high neuroticism compared with less acculturated Mexican Americans with greater Mexican orientation and lower neuroticism. Findings suggest that some Mexican Americans with high levels of neuroticism may be particularly susceptible to certain challenges and stressors associated with acculturation leading over time to the development of allostatic load, desensitization of the Hypothalamic CRF system and attenuation of the CAR.
Keywords: Neuroticism, Acculturative Stress, Acculturation, HPA axis, Cortisol, Mexican-Americans
Introduction
Neuroticism is one of five independent personality dimensions that are heritable and stable over the adult life span (Costa and McCrae, 1992). Neuroticism is defined as the proneness of the individual to experience negative affective states, and is associated with increased exposure to stressful life events (Bolger and Zuckerman, 1995; Felsten, 2004, 2002) greater susceptibility to the adverse effects of stress (Kendler et al., 2004; Ormel et al., 2001) and thus, a major risk factor for mood and anxiety disorders (DeGraaf et al., 2002; Fanous et al., 2002; Roberts and Kendler, 1999).
The relationship between neuroticism and stress may be of particular importance in Mexican Americans given that studies suggest a number of Hispanics face unique stressors associated with some aspects of acculturation (Finch et al., 2000; Huebner et al., 2005; Mena et al., 1987; Miranda and Matheny, 2000). Acculturation involves adaptation into a host culture (Mena et al., 1987) and acculturative stress includes the psychological, somatic, and social stressors that are associated with the process of acculturation (Bernal and Santiago, 2006; Berry, 1980; Cervantes and Castro, 1985). Neuroticism is associated with more exposure to acculturative stress (Mangold et al., 2007) and greater exposure to acculturative stress is associated with increased risk for mood and anxiety disorders in U.S. born Mexican Americans (Finch, et al., 2000; Matheson et al., 2008; Romero and Roberts, 2003; Thoman and Suris, 2004). Indeed, exposure to acculturative stress is more likely to predict depressive symptomatology in Mexican Americans with high neuroticism (Hovey and King, 1996; Huebner et al., 2005; Salgado de Snyder, 1987). These findings raise the possibility that neuroticism may be a risk factor that significantly increases susceptibility to the adverse effects of stressors associated with acculturation.
There is growing interest in the extent to which chronic exposure to stressful life events leads to dysregulation of crucial biological stress response systems associated with increased vulnerability for mood and anxiety disorders. Stress-induced dysregulation of the Hypothalamic-Pituitary-Adrenal (HPA) axis is one neurobiological pathway that may link neuroticism with increased risk for mood and anxiety disorders in Mexican Americans exposed to high levels of acculturative stress. The cortisol awakening response (CAR) has received increasing attention as a useful method to assess the integrity of the HPA axis. The CAR is a reliable biological marker of HPA activity, dependent on a moderate genetic influence (Bartels et al., 2003; Schmidt-Reinwald et al., 1999; Wust et al., 2000a) and changes in the CAR can yield important information regarding the relationship between altered stress responsivity and attenuation of the awakening portion of the cortisol circadian rhythm. The sensitivity/capacity of the adrenal cortex is proposed to play a crucial role in the magnitude of the CAR (Kudielka and Kirschbaum, 2003; Pruessner et al., 1997, 1999). Thus, examinations of the extent to which neuroticism may link high levels of acculturative stress with dysregulations of the CAR and greater risk for mood and anxiety disorders in Mexican Americans, may be of particular interest.
While studies examining the effects of neuroticism on the CAR in Mexican Americans are non-existent, results from studies in non-Hispanics are mixed showing an enhanced CAR (Polk et al 2005; Portella et al, 2005; Vedhara et al., 2006), diminished CAR (Hauner et al., 2008), and no association between neuroticism and the CAR (Chan et al., 2007; van Santen et al., 2010). Inconsistent results may be due to differences in cortisol measurement and the diagnostic status of the samples examined (i.e., subjects with and without a formal diagnosis of depression versus subclinical symptomatology). Moreover, no available studies have examined the effects of neuroticism on the CAR while controlling for childhood trauma, a factor known to alter the HPA axis and the CAR (Heim and Nemeroff, 2001; Stetler and Miller, 2005; Mangold et al., 2010).
Equally lacking are studies examining the effects of unique forms of cultural stress on the CAR. Studies conducted in our laboratory showed a positive association between acculturative stress and neuroticism in Mexican Americans (Mangold et al., 2007 ) and that greater acculturative stress and more Anglo-orientation is associated with attenuation of the CAR, after controlling for the effects of childhood trauma and independent of a formal diagnosis of depression (Mangold et al., 2010). These findings suggest that similar to childhood trauma (Mangold et al., 2010), and subclinical symptomatology (Mangold, et al., 2011; Dedovic et al., 2010), acculturative stress is associated with attenuation of the CAR in adults. This perhaps is not surprising given that theorists propose greater acculturation and more Anglo orientation may contribute to the erosion of crucial protective factors in some individuals, resulting in greater susceptibility to the adverse effects of chronic exposure to acculturative stress (Finch and Vega, 2003; Vega and Amaro, 1994). The absence of protective factors, in turn, may lead to the development of allostatic load, decreased adrenal capacity over time (McEwen and Lasley, 2003) and attenuation of the CAR. Indeed, theorists have proposed the “attenuation hypothesis” as one possible explanation that is consistent with the differences observed in the CAR in these studies. The “attenuation hypothesis” suggests that following chronic exposure to stress the hypothalamic CRF system may undergo desensitization, transitioning over time from a system with increased stress responsivity to a system with attenuated cortisol responses to stress and an attenuated CAR (Gunnar and Vazquez, 2001; Heim et al., 2008; Susman, 2006; Trickett et al., 2010). Results from a meta-analysis showing a negative correlation between length of time following the onset of chronic stress and the magnitude of the CAR provide additional support for this contention (Miller et al., 2007).
To date, there is a notable absence of studies examining the effects of neuroticism on the CAR in Mexican Americans and the majority of previous studies have failed to examine the effects of neuroticism while adequately controlling for factors known to alter the CAR (e.g., depression and childhood trauma, cortisol measurement with respect to time of awakening). Moreover, studies designed to distinguish the effects of neuroticism from the effects of acculturative stress on the CAR in Mexican Americans may be of particular importance. In view of the fact that neuroticism is associated with increased reactivity and susceptibility to the negative effects of psychosocial stressors, it is plausible that Mexican Americans with high levels of neuroticism, who are exposed to chronic acculturative stress, may represent an extraordinarily vulnerable group at high risk for attenuation of the CAR. Therefore, the aims of the current investigation were to: (1) examine the effects of neuroticism on the CAR in Mexican Americans utilizing a carefully constructed sample monitoring system; (2) examine the effects of neuroticism on the CAR while carefully screening for a diagnosis of depression and controlling for the effects of childhood trauma, age and sex and; (3) distinguish the effects of neuroticism from the effects of acculturative stress on the CAR. Based on previous findings supporting the “attenuation hypothesis”, we hypothesized a significant time × neuroticism × acculturation interaction where neuroticism would be associated with a significantly attenuated CAR in highly acculturated Mexican Americans with greater Anglo orientation after controlling for childhood trauma, depression, age and sex.
Materials and Methods
Participants and Study Design
The study was approved by the University of Texas Institutional Review Board, and all participants gave written, informed consent prior to participation. Participants of Mexican descent (n=59), aged 18 to 38, were recruited from the San Antonio metropolitan area, through advertisements in the community and local college campuses. Specific details of procedures for the current study have been reported elsewhere (Mangold et al., 2010). During an initial visit to the laboratory participants underwent a screening interview and a battery of self-report assessments designed to identify and exclude factors known to potentially affect the HPA axis including: lifetime depression (Bhagwager et al., 2005; Shea et al., 2007); use of oral contraceptives in the past 60 days (Meulenberg and Hofman, 1990; Pruessner et al., 1997, 1999); current pregnancy (Meulenberg and Hofman, 1990); menstrual cycle abnormalities (Bao et al., 2003, 2004; Suh et al., 1988); strenuous aerobic exercise (Hansen et al., 2008; Kanaley et al., 2001; Kelly et al., 2008); major medical conditions or history of head trauma; use of medications; severe obesity and current alcohol or other drug use disorders (Hansen et al., 2008; Huizink et al., 2006; Wand and Dobs, 1991). In addition, participants reporting abnormal sleeping patterns (Lasikiewicz et al., 2008), shift work or overtime (Lundberg and Hellstrom, 2002) were excluded from participation in the study (Clow et al., 2004; Hanrahan et al., 2006).
Psychometric Assessment
Personality/Neuroticism
Personality was measured with the Revised NEO Personality Inventory (NEO PI-R; Costa and McCrae, 1992) a 240-item self-report measure of the five factors of personality including Neuroticism, Extraversion, Openness to Experience, Agreeableness and Conscientiousness (Costa et al., 1991; Costa and McCrae, 1992a, b; McCrae and Costa, 1983). Reliability for the current sample was strong (α =.92) and consistent with reliability reported in a previous study conducted in our laboratory, employing the NEO with healthy, English-speaking Mexican and Mexican American young adults (Mangold et al., 2007).
Acculturation
Acculturation was measured with the revised Acculturation Rating Scale for Mexican Americans (ARSMA-II; Cuéllar et al., 1995). The ARSMA-II measures acculturation by assessing scores on two subscales: The Anglo Orientation Scale (AOS) and the Mexican Orientation Scale (MOS). The ARSMA-II has strong psychometric properties with good reliability for the current sample α =.89).
Depression
Participants were screened for depression using the Hamilton Depression Inventory Short Form (HDI-SF; Reynolds and Kobak, 1995a) and excluded from participation based on a score of 10 or greater as recommended by Reynolds and Kobak (1995b) indicating a strong likelihood of mild, moderate or severe depression. Reliability for the current sample was α=.78.
Substance Abuse/Dependence
Participants were screened and excluded for alcohol and other drug use disorders using the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (WHO ASSIST; Humeniuk et al., 2008). The ASSIST assesses the frequency of lifetime and recent use (within the past 3 months) and problems associated with the use of alcohol and other drugs of abuse (e.g., cannabis, cocaine, stimulants, etc.). Reliability for the current sample was α=.80.
Childhood Trauma
The current study assessed exposure to severe childhood stress prior to the age of 16, using the Early Trauma Inventory–Short Form adapted from the clinician administered Early Trauma Inventory (ETISR-SF; Bremner et al., 2007). The ETISR-SF has strong psychometric properties (Bremner et al., 2007; Hyman et al., 2005) with good reliability for the current sample (α = .82).
Ethnicity/Nativity
Participants reporting an Hispanic origin other than Mexican or Mexican-American were excluded from the study. Participants were determined to be of Mexican descent if they reported that both biological parents and both maternal and paternal grandparents were of Mexican descent.
Measurement of the CAR
Participants were free to wake up as usual (using an alarm or spontaneously), and instructed to start sampling immediately at awakening (T0), and T30, T45 and T60 minutes following awakening. Sampling was completed on two consecutive weekdays. Wake-up times were logged along with sampling times for comparison with electronic monitoring data. All participants completed the cortisol protocol within 3 weeks of screening. To avoid contamination of cortisol samples, participants were instructed to refrain from brushing their teeth, eating/drinking, strenuous exercise, alcohol, smoking and caffeine during sampling (Badrick et al., 2007; Clow et al., 2004). Participants placed a roll of cotton in their mouths, chewing on it until it became saturated, before returning to the salivette (Sarstedt; Rommelsdorft, Germany). Participants were instructed to remain sitting upright in bed until the second saliva sample was obtained, when they were free to follow their normal weekday routine during the sampling period. Cortisol was assessed in females during the early follicular phase of their menstrual cycle (determined by menstrual diary) employing a conservative approach to control for possible effects of menstrual cycle phase on the CAR (Bao et al., 2003, 2004).
We utilized electronic monitoring devices together with self-reported time of sampling to determine concordance between monitored times and self-reported times, detect deviations from the protocol and maximize accuracy in the documentation of sample times (Jacobs et al., 2005; Mangold et al., 2010, 2011). Participants collected saliva samples according to identical protocols on two separate, consecutive days. Salivettes were stored in a vial equipped with an electronic medication event monitoring system (MEMS; Aardex; Zug, Switzerland) as recommended for use in an ambulatory setting by (Kudielka et al., 2003). The MEMS is designed to compile the dosing/sampling-compliance histories of participants. This system includes a standard plastic vial with cap that contains a threaded opening and a closure that contains a micro-electronic circuit that registers times when the closure is opened and when it is closed. Time-stamped medication events are stored in the unit for later software analyses to maximize accuracy of recorded awakening/sampling times included in later analyses.
Hormonal Assays
Participants stored the samples in their home freezers until they were returned to the laboratory the next day and stored at −80 °C until analyzed, when they were thawed at room temperature and then centrifuged at 2500 g for 15 minutes at −4°C. Salivary cortisol levels were assayed in duplicate using high sensitivity enzyme immunoassays (Diagnostic Systems Laboratories; Webster, Texas) with a mean lower sensitivity limit of 0.11 µg/dL, standard curve range from 0.1 to 10µg/dL. The intra-assay and inter-assay coefficients of variation were less than 5% at all levels of the calibrator curve. The concentration of cortisol in saliva was expressed as µg/dL. To minimize the potential effects of exposure to stressful events during the sampling period participants who were currently students were not sampled the week prior to scheduled class examinations. Participants were asked to record stress exposures/daily hassles (Van Eck et al., 1996), sleep disturbances (Lasikiewicz et al., 2008) and protocol noncompliance (teeth brushing, eating, etc) known to affect HPA axis activity during the sampling period. However, there were no problems recorded during the sampling period and thus; analyses of data were performed on a final sample of 59 subjects.
Statistical Analyses
Details of the sample and the analytic approach were presented in a prior publication based on the same sample (Mangold et al., 2010). A total of 65 participants were screened for enrollment in the study, and six participants were excluded based on use of oral contraceptives, prescription medication use, and a diagnosis of major depression. The analyses are based on data for 59 participants. Descriptive statistics are reprinted here to characterize the sample. Cortisol data were log-transformed to correct positive skew. The primary statistical design was a mixed effects regression model with repeated measures at four time points (awakening and 30, 45, and 60 minutes thereafter). Fixed design effects were self-reported Neuroticism (NEO-PI-R; Costa and McCrae, 1992), used as a continuous, dimensional variable (raw scores ranged from 31 to146 and T scores ranged from 22.82 to 85.58), Acculturation (ARSMA-II; Cuéllar et al., 1995), a continuous variable used in our prior study, and Time (a fixed classification factor with four levels), and their interaction. We used a fully crossed design (Neuroticism × Acculturation × Time), focusing on interactions with Time which test effects on the changes in cortisol over time. Continuous variables were centered by subtracting the mean to reduce confounding of lower and higher order effects (Kraemer and Blasey, 2004).
Modeling time as a fixed classification factor with 4 levels simplified specification of the covariance structure and presentation of the results, and was supported by the observation of relatively little intra-individual variability in the timing of cortisol sampling: 89% of the samples were obtained within five minutes of the scheduled time, and 95% within 10 minutes of protocol. We specified the Kenward-Rogers adjustment for degrees of freedom and a spatial power structure for the covariance matrix with measures at 0, 30, 45 and 60 minutes. This is a generalization of first-degree autoregressive structure to the case of unequally spaced measures. It was preferred to autoregressive, compound symmetry, or an unstructured matrix by the BIC likelihood-criterion provided by SAS MIXED (Littell, et al., 1996, pp. 126–130).
All but three participants provided cortisol data following the same measurement protocol on two consecutive weekdays. We used the average of the day one and day two data because averaging over a period from two to six days increases trait specificity (Clow et al., 2004; Hellhammer et al., 2007; Kunz-Ebrecht et al., 2004, Scholtz et al., 2004; Thorn et al., 2006, 2009) and considerably simplifies the covariance structure and analysis. We had no expectation of meaningful day-to-day variability, and confirmed this by performing preliminary mixed effects analyses including Day as an additional design factor. These did not reveal any significant effects involving Day or meaningfully change the results, and are not reported further. Statistical analyses were done using the SAS 9.2 statistical library. Tests were at unadjusted two-tailed p =.05. To aid interpretation of the Neuroticism by Acculturation interaction, we graphed means at each time point for three patterns of the dimensions: one SD below the mean, at the mean, and one SD above the mean on both scales.
Results
Participant Characteristics
Table 1 presents demographic characteristics of the sample. Participants were single (n = 59), healthy, adult males (44 percent) and females (56 percent). Most were first or second year college students. They ranged in age from 18–38 years (median = 20 years, M = 22.0, SD = 5.4) and Body Mass Index (BMI) within normal parameters (M = 24.4, SD = 4.2). A little over half the sample reported an annual family income of $40,000 or less, and less than one-fifth reported a household income at or above $80,000. Approximately one-third of the sample were first generation Mexican Americans (born in Mexico and immigrated as a child or as an adult to the United States), approximately one-fourth of the sample were second generation (born in the U.S. of Mexican born parents, and a little over forty percent were third generation or more (parents and self were born in the United States). The majority were bilingual. Participants were generally healthy, with mean scores on the General health subscale of the RAND-36 in line with those previously reported for healthy, young adults (M = 77.6, SD = 20.1; Vander Zee et al., 1996). No current or lifetime psychiatric diagnoses or current use of psychotropic medication was reported. Individuals with HDI scores higher than 10, indicating depressive symptomatology, were excluded from the study, and therefore, the sample HDI mean (M =2.8, SD =2.4) is lower than normative means reported for college-aged participants (M = 5.16, SD = 4.48), reported by Reynolds and Kobak (1995b) and Vander Zee et al. (1996). However, scores on the HDI ranged from 0 to 9.50, suggesting subjects endorsed a range of depressive symptoms.
Table 1. Sample demographics for Mexican American adult, males and females.
Table 1 presents demographic characteristics of the sample. Participants were single, bilingual, healthy, young adult male and female college students, with an even distribution among first, second and third generational groups.
| Overall Sample |
Females | Males | |
|---|---|---|---|
| Gender | (n=59) | 33 (56%) | 26 (44%) |
| Age (Median and Range) | 20 (18–38) | 21(18–38) | 19 (18–37) |
| Body Mass Index (Mean and SD) | 24.39 ± 4.19 | 24.54 ± 4.5 | 24.20 ± 3.8 |
| Parental/Participant Income | |||
| Below 40K | 33 (55.9%) | 22 (66.7%) | 11 (42.3%) |
| 40K–80K | 16 (27.1%) | 7 (21.2%) | 9 (34.6%) |
| 80K and above | 10(17.0%) | 4 (12.1%) | 6 (23.1%) |
| Parental Occupational Status | |||
| Executive | 10 (16.9%) | 5(15.2%) | 5 (19.2%) |
| Administrative/Management | 18 (30.5%) | 11 (33.3%) | 7 (27.0%) |
| Clerical/Skilled Manual Labor | 20 (34.0%) | 11 (33.3%) | 9 (34.6%) |
| Semi-skilled or Unskilled Labor | 11 (18.6%) | 6 (18.2%) | 5 (19.2%) |
| Participant Education Level | |||
| ≤12 years | 4 (6.8%) | 2 (6.1%) | 2(7.7%) |
| 13 years | 23 (39.0%) | 11 (33.3%) | 12 (46.1%) |
| 14 years | 13 (22.0%) | 11 (33.3%) | 2 (7.7%) |
| 15 years | 6 (10.2%) | 2 (6.1%) | 4 (15.4%) |
| ≥16 year | 13 (22.0%) | 7 (21.2%) | 6 (23.1%) |
Exposure to Early Trauma
Participants, on average, reported exposure to more than five different trauma items across all trauma dimensions (M = 5.32, SD = 4.25). This is consistent with epidemiological evidence suggesting that over 56% of Americans experience a lifetime trauma, and risk of exposure to general or complex traumas are reported to be as high as 72% among Latino/Hispanic children (NCTSN; 2005).
Neuroticism
Table 2 reports mean raw scores for the NEO PI-R personality factor of Neuroticism and neuroticism facets for the Mexican-American male and female subjects in the current sample. To our knowledge, normed scores for Mexican-American samples are not yet available. Overall raw scores for the current sample were consistent with norms published for a predominately Caucasian sample (Costa and McCrae, 1992a) and with scores previously reported for a sample of Mexican American college students (Mangold et al., 2007). There were no significant differences in the neuroticism factor and facets scores among males and females.
Table 2. Neuroticism factor and facet scores for Mexican American adults (NEO-PI-R; Means and SD).
Table 2 reports Neuroticism factor and facet scores for the sample of Mexican American adults (NEO-PI-R; Costa and McCrae, 1992). Overall raw scores for the current sample were consistent with norms published for predominately Caucasian college samples (Costa and McCrae, 1992a) and with scores previously reported for a Mexican American, college sample (Mangold et al., 2007). There were no significant differences on the neuroticism factor and facet scores among males and females
| N=59 | College Aged Males | College Aged Females |
|---|---|---|
| Factors | ||
| Neuroticism (N) | 92.24 (26.5) | 86.9 (23.3) |
| Facets | ||
| Anxiety (N1) | 14.8 (5.3) | 16.9 (5.2) |
| Angry Hostility (N2) | 15.4 (5.2) | 13.7 (5.5) |
| Depression (N3) | 14.8 (6.3) | 13.5 (6.0) |
| Self-Consciousness (N4) | 16.6 (5.3) | 14.6 (5.2) |
| Impulsiveness (N5) | 19.0 (4.3) | 16.2 (4.3) |
| Vulnerability (N6) | 13.2 (5.5) | 12.1 (4.1) |
Characterization of the Cortisol Awakening Response
Salivary cortisol response for the subject sample was characterized with respect to overall cortisol secretory activity measured in the period 1hr after awakening and the change in concentration from awakening to 30 min post-awakening (Clow et al., 2004). Calculation of the mean changes in cortisol concentration from awakening to 30 min post-awakening for trial one (12.4 ± 16.5 nmol/l) and trial two (9.9 ± 16.1 nmol/l) compare to what is previously reported (9.3 ± 3.1 nmol/l), although with more variation (Clow et al., 2004). The correlation between delta cortisol for days one and two was r = 0.47 (df = 54, p = .0003; Spearman rho = 0.34, p=.01). The simple correlation across all data points (i.e., using 4 values per person with N = 59) was r = 0.54 for raw cortisol, and r = 0.42 for log cortisol.
Neuroticism, Acculturation and Cortisol Awakening Response
Table 3 presents the mixed effects regression analysis. The two factor Acculturation by Time interaction was significant (p = 0.0014), although the Neuroticism by Time interaction was not. The three-factor Neuroticism by Acculturation by Time interaction was highly significant (p = 0.0026) when the Neuroticism and Acculturation scales were included as dimensional covariates. Moreover, when the three subjects who did not provide cortisol data on Day 2 were omitted from the analyses, the three-factor interaction remained significant (F=4.99, df=3, 154, p=0.0025).
Table 3. The effects of neuroticism and acculturation on the Cortisol Awakening Response (CAR) in Mexican Americans.
Table 3 presents results from the mixed effects regression model examining the effects of neuroticism and acculturation on the Cortisol Awakening Response (CAR) in Mexican Americans. Results showed a significant Neuroticism × Acculturation × Time interaction (p = .0026). Mexican Americans with greater Mexican orientation and low neuroticism demonstrated a healthy, robust CAR, while the CAR was significantly attenuated in Mexican Americans with relatively greater Anglo orientation and high neuroticism.
| Effect | df | F | P |
|---|---|---|---|
| Neuroticism (N) | 1,59 | 2.01 | 0.16 |
| Acculturation (A) | 1,59 | 3.00 | 0.09 |
| N × A | 1,59 | 0.09 | 0.76 |
| Time | 3,163 | 28.02 | < 0.0001 |
| N × Time | 3,163 | 1.18 | 0.32 |
| A × Time | 3,163 | 5.42 | 0.0014 |
| N × A × Time | 3,163 | 4.93 | 0.0026 |
To display the result, we split both the Neuroticism and Acculturation scales at their means to create dichotomous indicators (low, high), and reanalyzed the data using these classifications in a 2 (Neuroticism) × 2 (Acculturation) × 4 (Time) mixed effects regression model. Figure 1 depicts the estimated CAR in the four groups created by crossing these dichotomous classifications. Participants who were low on both dimensions had a greater CAR in comparison to the other groups (a test of change over time in this group yielded (F = 14.75, df = 3,162, p<0.0001). Those high on one of the two dimensions but low on the other had a somewhat attenuated CAR. Their CAR profiles were quite similar, and both indicated significant change over time (F = 10.46 and 8.58 respectively, df = 3, 162, p<0.0001). In contrast, those with high levels of both Neuroticism and Acculturation showed almost no CAR at all (test for change over time yielded F =1.07, df = 3, 162, p = 0.36). The combined effect of being high on both dimensions was particularly evident in the virtual lack of change in cortisol seen in this group at 30 minutes after awakening.
Figure 1. The effects of the interaction of Neuroticism (N) by Acculturation (A) by Time on the Cortisol Awakening Response (CAR) in Mexican Americans.
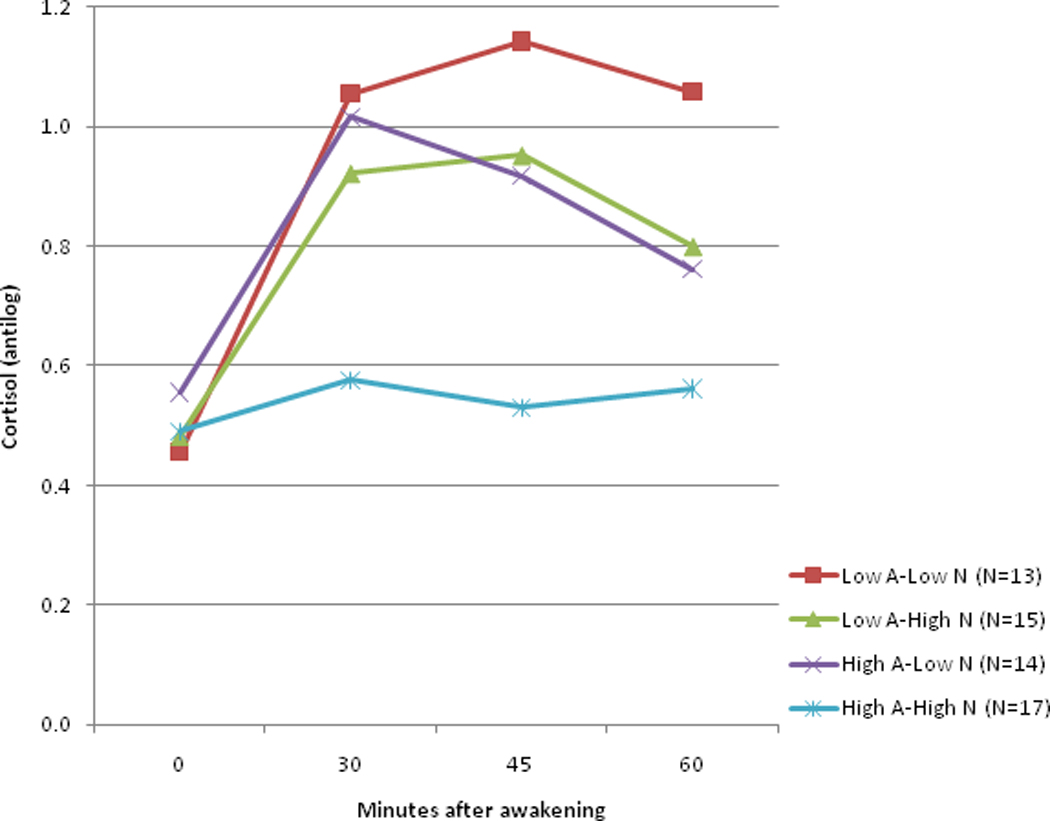
The effects of the Time × Neuroticism × Acculturation interaction on the Cortisol Awakening Response (CAR). The three-factor Neuroticism by Acculturation by Time interaction was significant (p = 0.0026) when the Neuroticism and Acculturation scales were included as dimensional covariates. To display the result, Neuroticism and Acculturation scales were split at their means to create dichotomous indicators (low, high), and the data reanalyzed using these classifications in a 2 (Neuroticism) × 2 (Acculturation) × 4 (Time) mixed effects regression model. Participants who were low on both dimensions had a greater CAR (a test of change over time in this group yielded (F = 14.75, df = 3,162, p<0.0001). In contrast, greater neuroticism was associated with an attenuated CAR in highly acculturated Mexican Americans with greater Anglo orientation (test for change over time yielded (F =1.07, df = 3, 162, p = 0.36). The graphic displays the exponentiated means of the log-transformed data (antilogs are presented to put the data in its original scale).
Simple effects analyses indicate a non-significant group difference at Time 0 (F=0.31, p=0.82), and significant differences at 30 (F=3.98, p=0.010), 45 (F=5.51, p=0.0015) and 60 minutes (F=3.21, p=0.0263, all numerator df=3). Examination of effect size using Cohen’s d, calculated for the data displayed in Figure 1, suggest differences between the Low Acculturation-Low Neuroticism group (most robust CAR) and the High Acculturation-High Neuroticism group (most attenuated CAR) are very large (large effect size) at all three time points (d=1.24, 1.30, 1.10). Differences between the Low Acculturation-Low Neuroticism group and the intermediate groups with mixed features (high on one dimension only) are moderate (medium effect size) only by 60 minutes (d=0.49, 0.57). Differences between those intermediate groups and the High Acculturation – High Neuroticism group are large, initially at 30 minutes (d=0.96, 1.17).
Finally, we evaluated the robustness of these results when adding age, sex, level of family income and a measure of early trauma to the analysis as covariates. The three-factor interaction of Neuroticism by Acculturation by Time remained significant (F = 4.82, df = 3, 159, p =.0031) and the pattern of estimated means was virtually unchanged (the mean difference in estimated means was zero). Moreover, although generational status was highly correlated with the ARSMA (r=0.605, df=57, p<.0001) in our sample, when we added Generation to the regression model both as a main effect and interaction with time (to capture effects of Generation on the CAR), Generation had no effect on the three factor interaction, which was slightly more significant with these two Generation effects in the model (F=5.12, df=3, 160, p=.0021). Generation itself was not a significant predictor in this model (main effect F=0.02, df=1, 57.8, p=0.91, interaction with time F=1.44, df=3, 160, p=0.23). Income had no meaningful effects when added as a covariate (p<0.0003 for the interaction).
Discussion
The current study assessed the effects of neuroticism and acculturation on the CAR in Mexican Americans while utilizing a carefully constructed sample monitoring system. This is the first study to distinguish the effects of neuroticism from the effects of acculturative stress on the CAR in Mexican Americans, and thus the first to demonstrate that less acculturated Mexican Americans with greater Mexican orientation who were low in neuroticism demonstrated a magnitude of response and pattern of change over time indicating a greater CAR compared to their highly acculturated counterparts with high neuroticism. Greater neuroticism was associated with an attenuated CAR in highly acculturated Mexican Americans with greater Anglo orientation. Particularly dramatic effects on the CAR were seen in highly acculturated Mexican Americans with high neuroticism. In that group, the CAR virtually disappeared, and changes in cortisol over time were not significant. Notably, attenuation of the CAR was independent of the effects of clinical depression and after controlling for childhood trauma, age and gender.
The current study has a number of strengths. Neuroticism increases the risk for depression in adulthood (Duggan et al., 1995; Fanous et al., 2002; Kendler et al., 1993, 2004; Roberts and Kendler, 1999) and depression alters HPA axis dynamics including the CAR (Heim and Nemeroff, 2001; Huber et al., 2006; Pruessner et al., 2003; Stetler and Miller, 2005). However, the current findings are not attributable to the effects of clinical depression given that participants in the study were excluded if they met diagnostic criteria for current or lifetime depression. In addition, we statistically controlled for the potential effects of childhood trauma, age, and gender on the CAR, all of which are known to influence the CAR. Participants were excluded for current antidepressant use, alcohol, nicotine and other drug use disorders to minimize the effects of these potential confounds on the HPA axis (Aihara et al., 2007; Badrick et al., 2007; Harris et al., 2007; Steptoe and Ussher, 2006; Wand and Dobs, 1991). To mitigate the effects of estrogen and progesterone on the HPA axis female participants were excluded for oral contraceptive use and sampled during the early follicular phase (Kirschbaum et al., 1999; Kudielka and Kirschbaum, 2005). Measurements were performed with strict reference to time of awakening (Federenko et al., 2004; Williams et al., 2005; Wust et al., 2000b) and sampling error was minimized through the use of electronic monitoring devices (Broderick et al., 2004; Kudielka et al., 2003; review in Hansen et al., 2008). Moreover, the current study measured the CAR across two separate sampling sessions to minimize the influence of fluctuating situational factors on the CAR, and to maximize trait specificity (Buske-Kirschbaum et al., 2007; Clow et al., 2004; Hellhammer et al., 2007; Kunz-Ebrecht et al., 2004; Scholtz et al., 2004; Thorn et al., 2006).
Our finding that greater neuroticism is associated with an attenuated CAR in highly acculturated Mexican Americans with greater Anglo orientation is consistent with findings showing an attenuated CAR in individuals with high neuroticism (Hauner et al., 2008), severe stress (Thorn et al., 2006) and burnout (Pruessner et al., 1999; Sonnenschein et al., 2007). Findings support previous studies showing the relationship between greater acculturative stress and negative health outcomes may be modulated by personality variables in various cultural groups (Lazarus and Opton, 1966) including Mexican-Americans (Cervantes & Castro, 1985; Mena, et al., 1987; Miranda & Matheny, 2000; Padilla et al., 1986; Pearlin & Schooler, 1978). However, findings from the current study are in contrast with studies showing neuroticism is associated with an enhanced CAR. For example, in a study of 30, healthy males and females, aged 21 to 57, neuroticism was associated with an enhanced CAR after controlling for past and current Axis 1 disorders (depression), medical illness and medication use, but not for childhood trauma, oral contraceptives, age or determination of sampling compliance (Portella et al., 2005). Similar results were reported in a study of 144 newly diagnosed, female breast cancer patients and controls, aged 30 to 75, in which neuroticism was associated with greater early morning peak cortisol levels, but only in the breast cancer patients, and with no controls for childhood trauma, age, oral contraceptive use, menstrual cycle phase, or determination of sampling compliance (Vedhara et al., 2006). Additionally, the effects of traits highly correlated with neuroticism (e.g., negative affect and attachment anxiety) on the CAR were assessed in a study of 334 healthy males and females, aged 18 to 54, in which greater negative affect was associated with a larger rise in CAR, but only in males, after controlling for depression, medical illness, age, and sampling compliance, but not for childhood trauma, oral contraceptive use, menstrual phase or hormone replacement (Polk et al., 2005). Conflicting results may be due to failure to control for crucial factors known to influence the CAR (e.g., childhood trauma, age, gender, variations in the measurement of cortisol, determination of sampling compliance and the potential moderating effects of exposure to chronic stressors), that were controlled in the current study.
Current results showing attenuation of the CAR in highly acculturated Mexican Americans with greater Anglo orientation suggests that acculturation can lead to the dysregulation of crucial physiological stress response systems for some Mexican Americans. Consistent with the attenuation hypothesis, it is plausible that, severe challenges and stressors associated with acculturation are associated with the development of allostatic load over time in particularly vulnerable individuals leading to alterations of the circadian rhythm and decreased adrenal capacity (Pruessner et al., 1999; Sapolsky, 2004). Similar effects on the axis are observed in individuals exposed to discrimination (Steptoe et al., 2007) and downward socioeconomic trajectories (Gustafsson et al., 2009).
There are some limitations to the current study worth noting. The retrospective design prohibits determination of causal pathways between neuroticism, acculturation and attenuation of the CAR. A significant portion of the sample reported a relatively low household income; therefore it is possible that family income contributed to a diminished CAR, possibly due to material hardship (Anderson and Armstead, 1995; Ranjit et al., 2005). However, we believe it is unlikely that our findings are due to the overall lower income in our sample because there was no correlation between parental income and either neuroticism (r=0.08, df=55, p=0.52) or acculturation (r = 0.21, df = 55, p = 0.11) and the effects of socioeconomic parameters on cortisol secretion and the CAR indicate an inverse relationship (Bennett et al., 2004; Kapuku et al., 2002; Kunz-Ebrecht et al., 2004; Lupien et al., 2000, 2001; Rosmond and Bjorntorp, 2000; Wright and Steptoe, 2005). However, it is possible that the burden imposed by increased economic burden contributed to an initial enhancement transitioning over time to attenuation of the CAR.
Determination of the precise developmental mechanisms underlying the relationship between neuroticism and attenuation of the CAR in highly acculturated Mexican Americans is beyond the scope of the current study. However, it is possible that individuals with high levels of neuroticism are particularly susceptible to increased neuroendocrine reactivity following exposure to the chronic stressors associated with acculturation leading to significant attenuation of the CAR. Consistent with the attenuation hypothesis, exposure to chronic stress may lead to enhanced stress reactivity, resulting in down regulation of central glucocorticoid receptors over time. The hypothalamic CRF system may undergo desensitization transitioning from a system with increased stress responsivity to a system with attenuated cortisol responses to stress and an attenuated CAR (Gunnar and Vazquez, 2001; Heim et al., 2008; Susman, 2006; Trickett et al., 2010). Alternatively, it is also plausible that exposure to stressors associated with acculturation in Mexican Americans with high levels of neuroticism produces a blunted CAR with no antecedent HPA axis hyperactivity.
Taken together, these findings show that less acculturated Mexican Americans with greater Mexican orientation who were low in neuroticism demonstrated a greater CAR compared to their more acculturated counterparts with higher neuroticism. In highly acculturated Mexican Americans with greater Anglo orientation and high neuroticism, the CAR was significantly attenuated.
RESEARCH HIGHLIGHTS.
We examined the effects of neuroticism, and acculturation on cortisol awakening response (CAR).
59 healthy Mexican American adults, ages 18 to 38 years, enrolled in the study.
Salivary samples were collected at awakening, and 30, 45, and 60 minutes thereafter on 2 days.
Results showed a significant Neuroticism × Acculturation × Time interaction.
The CAR was attenuated in highly acculturated Mexican Americans with high neuroticism.
ACKNOWLEDGEMENTS
This work was supported by NIH grant R24-MH070636-01A1 (DLM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K, Fukuda M, Oriuchi N, Endo K, Matsuda H, Mikuni M. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res. 2007;155:245–256. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Anderson NB, Armstead CA. Toward understanding the association of socioeconomic status and health: A new challenge for the biopsychosocial approach. Psychosom. Med. 1995;57:213–225. doi: 10.1097/00006842-199505000-00003. [DOI] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J. Clin. Endocrinol. Metab. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Bao AM, Ji YF, Van Someren EJW, Hofman MA, Liu RY, Zhou JN. Diurnal rhythms of free estradiol and cortisol during the normal menstrual cycle in women with major depression. Horm. Behav. 2004;45:93–102. doi: 10.1016/j.yhbeh.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bao AM, Liu RY, van Someren EJW, Hofman MA, Cao YX, Zhou JN. Diurnal rhythm of free estradiol during the menstrual cycle. European Journal of Endocrinology. 2003;148:227–232. doi: 10.1530/eje.0.1480227. [DOI] [PubMed] [Google Scholar]
- Bartels M, Van den Berg M, Sluyter F, Boomsma DI, de Geus EJC. Heritability of cortisol levels: Review and simultaneous analysis of twin studies. Psychoneuroendocrinology. 2003;28:121–137. doi: 10.1016/s0306-4530(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Bennett GG, Merritt MM, Wolin KY. Ethnicity, education, and the cortisol response to awakening: A preliminary investigation. Ethn. Health. 2004;9:337–347. doi: 10.1080/1355785042000285366. [DOI] [PubMed] [Google Scholar]
- Bernal G, Saez-Santiago E. Culturally centered psychosocial interventions. J. Comm. Psychol. 2006;34:121–132. [Google Scholar]
- Berry JW. Acculturation as varieties of adaptation. In: Padilla A, editor. Acculturation: Theory, models and some new findings. Boulder, CO: Westview; 1980. [Google Scholar]
- Bhagwagar Z, Hafizi S, Cowen PJ. Psychopharmacology. Vol. 182. 2005. Increased salivary cortisol after waking in depression; pp. 54–57. [DOI] [PubMed] [Google Scholar]
- Bolger N, Zuckerman A. A framework for studying personality in the stress process. JPSP. 1995;69:890–902. doi: 10.1037//0022-3514.69.5.890. [DOI] [PubMed] [Google Scholar]
- Broderick JE, Arnold D, Kudielka BM, Kirschbaum C. Salivary cortisol sampling compliance: Comparison of patients and healthy volunteers. Psychoneuroendocrinology. 2004;29:636–650. doi: 10.1016/S0306-4530(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Kern S, Ebrecht M, Hellhammer DH. Altered distribution of leukocyte subsets and cytokine production in response to acute psychosocial stress in patients with psoriasis vulgaris. Brain. Behav. Immun. 2007;21:92–99. doi: 10.1016/j.bbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Cervantes RC, Castro FG. Stress, coping and Mexican-American mental health: A systematic review. Hispanic J. Behav. Sci. 1985;7:1–73. [Google Scholar]
- Chan SW, Goodwin GM, Harmer CJ. Highly neurotic never-depressed students have negative biases in information processing. Psychol. Med. 2007;37:1281–1291. doi: 10.1017/S0033291707000669. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: Methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Costa P, Jr, McCrae R. Revised NEO Personality Inventory (NEO-PIR) and NEO Five-Factor Inventory professional manual. Odessa, Florida: Psychological Assessment Resources; 1992a. [Google Scholar]
- Costa P, Jr, McCrae R. Sonderegger TB. Psychology and aging: Nebraska Symposium on Motivation 1991. Lincoln: University of Nebraska; 1992b. Trait psychology comes of age; pp. 169–204. [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR, Dye DA. Facet scales for Agreeableness and Conscientiousness: A revision of the NEO Personality Inventory. Pers. Indiv. Diff. 1991;12:887–898. [Google Scholar]
- Cuellar I, Arnold B, Maldonado R. Acculturation rating-scale for Mexican-Americans II - A revision of the original ARSMA scale. Hispanic J. Behav. Sci. 1995;17:275–304. [Google Scholar]
- Dedovic K, Engert V, Duchesne A, Lue SD, Andrews J, Efanov SI, Beaudry T, Pruessner JC. Cortisol awakening response and hippocampal volume: Vulnerability for major depressive disorder? Biol. Psychiatry. 2010;68:847–853. doi: 10.1016/j.biopsych.2010.07.025. [DOI] [PubMed] [Google Scholar]
- De Graaf R, Bijl RV, Ravelli A, Smit F, Vollebergh AM. Predictors of first incidence of DSM-III-R psychiatric disorders in the general population: Findings from the Netherlands Mental Health Survey and Incidence Study. Acta Psychiatrica Scannavica. 2002;106:303–313. doi: 10.1034/j.1600-0447.2002.01397.x. [DOI] [PubMed] [Google Scholar]
- Duggan C, Sham P, Lee A, Minne C, Murray R. Neuroticism: A vulnerability marker for depression evidence from a family study. J. Affect. Disord. 1995;35:139–143. doi: 10.1016/0165-0327(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Fanous A, Gardner CO, Prescott CA, Cancro R, Kendler KS. Neuroticism, major depression and gender: A population-based twin study. Psychol. Med. 2002;32:719–728. doi: 10.1017/s003329170200541x. [DOI] [PubMed] [Google Scholar]
- Federenko I, Wust S, Hellhammer DH, Dechoux R, Kumsta R, Kirschbaum C. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004;29:174–184. doi: 10.1016/s0306-4530(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Felsten G. Minor stressors and depressed mood: Reactivity is more strongly correlated than total stress. Stress Health. 2002;18:75–81. [Google Scholar]
- Felsten G. Stress reactivity and vulnerability to depressed mood in college students. Pers. Individ. Dif. 2004;36:789–800. [Google Scholar]
- Finch BK, Kolody B, Vega WA. Perceived discrimination and depression among Mexican-origin adults in California. J. Health Soc. Behav. 2000;41:295–313. [PubMed] [Google Scholar]
- Finch BK, Vega WA. Acculturation stress, social support, and self-rated health among Latinos in California. J. Immigr. Health. 2003;5:109–117. doi: 10.1023/a:1023987717921. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev. Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Janlert U, Theorell T, Hammarstrom A. Life-course socioeconomic trajectories and diurnal cortisol regulation in adulthood. Psychoneuroendocrinology. 2009;35:613–623. doi: 10.1016/j.psyneuen.2009.09.019. [DOI] [PubMed] [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Appl. Nurs. Res. 2006;19:95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hansen AM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: A review. Scand. J. Clin. Lab. Invest. 2008;68:448–458. doi: 10.1080/00365510701819127. [DOI] [PubMed] [Google Scholar]
- Harris A, Ursin H, Murison R, Eriksen HR. Coffee, stress and cortisol in nursing staff. Psychoneuroendocrinology. 2007;32:322–330. doi: 10.1016/j.psyneuen.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Hauner KK, Adam EK, Mineka S, Doane LD, DeSantis AS, Zinbarg R, Craske M, Griffith JW. Neuroticism and introversion are associated with salivary cortisol patterns in adolescents. Psychoneuroendocrinology. 2008;33:1344–1356. doi: 10.1016/j.psyneuen.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2007;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hovey JD, King CA. Acculturative stress, depression and suicidal ideation among immigrant and second-generation Latino adolescents. J. Am. Acad. of Child and Adolesc. Psychiatry. 1996;35:1183–1192. doi: 10.1097/00004583-199609000-00016. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Issa K, Schik G, Wolf OT. The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrinology. 2006;31:900–904. doi: 10.1016/j.psyneuen.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Huebner DM, Nemeroff CJ, Davis MC. Do hostility and neuroticism confound associations between perceived discrimination and depressive Symptoms? J. Soc. Clin. Psychol. 2005;24:723–740. [Google Scholar]
- Huizink AC, Ferdinand RF, Ormel J, Verhulst FC. Hypothalamic-pituitary-adrenal axis activity and early onset of cannabis use. Addiction. 2006;101:1581–1588. doi: 10.1111/j.1360-0443.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, de Lacerda RB, Ling W, Marsden J, Monteiro M, Nhiwatiwa S, Pal H, Poznyak V, Simon S. Validation of the alcohol, smoking and substance involvement screening test (ASSIST) Addiction. 2008;103:1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- Jacobs N, Nicolson NA, Derom C, Delespaul P, van Os J, Myin-Germeys I. Electronic monitoring of salivary cortisol sampling compliance in daily life. Life Sci. 2005;76:2431–2443. doi: 10.1016/j.lfs.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Kanaley JA, Sames C, Swisher L, Swick AG, Ploutz-Snyder LL, Steppan CM, Sagendorf KS, Feiglin D, Jaynes EB, Meyer RA, Weinstock RS. Abdominal fat distribution in pre- and post-menopausal women: The impact of physical activity, age, and menopausal status. Metabolism. 2001;50:976–982. doi: 10.1053/meta.2001.24931. [DOI] [PubMed] [Google Scholar]
- Kapuku GL, Treiber FA, Davis HC. Relationships among socioeconomic status, stress induced changes in cortisol, and blood pressure in African American males. Ann. Behav. Med. 2002;24:320–325. doi: 10.1207/S15324796ABM2404_08. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Young R, Sweeting H, Fischer JE, West P. Levels and confounders of morning cortisol collected from adolescents in a naturalistic (school) setting. Psychoneuroendocrinology. 2008;33:1257–1268. doi: 10.1016/j.psyneuen.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex and stressful life events in the prediction of episodes of major depression. Am. J. Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- Kendler K, Neale M, Kessler R, Heath C, Eaves L. A longitudinal twin study of personality and major depression in women. Arch. Gen. Psychiatry. 1993;50:853–862. doi: 10.1001/archpsyc.1993.01820230023002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom. Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Blasey CM. Centering in regression analyses: A strategy to prevent errors in statistical inference. Int. J. Methods Pscyhiatr. Res. 2004;13:141–151. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: A review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Lasikiewicz N, Hendrickx H, Talbot D, Dye L. Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: Associations with sleep quality and metabolic parameters. Psychoneuroendocrinology. 2008;33:143–151. doi: 10.1016/j.psyneuen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Lazarus RS, Opton E. A cross-cultural study of stress-reaction patterns in Japan. J. Pers. Soc. Psychol. 1966;4:622–633. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW. SAS System for Mixed Models. Cary, NC: SA; 1996. [Google Scholar]
- Lundberg U, Hellstrom B. Workload and morning salivary cortisol in women. Work & Stress. 2002;16:356–363. [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child's stress hormone levels correlate with mother's socioeconomic status and depressive state. Biol. Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien S, King S, Meaney M, McEwen B. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev. Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Mangold D, Veraza R, Kinkler L, Kinney N. Neuroticism as a predictor of acculturative stress in Mexican-American college students. Hispanic J. Behav. Sci. 2007;29:366–383. [Google Scholar]
- Mangold D, Wand G, Javors M, Mintz J. Acculturation, childhood trauma and the cortisol awakening response in Mexican-American adults. Horm. Behav. 2010;58:637–646. doi: 10.1016/j.yhbeh.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold DL, Marino E, Javors M. The cortisol awakening response predicts subclinical depressive symptomatology in Mexican Americans. J Psychiatric Research. 2011;45:902–909. doi: 10.1016/j.jpsychires.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson K, Jorden S, Anisman H. Relations between trauma experiences and psychological, physical and neuroendocrine functioning among Somali refugees: Mediating role of coping with acculturation stressors. J. Immigr. Minor. Health. 2008;10:291–305. doi: 10.1007/s10903-007-9086-2. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Joint factors in self-reports and ratings: Neuroticism, extroversion, and openness to experience. Pers. Indiv. Diff. 1983;4:245–255. [Google Scholar]
- McEwen B, Lasley EN. Allostatic load: When protection gives way to damage. Adv. Mind Body Med. 2003;19:28–33. [PubMed] [Google Scholar]
- Mena FJ, Padilla AM, Maldonado M. Acculturative stress and specific coping strategies among immigrant and later generation college students. Hispanic J. Behav. Sci. 1987;9:207–225. [Google Scholar]
- Meulenberg PMM, Hofman JA. The effect of oral-contraceptive use and pregnancy on the daily rhythm of cortisol and cortisone. Clin. Chim. Acta. 1990;190:211–221. doi: 10.1016/0009-8981(90)90175-r. [DOI] [PubMed] [Google Scholar]
- Miranda AO, Matheny KB. Socio-psychological predictors of acculturative stress among Latino adults. J. M. Health Couns. 2000;22:306–317. [Google Scholar]
- Ormel J, Oldehinkel AJ, Brilman EI. The interplay and etiological continuity of neuroticism, difficulties, and life events in the etiology of major and subsyndromal, first and recurrent depressive episodes in later life. Am. J. Psychiatry. 2001;158:885–891. doi: 10.1176/appi.ajp.158.6.885. [DOI] [PubMed] [Google Scholar]
- Padilla AM, Alvarez M, Lindholm KJ. Generational status and personality factors as predictors of stress in students. Hispanic J. Behav. Sci. 1986;8:275–288. [Google Scholar]
- Pearlin L, Schooler C. The structure of coping. J. Health and Soc. Behav. 1978;19:2–21. [PubMed] [Google Scholar]
- Polk DE, Cohen S, Doyle WJ, Kirschbaum C. State and trait affect as predictors of salivary cortisol in healthy adults. Psychoneuroendocrinology. 2005;30:261–272. doi: 10.1016/j.psyneuen.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Portella M, Harmer C, Flint J, Cowen P, Goodwin G. Enhanced early morning salivary cortisol in neuroticism. Am. J. Psychiatry. 2005;162:807–809. doi: 10.1176/appi.ajp.162.4.807. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom. Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosom. Med. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Young EA, Kaplan GA. Material hardship alters the diurnal rhythm of salivary cortisol. Int. J. Epidemiol. 2005;34:1138–1143. doi: 10.1093/ije/dyi120. [DOI] [PubMed] [Google Scholar]
- Reynolds WM, Kobak KA. Reliability and validity of the Hamilton Depression Inventory: A paper-and-pencil version of the Hamilton Depression Rating Scale Clinical Interview. Psychological Assessment. 1995a;7:472–483. [Google Scholar]
- Reynolds WM, Kobak KA. A Self-Report Version of the Hamilton Depression Rating Scale (HDRS) Professional Manual. Lutz, FL: Psychological Assessment Resources; 1995b. [Google Scholar]
- Roberts S, Kendler K. Neuroticism and self-esteem as indices of the vulnerability to major depression in women. Psychol. Med. 1999;29:1101–1109. doi: 10.1017/s0033291799008739. [DOI] [PubMed] [Google Scholar]
- Romero AJ, Roberts RE. The impact of multiple dimensions of ethnic identity on discrimination and adolescents’ self-esteem. J. Applied Soc. Psychol. 2003;33:2288–2305. [Google Scholar]
- Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J. Intern. Med. 2000;247:188–197. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Salgado de Snyder VN. Factors associated with acculturative stress and depressive symptomatology among married Mexican immigrant women. Psychol. Women Q. 1987;1:475–488. [Google Scholar]
- Sapolsky RM. Social status and health in humans and other animals. Ann. Rev. Anthropol. 2004;33:393–418. [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurmeyer TH, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999;64:1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Scholtz W, Hellhammer J, Schulz P, Stone AA. Perceived work overload and chronic worrying predict weekend-weekday differences in the cortisol awakening response. Psychosom. Med. 2004;66:207–214. doi: 10.1097/01.psy.0000116715.78238.56. [DOI] [PubMed] [Google Scholar]
- Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: Preliminary results. Psychoneuroendocrinology. 2007;32:1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Sonnenschein M, Mommersteeg PMC, Joutveen JH, Sorbi MJ, Schaufeli WB, Doornen LJP. Exhaustion and endocrine functioning in clinical burnout: An in-depth study using the experience sampling method. Biol. Psychol. 2007;75:176–184. doi: 10.1016/j.biopsycho.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain, Behav., Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Ussher M. Smoking, cortisol and nicotine. Int. J. Psychophysiology. 2006;59:228–235. doi: 10.1016/j.ijpsycho.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Blunted cortisol response to awakening in mild to moderate depression: Regulatory influences of sleep patterns and social contacts. JAP. 2005;114:697–705. doi: 10.1037/0021-843X.114.4.697. [DOI] [PubMed] [Google Scholar]
- Suh BY, Liu JH, Berga SL, Quigley ME, Laughlin GA, Yen SS. Hypercortisolism in patients with functional hypothalamic-amenorrhea. J. Clin. Endocrinol. Metab. 1988;66:733–739. doi: 10.1210/jcem-66-4-733. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: Stress, early vulnerabilities and the attenuation hypothesis. Neurosci. Biobehav. Rev. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Thoman L, Suris A. Acculturation and acculturative stress as predictors of psychological distress and quality-of-life functioning in Hispanic psychiatric patients. Hispanic J. Behav. Sci. 2004;26:293–311. [Google Scholar]
- Thorn L, Hucklebridge F, Evans P, Clow A. Suspected non-adherence and weekend versus week day differences in the awakening cortisol response. Psychoneuroendocrinology. 2006;31:1009–1018. doi: 10.1016/j.psyneuen.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Thorn L, Hucklebridge F, Evans P, Clow A. The cortisol awakening response, seasonality, stress and arousal: A study of trait and state influences. Psychoneuroendocrinology. 2009;34:299–306. doi: 10.1016/j.psyneuen.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Trickett PK, Noll JG, Susman EJ, Shenk CE, Putnam FW. Attenuation of cortisol across development for victims of sexual abuse. Dev. Psychopathol. 2010;22:165–175. doi: 10.1017/S0954579409990332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom. Med. 1996;58:447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- Van Santen A, Vreeburg SA, Van der Does AJ, Spinhoven P, Zitman FG, Penninx BW. Psychological traits and the cortisol awakening response: Results from the Netherlands Study of Depression and Anxiety. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Vander Zee K, Sanderman R, Heyink J, de Haes H. Psychometric qualities of the RAND 36-item health survey 1.0: A multidimensional measure of general health status. International Journal of Behavioral Medicine. 1996;3:104–122. doi: 10.1207/s15327558ijbm0302_2. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Tuinstra J, Miles JN, Sanderman R, Ranchor AV. Psychosocial factors associated with indices of cortisol production in women with breast cancer and controls. Psychoneuroendocrinology. 2006;31:299–311. doi: 10.1016/j.psyneuen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Vega WA, Amaro H. Latino outlook: good health, uncertain prognosis. Annu. Rev. Public Health. 1994;15:39–67. doi: 10.1146/annurev.pu.15.050194.000351. [DOI] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the Hypothalamic-Pituitary-Adrenal axis in actively drinking alcoholics. J. Clin. Endocrinol. Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Williams E, Magid K, Steptoe A. The impact of time of waking and concurrent subjective stress on the cortisol response to awakening. Psychoneuroendocrinology. 2005;30:139–148. doi: 10.1016/j.psyneuen.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30:582–590. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000a;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise Health. 2000b;2:79–88. [PubMed] [Google Scholar]


