Abstract
We have recently found that following complete Freund’s adjuvant (CFA) induced inflammation that cutaneous polymodal nociceptors (CPM) lacking the transient receptor potential vanilloid 1 (TRPV1) are sensitized to heat stimuli. In order to determine possible mechanisms playing a role in this change, we examined gene expression in the L2/L3 sensory ganglia following CFA injection into the hairy hindpaw skin and found that G-protein coupled purinoreceptor P2Y1 expression was increased. This receptor is of particular interest as most CPMs innervating mouse hairy skin bind isolectin B4 (IB4), which co-localizes with P2Y1. Additionally, our recent findings have shown that cutaneous CPMs in P2Y1−/− mice, displayed significantly reduced thermal sensitivity. Together these findings suggested a possible role for P2Y1 in inflammation-induced heat sensitization in these fibers. To test this hypothesis, we utilized our in vivo siRNA technique to knockdown the inflammation-induced increase in P2Y1 expression and then examined the functional effects using ex vivo recording. We found that the normal reduction of heat thresholds in CPM fibers induced by CFA was completely blocked by inhibition of P2Y1. Surprisingly, inhibition of P2Y1 during inflammation also significantly increased the number of CPM neurons expressing TRPV1 without a change in the total number of TRPV1 positive cells in the L2 and L3 DRGs. These results show that the inflammation-induced enhanced expression of P2Y1 is required for normal heat sensitization of cutaneous CPM fibers. They also suggest that P2Y1 plays a role in the maintenance of phenotype in cutaneous afferent fibers containing TRPV1.
1. INTRODUCTION
Peripheral inflammation induces an array of changes in dorsal root ganglion (DRG) neurons and within the targeted area, which may be responsible for sensory neuron sensitization [15; 10; 14] leading to hyperalgesia [15; 2]. The inflamed area is known to produce changes in inflammatory mediators, signaling molecules, calcitonin gene-related peptide (CGRP), bradykinin, prostaglandins, serotonin, histamine, H+, ATP, and neurotrophic factors [18; 15; 23; 20; 10; 14], while the DRGs have been shown to contain enhanced levels of neurotrophic factor receptors, ion channels and other signaling molecules [26; 31; 23].
Previous reports have shown that isolectin B4 (IB4) binding, transient receptor potential vanilloid type 1 (TRPV1) lacking, polymodal C-fibers (CPMs) have a decreased threshold after injection of complete Freund’s adjuvant (CFA) into the skin as measured by an ex vivo skin-nerve-DRG-spinal cord preparation [14], similar to what has been shown after axotomy and regeneration [10]. One particular receptor that has been hypothesized to be involved in CPM neuron heat sensitivity is the purinergic receptor P2Y1. While reports of P2Y1 distribution in DRG vary, several studies have reported that P2Y1 is expressed in the subpopulation of small diameter nociceptive sensory afferents that contain P2X3, bind to the isolectin B4 (IB4), lack TRPV1 and respond to adenosine diphosphate (ADP) [29; 28; 30; 7; 8; 13; 1; 21; 22]. In mice lacking the P2Y1 receptor, CPM neurons specifically were found to have significantly elevated thresholds to heat stimuli and lower thresholds to cold stimuli compared to wildtype mice [27]. Peripheral inflammation also caused changes in the behavioral paw withdraw thresholds to radiant heat stimuli that was reduced in P2Y1 knockout mice compared to wildtypes [22]. We have also recently shown that this population of cutaneous CPM fibers undergoes a very similar decrease in heat thresholds following peripheral nerve injury and regeneration, and that this decrease was correlated with changes in the expression of the two purinergic receptors, P2X3 and P2Y1 [10].
Although data may suggest that changes in sensory neurons themselves or in the periphery could lead to the alterations in sensory neuron response properties, there has not been a direct test of these hypotheses in vivo. In order to determine if the CFA-induced decrease in CPM heat threshold was due to dynamic changes in P2Y1 signaling in cutaneous sensory neurons, we used an ex vivo skin/nerve/DRG/spinal cord preparation to quantitatively characterize peripheral response properties of saphenous afferents after injection of CFA into the hairy hindpaw skin. We then coupled this preparation with our newly developed in vivo small interfering RNA (siRNA)-mediated knockdown strategy [11] to target P2Y1 mRNA degradation specifically in saphenous neurons during peripheral inflammation. Additionally, we examined mRNA and protein levels for P2Y1 (among others) in DRGs during inflammation to correlate changes in expression with potential mechanisms associated with CPM neuron sensitization.
2. METHODS
2.1. Animals
Electrophysiology and molecular biology experiments were conducted using age-matched adult (4–6 weeks) male Swiss Webster mice (Hilltop Farms, Scottdale, PA). All animals were housed in group cages, maintained in a 12h light-dark cycle with a temperature controlled environment and given food and water ad libitum. All procedures used in these experiments were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. Animals were cared for and used in accordance with guidelines of the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals and following institutional AAALAC approved practices.
2.2. Injection of Complete Freund’s Adjuvant (CFA)
Mice were anesthetized by isofluorane. A syringe containing a 1:1 mixture of CFA and saline and a 30g needle was used to inject CFA into the hairy hindpaw skin. Approximately 20 μL of CFA mixture was injected directly under the hairy skin starting slightly above the ankle and slowly retracting the needle as the CFA was introduced under the skin. The wound was cleaned and animals were allowed to survive for 3 and 5 days after nerve injection for immunocytochemical, electrophysiological, western blotting and/or realtime PCR analysis.
2.3. siRNA injections
siRNAs were designed and conjugated to Penetratin-1 as described previously [4; 9; 12]. Mice were anesthetized as described. A small incision made in the mid-thigh region exposed the saphenous nerve to be injected. Pen-siRNAs were heated to 65°C for 5 min prior to injection. 0.1–0.2 μL of 90 μM Penetratin-1 linked control (PenCON) or P2Y1 targeting (PenY1) siRNAs were pressure injected into the saphenous nerve using a quartz microelectrode connected to a pico-spritzer immediately prior to CFA injection. This strategy for in vivo siRNA-mediated inhibition does not cause significant injury to the sensory afferents being studied even though it requires direct injection of Penetratin-1 modified siRNAs into the saphenous nerve [12].
2.4. RNA Isolation and Realtime PCR
Animals were deeply anesthetized with a mixture of ketamine and xylazine (Ketamine: 90 mg/kg and Xylazine: 10 mg/kg). The mice were then intracardially perfused with ice cold 0.9% NaCl prior to dissection of DRGs. RNA isolation from the L2 and L3 DRGs was performed using Qiagen RNeasy mini kits for animal tissues using the supplied protocol. Purified RNA was treated with DNase I (Invitrogen) and 1 μg of DNased RNA was reverse transcribed using Superscript II Reverse Transcriptase (Invitrogen). For realtime PCR, 20 ng samples of cDNA were added to a SYBR Green MasterMix (Applied Biosystems) and run in triplicate on an Applied Biosystems Imager. Forward and reverse primer sequences used in realtime PCR reactions for TRPV1 and GAPDH were obtained from Elitt et al. [6], and primer sequences for P2X3, P2Y2 and P2Y1 were obtained from Jankowski et al., [10]. mx2 primer sequences were obtained from Jankowski et al. [11]. This gene was chosen for analysis under the experimental conditions in order to verify that there was no anti-viral related off-target effects associated with the siRNA injections [11]. Values were normalized to GAPDH and changes in expression are calculated as a ΔΔCt value that is determined by subtracting the cycle time (Ct) values of the gene of interest from the GAPDH internal control for each sample and compared among samples. Fold change is described as 2ΔΔCt (Applied Biosystems) and 2-fold change equals 100% change.
2.5. Western Blot
DRGs from naïve and inflammed mice were homogenized in lysis buffer containing 1% sodium dodecyl sulfate (SDS), 10 mM Tris–HCl (pH 7.4), and protease inhibitors (1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM sodium orthovanadate and 100 μg/ml phenylmethylsulfonyl fluoride; Sigma Biochemicals). Samples (10 μg) were centrifuged, boiled 10 min in a denaturing buffer containing β-mercaptoethanol and SDS, separated on a 10% polyacrylamide SDS-PAGE gel and transferred to a PVDF (Hybond) membrane (Amersham) that was blocked in 5% milk (in 0.1M tris buffered saline with 0.1% tween-20) and then incubated with primary antibodies overnight at 4°C (P2Y1: 1:800; Alamone; actin: 1:500; Ab Cam). Antibody binding was visualized using horseradish peroxidase-conjugated goat anti-rabbit or donkey anti-goat secondary antibodies (1:10,000) and chemiluminescent detection (Pierce Biochemical). Immunoreactive bands were analyzed by densitometry and intensity quantified using NIH Image J software. Band intensity was normalized to actin and reported as a percent change.
2.6. Ex-vivo preparation
The ex-vivo somatosensory system preparation has been described in detail previously [19; 10; 12]. Briefly, mice were anesthetized via injection of ketamine and xylazine (90 and 10 mg/kg, respectively) and perfused transcardially with oxygenated (95% O2–5% CO2) artificial CSF (aCSF; in mM: 1.9 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26.0 NaHCO3, and 10.0 D-glucose) containing 253.9 mM sucrose at 12–15°C. The spinal cord and the right hindlimb was excised and placed in a bath of aCSF. Hairy skin of the right hindpaw, saphenous nerve, DRGs and spinal cord were isolated. Following dissection, the preparation was transferred to a separate recording chamber containing chilled oxygenated aCSF in which the sucrose was replaced with 127.0 mM NaCl. The skin was pinned out on a stainless steel grid located at the bath/air interface, such that the dermal surface remained perfused with the aCSF while the epidermis stayed dry. The platform served to provide stability during applied thermal and mechanical stimuli. The bath was then slowly warmed to 31°C before recording.
2.7. Recording and Stimulation
Sensory neuron somata were impaled with quartz microelectrodes (impedance >150 MΩ) containing 5% Neurobiotin (Vector Laboratories, Burlingame, CA) in 1 M potassium acetate. Orthograde electrical search stimuli were delivered through a suction electrode on the nerve to locate sensory neuron somata innervating the skin. Peripheral receptive fields (RF) were localized with a blunt glass stylus and von Frey hairs. When cells were driven by the nerve but had no mechanical RF, a thermal search was conducted. This was accomplished by applying hot (~ 52°C) and/or cold (~ 0°C) physiological saline to the skin. There was some concern that the brief but multiple applications of hot saline might cause sensitization of nociceptors during the course of an experiment. We examined this possibility in two recent studies [19; 12] and found no change in average heat thresholds obtained at the onset of the experiment when compared to the average heat thresholds of the last fibers recorded. We have made a similar comparison of the data from these experiments in mice following CFA and also found no change in the average heat thresholds during the course of these experiments (data not shown).
The response characteristics of DRG cells were determined by applying digitally controlled mechanical and thermal stimuli. The mechanical stimulator consisted of a tension/length controller (Aurora Scientific) attached to a 1 mm diameter plastic disc. Computer controlled 5s square waves of 1, 5, 10, 25, 50 and 100 mN were applied to the cell’s RF. After mechanical stimulation, a controlled thermal stimulus was applied using a 3 mm2 contact area peltier element (Yale Univ. Machine Shop). The temperature stimulus consisted of a 12s heat ramp from 31–52°C followed by a 5s plateau at 52°. The stimulus then ramped back down to 31°C in 12s. Adequate recovery times (approx. 30s) were employed between stimulations. While recording from myelinated nociceptors in many cases, multiple heat applications were made and in some cases the heat ramp was continued to 54°C and held for 5s. In other instances, fibers that were unable to be characterized by computer controlled mechanical or thermal stimulation but were characterized by von Frey and/or saline stimuli were not included in the determination of thresholds. All elicited responses were recorded digitally for offline analysis (Spike2 software, Cambridge Electronic Design). After physiological characterization, select cells were labeled by iontophoretic injection of Neurobiotin (2–3 cells per DRG). Peripheral conduction velocity was then calculated from spike latency and the distance between stimulating and recording electrodes (measured directly along the nerve). Thermal thresholds were determined to be the temperature at which the first spike was elicited during the temperature change for fibers that did not exhibit ongoing activity prior to thermal stimulation. For those fibers that did have some degree of ongoing activity, threshold was determined as the temperature at the second spike of two where the instantaneous frequency exceeded that present in a 30 second window prior to the thermal stimulation.
2.8 Classification of cutaneous A- and C-fiber sensory neurons
Sensory neurons with a conduction velocity of < 1.2 m/s were classified as C-fibers [17], and all others were classified as A-fibers. Conduction velocities between 1.2 and 10 m/s were considered to be in the Aδ range and those ≥10 m/s were in classified as conducting in the Aβ range [16; 24]. For the purposes of these experiments we have focused our recordings and analyses specifically on the population of inflamed C-fibers. In addition, in all inflamed preparations we encountered a number of cells that were driven by the electrical nerve stimulus, but were found to be both mechanically and thermally unresponsive; however, only cells that had a response to cutaneous stimulation (mechanical or thermal) were included in the analysis.
The C-fiber classes were as follows: 1) C-polymodal (CPM), meaning those that responded to mechanical and heat stimuli (CMH) and sometimes cool/cold stimuli (CMHC); 2) C-mechano (CM), those that responded only to mechanical stimulation of the skin; 3) C-mechano cool/cold (CMC), those that responded to mechanical and cooling stimuli (but not heating); 4) C-heat (CH), those that were mechanically insensitive but heat sensitive, and 5) C-cooling/cold (CC), those that were mechanically insensitive but responded to cooling of the skin.
2.9. Tissue processing and analysis of recorded cells
Once a sensory neuron was characterized and intracellularly filled with Neurobiotin, the DRG containing the injected cell was removed and immersion fixed with 4% paraformaldehyde in 0.1 M phosphate buffer (PB) for 30 min at 4°C. Ganglia were then embedded in 10% gelatin, postfixed in 4% paraformaldehyde, and cryoprotected in 20% sucrose. Frozen sections (60 μm) were collected in PB and reacted with fluorescently-tagged (FITC) avidin to label Neurobiotin-filled cells (Vector Laboratories). Next, each section was processed for IB4 binding (AlexaFluor 647; Molecular Probes, Eugene, OR) and/or TRPV1 (1:500; CalBiochem), or CGRP (1:2000; Chemicon) immunohistochemistry. After incubation in primary antiserum, tissue was washed and incubated in Cy3 or Cy5 conjugated donkey anti-rabbit secondary antisera (1:200; Jackson Immunoresearch). Distribution of fluorescent staining was determined using Olympus FluoView™ 500 laser scanning confocal microscope (Olympus America Inc.). Sequential scanning was performed to prevent bleed-through of the different fluorophores.
2.10. Cell counts
The expression of TRPV1 and IB4 binding was analyzed in 3 L3 DRGs from naïve mice and 3 L3 DRGs from each CFA injected group at each time point. The DRGs taken after electrophysiological experiments and were processed for imunohstochemical analysis as described above. The numbers of positive cells were determined as previously reported [3; 10]. In brief, 3 non-consecutive sections were randomly chosen and 15 μm stacks with 3 μm thick optical sections were captured using a 40x oil immersion objective. Multiple optical stacks were taken of each selected tissue section and visual confirmation was used to avoid analyzing cells twice. The number of TRPV1 positive cells or those that bound IB4 were counted and averaged in the top and bottom optical section of each stack and reported as mean ± SEM.
2.11. Primary Neuron Culture
DRGs from adult male mice were dissected into cold HBSS and then treated with 60U papain (Worthington), 1 mg of cysteine and 3 μL of NaHCO3 at 37°C for 10 min. Ganglia were then treated with 12 mg of collagenase II (Worthington) at 37°C for 10 min., washed and triturated with fire polished glass Pasteur pipettes in 1 mL of F12 complete media. Cells were then plated into poly-d-lysine/laminin coated wells, allowed to sit at 37°C/5% CO2 for 2h and then flooded with 1 mL of F12 media containing 10% fetal calf serum. Cultures were allowed to grow for 18–24 hours prior to calcium imaging analysis.
2.12. Calcium Imaging
Ca++ imaging was performed as previously described [22]. Cells were loaded with 2 mM fura-2-AM in HBSS with 5 mg/ml bovine serum albumin for 30 min at 37°C. The cells were then placed onto a microscope stage with constantly flowing HBSS at 5 ml/min. Temperature was maintained at 30°C using heated stage and in-line heating system (Warner Instruments). Treatments (capsaicin, ADP and K+) were delivered with a computer-controlled rapid-switching local perfusion system. Cells in regions of interest were identified with the Simple PCI, C-Imaging software. Absorbance data at 340 and 380 nm were collected once per second and the relative fluorescence (ratio 340/380) was plotted over time. A 50 mM K+ stimulus in HBSS was applied to identify healthy neurons. To evaluate the activation of cells by P2Y1 and/or TRPV1, the percentage of neurons responding to capsaicin and/or ADP was calculated. Capsaicin was dissolved in 1-methyl-2-pyrrolidinone as a 10 mM stock solution; a working solution of 1 μM capsaicin was made fresh daily in HBSS. A 10 mM stock solution of ADP in HBSS was made fresh daily and applied as a 100 μM working solution.
2.13. Data analysis
One-way ANOVA tests and posthoc analysis (Tukey) were used to analyze differences in firing rate and instantaneous frequency along with mechanical and thermal thresholds of both A- and C-fibers. Average firing rates, maximal firing rates and firing rates during individual temperatures during the heat ramp were specifically analyzed. In order to test individual temperatures during the heat ramp, Student’s t test with Bonferroni correction was used. This information was sorted by neuronal functional type to examine whether or not certain classes of neurons have coherence with regard to the expression of any of the markers tested. Data recorded in response to the heat ramp was normalized as in our previous publications by multiplying the average spikes per degree by the percentage of cells responding at that temperature [e.g. 14]. Differences in fiber prevalence and immunostaining were determined by Fisher’s Exact analysis. Immunolabeling data from functionally identified cells was not found to be statistically different between the two time points analyzed and were combined for ease of presentation into a single table. Percent changes in mRNA were also determined to be statistically significant by ANOVA with post hoc analysis (Tukey). Statistical analyses were performed on the raw Ct data and represented for ease of presentation as a percent change. P-values were set at p < 0.05.
3. RESULTS
3.1. In vivo inhibition of CFA-induced P2Y1 expression
We first verified that peripheral inflammation caused a change in P2Y1 expression in DRGs. P2Y1 mRNA and protein were found to be significantly enhanced in the L2/L3 DRGs after inflammation of the hairy hindpaw skin at three (mRNA: 49.3% +/− 7%; protein: 106% +/− 21%) and five (mRNA: 59% +/− 8%; protein: 48% +/− 16%) days after CFA injection (Fig. 1). We then wanted to verify that P2Y1 gene expression could be specifically inhibited by P2Y1 targeting (PenY1) siRNAs injected into the saphenous nerve during peripheral inflammation. At three days following CFA injection, P2Y1 mRNA was found to be significantly increased in the DRGs after injection of control, non-targeting (PenCON) siRNA (54% +/− 7%; Fig. 1A). Injection of targeting PenY1 siRNA significantly inhibited the inflammation induced increase in P2Y1 mRNA (22% +/− 7%) (Fig. 1A). Effects that were seen at the five day time point were slightly different. As before, CFA alone (59% +/− 8%) and PenCON injection plus CFA (54% +/− 8%) showed enhanced levels of P2Y1 in the DRGs. While inflamed mice that received P2Y1 targeting siRNA injection plus CFA (36% +/− 8%) did not show a significant increase in mRNA expression compared to naïve mice, the levels were also not significantly different from that seen in the two control groups (Fig. 1B). Results obtained from RT-PCR analysis were then verified at the protein levels (Fig. 1c,d; PenCON plus CFA: 116% +/− 59% (3d) and 86% +/− 14% (5d); and PenY1 plus CFA: 26% +/− 34% (3d) and 35% +/− 18% (5d)).
Figure 1. Realtime PCR and western blotting analysis of dorsal root ganglia (DRGs) three and five days after injection of CFA into the hairy hindpaw skin with or without injection of non-targeting control (PenCON) or P2Y1 targeting (PenY1) siRNAs.
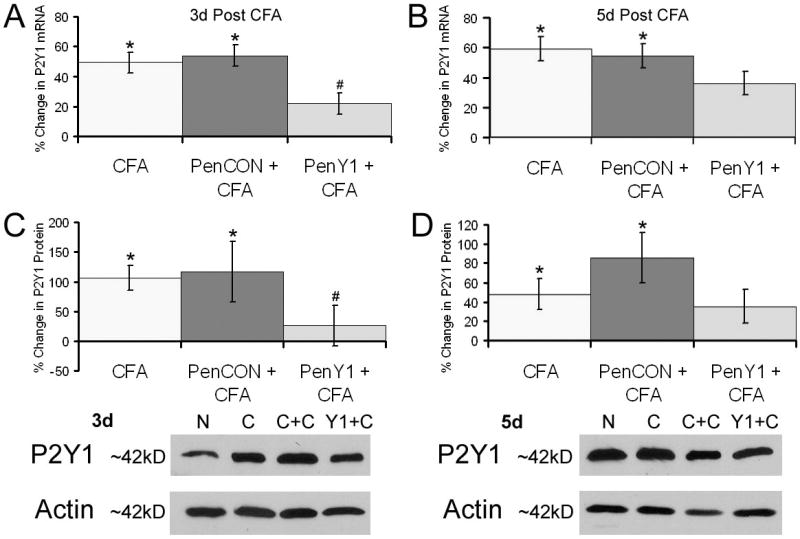
P2Y1 mRNA is significantly increased in the L2/L3 DRGs seven days after CFA alone (49% +/− 7%), and after CFA plus PenCON injection (54% +/− 7%); however, injection of PenY1 siRNAs prevented the CFA induced increase in P2Y1 mRNA (22% +/− 7%) at this time point relative to naïve mice (Panel A). mRNA levels of P2Y1 remained elevated at five days under all conditions (Panel B). No statistical differences were detected between any of the three conditions at this timepoint. Western blot analysis verified that an increase in mRNA expression corresponded to an increase in protein expression three days after CFA (106% +/− 21%) and after PenCON siRNA injection plus CFA (216% +/− 59%) whereas injection of PenY1 siRNAs plus CFA (26% +/− 34%) displayed little increase in P2Y1 protein relative to naïves (Panel C). Similar results were obtained at five days under both control conditions (CFA: 48% +/− 16% and PenCON plus CFA: 86% +/− 26%) and even after injection of P2Y1 siRNAs plus CFA (35% +/− 18%) there was a rebound in protein expression (Panel D). Representative western blots are shown for P2Y1 protein normalized to actin at three days and five days after CFA with or without injection of PenCON or PenY1 siRNAs (Panel C,D inserts; N= Naïve, C= CFA; C+C= PenCON + CFA; Y1+C= PenY1 + CFA). *statistically different than naïves, p value < 0.05; # statistically different than CFA alone and PenCON + CFA, p value < 0.05.
To examine whether the inhibition of P2Y1 expression was selective, we analyzed the expression of additional genes following siRNA-targeted knockdown. First, we examined the expression of two other purinergic receptors. mRNA for the ATP receptor P2X3, which was increased in the DRGs seven days after inflammation, was unchanged following control and P2Y1 targeting siRNA injections three days after CFA-induced inflammation (CFA: −29% +/− 17%; PenCON plus CFA: 3% +/− 31%; PenY1 plus CFA: −8% +/− 20%). Next we analyzed the expression of P2Y2 three days after inflammation. Following the injection of P2Y1-targeting siRNAs, we found no change in P2Y2 mRNA levels (CFA: 9% +/− 23%; PenCON plus CFA: −16% +/− 12%; PenY1 plus CFA: −18% +/− 13%) at this time point after CFA injections. Then, in order to confirm that there were no anti-viral-related off-target effects of our siRNA injections, we examined the expression levels of myxovirus resistance 2 (mx2; [11]). We found that its expression was not altered by the presence of siRNAs (not shown).
3.2. Summary of results from recording experiments
A total of 408 primary cutaneous neurons were intracellularly recorded and physiologically characterized from 56 inflamed adult Swiss-Webster mice. Of these, 163 cells came from 13 mice at the three day time point and 10 mice at the five day time point in animals that only received CFA injection. An additional 125 cells were derived from 7 mice at the three day time point and 7 mice at the five day time point in animals that received CFA plus control, non-targeting (PenCON) siRNA injection. The final 120 cells came from 10 mice at the three day time point and 9 mice at the five day time point in animals that received CFA plus P2Y1 targeting (PenY1) siRNA injection. The results from these three experimental groups were compared with each other and to previously published results from naïve SW mice [10].
3.3. Immunohistochemical characterization of CPM and CH neurons during peripheral inflammation
A total of 68 cutaneous C-fibers were intracellularly labeled, recovered for processing and immunohistochemically characterized in inflamed mice from the three experimental conditions. There were no differences in immunolabeling for any marker between the two time points under any experimental condition. Therefore, we combined immunostaining results from the two time points for ease of presentation. We found that the distribution of immunohistochemical phenotypes in these single characterized sensory neurons was very similar to that observed in naïve mice where most (75%) of the CPM fibers were IB4 posiitive and none were TRPV1 positive [19]. This differs from the results observed following saphenous nerve regeneration where some regenerated CPM neurons (16%) were occasionally found to contain TRPV1 [10]. We did find that two of four CPM neurons in the CFA plus PenCON injected mice did label positively for TRPV1. However, this result was not significantly different from CFA alone (p value < 0.1). Surprisingly, 71% (5/7) of CPM neurons in the P2Y1 siRNA injected and inflamed mice were immunoreactive for TRPV1. This was found to be significantly different than the control conditions (Table 1; p value < 0.05).
Table 1.
Immunstaining Results for Cutaneous CPM Neurons During Peripheral Inflammation
| Condition | IB4+ | TRPV1+ | CGRP+ | IB4+/TRPV1+ | IB4+/CGRP+ |
|---|---|---|---|---|---|
| CFA | 9/12 | 0/7 | 2/5 | 0/7 | 0/5 |
| CFA + PenCON | 12/15 | 2/4 | 2/9 | 1/4 | 0/9 |
| CFA + PenY1 | 9/19 | 5/7 | 6/12 | 1/7 | 1/12 |
This change does not appear to be due to an increase in TRPV1 expression as injection of CFA into the hairy hindpaw skin did not alter whole L2, 3 DRG TRPV1 mRNA levels under any of the experimental conditions (CFA: −18% +/− 23%; PenCON plus CFA: 19% +/− 25%; PenY1 plus CFA: −7% +/− 22%). However, this assay may not pick up expression changes in a small fraction of sensory neurons in the DRGs. This change was also apparently not due to de novo TRPV1 expression as there was no difference in the numbers of TRPV1-containing DRG neurons under any of these experimental conditions (3d: Naïve: 32.6 +/− 1.2; CFA: 33.8 +/− 1.5; PenCON plus CFA: 33.5 +/− 1.2; PenY1 plus CFA: 30.5 +/− 2.1; 5d: CFA: 30.9 +/− 1.3; PenCON plus CFA: 23.8 +/− 1.3; PenY1 plus CFA: 31.6 +/− 1.9). IB4 binding was also unaffected under any condition (3d: Naïve: 33.8 +/− 1.4; CFA: 38.2 +/− 1.8; PenCON plus CFA: 39.2 +/− 1.4; PenY1 plus CFA: 36.3 +/− 2.2; 5d: CFA: 34.4 +/− 1.9; PenCON plus CFA: 33.9 +/− 1.1; PenY1 plus CFA: 35.1 +/− 1.5)
Overall, 30 of 46 CPM fibers (65%) bound IB4; nine of 12 (75%) in mice that only received CFA injection and 12 of 15 (80%) CPM neurons were IB4 binding in the PenCON plus CFA condition. Interestingly, only nine of 19 (47%) CPM neurons were IB4 binding in the P2Y1 siRNA plus CFA mice. However this apparent decrease in the percentage of IB4-positive CPM fibers was not statistically significant (p = 0.07). Taken together, these results suggest the possibility that under these conditions (CFA + siRNA knockdown of P2Y1 expression), some TRPV1-positive CH fibers gain mechanical sensitivity (Table 1).
The proportion of CPM neurons stained for calcitonin gene related peptide (CGRP) labeling during inflammation was not different among the three experimental groups. Nine of 26 (35%) cells were immunoreactive for CGRP (Table 1). We also were able to verify previous data on CH neurons as all of these fibers (six) under the three conditions stained positively for TRPV1 but were negative for IB4 (not shown; [19; 10; 12]).
3.4. P2Y1 knock-down does not affect recruitment of CH neurons induced by peripheral inflammation
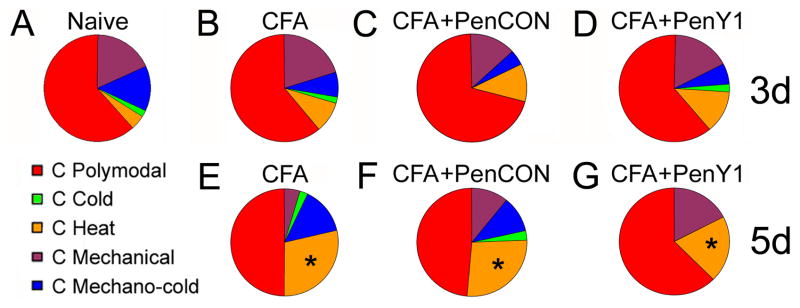
The TRPV1-immunopositive, IB4-negative CH fibers showed no significant changes in thermal threshold at any time points tested during inflammation under any condition (Thresholds- Naïve: 41.0 +/− 1.6 °C; Combined Time Points: CFA: 42.5 +/− 1.2°C; PenCON + CFA: 42.9 +/− 1.7°C; PenY1 + CFA: 41.7 +/− 1.4°C; p value < 0.5). In addition, although there was a near doubling or greater in the percentage of CH fibers at the 3 day time point in all three conditions, (naïve 5%; CFA alone 9%; CFA plus PenCon siRNA (11%); CFA plus PenY1 siRNA (13%), this increase did not reach statistical significance. At the 5 day time the percentage of CH increased further and reaching statistical significance (CFA alone, 30%) compared to naïve mice (Fig 2a,b; p value < 0.05; Fisher’s Exact). Similar significant increase in CH fiber prevelance was also found after CFA plus PenCON siRNA injection (27%) and PenY1 siRNA injection plus CFA (20%; Fig. 2c,d). Thus the injection of PenCon and PenY1 siRNAs had no effect on the normal changes in the CH subset of cutaneous fibers following CFA induced inflammation.
Figure 2. Phenotypes of C-fibers before and after injection of CFA into the hairy hindpaw skin as characterized by ex vivo recording.
A Most of the C-fibers are polymodal (CPMs) under normal conditions. The remaining C-fibers are approximately of equal representation comprised of those that respond to mechanical and cold stimulation (CMC), only mechanically sensitive (CM), mechanically insensitive but cold/cooling sensitive (CC) and mechanically insensitive but heat sensitive (CH). B, C, D: Inflamed C-fibers display no shift in the distribution of fiber types three days after CFA injection under any experimental condition. E: However, at five days post CFA, there is a significant increase (Naïve= 5%; CFA= 30%) in the percentage of mechanically insensitive C-fibers that respond to heat (CH). F: These findings are also observed after injection of control, non-targeting (PenCON) siRNAs (27%) and CFA at five days. G: The CFA induced recruitment of CH neurons at five days is unaffected by injection of P2Y1 targeting (PenY1) siRNAs (20%). p value < 0.01.
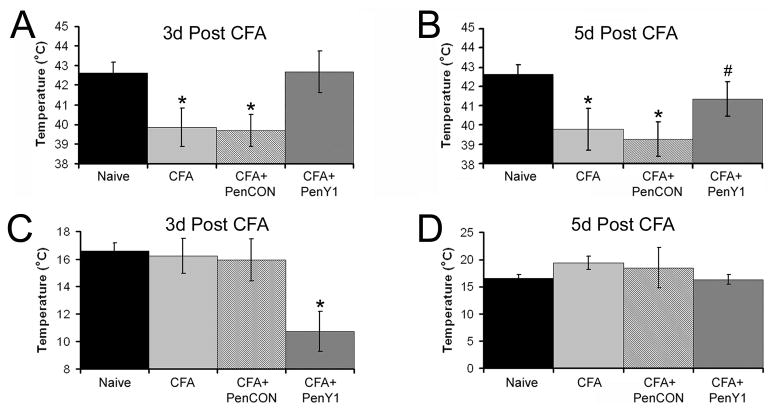
3.5. P2Y1 knock-down blocks the CFA-induced decrease in CPM heat threshold
Within three days of injection of CFA into the cutaneous target, CPM thermal thresholds were significantly reduced to 39.9 +/− 1.0°C (p = 0.01) compared to previous naïve mouse data (42.6 +/− 0.5°C). Similar reduction in threshold was detected in mice that received PenCON siRNA injection (39.7 +/− 0.8°C; p value < 0.003). However, inhibition of P2Y1 completely blocked the CFA-induced decrease in CPM heat threshold at the three day time point (42.7°C +/− 1.1°C; Fig. 3a). At five days, CPMs still showed a significant decrease in thermal threshold (CFA: 39.8°C +/− 1.1; PenCON plus CFA: 39.3°C +/− 0.9°C; p = 0.01) that remained partially blocked by inhibition of P2Y1 (41.4°C +/− 0.9°C; Fig. 3b). Although heat thresholds of CPM neurons from mice that received P2Y1 siRNA and CFA at this time point were not statistically different than naïve CPMs, these fibers were also not statistically different than either control condition (p value = 0.1).
Figure 3. Heat and cold thresholds of CPM neurons before and after CFA injection into the hairy hindpaw skin.
Polymodal C-fibers (CPM) showed a significant reduction in thermal threshold at three and five days after CFA (n=32, three days and n=19, five days) or with injection or non-targeting control (PenCON; n=31, three days and n=18, five days) siRNAs. p value < 0.02*. However injection of P2Y1 targeting siRNAs plus CFA (PenY1; n=29, three days and n=18, five days) resulted in a complete block of the CFA induced decrease in CPM heat threshold (Panel A) compared to naïve (n=60) mice at three days. p value < 0.2 compared to naïve and p value < 0.05 compared to inflamed conditions. The decrease in the CPM heat threshold remained under the control conditions at five days (p value < 0.05) but CPM fibers in mice that received injection of P2Y1 siRNAs during inflammation were also beginning to decrease as heat thresholds were not statistically different between any of the other three conditions at this time point (Panel B). Cold threshold in CPM neurons is unaffected by CFA (n=8, three days and n=8, five days) or PenCON plus CFA (n=12, three days and n=4, five days) at both time points tested; however inhibition of P2Y1 at three days (n=10) significantly reduced the cold threshold of these fibers (Panel C), which recovered by five days (n=9; Panel D). *p value < 0.05 relative to naives, # p value < 0.05 relative to PenCON plus CFA but not different from naïve or CFA alone.
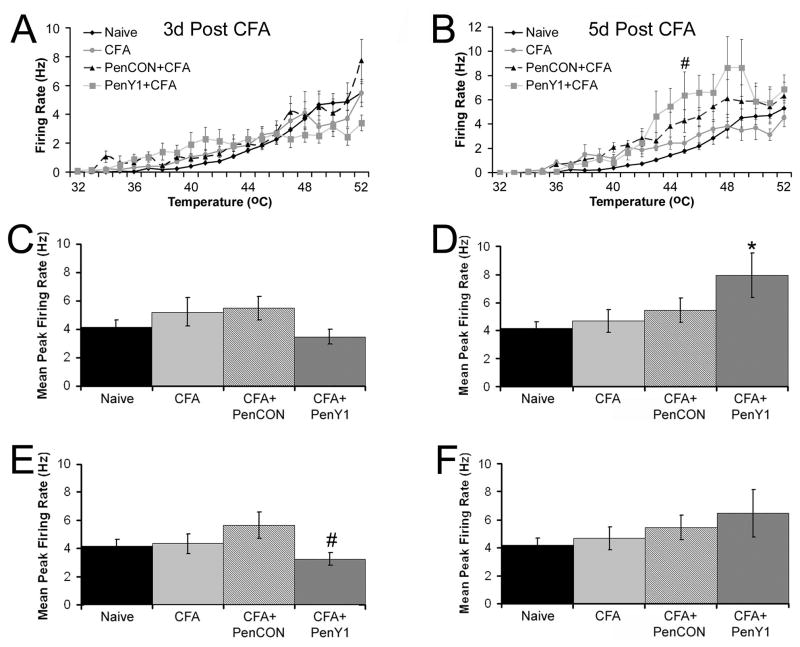
Unlike our previous study using different mouse strains [14], the average firing rate of CPM neurons during the heat ramp was unaffected by CFA at both time points and under all conditions (Fig. 4a,b). The only exception to this was at the five day time point where inhibition of P2Y1 during inflammation caused a significant increase in firing rate at 45°C relative to naïve mice (p value < 0.01); however, this was not statistically different than the CFA alone or PenCON plus CFA groups at this temperature. We also determined the maximal firing rate of CPM neurons and found no differences in firing rate between any experimental groups at the three day time point (Fig. 4c). However, inhibition of P2Y1 did cause a significant increase in maximal firing rate at the five day time point relative to naives (Fig. 4d; p value < 0.05).
Figure 4. Firing rates of CPM neurons during heat stimuli before and after injection of CFA into the hairy hindpaw skin.
Firing rate per degree to heat stimuli in CPM neurons is unaffected by CFA at three (Panel A) and five (Panel B) days under all three conditions compared to naïves with the exception of the 45°C temperature at five days where inhibition of P2Y1 during inflammation was significantly higher than naives. However, this increase was not statistically different than CFA alone or PenCON plus CFA groups. #p value < 0.05 relative to naïves. When calculating maximal firing rate over the entire heat ramp, there was no difference in the maximal firing rate to heat stimuli at three days after CFA injection (Panel C) under any condition; however, inhibition of P2Y1 did cause a significant increase in the maximal firing rate of CPM neurons to heat stimuli (Panel D). * p value < 0.05 compared to naives. Removal of the TRPV1 immunopositive/peptidergic CPM neurons from these data reveal that increases in firing rate by inhibition of P2Y1 five days after CFA injection (Panel F) is due to this small population of CPM neurons. In fact at the three day time point, removal of these few CPM neurons reduced the maximal firing rate below that of the PenCON plus CFA group but this was not different than naives or CFA alone (Panel E). # p value < 0.05 relative to PenCON plus CFA.
Immunohistochemical analysis of single characterized cells indicated that there was a significant increase in TRPV1 positive cells with both heat and mechanical sensitivity. Given our previous results showing that TRPV1 positive CH fibers fire at significantly higher rates than CPM fibers [19], we have re-analyzed these results subtracting the TRPV1/peptidergic fibers from the analysis. Removal of these few cells from the data eliminates the effect of P2Y1 inhibition during inflammation on maximal firing rate (Fig. 4e,f), in addition to the effect of inhibition at the 45°C data point (Fig 4b, not shown). Interestingly, we have also observed lack of increased firing rate in TRPV1−/− mice [14]. In this previous study we suggested that this reduced sensitization may be due to the lack of neurogenic inflammation. Therefore, in these strains, there may be a greater degree of neurogeneic inflammation from the peptidergic/TRPV1+ cells causing alterations (changes in firing rate) on the CPM population to a larger degree. This does not appear to be the case in the SWs and may be the reason we do not see the effect of inflammation on overall firing rate in this study.
Although CFA did not induce any changes in CPM cold threshold, inhibition of P2Y1 did result in a significant reduction in cold threshold three days after CFA injection (Naïve: 16.6°C +/− 0.6°C; CFA: 16.2°C +/− 1.1°C; PenCON plus CFA: 15.9°C +/− 1.5°C; PenY1 plus CFA: 10.7°C +/− 1.4°C), which recovered at the five day time point (CFA: 19.4°C +/− 1.3°C; PenCON plus CFA: 18.5°C +/− 3.7°C; PenY1 plus CFA: 16.3°C +/− 0.9°C; Fig. 3c.d). CPM mechanical thresholds were not altered at either time point under any condition after peripheral inflammation (Naïve: 21.2 mN +/− 2.2 mN; CFA: 23.4 mN +/− 6.9 mN; PenCON plus CFA: 29.0 mN +/− 5.1 mN; PenY1 plus CFA: 28.7 mN +/− 6.5 mN; combined time points).
3.6. ADP and capsaicin responsiveness of DRG neurons
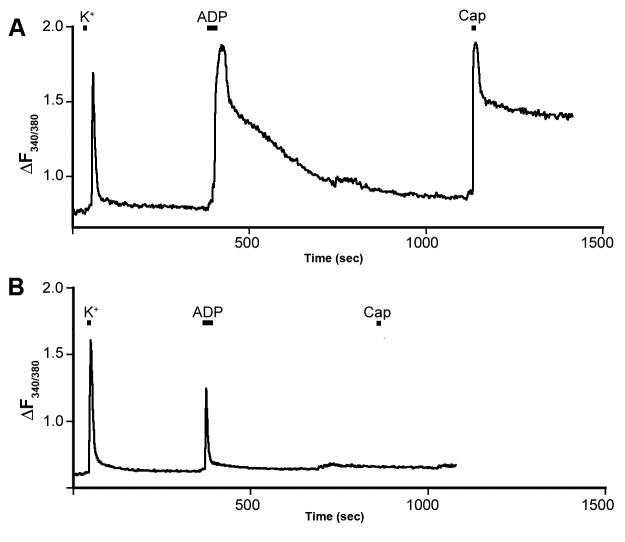
The results of the immunostaining suggest the possibility that inhibition of P2Y1 levels results in changes in phenotype of CH cells containing TRPV1/CGRP (i.e. they gained mechanical sensitivity). This in turn suggests the possibility that this population or a subpopulation of these cells normally contain P2Y1. However, previous studies have suggested that P2Y1 and TRPV1 were located in different populations of DRG neurons [28; 21; 22]. In order to determine if any mouse sensory neurons contained both functional TRPV1 and P2Y1 receptors, we performed calcium imaging on dissociated DRG neurons and analyzed responsiveness to ADP (P2Y1) and capsaicin (TRPV1). We found that 43% (38/88) of dissociated DRG neurons that responded to ADP were also responsive to capsaicin (Fig 5). These results demonstrate that a significant population of sensory neurons contains both functional TRPV1 and P2Y1 receptors. This finding suggests that the change in phenotype of these TRPV1 containing cells following inflammation could be a direct affect of the P2Y1 knockdown and not an off target effect.
Figure 5. ADP and capsaicin responses of DRG neurons.
Two examples of neuronal responses to ADP (100 μM) and capsaicin (Cap, 1.0 μM) measured by Fura-2 Ca++ imaging. A) A DRG neuron responsive to both ADP and capsaicin. B) A neuron responsive to ADP, but not capsaicin. The magnitude of the ADP response was not correlated with capsaicin responsiveness. All cells were challenged with 50 mM K+ to verify neuronal identity and health. Overall, 43% of ADP-responsive neurons also responded to capsaicin.
4. DISCUSSION
4.1. P2Y1 knockdown in vivo inhibits the CFA-induced decrease in CPM heat threshold
In agreement with our previous study, we found that CFA-induced inflammation resulted in a decrease in the heat threshold of cutaneous CPM fibers without affecting mechanical thresholds in the same fibers [14]. We also found that siRNA inhibition of the normal CFA-induced increase in P2Y1 expression blocked the decrease in CPM heat threshold. These results are also consistent with our previous study showing that CPM fibers in mice lacking the P2Y1 receptor have significantly increased heat thresholds [27]. Together these findings suggest that this ADP-binding G-protein coupled receptor is important for regulating heat signaling in this specific type of cutaneous sensory neuron.
The specificity and duration of the in vivo knockdown of P2Y1 mRNA and protein levels is an important consideration in the interpretation of these results. While it is not feasible to determine all possible off target effects, we have determined that there were no effects on the expression other purinergic receptors (P2X3 and P2Y2) found in CPMs and other sensory neurons. In addition to our previous study [12], we have also confirmed here that siRNA injection does not induce anti-viral related off target effects by monitoring mx2 expression levels (not shown). It is important to note that heat thresholds of CPM neurons in the PenY1 injected mice began to return to control levels at the five day time point when the RNA and protein levels in the DRGs were also returning to levels detected under inflamed conditions. This temporal correlation between P2Y1 levels and functional properties would also suggest that the effect is specific.
Although CFA did not alter the cold threshold of CPM neurons, inhibition of P2Y1 during peripheral inflammation did reduce the cold threshold consistent to what had been shown in mice lacking the P2Y1 receptor [27]. Thus, inhibition of P2Y1 greatly reduced the range of thermal stimuli able to be detected in CPM neurons during CFA-induced inflammation. The greatest change appears to be the decrease in heat threshold, but it is apparent that changes in P2Y1 expression can modulate the cold/cooling thresholds of these fibers as well. Similar to the effects on heat thresholds, there is a recovery of cold thresholds that is correlated with the recovery of P2Y1 expression at the five-day time point. The rebound in both cold and heat thresholds five days after P2Y1 siRNA injection in inflamed mice serves as a good internal control for our siRNA-mediated knockdown strategy and strengthens the conclusion that P2Y1 is involved in setting thermal thresholds in this population of cutaneous sensory neurons.
The lack of an increase in firing rate to heat stimulation following inflammation during the heat ramp; however, differs from our earlier findings in wildtype mice [14]. Interestingly, in the same study we found that CPM fibers in TRPV1−/− mice also did not exhibit this increase in firing rate, suggesting an essential role for TRPV1 in this process, potentially involving a reduction in neurogenic inflammation [14]. The earlier study was carried out in C57Bl/6 and C3HBl/6 mice compared with Swiss Webster mice used here. We had previously shown that many more sensory neurons stain positively for TRPV1 in C3HBl/6 than in BALB/c mice [33]. Similarly we have also observed that C57Bl/6 and C3HBl/6 mice have more than twice as many TRPV1 positive cells in the L2/L3 DRGs than SW mice (unpublished observations). Taken together these findings suggest the possibility that the increased firing rate to heat following inflammation is driven by relative number of TRPV1 containing fibers. Notably, this apparent difference in inflammation-induced sensitization between strains could be one reason why these breeds show different behavioral sensitivities to inflammatory pain tests [25].
4.2. Effects of siRNA knockdown on changes in sensory neuron phenotypes during inflammation
In agreement with our recent study following saphenous nerve axotomy and regeneration, we found that peripheral inflammation caused a significant increase in the numbers of functional TRPV1-positive CH fibers [10]. Neither control siRNA injections nor targeted P2Y1 siRNA injections had any effect on this increase during inflammation. Peripheral inflammation also did not result in changes in the total DRG expression of neurochemical markers such as IB4 and TRPV1 in sensory neurons. However, immunolabeling of individually characterized fibers does suggest a possible unexpected effect of the P2Y1-targeting siRNAs on the TRPV1-expressing, peptidergic fibers, as inhibition of the normal increase in P2Y1 expression significantly increased the percentage of CPM neurons expressing TRPV1 following inflammation (Table 1). In our previous studies [10; 14] we suggested that the appearance of TRPV1 positive CPM fibers reflected the acquisition of mechanical sensitivity of previous mechanically insensitive CH fibers. The findings here would suggest that P2Y1 levels may play a role in this phenotypic change. Previous studies have suggested that TRPV1-expressing sensory neurons lacked P2Y1 [30] raising the possibility that the effect observed here on TRPV1-expressing cutaneous fibers could be indirect, or possibly the result of an “off-target” effect of the siRNA injections. However, the results of our in vitro experiments demonstrating that a significant population of dissociated sensory neurons showed responses to both ADP and capsaicin suggest that the effects are likely direct.
This finding could also explain the significant increase in the maximal firing rate of CPM neurons after inflammation in mice that received PenY1 injections (Fig. 4d), as TRPV1 positive CH have significantly higher firing rates to heat when compared to non-peptidergic CPM fibers lacking TRPV1 [19]. To examine this possibility, we removed the relatively few TRPV1/peptidergic CPM fibers from the analysis and the significant effects on firing rate were lost (Fig. 4F).
These differing effects of P2Y1 knockdown on two distinct populations of cutaneous sensory neurons suggest that P2Y1 may have different roles in functionally discrete types of cutaneous sensory neurons. One role regulates the changes in thermal activation of non-peptidergic, IB4-binding CPM neurons and the other regulates mechanical sensitivity in heat sensitive, peptidergic, TRPV1-containing CH-fibers. These results also suggest the possibility that the increase in P2Y1 expression following inflammation is not uniform across all types of cutaneous sensory neurons. For example, normally following inflammation whole DRG expression of P2Y1 is significantly increased. This increase is correlated with both a decrease in the heat threshold of the CPM fibers and the appearance of TRPV1 positive cutaneous CPM fibers. However, while in vivo knockdown of this increase in P2Y1 expression blocks the inflammation induced decrease in heat threshold in the CPM fibers, it increases the recruitment of mechanical sensitivity in the TRPV1 population. Thus P2Y1 levels are positively correlated with heat sensitivity in the nonpeptideric - TRPV1 negative CPM fibers and negatively correlated with mechanical sensitivity in the peptidergic -TRPV1 positive CH population. One possible explanation for these results is that normally following inflammation P2Y1 is significantly increased in the nonpeptidergic CPM population but is either unchanged or decreased in the TRPV1 positive peptidergic CH fibers.
Although the mechanism of how P2Y1 may be regulating phenotypic maintenance in the TRPV1 containing cutaneous C-fibers is unclear, signaling downstream of Gq coupled receptors (like P2Y1) include regulation of TRPV1 signaling [21; 32]. Although these are thought to be positive regulators of TRPV1, our results suggest that P2Y1 levels do not affect heat sensitivity in this population. Once again it is possible that levels of P2Y1 are normally decreased in cells that contain both TRPV1 and P2Y1 during cutaneous inflammation. This would be consistent with the idea that P2Y1 signaling in this population of fibers is a negative regulator of mechanical sensitivity and when the normal decrease in P2Y1 expression is augmented by the further in vivo siRNA knockdown, any remaining regulation is released resulting in the acquisition of mechanical sensitivity. However, this will need to be specifically tested in single cells in the future.
4.3. Summary
The results presented here confirm our previous report showing that inflammation results in the decrease in heat threshold of CPM fibers, the increase in the prevalence of CH fibers and the acquisition of mechanical sensitivity by some normally mechanically insensitive CH fibers. These results also suggest that while the increase in heat and mechanical sensitivity in these different populations of cells is P2Y1 dependent, the recruitment of mechanically insensitive CH fibers is P2Y1 independent.
Malin and Molliver [22] recently reported that P2Y1−/− mice had normal behavioral responses to acute heat. However, similar to that seen in mice lacking TRPV1 the effect on heat signaling was only evident following inflammation [2; 5]. In addition, we have recently shown that TRPV1−/− mice lack functional CH fibers and that following inflammation in TRPV1−/− mice, CPM fibers undergo the same decrease in heat threshold seen here, but this that change was not sufficient to drive heat hyperalgesia in the absence of TRPV1 [14]. The results of these studies, taken together with the current findings, suggest that the increase in P2Y1 signaling following inflammation and the subsequent decrease in CPM heat threshold may also be required for the normal development of heat hyperalgesia. Thus it is possible that both TRPV1-positive CH fibers and the non-peptidergic CPM fibers contribute to the development of heat hyperalgesia following inflammation.
Summary.
The purinergic receptor P2Y1 modulates the thermal sensitivity of polymodal fibers and the functional phenotype of TRPV1 containing cutaneous fibers following inflammation.
Acknowledgments
This study was supported by NIH grants NS023725 and NS052848 to HRK and NS056122 to DCM.
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64(12):1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 3.Christianson JA, McIlwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon afferents compared to skin and muscle afferents. Neuroscience. 2006;140(1):247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, Troy CM. Highly efficient small interfering RNA delivery to primary mammalian neurons induces MicroRNA-like effects before mRNA degradation. J Neurosci. 2004;24(45):10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405(6783):183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 6.Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26(33):8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerevich Z, Borvendeg SJ, Schroder W, Franke H, Wirkner K, Norenberg W, Furst S, Gillen C, Illes P. Inhibition of N-type voltage-activated calcium channels in rat dorsal root ganglion neurons by P2Y receptors is a possible mechanism of ADP-induced analgesia. J Neurosci. 2004;24(4):797–807. doi: 10.1523/JNEUROSCI.4019-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerevich Z, Muller C, Illes P. Metabotropic P2Y1 receptors inhibit P2X3 receptor-channels in rat dorsal root ganglion neurons. Eur J Pharmacol. 2005;521(1–3):34–38. doi: 10.1016/j.ejphar.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Jankowski MP, Cornuet PK, McIlwrath S, Koerber HR, Albers KM. SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience. 2006;143(2):501–514. doi: 10.1016/j.neuroscience.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jankowski MP, Lawson JJ, McIlwrath SL, Rau KK, Anderson CE, Albers KM, Koerber HR. Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J Neurosci. 2009;29(6):1636–1647. doi: 10.1523/JNEUROSCI.3474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, Albers KM. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009;1256:43–54. doi: 10.1016/j.brainres.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankowski MP, Rau KK, Soneji DJ, Anderson CE, Koerber HR. Enhanced Artemin/GFR{alpha}3 Levels Regulate Mechanically Insensitive, Heat-Sensitive C-Fiber Recruitment after Axotomy and Regeneration. J Neurosci. 2010;30(48):16272–16283. doi: 10.1523/JNEUROSCI.2195-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi K, Fukuoka T, Yamanaka H, Dai Y, Obata K, Tokunaga A, Noguchi K. Neurons and glial cells differentially express P2Y receptor mRNAs in the rat dorsal root ganglion and spinal cord. J Comp Neurol. 2006;498(4):443–454. doi: 10.1002/cne.21066. [DOI] [PubMed] [Google Scholar]
- 14.Koerber HR, McIlwrath SL, Lawson JJ, Malin SA, Anderson CE, Jankowski MP, Davis BM. Cutaneous C-polymodal fibers lacking TRPV1 are sensitized to heat following inflammation, but fail to drive heat hyperalgesia in the absence of TRPV1 containing Cheat fibers. Mol Pain. 2010;6(1):58. doi: 10.1186/1744-8069-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koltzenburg M. The changing sensitivity in the life of the nociceptor. Pain. 1999;(Suppl 6):S93–102. doi: 10.1016/S0304-3959(99)00142-6. [DOI] [PubMed] [Google Scholar]
- 16.Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78(4):1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- 17.Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992;68(2):581–595. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- 18.Kress M, Riedl B, Reeh PW. Effects of oxygen radicals on nociceptive afferents in the rat skin in vitro. Pain. 1995;62(1):87–94. doi: 10.1016/0304-3959(94)00254-C. [DOI] [PubMed] [Google Scholar]
- 19.Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9(4):298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D, Ren Y, Xu X, Zou X, Fang L, Lin Q. Sensitization of primary afferent nociceptors induced by intradermal capsaicin involves the peripheral release of calcitonin gene-related Peptide driven by dorsal root reflexes. J Pain. 2008;9(12):1155–1168. doi: 10.1016/j.jpain.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malin SA, Davis BM, Koerber HR, Reynolds IJ, Albers KM, Molliver DC. Thermal nociception and TRPV1 function are attenuated in mice lacking the nucleotide receptor P2Y2. Pain. 2008;138(3):484–496. doi: 10.1016/j.pain.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malin SA, Molliver DC. Gi- and Gq-coupled ADP (P2Y) receptors act in opposition to modulate nociceptive signaling and inflammatory pain behavior. Mol Pain. 2010;6:21. doi: 10.1186/1744-8069-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malin SA, Molliver DC, Koerber HR, Cornuet P, Frye R, Albers KM, Davis BM. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26(33):8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIlwrath SL, Lawson JJ, Anderson CE, Albers KM, Koerber HR. Overexpression of neurotrophin-3 enhances the mechanical response properties of slowly adapting type 1 afferents and myelinated nociceptors. Eur J Neurosci. 2007;26(7):1801–1812. doi: 10.1111/j.1460-9568.2007.05821.x. [DOI] [PubMed] [Google Scholar]
- 25.Mogil JS, Breese NM, Witty MF, Ritchie J, Rainville ML, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci. 2005;25(43):9893–9901. doi: 10.1523/JNEUROSCI.2019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molliver DC, Lindsay J, Albers KM, Davis BM. Overexpression of NGF or GDNF alters transcriptional plasticity evoked by inflammation. Pain. 2005;113(3):277–284. doi: 10.1016/j.pain.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Molliver DC, Rau KK, McIlwrath SL, Jankowski MP, Koerber HR. The ADP receptor P2Y1 is necessary for normal thermal sensitivity in cutaneous polymodal nociceptors. Mol Pain. 2011;7:13. doi: 10.1186/1744-8069-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moriyama T, Iida T, Kobayashi K, Higashi T, Fukuoka T, Tsumura H, Leon C, Suzuki N, Inoue K, Gachet C, Noguchi K, Tominaga M. Possible involvement of P2Y2 metabotropic receptors in ATP-induced transient receptor potential vanilloid receptor 1-mediated thermal hypersensitivity. J Neurosci. 2003;23(14):6058–6062. doi: 10.1523/JNEUROSCI.23-14-06058.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura F, Strittmatter SM. P2Y1 purinergic receptors in sensory neurons: contribution to touch-induced impulse generation. Proc Natl Acad Sci U S A. 1996;93(19):10465–10470. doi: 10.1073/pnas.93.19.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruan HZ, Burnstock G. Localisation of P2Y1 and P2Y4 receptors in dorsal root, nodose and trigeminal ganglia of the rat. Histochem Cell Biol. 2003;120(5):415–426. doi: 10.1007/s00418-003-0579-3. [DOI] [PubMed] [Google Scholar]
- 31.Tamura S, Morikawa Y, Senba E. TRPV2, a capsaicin receptor homologue, is expressed predominantly in the neurotrophin-3-dependent subpopulation of primary sensory neurons. Neuroscience. 2005;130(1):223–228. doi: 10.1016/j.neuroscience.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Woo DH, Jung SJ, Zhu MH, Park CK, Kim YH, Oh SB, Lee CJ. Direct activation of transient receptor potential vanilloid 1(TRPV1) by diacylglycerol (DAG) Mol Pain. 2008;4:42. doi: 10.1186/1744-8069-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J Neurosci. 2004;24(28):6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]