Abstract
The host response and remodeling of ECM scaffolds are believed to be critical determinants of success or failure in repair or reconstructive procedures. Host response has been investigated in subcutaneous or abdominal wall implantation models. The extent to which evaluation of the host response to ECM intended for tendon or ligament repair should be performed in an orthotopic site is not known. This study compared the host response to human-derived fascia lata ECM among various implantation sites in the rat model. Results showed that a xenograft in the rat shoulder does not exhibit a different host response at 7 days from xenograft in the body wall, suggesting that either site may be appropriate to study the early host response to biologic grafts as well as the effect of various treatments aimed to modify the early host response. By 28 days, a xenograft in the rat shoulder does elicit a unique host response from that seen in the body wall. Therefore, it may be more appropriate to use an orthotopic shoulder model for investigating the long-term host response and remodeling of biologic grafts to be used for rotator cuff repair.
Keywords: fascia lata ECM, quantitative real time PCR, host response, pro-inflammatory, pro-remodeling, histology
Natural extracellular matrix (ECM) scaffolds are used for tissue repair and reconstruction in many applications including skin, heart, and blood vessels, urinary tract, dura mater, laryngeal/esophageal structures, and musculoskeletal structures such as body wall, cartilage, bone, tendon, and ligament.1-3 Scaffolds derived from ECM are believed to provide a chemically and structurally instructive environment for host cells, via their natural composition, three-dimensional structure, and/or remodeling byproducts1-3 and thus may improve the biology of repair healing. However, the type of ECM that is best suited for a given clinical application is not known and the topic of much current research.
The host response and remodeling of ECM scaffolds are believed to be critical determinants of success or failure in repair or reconstructive procedures4 and has been investigated using subcutaneous5-7 or abdominal wall8-10 implantation models. A histologic study in a rat model demonstrated distinct inflammatory cell responses to various ECM scaffolds over time.9 Further, immunohistochemical and gene analysis in mouse and rat models showed that ECM scaffolds derived from small intestinal or urinary bladder submucosa demonstrated a Th2-like lymphocyte and M2 type macrophage response, suggestive of a remodeling rather than a rejection response.11,12 Although these evaluations did not take place at the intended site of clinical application, it is assumed that subcutaneous or body wall implantation models allow for fundamental evaluation of material–tissue interactions.
However, in tendon and ligament repair, mechanical loading13-15 and the presence of joint synovial fluid16,17 are known to profoundly influence the form and function of the repair tissue. Hence it is reasonable to assume that these environmental conditions could also influence the manner in which the host responds to and remodels an ECM used for these applications. While ECM scaffolds are being used for tendon and ligament repair in human patients,18-20 the host response and remodeling of ECM scaffolds have not been systematically investigated in these sites. The extent to which the host response to an ECM scaffold intended for tendon or ligament repair differs between subcutaneous/body wall sites and an orthotopic site is not known and may have implications for the models used to evaluate these scaffold materials.
The purpose of this work was to compare the host response to an ECM scaffold among various implantation sites and injury conditions (abdominal wall fascial defect, lumbar wall fascial defect, shoulder no injury, and shoulder with capsule and tendon defect) in a rat model. In this case human-derived fascia lata ECM, a xenograft in this animal model, was used. We hypothesized that the expression of pro-inflammatory genes and the presence of inflammatory cells within fascia xenografts would be greater in the shoulders than in either of the body wall sites. Secondly, we hypothesized that the expression of pro-inflammatory genes and the presence of inflammatory cells within fascia xenografts would be greater in shoulders with a capsule/tendon defect than without. Thirdly, we hypothesized the expression of pro-inflammatory genes would be greater within fascia xenografts than fresh fascia autografts, which served as a baseline for the host response to surgical intervention and tissue injury.
METHODS
Study Design
Sixteen male Lewis rats (retired breeders) were used for this study. Each rat underwent graft implantation at six surgical sites: xenografts in the shoulder (1) with and (2) without capsule and tendon injury, xenografts in (3) abdominal and (4) lumbar wall fascial defects, and fresh fascia autografts taken from and replaced in (5) abdominal and (6) lumbar wall fascial defects as a baseline for the host response to surgical intervention and tissue injury. Rats were sacrificed at seven (n = 8) and 28 days (n = 8). Samples were harvested and analyzed for expression of 16 host response genes (Table 1) and 10 matrix-related genes (Table 2), inflammatory cells, fibroblast-like cells, total cellularity and vascularity.
Table 1.
Names and Abbreviations for Host Response Genes
| Pro-Inflammatory |
Pro-Remodeling |
||
|---|---|---|---|
| Name | Abbrev. | Name | Abbrev. |
| Interleukins | IL-1α | Arginase | ARG |
| IL-1β | Interleukins | IL-1raa | |
| IL-6 | IL-4 | ||
| IL-8 | IL-5 | ||
| IL-12β | IL-10 | ||
| IL-18 | IL-13 | ||
| Interferon γ | INFγ | Transforming growth |
TGFβ |
| Tumor necrosis factor α |
TNFα | Factorbβ | |
| Inducible nitric oxide synthase |
iNOS | ||
Interleukin 1 receptor antagonist.
Table 2.
Names and Abbreviations for Matrix-Related Genes
| ECM/Tendon |
Enzyme |
||
|---|---|---|---|
| Name | Abbrev. | Name | Abbrev. |
| Decorin | Dec | Cathepsin K | Cat K |
| Collagen type I | Col I | Matrix metallo-proteinases (MMP) |
MMP2 |
| Collagen type III | Col III | MMP3 | |
| Thrombospondin 4 | TSP4 | MMP13 | |
| Scleraxis | SCX | ||
| Tenomodulin | TNMD | ||
Human Fascia Xenografts
Sixty-four implants (0.7 cm × 0.7 cm) were obtained from fascia lata derived from four human donors (Musculoskeletal Transplant Foundation, Edison, NJ, age range 18–55 years). The fascia was processed in manner similar to the antibiotic only treatment method (ABX) described previously, in which processed fascia lata demonstrated minimal genetic material and minimal between donor variability in biochemical or mechanical properties.21 All grafts within a given rat were derived from fascia from the same human donor.
Surgical Procedure
Surgical procedures were approved by the IACUC at the Cleveland Clinic.
Bilateral Shoulder Implantation
Via a posterior skin incision, superficial muscle layers were dissected off the acromion, the acromio-clavicular ligament was cut, and the acromion was removed. A fascia xenograft was attached to the supraspinatus and infraspinatus at the myotendinous junction with two 5-0 Prolene mattress sutures. On shoulders designated for the capsule/tendon defect, the supraspinatus and infraspinatus tendons were released from the humerus and the capsule was excised.22,23 In both shoulders, the graft was tied to the humerus via a transosseous tunnel with a 5-0 Prolene mattress suture. The overlying muscles were approximated with 3-0 Vicryl suture and the skin closed with running 4-0 Monocryl suture.
Abdominal Wall Implantation
Via a ventral midline incision, a full-thickness 0.7 cm × 0.7 cm fascial defect was created in the anterior sheath adjacent to the linea alba, leaving the underlying rectus muscle, transversalis fascia, and peritoneum intact.24 A fascia xenograft was secured into the defect at its corners using 5-0 Prolene. On the contralateral side, a second 0.7 cm × 0.7 cm anterior sheath defect was created and repaired with fresh autograft fascia (control). The skin incision was closed using 4-0 Vicryl suture.
Lumbar Wall Implantation
Via a dorsal midline incision the areolar layer was detached from the spinal processes and a full-thickness 0.7 cm × 0.7 cm fascial defect was created in the lumbar fascia without violating the underlying paraspinal muscles. A fascia xenograft was secured into the defect at its corners using 5-0 Prolene. On the contralateral side, a defect with autograft repair was performed as described above. The surgical site was closed in layers with 4-0 Vicryl suture.
Euthanasia and Tissue
Harvest At 7 and 28 days, rats were sacrificed (n = 8 per time point). Autografts had tended to contract and pull away from the fixation sutures resulting in a small ball of tissue that was not usable for histologic evaluation. Hence all autograft tissue was placed immediately in Trizol at 4°C for gene expression analysis. The xenografts were harvested free from underlying host tissue for assessment of host response within the graft. Xenografts from a subset of animals (three at 7 days and five at 28 days), were processed for both histology and gene expression. In this subset of animals, xenografts were bisected longitudinally and half of each xenograft fixed in 10% neutral buffered formalin for 24 h and processed for paraffin embedding. The second half of these samples and the full piece of all other samples from the remaining rats were placed immediately in Trizol at 4°C for gene expression analysis.
RNA Harvest and RT-PCR
Tissue samples were processed as described previously.25 18S was measured as a housekeeping gene using TaqMan® rRNA Control Reagents (VIC® dye) and TaqMan® Universal PCR Master Mix (ABI, Foster City, CA). All other genes were measured using Power SYBR® Green PCR Master Mix (ABI). The primer sets for all experimental genes (Supplemental Table S1) were designed using PerlPrimer v1.1.18 software (http://perlprimer.sourceforge.net) with one primer spanning an exon junction. The relative expression for each sample was determined from a relative standard curve (R2 = 0.99) created for each gene25-27 using cDNA made from either IL1-β stimulated rat tail tendon fibroblasts or normal rat spleen. All assays were performed in triplicate, averaged, and the relative expression for each gene normalized to the relative 18S expression, giving a normalized relative expression value.25-27
Histologic Analysis
One representative hematoxylin and eosin (H&E) section from each xenograft site in three rats at 7 days and five rats at 28 days was scored blindly by two board-certified pathologists (RR and CT) for inflammatory cells, fibroblast-like cells, total cellularity and vascularity, according to a semiquantitative scoring system adapted from the International Organization for Standardization (ISO) Standard 10993-6.24
Statistical Analysis
Normalized relative gene expression values were natural log (ln) transformed to correct for extreme skewness of their distributions. (Non-parametric procedures were not used due to a large percentage of undetectable expressions and the unsuitability of the median as a location measure.) To facilitate ln transformation, samples with undetectable expression were assigned a relative expression of 1 × 10−12 (two orders of magnitude lower than lowest measured value for any gene). Analysis of variance (ANOVA) was used to test for differences between sites for each gene and histologic outcome. Using a Bonferroni adjustment for multiple comparisons, a p-value of 0.002 for gene expression analysis (0.05/25 genes evaluated) and 0.006 for histologic analysis (0.05/8 histologic outcomes) was considered significant. Where ANOVA showed significant differences, a Tukey HSD post hoc test (p = 0.05) was used to identify differences between sites. The statistical analyses were performed using SAS version 9.2.
RESULTS
Post-surgical complications necessitated early sacrifice of two rats in the 7-day group, leaving six rats in the 7-day group and eight rats in the 28-day group.
Gene Expression
At neither time point were differences in the expression of any gene found between xenografts in shoulders (1) with versus (2) without capsule and tendon injury, xenografts in (3) abdominal versus (4) lumbar wall defects, or autografts in (5) abdominal versus (6) lumbar wall defects. Data were therefore reduced to three groups (at two time points): shoulder xenografts (1 and 2), body wall xenografts (3 and 4), and body wall autografts (5 and 6). Only where significant differences existed are gene expression data presented graphically, however, all data for 7- and 28-day genes is provided in Supplemental Table S2.
Pro-Inflammatory Genes
At 7 days, expression levels of two pro-inflammatory genes (IL-1β and iNOS) were significantly higher in xenografts (shoulder and body wall) than in body wall autografts (Fig. 1). At 28 days, six (IL-1α, IL-1β, IL-6, IL-18, iNOS, TNFα) out of nine pro-inflammatory genes were expressed at significantly higher levels in shoulder xenografts than in body wall grafts (xeno- or auto-) (Fig. 2). There were no differences in pro-inflammatory gene expression between xenografts and autografts in the body wall at 28 days.
Figure 1.
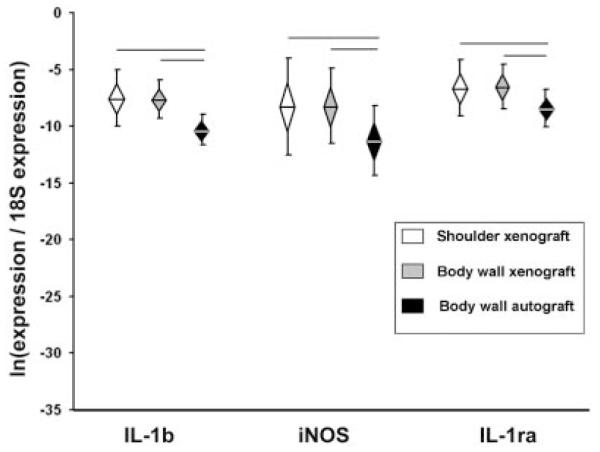
Gene expression, Day 7. Normalized relative gene expression, ln transformed, plotted as mean (n = 6), error bars = standard deviation, diamond = 95% confidence interval. Significant differences between groups are denoted by the solid horizontal lines.
Figure 2.
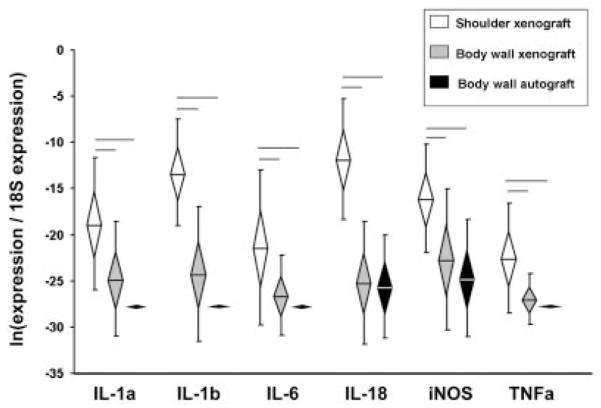
Pro-inflammatory gene expression, Day 28. Normalized relative gene expression, ln transformed, plotted as mean (n = 8), error bars = standard deviation, diamond = 95% confidence interval. Significant differences between groups are denoted by the solid horizontal lines.
Pro-Remodeling Genes
The pro-remodeling gene IL-1ra was significantly higher in xenografts (shoulder and body wall) than body wall autografts at 7 days (Fig. 1). At 28 days, IL-1ra and TGFβ were significantly higher in shoulder xenografts than in body wall grafts (xeno- or auto-) (Fig. 3). In contrast, IL-5 expression was significantly lower in shoulder xenografts than in body wall grafts (xeno- or auto-). There were no differences in pro-remodeling gene expression between xenografts and autografts in the body wall at 28 days.
Figure 3.
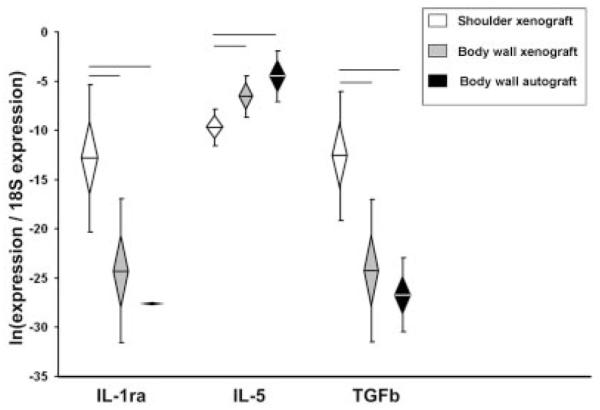
Pro-remodeling gene expression, Day 28. Normalized relative gene expression, ln transformed, plotted as mean (n = 8), error bars = standard deviation, diamond = 95% confidence interval. Significant differences between groups are denoted by the solid horizontal lines.
ECM/Tendon Genes
There were no differences among groups in the expression of ECM/tendon genes at 7 days (Supplemental Table S2). At 28 days, decorin and collagen I expression levels in shoulder xenografts were not different from body wall xenografts, but were significantly higher than body wall autografts (Fig. 4). Scleraxis and tenomodulin expression were significantly higher in shoulder xenografts than body wall grafts (xeno- or auto-) at 28 days (Fig. 4). There were no differences in ECM/tendon gene expression between xenografts and autografts in the body wall at 28 days.
Figure 4.
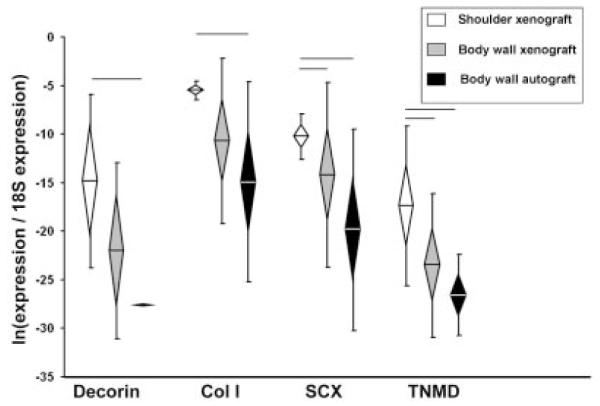
ECM/tendon gene expression, Day 28. Normalized relative gene expression, ln transformed, plotted as mean (n = 8), error bars = standard deviation, diamond = 95% confidence interval. Significant differences between groups are denoted by the solid horizontal lines.
ECM Catabolic Genes
There were no differences among groups in the expression of ECM catabolic genes at 7 days (Supplemental Table S2). At 28 days cathepsin K and MMP3 were significantly higher in shoulder xenografts than in body wall grafts (xeno- or auto-) (Fig. 5). The expression of cathepsin K in body wall xenografts was also significantly higher than in body wall autografts.
Figure 5.
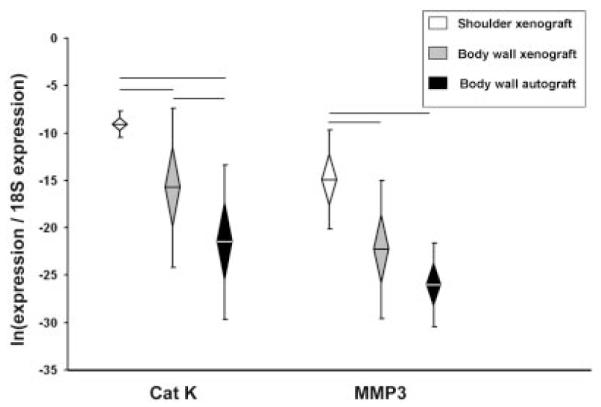
ECM catabolic gene expression, Day 28. Normalized relative gene expression, ln transformed, plotted as mean (n = 8), error bars = standard deviation, diamond = 95% confidence interval. Significant differences between groups are denoted by the solid horizontal lines.
Histology
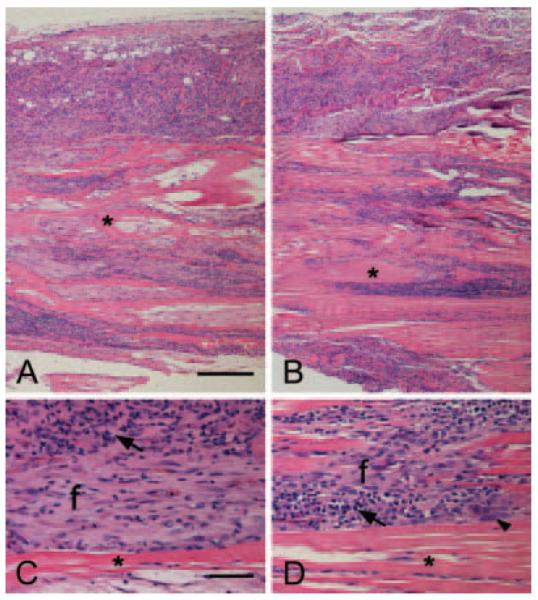
Xenografts in all sites at both time points elicited a chronic inflammatory response composed of neutrophils, lymphocytes, plasma cells, macrophages, and giant cells. By 28 days, the neutrophil response had largely resolved and the fascia matrix exhibited variable regions of cellularity (Fig. 6). The original fascia matrix is identified as bands of eosinophilic collagenous tissue (asterisks) infiltrated by variable amounts of inflammatory and spindle-shaped cells (Fig. 6A,B). Higher magnification shows comparable cellular composition in the abdominal wall (Fig. 6C) and shoulder (Fig. 6D) xenografts. Neovascularization is seen within areas of spindle-shaped fibroblast-like cells (f). The smaller and darker cells with scant cytoplasm (arrows) are mostly lymphocytes with admixed macrophages, plasma cells and occasional multinucleated giant cells (arrowhead). Semi-quantitative evaluation of the histologic slides revealed no differences in histologic outcomes between xenografts at the shoulder versus the body wall at either time point (Table 3).
Figure 6.
Histology, Day 28. Representative H&E stained sections of fascia xenograft from the abdominal wall (A,C) and shoulder (B,D). (A,B) Scale bar = 200 μm (×100); (C,D) scale = bar = 50 μm (×400); original fascia matrix (asterisks), fibroblast-like cells (f), lymphocytes (arrows), multinucleated giant cells (arrowhead).
Table 3.
Histologic Scores; Mean (Range), n = 6
| Inflammatory Cell Outcomes |
Non-Inflammatory Cell Outcomes |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time | Group | Lymphocyte | Plasma Cells |
Macrophage | Giant Cells |
PMNs | Vascularity | Fibroblast | Cellularity |
| 7 days | Body wall xenograft |
0.83 (0–1) | 0.67 (0–1) | 1.5 (1–2) | 0.33 (0–1) | 1.17 (0–3) | 0.5 (0–1) | 1.17 (1–2) | 1 |
| Shoulder xenograft |
1 | 1.25 (1–2) | 2 (1–3) | 0.5 (0–1) | 2 (1–3) | 0.5 (0–1) | 1.25 (1–2) | 1.17 (1–2) | |
| 28 days | Body wall xenograft |
1.8 (1–3) | 1.8 (1–2) | 2.7 (2–3) | 1.8 (1–3) | 0.4 (0–4) | 1.1 (1–2) | 1.6 (1–2) | 2.55 (1–4) |
| Shoulder xenograft |
1.8 (1–3) | 1.3 (1–2) | 2.55 (2–3) | 1.35 (1–2) | 0.4 (0–4) | 1.1 (1–2) | 2.05 (1–3) | 3.15 (2–4) | |
Histologic scoring was adapted from ISO 10993-6 standard.24 Inflammatory cell outcomes and vascularity: 1 = 1–5/hpf; 2 = 5–10/hpf; 3 = heavy infiltrate; 4 = packed. Fibroblasts: 1 = rare-100/hpf; 2 = 100–1,000/hpf; 3 = heavy infiltrate; 4 = packed. Cellularity: 1 ≤ 25%; 2 = 26-50%; 3 = 51-75%; 4 = >75%. hpf = high powered field (40×).
DISCUSSION
The purpose of this work was to compare the host response to human-derived fascia lata ECM (in this case a xenograft) among various implantation sites and injury conditions in the rat model. We evaluated host response by examining the expression of several pro-inflammatory and pro-remodeling genes that have been identified in previous musculoskeletal injury and treatment models.11,12,28-32 For instance, IL-1α and Il-1β have been identified in the inflammatory response to bone fracture29-32 and osteoarthritis (OA)28 as well as in the host response to biologic scaffolds.11,12 IL-1 receptor antagonist (IL-1ra), which attenuates the effects of both IL-1α and IL-1β, has been investigated as a therapeutic agent for the treatment of OA.28 Further, evaluation of macrophage polarization in response to implantation of biologic scaffolds has demonstrated that iNOS is associated with M1 pro-inflammatory macrophages and ARG is indicative of M2 or pro-remodeling macrophages.12
At 28 days there were large differences in the gene expression profiles between xenografts implanted in the shoulder versus the body wall. Six pro-inflammatory genes (IL-1α, IL-1β, IL-6, IL-18, iNOS, TNFα), two pro-remodeling genes (IL-1ra, TGFβ), three ECM/ tendon genes (decorin, scleraxis, tenomodulin) and two ECM catabolic genes (cathepsin K, MMP3) were significantly higher in shoulder xenografts than in body wall xenografts. Further, the expression of IL-5 (a pro-remodeling gene) was significantly lower in shoulder xenografts than in body wall xenografts at 28 days. The similarities in 28-day gene expression between body wall xenograft and autograft suggest that differences between shoulder and body wall xenografts at 28 days are attributed to differences associated with the site of implantation.
The host response to grafts implanted at the shoulder site could be uniquely influenced by the large transosseous bone tunnel utilized for graft attachment to the humerus. This tunnel was disproportionately larger than any bone perforation that might occur in a human undergoing rotator cuff repair, potentially resulting in a particularly dominating bone healing response. It has been shown that after fracture, the expression of IL-1, TNFα, and TGFβ in the bone peaks in the fracture callus at 21 days,29,30,33 perhaps corroborating the higher expression of these genes at the shoulder site in our model. In contrast, during fracture healing IL-6 dropped after 7 days and remained undetectable and IL-12β and INFγ expression were elevated at 28 days.31,32 In our study, however, IL-6 remained elevated and Il-12β and INFγ expression were not elevated at 28 days in the shoulder xenografts. Further investigation is warranted to obtain a clearer understanding of the influence of bone tunnel bleeding/healing on the host response to ECM grafts.
The presence of mechanical factors at the shoulder site could also contribute to the differences in geneexpression between the shoulder and body wall sites, as the influence of mechanical load on gene expression is well-documented.14,15 Although the grafts were not placed in the shoulders under tension, the rats were allowed unrestricted activity following surgery. It is possible the shoulder grafts experienced some degree of tensile or shear load due to active and passive motion of the joint and/or the overlying musculature. However, the extent to which tensile load may have contributed to differences in gene expression between the shoulder and body wall sites in this study could be under-represented because nearly half the graft was outside the region that was affixed to the host with sutures.
It also is possible that differences in gene expression between the shoulder and body wall sites at 28 days reflects a delay in resolution of the inflammatory response in the shoulder, but not a fundamentally different host response between sites. This conclusion is supported by the finding that there were no histologic differences in the number or type of immune cells between the shoulder and body wall grafts at either time point. A longer duration study would be required to determine the extent to which the differences in gene expression between the shoulder and body wall sites persist beyond 28 days and result in a clinically important difference in remodeling outcome.
It is important to note that at 7 days there was no difference in the gene expression profiles between xenografts implanted in the shoulder versus the body wall, and both xenograft groups showed increased expression of two pro-inflammatory genes (IL-1β, iNOS) and one pro-remodeling gene (IL-1ra) compared to autografts. The absence of a site-dependent xenograft gene response at 7 days suggests that the body wall model may be appropriate to study the early host response to biologic grafts, as well as the effect of various treatments aimed to modify the early host response.
Contrary to our second hypothesis, the presence of a capsule and tendon defect in the rotator cuff did not influence the host response to the xenografts. We expected that opening the shoulder joint capsule and exposing the injury site to synovial fluid would create a different wound healing environment and thus elicit a different host response to the graft. It is possible that we were unable to detect a difference even if it existed because the graft was approximately twice the size of the capsule/tendon defect. Thus, the gene expression data may have reflected equal numbers of cells that were and were not exposed to synovial fluid. Further, the healing response associated with the large transosseous bone tunnel utilized for graft attachment in both shoulder sites may have dominated the host response. To allow for a more conclusive evaluation of the influence of the synovial environment on host response to biomaterials, future studies should utilize a graft that is more proportional to the size of the capsule/tendon defect and incorporate a bone injury proportional to that incurred during graft fixation in human rotator cuff repair.
Contrary to our third hypothesis, no differences in gene expression were seen between xenografts and autografts placed in the body wall sites at 28 days. The higher expression of IL-1b, iNOS, and IL-1ra in xenografts noted at 7 days had resolved by 28 days. These results demonstrate that the long-term host response to the xenograft material at the body wall site is not different than the baseline response to the surgical intervention.
The interpretation of our study is limited by the following considerations. First, as previously noted, the shoulder grafts were approximately twice the size of the capsule and tendon defect, thus any differences in host response due to the presence of a joint injury may have been masked. Second, portions of the shoulder grafts that were outside of the suture attachment point and thus incapable of experiencing any tensile loads were included in the gene analysis. Third, the bone tunnel in the rat shoulder is disproportionately larger than it would be in a human undergoing rotator cuff repair, therefore, any influence of bone healing on the host response to the graft may be exaggerated in this rat shoulder model. Finally, the surgical challenge to the animals in our study was significant, however, the various surgical sites were reasonably isolated from each other and any systemic effects of the surgical insult would have likely affected all sites uniformly. Hence we do not believe confounding effects were introduced due to the multiple procedures performed.
In summary, implantation of a xenograft in the rat shoulder does not exhibit a different host response at 7 days from xenografts in the body wall, suggesting the body wall model may be appropriate to study the early host response to biologic grafts as well as the effect of various treatments aimed to modify the early host response. By 28 days, a xenograft implanted in the rat shoulder does elicit a unique host response from that seen in the body wall. To the extent that gene expression differences between the shoulder and body wall are shown to manifest for clinically meaningful reasons (e.g., bony fixation, mechanical load, and/or synovial fluid), it may be more appropriate to use an orthotopic shoulder model for investigating the long-term host response and remodeling of biologic grafts to be used for rotator cuff repair.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the NIH (AR056633-01). One or more of the authors receives research funding and royalty from the Musculoskeletal Transplant Foundation.
Footnotes
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377–383. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 2.Badylak SF, Freytes DO, Gilbert TW. Extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2009;5:1–13. doi: 10.1016/j.actbio.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 3.Reing JE, Zhang L, Myers-Irvin J, et al. Degradation products of extracellular matrix affect cell migration and proliferation. Tissue Eng Part A. 2009;15:605–614. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 4.Badylak SF, Valentin JE, Ravindra AK, et al. Macrophage phenotype as a determinant of biologic scaffold remodeling. Tissue Eng Part A. 2008;14:1835–1842. doi: 10.1089/ten.tea.2007.0264. [DOI] [PubMed] [Google Scholar]
- 5.Kidd KR, Dal Ponte DB, Kellar RS, et al. A comparative evaluation of the tissue responses associated with polymeric implants in the rat and mouse. J Biomed Mater Res. 2002;59:682–689. doi: 10.1002/jbm.10032. [DOI] [PubMed] [Google Scholar]
- 6.Patil SD, Papadmitrakopoulos F, Burgess DJ. Concurrent delivery of dexamethasone and VEGF for localized inflammation control and angiogenesis. J Control Release. 2007;117:68–79. doi: 10.1016/j.jconrel.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Laschke MW, Haufel JM, Thorlacius H, et al. New experimental approach to study host tissue response to surgical mesh materials in vivo. J Biomed Mater Res A. 2005;74:696–704. doi: 10.1002/jbm.a.30371. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen P, Bjursten LM, Ericson LE. Implants in the abdominal wall of the rat. Scand J Plast Reconstr Surg. 1986;20:173–182. doi: 10.3109/02844318609006316. [DOI] [PubMed] [Google Scholar]
- 9.Valentin JE, Badylak JS, McCabe GP, et al. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88:2673–2686. doi: 10.2106/JBJS.E.01008. [DOI] [PubMed] [Google Scholar]
- 10.Cook JL, Fox DB, Kuroki K, et al. In vitro and in vivo comparison of five biomaterials used for orthopedic soft tissue augmentation. Am J Vet Res. 2008;69:148–156. doi: 10.2460/ajvr.69.1.148. [DOI] [PubMed] [Google Scholar]
- 11.Allman AJ, McPherson TB, Badylak SF, et al. Xenogeneic extracellular matrix grafts elicit a TH2-restricted immune response. Transplantation. 2001;71:1631–1640. doi: 10.1097/00007890-200106150-00024. [DOI] [PubMed] [Google Scholar]
- 12.Brown BN, Valentin JE, Stewart-Akers AM, et al. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials. 2009;30:1482–1491. doi: 10.1016/j.biomaterials.2008.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimbel JA, Van Kleunen JP, Williams GR, et al. Long durations of immobilization in the rat result in enhanced mechanical properties of the healing supraspinatus tendon insertion site. J Biomech Eng. 2007;129:400. doi: 10.1115/1.2721075. [DOI] [PubMed] [Google Scholar]
- 14.Thomopoulos S, Williams GR, Soslowsky LJ. Tendon to bone healing: differences in biomechanical, structural, and compositional properties due to a range of activity levels. J Biomech Eng. 2003;125:106–113. doi: 10.1115/1.1536660. [DOI] [PubMed] [Google Scholar]
- 15.Thomopoulos S, Das R, Birman V, et al. Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression. Tissue Eng Part A. 2011;17:1039–1053. doi: 10.1089/ten.tea.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bedi A, Kawamura S, Ying L, et al. Differences in tendon graft healing between the intra-articular and extraarticular ends of a bone tunnel. HSS J. 2009;5:51–57. doi: 10.1007/s11420-008-9096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amiel D, Harwood FL, Gelberman RH, et al. Autogenous intrasynovial and extrasynovial tendon grafts: an experimental study of pro alpha 1(I) collagen mRNA expression in dogs. J Orthop Res. 1995;13:459–463. doi: 10.1002/jor.1100130321. [DOI] [PubMed] [Google Scholar]
- 18.Badhe SP, Lawrence TM, Smith FD, et al. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. J Shoulder Elbow Surg. 2008;17:35S–39S. doi: 10.1016/j.jse.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Bond JL, Dopirak RM, Higgins J, et al. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24:403–409. doi: 10.1016/j.arthro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 20.Noyes FR, Barber-Westin SD. Reconstruction of the anterior cruciate ligament with human allograft. Comparison of early and later results. J Bone Joint Surg Am. 1996;78:524–537. doi: 10.2106/00004623-199604000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Derwin KA, Baker AR, Spragg RK, et al. Regional variability, processing methods, and biophysical properties of human fascia lata extracellular matrix. J Biomed Mater Res A. 2008;84:500–507. doi: 10.1002/jbm.a.31455. [DOI] [PubMed] [Google Scholar]
- 22.Dourte LM, Perry SM, Getz CL, et al. Tendon properties remain altered in a chronic rat rotator cuff model. Clin Orthop Relat Res. 2010;468:1485–1492. doi: 10.1007/s11999-009-1206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsu JE, Reuther KE, Sarver JJ, et al. Restoration of anterior-posterior rotator cuff force balance improves shoulder function in a rat model of chronic massive tears. J Orthop Res. 2011;29:1028–1033. doi: 10.1002/jor.21361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chin L, Calabro A, Rodriguez ER, et al. Characterization of and host response to tyramine substituted-hyaluronan enriched fascia extracellular matrix. J Mater Sci Mater Med. 2011;22:1465–1477. doi: 10.1007/s10856-011-4325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leigh DR, Abreu EL, Derwin KA. Changes in gene expression of individual matrix metalloproteinases differ in response to mechanical unloading of tendon fascicles in explant culture. J Orthop Res. 2008;26:1306–1312. doi: 10.1002/jor.20650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.User Bulletin No 2. 1997 ABI PRISM 7700 Sequence Detection System. 1-36.
- 27.Johnson MR, Wang K, Smith JB, et al. Quantitation of dihydropyrimidine dehydrogenase expression by real-time reverse transcription polymerase chain reaction. Anal Biochem. 2000;278:175–184. doi: 10.1006/abio.1999.4461. [DOI] [PubMed] [Google Scholar]
- 28.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 29.Kon T, Cho TJ, Aizawa T, et al. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J Bone Miner Res. 2001;16:1004–1014. doi: 10.1359/jbmr.2001.16.6.1004. [DOI] [PubMed] [Google Scholar]
- 30.Lange J, Sapozhnikova A, Lu C, et al. Action of IL-1beta during fracture healing. J Orthop Res. 2010;28:778–784. doi: 10.1002/jor.21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 2008;14:179–186. doi: 10.1089/ten.teb.2008.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pape HC, Marcucio R, Humphrey C, et al. Traumainduced inflammation and fracture healing. J Orthop Trauma. 2010;24:522–525. doi: 10.1097/BOT.0b013e3181ed1361. [DOI] [PubMed] [Google Scholar]
- 33.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.