Abstract
Alström Syndrome is a clinically complex disorder characterized by childhood retinal degeneration leading to blindness, sensorineural hearing loss, obesity, type 2 diabetes mellitus, cardiomyopathy, systemic fibrosis, and pulmonary, hepatic, and renal failure. Alström Syndrome is caused by recessively inherited mutations in the ALMS1 gene, which codes for a putative ciliary protein. Alström Syndrome is characterized by extensive allelic heterogeneity, however founder effects have been observed in some populations. To date, more than 100 causative ALMS1 mutations have been identified, mostly frameshift and nonsense alterations resulting in termination signals in ALMS1. Here we report a complex Turkish kindred in which sequence analysis uncovered an insertion of a novel 333 basepair Alu Ya5 SINE retrotransposon in the ALMS1 coding sequence, a previously unrecognized mechanism underlying mutations causing Alström Syndrome. It is extraordinarily rare to encounter the insertion of an Alu retrotransposon in the coding sequence of a gene. The high frequency of the mutant ALMS1 allele in this isolated population suggests that this recent retrotransposition event spread quickly, and may be used as a model to study the population dynamics of deleterious alleles in isolated communities.
Keywords: Alström Syndrome, ALMS1, Alu Ya5, Insertion Mutation, Short Interspersed Nuclear Elements (SINE)
INTRODUCTION
Alström Syndrome (ALMS; OMIM 203800) is a multi-systemic autosomal recessive disorder with a broad spectrum of clinical signs (Alström, et al. 1959; Marshall et al. 2007). Patients with ALMS typically present in the first months of life with progressive retinal cone-rod dystrophy that leads to blindness by the end of the second decade. Bilateral sensorineural hearing impairment develops during childhood or adolescence. Other hallmarks of ALMS that develop in early childhood include truncal obesity, significant insulin resistance, hyperinsulinemia, type 2 diabetes mellitus (T2DM), and hypogonadism in both male and female patients. Dilated cardiomyopathy (DCM) and congestive heart failure (CHF) may develop suddenly in infants, whereas the gradual onset of restrictive cardiomyopathy can occur during adolescence or adulthood. Multi-organ fibrosis ultimately leads to major organ failure and premature death (Marshall et al. 2005, 2007). Serious manifestations of the fibrosis that affect patients' prognosis and survival include pulmonary fibrosis and restrictive lung disease, steatosis leading to cirrhosis and hepatic failure, and progressive glomerulosclerosis and renal failure. Other symptoms, not always present, include urological abnormalities, advanced bone age, adult short stature and scoliosis, hypothyroidism, mixed dyslipidemia, and hypertension. The clinical features emerge at different times throughout childhood making correct diagnosis in children problematic. To address the difficulty in obtaining an accurate molecular diagnosis due to phenotypic variability and the progressive nature of the clinical features, a set of diagnostic criteria has been developed to allow for the patient's age (Marshall et al. 2007). The differential diagnosis includes the more common Bardet-Biedl Syndromes (BBS), a genetically heterogeneous disorder that manifests principally in later-onset rod-cone dystrophy, postaxial polydactyly, central obesity, developmental disabilities and cognitive impairment, hypogonadism, and structural and functional renal abnormalities (Waters and Beales 2010).
ALMS is caused by mutations in ALMS1, a large gene on chromosome 2p13 comprised of 23 exons. ALMS1 encodes a protein of 4,169 amino acids (Collin et al. 2002; Hearn et al. 2002). The ALMS1 protein localizes to centrosomes and basal bodies of ciliated cells and is ubiquitously expressed in all tissues reported to be pathologically affected in patients, implicating ALMS as one of the emerging diseases falling under the class of ciliopathies. In general, the clinical picture of ciliopathies is variable with a wide range and growing number of clinically distinct phenotypes and causative genes (Baker and Beales 2010). Although the exact biological function of ALMS1 remains elusive, the pathophysiology of ALMS is thought to involve aberrant intracellular trafficking and ciliary dysfunction (Collin et al. 2005; Li et al. 2007). There are over a hundred recognized mutations causing Alström Syndrome, most of which occur in exons 8,10, and 16 of ALMS1 (Marshall et al. 2007b; Joy et al. 2002; Pereiro et al. 2011).
In the present study, we report a novel mutation characterized by the insertion of a large Alu Ya5 SINE retrotransposon into the coding sequence of ALMS1. This is the first report of a pathogenic ALMS1 mutation resulting from an Alu insertion.
MATERIALS AND METHODS
Clinical assessment of patients
The patients were diagnosed with ALMS at the Pediatric Department of Dicle University, based upon the clinical features and the diagnostic criteria for children aged 3–14 years proposed by Marshall et al. (2007). Written informed consent to participate was obtained from the parents, and the patients and siblings gave their verbal consent. The study protocol was conducted in accordance with the tenets of the Declaration of Helsinki, and the institutional review boards of the participating institutions approved the study protocols. No financial remuneration was given to patients or families for participation in this study.
Patient assessment included ophthalmologic, audiologic, and general physical examinations according to standard hospital protocols. Serum chemistries and routine urinalysis were performed using traditional methods in licensed clinical laboratories.
Extraction of Genomic DNA for sequencing
We collected peripheral blood samples from the patients, their unaffected siblings, and their parents, after obtaining appropriate informed consent. Genomic DNA from the propositus and his extended family was amplified with ALMS1 exon 16-specific primers as described previously (Collin et al. 2002). Products were purified and sequenced using the 3730×l DNA Analyzer (Applied Biosystems).
Accession Numbers
The GenBank accession number for the ALMS1 sequence reported in this paper is NM_015120.4)
RESULTS
Clinical Presentation of Patients
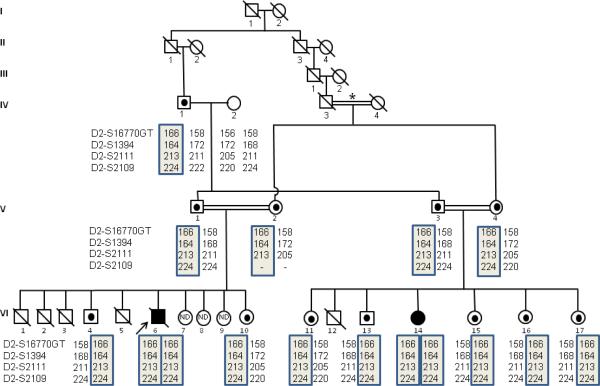
Here, we describe two patients from a highly consanguineous kindred residing in a small village near Kızıltepe, Mardin in southeastern Turkey. The fathers are brothers (V-1 and V-3) and the mothers are sisters (V-2 and V-4). Additionally, the great grandparents of the affected children descend from a common ancestral couple (I-1, and I–2) (Fig. 1).
Fig. 1.
A six generation Turkish family segregating for the ALMSAlu allele. Squares indicate male family members, circles indicate female family members. A strike-through indicates deceased. Double slashed lines indicate marriage consanguinity, with the relationship between IV-3 and IV-4, marked by an asterisk, being first degree. Black solid symbols indicate patients who are homozygous for the ALMSAlu allele. Heterozygous carriers of the Alu insertion are depicted by a black dot in the center of the symbol. ND indicates that the individual was unaffected, and not genotyped. The propositus (VI-6) is indicated by an arrow. The fathers (V-1 and V-3) are brothers and mothers (V-2 and V-4) are sisters). Additionally, the great grandparents of the affected children descend from a common ancestral couple (I-1, and I-2). This pedigree pinpoints that original transposition event occurred not less than 7 generations ago (I-1and I-2), and the allele has persisted in the families since then.
Patient #1
The proband (VI-6), a 13 year-old boy, was one of 10 children from a first degree consanguineous marriage. Three of his brothers (VI-1–3) died at 6–7 months of age, and one brother (VI-5) died at 12 months - all of unknown etiology, as medical evaluation was not available to the family. The proband had progressive vision loss since birth and was totally blind with no light perception. He also had bilateral sensorineural hearing loss. He exhibited truncal obesity, with a BMI of 28.9 (>95% for age and gender), short stature (<25 centile), kyphosis, pes planus, thin hair, thick ears, mild hypertension, and hypogonadism (low testicular volume, delayed puberty, Tanner grade 1). He had normal intelligence and no digital anomalies. This patient did not have the advanced bone age that is typical for children with ALMS, but instead had retarded growth with a bone age 12.5 years (Fig. 2).
Fig. 2.
Summary of clinical features of the patients with Alström Syndrome. A. Proband (VI-6) at 13 years of age. B. Proband's cousin (VI-14) at 6 years of age. C. Summary of clinical presentation of the two patients. NA-not assessed.
The patient had insulin resistance, hyperinsulinemia (194 pmol/L) and T2DM (fasting glucose: 45.1 mmol/L; HbA1C: 14.4%), He had no metabolic acidosis and was urine ketone-negative. He was hyperlipidemic with a total cholesterol of 17.6 mmol/L, an HDL of 0.62 mmol/L, VLDL of 7.3 mmol/L, and triglycerides > 16 mmol/L. He had sub-clinical hypothyroidism (TSH: 7.8 μIU/ml, free T4: 913.7 nmol/L). He did not have cardiomyopathy as an infant, but left ventricular hypertrophy without cardiac failure was detected by echocardiography at 11 years of age. He had hepatosplenomegaly and renal failure (urea: 59.2mmol/L, creatinine: 266.9umol/L, parathormone: 216 ng/L, urine density: 1005). He died from complications of CHF, hypertension, intracranial hemorrhage, and multiple organ failure (renal, hepatic, and pulmonary) after an episode of severe acute gastroenteritis at age 14 years.
Patient #2
This girl (VI–14), age 6 years, is a first cousin of Patient #1. She is one of seven children from a first degree consanguineous marriage. She had 5 unaffected siblings, and one sibling (VI–12) who died at the age of 6 months of undocumented causes. Her birth and perinatal period was normal, but photodysphoria and nystagmus were noticed at birth. Vision loss and obesity (BMI 29.2; >97th centile) manifested in early childhood. She has normal hearing. Her glucose, hepatic, pulmonary, urological, cardiac, and renal functions are normal. Her intelligence is within normal range and she lacks digital anomalies. She has hypertriglyceridemia (4.11mmol/L) (Fig. 2).
Detection of ALMS1 Mutations
Upon genetic evaluation, both patients were homozygous for an identical insertion of 333 bp in exon 16 of ALMS1. Comparison of the insertion sequence to the catalog of repetitive sequences in Repbase (Repbase, Genetic Information Research Institute, http://www.girinst.org/; Jurka et al. 2005) revealed that it belongs to the class of Alu Ya5 elements, an evolutionary recent transposition competent Alu family in the human lineage (Comeaux et al. 2009). In comparison to the wild-type ALMS1 allele (Fig. 3a), the ALMS1Alu allele (Fig. 3b) bears a target site duplication, which reflects the Alu insertion mechanism via target-site primed reverse transcription insertion (Perez-Stable et al. 1984). The target site correlates exactly with the proposed consensus 5'-TT^AAAA-3' of a primary nicking site for Alu insertions (Jurka, 1997). The long uninterrupted stretch of adenosines (76 nts) suggests that the Alu sequence was inserted by a recent retrotransposition event.
Fig. 3.
Alu retrotransposon insertion in the ALMS1 locus. Panel a shows the region in exon 16 where the insertion was discovered. The site targeted by the Alu insertion is highlighted in yellow. Open reading frame (ORF) is shown in bold. The target site (underlined) exactly matches the proposed consensus 5'-TT^AAAA-3' of a primary nicking site for Alu insertions (Jurka, 1997). Panel b shows the nucleotide sequence of the 333 base pair Alu insertion mutation introducing a premature stop codon, denoted with an asterisk. The Alu element is highlighted in red, and target site duplication in yellow. The alternative C-terminus of the ORF is in blue font. Panel c shows the sequence alignments of the Alu discovered in this study (Alu ALMS1), the Alu element at the CLCN5 locus (Alu CLCN5) confirmed as a recent de novo germline transposition, and the Alu Ya5 sequence from Repbase (RepBase ID: AluYa5). Truncation of 34 nucleotides at the 5' end of the Alu ALMS1 element, which contains the A-box of the Alu bipartite promoter, is likely due to incomplete reverse-transcription of original RNA copy. Identical bases are highlighted in red. Interestingly, C-->A transversion (highlighted in green) is a unique SNP in publicly available human genomic databases and likely reflects a rare polymorphism among active Alu elements.
Interestingly, we did not identify a 100% identical sequence anywhere in the human genome assembly, indicating a hitherto unknown polymorphism of active Alu Ya5 elements. Another distinguishing feature of the ALMS1Alu allele is a truncation of 34 nucleotides at the 5' end, likely a result of incomplete reverse transcription during transposition. Therefore, this particular element is likely no longer transcription- and retrotransposition-competent.
Heterozygous carriers were identified by haplotype analysis with chromosome 2p13-specific microsatellite markers and confirmed by PCR genotyping for the presence of the Alu allele (forward, TGATGATAGCAGAGGGGAAC; reverse, GGAAAGGATGTTGGTGGTAGT). The wild-type allele produces a PCR product size of 313 bps, while the Alu allele produces a PCR product size of 646 bps. As heterozygous carriers have been identified in both branches of the pedigree, at least one of the great-great-grandparents (I-1, or I-2) was likely also heterozygous for the ALMS1Alu allele. No clinical features of ALMS were reported in previous generations of this pedigree and no other affected individuals have yet been identified in this village.
Frequency of the ALMS1Alu Allele
DNA from 29 unaffected individuals from the Kiztepe village and 50 additional unaffected individuals from a random, unrelated Turkish population was tested for the presence of ALMS1Alu allele. We did not identify the insertion in any individuals in the “pan-Turkey” cohort. In contrast, in the local village population, the frequency of the mutation was 6.9% (2 out of 29 tested were heterozygous carriers of ALMS1Alu).
DISCUSSION
Mobile genetic elements, or transposons, are powerful modulators of genome variation and the biological implications of their activity are considerable, ranging from genomic instability in cancers to regulation of gene expression during development to speciation (Jurka et al. 2005; Comeaux et al. 2009; Perez-Stable et al. 1984). In mammalian genomes, all transposons belong to the group of retrotransposons which generate new copies and multiply by reverse-transcription of their RNA and integration of this cDNA into new genomic locations. Among the many types of retrotransposons present in humans, the short interspersed nuclear elements (SINEs) of the Alu class, originally derived from a signal recognition particle, 7SL RNA, have a high mutagenic potential in humans, and represent the largest group of repetitive sequences, accounting for approximately 20% of the human genome (Lander et al. 2001). Alu elements have propagated to an estimated 1–2×106 copies in primate genomes, and have generally achieved a balance between detrimental consequences for the individual and beneficial outcomes for genetic variation and speciation.
These Alu transpositions affect the genome in several ways, such as causing insertion mutations, recombination between elements, gene adaptation, and alterations in gene expression. Throughout 65 million years of primate evolution, these elements have spread over the entire genome by retrotransposition, contributing significantly to the size of the genome. Over the evolutionary timescale, most Alu subfamilies have lost their ability for retrotransposition and reside within the genomes as phenotypically neutral elements that are located in the intergenic, intronic, and untranslated regions, but rarely in the coding regions of genes and genomes (Batzer and Deininger 2002). However, some have preserved their retrotransposition capability, and have been shown to be the causative alteration in some diseases (Callinan and Batzer 2006; Claverie-Martín et al.,2003, 2005).
The Alu element identified in these patients belongs to the still-active Ya5 family, and thus features of the insertion suggest a very recent retrotransposition event. Interestingly, this is one of very few examples for an Alu element causing a direct disruption of an open reading frame by insertion in a coding exon.
The mutagenic potential of these retrotransposons has led to the development of host genome defense mechanisms aimed to suppress “retrotransposons life cycle”, mainly at the stage of transcription via DNA methylation of their promoters (Yoder et al. 1997). At the molecular level, Alu-mediated recombination can lead to deletion of genomic loci flanked by identical Alu repeats and is common in some hereditary cancers (Moolhuijzen et al.; Konkel and Batzer 2010). Another phenomenon, exon skipping, occurs when Alu insertions interfere with the assembly of the splicing machinery upon premRNA. Finally and infrequently, as in the case reported here, de novo Alu insertions may disrupt a coding exon of an essential gene, leading to an inheritable disease in people homozygous for the disrupted allele.
In summary a novel mutation was identified to be caused by a recent Alu insertion in the coding sequence of human ALMS1 in a Turkish family with Alström Syndrome. The large insertion leads to a disruption of the open reading frame and thus represents a unique disease-causing variant among ALMS1 mutations and extends the spectrum of mutations causative for Alström Syndrome. Additionally, this new observation provides valuable evidence for the devastating mutagenic potential of transposition-competent Alu elements.
ACKNOWLEDGEMENTS
The authors are grateful to the individuals with Alström Syndrome and their families who continue to show enthusiastic support of research efforts. This work was supported by National Institutes of Health grant HD036878 (JDM, GBC, JKN) and by The Jackson Laboratory institutional multimedia, allele typing, and sequencing services supported by US PHS National Institutes of Health grant CA34196.
REFERENCES
- Alström CH, Hallgren B, Nilsson LB, Åsander H. Retinal degeneration combined with obesity, diabetes mellitus and neurogenous deafness. A specific syndrome (not hitherto described) distinct from Laurence-Moon-Biedl syndrome. A clinical endocrinological and genetic examination based on a large pedigree. Acta Psychiatr Neurol Scand. 1959;34(Suppl. 129):1–35. [PubMed] [Google Scholar]
- Baker K, Beales PL. Making sense of cilia in disease: the human ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C:281–295. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nat Rev Genet. 2002;3:370–379. doi: 10.1038/nrg798. [DOI] [PubMed] [Google Scholar]
- Callinan PA, Batzer MA. Retrotransposable elements and human disease. Genome Dyn. 2006;1:104–115. doi: 10.1159/000092503. [DOI] [PubMed] [Google Scholar]
- Claverie-Martin F, González-Acosta H, Flores C, Antón-Gamero M, García-Nieto V. De novo insertion of an Alu sequence in the coding region of the CLCN5 gene results in Dent's disease. Hum Genet. 2003;113:480–485. doi: 10.1007/s00439-003-0991-8. [DOI] [PubMed] [Google Scholar]
- Claverie-Martín F, Flores C, Antón-Gamero M, González-Acosta H, García-Nieto V. The Alu insertion in the CLCN5 gene of a patient with Dent's disease leads to exon 11 skipping. J Hum Genet. 2005;50:370–374. doi: 10.1007/s10038-005-0265-5. [DOI] [PubMed] [Google Scholar]
- Collin GB, Marshall JD, Ikeda A, So WV, Russell-Eggitt I, Maffei P, Beck S, Boerkoel CF, Sicolo N, Martin M, Nishina PM, Naggert JK. Mutations in ALMS1 cause obesity, type 2 diabetes and neurosensory degeneration in Alström syndrome. Nat Genet. 2002;31:74–78. doi: 10.1038/ng867. [DOI] [PubMed] [Google Scholar]
- Collin GB, Cyr E, Bronson R, Marshall JD, Gifford EJ, Hicks W, Murray SA, Zheng QY, Smith RS, Nishina PM, Naggert JK. Alms1-disrupted mice recapitulate human Alström syndrome. Hum Mol Genet. 2005;14:2323–2333. doi: 10.1093/hmg/ddi235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeaux MS, Roy-Engel AM, Hedges DJ, Deininger PL. Diverse cis factors controlling Alu retrotransposition: what causes Alu elements to die? Genome Res. 2009;19:545–555. doi: 10.1101/gr.089789.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn T, Renforth GL, Spalluto C, Hanley NA, Piper K, Brickwood S, White C, Connolly V, Taylor JFN, Russell-Eggitt I, Bonneau D, Walker M, Wilson DI. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alström syndrome. Nat Genet. 2002;31:79–83. doi: 10.1038/ng874. [DOI] [PubMed] [Google Scholar]
- Joy T, Cao H, Black G, Malik R, Charlton-Menys V, Hegele RA, Durrington PN. Alström syndrome (OMIM 203800): a case report and literature review. Orphanet J Rare Dis. 2002;1:49. doi: 10.1186/1750-1172-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J. Sequence patterns indicate an enzymatic involvement in integration of mammalian retroposons. Proc Natl Acad Sci USA. 1997;94:1872872dc. doi: 10.1073/pnas.94.5.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J. Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Gen Res. 2005;110:462–467. doi: 10.1159/000084979. [DOI] [PubMed] [Google Scholar]
- Konkel MK, Batzer MA. A mobile threat to genome stability: The impact of non-LTR retrotransposons upon the human genome. Semin Cancer Biol. 2010;20:211–221. doi: 10.1016/j.semcancer.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K, Meldrim J, Mesirov JP, Miranda C, Morris W, Naylor J, Raymond C, Rosetti M, Santos R, Sheridan A, Sougnez C, Stange-Thomann N, Stojanovic N, Subramanian A, Wyman D, Rogers J, Sulston J, Ainscough R, Beck S, Bentley D, Burton J, Clee C, Carter N, Coulson A, Deadman R, Deloukas P, Dunham A, Dunham I, Durbin R, French L, Grafham D, Gregory S, Hubbard T, Humphray S, Hunt A, Jones M, Lloyd C, McMurray A, Matthews L, Mercer S, Milne S, Mullikin JC, Mungall A, Plumb R, Ross M, Shownkeen R, Sims S, Waterston RH, Wilson RK, Hillier LW, McPherson JD, Marra MA, Mardis ER, Fulton LA, Chinwalla AT, Pepin KH, Gish WR, Chissoe SL, Wendl MC, Delehaunty KD, Miner TL, Delehaunty A, Kramer JB, Cook LL, Fulton RS, Johnson DL, Minx PJ, Clifton SW, Hawkins T, Branscomb E, Predki P, Richardson P, Wenning S, Slezak T, Doggett N, Cheng JF, Olsen A, Lucas S, Elkin C, Uberbacher E, Frazier M, Gibbs RA, Muzny DM, Scherer SE, Bouck JB, Sodergren EJ, Worley KC, Rives CM, Gorrell JH, Metzker ML, Naylor SL, Kucherlapati RS, Nelson DL, Weinstock GM, Sakaki Y, Fujiyama A, Hattori M, Yada T, Toyoda A, Itoh T, Kawagoe C, Watanabe H, Totoki Y, Taylor T, Weissenbach J, Heilig R, Saurin W, Artiguenave F, Brottier P, Bruls T, Pelletier E, Robert C, Wincker P, Smith DR, Doucette-Stamm L, Rubenfield M, Weinstock K, Lee HM, Dubois J, Rosenthal A, Platzer M, Nyakatura G, Taudien S, Rump A, Yang H, Yu J, Wang J, Huang G, Gu J, Hood L, Rowen L, Madan A, Qin S, Davis RW, Federspiel NA, Abola AP, Proctor MJ, Myers RM, Schmutz J, Dickson M, Grimwood J, Cox DR, Olson MV, Kaul R, Raymond C, Shimizu N, Kawasaki K, Minoshima S, Evans GA, Athanasiou M, Schultz R, Roe BA, Chen F, Pan H, Ramser J, Lehrach H, Reinhardt R, McCombie WR, de la Bastide M, Dedhia N, Blöcker H, Hornischer K, Nordsiek G, Agarwala R, Aravind L, Bailey JA, Bateman A, Batzoglou S, Birney E, Bork P, Brown DG, Burge CB, Cerutti L, Chen HC, Church D, Clamp M, Copley RR, Doerks T, Eddy SR, Eichler EE, Furey TS, Galagan J, Gilbert JG, Harmon C, Hayashizaki Y, Haussler D, Hermjakob H, Hokamp K, Jang W, Johnson LS, Jones TA, Kasif S, Kaspryzk A, Kennedy S, Kent WJ, Kitts P, Koonin EV, Korf I, Kulp D, Lancet D, Lowe TM, McLysaght A, Mikkelsen T, Moran JV, Mulder N, Pollara VJ, Ponting CP, Schuler G, Schultz J, Slater G, Smit AF, Stupka E, Szustakowski J, Thierry-Mieg D, Thierry-Mieg J, Wagner L, Wallis J, Wheeler R, Williams A, Wolf YI, Wolfe KH, Yang SP, Yeh RF, Collins F, Guyer MS, Peterson J, Felsenfeld A, Wetterstrand KA, Patrinos A, Morgan MJ, de Jong P, Catanese JJ, Osoegawa K, Shizuya H, Choi S, Chen YJ, International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- Li G, Vega R, Nelms K, Gekakis N, Goodnow C, McNamara P, Wu H, Hong N, Glynne R. A Role for Alström Syndrome Protein, Alms1, in Kidney Ciliogenesis and Cellular Quiescence. PLoS Genet. 2007;3:e8. doi: 10.1371/journal.pgen.0030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JD, Bronson RT, Collin GB, Nordstrom AD, Maffei P, Paisey RB, Carey C, Macdermott S, Russell-Eggitt I, Shea SE, Davis J, Beck S, Shatirishvili G, Mihai CM, Hoeltzenbein M, Pozzan GB, Hopkinson I, Sicolo N, Naggert JK, Nishina PM. New Alström syndrome phenotypes based on the evaluation of 182 cases. Arch Intern Med. 2005;165:675–683. doi: 10.1001/archinte.165.6.675. [DOI] [PubMed] [Google Scholar]
- Marshall JD, Beck S, Maffei P, Naggert JK. Alström Syndrome. Eur J Hum Genet. 2007;15:1193–1202. doi: 10.1038/sj.ejhg.5201933. [DOI] [PubMed] [Google Scholar]
- Marshall JD, Hinman EG, Collin GB, Beck S, Cerqueira R, Maffei P, Milan G, Zhang W, Wilson DI, Hearn T, Tavares P, Vettor R, Veronese C, Martin M, So WV, Nishina PM, Naggert JK. Spectrum of ALMS1 variants and evaluation of genotype-phenotype correlations in Alström syndrome. Hum Mutat. 2007;28:1114–1123. doi: 10.1002/humu.20577. [DOI] [PubMed] [Google Scholar]
- Moolhuijzen P, Kulski JK, Dunn DS, Schibeci D, Barrero R, Gojobori T, Bellgard M. The transcript repeat element: the human Alu sequence as a component of gene networks influencing cancer. Funct Integr Genomics. 2010 Apr 15; doi: 10.1007/s10142-010-0168-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Pereiro I, Hoskins BE, Marshall JD, Collin GB, Naggert JK, Piñeiro-Gallego T, Oitmaa E, Katsanis N, Valverde D, Beales PL. Arrayed Primer Extension (APEX) technology simplifies mutation detection in Bardet Biedl and Alström Syndrome. Eur J Hum Genet. 2011;19:485–488. doi: 10.1038/ejhg.2010.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Stable C, Ayres TM, Shen CK. Distinctive sequence organization and functional programming of an Alu repeat promoter. Proc Natl Acad Sci USA. 1984;81:5291–5295. doi: 10.1073/pnas.81.17.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Beales PL. Bardet-Biedl Syndrome. In: Pagon RA, Bird TC, Dolan CR, Stephens K, editors. GeneReviews [Internet] University of Washington, Seattle; Seattle (WA): 2010. 1993-.2003 Jul 14 [updated 2010 Nov 18] [Google Scholar]
- Yoder JA, Walsh CP, Bestor TH. Cytosine methylation and the ecology of intragenomic parasites. Trends Genet. 1997;13:335–340. doi: 10.1016/s0168-9525(97)01181-5. [DOI] [PubMed] [Google Scholar]