Abstract
Background
Patients with eucalcemic parathyroid hormone elevation (ePTH) after parathyroidectomy for primary hyperparathyroidism (HPT) may be at risk of recurrence. We aimed to examine risk factors, trend of PTH level, and outcome of patients with ePTH 6 months after parathyroidectomy.
Methods
A total of 161 primary HPT were analyzed. The 6-month postoperative calcium and PTH levels were obtained. ePTH was defined as an elevated PTH level in the presence of normocalcemia. At 6 months, 98 had eucalcemic normal PTH and 63 (39.1%) had ePTH. Perioperative variables, PTH trend, and outcome were compared between 2 groups. Multivariable analyses were performed to identify independent preoperative and operative/postoperative risk factors for ePTH.
Results
Among preoperative factors, advanced age (odds ratio [OR] = 1.042, P = .027) and low 25-hydroxyvitamin D3 (25OHD3) (OR = 1.043, P = .009) were independently associated with ePTH, whereas among operative/postoperative factors, high 10-min intraoperative PTH level (OR = 1.015, P = .040) and high postoperative 3-month PTH (OR = 1.048, P < .001) were independently associated with ePTH. After a mean follow-up of 38.7 months, recurrence rate was similar between the 2 groups (P = 1.00). In the first 2 postoperative years, 75 (46.6%) had ePTH on at least 1 occasion and 8 (5.0%) had persistently ePTH on every occasion.
Conclusions
Advanced age, low 25OHD3, high 10-min intraoperative PTH, and high postoperative 3-month PTH were independently associated with ePTH at 6-month. Although 39.1% of patients had ePTH at 6 months, more than 50% had at least 1 ePTH within the first 2 years of follow-up. Recurrence appeared similar between those with or without ePTH at 6 months.
Although primary hyperparathyroidism (pHPT) remains a relatively uncommon endocrine disease among Asian countries with an incidence ranging between 0.5 and 1 per 3000 of the general population, it has been increasing over the last decade.1–3 This is related to better patient education, increased disease awareness, and more frequent serum calcium screening.2,3 Similar to the West, parathyroidectomy proves to be a cost-effective and curative treatment for the majority pHPT patients.4 In experienced hands, the surgical cure rate approaches 95%–100% with minimal morbidity.4 However, despite attaining postoperative normocalcemia, parathyroid hormone (PTH) elevation is not uncommonly observed after surgery. In the literature, this phenomenon of eucalcemic parathyroid hormone elevation (ePTH) has been well described and studied, although the exact etiology remains poorly understood.5 A number of explanations or causes have been suggested, and they include relative postoperative hypocalcemia/vitamin D deficiency leading to secondary HPT, chronic renal impairment, renal leak of calcium, bone remineralization or bone hunger, changes in the calcium-sensing receptors, decreased vitamin D 1-alpha-hydroxylation, and PTH receptor downregulation as a response to the high preoperative PTH level.5–11 Our clinical experience indicates that ePTH tends to concern referring physicians and treating surgeons alike.9,12 Furthermore, the natural history of postoperative ePTH and the optimal testing schedule following parathyroidectomy remains not entirely defined.5 More importantly, some authors have suggested that perhaps ePTH may imply “incomplete” parathyroidectomy or an early sign of future HPT recurrence.11,12 This issue is particularly relevant when an increasing number of surgeons are now performing focused or limited parathyroidectomy. Several studies so far have observed that when compared with patients with eucalcemic normal PTH level, patients with ePTH are at significantly higher rate of recurrence.9,12 Furthermore, it was found that 1-week serum calcium levels ≥9.7 mg/dL and a persistent PTH elevation predicted future recurrence.9 As a result, our study aimed to examine the risk factors, the trend of postoperative PTH level, and the long-term outcome in patients with ePTH 6 months after parathyroidectomy for pHPT by comparing perioperative variables and outcomes between pHPT patients with and without ePTH at 6-month after parathyroidectomy.
Patients and Methods
From 2004–2009, a total of 193 consecutive pHPT patients were managed at our institution. All were operated on and managed by the same team of surgeons. Of these, 16 (8.3%) were either lost to follow-up or had less than 6 months of follow-up, 3 (1.6%) had persistent disease, 6 (3.1%) patients had familial HPT or multiple endocrine neoplasia (MEN), and 7 (3.6%) patients required calcium ± vitamin D supplement for >6 months after surgery. For the purpose of the study, they were excluded leaving 161 (83.4%) patients eligible for the analysis. The mean follow-up period was 38.7 ± 23.7 months. An ePTH was defined as elevated PTH (i.e., >54 pg/mL) in combination with normal adjusted calcium (Ca) (i.e., ≤2.55 mmol/L) at 6 months after surgery. At 6-month follow-up, there were 98 (60.9%) with eucalcemic normal PTH (group A) and 63 (39.1%) with ePTH (group B). Demographics, biochemical profile, operative findings, and postoperative outcomes were compared between the 2 groups.
Surgical Procedure
Details on the surgical management of pHPT were described previously.4 Patients with 1 unequivocal preoperative localization study by Tc-99m-Sestamibi (MIBI) scan and/or high-frequency ultrasonography were eligible for focused or unilateral neck exploration. The focused or unilateral neck exploration was carried out by making a 2.5-cm lateral skin incision under general or regional cervical block with intravenous sedation. Quick intraoperative parathyroid hormone assay (IOPTH) and fasting 25-hydoxyvitamin D3 (25OHD3) were obtained by peripheral venous sampling on induction of anesthesia (i.e., before skin incision). IOPTH was repeated at 0 and 10 min after adenoma excision. A decline in IOPTH > 50% at 10 min compared with that at either induction or 0 min was defined as biochemical cure.
Laboratory Methods
All measurement of Ca, phosphate, alkaline phosphatase, and creatinine levels were measured in the same laboratory by standardized methods using the Roche Diagnostics Modular Analytic System (Roche Diagnostics, Indianapolis, IN).13 Serum 25OHD3 was measured using a direct electrochemiluminescence immunoassay procedure (Roche Hitachi Elecsys 2010, Minato-ku, Tokyo, Japan), and the interassay and intraassay coefficients of variations (CVs) were 6.6 and 4.0%, respectively. IOPTH was measured by Access 2 immunoassay system (Beckman Coulter, Brea, CA) and the interassay and intraassay CVs were 5.8% and 4.5%, respectively. Bone mineral density (BMD) was measured by the DXA technique using the Hologic QDR 4500 and Delphi W (Hologic, Bedford, MA).
Follow-up Evaluation
Postoperative serum Ca was measured on Day 0, Day 1, at 3 months, 6 months, 12 months, and annually, and PTH level was measured on Day 1, at 3 months, 6 months, 12 months, and annually. Those with hypocalcemic symptoms and/or a serum Ca < 2.00 mmol/L were given 2500 mg Oscal tablets twice daily. Rocaltrol 0.25 mcg twice daily was added if Oscal tablets alone failed to maintain normocalcemia. Postoperative serum 25OHD3 was not routinely checked. Biochemical cure was defined as a sustained reduction in serum Ca to a normal level (i.e., ≤2.55 mmol/L) at the latest follow-up, whereas biochemical recurrence was defined as normocalcemia for at least 6 months after surgery, followed by hypercalcemia (adjusted Ca > 2.55 mmol/L) some time thereafter. The present study protocol was approved by the local institutional review board.
Statistical Analysis
Statistical analysis was performed by chi-square or Fisher exact test to compare categorical variables, and Mann-Whitney U test was used to compare continuous variables between groups. Continuous variables were expressed as mean ± SD. Since there were 63 patients with ePTH at 6 months and using the rule of thumb of 10 events per covariate, the 5 most statistically significant covariates (based on lowest P values) in the univariate analysis were entered into multivariable analysis. Two binary logistic regression analyses with a variable entrance criterion <.05 were conducted to identify preoperative and operative/postoperative factors associated with ePTH 6 months after surgery, respectively. To evaluate the changes in postoperative PTH levels over time within each group, the Wilcoxon paired-sample rank test was used. All statistical analyses were performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL).
Results
Table 1 shows a comparison of patient demographics, preoperative biochemical profile, and BMD between groups A and B. Group B was significantly older at the time of parathyroidectomy (P = .003), had higher preoperative Ca (P = .020), higher PTH (P < .001), higher creatinine levels (P = .015), and lower 25OHD3 (P = .007) levels than group A. However, preoperative serum alkaline phosphatase and 24-hour urine calcium daily excretion were similar between the 2 groups. Of the 137 patients (85.1%) who had BMD measured <4 months before surgery, the BMD at the forearm, femoral neck, and lumbar spine were significantly lower in group B than in A.
Table 1.
Comparison of patient demographics, preoperative biochemical profile, and bone mineral density between those who had normal PTH level 6 months after parathyroidectomy (group A) and those who had elevated PTH 6 months after parathyroidectomy (group B)
| Group A (n = 98) | Group B (n = 63) | P value | |
|---|---|---|---|
| Age of parathyroidectomy (years) | 59.0 ± 13.1 | 65.6 ± 13.8 | .003 |
| Sex | .949 | ||
| Male | 26 (26.5) | 17 (27.0) | |
| Female | 72 (75.5) | 46 (73.0) | |
| Body mass index (kg/m2) | 24.8 ± 4.9 | 23.4 ± 3.7 | .388 |
| Preoperative adjusted calcium level (mmol/L) | 2.86 ± 0.2 | 2.94 ± 0.3 | .020 |
| Preoperative PTH level (pg/mL) | 169.3 ± 257.9 | 249.5 ± 302.6 | <.001 |
| Preoperative creatinine level (umol/L) | 82.0 ± 24.9 | 103.9 ± 59.9 | .015 |
| Preoperative serum alkaline phosphatase (U/L) | 112.8 ± 69.4 | 116.8 ± 78.1 | .898 |
| Preoperative 24-h urine calcium (mmol/day) | 6.7 ± 3.7 | 5.7 ± 3.4 | .150 |
| Preoperative 25OHD3 (ng/mL)a | 29.9 ± 18.1 | 22.2 ± 13.7 | .007 |
| Preoperative bone mineral densityb | |||
| Forearm (g/cm2) | 0.6 ± 0.1 | 0.5 ± 0.1 | .024 |
| Femoral neck (g/cm2) | 0.6 ± 0.1 | 0.6 ± 0.1 | .040 |
| L1–4 lumbar spine (g/cm2) | 0.8 ± 0.2 | 0.8 ± 0.2 | .040 |
PTH parathyroid hormone, 25OHD 3 25-hydroxyvitamin D3
aAvailable in 121 patients
bAvailable in 137 patients
Table 2 shows a comparison of operative findings between groups A and B. Group B had significantly heavier mean total parathyroid tissue excised (1467.5 ± 1946.0 mg vs 937.5 ± 2113.9 mg, P < .001) and adenoma (1461.1 ± 1947.9 mg vs 924.4 ± 2118.2 mg, P < .001) than group A. Although group B had significantly more focused or unilateral parathyroid exploration (92.1% vs 79.6%, P = .044), the number of parathyroid glands excised and total operating time were similar in the 2 groups. Consistent with the higher preoperative PTH level, the IOPTH level at induction, 0 min, and 10 min after excision of abnormal parathyroid tissue were also significantly higher in group B than in A. However, the percentage drop in IOPTH at 10 minutes from its highest level was not different between the 2 groups (P = .412).
Table 2.
Comparison of operative findings between those who had normal PTH level 6 months after parathyroidectomy (group A) and those who had elevated PTH 6 months after parathyroidectomy (group B)
| Group A (n = 98) | Group B (n = 63) | P value | |
|---|---|---|---|
| Total parathyroid tissue excised (mg) | 937.5 ± 2113.9 | 1467.5 ± 1946.0 | <.001 |
| Weight of adenoma (mg) | 924.4 ± 2118.2 | 1461.1 ± 1947.9 | <.001 |
| Operating time (minutes) | 74.6 ± 33.8 | 65.3 ± 30.0 | .076 |
| Surgical approach | .044 | ||
| Focused/unilateral | 78 (79.6%) | 58 (92.1%) | |
| Bilateral exploration | 20 (20.4%) | 5 (7.9%) | |
| No. of parathyroid glands excised | .285 | ||
| 1 | 86 (87.8%) | 59 (93.7%) | |
| ≥2 | 12 (12.2%) | 4 (6.3%) | |
| IOPTH (pg/mL) | |||
| Induction/preincision | 124.2 ± 127.5 | 205.3 ± 287.5 | <.001 |
| 0 min after excision | 64.6 ± 73.4 | 146.5 ± 225.7 | .001 |
| 10 min after excision | 26.3 ± 21.4 | 73.0 ± 139.0 | <.001 |
| Drop at 10 min (%) | 75.0 ± 14.8 | 69.7 ± 21.9 | .412 |
IOPTH quick intraoperative parathyroid hormone level
Table 3 shows a comparison of postoperative PTH, Ca levels, follow-up period, and recurrence rate between groups A and B. A total of 21 patients (21.4%) in group A and 14 patients (22.2%) in group B had undetectable PTH level (≤ 3.0 pg/mL) on postoperative Day 1, and this was not significantly different between the 2 groups (P = .848). Although the postoperative mean PTH level on Day 1 was similar between the 2 groups, the mean PTH level at 3, 6, 12, and 24 months, and latest follow-up were significantly higher in group B than in A. Postoperative Day 0 and Day 1 Ca were significantly higher in group B than in A. However, the proportion of patients requiring oral Oscal ± rocaltrol supplements was similar between groups B and A (4.8% vs 7.1%, P = .741, respectively). The mean duration of supplementation for groups B and A was 2.2 ± 1.4 and 2.9 ± 1.5 months, respectively. Of these 10 patients (6.2%), the mean duration of supplementation was 2.7 ± 1.5 months and 4 patients required rocaltrol supplements. The mean follow-up period was similar in groups A and B (P = .318). One patient in group A and B suffered biochemical recurrence at 24.2 and 46.7 months, respectively. Recurrence rate was not significantly different between the 2 groups (1.0% vs 1.6%, P = 1.000).
Table 3.
Comparison of postoperative parathyroid hormone, adjusted calcium levels, follow-up period, and recurrence rate between those who had normal PTH level 6 months after parathyroidectomy (group A) and those who had elevated PTH 6 months after parathyroidectomy (group B)
| Group A (n = 98) | Group B (n = 63) | P value | |
|---|---|---|---|
| Postoperative Day 1 PTH (pg/mL) | 19.7 ± 15.3* | 34.7 ± 73.8* | .795 |
| Postoperative 3-month PTH (pg/mL) | 40.2 ± 21.0+ | 91.1 ± 75.8+ | <.001 |
| Postoperative 6-month PTH (pg/mL) | 35.6 ± 13.3 | 90.1 ± 46.3 | <.001 |
| Postoperative 12-month PTH (pg/mL) | 40.1 ± 16.9* | 75.1 ± 53.1* | <.001 |
| Postoperative 24-month PTH (pg/mL) | 39.3 ± 19.0+ | 69.4 ± 48.4* | <.001 |
| Latest postoperative PTH level (pg/mL) | 38.6 ± 20.5+ | 67.8 ± 49.9* | <.001 |
| Postoperative Day 0 Ca level (mmol/L) | 2.56 ± 0.2 | 2.64 ± 0.2 | .011 |
| Postoperative Day 1 Ca level (mmol/L) | 2.35 ± 0.2 | 2.42 ± 0.2 | .021 |
| No. of patients requiring oral Oscal ± Rocaltrol supplements on discharge | 7 (7.1) | 3 (4.8) | .741 |
| Latest postoperative serum Ca level (mmol/L) | 2.25 ± 0.1 | 2.25 ± 0.1 | .486 |
| Drop in serum Ca from the highest preoperative level to 6-month postoperative level (%) | 19.1 ± 6.7 | 20.9 ± 7.5 | .107 |
| Follow-up period (months) | 39.9 ± 24.4 | 36.7 ± 22.6 | .318 |
| Biochemical recurrence (%) | 1 (1.0) | 1 (1.6) | 1.000 |
PTH parathyroid hormone, Ca adjusted Ca
* P value < .05 when compared with the relative postoperative 6-month PTH
+ P value > .05 when compared with the relative postoperative 6-month PTH
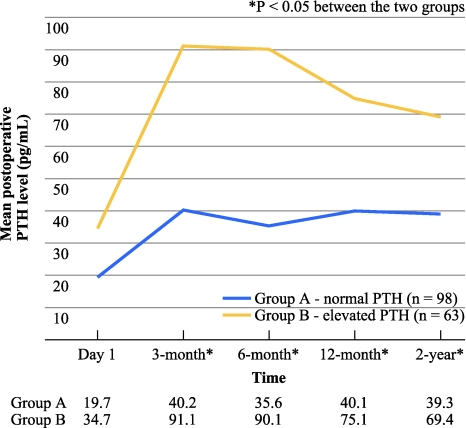
Figure 1 shows the change in mean postoperative PTH level on Day 1, at 3 months, 6 months, 12 months, and 2 years after parathyroidectomy in group A and B. In both groups, there was a significant rise in PTH from postoperative Day 1 to 3 months. However, after 6 months, there were no significant changes in PTH in group A, whereas there was a significant drop in group B. Postoperative PTH elevation appeared to peak at 3–6 months in group B. In the first 2 postoperative years, only 78 patients (48.4%) maintained normal postoperative PTH levels over the entire cohort. Also, 75 patients (46.6%) had elevated PTH level on at least 1 occasion and 8 (5.0%) had persistently elevated PTH on all occasions. On further analysis, in group B, 26 of 55 patients (47.3%) showed a normalized PTH at 12 months, while in group A, 10 of 86 patients (11.6%) had an elevated PTH at 12 months. Overall, the proportion of patients with normalized PTH increased from 62.5% at 6 months to 78.7% at 2 years, although at 2 years the difference in PTH level was still significant between the 2 groups.
Fig. 1.
Change in mean postoperative serum parathyroid hormone (PTH) level on Day 1, at 3 months, 6 months, 12 months, and 2 years after parathyroidectomy in those with normal PTH level at 6 months (group A) and those with elevated PTH at 6 months (group B); blue line represents group A (n = 98); yellow line represents group B (n = 63)
Table 4 shows the multivariable analysis of the 5 most statistically significant preoperative and perioperative risk factors for ePTH 6 months after parathyroidectomy. Adenoma weight (instead of total parathyroid weight) was entered into the multivariable analysis. After adjusting for preoperative PTH, creatinine level and adenoma weight, advanced age (odds ratio [OR] = 1.042) and low 25OHD3 (OR = 1.043) were independent preoperative factors for ePTH 6 months after surgery.
Table 4.
A multivariable analysis of preoperative and perioperative factors for eucalcemic parathyroid hormone elevation 6 months after parathyroidectomy
| Covariates | β coefficient | Odds ratios (95% confidence interval) | P value |
|---|---|---|---|
| Age of parathyroidectomy (years) (n = 161) | 0.041 | 1.042 (1.005–1.080) | .027 |
| Preoperative PTH level (pg/mL) (n = 161) | 0.001 | 1.001 (0.998–1.003) | .646 |
| Preoperative creatinine level (umol/L) (n = 161) | 0.012 | 1.013 (0.999–1.026) | .071 |
| Adenoma weight (mg) (n = 161) | 0.001 | 1.000 (0.999–1.001) | .099 |
| Preoperative 25OHD3 (ng/mL) (n = 121) | 0.042 | 1.043 (1.010–1.076) | .009 |
PTH parathyroid hormone, 25OHD 3 25-hydroxyvitamin D3
Table 5 shows the multivariable analysis of the 5 most statistically significant operative and postoperative risk factors for ePTH 6 months after parathyroidectomy. Factors beyond the 6-month mark were not considered in the model and only the 10-minute IOPTH was entered instead of all 3 measurements. After adjusting for surgical approach, postoperative Day 0 Ca level and postoperative Day 1 Ca level, high 10-minute IOPTH level (OR = 1.015), and high postoperative 3-month PTH (OR = 1.048) were independent postoperative factors for ePTH 6 months after surgery
Table 5.
Multivariable analysis of operative and postoperative factors for eucalcemic parathyroid hormone elevation 6 months after parathyroidectomy
| Covariates | β coefficient | Odds ratios (95% confidence interval) | P value |
|---|---|---|---|
| Unilateral neck exploration (n = 161) | −0.006 | 0.994 (0.278–3.557) | .992 |
| IOPTH 10-min after excision (pg/mL) (n = 161) | 0.015 | 1.015 (1.001–1.029) | .040 |
| Postoperative 3-month PTH level (pg/mL) (n = 161) | 0.048 | 1.048 (1.028–1.069) | <.001 |
| Postoperative Day 0 Ca level (n = 161) | 1.873 | 6.508 (0.189–224.0) | .300 |
| Postoperative Day 1 Ca level (mmol/L) (n = 161) | 3.339 | 8.329 (0.273–3.557) | .224 |
IOPTH quick intraoperative PTH level, PTH parathyroid hormone, Ca adjusted serum calcium
Discussion
Despite the fact that the exact etiology remains poorly understood, the phenomenon of postoperative ePTH in pHPT has been extensively described and studied.6–12,14–18 In the literature, the reported incidence ranges between 10% and 60%, depending on patient selection and definition of ePTH.5–12 Unlike previous studies that defined ePTH as an elevated PTH within weeks after surgery, our study defined ePTH as an elevated PTH level 6 months after surgery. This was because surgical cure or success of parathyroidectomy in pHPT often depends on attaining normocalcemia at 6 months, and in practice both surgeons and/or endocrinologists often check Ca and PTH around this time period.4,13 In our series, the incidence of eucalcemic PTH elevation at 6 months was 39.1%, and this appeared consistent to those previously reported.6–11,14–17 Similar to other studies, although postoperative PTH tends to slowly normalize over time, it also fluctuates.7,12 Over the first 2 years, only 78 patients (48.4%) maintained normal PTH levels throughout the entire cohort. Also, 75 patients (46.6%) had elevated PTH level on at least 1 occasion and 8 (5.0%) had persistently elevated PTH on all occasions. On closer analysis, in group B, 26 of 55 patients (47.3%) showed a normalized PTH at 12 months, while in group A, 10 of 86 patients (11.6%) had an elevated PTH at 12 months.
To examine the possible relationship between perioperative factors and ePTH at 6 months, two binary regression analyses were performed; one for preoperative factors and the other for operative/postoperative factors. In the univariate analysis of preoperative factors, similar to previous studies, advanced age, higher PTH, higher creatinine, heavier adenoma, and low 25OHD3 were significantly associated with ePTH; in the multivariable analysis, advanced age and low 25OHD3 were factors independently associated with ePTH at 6 months.7–9,11,12,17,18 Although age and 25OHD3 would seem interrelated as the latter tends to decrease with age, our data seemed to support the hypothesis that preexisting vitamin D deficiency is a possible cause of postoperative ePTH. This is consistent with previous studies.7,13,19,20 The implication is that perhaps patients with advanced age or with low preoperative 25OHD3 may benefit from a course of vitamin D supplementation after surgery. Beyer et al. reported that by giving 0.25 mcg oral rocaltrol supplementation after parathyroidectomy, the incidence of postoperative ePTH was significantly decreased compared with those who received oral calcium alone.15 However, it was interesting that Grubbs et al. demonstrated that giving ergocalciferol for a median duration of 28 days before parathyroidectomy did not correct postoperative ePTH.21 That means that if postoperative ePTH is to be corrected by vitamin D supplementation, it should be administered for some time after surgery, although the optimal duration remains unknown. To further confirm these findings, perhaps measuring postoperative 25OHD3 level in both groups would be useful, but such data were not available in our study. The authors believed this would definitely be an area for future studies. When both operative and postoperative factors were analyzed in the univariate and multivariable analyses, high 10-min IOPTH and high postoperative 3-month PTH level were independently associated with ePTH. However, given the nature of these factors, they probably acted as surrogates rather than risk factors for ePTH. Nevertheless, they would be useful indicators for possible ePTH in the future.
In terms of etiology, although the vitamin D deficiency hypothesis remains the most plausible mechanism for postoperative ePTH, other hypotheses including a multifactorial model are still possible given the interrelationship of these factors. For example, the finding of lower preoperative BMD at the forearm, femoral neck, and lumbar spine in group B seems to support the hungry bone syndrome hypothesis as more severe bone mineral loss leads to more severe bone hunger and higher PTH. However, unlike previous studies, our study did not observe the higher level of alkaline phosphatase that is a marker for bone turnover in group B.9,17,22 Perhaps, a future study could assess other bone markers such as osteocalcin and propeptide type I collagen to see if they also remain similar in the 2 groups. Although BMD improves after parathyroidectomy, it is unclear whether similar improvement is observed in those with and without ePTH.23 Although the higher creatinine level or worse renal impairment is one particular state in which PTH resistance is frequently observed, our study was unable to establish whether the PTH elevation was specifically related to PTH resistance.24 Perhaps, measuring the level of nephrogenous cyclic adenosine monophosphate or the 1,25-dihyroxyvitamin D/PTH ratios would shed light on this issue.7,8
In terms of operative findings, it was somewhat expected to find that patients with ePTH were significantly more likely to undergo focused or unilateral exploration because heavier adenoma is generally more likely to localize unequivocally.4 In contrast to one previous study that found a less significant drop of PTH in the elevated PTH group, our data showed the percentage drop was similar in the 2 groups.18
There have been suggestions that perhaps ePTH after parathyroidectomy may imply a “less complete” parathyroidectomy.9,11,12 Siperstein et al. showed that preoperative localizing studies and IOPTH failed to identify multiglandular disease (MGD) in at least 16% of pHPT patients.25 One postulation is that perhaps those with ePTH may suffer from undiagnosed MGD. However, so far, our data do not support this. After a mean follow-up of 38.7 months, the recurrence rate appeared similar in the 2 groups, although the number of recurrences was small and longer follow-up period might have been necessary.
In terms of the trend of ePTH, our study found the ePTH peaked sometime between 3 and 6 months. This differs from one study which observed the peak occurring at 2 months.17 However, similar to previous reports, the trend of PTH slowly normalized over time in patients with ePTH with more than 3/4 of the entire cohort having normal PTH.6,10,12,14 Future studies could focus on which factors influence the duration of normalization. One obvious factor would be vitamin D supplementation.15 However, since only 6.2% of patients received vitamin D supplementation lasting a mean duration of 2.7 ± 1.5 months, we did not exclude them from the binary logistic regression model. The authors believe that the trend of PTH may represent the trend of postoperative PTH in an untreated vitamin D group.
Despite our findings, our data should be interpreted cautiously as there were limitations. Firstly, because of the retrospective nature, some preoperative variables such as 25OHD3 and BMD as well as postoperative PTH levels were incomplete, and this limits the power of the multivariable analysis. Secondly, because of the relatively short follow-up, our data only had 2 patients with recurrence, and that limits the power of the study. Our data came from one university hospital and so were subjected to both institutional and referral biases. Future studies could assess the association between ePTH and other outcome variables such as quality of life, BMD, or fracture risk.
In conclusion, advanced age (OR = 1.042, P = .027) and low 25-OHD3 (OR = 1.043, P = .009) were independent preoperative factors associated with ePTH, whereas high 10-minute intraoperative PTH level (OR = 1.015, P = .040) and high postoperative 3-month PTH (OR = 1.048, P < .001) were independent postoperative factors associated with ePTH at 6 months. Given these findings, perhaps patients with these factors could receive a postoperative course of vitamin D supplementation in an attempt to normalize ePTH. However, the optimal duration remains unknown and is an area for further study. In addition, our data showed that in the first 2 postoperative years, more than half had elevated PTH level on at least one occasion and 8 (5.0%) had persistently elevated PTH level on all occasions. After a mean follow-up of 38.7 months, recurrence rate appeared similar between those with ePTH and without ePTH at 6 months.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bilezikian JP, Meng X, Shi Y, Silverberg SJ. Primary hyperparathyroidism in women: a tale of two cities—New York and Beijing. Int J Fertil Womens Med. 2000;45:158–165. [PubMed] [Google Scholar]
- 2.Lo CY, Chan WF, Kung AW, Lam KY, Tam SC, Lam KS. Surgical treatment for primary hyperparathyroidism in Hong Kong. Arch Surg. 2004;139:77–82. doi: 10.1001/archsurg.139.1.77. [DOI] [PubMed] [Google Scholar]
- 3.Chen HH, Chen YW, Wu CJ. Primary hyperparathyroidism in Taiwan: clinical features and prevalence in a single-center experience. Endocrine. 2010;37:373–378. doi: 10.1007/s12020-010-9315-7. [DOI] [PubMed] [Google Scholar]
- 4.Lo CY, Lang BH, Chan WF, Kung AW, Lam KS. A prospective evaluation of preoperative localization by technetium-99 m sestamibi scintigraphy and ultrasonography in primary hyperparathyroidism. Am J Surg. 2007;193:155–159. doi: 10.1016/j.amjsurg.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Oltmann SC, Maalouf NM, Holt S. Significance of elevated parathyroid hormone following parathyroidectomy for primary hyperparathyroidism. Endocr Pract. 2011;17(Suppl 1):57–62. doi: 10.4158/EP10324.RA. [DOI] [PubMed] [Google Scholar]
- 6.Nordenstrom E, Westerdahl J, Isaksson A, Lindblom P, Bergenfelz A. Patients with elevated serum parathyroid hormone levels after parathyroidectomy: showing signs of decreased peripheral parathyroid hormone sensitivity. World J Surg. 2003;27:212–215. doi: 10.1007/s00268-002-6600-5. [DOI] [PubMed] [Google Scholar]
- 7.Yamashita H, Noguchi S, Moriyama T, Takamatsu Y, Sadanaga K, Uchino S, et al. Reelevation of parathyroid hormone level after parathyroidectomy in patients with primary hyperparathyroidism: importance of decreased renal parathyroid hormone sensitivity. Surgery. 2005;137:419–425. doi: 10.1016/j.surg.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon K, Cohan P, Darwin C, Van Herle A, Chopra IJ. Elevated serum parathyroid hormone concentration in eucalcemic patients after parathyroidectomy for primary hyperparathyroidism and its relationship to vitamin D profile. Metabolism. 2004;53:1101–1106. doi: 10.1016/j.metabol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Ning L, Sippel R, Schaefer S, Chen H. What is the clinical significance of an elevated parathyroid hormone level after curative surgery for primary hyperparathyroidism? Ann Surg. 2009;249:469–472. doi: 10.1097/SLA.0b013e31819a6ded. [DOI] [PubMed] [Google Scholar]
- 10.Carty SE, Roberts MM, Virji MA, Haywood L, Yim JH. Elevated serum parathormone level after “concise parathyroidectomy” for primary sporadic hyperparathyroidism. Surgery. 2002;132:1086–1093. doi: 10.1067/msy.2002.128479. [DOI] [PubMed] [Google Scholar]
- 11.Wang TS, Ostrower ST, Heller KS. Persistently elevated parathyroid hormone levels after parathyroid surgery. Surgery. 2005;138:1130–1136. doi: 10.1016/j.surg.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Solorzano CC, Mendez W, Lew JI, Rodgers SE, Montano R, Carneiro-Pla DM, et al. Long-term outcome of patients with elevated parathyroid hormone levels after successful parathyroidectomy for sporadic hyperparathyroidism. Arch Surg. 2008;143:659–663. doi: 10.1001/archsurg.143.7.659. [DOI] [PubMed] [Google Scholar]
- 13.Lang BH, Lo CY. Vitamin D3 deficiency is associated with late-onset hypocalcemia after minimally invasive parathyroidectomy in a vitamin D borderline area. World J Surg. 2010;34:1350–1355. doi: 10.1007/s00268-009-0377-8. [DOI] [PubMed] [Google Scholar]
- 14.Mittendorf EA, McHenry CR. Persistent parathyroid hormone elevation following curative parathyroidectomy for primary hyperparathyroidism. Arch Otolaryngol Head Neck Surg. 2002;128:275–279. doi: 10.1001/archotol.128.3.275. [DOI] [PubMed] [Google Scholar]
- 15.Beyer TD, Solorzano CC, Prinz RA, Babu A, Nilubol N, Patel S. Oral vitamin D supplementation reduces the incidence of eucalcemic PTH elevation after surgery for primary hyperparathyroidism. Surgery. 2007;141:777–783. doi: 10.1016/j.surg.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Mazzaglia PJ, Milas M, Berber E, Siperstein A, Monchik JM. Normalization of 2-week postoperative parathyroid hormone values in patients with primary hyperparathyroidism: four-gland exploration compared to focused-approach surgery. World J Surg. 2010;34:1318–1324. doi: 10.1007/s00268-010-0557-6. [DOI] [PubMed] [Google Scholar]
- 17.Denizot A, Pucini M, Chagnaud C, Botti G, Henry JF. Normocalcemia with elevated parathyroid hormone levels after surgical treatment of primary hyperparathyroidism. Am J Surg. 2001;182:15–19. doi: 10.1016/S0002-9610(01)00664-X. [DOI] [PubMed] [Google Scholar]
- 18.Mizrachi A, Gilat H, Bachar G, Feinmesser R, Shpitzer T. Elevated parathyroid hormone levels after parathyroidectomy for primary hyperparathyroidism. Head Neck. 2009;31:1456–1460. doi: 10.1002/hed.21119. [DOI] [PubMed] [Google Scholar]
- 19.Redman C, Bodenner D, Stack B. Role of vitamin D deficiency in continued hyperparathyroidism following parathyroidectomy. Head Neck. 2009;31:1164–1167. doi: 10.1002/hed.21082. [DOI] [PubMed] [Google Scholar]
- 20.Untch BR, Barfield ME, Dar M, Dixit D, Leight GS, Olson JA. Impact of 25-hydroxyvitamin D deficiency on perioperative parathyroid hormone kinetics and results in patients with primary hyperparathyroidism. Surgery. 2007;142:1022–1026. doi: 10.1016/j.surg.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 21.Grubbs EG, Rafeeq S, Jimenez C, Feng L, Lee JE, Evans DB, et al. Preoperative vitamin D replacement therapy in primary hyperparathyroidism: safe and beneficial? Surgery. 2008;144:852–859. doi: 10.1016/j.surg.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Westerdahl J, Valdemarsson S, Lindblom P, Bergenfelz A. Postoperative elevated serum levels of intact parathyroid hormone after surgery for parathyroid adenoma: sign of bone mineralization and decreased calcium absorption. World J Surg. 2000;24:1323–1329. doi: 10.1007/s002680010219. [DOI] [PubMed] [Google Scholar]
- 23.Ambrogini E, Cetani F, Cianferotti L, Vignali E, Banti C, Viccica G, et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective randomized clinical trial. J Clin Endocrinol Metab. 2007;92:3114–3121. doi: 10.1210/jc.2007-0219. [DOI] [PubMed] [Google Scholar]
- 24.Souberbielle JC, Roth H, Fouque DP. Parathyroid hormone measurement in CKD. Kidney Int. 2010;77:93–100. doi: 10.1038/ki.2009.374. [DOI] [PubMed] [Google Scholar]
- 25.Siperstein A, Berber E, Barbosa GF, Tsinberg M, Greene AB, Mitchell J, et al. Predicting the success of limited exploration for primary hyperparathyroidism using ultrasound, sestamibi, and intraoperative parathyroid hormone: analysis of 1158 cases. Ann Surg. 2008;248:420–428. doi: 10.1097/SLA.0b013e3181859f71. [DOI] [PubMed] [Google Scholar]