Abstract
We report a case of primary malignant melanoma (MM) of the stomach. The patient, a 73-year-old man, was referred to our hospital for investigation of an elevated lesion in the stomach, detected by gastroscopy. On admission, physical examinations and laboratory data were unremarkable. Gastroscopy revealed a pigmented, elevated tumor, approximately 2 cm in diameter, in the posterior wall of the stomach. A biopsy was taken, which resulted in a diagnosis of MM, based on the presence of melanin in tumor cells. F-18 fluorodeoxyglucose positron emission tomography showed no accumulation of tracer except for the tumor in the stomach, indicating that it was a primary MM of the stomach. The patient underwent distal gastrectomy, but died of recurrence 1 year later. Very few cases of primary MM of the stomach have been reported. Thus, we report this case, followed by a review of the literature.
Keywords: Malignant melanoma, Primary lesion, Stomach
Introduction
Occasionally, ectopic neoplasms arise in the gastrointestinal (GI) tract [1]. Malignant melanoma (MM) usually arises from typical sites where there are melanocytes: namely, the skin, eyes, meninges, and anal region. While it is found occasionally in the GI tract, the vast majority of GI melanomas are metastases from a cutaneous primary tumor [2]. In fact, clinical GI tract involvement secondary to cutaneous melanoma has been reported in up to 4% of living patients and up to 60% at autopsy [3, 4]. Conversely, primary MM of the GI tract, particularly in the stomach, is extremely rare, although sporadic cases have been reported [5–14]. We present another case of primary MM of the stomach, which to our knowledge, is the first documented case of a primary MM of the stomach, from Japan.
Case report
A 73-year-old man was referred to our hospital after gastroscopy had shown an elevated lesion in the posterior wall of the stomach. On admission, he appeared in good health, without peripheral adenopathy, and laboratory data, including serum lactate dehydrogenase (LDH), were all within normal limits. Gastroscopy showed a pigmented, elevated lesion, approximately 2 cm in diameter, in the posterior wall of the stomach (Fig. 1). A biopsy was performed and histologic examination revealed sheet-like malignant cells with large nuclei and eosinophilic cytoplasms containing dark brown pigment (Fig. 2a). Immunohistochemically, the tumor cells were positive for S-100 proteins (Fig. 2b) and HMB-45 antibodies (Fig. 2c), and negative for pan-cytokeratin antibodies (AE1/AE3) and leukocyte common antigen. Based on these findings, we diagnosed MM. Ophthalmologic, dermatologic, and oral examinations were negative, as were computed tomography of the chest and anoscopy. Furthermore, F-18 fluorodeoxyglucose positron emission tomography (FDG-PET) showed no accumulation of tracer, except in the tumor of the stomach (Fig. 3). Therefore, we performed distal gastrectomy for assumed primary MM of the stomach without metastases. The resected specimen contained a brown-pigmented fungiform tumor, 2 cm in diameter, in the posterior wall of the stomach (Fig. 4). Postoperative histological and immunohistochemical examinations confirmed the diagnosis. Tumor cells were spreading through the submucosal layer of the stomach, but the resection margins were clear of tumor. Six of 27 resected lymph nodes were positive for metastases. The patient was discharged from our hospital after an uneventful recovery, and was followed up at another hospital. He was readmitted to our hospital 9 months later with abdominal pain, general fatigue, and anorexia. A subcutaneous tumor in his back was resected and pathological examination revealed a metastasis of MM (Fig. 5). Computed tomography showed ascites and pleural effusions, but cytological examinations of the fluid were negative. He became extremely cachectic and died approximately 2 months later; 1 year after the gastrectomy.
Fig. 1.
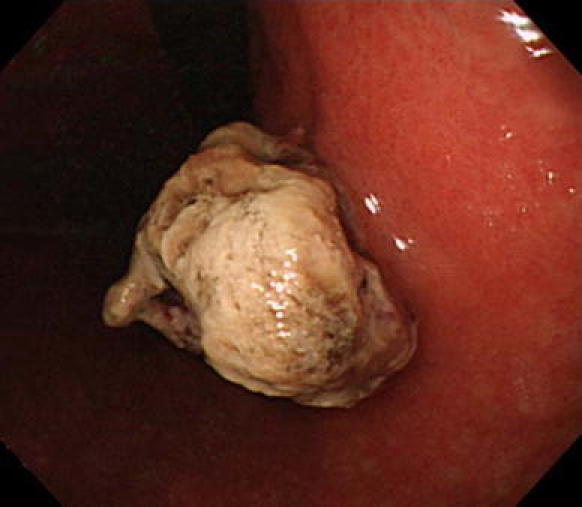
Endoscopic examination showed a pigmented elevated lesion in the posterior wall of the stomach
Fig. 2.
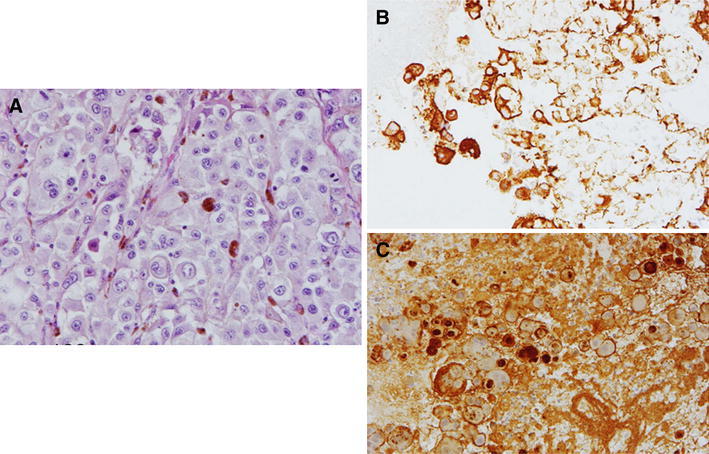
a Histology showed sheet-like malignant cells with large nuclei and eosinophilic cytoplasms containing dark brown pigment. b Tumor cells were positive for HMB-45 antibody. c Tumor cells were also positive for S-100 protein. a H&E, ×400; b and c immunohistochemistry, ×200
Fig. 3.
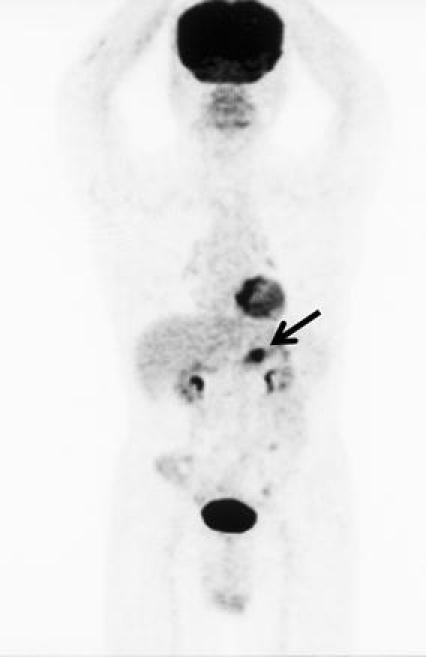
FDG-PET showed accumulation of tracer in the gastric tumor (arrow). No accumulation was observed in any other site
Fig. 4.
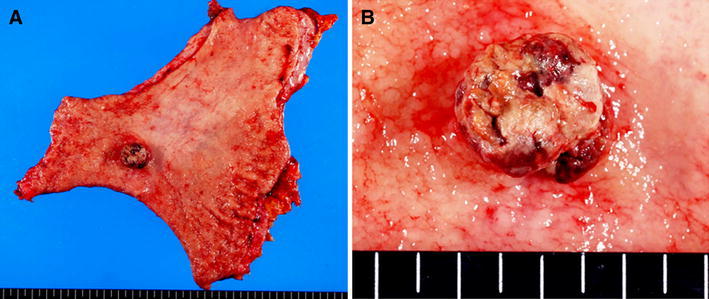
Surgical specimen from the distal gastrectomy. a There was a tumor in the posterior wall of the stomach. b The tumor was 2.0 × 1.9 × 0.9 cm in size
Fig. 5.
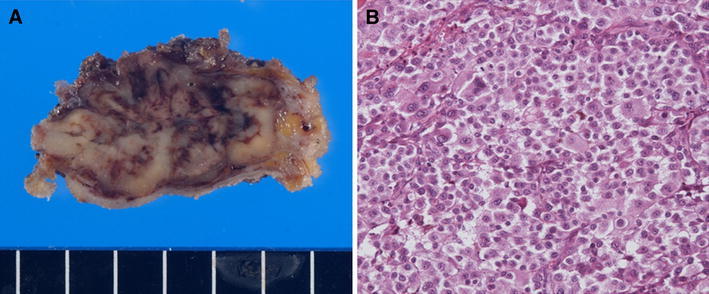
a Resected subcutaneous tumor. b Histological examination revealed sheet-like malignant cells similar to those in the tumor of the stomach. H&E, ×200
Discussion
Primary MM of the stomach is extremely rare. Since normal stomach epithelium lacks melanocytes, the cell of origin remains obscure, although possible etiologies of primary MM have been suggested. For example, ectopic migration of melanocyte precursors or differentiation of the APUD cells (amine precursor uptake and decarboxylation cells) to melanocytes has been suggested as a possible mechanism of the development of MM [8, 15, 16]. Criteria for the diagnosis of primary MM include the absence of other primary site melanomas and no history of the removal of a melanoma or atypical melanocytic lesion from the skin or other organs [5, 17]. It is recognized that spontaneous regression of primary melanoma occasionally occurs [5, 7, 18, 19]. Moreover, metastatic melanoma of the GI tract is found in up to 60% of autopsies of patients who have died with primary cutaneous melanoma [3, 4]. Therefore, it is important to be aware of the danger of misinterpreting metastasis to the gastric wall. Our patient had no remarkable history and FDG-PET showed no accumulation of tracer, other than the tumor in the stomach. Therefore, occult primary MM in another site seems highly unlikely. A definitive diagnosis of MM was made by immunohistological examination of harvested tissues. Since melanocytes are very sensitive to S-100 proteins and HMB-45 antibodies, immunohistochemical staining with those pigments is important to confirm the presence of melanocytes in a tumor [5, 7, 20]. Immunohistochemical examination of the tumor from our patient revealed a positive reaction with S-100 proteins and HMB-45 antibodies, which led us to assume a primary MM of the stomach. MMs that arise in mucosal surfaces appear to be more aggressive and associated with a worser prognosis than cutaneous MMs. This poorer prognosis may be related to late diagnosis, an inherently more aggressive behavior of mucosal MM, or earlier dissemination because of the rich lymphatic and vascular supply of GI tract mucosa [5, 7, 8, 10]. It has been reported that the median overall survival associated with primary MM of the GI tract is 17 months, whereas that associated with primary MM of the stomach is only 5 months [21]. Although there are no widely accepted treatment protocols for primary MM of the GI tract, surgical resection is the only identifiable treatment modality for which independent predictive prognostic values have been demonstrated [21]. It was also reported that age >70 years, stage (localized or distant), tumor location, and lymph node involvement are independently predictive of the prognosis of patients with primary MM of the GI tract [21]. The factors of age, tumor location, and lymph node involvement were applicable to our patient. On the other hand, some serum substances are known tumor markers of cutaneous MM. Although there are few markers that are useful for early detection of MM [22], some markers such as LDH, S-100B protein, neuron-specific enolase, and 5-S-cysteinyldopa are recognized as significant prognostic factors [23, 24]. We examined only serum LDH levels, which were within normal limits in our patient. To our knowledge, there are no reports on tumor markers of primary MM of the GI tract. Therefore, clinical studies are required to identify markers that could be used for the early detection or prognosis of MM of the GI tract. A number of randomized controlled trials (RCTs) have been conducted to evaluate the efficacy of chemotherapy, non-specific immune stimulants such as bacillus Calmette–Guerin, Corynebacterium parvum, levamisole, or combinations of these agents with dacarbazine chemotherapy [25], but none of these therapies is recognized as efficient. However, the use of interferon alpha (INF-α) in the adjuvant setting has been approved recently for prolonging disease-free survival in patients with high-risk cutaneous MM [26]. Although RCTs have not shown their significant effect on overall survival, a meta-analysis of the results of 14 RCTs indicated that IFN-α adjuvant treatment achieved significant improvement in both the disease-free survival and the overall survival of patients with high-risk cutaneous MM [27]. It is possible that the prognosis of our patient could have been improved if we had given him adjuvant therapy after gastrectomy. Future studies are required to assess whether adjuvant therapies using agents such as INF-α can prolong the survival of patients with MM of the GI tract.
Conflict of interest
None of the authors have a conflict of interest to disclose.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Song SE, Lee CH, Kim KA, Lee HJ, Park CM. Malignant glomus tumor of the stomach with multiorgan metastases: report of a case. Surg Today. 2010;40(7):662–667. doi: 10.1007/s00595-008-4113-z. [DOI] [PubMed] [Google Scholar]
- 2.Reintgen DS, Thompson W, Garbutt J, Seigler HF. Radiologic, endoscopic, and surgical considerations of melanoma metastatic to the gastrointestinal tract. Surgery. 1984;95:635–639. [PubMed] [Google Scholar]
- 3.Liang KV, Sanderson SO, Nowakowski GS, Arora AS. Metastatic malignant melanoma of the gastrointestinal tract. Mayo Clin Proc. 2006;81:511–516. doi: 10.4065/81.4.511. [DOI] [PubMed] [Google Scholar]
- 4.Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-X. [DOI] [PubMed] [Google Scholar]
- 5.Alazmi WM, Nehme OS, Regalado JJ, Rogers AI. Primary gastric melanoma presenting as a nonhealing ulcer. Gastrointest Endosc. 2003;57:431–433. doi: 10.1067/mge.2003.120. [DOI] [PubMed] [Google Scholar]
- 6.Germano D, Rosati G, Romano R, Vita G, Lepore G, Sanctis D, et al. Primary gastric melanoma presenting as a double ulcer. J Clin Gastroenterol. 2004;38:828. doi: 10.1097/01.mcg.0000139030.08074.da. [DOI] [PubMed] [Google Scholar]
- 7.Jelincic Z, Jakic-Razumovic J, Petrovic I, Cavcic AM, Unusic J, Trotic R. Primary malignant melanoma of the stomach. Tumori. 2005;91:201–203. doi: 10.1177/030089160509100219. [DOI] [PubMed] [Google Scholar]
- 8.Lagoudianakis EE, Genetzakis M, Tsekouras DK, Papadima A, Kafiri G, Toutouzas K, et al. Primary gastric melanoma: a case report. World J Gastroenterol. 2006;12:4425–4427. doi: 10.3748/wjg.v12.i27.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan-Mou Yang J, Krishna GS, Macleod C, Oosthuysen W. Primary gastric mucosal melanoma. NZ Med J. 2008;121:96–99. [PubMed] [Google Scholar]
- 10.Castro C, Khan Y, Awasum M, Belostocki K, Rosenblum G, Belilos E, et al. Case report: primary gastric melanoma in a patient with dermatomyositis. Am J Med Sci. 2008;336:282–284. doi: 10.1097/MAJ.0b013e318159cc21. [DOI] [PubMed] [Google Scholar]
- 11.Doran H, Patrascu T, Catrina E, Mihalache O, Degeratu D, Predescu G. Gastric melanoma—clinical case. Chirurgia (Bucur) 2009;104:641–644. [PubMed] [Google Scholar]
- 12.Macak J. Melanoma of the stomach: reality or fiction? Pathologica. 1998;90:388–390. [PubMed] [Google Scholar]
- 13.Rao GM, Satyanarayana Y, Janaki M, Hayath MS. Primary melanocarcinoma of stomach. Indian J Gastroenterol. 1999;18:176. [PubMed] [Google Scholar]
- 14.Stringer P, Pike C. Gastric melanoma. Br J Surg. 1954;41:425–426. doi: 10.1002/bjs.18004116824. [DOI] [PubMed] [Google Scholar]
- 15.Tabaie HA, Citta RJ, Gallo L, Biondi RJ, Meoli FG, Silverman D. Primary malignant melanoma of the small intestine: report of a case and discussion of the APUD cell concept. J Am Osteopath Assoc. 1984;83:374–377. [PubMed] [Google Scholar]
- 16.Krausz MM, Ariel I, Behar AJ. Primary malignant melanoma of the small intestine and the APUD cell concept. J Surg Oncol. 1978;10:283–288. doi: 10.1002/jso.2930100402. [DOI] [PubMed] [Google Scholar]
- 17.Christova S, Meinhard K, Mihailov I, Alexiev B. Three cases of primary malignant melanoma of the alimentary tract. Gen Diagn Pathol. 1996;142:63–67. [PubMed] [Google Scholar]
- 18.Ducic Y. Spontaneous regression of cutaneous melanoma with subsequent metastasis. J Oral Maxillofac Surg. 2002;60:588–591. doi: 10.1053/joms.2002.31862. [DOI] [PubMed] [Google Scholar]
- 19.Printz C. Spontaneous regression of melanoma may offer insight into cancer immunology. J Natl Cancer Inst. 2001;93:1047–1048. doi: 10.1093/jnci/93.14.1047. [DOI] [PubMed] [Google Scholar]
- 20.Schuchter LM, Green R, Fraker D. Primary and metastatic diseases in malignant melanoma of the gastrointestinal tract. Curr Opin Oncol. 2000;12:181–185. doi: 10.1097/00001622-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Cheung MC, Perez EA, Molina MA, Jin X, Gutierres JC, Franceschi D, et al. Defining the role of surgery for primary gastrointestinal tract melanoma. J Gastrointest Surg. 2008;12:731–738. doi: 10.1007/s11605-007-0417-3. [DOI] [PubMed] [Google Scholar]
- 22.Brochez L, Naeyaert JM. Serological markers for melanoma. Br J Dermatol. 2000;143:256–268. doi: 10.1046/j.1365-2133.2000.03649.x. [DOI] [PubMed] [Google Scholar]
- 23.Wibe E, Hannisdal E, Paus E, Aamdal S. Neuron-specific enolase as a prognostic factor in metastatic malignant melanoma. Eur J Cancer. 1992;28A:1692–1695. doi: 10.1016/0959-8049(92)90070-I. [DOI] [PubMed] [Google Scholar]
- 24.Banfalvi T, Boldizsar M, Gergye M, Gilde K, Kremmer T, Otto S. Comparison of prognostic significance of serum 5-S-cysteinyldopa, LDH and S-100B protein in stage III–IV malignant melanoma. Pathol Oncol Res. 2002;8:183–187. doi: 10.1007/BF03032392. [DOI] [PubMed] [Google Scholar]
- 25.Eggermont AM, Testori A, Marsden J, Hersey P, Quirt I, Petrella T, et al. Utility of adjuvant systemic therapy in melanoma. Ann Oncol. 2009;20(Suppl 6):vi30–vi34. doi: 10.1093/annonc/mdp250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirkwood JM, Tarhini AA, Moschos SJ, Panelli MC. Adjuvant therapy with high-dose interferon alpha 2b in patients with high-risk stage IIB/III melanoma. Nat Clin Pract Oncol. 2008;5:2–3. doi: 10.1038/ncponc1004. [DOI] [PubMed] [Google Scholar]
- 27.Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102:493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]


